Abstract
In eukaryotic cells, the nucleus contains the genome and is the site of transcriptional regulation. The nucleus is the largest and stiffest organelle and is exposed to mechanical forces transmitted through the cytoskeleton from outside the cell and from force generation within the cell. Here, we discuss the effect of intra- and extracellular forces on nuclear shape and structure and how these force induced changes could be implicated in nuclear mechanotransduction, i.e. force-induced changes in cell signaling and gene transcription. We review mechanical studies of the nucleus and nuclear structural proteins, such as lamins. Dramatic changes in nuclear shape, organization, and stiffness are seen in cells where lamin proteins are mutated or absent, as in genetically engineered mice, RNAi studies, or human disease. We examine the different mechanical pathways from the force-responsive cytoskeleton to the nucleus. We also highlight studies which link changes in nuclear shape with cell function during developmental, physiological and pathological modifications. Together, these studies suggest that the nucleus itself may play an important role in the cell’s response to force.
Keywords: nucleus, lamins, gene regulation, force
Introduction
Mechanotransduction describes the molecular mechanisms by which cells respond to changes in their physical environment by translating mechanical stimuli into biochemical signals. These mechanical changes or stimuli can be either forces exerted on the cell from the extracellular environment such as compression, tension and fluid shear stress, or intracellular forces arising from cellular responses to changes in extracellular matrix stiffness. For example, cells are able to adjust their internal stiffness to the stiffness of the extracellular matrix, clearly indicating mechanical feedback between the cell and its environment1. In many cases, force-responses are acute and may only transiently affect the cytoskeleton and local focal adhesions or intracellular messengers such as calcium concentration. However, mechanotransduction often refers to long-term phenotypic changes in the cell, commonly arising from mechanically induced changes in gene expression. Cells can sense mechanical stimulation and changes in their physical environment through force induced conformational changes on the molecular level, but many of the molecular mechanisms are still incompletely understood. Extracellular forces can stimulate stretch sensitive ion channels, activate integrins and other focal adhesion proteins, modify concentration and conformation of cytoskeletal crosslinking proteins and myosin2, or reorder the cytoskeleton through conformational changes in the actin, intermediate filament, or microtubule structures (See Janmey et al.3 and Vogel et al.4 for recent reviews).
For many mechanotransduction events, the downstream cellular pathways for force-sensed gene transcription, e.g. the activation of the transcription factors, have been well characterized. Opening of stretch sensitive ion channels can result in changes in intracellular ion concentrations, most commonly calcium, inside the cell both by ion influx and by release of ions from intracellular stores3. These changes in ion flux are widespread among cellular populations and can have different downstream effects including activation of signaling pathways which lead to changes in gene transcription. Similarly, cytoplasmic proteins can directly or indirectly affect transcription following activation of integrins, reorganization of cytoskeletal cross-linking proteins, or force-induced changes in cytoskeletal conformation and/or organization. Transcription factors, such as nuclear factor-κB (NF-κB), translocate from the cytoplasm to the nucleus upon mechanical stimulation, and protein cascades such as the mitogen-activated protein kinase (MAPK) cascade can activate transcription factors following cytoskeletal events5.
There are other more recently discovered examples where gene transcription is affected both by cytoskeletally-activated elements as well as nuclear proteins associated with nucleoskeletal structure. R-Smad proteins, which are activated by ligand binding to TGF-β, in turn interact with a nuclear organizational protein MAN-16. Loss of the nuclear envelope proteins lamin A and C can result in impaired NF-kB-regulated transcription7. The cell cycle regulator and tumor suppressor retinoblastoma protein (pRb) interacts with nucleoplasmic lamin binding proteins and lamin A8,9, and expression levels of lamin A/C correlate with the DNA-binding and transcriptional activity of activating protein 1 (AP-1), which in turn affects cellular proliferation10. Aside from these and other lamin-dependent changes in gene transcription in the nucleus, there are many other hypothesized mechanisms correlating nuclear shape to a mechanotransduction response of cells.
