Abstract
Cone photoreceptors of the vertebrate retina terminate their response to light much faster than rod photoreceptors. However, the molecular mechanisms underlying this rapid response termination in cones are poorly understood. The experiments presented here tested two related hypotheses: first, that the rapid decay rate of metarhodopsin (Meta) II in red-sensitive cones depends on interactions between the 9-methyl group of retinal and the opsin part of the pigment molecule, and second, that rapid Meta II decay is critical for rapid recovery from saturation of red-sensitive cones after exposure to bright light. Microspectrophotometric measurements of pigment photolysis, microfluorometric measurements of retinol production, and single-cell electrophysiological recordings of flash responses of salamander cones were performed to test these hypotheses. In all cases, cones were bleached and their visual pigment was regenerated with either 11-cis retinal or with 11-cis 9-demethyl retinal, an analogue of retinal lacking the 9-methyl group. Meta II decay was four to five times slower and subsequent retinol production was three to four times slower in red-sensitive cones lacking the 9-methyl group of retinal. This was accompanied by a significant slowing of the recovery from saturation in cones lacking the 9-methyl group after exposure to bright (>0.1% visual pigment photoactivated) but not dim light. A mathematical model of the turn-off process of phototransduction revealed that the slower recovery of photoresponse can be explained by slower Meta decay of 9-demethyl visual pigment. These results demonstrate that the 9-methyl group of retinal is required for steric chromophore–opsin interactions that favor both the rapid decay of Meta II and the rapid response recovery after exposure to bright light in red-sensitive cones.
INTRODUCTION
A remarkable feature of our visual system is the ability to resolve changes in light intensity that span more than 12 orders of magnitude. The upper end of this range is mediated by cone photoreceptors, which can report fast changes in ambient illumination and come in several types that are sensitive to different parts of the wavelength spectrum. A key requirement for cones to encode this wide range of light intensities is their ability to adapt sensitivity based both on the background light level (light adaptation) and on the extent of bleaching of their visual pigment (bleaching adaptation).
Both light adaptation and bleaching adaptation allow the cones to reduce their sensitivity as the background light intensity increases, the goal of which is to prevent response saturation and extend the operating range of the cell (for review see Fain et al., 2001 and Burns and Arshavsky, 2005). Light adaptation is subserved by mechanisms that are mediated by intracellular Ca2+ and counteract the effect of steady light by reducing the rate of cGMP hydrolysis and accelerating the rate of its synthesis. Brighter background lights that cause a significant bleaching of the visual pigment can also induce bleaching adaptation, which like background adaptation results in reduced sensitivity and speeded response time course, and occurs by residual activation of phototransduction by bleached pigment (Cornwall and Fain, 1994; Leibrock et al., 1998). Under these conditions, the recovery of sensitivity ultimately requires the regeneration of the visual pigment, a process called dark adaptation (for review see Lamb and Pugh, 2004).
Pigment regeneration takes place via a series of reactions collectively referred to as the visual cycle, which in cones has been recently shown to occur through reciprocal interactions with the Müller cells (Mata et al., 2002; Wang et al., 2009) as well as the retinal pigment epithelium (Wang et al., 2009). In this process, the all-trans retinol (ROL) generated during the bleaching of the cone visual pigment is transported to both cell types. In Müller cells, ROL is reconverted to 11-cis retinol and sent back to the cones, which first oxidize it and then use it to regenerate pigment. In the retinal pigment epithelium, 11-cis retinal is produced directly, which after translocation of receptors, is used to regenerate visual pigment. Key to the recovery of sensitivity is ultimately the regeneration of the pigment, a process that can take minutes (Lamb and Pugh, 2004); however, cones are known to recover fully their circulating current and thus responsiveness after exposure to bleaching light on a much faster time scale (20–100 ms) (Kenkre et al., 2005). It should be noted that this time is substantially faster than the 20-min recovery time for rod photoreceptors measured under similar light conditions (Thomas and Lamb, 1999). We sought to understand mechanistically how the fast recovery of the cone-circulating current occurs before the pigment is regenerated.
It should be noticed that the speed of recovery, as one indication of dark adaptation, can be characterized by various parameters. Thus, faster recovery may mean shorter time in saturation measured between the moment of strong bleaching flash and first sign of recovery of circulating current. A closely related but not identical measure is the steepness of recovery of preflash “dark” current after the saturation ceased. Finally, full recovery demands complete restoration of dark-adapted sensitivity, the process that can take a substantially longer time than the restoration of the dark current (Krispel et al., 2003). In the present study, we use the term “accelerated recovery” to designate both shortening time in saturation and steeper restoration of the circulating current (recovery of response amplitude to saturating flashes).
Recently, we examined the role of cone visual pigment structure in recovery of response amplitude of red-sensitive cones (hereafter referred to as red cones) exposed to bright light (Estevez et al., 2006). In this study, we observed that red cones regenerated with 11-cis 9-demethyl (9-DM) retinal, an analogue of retinal lacking the 9-methyl group, were up to 10 times slower to recover their circulating current after intense light exposure compared with cones containing native visual pigment. This correlated with slower recovery of steady-state guanylyl cyclase rates in these cells, suggesting that slow response recovery was due to sluggish termination of phototransduction activation. We concluded that visual pigment structure, particularly the steric interaction of red cone opsin with the 9-methyl group of retinal, plays an important role in ensuring the rapid response recovery after bright light exposure. However, the specific mechanisms of that role remained unresolved.
Early biochemical studies that examined the effect of the 9-methyl group of retinal on opsin had suggested that this group may play a steric role in the efficient hydrolysis of the Schiff base linkage between retinal and red cone opsin (metarhodopsin [Meta] II decay), followed by the efficient release of all-trans retinal from the opsin binding pocket (Das et al., 2004). Our results were consistent with this hypothesis; however, no direct measurements of Meta II decay in intact cone photoreceptors containing 9-demethyl–based visual pigment had yet been made. The experiments presented here were designed to directly test this hypothesis. Accordingly, we have made microspectrophotometric measurements of the rates of visual pigment photolysis as well as the time course of ROL formation in isolated salamander red cones containing normal visual pigment or pigment lacking the 9-methyl group of retinal. Direct measurements of the reduction of all-trans retinal to ROL by retinol dehydrogenase were also made by single-cell microfluorometry. All of these results were correlated with single-cell electrophysiological measurements of flash response kinetics and the time course of recovery of circulating current in darkness after bleaching exposure. These results have been fitted with a kinetic model of quenching of the phototransduction cascade. Accordingly, we conclude that the 9-methyl group of retinal is critical for rapid Meta II decay as well as rapid flash response recovery in salamander red cone photoreceptors.
MATERIALS AND METHODS
Animals and preparation
All experiments were performed on red cone photoreceptors isolated from the retinae of larval salamanders. Two different species of salamanders were used in this study: Ambystoma tigrinum and Ambystoma mexicanum. Physiological, microspectrophotometric, and microfluorometric experiments on cones from both species were performed in the laboratory at Boston University School of Medicine. Microspectrophotometric experiments conducted in the laboratory at The Russian Academy of Sciences in St. Petersburg, Russia, were performed only on Amybstoma mexicanum cones, as only that species was available. When data from a single species are illustrated or discussed, appropriate notation is provided in the text or figure legends. Data derived from experiments on both species were compared to ensure that species differences did not introduce confounding variables. Ambystoma tigrinum were purchased from Charles D. Sullivan, Inc. Ambystoma mexicanum used for experiments in the St. Petersburg laboratory were purchased from a local breeder; those used for experiments in Boston were purchased from the Ambystoma Genetic Stock Center. All experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine and in accordance with the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act.
Salamanders were dark-adapted overnight, killed by decapitation, and double-pithed under dim red illumination. Eyes were enucleated and hemisected in physiological solution. These as well as all subsequent manipulations were viewed under infrared illumination. The physiological solution used for all dissections and subsequent measurements contained (in mM): 110 NaCl, 2.5 KCl, 1.6 MgCl2, 1.0 CaCl2, 10 dextrose, 10 HEPES, and 100 mg/l bovine serum albumin. The pH was adjusted to 7.8. Retinae were separated from the retinal pigment epithelium by dissection with fine forceps, and photoreceptors were isolated by chopping the retina with a small piece of razor blade. The resulting suspension containing intact cells was transferred to a chamber mounted on a microscope stage designed for microspectrophotometric, electrophysiological, or microfluorometric experiments. Individual cells were viewed using infrared-sensitive CCD cameras connected to the microscopes. All experiments were performed at 20°C. The methods are similar to those described previously (Cornwall et al., 1983, 1990, 2000; Ala-Laurila et al., 2006; Estevez et al., 2006).
