Abstract
Particulate matter less than 10 μm (PM10) has been shown to be associated with aggravation of asthma and respiratory and cardiopulmonary morbidity. There is also great interest in the potential health effects of PM 2.5. Particulate matter (PM) varies in composition both spatially and temporally depending on the source, location and seasonal condition. El Paso County which lies in the Paso del Norte airshed is a unique location to study ambient air pollution due to three major points: the geological land formation, the relatively large population and the various sources of PM. In this study, dichotomous filters were collected from various sites in El Paso County every seven days for a period of one year. The sampling sites were both distant and near border crossings, which are near heavily populated areas with high traffic volume. Fine (PM2.5) and Coarse (PM10-2.5) PM filter samples were extracted using dichloromethane and were assessed for biologic activity and polycyclic aromatic (PAH) content. Three sets of marker genes human BEAS2B bronchial epithelial cells were utilized to assess the effects of airborne PAHs on biologic activities associated with specific biological pathways associated with airway diseases. These pathways included in inflammatory cytokine production (IL-6, IL-8), oxidative stress (HMOX-1, NQO-1, ALDH3A1, AKR1C1), and aryl hydrocarbon receptor (AhR)-dependent signaling (CYP1A1). Results demonstrated interesting temporal and spatial patterns of gene induction for all pathways, particularly those associated with oxidative stress, and significant differences in the PAHs detected in the PM10-2.5 and PM 2.5 fractions. Temporally, the greatest effects on gene induction were observed in winter months, which appeared to correlate with inversions that are common in the air basin. Spatially, the greatest gene expression increases were seen in extracts collected from the central most areas of El Paso which are also closest to highways and border crossings.
Keywords: asthma, lung oxidative stress, PM, polycyclic aromatic hydrocarbons, border air samples
INTRODUCTION
The incidence of asthma has been increasing for certain groups of children in the U.S. and elsewhere for the past decade or more (Gilmour et al., 2006; Ginde et al., 2008). Exposure to particulate matter (PM) from vehicle emissions and other sources appears to be important in cardiopulmonary diseases (Delfino, 2002; Nel, 2007). Children living near highways and high traffic areas have increased risk for asthma, and their asthma is less well-controlled (Brugge 2007; Jerrett et al., 2008; Meng et al., 2008). There appear to be numerous environmental and genetic factors that play major roles in the incidence and exacerbation of asthma (Selgrade et al., 2006). An important group of environmental chemicals that may be associated with asthma exacerbation and bronchitis in children are the polycyclic aromatic hydrocarbons (PAHs) (Hertz-Picciotto et al., 2007). PAHs are associated with the combustion of fossil fuels from mobile and fixed sources, and are present in cigarette smoke (Bostrom et al., 2002; Lewtas, 2006).
Our previous studies (Arrieta et. al., 2003), have shown that chemical extracts from PM10 filters collected in the Paso del Norte airshed located on the U.S.-Mexico border between El Paso, TX, U.S. and Cd. Juarez, Chihuahua, MX are rich in PAHs. The PAHs found in air samples vary in size/mass based upon their number of fused aromatic rings. Small PAHs are most prevalent in combustion emissions, but they are volatile and are not retained on high volume or medium volume dichotomous air sampling filters, such as those used in the present studies. Semi-volatile PAHs are retained on PM trapped on air filters and can be extracted with organic solvents for chemical and biologic characterization. Previous work has suggested important seasonal and regional variation in the amount and types of PAHs that are present in ambient air samples. Therefore, the purpose of the present studies was to perform long term monitoring of up to 10 sites in the Paso del Norte Air Basin to determine the nature of PAH exposures that may play a role in the induction or exacerbation of asthma.
In the present studies we also utilized a set of cell-based biomarkers for genes which are biomarkers of exposures. We examined seven genes expressed in a human bronchial epithelial cell line that are associated with three biologic pathways: oxidative stress, inflammatory cytokine production, and AhR-dependent signaling pathways. Arietta et al (2003) showed that PAHs extracted from PM10 activate Ah receptor response elements, leading to activation of genes including cytochrome P450 1A1 (CYP1A1). Our previous work has shown that PAH-quinones formed environmentally or through cell metabolism are inducers of oxidant stress, and that several marker genes are activated, including HMOX-1, NQO1, AKR1C1, and ALDH3A1 (Burchiel et al.,2007). We also added two genes that are markers of inflammatory pathways that are induced by epithelial cell-derived cytokines, IL-6 and IL-8 (Nel, 2005).
Results of these studies demonstrate significant spatial and temporal variation in the activity of PM10-2.5 (Coarse) and PM 2.5 (Fine) filters extracts that is associated with the winter months in the Paso del Norte airshed. Significant concentrations of PAHs were detected in both the PM10-2.5 and PM2.5 fractions. Sample extracts has significant gene induction activity for CYP1A1, NQO1, ALDH3A1, AKR1C1, and HMOX-1. However, there was only marginal activity noted for the induction of IL-6 and IL-8. These results suggest that the main action of PAHs on human bronchial epithelial cells is the induction of oxidative stress.
MATERIALS AND METHODS
Air Monitoring and Analysis
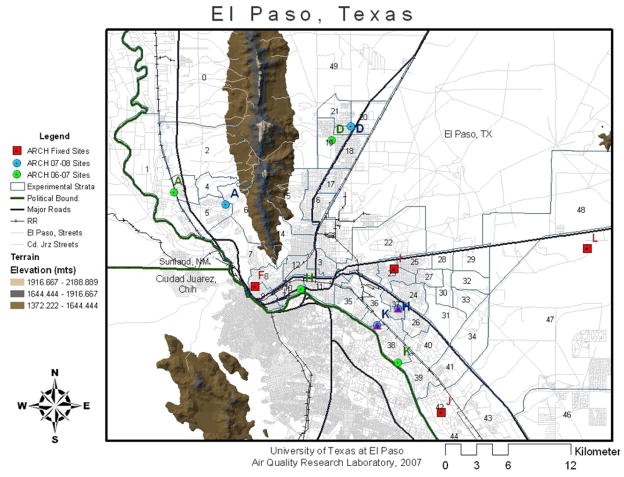
Eight sites were selected in El Paso in accordance with the EPA guidelines (U.S. EPA 1997) for collecting representative PM exposure concentrations for the 50 geographic strata identified in the childhood asthma study. These sites are designated as “primary” sites in Figure 1. In addition, four more sites were selected and designed as “secondary” sites for passive sampling of NOx, O3 and VOCs for the study.
Figure 1.
Geologic map of the land areas that represent the Paso del Norte airshed located in El Paso, TX, U.S., Cd. Juarez, Chihuahua, MX, and the Sunland Park region of southern New Mexico, U.S.. El Paso City-County was divided into 50 equally populated strata (approximately 13,000 people per strata in the year 2000) and air monitors were located at various times in these strata for up to one year, with dichotomous PM10-2.5 and PM2.5 filters being collected continually and retrieved on a weekly basis for analysis. Some of the dichotomous air samplers were co-located at sites monitored by the State of Texas Commission on Environmental Quality (TCEQ) air samplers to allow for comparison of results.
One dichotomous air sampler (Anderson Inst., 1990) was placed at each site and 1 additional air sampler was used for duplicate samples among the eight sites. The air sampler’s inlet head allows particles with aerodynamic diameter of 10-μm or less (PM10) to enter the sampling air stream. The air stream then enters a virtual impactor where fine (PM2.5) and coarse (PM10-2.5) particles are separated and collected on selected filter media.
Seven-days samples were collected on 37-mm-diameter ringed Teflon filters (Gelman Science Inc., ID No. R2PJ037) at a flow rate of 1 m3/hr. Quality control was managed by following EPA guidelines and procedures for particulate matter monitoring (U.S. EPA, 1994) and gravimetric weighing (U.S. EPA, 1998). A mini-Buck bubble calibrator, Model M-30, a primary standard calibration device traceable to NIST, was used to calibrate the rotameters on the dichotomous samplers (A.P. Buck, 1987). One set of duplicate samples were collected at one of the eight sites each week.