The nucleus itself has been proposed to act as a cellular mechanosensor, with changes in nuclear shape causing conformational changes in chromatin structure and organization and directly affecting transcriptional regulation. This review will concentrate on alterations in nuclear structure associated with induced mechanical force, independent of any chemical signals from the cytoplasm. To this end, we will describe the structural, load-bearing and force-sensitive components of the nucleus and review studies of their mechanical properties. We will then discuss the proposed mechanisms for force transmission between the extracellular matrix, the cytoskeleton, and the nucleus and how the induced changes in nuclear shape and structure can modulate cellular signaling and function to adapt to the altered physical environment of the cell.
Structural components of the nucleus
The cell nucleus can be structurally and functionally divided into at least two separate regions, the nuclear envelope and the nuclear interior. The nuclear envelope consists of two phospholipid bilayer membranes (i.e. the outer nuclear membrane, which is continuous with the endoplasmic reticulum, and the inner nuclear membrane) and the nuclear lamina. The inner and outer nuclear membranes join at the nuclear pore complexes, which allow nuclear-cytoplasmic transport. Underlying the inner nuclear membrane is the nuclear lamina, a dense protein network consisting mostly of lamin proteins and lamin-associated proteins. These lamin binding proteins help connect the lamina to the inner nuclear membrane and stabilize the lamina network in addition to connecting lamins to chromatin structures and gene regulatory components.
The nuclear interior is less well defined. Within the nucleoplasm, DNA is wound onto histones which are organized into chromatin fibers. These fibers in turn are organized into chromosomes that occupy distinct, non-random chromosome territories within the interphase nucleus11. Nuclear structures such as nucleoli, Cajal bodies, and PML bodies are also present as distinct structural and functional elements, and these structures could be influenced by mechanical forces. Several structural proteins are found in the nuclear interior, e.g. nucleoplasmic lamin A and lamin C proteins12, nuclear actins13, nuclear myosin14, and nuclear spectrins15. Despite the presence of these structural proteins in the nuclear interior, the existence of a structural, force-bearing nuclear matrix throughout the nuclear interior is a matter of open debate11,16.
Nuclear lamins
Lamins are the main components of the nuclear lamina, but also form stable structures in the nuclear interior. Lamins regulate and support protein complexes involved in gene expression17, DNA replication, transcription and repair18, nuclear positioning17, and aging19. Lamins are type V intermediate filament proteins divided into two different subtypes: A-type lamins, which are all products of alternative splicing from the LMNA gene, and B-type lamins, encoded by two separate genes, LMNB1 and LMNB2.
A-type lamins, the most common of which are lamins A and C, are developmentally regulated proteins found in varying levels in almost all differentiated cells, with high levels in skeletal and cardiac muscle20-24. A-type lamins are absent in human embryonic stem cells and are present only after cells begin differentiation25. Cells are able to survive and proliferate without A-type lamins26, but mutations in the LMNA gene are responsible for a group of human diseases referred to as laminopathies (described in detail below). Mice deficient in lamin A and C (lmna-/-) develop severe muscular dystrophy and die prematurely at 6-8 weeks of age27. Lamins A and C are in dynamic equilibrium between the nuclear lamina at the periphery and the nuclear interior28,29 and are hypothesized to modulate gene expression both at the nuclear periphery and interior 19,30,31. A-type lamins also play a major role in the maintenance of nuclear shape19,32,33, stability7,34 and structure12,33,35. In contrast to the lmna-/- mice, transgenic mice expressing lamin C but not lamin A show no overt phenotype, indicating that lamin A might be dispensable, at least in the mouse36. Also, nuclei from these lamin C-only mice show only slight alterations in shape and mechanics33. These recent studies highlight the complexity associated with nuclear lamina composition based on differential expression of lamins A, B and C.