Pigment regeneration
The native visual pigment chromophore contained in dark-adapted cones of both species is a mixture of 11-cis retinal (A1) and 11-cis-3,4-dehydroretinal (A2). This native pigment was removed by exposure to bright light (bleaching) and replaced with an exogenous chromophore, either 11-cis retinal or 9-DM retinal. 11-cis retinal and the 9-DM analogue used in these studies were exclusively in the A1 form. These were synthesized and purified in the Department of Ophthalmology at the Medical University of South Carolina (Crouch et al., 2002). Retinoid solutions were prepared by dissolving 50 µg retinoid in 3 µl ethanol and diluting in 3 ml of physiological solution to create a working concentration of 30–50 µM retinoid in solution containing <0.3% ethanol as a retinoid carrier. This retinoid solution was added directly to the bath containing bleach-adapted cells by application with a pipetter. The final concentration of retinoid in the bath was 18–30 µM, and cells were exposed to this solution for ∼5–8 min before washing with normal physiological solution.
The preparation was monitored for ∼20 min before subsequent microspectrophotometric, electrophysiological, or microfluorometric experiments were performed. Finally, after this 20-min period, the cell was tested to ensure a fully recovered steady state of regeneration by measuring flash response sensitivity or absorbance spectra. The rates of chromophore binding and pigment regeneration by 11-cis retinal and 9-DM retinal have been shown previously to be roughly the same (Kono, M., personal communication). This technique is similar to methods described previously (Jin et al., 1993; Kefalov et al., 1999; Corson et al., 2000; Crouch et al., 2002; Estevez et al., 2006). In experiments during which multiple visual pigment regenerations were performed on a single cell, interphotoreceptor retinoid binding protein (IRBP) was added to the superfusate to facilitate chromophore exchange. IRBP was provided by B. Wiggert (National Eye Institute, National Institutes of Health, Bethesda, MD).
The analysis of spectral data from cones in the dark-adapted condition (native visual pigment) is complicated by the varying ratio of two visual pigment chromophores (A1 and A2 retinal), which varies seasonally and from animal to animal among larval salamanders but can be determined by fitting the dark-adapted spectra with a combination of A1- and A2-based visual pigment templates (Govardovskii et al., 2000). Because of this A1/A2 mixture in native dark-adapted pigment, experiments throughout the entire study were designed to mostly compare cones bleached and regenerated with 9-DM retinal to cones bleached and regenerated with 11-cis retinal. However, often data from native cones are also illustrated for comparison (e.g., see Figs. 1–5).
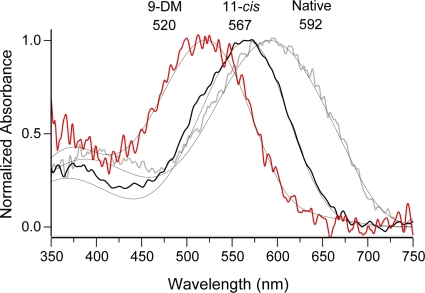
Figure 1.
Normalized absorbance spectra of Ambystoma mexicanum red cones containing different visual pigment chromophores. Averaged T-spectra of red cones in their native dark-adapted state (containing a mixture of A1 and A2 retinal [gray curve]; λmax = 592 nm; n = 11), red cones regenerated with 11-cis retinal (11-cis [black curve]; λmax = 567 nm; n = 11), or red cones regenerated with 9-DM retinal (red curve; λmax = 520 nm; n = 9). Smooth lines show corresponding template fittings. Native pigment is best approximated with a 30/70% mixture of A1/A2 chromophore (λmax = 567 and 615 nm, respectively). 11-cis–regenerated pigment is pure 567 nm A1. 9-DM spectrum can be represented by the sum of 93% 520-nm 11-cis A2 pigment and 7% of native 11-cis A2, 615 nm chromophore.
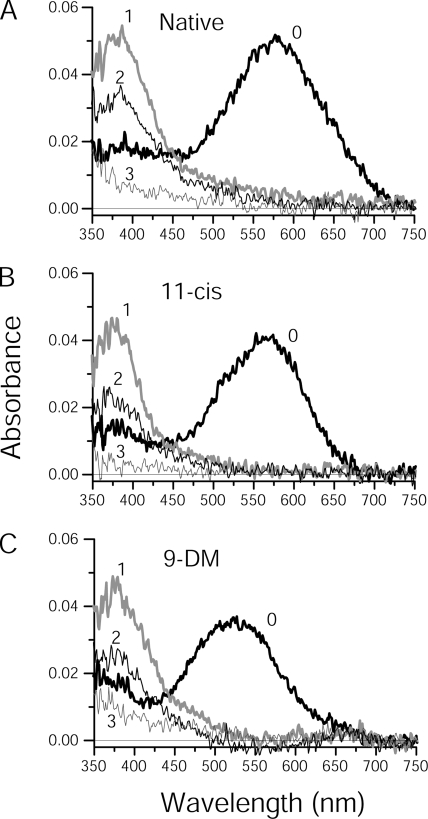
Figure 2.
Series of post-bleach spectra of Ambystoma mexicanum red cones containing different visual pigment chromophores. Spectra were recorded at two polarizations (only T-polarizations are shown) and averaged from native red cones (A; n = 11), or red cones regenerated with either 11-cis retinal (B; n = 11) or 9-DM retinal (C; n = 9). After recording the dark spectra (curves labeled 0 in each case), a 1-s 525-nm flash was applied and post-bleach spectra were recorded immediately after the bleach (curves labeled 1 in each case) and at various time increments thereafter. Curves 2 represent recordings at either 5 s (A and B) or at 30 s (C) post-bleach and illustrate the approximate half-maximum MI + MII absorbance (peak at 380 nm). Curves 3 were recorded at 600 s post-bleach in all cases. Intermediate spectra recorded at 10, 20, 60, 100, 200, and 300 s post-bleach are excluded to avoid clutter.
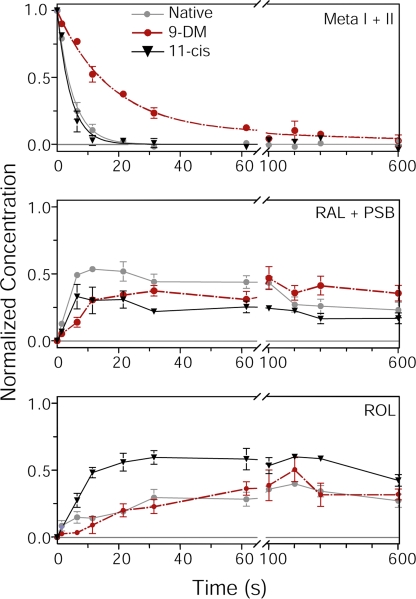
Figure 3.
The time course of bleaching products in Ambystoma mexicanum red cones containing different visual pigments as assessed by microspectrophotometry. The time course of metapigment decay (top; Meta I + II), the appearance of all-trans retinal and its protonated Schiff-bases (middle; RAL + PSB), and the formation of ROL (bottom) are plotted. Concentrations of bleaching products are expressed as a fraction of bleached visual pigment. Measurements were made from native red cones (gray; n = 11), or red cones regenerated either with 11-cis retinal (black; n = 11) or 9-DM retinal (red; n = 9). All curves were generated from the spectra shown in Fig. 2. The data in the top panel were fitted with a single-exponential decay function in the case of native and 11-cis pigments; however, a two-exponential approximation was required to fit the decay of 9-DM pigment. The data points in the middle and bottom panels were connected by straight lines. Note in the top panel, the time course of Meta decay in red cones containing 9-DM pigment (half-decay time τ1/2 = 14.1 s) is four to five times slower than in red cones containing native or 11-cis–regenerated pigment (τ1/2 = 3.5 and τ1/2 = 3 s, respectively). Error bars show ± SEM.
Figure 4.
The time course of retinol formation in salamander red cones containing different visual pigments as assessed by microfluorometry. The normalized relative fluorescence intensity is plotted as a function of time after a >95% bleach in red cones from Ambystoma mexicanum under different visual pigment conditions. Data were normalized relative to the peak value and fitted with a single-exponential function. Red cones contained their native A1/A2 mixture (gray; n = 6; τ = 17.5 ± 1.2 s), or were regenerated with either 11-cis retinal (black; n = 5; τ = 20.8 ± 0.9 s) or 9-DM retinal (red; n = 6; τ = 70.9 ± 6.7 s). Error bars show ± SEM. Note that between the first and second measurements in all three cases, there is a rapid initial increase in fluorescence that is present in all fluorescence recordings; this has been reported previously (Tsina et al., 2004; Ala-Laurila et al., 2006) and is of unknown origin. The inset illustrates an experiment in which the rise and fall of retinol fluorescence were measured in a single red cone from Ambystoma tigrinum under all three visual pigment conditions. First, the red cone was measured with its native pigment (gray squares), then containing 9-DM retinal visual pigment (red circles), and finally containing 11-cis retinal visual pigment (black triangles). 100 µM IRBP was present to facilitate the exchange of chromophore. Data are normalized relative to the peak value.