Filters were conditioned, pre-weighed, and stored in petri dishes prior to the deployment. Loaded filters were carefully removed from the field and transported to the laboratory at the University of Texas at El Paso (UTEP) for gravimetric analysis. A CAHN model C-33 microbalance, which has an accuracy of 2 μg and an ultimate precision of 1 μg was used in the analysis (Orion Research, 1997). All samples were pre-conditioned to room temperature (25 ± 3 °C) and humidity (30± 5 %) for at least twenty-four hours by storing them in a custom-made storage cabinet in the laboratory prior to weighing. Mass concentrations were reported as micrograms of PM per cubic meter of air (μg/m3), adjusted for the 10% of fine mass in the coarse mass, due to the use of virtual impactor in the dichotomous samplers (Li et al 2001).
Extraction method
PM 2.5 and PM 10-2.5 Teflon filters were analyzed using the MarsX (CEM Corp.) microwave accelerated solvent extraction system (MAE). Filters from the same site and of the same type (either PM10-2.5 or PM 2.5) were combined into one composite sample. The Teflon ring on each filter was cut on cleaned filter paper before being put into the extraction vessel. Forty mL of dichloromethane was added to each vessel. The vessels were sealed, and placed into the microwave. The MAE method was programmed as follows: energy at 600 W, initial temperature 110 °C and hold for 14 min. The vessels were allowed to cool then the solvent from each vessel was transferred to a K-D condenser individually. The filter samples were extracted a second time with 40 mL of Acetone/Methanol (v:v 1:1) using the same MAE program. Two extracts for the same sample were combined and condensed to 10mL. Each extract was split into 2 aliquots for chemical and biological analysis respectively. For biological analysis, the 5-mL extract was further condensed to 100 uL and solvent exchanged to 25 uL in DMSO. We compared extraction efficiencies for several different PAH standards and found that extraction efficiencies ranged from 70–135% for different PAHs. We did not correct for extraction efficiencies because we did not want to introduce additional bias into sample analysis.
Cell culture
Human bronchial epithelial cell line, BEAS2B were used for biological assay. The cells were purchased from American Type Cell Culture (Marassas, VA) and cultured in LHC-9 media (Gibco, Grand Island, NY). Cells were grown in a humidified incubator at 37°C with a 5% CO2 atmosphere and subcultured weekly by dissociating with PET (Athena ES, Baltimore, MD). Cells were plated in 6 well tissue culture plates that had be coated with FNC coating mix (Athena ES, Baltimore, MD) at 5 × 103 cells per well. Cells were allowed to attach and grow for 3 days at which time the media was removed and replaced with treatment media. BEAS2B are a common model cell line that has been found to be useful for examining the effects of environmental agents (Veranth et al., 2004, 2006). They are a human epithelial cell line that responds to environmental stressors through the production of cytokines and are useful for monitoring oxdative stress and AhR controlled genes. While our long term goal was to understand the influence of environmental factors, and in particular airborne PM and PAHs, on the incidence of asthma and children, BEAS2B cells are only one of the numerous types of cells in the lung that contribute to asthma and therefore our findings are mostly for the purpose of comparing the effects of air samples obtained from different areas at various times.
Treatment
A dose of 100 μg was calculated using the “Collected mass” total from each filter (Table 1) and the volume of sample that was provided for analysis. Previous studies have shown that the amount of PAH is correlated with the amount of mass collected at each site (Arrieta et al., 2003). Therefore, we wanted to compare sample collected from different sites based on an equal mass comparison. Treatments were made at 50 μg extracted mass per ml growth media, two ml of treatment media was then added to each of 6 wells of a 6 well plate. Some samples required > 0.1% DMSO, matching DMSO controls were run. In cases where the amount of DMSO varied the highest concentration of DMSO was used. Each treatment was run in duplicate. Following 18 hr exposure to treatment, RNA was isolated from each sample.
RNA isolation
Media was aspirated from cell culture wells and wells were rinsed one time with phosphate buffered saline without calcium or magnesium (PBS−). Qiagen RNeasy Mini Kit ™ (Qiagen Cat. No. 74104) and QIAshredder™ (Qiagen Cat. No. 79654) were used according to provided instructions to isolate RNA. RNA was then quantified on an Agilent Nanodrop spectrophotometer. All samples were stored at −20°C until assayed.
Real time polymerase chain reaction (PCR)
All reagents for real time PCR were purchased from Applied Biosystems (Foster City, CA) unless otherwise noted. A reverse transcriptase (RT) reaction, 60 μL containing a minimum of 1,080 ng, was run on each sample using the High Capacity cDNA Reverse Transcription Kit. We followed the incubation conditions provided: 25°C for 10 min, 37°C for 2 hr. Samples were then diluted to 9 ng per μl with RNase, and DNase free water and were stored at −20°C. Real time PCR reactions utilizing TaqMan® Universal PCR Master Mix were carried out in 384 well plates at 10 μL per reaction and 1 μL cDNA (9 ng total RNA) was added to each well. NQ01 (Assay ID HS00168547_m1), HMOX1 (Assay ID HS00157965_m1), IL-6 (Assay ID HS00174131_m1), IL-8 (Assay ID HS00174103_m1), ALDH3A1 (Assay ID HS00167469_m1), AKR1C1 (Assay ID HS00413886_m1), and CYP1A1 (Assay ID HS00153120_m1) were purchased as ready to use Assays on Demand and were added to the master mix prior to adding cDNA. PCR thermal cycling parameters used were AmpErase UNG activation 2 min at 50°C, polymerase activation 10 min 95°C, melt 15 s at 95°C, anneal and extend 1 min at 60°C (melt and anneal/extend for 40 cycles). Comparative CT (first amplification cycle exceeding threshold) method was used for relative quantification. GADPH was the endogenous reference and DMSO (or DMSO high) served as the calibrator. Real-time PCR was run on Applied Biosystem’s 7900HT system with a standard 384 well block. To calculate the comparative CT, briefly, we determined the difference in CT ( Δ CT) values between target and endogenous control, then determined the fold difference in gene expression (Δ Δ CT) and the Δ CT of the calibrator was subtracted from the Δ CT of the target (Δ CT target - Δ CT calibrator). This calculation is described in detail by Applied Biosystems.
Desert Research Institute Analysis of PAHs
Desert Research Institute, Nevada, performed the analysis of the extracts using a gas chromatography/mass spectrometry (GC/MS) method. Mass spectrometry provided definitive identification of PAHs. Briefly, the samples were analyzed by the electron impact (EI) GC/MS technique, using a Varian CP-3800 GC equipped with a CP8400 autosampler and interfaced to a Varian 4000 Ion Trap mass spectrometer. Injections were 1μl in size in the splitless mode onto a 30 m long 5% phenylmethylsilicone fused-silica capillary column (DB-5ms, J&W Scientific, 30m × 0.25 mm ×0.25 μm).
Quantification of the individual compounds is obtained by selective ion storage (SIS) technique, monitoring the molecular (or the most characteristic) ion of each compound of interest and the corresponding deuterated internal standard. Calibration curves for the GC/MS quantification are made for the most abundant and characteristic ion peaks of the compounds of interest using the deuterated species most closely matched in volatility and retention characteristics as internal standards. National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 1647 (certified PAH) with the addition of deuterated internal standards and of those compounds not present in the SRM, is used to make calibration solutions. A six- to eight-level calibration are performed for each compound of interest and the calibration check (using median calibration standards) is run every 10 samples to check for accuracy of analyses. If the relative accuracy of measurement (defined as a percentage difference from the standard value) is less than 20%, the instrument is recalibrated.