B-type lamins are constitutively expressed in all cell types of metazoans37. In contrast to A-type lamins, only a single disease has been attributed to the LMNB1 gene, namely an autosomal dominant leukodystrophy caused by gene duplication38. Knockdown of B-type lamins is lethal in C. elegans39 and mice40, suggesting that mutations in B-type lamins may be embryonic lethal. RNAi gene silencing of LMNB1 and LMNB2 in cultured mammalian cells induces apoptosis26, indicating that these genes are essential to cell survival and not just organism survival. However, fibroblasts derived from a genetically engineered mouse with a severely truncated lamin B1 gene are viable, but show severe nuclear blebbing40 and defects in interphase chromosome positioning and gene regulation41.
Lamin binding proteins
Inner nuclear membrane lamin binding proteins such as LBR, emerin, LAP2α and MAN1 contain at least one transmembrane domain and a lamin binding domain42. These lamin binding proteins are dynamic and interact with many different partners which may provide the opportunity for changes in nuclear structure in response to biochemical and physical factors35,43. Emerin, which has been shown to bind lamin A/C in vitro and in vivo, can directly interact with numerous other structural proteins such as actin, nesprins as well as transcription factors such as Btf, GCL, and others44. It is unlikely that emerin binds all of these proteins simultaneously; most likely, there is a dynamic association of emerin with different protein complexes. Given the large number of lamin binding proteins and their many interactions, there is a complex web of possible structural and transcriptional interactions associated with the lamin network in the nucleus.
Lamin binding proteins also help connect the lamina with peripheral DNA and chromatin and are involved in gene expression. Lamins can directly bind to naked DNA via 30-40 base pair-long nonspecific segments45. However, most lamin-DNA interactions occur via lamin binding proteins46. As one example of many LEM-domain proteins, the inner nuclear membrane-spanning protein emerin can bind A-type lamins and the protein BAF, which in turn directly interacts with double stranded DNA, histone H3, histone H1.1, and possibly other transcription factors.
Other structural proteins in the nucleus
Recently, a number of structural proteins which are traditionally considered typical components of the cytoskeleton have also been identified inside the nucleus. The existence of nuclear actin in particular is now widely accepted, although it remains unclear what structures actin forms inside the nucleus47. Recent evidence suggests that aside from stores of globular actin, nuclear filamentous actin is primarily found as short oligomers13. Nuclear actin does not stain with phalloidin, and it is hypothesized that nuclear actin may polymerize in a unique conformation48 which is resistant to phalloidin labeling49. The functions of nuclear actin are also poorly understood, but several data imply that actin is involved in transcription50. Also, actin can bind lamins and lamin binding proteins51 and electron microscopy of Xenopus oocyte nuclei shows actin oligomers interacting with nuclear pores and Cajal bodies at the nuclear periphery52. These interactions suggest mechanical or structural function, but none have been determined yet. Interestingly, actin associated proteins such as protein 4.153, myosin14 and αII-spectrin15 have recently been identified in the nucleus as well and might be implicated in movement of DNA within the nucleus. αII-spectrin binds lamin complexes54 and aids in DNA repair55, but the mechanical function of αII-spectrin has not been elucidated. Other putative spectrin repeat proteins such as nesprin proteins (also called myne or syne) are also found at the nuclear envelope, as discussed in the sections below.
Chromatin
Chromatin, a complex of mainly DNA and histone proteins, is the major component of the nuclear interior and is critical to pack the approximately two meters of DNA (in humans) into a nucleus of 5-20 μm diameter. At least three architectural motifs have been characterized in higher-order organization of interphase chromatin56,57: (i) 30 nm fibers and other configurations resulting from nucleosome packing and stacking; (ii) loops of chromatin fibers ranging in size from several kbp to > 10 Mbp able to interact with distant genome regions; (iii) particular areas of the genome that are tethered to scaffolding structures like the nuclear lamina or the nucleolus. Chromatin is further organized into chromosomes, each ranging in size from 51 - 245 Mbp which occupy non-random chromosome territories in the interphase nucleus11.