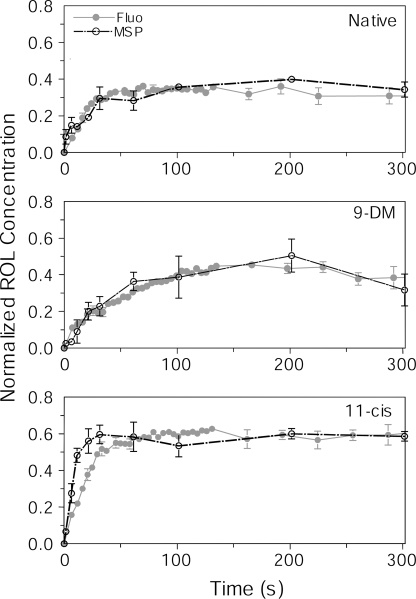
Figure 5.
A comparison of time courses of ROL production derived from microspectrophotometry versus microfluorometry. Data from Figs. 3 (bottom) and 4 have been replotted on the same graph for comparison. Data obtained by microspectrophotometry (MSP) are shown in black: n = 11 cells (native), 9 cells (9-DM), and 11 cells (11-cis). Fluorescence data are shown in gray: n = 6 cells (native), 6 cells (9-DM), and 5 cells (11-cis). Concentration of ROL in microspectrophotometry data are expressed as a fraction of bleached cone pigment. Fluorescence data are scaled for best visual match to microspectrophotometry. Error bars show ± SEM.
Microspectrophotometry
A high-speed dichroic microspectrophotometer was used to measure the time course of photolysis of visual pigment and ROL production in salamander red cones containing different visual pigments (Kolesnikov et al., 2003; Ala-Laurila et al., 2006). In brief, this instrument operates over the range of 330 to 800 nm and at two measuring beam polarizations: either in the transverse direction (orthogonal to the long axis of the outer segment, hereafter labeled T) or in the longitudinal direction (parallel to the long axis of the outer segment, hereafter labeled L). This allows post-bleach photoproducts to be distinguished from one another by their orientation in the plane of the photoreceptor disk membrane, because the absorbance of the photoproduct is maximal when the polarization plane of the measuring light beam matches the plane of the double-bond carbon chain of the chromophore. A single measurement, including the T and L scan, is completed within 1 s. Typical bleach is ∼0.5% per scan in both directions.
The specific protocol for microspectrophotometric measurements was as follows. First, absorbance measurements were made in the dark-adapted condition to characterize the spectral properties of the particular pigment to be tested (either native pigment or pigment that had been regenerated with 11-cis retinal or 9-DM retinal). Then, the cone was bleached with a 1-s flash from a 525-nm high-intensity light-emitting diode (LED; Marl International, Ltd.) that was estimated to bleach >90% of the pigment. Post-bleach spectra were recorded immediately after the flash, and then at the following intervals (in s): 5, 10, 20, 30, 60, 100, 200, 300, and 600. Next, a second exhaustive bleach was applied to determine the small fraction of unbleached and photoregenerated pigment remaining. After bleaching the photoreceptor, five distinct photoproducts were observed: Meta I and II, all-trans retinal and its nonspecific protonated Schiff bases (PSBs), and ROL. Meta III is not observed in salamander cones (Ala-Laurila et al., 2006; Golobokova and Govardovskii, 2006). Meta II and all-trans retinal both have absorbance peaks near 380 nm, but can be distinguished by their different orientations within the disk membrane (Meta II is predominately oriented in the disk plane and best observed in the T orientation, whereas all-trans retinal is oriented across the disk membrane and therefore best observed in the L orientation). PSBs have an absorbance maximum near 440 nm, are oriented across the disk plane, and are best observed in the L orientation. Finally, all-trans A1 retinol has an absorbance peak at 325 nm and is best observed in the L orientation. Therefore, the absorbances at three wavelengths chosen close to absorbance maxima of PSB and Meta I (450 nm), Meta II and all-trans retinal (380 nm) and retinol (340 nm), and two polarizations can be analyzed by a series of linear equations whose solutions at any moment of time yield the contribution of each photoproduct to the recorded spectra (Kolesnikov et al., 2003; Ala-Laurila et al., 2006). The absorbances can further be converted to concentrations normalized to bleached pigment by taking into account relative molar extinctions of the products. Meta I and II are in rapid equilibrium, and for kinetic computations can be considered as a single product, hereafter referred to as Meta (Ala-Laurila et al., 2006).
Electrophysiology
Extracellular membrane current recordings of salamander red cones were performed by methods that have been described previously (Cornwall et al., 1983, 2000). Protocols for light stimulation, identification of cell types, and measurements of visual pigment bleaching for electrophysiological experiments were identical to those reported previously (Estevez et al., 2006). Responses to test flashes were recorded at various light intensities, adjusted with neutral density filters. Dim flash responses were elicited with 20-ms flashes. For bleaching protocols (as in Fig. 6), a 500-ms flash was used to allow light exposures that bleached visual pigment over a broad range (0.001–45%). The wavelength of the stimulation light was 560 nm for red cones regenerated with 11-cis retinal and 520 nm for red cones regenerated with 9-DM retinal. These wavelengths are close to λmax of corresponding pigments (Fig. 1). For electrophysiological experiments, the fraction of visual pigment bleached was estimated according to the relation:
| (1) |
where F is the fraction of pigment bleached, I is the bleaching light intensity in photons µm−2s−1, P is the photosensitivity of cones at the wavelength of peak absorbance (µm2), and t is the time of light exposure (s). The photosensitivity determined for A2 visual pigment by Jones et al. (1993) (6.0 × 10−9 µm2) is assumed to be valid for the 9-DM retinal chromophore as well because both pigments have similar molar absorbances (30,000 M−1cm−1) (Estevez et al., 2006). A 33% higher photosensitivity was used for A1-regenerated visual pigment (molar absorbance of 40,000 M−1cm−1). Calculations of bleaching exposure made in this way have been directly compared and are in good agreement with direct measurement of the bleached pigment fraction in single salamander rods (Ala-Laurila et al., 2007).
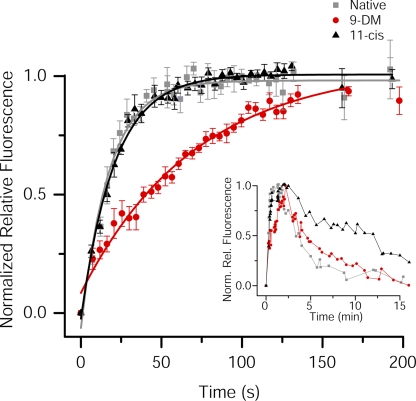
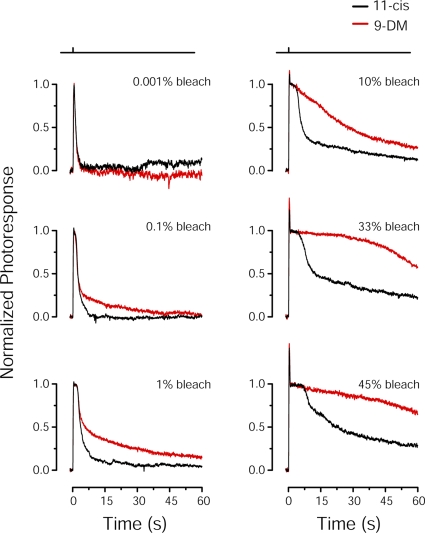
Figure 6.
Flash response properties of Ambystoma mexicanum red cones containing different visual pigments. Shown are comparisons of average normalized flash responses to 500-ms test flashes that bleached various pigment fractions (pigment fraction shown in top right corner of each panel) in red cones. Cones were regenerated with 11-cis retinal (black traces; n = 6) or 9-DM retinal (red traces; n = 4). All traces have been smoothed by the Savitsky-Golay method (5 points to left and right). A significant difference in the photoresponse recovery between red and black traces is first observed at 0.1% bleach.
Microfluorometry
The appearance of ROL in photoreceptor outer segments after reduction of all-trans retinal can be observed directly by measuring its intrinsic fluorescence emitted after bleaching with bright light (Tsina et al., 2004; Chen et al., 2005; Ala-Laurila et al., 2006). Measurements of this kind were made from the outer segments of isolated intact red cones of both Ambystoma tigrinum and Ambystoma mexicanum. The instrumentation and methodology for making these measurements have been described previously (Tsina et al., 2004; Chen et al., 2005). In brief, a 150-ms step of 360 nm light induced fluorescence of retinol from within the outer segment. This emitted fluorescence was collected by a microscope objective passed through a dichroic filter that transmits in the range of 465 to 550 nm and focused on the face of an image intensifier attached to a high-sensitivity digital CCD camera. The spatial distribution of fluorescence in the video image was measured before and at various times after exposure to bleaching light that was calculated to have bleached in excess of 95% of the visual pigment. This image was converted to pseudo color and used to evaluate the time course of fluorescence increase due to ROL formation as it was reduced from all-trans retinal.