Correlation analysis
Pearson correlation coefficients were computed for each combination of gene expression level and PAH compound. Separate analyses were performed for the two filter sizes (PM10-2.5, PM 2.5) on the data collected in October 2006. The correlations were based on available data at the eight sites. The matrix of pairwise correlations was summarized as a heat map. Calculations were performed in a commercial software package (Matlab, version 7.0.4, The MathWorks Inc., Natick, MA, 2005). Correlations that exceeded 0.76 and 0.82 were considered statistically significant at the 5% level for the PM 2.5 and PM 10-2.5 data, respectively. Correlations that were less than zero were represented as zero on the heat map.
RESULTS
Temporal and Spatial Gravimetric Analysis of PM10-2.5 and PM 2.5 Filters
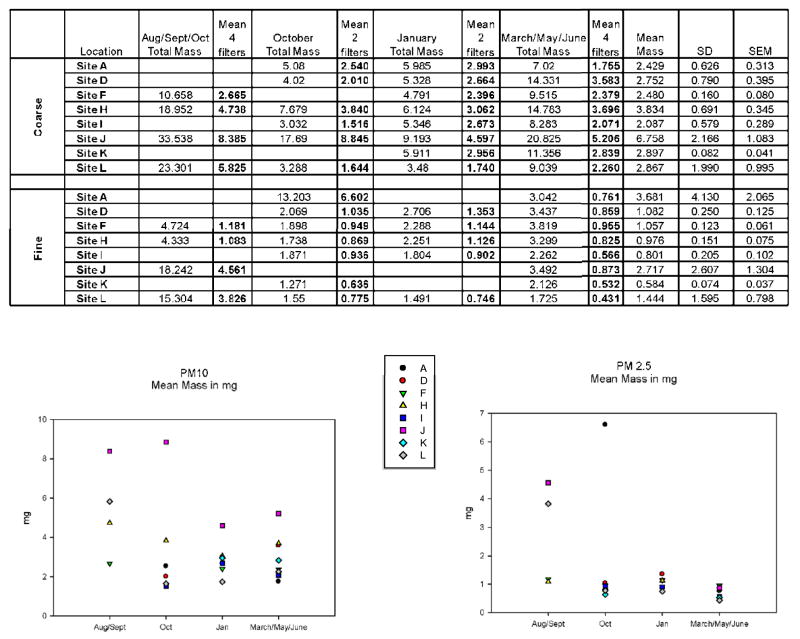
Teflon filters collected weekly from up to 12 different sites (labeled sites A-L) in El Paso County (Figure 1). Filter weights determined over a one year’s period revealed significant differences between the sample collection sites (Figure 2). For combined seasons, the PM10-2.5-2.5 filters (3.3 mg/filter) demonstrated higher overall masses than the PM 2.5 filters (1.5 mg/filter). The highest masses were found near highways, with the highest total masses for all seasons combined being observed at the H and J sites for PM10-2.5 and A and J sites for PM 2.5. The A site is located near highly populated area of El Paso, near Interstate Highway 10 and busy railroad tracks, and west of the Franklin Mountains where air pollutants have previously been shown to accumulate. The H and J sites are in the South Valley of El Paso along the Rio Grande near industry (H) and border farming areas (J).
Figure 2.
Gravimetric results for air filters obtained from various sites in El Paso, TX during the time period August 2006 through June 2007 for Sites A, D, E, H, I, J, K, and L shown on the map in Figure 1. Because individual filters did not usually contain sufficient amounts of PAHs for chemical analysis, extracts were made from 4 pooled filters using the one week filter collection from each site for the months listed. Descriptions of these sites are included in the text.
Analysis of CYP1A1 Gene Induction PM10-2.5 and PM 2.5 Extracts in BEAS2B Cells
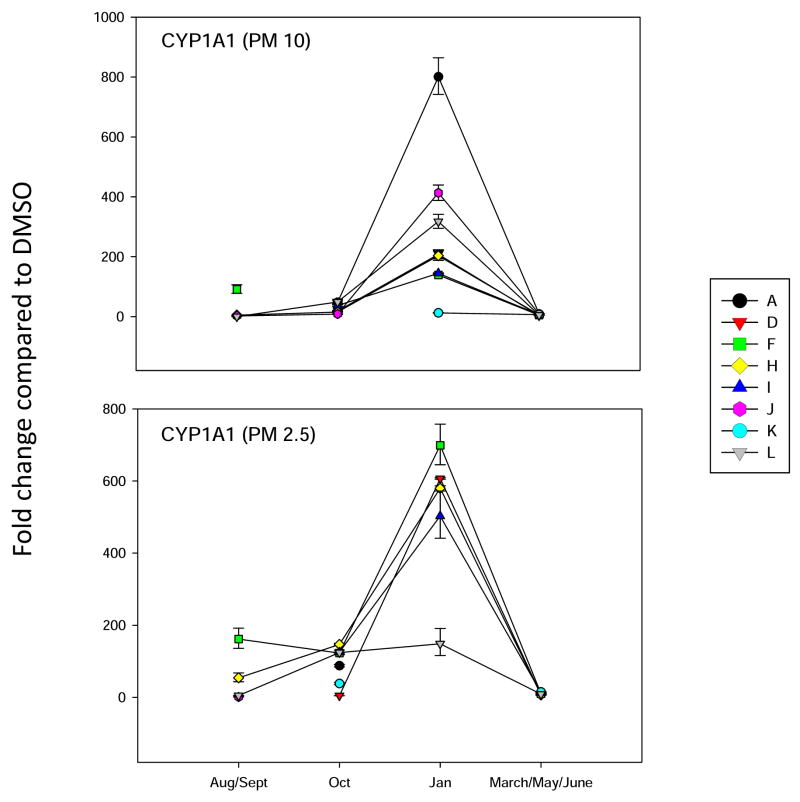
Pilot studies showed that biologic activity of dichloromethane extracts of PM filters could be detected using real time PCR assays of various marker genes. CYP1A1 (Figure 3) was used as a marker of Ah receptor activity, usually indicative of high molecular weight PAHs and halogenated aromatic compounds (Note: we did not detect and halogenated aromatic hydrocarbons in our samples.). Significant CYP1A1 induction was detected in all samples ranging from 0–800 fold in PM10-2.5 extracts and 0–700 fold in PM 2.5 extracts. The highest levels of gene induction were seen in the A and H site filters during the month of January 2007 for the PM10-2.5 extracts and in the site F and D sites for January 2007 PM 2.5 filter extracts.
Figure 3.
Analysis of CYP1A1 expression in BEAS2B cell treated for 18 hrs with dichloromethane extracts of PM10-2.5 (shown as PM10) or PM2.5 filters obtained from filters pooled from 2 weeks during Aug/Sept 2006, Oct 2006, Jan 2007, or 3 weeks during March/May/June 2007. Results shown are for the fold induction of gene expression determined using RT-PCR for three replicate cell treatments (Mean + SD) compared to DMSO solvent controls.