Chromatin itself is not homogeneous, and chromatin structure, location, and function are correlated. Heterochromatin is densely packed chromatin, which usually reflects modifications of DNA, histones and other DNA binding proteins, and is typically transcriptionally inactive. Heterochromatin is often located at the periphery of the nucleus or close to the nucleolus; both of these genome regions present low activity in gene expression. Several specific proteins and characteristic histone modifications present in heterochromatin are responsible for silencing genes58. Conversely, euchromatin is gene rich with more transcriptional activity and is often located at the nuclear interior in more open chromatin structures. Recent micropipette aspiration experiments suggest that open euchromatin structures are more deformable than tightly packed heterochromatin structures in embryonic stem cells and model systems59, so one can imagine that external or intracellular forces could reorganize gene rich areas relatively easily.
Nuclear bodies and intranuclear structures
Nucleoli, regions of ribosome biogenesis, are the largest subnuclear structures. Nucleoli are distinct structures within nuclei, but nucleolar proteins exist in dynamic equilibrium with the nucleoplasm with transition times on the order of seconds60. Still, the fidelity of nucleolar structure appears to be driven by complex molecular interactions within the nucleolus61. As such, nucleoli appear structurally and mechanically distinct within the nucleoplasm. Nucleoli can be visualized in nuclei in whole cells using atomic force microscopy (AFM), suggesting that they are stiffer than the surrounding nucleoplasm62. Similarly, nucleoli appear as fluid structures which deform cohesively in cells which are deformed by micropipette aspiration, and they show permanent deformation under high stress59. While the importance of nucleolar stiffness is unknown, the compact nucleolar structure maintains its shape during short-term mechanical stress and can act as fiducial markers within the nucleoplasm to study sub-nuclear deformations59,63.
Cajal bodies, also called coiled bodies, are dynamic structures which associate with small nuclear ribonucleoproteins (snRNPs) and nucleoli64. Cajal bodies are regulated by cellular stresses such as heat shock, heavy metal exposure, viral infection, and DNA damage65, and numerous stimuli can cause Cajal bodies to translocate within the nucleoplasm66. Promyelocytic leukaemia (PML) bodies are involved with many aspects of nuclear function, including transcriptional regulation and senescence-associated changes in chromatin structure; PML bodies also respond to chemical cellular stresses65, but many of their functions remain unclear. PML bodies are typically located close to transcriptionally active genes67 and associate with nuclear structural proteins68. PML bodies increase in number and size in response to cellular mechanical stress, and are therefore thought to be stress-responsive structures69.
Laminopathies: Diseases associated with nuclear structure
Many physiological functions of nuclear structure and organization have recently been elucidated by studying pathophysiological changes associated with human diseases involving mutations in nuclear envelope proteins. Laminopathies are diseases caused by mutations in the LMNA gene encoding lamins A and C. This group of more than 12 diseases includes Emery-Dreifuss muscular dystrophy, limb girdle muscular dystrophy, dilated cardiomyopathy, Dunnigan-type familial partial lipodystrophy, and Hutchinson-Gilford progeria syndrome (see Worman and Bonne70 for a recent review). Even though lamins A and C are expressed in almost all differentiated cells, many of the laminopathies have tissue-specific phenotypes. To date, over 200 mutations in the LMNA gene have been identified; most of these mutations are linked to muscular dystrophies, but some mutations have little or no effect on muscle tissue. Thus, it remains unclear how different mutations in the same protein can cause such a broad spectrum of diseases. The molecular mechanism underlying these diseases remains unclear, in part because the function of the nuclear envelope is not completely understood. Cells derived from laminopathy patients often have abnormally shaped nuclei and changes in chromatin organization. One proposition to explain at least some of the tissue-specific defects associated with laminopathies has been the “structural hypothesis”, which proposes that functional loss of lamins A and C could increase nuclear fragility and result in increased cell death in mechanically stressed