RESULTS
Microspectrophotometric measurements of pigment photolysis
Fig. 1 compares average absorbance spectra of the native visual pigment of dark-adapted Ambystoma mexicanum red cone outer segments and spectra measured after exhaustive bleaching and regeneration with either 11-cis retinal or 9-DM retinal. The native dark-adapted spectrum had an absorbance maximum at 592 nm (Fig. 1, gray line). This represents a mixture of pigments, each composed of the same opsin, one containing the A1 form of 11-cis retinal and the other with the A2 form. The pure A1 form has a spectral maximum at 567 nm, and the A2 form has its maximum at 615 nm. Native dark-adapted spectra were fitted by a procedure that convolved the two pure pigment spectra into one with the measured spectral shape as described in Materials and methods. This procedure allowed an estimate of the proportion of each form; the average native spectrum shown represents a mixture of 0.33 A1/0.67 A2.
Bleaching and subsequent regeneration with 11-cis retinal yields the visual pigment whose absorbance spectrum (Fig. 1, black line) can well be fitted with the A1-based template with λmax = 567 nm. On the other hand, the 9-DM–based pigment (Fig. 1, red line) peaks at 520 nm, and the spectrum can be described with the A2-based 520-nm template plus a small (≈7%) admixture of the native A2 chromophore. This value is significantly blue-shifted with respect to 11-cis–based pigment, a finding consistent with previous studies (Das et al., 2004; Estevez et al., 2006). Variation in A1/A2 ratios among animals, and possible admixture of native chromophore(s) in regenerated pigment, may account for a small difference in λmax values of salamander red cone pigment reported here and elsewhere (Makino et al., 1999; Estevez et al., 2006).
After bleaching, all three pigments show the same sequence of photolysis products as illustrated in Fig. 2. Immediately after the bleaching flash (curves 1 in all panels), Meta (main peak at 370–380 nm) appears. Then, the Meta decays to all-trans retinal and opsin. The decay of Meta to approximately half its original value is represented in curves 2 of all panels and can be visualized by the reduced amplitude of the initial 380-nm peak. The all-trans retinal released from metaproducts is then converted to ROL, as indicated by the progressive short-wave shift of the absorbance maximum, finally to a peak below 350 nm (curves 3 in all panels; peak not shown). Both retinal and retinol can be followed more clearly with the spectra recorded at L-polarization (not depicted).
A remarkable feature of the photolysis of the 9-DM pigment is its substantially slower rate compared with both the A1 and A2 pigments containing methylated chromophores. Although the Meta peak in the native and the 11-cis retinal–regenerated pigments decays to a half of its original height at ∼5 s post-bleach, the half-decay time of the 9-DM metapigment is near 30 s (Fig. 2, curves 2 in all panels).
As explained in Materials and methods, recording spectra at two polarizations and reading absorbances at three wavelengths allow computation of the concentrations of all bleaching products. The time courses of formation and decay of Meta, retinal plus its PSB, and retinol are shown in Fig. 3. The decay of metaproducts in native cones and those with the 11-cis chromophore can satisfactorily be described with a single exponent having the time constant of 5.1 and 4.4 s, respectively (Fig. 3, top, gray and black curves, respectively). A two-exponential approximation was necessary for the decay of 9-DM metapigment, with time constants of 18 and 830 s, and amplitudes of 0.91 and 0.09 (Fig. 3, top, red curve). The half-decay times (τ1/2) were 3.5, 3.0, and 14 s for native, 11-cis–, and 9-DM–regenerated pigment, respectively. Collectively, these data show that the decay of metapigments (and hence retinal release from opsin) containing the 9-DM chromophore is roughly fivefold slower than with its methylated counterparts.
Microfluorometric measurements of ROL
A primary goal of the present study was to determine if the sluggish response recovery of red cones containing 9-DM visual pigment after their exposure to bright light is due to slowed hydrolysis of activated visual pigment. However, the decay of metaproducts may not be the sole factor that controls the recovery from saturation after extensive bleach. All-trans retinal released from the chromophore pocket may secondarily bind to opsin forming complexes that retain some residual activity (Buczylko et al., 1996; Jäger et al., 1996). Hence, the reduction of retinal to retinol may be important for further quenching of the light-activated visual pigment. Our microspectrophotometric data allow determining the time course of retinol production, yet the calculations need an analysis of a complex mixture of photoproducts based on absorbance measurements in the spectral range where the performance of the instrument is rather poor and effects of possible impurities can interfere with the measurement. To address this issue, we also used an alterative approach to measure the rate of retinol formation: microfluorometry. This method monitors the increase in intrinsic retinol fluorescence within bleached outer segments of isolated cones as all-trans retinal is reduced to ROL by retinol dehydrogenase, and then cleared from the outer segment (see Materials and methods). This method has the advantage that it does not rely on absorbance measurements of a mixture of bleached photoproducts, but rather on the production of ROL alone.
Microfluorometric experiments were performed on cones of both species. Fig. 4 (inset) shows an example of fluorescence production and clearance measured in a single red cone from Ambystoma tigrinum. First, the dark-adapted cone was drawn inner segment first into a pipette filled with the same physiological solution as the extracellular bath. The pipette was attached to a current amplifier to permit electrophysiological recording. The pipette served to stabilize mechanically the cell and permitted extracellular membrane current recordings of flash responses. Responses were recorded from a cell at two different wavelengths of light (440 vs. 620 nm) to establish that the cone was red sensitive (not depicted). A fluorescence measurement was recorded (150 ms exposure to 360 nm excitation light) to establish the dark-adapted level of ROL in the cell. Then, the cone was bleached (>95%) for 10 s with 620 nm light in the presence of 100 µM IRBP. Immediately after the bleach, the rise and fall of outer segment fluorescence, corresponding to the formation and clearance of ROL, were recorded (Fig. 4, inset, gray squares). The dark current and sensitivity of the cone were monitored after the bleach until the cell had reached a new steady state. Then, the bleached cone was regenerated with exogenous 9-DM retinal, and again the dark current and sensitivity were monitored until the cell reached a fully dark-adapted state. The cell was then exposed to second identical bleach, again in the presence of 100 µM IRBP, and the rise and fall of outer segment fluorescence were again recorded (Fig. 4, inset, red circles). It is apparent from comparison of the rising phases of these recordings that fluorescence production was considerably slowed when the pigment was in its 9-demethyl form relative to that when it contained native chromophore. IRBP was again added to the bath during the bleach to facilitate removal of excess exogenous 9-DM retinal from the preparation. After washing the bleached cone with IRBP, the dark current and sensitivity were monitored until the cell again reached a new steady state. Then, the bleached cone was regenerated with exogenous 11-cis retinal, and the dark current and sensitivity were monitored. Finally, the cell was exposed to a third bleach in the presence of IRBP, and the rise and fall of outer segment fluorescence were recorded (Fig. 4, inset, black triangles). Here, the fluorescence rose rapidly, similar to the original condition in which the cell contained native visual pigment; however, it decayed somewhat more slowly than was the case after the first bleach. The reason for this is not known.
Thus, the production and clearance of fluorescence (ROL) was recorded from the outer segment of a single red cone under three different conditions: first when the cell contained its native complement of visual pigment, then when the cell contained regenerated 9-demethyl visual pigment, and finally when the cell contained regenerated 11-cis visual pigment. In the cases in which the cone contained either native pigment or pigment regenerated with exogenous 11-cis retinal, the rate of rise of retinol fluorescence was very similar, reaching a maximum value with time constants τ = 15.0 s or τ =19.8 s (fitted curves not shown). This is consistent with the mean time constant for the post-bleach rise of retinol fluorescence in tiger salamander red cones (τ = 23.9 ± 4.8 s) reported previously (Ala-Laurila et al., 2006). In contrast, when the cone contained pigment regenerated with 9-DM retinal, the rise of retinol fluorescence was significantly slowed, reaching a peak with a time constant τ = 95.9 s.
Fluorescence experiments performed on red cones from Ambystoma mexicanum produced essentially identical results. These average data are plotted in the main panel of Fig. 4, which has been shown on an expanded time scale to highlight the rising phase of the fluorescence. Here, the time constant of post-bleach retinol formation in red cones containing 9-DM retinal (τ = 70.9 ± 6.7 s; n = 6) was ∼3.4 times slower than in cones regenerated with 11-cis retinal (τ = 20.8 ± 0.9 s; n = 5), and 4 times slower than in native cones (τ = 17.5 ±1.2 s; n = 6).