Analysis of Oxidative Stress Genes Induced by PM10-2.5 and PM 2.5 Extracts
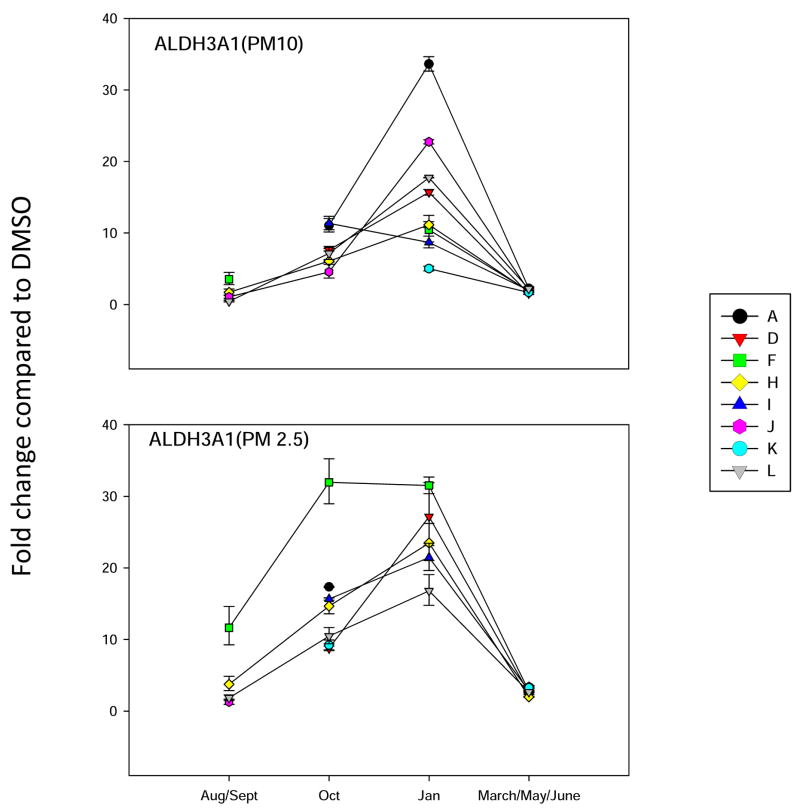
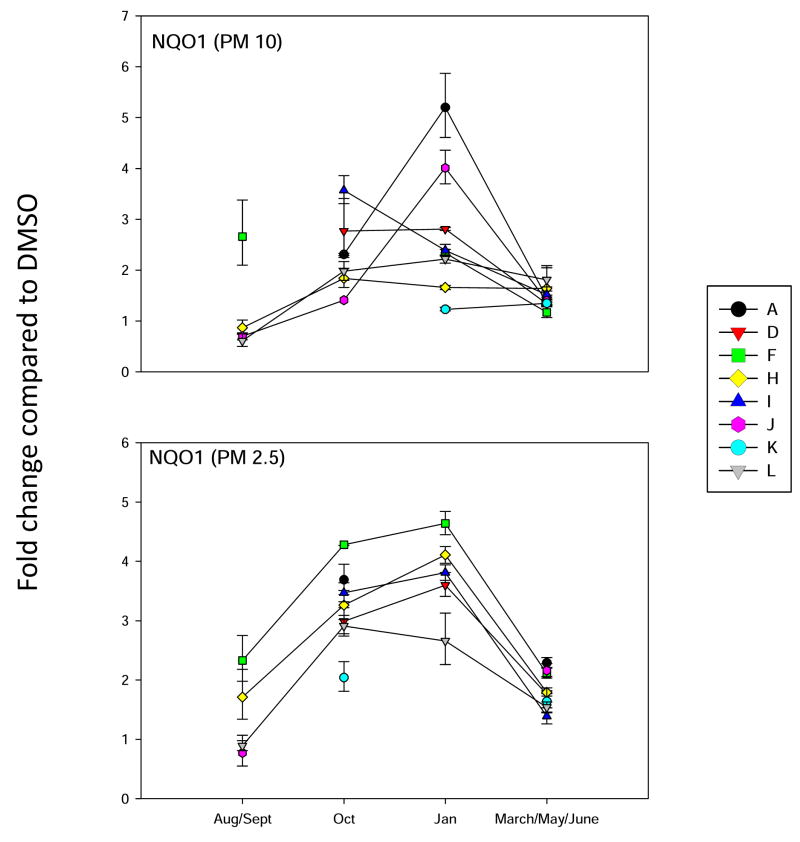
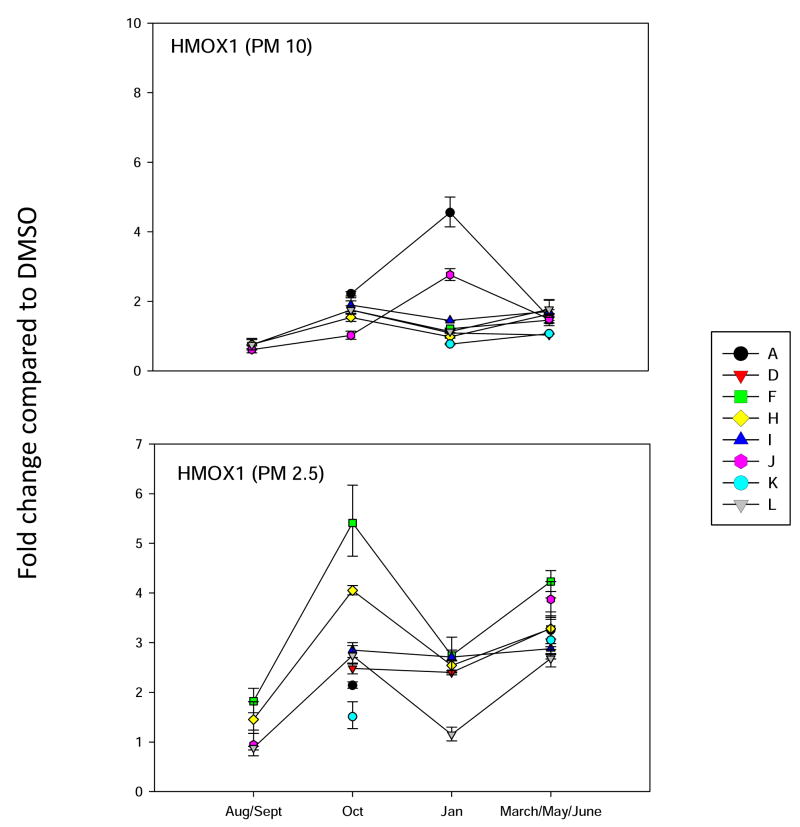
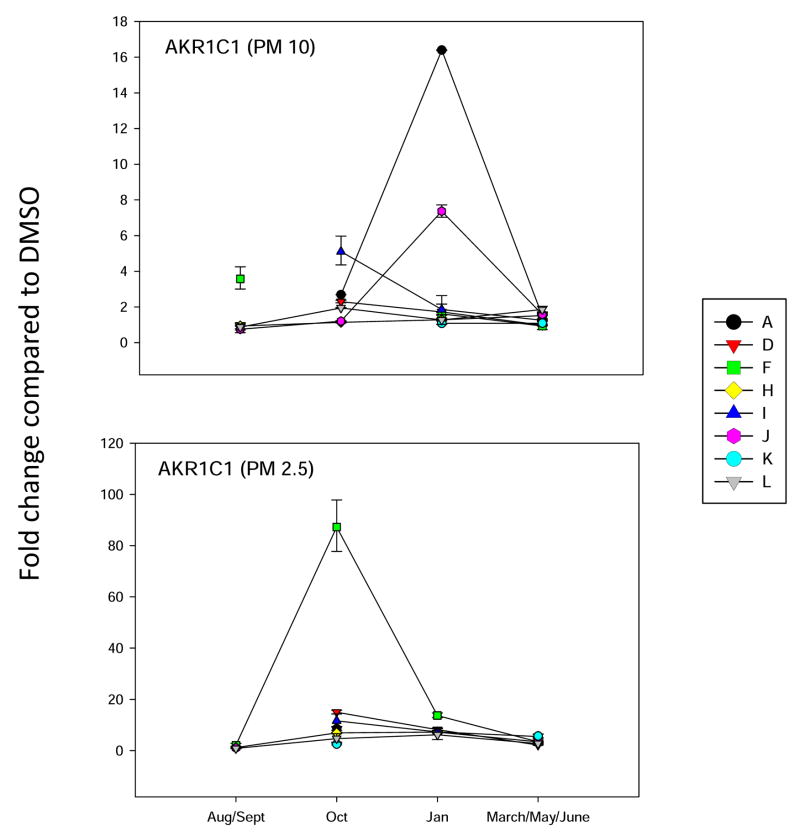
Since it is well known that PM extracts can induce oxidative stress in cells (Risom et al., 2005; Li et al., 2008), we analyzed several different marker genes that are activated via different mechanisms. Hemeoxygenase-1 (HMOX-1) has been used a marker for oxidative stress in cells (Chahine et al, 2007). It’s mechanism of induction is quite complicated and can involve several signaling pathways. The activation of antioxidant receptor element (ARE) signally pathways is associated with the induction of a number of Phase 2 enzymes, including aldehyde dehydrogenase 3A1 (ALDH3A1) and N-quinone oxidoreductase (NQO1) (Kong et al., 2001). Recently, the aldoketoreducatse 1C (AKR1C) family of enzymes (Penning et al., 2007) has also been shown to be induced by ARE signaling pathways (Lou et al., 2006). We have found that PAHs increase the expression of all four of the above genes in mammary epithelial cells (Burchiel et al, 2007). Therefore, all four genes were included in the oxidative stress analysis of PM10 and PM 2.5 extracts on BEAS2B cells. Results showed that ALDH3A1 gene was induced more that 30 fold by site A January 2007 PM10 extracts, and by October and January extracts of PM 2.5 obtained from site F (Figure 4). This is a similar pattern for that observed with the induction of NQO1 (Figure 5) and HMOX-1 (Figure 6), with the exception that the fold changes were lower for both of these genes. Interestingly, the AKR1C1 gene was induced 80 fold primarily in the October F site PM 2.5 samples (Figure 7). PM10 extracts induced AKR1C1 by 20 fold, mainly in Jan samples obtained from site A. Collectively, these results demonstrate that oxidative stress genes have mixed properties of induction that have some commonalities within the group and that may overlap pathways associated with CYP1A1 induction.