Fig. 5 compares measurements of retinol formation obtained by the fluorescence technique (Fig. 4, main panel) with those obtained by microspectrophotometry (Fig. 3, bottom). Notice that in Fig. 4, the fluorescence data are scaled to unity at their maximum to facilitate comparison of their time courses. However, the fluorescence signal has no intrinsic standard. To convert the levels of fluorescence into relative concentrations of retinol normalized with respect to bleached visual pigment, fluorescence curves were scaled for best visual fit to the concentration time courses deduced from microspectrophotometry (Fig. 5). This provides the concentration of retinol as a fraction of bleached visual pigment, that is, expressed as mol retinol per mol rhodopsin. The average time course of retinol concentration estimated from microfluorometry is illustrated by closed gray circles; retinol concentrations measured from microspectrophotometry are shown by open black circles. Data obtained with the two methods in native and 9-DM–regenerated cells are in a good agreement (Fig. 5, top and middle). The concentration of retinol in cells regenerated with the 11-cis chromophore increased more rapidly as judged from microspectrophotometry than that measured by microfluorometry (Fig. 5, bottom). The reason for this discrepancy is unclear.
Because a saturating exponential function provides a good description of retinol time course (Fig. 4), we can estimate the initial rate of retinol production (in mol ROL/mol Rh per second) as kROL = ROLmax/τ. Here, ROLmax is maximum retinol concentration with respect to bleached visual pigment, as judged from microspectrophotometry (Figs. 3 and 5), and τ is the time constant of the front of the ROL curve (as assessed with microfluorometry; see above). This yields the values of 0.022 s−1, 0.007 s−1, and 0.029 s−1 for native, 9-DM–, and 11-cis–regenerated visual pigment, respectively. Thus, the retinol production in 9-DM cones proceeds three- to fourfold slower than in cones containing methylated chromophore.
Electrophysiological recordings of Ambystoma mexicanum red cones under different visual pigment conditions
The rationale for performing the microspectrophotometric measurements of visual pigment photolysis (Figs. 2 and 3) and the microfluorometric measurements of ROL formation (Fig. 4) was motivated by our previous work (Estevez et al., 2006) in which salamander red cones regenerated to contain 9-DM visual pigment demonstrated sluggish flash response recovery after exposure to light that bleached >0.2% visual pigment. These experiments were performed on red cones isolated from Ambystoma tigrinum (Fig. 6) (Estevez et al., 2006). To provide a direct comparison between photoproduct decay rate and recovery of physiological responses in the same species, analogous electrophysiological measurements were made on red cones of Ambystoma mexicanum. These results are shown in Fig. 6. Cells were pre-bleached and regenerated with either 11-cis retinal (black traces; n = 6) or 9-DM retinal (red traces; n = 4). The average photoresponses to 500-ms test flashes calculated to have bleached from 0.001 to 45% of the cone visual pigment are illustrated. Light intensities were adjusted with neutral density filters so that test flashes produced equivalent levels of photoactivation of the two chromophores, based on calculations described in Materials and methods. After each flash exposure, visual pigment was regenerated by excess retinoid partitioned in outer segment membranes (see Estevez et al., 2006). Flash responses elicited in cells containing 11-cis retinal and 9-DM retinal had identical time courses at low flash strengths (bleaching <0.1% of the pigment). However, as can be seen from comparisons of responses, exposure to flashes of increased light intensity that bleached ≥0.1% of the pigment resulted in significant differences in flash response shape; flash responses from cells containing 9-DM visual pigment were progressively slower to recover from saturation and restore dark current as flash strength increased.
Kinetic scheme of quenching of cone PDE activity
Firsov et al. (2005) have shown that the progressive retardation of the recovery from saturation with increasing stimulus strength, similar to that seen in Fig. 6, can be explained by accumulation of metaproducts that stimulate, with a low efficiency, the phototransduction cascade. According to this idea, responses to low-intensity flashes are turned off by fast rhodopsin phosphorylation and transducin/phosphodiesterase (PDE) quenching. At supersaturating flash intensities, this fast phase is completed while the cell response still remains in saturation, and the recovery is now controlled by long-lived metaproducts. This idea is in general agreement with the findings presented here that show that slower photolysis of 9-demethyl metaproducts is accompanied by slower recovery of photoresponses to bright flashes. To quantitatively test the idea, we applied the model of cascade turn-off suggested by Firsov et al. (2007) as illustrated in Fig. 7.
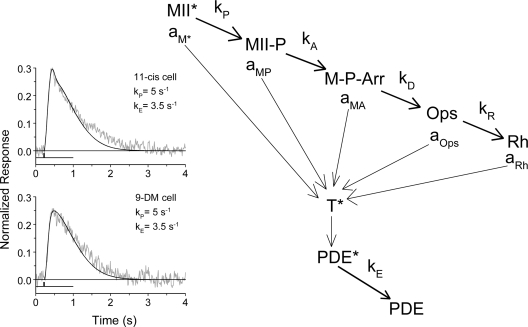
Figure 7.
Kinetic scheme of quenching of the phototransduction cascade according to Firsov et al. (2005). The photoexcited visual pigment, MII*, is quenched in a series of reactions: the phosphorylation of MII* (formation of MII-P), subsequent arrestin binding (formation of M-P-Arr), decay of phosphorylated and arrestin-bound metaproducts to all-trans retinal and opsin (Ops), and eventual regeneration of the dark pigment (Rh). Each reaction is assumed to proceed accordingly to first-order kinetics with rate constants kP, kA, kD, and kR, respectively. GTPase (operating with rate constant kE) quenches the active transducin T*–PDE* complex. Fractional (with respect to fully active MII*) activities of the intermediates toward transducin are aM* = 1 > aMP > aMA > aOps > aRh. This is adapted from Firsov et al. (2005), with permission. (Insets) Cone responses to a dim 20-ms flash (noisy gray curves) with superimposed phototransduction model response (smooth black line) for regenerated 11-cis (top) and regenerated 9-DM (bottom) red cones. kP and kE were obtained from modeling. Light stimulus marks are shown below the curves.
The model assumes that the photoexcited visual pigment, MII*, is quenched in a series of reactions, each step resulting in a progressive decline of MII*'s ability to activate transducin (T). These steps include the phosphorylation of MII* (formation of MII-P), subsequent arrestin binding (formation of M-P-Arr), decay of phosphorylated and arrestin-bound metaproducts to all-trans retinal and opsin (Ops), and eventual regeneration of the dark pigment (Rh). Each reaction is assumed to proceed accordingly to first-order kinetics and is characterized by a corresponding rate constant (kP, kA, kD, and kR). (Assumed first-order kinetics of phosphorylation of the visual pigment does not necessary imply that it is a random one-step process. Sequential multiple phosphorylation may also result in approximately single-exponential quenching, as seen from detailed modeling by Hamer et al., 2005 [their Fig. 5]. This is irrelevant for our model. A second issue is that we do not know the level of phosphorylation of the 9-DM pigment relative to that of pigment regenerated with 11-cis retinal. The rod 9-DM pigment only has ∼25% the phosphorylation level as the native pigment [Morrison et al., 1995]. Phosphorylation levels for the 9-DM cone pigments have not yet been measured.) In turn, the active transducin T*–PDE* complex is quenched by GTPase operating with the rate constant kE. Fractional (with respect to fully active MII*) activities of the intermediates toward transducin are:
The kinetic scheme of Fig. 7 is described by a system of five linear differential equations whose solution provides the time course of the light-induced activity PDE*(t). On the other hand, the PDE*(t) curve can also be deduced from electrical photoresponses by analyzing the saturation time versus intensity function. It has been shown by Firsov et al. (2005) that
| (2) |
where p(t) is the wave of PDE activity elicited by a flash of unit intensity, c is a constant (the criterion level to which PDE*(t) should decay to result in a fixed recovery of the circulating current), and I is light intensity (Firsov et al., 2005). In other words, fractional activity of PDE at a certain time t is inversely proportional to the intensity of the flash that results in saturation time tsat = t. Hence, plotting the inverse of the stimulus intensity versus time of response saturation provides the time course of PDE turn-off scaled by the factor c.
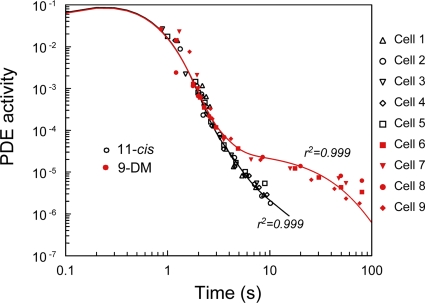
Data from six cones regenerated with 11-cis retinal and four cones regenerated with 9-DM retinal processed this way are shown in Fig. 8. It is seen that up to ∼2 s, the PDE quenching with both types of the chromophore follow a common curve. At longer times, however, PDE activity decays substantially more slowly in cells containing 9-DM visual pigment (red symbols) compared with cells containing normal visual pigment (black symbols).
Figure 8.
Comparison of quenching of the phototransduction cascade in salamander red cones containing different visual pigments. Experimental points for 11-cis–regenerated cones are shown in black; those for 9-DM cones are in red. The solid line is drawn as a five-exponential least-square approximation with four free parameters. It yields the time course of quenching of flash-induced PDE activity for red cones of Ambystoma mexicanum containing 11-cis (black) or 9-DM (red) visual pigment, as follows from the theoretical prediction of the kinetic scheme of Fig. 7. Numbers near the curves show coefficient of determination of the model fits. The fits were further used to obtain the parameters of reactions given in Table I, as explained in the text.
To see whether slower decay of Meta can explain slower turn-off of the cascade in 9-DM cones, we fitted the experimental data with the model depicted in Fig. 7. The fitting procedure was based on the fact that the solution of the system of the equations comprising the model is a sum of five exponentials, with five amplitudes and five rate constants. Rate constants are equal to corresponding rate constants of the kinetic scheme, whereas amplitudes can be expressed via a- and k-values. The amplitudes are bound to the condition that their sum is zero to ensure that PDE*(t) starts from 0. Therefore, formal five-exponential approximation of the experimental data ideally yields entire information necessary to calculate all rate constants and catalytic activities of the bleaching products. Resulting formulas relating kinetic parameters of the scheme to the parameters of fitting are cumbersome and are not given here.
Of course, a straightforward nine-parametric fitting of this sort would mostly yield meaningless results. To reduce the number of free parameters, some of them should be estimated from independent experimental data. The rate of Meta decay kD was obtained by microspectrophotometric measurements (Fig. 3, top). The value of 0.02 s−1 was accepted as a crude estimate of the rate of recombination of opsin with 11-cis retinal (kR), based on the fact that complete recovery of the circulating current after strong bleaches takes ∼3 min. We found that uncertainty of this value does not markedly affect values of other significant parameters derived from modeling. aRh was set to 0 because the activity of regenerated pigment is probably well below the levels detectable in our experiments. To further reduce the freedom of fitting, we estimated kP and kE by fitting nonsaturated flash responses with a complete model of phototransduction by Kuzmin et al. (2004). The model is similar to that by Hamer et al. (2005) and includes all firmly established mechanisms of activation, quenching, and Ca2+ feedback regulation of the phototransduction cascade. It appeared that nonsaturated flash responses in 11-cis– and 9-DM–regenerated cones can be fitted with the same values of kP and kE after just a small adjustment of the kinetics of Ca2+ feedback (Fig. 7, inset). The values shown in Fig. 7 were therefore fixed in further analysis.
Thus, only four parameters, namely the rate of arrestin binding to phosphorylated metaproducts (kA), fractional activity of phosphorylated but arrestin-unbound metaproducts (aMP), fractional activity of metaproducts phosphorylated and capped with arrestin (aMA), and fractional activity of “naked” opsin (aOps), have to be determined from fitting. An additional consideration during fitting is that the values of PDE*(t) encompass a range of ∼5 log units. Therefore, points at the low-amplitude slow tail should be assigned higher weights to be properly treated with the fitting algorithm. We assigned to each experimental point the weight inverse to the point's value.
Weighted least-square fits of experimental data with a sum of five exponentials made under constraints discussed above are shown in Fig. 8 by smooth bold lines. The parameters of the kinetic scheme derived from fitting are provided in Table I. The model fits are consistent with the assumption that fivefold slower Meta decay in 9-DM–regenerated cones is the main cause of the differences observed in the recovery from saturation after exposure to bright light. In addition, the model suggests that the residual activity of partially quenched 9-DM Meta intermediates is several-fold higher than in methylated 11-cis pigment (Table I).
TABLE I.
First-order rate constants of decay (k, s−1) and fraction activities (a) of visual pigment intermediates in salamander red cones
| MII | MII-P | M-P-Arr | Ops | kPDE s−1 | aRH | |||||
| kP s−1 | aM* | kA s−1 | aMP | kD s−1 | aMA | kR s−1 | aOps | |||
| 11-cis | 5 | 1 | 1 | 0.002 | 0.21 | 2.7 × 10−5 | 2 × 10−2 | 4 × 10−6 | 3.5 | nd |
| 9-DM | 5 | 1 | 1.05 | 0.004 | 0.042 | 1.1 × 10−4 | 2 × 10−2 | 4 × 10−6 | 3.5 | nd |
kP and kE are determined from fitting the full model of phototransduction to nonsaturated flash responses; kD values are obtained by single-exponential fitting to Meta decay in Fig. 3; kA, aMP, aMA, and aOps from fitting the kinetic scheme above to Tsat versus bleach data, using previously found kP, kE, and kD. These results, together with Fig. 7, suggest that, in addition to slower decay, the residual activity of 9-demethyl metaproducts (aMP and aMA) is substantially higher than that of 11-cis pigment. nd, not determined.
DISCUSSION
The experiments performed here were designed to test two related hypotheses. The first is that removal of the 9-methyl group from the retinal moiety that is covalently attached to opsin in red cones results in a visual pigment whose activated state (Meta II) decays unusually slowly compared with that of native visual pigment. The second related hypothesis is that this slowed Meta decay results in sluggish recovery of flash responses elicited by bright light. To test these hypotheses, we correlated microspectrophotometric measurements of post-bleach visual pigment photolysis and microfluorometric measurements of ROL formation with electrophysiological measurements of flash response kinetics in intact red cone photoreceptor cells isolated from salamander retina. Measurements were made under dark-adapted conditions when cells contained a full complement of native visual pigment, or when the visual pigment had been fully bleached and regenerated to contain either 11-cis retinal or 9-DM retinal.
We summarize our results as follows. First, visual pigment was successfully regenerated in the outer segments of bleached red cones of Ambystoma mexicanum to contain 9-DM retinal. The absorbance spectrum of the pigment was significantly blue-shifted (λmax = 520 nm) compared with visual pigment either containing the native 11-cis retinal/dehydroretinal mixture (λmax = 592 nm) or 11-cis retinal (λmax = 567 nm) (Fig. 1). Second, the time course of Meta decay measured in situ in red cones containing 9-DM retinal (τ1/2 = 14 s) was approximately five times slower than that regenerated with 11-cis retinal (τ1/2 = 3 s), and four times slower than in native pigment (τ1/2 = 3.5 s) (Fig. 3). Almost no Meta III was observed in red cones, consistent with earlier reports (Ala-Laurila et al., 2006; Golobokova and Govardovskii, 2006). Third, microspectrophotometric and microfluorometric measurements revealed that the initial rate of retinol production was several-fold slower in cone outer segments containing pigment lacking the 9-methyl group of retinal (Figs. 4 and 5).
We have compared these microspectrophotometric and microfluorometric results to electrophysiological results obtained under identical conditions of pigment bleaching and regeneration. Consistent with earlier results obtained on red cones from Ambystoma tigrinum (Estevez et al., 2006), red cones regenerated with 9-DM retinal had photoresponses to dim flashes that appeared to be identical in time course to those of red cones regenerated with 11-cis retinal. However, at brighter flashes that bleached from ∼0.1 to 45% of the visual pigment, red cones lacking the 9-methyl group had progressively slower response termination. For example, after a 33% bleach, red cones containing visual pigment lacking the 9-methyl group exhibited a flash response plateau that was approximately seven times longer than cones regenerated with 11-cis retinal; further restoration of dark current was also greatly retarded (Fig. 6). Slower recovery from saturation paralleled slower decay of Meta in 9-DM cones.
The role of decay of metaproducts in regulation of quenching of responses in rods and cones
The importance of Meta decay in regulating the time course of dark adaptation of rods is well established. Exposure of rods to bright bleaching light leads to the persistence of long-lived photolysis intermediates that are able to activate, with low gain, the phototransduction cascade for a time beyond the end of light stimulation. These persistent effects have been observed in physiological (Donner and Hemilä, 1975; Lamb, 1980; Leibrock and Lamb, 1997; Leibrock et al., 1998; Kolesnikov et al., 2003) as well as biochemical (Okada et al., 1989; Jäger et al., 1996) experiments in rods. They are understood to provide at least a partial basis for the slowness of photoresponse recovery that occurs after exposure to bright light.