Figure 4.
Analysis of ALDH3A1 gene expression in BEAS2B cells, as described in Figure 3.
Figure 5.
Analysis of NQO1 gene expression in BEAS2B cells, as described in Figure 3.
Figure 6.
Analysis of HMOX-1 gene expression in BEAS2B cells, as described in Figure 3.
Figure 7.
Analysis of AKR1C1 gene expression in BEAS2B cells, as described in Figure 3.
Analysis of Inflammatory Cytokine Gene Expression
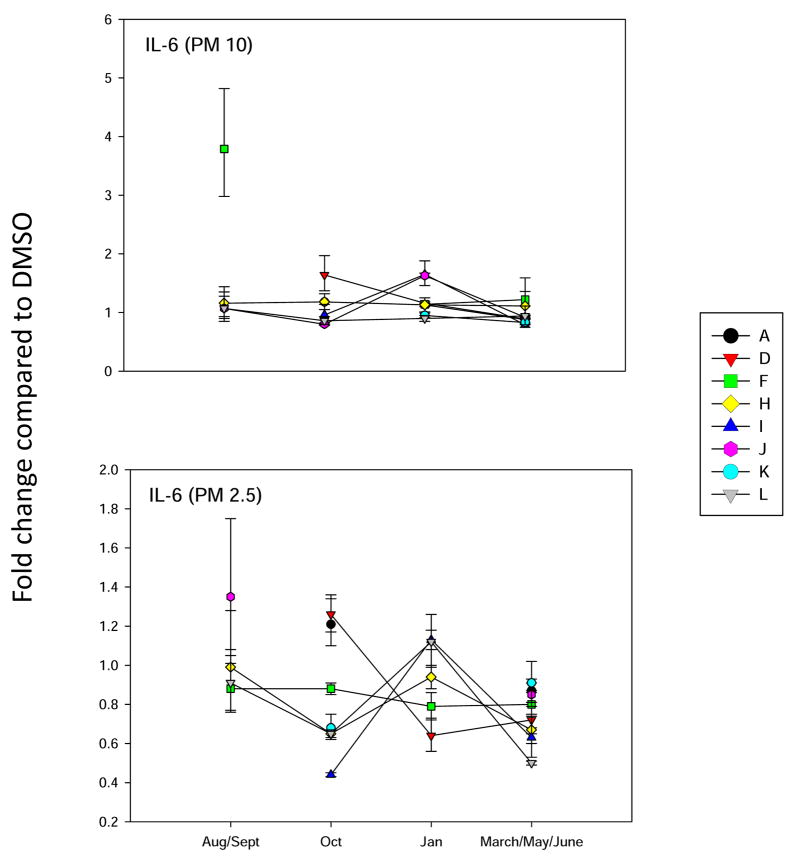
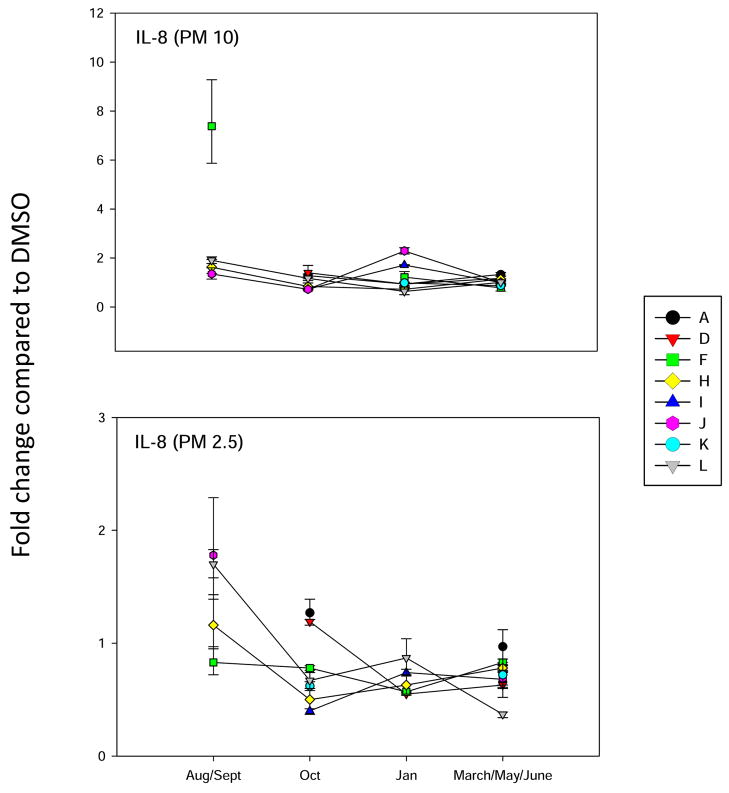
Two cytokines that have been associated with inflammatory lung diseases induced by PM are interleukin-6 (IL-6) and interleukin-8 (IL-8) (Becker et al., 2005; Zhao et al, 2008). The August/Sept 2006 PM10 and PM 2.5 demonstrated small increases in IL-6 (Figure 8) and IL-8 (Figure 9) gene induction. PM extracts from other months demonstrated minimal activity for IL-6 and IL-8 induction. Thus, it appears that the PAHs present in the PM10-2.5 and 2.5 fractions have minimal pro-inflammatory activities.
Figure 8.
Analysis of IL-6 gene expression in BEAS2B cells, as described in Figure 3.
Figure 9.
Analysis of IL-8 gene expression in BEAS2B cells, as described in Figure 3.