Alternative mechanisms have been suggested to explain the slowness of the cascade quenching at high bleaches. Lyubarsky and Pugh (1996) proposed that in this condition the cascade turn-off is retarded (kE decreased) due to local saturation of the PDE pool that serves as a GTP-ase activating factor for T* quenching. Alternatively, biochemical experiments on carp cone membranes provide evidence that the phosphorylation power (hence rhodopsin turn-off) of cones may be limited at high light intensities (Tachibanaki et al., 2005). However, these mechanisms do not account for parallel effect of 9-DM chromophore on the rate of Meta decay and recovery of saturation observed by us in salamander red cones.
An example illustrating this critical relationship between Meta II decay and photoresponse recovery was shown in a recent study by Imai et al. (2007). They accelerated Meta II decay 10-fold and Meta III twofold in mouse rods by replacing rhodopsin with its E122Q mutant, and observed no effect on the dim-flash response kinetics unless arrestin was concurrently knocked out (indicating that phosphorylation and subsequent binding of arrestin is fast enough to precede Meta II decay). However, after exposing to brighter flashes that bleached 20% of the pigment, flash responses elicited in mutant rods exhibited a faster rate of recovery. This included both reduced time in saturation and steeper return to the dark current level. Thus, these results on rod photoreceptors support the idea that different mechanisms dominate the response recovery depending on the amount of light present, and that it is Meta decay that controls the photoresponse recovery after bright illumination. The sluggish photoresponse recovery of red cones lacking the 9-methyl group of retinal observed in response to bright but not dim light suggests that this is also true for cones.
This idea is also consistent with conclusions from electrophysiological studies by Firsov and coworkers on goldfish cones (Firsov et al., 2007) and frog rods (Firsov et al., 2005). According to their model, at low intensities the cells recover to the prestimulus state by using the fast mechanisms that quench dim flash responses (phosphorylation, arrestin binding, and Ca2+ feedback). Apparently, these reactions in cones have no dependency on the presence of the 9-methyl group. However, after flashes that bleach larger fractions of the pigment (>3% in goldfish cones and >0.1% in rods), these fast steps have run their normal courses while the cell's response still remains in saturation, and thus, residual activity of decaying Meta and subsequent pigment regeneration control the time course of photoresponse recovery.
Mathematical modeling based on the Firsov et al. (2005) kinetic scheme of cascade quenching further supports this idea. Application of this model to our electrophysiological data (Fig. 6) shows that the difference in the time courses of PDE turn-off in cones containing normal and 9-DM chromophores can be predicted based on the kinetics of photolysis of corresponding visual pigments obtained microspectrophotometrically (Fig. 8). This idea is also consistent with the results from our previous work on Ambystoma tigrinum in which we demonstrated that slowness of response recovery in 9-DM cones was due to persistent activations of the transduction cascade (Estevez et al., 2006).
Thus, targeted modification of the visual pigment chromophore results in concomitant changes in the photolysis kinetics and the speed of photoresponse recovery. Our results indicate that the 9-methyl group of retinal is required for steric chromophore–opsin interactions that favor both the rapid decay of Meta and the rapid termination of the photoresponse after exposure to bright but not dim light in red cones. These findings provide a partial mechanistic explanation for the rapid time course of dark adaptation observed in red cone photoreceptors.
Steric interactions of opsin and the 9-methyl group
Previous biochemical studies of chicken rod and cone visual pigments have also investigated the opsin–chromophore interactions that influence the lifetime of Meta II in rod and cone visual pigments (Imai et al., 1997, 2007; Kuwayama et al., 2002, 2005). For instance, Kuwayama and coworkers replaced the proline at position 189 in chicken green cone pigment with the corresponding rhodopsin residue (isoleucine) (Kuwayama et al., 2002). They observed a 27-fold slower Meta II decay rate in these mutated cone pigments. Similarly, they demonstrated an acceleration of the Meta II decay rate by the reverse, that is, by replacing the isoleucine residue as position 189 in rhodopsin with the corresponding residue (proline) in chicken green cone pigment (Kuwayama et al., 2002). In other studies, an accelerated Meta II decay rate in rhodopsin was induced by mutation at residue 122 with the corresponding residue in cone pigments (Imai et al., 1997). This group concluded that the 50-fold difference in Meta II decay rate and the 700-fold difference in Meta III decay rate between chicken rhodopsin and chicken green cone pigment is regulated by the residues at positions 122 and 189 (Kuwayama et al., 2002, 2005).
Could these mutations at positions 122 and 189 that modulate Meta II decay rate in chicken green cone pigment also have their effects through the 9-methyl group of retinal? No definitive experiments have been performed to investigate this possibility. However, Kuwayama and coworkers note that the CG1 carbon atom of I189 in bovine rhodopsin is 3.8 Å from the 9-methyl group of retinal, whereas the peptide amine of I189 is 4.6 Å from the 9-methyl group (Kuwayama et al., 2002). They speculate that I189 may have steric interactions with the 9-methyl group in chicken rhodopsin that may regulate Meta II decay. However, the role of the 189 residue (proline) in salamander red cone pigment is not known, nor is it known if this residue interacts with the 9-methyl group of retinal in salamander rod or cone pigment. The chicken green cone pigment has higher homology in amino acid sequence with vertebrate rhodopsins (70%) than with other cone visual pigments (40%) (Wang et al., 1992). Based on these differences, the speculations of chromophore–opsin interactions between the 9-methyl group of retinal and residue 189 in chicken green cone pigment cannot be directly translated to salamander red cone pigment, but they provide impetus for further investigation.
Han et al. (1997) in a separate study have indicated that the 9-methyl group of retinal interacts with Gly121 of rhodopsin. In the salamander, the Gly121 in rhodopsin is conserved in red cone pigment. However, this residue may be in a different structural location in red cone pigment, and there is no evidence that it interacts with the 9-methyl group of retinal. How the 9-methyl group of retinal may interact with various amino acid residues in salamander red cone pigment is not known. The data presented here do not address this point. However, it is clear that the absence of this methyl group on retinal causes significant changes in the biochemical and physiological properties of the red cone opsins that strongly affect Meta II decay and flash response recovery kinetics under bright light exposure.
Acknowledgments
This work was supported by National Institutes of Health grants EY-001157 (to M.C. Cornwall) and EY-004939 (R.K. Crouch); unrestricted grant to MUSC from Research to Prevent Blindness, Inc., New York, NY; The Russian Foundation for Basic Research grant 06-04-48914 and the Biological Department of The Russian Academy of Sciences (to V.I. Govardovskii); and a grant from the Academy of Finland (grant 123231 to P. Ala-Laurila). R.K. Crouch is a Research to Prevent Blindness Senior Scientific Investigator.
Edward N. Pugh Jr. served as editor
Footnotes
Abbreviations used in this paper: 9-DM, 11-cis 9-demethyl; IRBP, interphotoreceptor retinoid binding protein; Meta, metarhodopsin; PDE, phosphodiesterase; PSB, protonated Schiff base; ROL, all-trans retinol.