PAH Analyses of October 2006 and January 2007 Sites
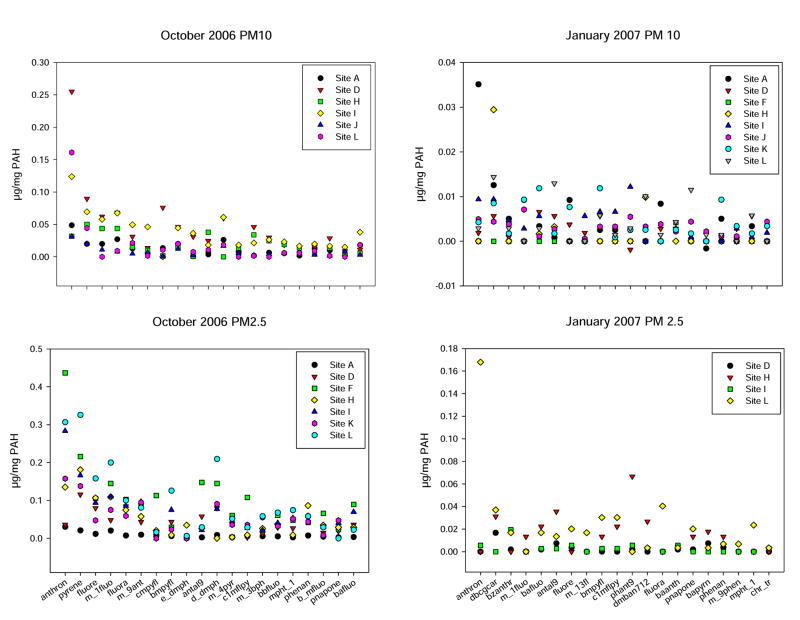
Dichloromethane extracts of two PM 10-2.5 and PM 2.5 filters from each of the sites for the months of October 2006 and January 2007 samples were analyzed for 150 PAHs by the Desert Research Institute (Reno, NV). These months were chosen for detailed analysis because of the strong gene induction profiles that were seen in these samples. As shown in Figure 9, the top 20 PAHs were compared for their spatial distributions at 10 sites in El Paso. Results are expressed on a ug/mg of total PM mass basis. October 2006 PM samples had overall greater amounts of PAHs than did January 2007 samples. Anthrone, pyrene, fluoranthene, and m-fluorene, were detected in highest amounts in October PM2.5 samples. Anthrone was also the predominant PAH detected in January PM10-2.5 samples.
Correlation of PAH Levels with Gene Expression in October 2006 and January 2007 Samples
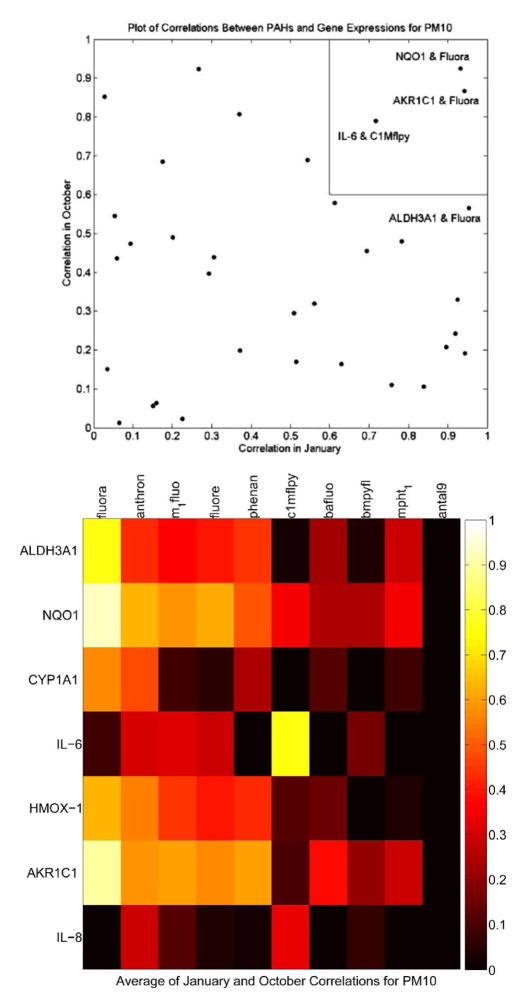
Calculation of Pearson correlation coefficients for individual PAHs from PM10-2.5 samples and compared to marker gene expression cross all sites examined revealed that the top 20 PAHs detected in October 2006 and January 2007 PM10 samples correlated roughly with the induction of oxidative stress genes NQO1 and AKR1C1 and the presence of fluoranthene in sample extracts. Not surprisingly, initial experiments with pure fluoranthene could not replicate the gene expression finding in relevant concentration ranges (nm-μm concentrations). Thus, current studies are underway to examine various combinations of pure PAHs in an attempt to replicate the findings obatained with sample extracts.
DISCUSSION
The Paso del Norte airshed is shared by two cities, El Paso, Texas, USA and Ciudad Juarez, Chihuahua, Mexico. About 2.2 million people live in the area with approximately 700,000 residing in El Paso. Over the past decades, increases in population have also led to increases in pollution. Both cities have failed to meet air quality standards for three pollutants; ozone, carbon monoxide, and particulate matter less than 10 μm in diameter (PM10). Some of the highest levels of PM10 are experienced during the winter months when temperature inversions trap PM10 in the ambient air. Industries including electronics, transportation, textile, and machinery, open burning, and the surrounding desert are major sources for the area’s pollution. Unregulated, least controlled open burnings, kilns for making bricks in particular, are reported to burn toxic waste fuels such as tires or used motor oil and considered one of the major contributors to PM10 pollution in the region.
Inhaled PM presents a major health concern due to its association with adverse effects on the respiratory system and cardiovascular disease as well as exacerbations of allergies, asthma, chronic bronchitis and respiratory infections (Delfino et al., 2002 Delfino et al., 2005; Gilmour et al., 2008; Nel, 2008). Atmospheric PM is found in urban air as a mixture of liquid and solid particles with various composition and dimension. PM may be composed of mixtures containing thousands of various compounds. Diesel exhaust particles (DEP) could be composed of up to 18,000 different high-molecular-weight organic compounds. Polycyclic aromatic hydrocarbons (PAHs) are reported to exist in significantly quantities in DEP and urban PM due to their adsorption to the carbon surfaces of PM (Risom et al., 2005).
The present studies were designed to provide environmental information on the variation in PM and PAHs in various regions and during different seasons in El Paso. These studies were conducted as part of a multifactorial analysis of environmental exposures in the Paso del Norte airshed combined with the development of a cohort of children who are being tracked for asthma conditions. Although PAHs have been well characterized for their carcinogenic effects (Lewtas, 2007), only recently have they been implicated in the induction of inflammation and oxidative stress seen in many animal models (Li et al, 2006). Oxidative stress by PAHs has previously been attributed to redox-cycling quinones (Li et al., 2002), which can form from numerous PAHs in the presence of u.v. light or cellular peroxidases (Reed et al., 2004). Oxidative stress produced by PM has previous also been attributed to the presence of metals (Risom et al., 2004). In the present studies, we extracted our PM samples with dichorlomethane, which is not effective in removing metals from PM, and our analysis demonstrated little (sub ppm levels) metal contamination. However, we cannot rule out the possibility that low levels of metals may contribute to our findings and that there may be important interaction with PAHs. In addition, there are other factors associated with PM, such as crustal silicates that are likely present in our samples, as well as endotoxin that may bind to PM and may contribute to our results.
In this study we investigated the PAH of composition PM2.5 and PM10-2.5 extracts and compared these finding to marker gene induction in human bronchial epithelial cells to determine possible associations with the activation biological signaling pathways. The results of the present studies demonstrated significant temporal and spatial variation in the amounts and compositions of PAH mixtures in El Paso air. As reported previously (Arietta et al., 2004), we found evidence that there are Ah receptor ligands present in air samples, as evidenced by the induction of CYP1A1 in BEAS2B cells. Based on these earlier studies we believe that the concentrations of PAHs obtained from air PM samples are representative of those that can be experienced in highly polluted areas near traffic and other sources. Nearly all samples obtained from all sites produced some level of CYP1A1 induction, but it was striking that some samples produced greater than 800-fold induction during certain time periods (January 2007). The reason for this peak in activity is unclear, but likely results from unique atmospheric and meteorologic conditions leading to higher PAHs in PM samples. It is a concern that certain air samples contain high levels of AhR ligand activity as the agents that bind and activate these receptors have been linked to lung cancer (Bostrom et al., 2002; Bock and Kohle, 2006). Induction of AhR activity has also been linked to oxidative stress in human cells (Dalton et al., 2002). The specific PAH responsible for the induction of CYP1A1 in our samples has not definitively identified in the present studies, but several candidate PAHs are currently under investigation.