References
- Ala-Laurila P., Kolesnikov A.V., Crouch R.K., Tsina E., Shukolyukov S.A., Govardovskii V.I., Koutalos Y., Wiggert B., Estevez M.E., Cornwall M.C. 2006. Visual cycle: dependence of retinol production and removal on photoproduct decay and cell morphology.J. Gen. Physiol. 128:153–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Laurila P., Donner K., Crouch R.K., Cornwall M.C. 2007. Chromophore switch from 11-cis-dehydroretinal (A2) to 11-cis-retinal (A1) decreases dark noise in salamander red rods.J. Physiol. 585:57–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczylko J., Saari J.C., Crouch R.K., Palczewski K. 1996. Mechanisms of opsin activation.J. Biol. Chem. 271:20621–20630 [DOI] [PubMed] [Google Scholar]
- Burns M.E., Arshavsky V.Y. 2005. Beyond counting photons: trials and trends in vertebrate visual transduction.Neuron. 48:387–401 [DOI] [PubMed] [Google Scholar]
- Chen C., Tsina E., Cornwall M.C., Crouch R.K., Vijayaraghavan S., Koutalos Y. 2005. Reduction of all-trans retinal to all-trans retinol in the outer segments of frog and mouse rod photoreceptors.Biophys. J. 88:2278–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M.C., Fain G.L. 1994. Bleached pigment activates transduction in isolated rods of the salamander retina.J. Physiol. 480:261–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M.C., Fein A., MacNichol E.F., Jr 1983. Spatial localization of bleaching adaptation in isolated vertebrate rod photoreceptors.Proc. Natl. Acad. Sci. USA. 80:2785–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M.C., Fein A., MacNichol E.F., Jr 1990. Cellular mechanisms that underlie bleaching and background adaptation.J. Gen. Physiol. 96:345–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M.C., Jones G.J., Kefalov V.J., Fain G.L., Matthews H.R. 2000. Electrophysiological methods for measurement of activation of phototransduction by bleached visual pigment in salamander photoreceptors.Methods Enzymol. 316:224–252 [DOI] [PubMed] [Google Scholar]
- Corson D.W., Kefalov V.J., Cornwall M.C., Crouch R.K. 2000. Effect of 11-cis 13-demethylretinal on phototransduction in bleach-adapted rod and cone photoreceptors.J. Gen. Physiol. 116:283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch R.K., Kefalov V., Gartner W., Cornwall M. Carter. 2002. Use of retinal analogues for the study of visual pigment function. Methods in enzymology. G Protein Pathways Part A: Ribonucleases. Iyengar R., Hildebrandt J.D., Academic Press; 29–48 [DOI] [PubMed] [Google Scholar]
- Das J., Crouch R.K., Ma J.X., Oprian D.D., Kono M. 2004. Role of the 9-methyl group of retinal in cone visual pigments.Biochemistry. 43:5532–5538 [DOI] [PubMed] [Google Scholar]
- Donner K.O., Hemilä S. 1975. Kinetics of long-lived rhodopsin photoproducts in the frog retina as a function of the amount bleached.Vision Res. 15:985–995 [DOI] [PubMed] [Google Scholar]
- Estevez M.E., Ala-Laurila P., Crouch R.K., Cornwall M.C. 2006. Turning cones off: the role of the 9-methyl group of retinal in red cones.J. Gen. Physiol. 128:671–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G.L., Matthews H.R., Cornwall M.C., Koutalos Y. 2001. Adaptation in vertebrate photoreceptors.Physiol. Rev. 81:117–151 [DOI] [PubMed] [Google Scholar]
- Firsov M.L., Kolesnikov A.V., Golobokova E.Y., Govardovskii V.I. 2005. Two realms of dark adaptation.Vision Res. 45:147–151 [DOI] [PubMed] [Google Scholar]
- Firsov M.L., Golobokova E.Y., Govardovskii V.I. 2007. Two-stage quenching of cone phototransduction cascade.Sensornye Sistemy. 21:55–59 [Google Scholar]
- Golobokova E.Y., Govardovskii V.I. 2006. Late stages of visual pigment photolysis in situ: cones vs. rods.Vision Res. 46:2287–2297 [DOI] [PubMed] [Google Scholar]
- Govardovskii V.I., Fyhrquist N., Reuter T., Kuzmin D.G., Donner K. 2000. In search of the visual pigment template.Vis. Neurosci. 17:509–528 [DOI] [PubMed] [Google Scholar]
- Hamer R.D., Nicholas S.C., Tranchina D., Lamb T.D., Jarvinen J.L. 2005. Toward a unified model of vertebrate rod phototransduction.Vis. Neurosci. 22:417–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Groesbeek M., Sakmar T.P., Smith S.O. 1997. The C9 methyl group of retinal interacts with glycine-121 in rhodopsin.Proc. Natl. Acad. Sci. USA. 94:13442–13447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H., Kojima D., Oura T., Tachibanaki S., Terakita A., Shichida Y. 1997. Single amino acid residue as a functional determinant of rod and cone visual pigments.Proc. Natl. Acad. Sci. USA. 94:2322–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H., Kefalov V., Sakurai K., Chisaka O., Ueda Y., Onishi A., Morizumi T., Fu Y., Ichikawa K., Nakatani K., et al. 2007. Molecular properties of rhodopsin and rod function.J. Biol. Chem. 282:6677–6684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger S., Palczewski K., Hofmann K.P. 1996. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin.Biochemistry. 35:2901–2908 [DOI] [PubMed] [Google Scholar]
- Jin J., Crouch R.K., Corson D.W., Katz B.M., MacNichol E.F., Cornwall M.C. 1993. Noncovalent occupancy of the retinal-binding pocket of opsin diminishes bleaching adaptation of retinal cones.Neuron. 11:513–522 [DOI] [PubMed] [Google Scholar]
- Jones G.J., Fein A., MacNichol E.F., Jr., Cornwall M.C. 1993. Visual pigment bleaching in isolated salamander retinal cones. Microspectrophotometry and light adaptation.J. Gen. Physiol. 102:483–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov V.J., Cornwall M.C., Crouch R.K. 1999. Occupancy of the chromophore binding site of opsin activates visual transduction in rod photoreceptors.J. Gen. Physiol. 113:491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkre J.S., Moran N.A., Lamb T.D., Mahroo O.A. 2005. Extremely rapid recovery of human cone circulating current at the extinction of bleaching exposures.J. Physiol. 567:95–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov A.V., Golobokova E.Y., Govardovskii V.I. 2003. The identity of metarhodopsin III.Vis. Neurosci. 20:249–265 [DOI] [PubMed] [Google Scholar]
- Krispel C.M., Chen C.K., Simon M.I., Burns M.E. 2003. Novel form of adaptation in mouse retinal rods speeds recovery of phototransduction.J. Gen. Physiol. 122:703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwayama S., Imai H., Hirano T., Terakita A., Shichida Y. 2002. Conserved proline residue at position 189 in cone visual pigments as a determinant of molecular properties different from rhodopsins.Biochemistry. 41:15245–15252 [DOI] [PubMed] [Google Scholar]
- Kuwayama S., Imai H., Morizumi T., Shichida Y. 2005. Amino acid residues responsible for the meta-III decay rates in rod and cone visual pigments.Biochemistry. 44:2208–2215 [DOI] [PubMed] [Google Scholar]
- Kuzmin D.G., Firsov M.L., Govardovskii V.I. 2004. Mathematical modeling of phototransduction and light adaptation in frog retinal rods.Sens. Syst. 18:305–316 [Google Scholar]
- Lamb T.D. 1980. Spontaneous quantal events induced in toad rods by pigment bleaching.Nature. 287:349–351 [DOI] [PubMed] [Google Scholar]
- Lamb T.D., Pugh E.N., Jr 2004. Dark adaptation and the retinoid cycle of vision.Prog. Retin. Eye Res. 23:307–380 [DOI] [PubMed] [Google Scholar]
- Leibrock C.S., Lamb T.D. 1997. Effect of hydroxylamine on photon-like events during dark adaptation in toad rod photoreceptors.J. Physiol. 501:97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrock C.S., Reuter T., Lamb T.D. 1998. Molecular basis of dark adaptation in rod photoreceptors.Eye. 12:511–520 [DOI] [PubMed] [Google Scholar]
- Lyubarsky A.L., Pugh E.N., Jr 1996. Recovery phase of the murine rod photoresponse reconstructed from electroretinographic recordings.J. Neurosci. 16:563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino C.L., Groesbeek M., Lugtenburg J., Baylor D.A. 1999. Spectral tuning in salamander visual pigments studied with dihydroretinal chromophores.Biophys. J. 77:1024–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata N.L., Radu R.A., Clemmons R.C., Travis G.H. 2002. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight.Neuron. 36:69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D.F., Ting T.D., Vallury V., Ho Y.K., Crouch R.K., Corson D.W., Mangini N.J., Pepperberg D.R. 1995. Reduced light-dependent phosphorylation of an analog visual pigment containing 9-demethylretinal as its chromophore.J. Biol. Chem. 270:6718–6721 [DOI] [PubMed] [Google Scholar]
- Okada D., Nakai T., Ikai A. 1989. Transducin activation by molecular species of rhodopsin other than metarhodopsin II.Photochem. Photobiol. 49:197–203 [DOI] [PubMed] [Google Scholar]
- Tachibanaki S., Arinobu D., Shimauchi-Matsukawa Y., Tsushima S., Kawamura S. 2005. Highly effective phosphorylation by G protein-coupled receptor kinase 7 of light-activated visual pigment in cones.Proc. Natl. Acad. Sci. USA. 102:9329–9334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.M., Lamb T.D. 1999. Light adaptation and dark adaptation of human rod photoreceptors measured from the a-wave of the electroretinogram.J. Physiol. 518:479–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsina E., Chen C., Koutalos Y., Ala-Laurila P., Tsacopoulos M., Wiggert B., Crouch R.K., Cornwall M.C. 2004. Physiological and microfluorometric studies of reduction and clearance of retinal in bleached rod photoreceptors.J. Gen. Physiol. 124:429–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.S., Estevez M.E., Cornwall M.C., Kefalov V.J. 2009. Intra-retinal visual cycle required for rapid and complete cone dark adaptation.Nat. Neurosci. 12:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.Z., Adler R., Nathans J. 1992. A visual pigment from chicken that resembles rhodopsin: amino acid sequence, gene structure, and functional expression.Biochemistry. 31:3309–3315 [DOI] [PubMed] [Google Scholar]