We found that PM extracts induced several markers of oxidative stress (ALDH3A1, NQO1, AKR1C1, and HMOX-1) in human lung epithelial cells. We believe that oxidative stress is associated with the activation of both the Ah receptors and subsequent binding to xenobiotic response elements (XRE), as well as activation of antioxidant response elements (ARE) and Nrf-2 signaling pathways (Kong et al., 2001; Risom et al., 2005; Li and Nel, 2006). Both pathways are important in the gene regulation of the most highly induced marker genes observed in this study ALDH3A1, NQO1, and AKR1C1 (Kohle and Bock, 2007). Previous studies in one of our labs have shown that benzo(a)pyrene quinones (BPQs) induce a similar battery of genes (Burchiel et al., 2007). In support of recent epidemiologic studies (Hertz-Picciotto et al., 2007), our continuing working hypothesis is environmental PAHs may play a role in producing oxidative stress that could be associated with the exacerbation of asthma.
The HMOX-1 gene was also induced in BEAS2B cells by PM extracts. The HMOX-1 gene is induced by carbon monoxide and nitric oxide in many cells, and also is under the control of numerous pathways, including the XRE and ARE pathways (Ryter et al., 2006). HMOX-1 is an important marker of environmental oxidative stress in many tissues (Na and Surh, 2007), and has been used in animal studies (Rouse et al., 2008) and epidemiologic studies as a marker of PM-induced oxidative stress (Chahine et al., 2007). In the present studies, we found that HMOX-1 was induced by both PM10-2.5 and PM2.5 extracts containing PAHs. The induction of HMOX-1 in BEAS2B cells appeared to correlate better with the PAHs present in October PM 2.5 samples rather than PM10-2.5. Further studies are necessary to determine the mechanism(s) of HMOX-1 activation by complex mixtures of PAHs.
The induction of lung inflammation is associated with asthma (Becker et al., 2005). Although we found a small but significant association of PAHs and the induction of IL-6, we were somewhat surprised to determine that PAHs extracted from ambient PM had minimal effects on the induction of inflammatory cytokines (IL-6 and IL-8) in bronchial epithelial cells. Previous studies that have examined PM extracts in cultured airway epithelial cells have found that IL-8 is induced (Becker et al., 2005; Duvall et al., 2008). Additionally, we have reported that redox-cycling quinones can induce histamine degranulation and IL-4 production in human peripheral blood basophils, and presumably similar pathways are activated in lung mast cells, that could contribute to atopic asthma (Kepley et al., 2004).
The geographic distribution of the activity of the extracts suggests that exposure to the causative agents varies significantly across neighborhoods. In Figures 3–9, Sites K and L generally exhibit the lowest fold changes, and Sites F and A, the highest. These relative activities of the PM extracts is consistent with the location of Site L in a rural desert, K in a residential and farming area proximal to the Rio Grande, and F in the urban core zone. The reasons for the high activity at Site A (suburban, commercial) call for further study.
In conclusion, we found that there is both temporal and spatial variation in the amount and nature of PAHs that are present in the Paso del Norte Air Basin, with the highest levels being measured during winter months. PM extracts were found to induce genes which are known markers of AhR ligand activity and oxidative stress. There were important differences between the activities detected in the PM10-2.5 extracts compared to the PM 2.5 extracts. We believe that the oxidative stress induction may play important roles in the exacerbation of asthma in especially children living in high exposure sites. Current studies are aimed at measuring asthma rates in these areas and then performing a multifactorial analysis to determine if PAHs, VOCs, ozone, and/or NOx levels are correlated with the incidence of asthma in children based on land use regression models and exposure source algorithms (Mukerjee et al., 2004; Gonzales et al., 2005; Smith et al., 2006).
Figure 10.
The levels of the top 20 PAHs detected in PM10-2.5 and PM2.5 filter extracts for each of the Paso del Norte airshed sampling sites measured during Oct 2006 and January 2007. Results shown are the mean for duplicate determinations of each PAH compared to reference samples expressed on a ug/mg total PM mass measured on each filter.
Figure 11.
Correlation of top 20 PAHs measured during October 2006 and January 2007 at all sites with the fold induction for each of the target genes measured at the various sites. In the top graph, the correlations for individual PAHs and genes are shown for both October 2006 and January 2007 samples. The PAH that showed significant correlation for both samples sets was fluoranthene. In the lower graph, the averaged correlations for all zones for the January and October samples are shown for the top ten PAHs as a function of the marker gene expression. Light colors indicate the highest positive correlations using Pearson correlation coefficients (highest correlation = 1.0 on y axis). Dark colors indicate either no correlation (correlation = 0) or negative correlations also shown as 0 on y axis.
Acknowledgments
This work was supported in part by NIEHS S11 ES013339-01A1, the New Mexico Center for Environmental Health Sciences P30 ES012072, and the University of Texas at El Paso
Abbreviations
- IL-6
interleukin-6
- IL-8
interleukin-8
- PAHs
polycyclic aromatic hydrocarbons
- PM
particluate matter
Footnotes
Competing Interests Declaration: The authors have no competing interests of conflicts to report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson Instruments Inc. Operators and Instruction Manual Dichotomous Sampler. Atlanta, Ga: May, 1990. [Google Scholar]
- A.P. Buck, Inc. Mini-Buck Calibrator Instruction Manual. 1987. [Google Scholar]
- Arrieta DE, Ontiveros CC, Li W-W, Garcia JH, Denison MS, McDonald JD, Burchiel SW, Washburn BS. Aryl Hydrocarbon Receptor-Mediated Activity of Particulate Organic Matter from the Paso del Norte Airshed Along the U.S.-Mexico Border. Environ Health Perspectives. 2003;111:1299–1305. doi: 10.1289/ehp.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Mundandhara S, Devlin RB, Madden M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: further mechanistic studies. Toxicol Appl Pharmacol. 2005;207(2 Suppl):269–275. doi: 10.1016/j.taap.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Bock KW, Köhle C. Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol. 2006;72:393–404. doi: 10.1016/j.bcp.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Boothe VL, Shendell DG. Potential health effects associated with residential proximity to freeways and primary roads: review of scientific literature, 1999–2006. J Environ Health. 2008;70:33–41. 55–56. [PubMed] [Google Scholar]
- Boström CE, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, Rannug A, Törnqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110(Suppl 3):451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge D, Durant JL, Rioux C. Near-highway pollutants in motor vehicle exhaust: a review of epidemiologic evidence of cardiac and pulmonary health risks. Environ Health. 2007;6:23. doi: 10.1186/1476-069X-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchiel SW, Thompson TA, Lauer FT, Oprea TI. Activation of dioxin response element (DRE)-associated genes by benzo(a)pyrene 3,6-quinone and benzo(a)pyrene 1,6-quinone in MCF-10A human mammary epithelial cells. Toxicol Appl Pharmacol. 2007;221:203–214. doi: 10.1016/j.taap.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick AD, Davis JD, Liu KJ, Hudson LG, Shi H, Monske ML, Burchiel SW. Benzo(a)pyrene Quinones Increase Cell Proliferation, Generate Reactive Oxygen Species, and Transactivate the Epidermal Growth Factor Receptor. Cancer Research. 2003;63:7825–7833. [PubMed] [Google Scholar]
- Chahine T, Baccarelli A, Litonjua A, Wright RO, Suh H, Gold DR, Sparrow D, Vokonas P, Schwartz J. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect. 2007;115:1617–1622. doi: 10.1289/ehp.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Fan Y, Karyala S, Schwemberger S, Tomlinson CR, Sartor MA, Puga A. Ligand-independent regulation of transforming growth factor beta1 expression and cell cycle progression by the aryl hydrocarbon receptor. Mol Cell Biol. 2007;27:6127–6139. doi: 10.1128/MCB.00323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton TP, Puga A, Shertzer HG. Induction of cellular oxidative stress by aryl hydrocarbon receptor activation. Chem Biol Interact. 2002;141:77–95. doi: 10.1016/s0009-2797(02)00067-4. [DOI] [PubMed] [Google Scholar]
- Delfino RJ. Epidemiologic evidence for asthma and exposure to air toxics: Linkages between occupational, indoor, and community air pollution research. Environ Health Perspect. 2002;110(suppl 4):573–589. doi: 10.1289/ehp.02110s4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, Kleinman MT, Vaziri ND, Longhurst J, Zaldivar F, Sioutas C. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Perspect. 2008;116:898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall RM, Norris GA, Dailey LA, Burke JM, McGee JK, Gilmour MI, Gordon T, Devlin RB. Source apportionment of particulate matter in the U.S. and associations with lung inflammatory markers. Inhal Toxicol. 2008;20:671–83. doi: 10.1080/08958370801935117. [DOI] [PubMed] [Google Scholar]
- Gilmour MI, Jaakkola MS, London SJ, Nel AE, Rogers CA. How exposure to environmental tobacco smoke, outdoor air pollutants, and increased pollen burdens influences the incidence of asthma. Environ Health Perspect. 2006;114:627–633. doi: 10.1289/ehp.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginde AA, Espinola JA, Camargo CA., Jr Improved overall trends but persistent racial disparities in emergency department visits for acute asthma, 1993–2005. J Allergy Clin Immunol. 2008;122:319–321. doi: 10.1016/j.jaci.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales M, Qualls C, Hudgens E, Neas L. Characterization of a Spatial Gradient of Nitrogen Dioxide Across a United States-Mexico Border City During Winter. Sci Total Environ. 2005;337:163–173. doi: 10.1016/j.scitotenv.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Baker RJ, Yap PS, Dostál M, Joad JP, Lipsett M, Greenfield T, Herr CE, Benes I, Shumway RH, Pinkerton KE, Srám R. Early childhood lower respiratory illness and air pollution. Environ Health Perspect. 2007;115:1510–1518. doi: 10.1289/ehp.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Shankardass K, Berhane K, Gauderman WJ, Künzli N, Avol E, Gilliland F, Lurmann F, Molitor JN, Molitor JT, Thomas DC, Peters J, McConnell R. Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ Health Perspect. 2008;116:1433–1438. doi: 10.1289/ehp.10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepley CL, Lauer FT, Oliver JO, Burchiel SW. Polycyclic aromatic hydrocarbons (PAHs) enhance IgE-mediated histamine release and IL-4 production in human basophils. Clin Immunol. 2003;107:100–109. doi: 10.1016/s1521-6616(03)00004-4. [DOI] [PubMed] [Google Scholar]
- Köhle C, Bock KW. Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem Pharmacol. 2007;73:1853–62. doi: 10.1016/j.bcp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Kong AN, Owuor E, Yu R, Hebbar V, Chen C, Hu R, Mandlekar S. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE) Drug Metab Rev. 2001;33:255–271. doi: 10.1081/dmr-120000652. [DOI] [PubMed] [Google Scholar]
- Lewtas J. Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat Res. 2007;636:95–133. doi: 10.1016/j.mrrev.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Nel AE. Role of the Nrf2-mediated signaling pathway as a negative regulator of inflammation: implications for the impact of particulate pollutants on asthma. Antioxid Redox Signal. 2006;8:88–98. doi: 10.1089/ars.2006.8.88. [DOI] [PubMed] [Google Scholar]
- Li WW, Orquiz R, Garcia JH, Espino TT, Pingitore NE, Gardea-Torresdey J, Chow J, Watson JG. Analysis of temporal and spatial dichotomous PM air samples in the El Paso-Cd. Juarez air quality basin. J Air Waste Manag Assoc. 2001;51:1551–1560. doi: 10.1080/10473289.2001.10464377. [DOI] [PubMed] [Google Scholar]
- Lou H, Du S, Ji Q, Stolz A. Induction of AKR1C2 by phase II inducers: identification of a distal consensus antioxidant response element regulated by NRF2. Mol Pharmacol. 2006;69:1662–1672. doi: 10.1124/mol.105.019794. [DOI] [PubMed] [Google Scholar]
- Meng YY, Wilhelm M, Rull RP, English P, Ritz B. Traffic and outdoor air pollution levels near residences and poorly controlled asthma in adults. Ann Allergy Asthma Immunol. 2007;98:455–63. doi: 10.1016/S1081-1206(10)60760-0. [DOI] [PubMed] [Google Scholar]
- Mukerjee S, Noris GA, Smith L, Nobel C, Neas LN, Özkaynak AH, Gonzales M. Receptor Model Comparisons and Wind Direction Analyses of Volatile Organic Compounds and Submicrometer Particles in an Arid, Binational, Urban Airshed. Environ Sci Technol. 2004;15;38(8):2317–27. doi: 10.1021/es0304547. [DOI] [PubMed] [Google Scholar]
- Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol. 2008;46:1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Nel A. Atmosphere. Air pollution-related illness: effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- Orion Research. Cahn Model C-33 Microbalance Instruction Manual. 1997. [Google Scholar]
- Penning TM, Drury JE. Human aldo-keto reductases: Function, gene regulation, and single nucleotide polymorphisms. Arch Biochem Biophys. 2007;464:241–250. doi: 10.1016/j.abb.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risom L, Møller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutation Research. 2005;592:119–137. doi: 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Rouse RL, Murphy G, Boudreaux MJ, Paulsen DB, Penn AL. Soot nanoparticles promote biotransformation, oxidative stress, and inflammation in murine lungs. Am J Respir Cell Mol Biol. 2008;39:198–207. doi: 10.1165/rcmb.2008-0057OC. [DOI] [PubMed] [Google Scholar]
- Smith LA, Mukerjee S, Gonzales M, Stallings C, Neas L, Norris G, Özkaynak H. Use of GIS and ancillary variables to predict particulate, volatile organic compound and nitrogen dioxide pollutant levels at unmonitored locations. Atmos Environ. 2006;40:3773–3787. [Google Scholar]
- U.S. EPA, Reference Method for the Determination of Particulate Matter as PM10 in the Atmosphere (Dichotomous Sampler Method). Quality Assurance Handbook for Air Pollution Measurements Systems, Vol. II, Section 2.10 (1994).
- U.S. EPA, Guidance for Network Design and Optimum Site Exposure for PM2.5 and PM10, EPA-454/R-99-022, EPA OAQPS, Research Triangle Park, NC December 1997.
- Veranth JM, Moss TA, Chow JC, Labban R, Nichols WK, Walton JC, Watson JG, Yost GS. Correlation of in vitro cytokine responses with the chemical composition of soil-derived particulate matter. Environ Health Perspect. 2006;114:341–349. doi: 10.1289/ehp.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veranth JM, Reilly CA, Veranth MM, Moss TA, Langelier CR, Lanza DL, Yost GS. Inflammatory cytokines and cell death in BEAS-2B lung cells treated with soil dust, lipopolysaccharide, and surface-modified particles. Toxicol Sci. 2004;82:88–96. doi: 10.1093/toxsci/kfh248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environmental Health Perspectives. 2005;113:1536–1541. doi: 10.1289/ehp.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Usatyuk PV, Gorshkova IA, He D, Wang T, Moreno-Vinasco L, Geyh AS, Breysse PN, Samet JM, Spannhake EW, Garcia JG, Natarajan V. Regulation of COX-2 Expression and IL-6 Release by Particulate Matter in Airway Epithelial Cells. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2008-0105OC. epub.ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]