tissue such as muscle. Indeed, muscle biopsies from Emery-Dreifuss muscular dystrophy patients often show fragmented nuclei71, and experiments on lmna-/- mouse embryo fibroblasts show that these cells have decreased nuclear stiffness, increased nuclear fragility, and an increased sensitivity to mechanical strain7,28. Conversely, Hutchinson Gilford progeria syndrome (HGPS) is caused by a LMNA mutation which results in increased presence of wild-type and mutant lamin A at the nuclear envelope due to defective protein processing19,72 and results in stiffer, less compliant nuclei32. Patients with HGPS have a severe premature aged phenotype in nearly all load bearing tissues: cartilage, bone, skin, cardiovascular, etc., but they show only minimal or no defects in soft tissues such as the brain and internal organs. The presence of deficiencies in load bearing tissues of organism-level mutations suggests the role of force in disease progression. However, lamins not only play an important role in nuclear structure and stability, but also interact with several transcriptional regulators directly and indirectly, as discussed in the sections above. Through these interactions, lamins can modulate transcriptional regulation but also contribute to chromatin organization and epigenetic changes. Lmna-/- mouse embryo fibroblasts have altered proliferation, and lmna-/- myoblasts have impaired differentiation73. Similarly, HGPS nuclei show changes in interior chromatin organization, loss of heterochromatin condensation74, and accumulation of DNA damage75. Consequently, the “gene regulation hypothesis” proposes that altered interactions of these transcriptional regulators are responsible for the plethora of diseases.
Importantly, the “structural hypothesis” and “gene regulation hypothesis” are not mutually exclusive, and could in fact be interrelated through nuclear mechanotransduction. Experiments on lmna-/- mouse embryo fibroblasts showed that these cells have reduced activation of mechanosensitive genes in response to mechanical strain and impaired transcriptional activation7. Thus, changes in nuclear structure and function could contribute both to increased cellular sensitivity to mechanical strain and to altered transcriptional regulation. Furthermore, since the mechanical environment can direct stem cell differentiation76, impaired mechanotransduction signaling could contribute to some of the differentiation defects seen in lmna-/- myoblasts77,78. Beyond the nucleus itself, lamins A and C and other nuclear envelope proteins are critical for physically connecting the nucleus to the surrounding cytoskeleton; see below for details.
Taken together, these observations lead us to a more differentiated look at laminopathies based on the type and location of the particular LMNA mutations. Mutations affecting skeletal and cardiac muscle are often missense mutations79,80 that affect the stability of the protein or its ability to polymerize81. Lmna-/- mice that completely lack A-type lamins develop severe muscular dystrophy and dilated cardiomyopathy73, serving as an animal model for Emery-Dreifuss muscular dystrophy82-84. Loss of A-type lamins results in reduced nuclear stiffness and increased nuclear fragility27,33 leading to increased cellular sensitivity to mechanical stress, which can cause further defects in nuclear-cytoskeletal coupling73,85, mechanotransduction signaling7, tissue regeneration73,85,86, cell proliferation73, and cell differentiation25,77,87. However, the majority of human LMNA mutations linked to muscular dystrophies are autosomal dominant79, suggesting dominant negative effects of those mutations. Interestingly, most mouse models (e.g. lmnaH222P, lmnaN195K) require homozygous expression of the mutant lamin to elicit a phenotype84, although a recent report indicates that haploinsufficiency in lmna+/- mice results in late-onset dilated cardiopmyopathy86. LmnaHG/HG and lmnaHG/+ mice expressing a progerin construct show dose dependent effects that can also be modulated by levels of wild-type lamin A88,89. Progerin can alter the segregation of A-type and B-type lamin homopolymers90 and affect diffusional mobility of wild-type lamins59. Photobleaching experiments of fluorescently labeled lamins reveal that most LMNA mutations increase the protein’s mobility, with the most severe effects seen in mutations in the central rod-domain12,91. Taken together, these findings suggest that at least some of the mutant lamins can modulate stability and polymerization of wild-type lamins and generally affect overall nuclear structure, stability, and function, providing a possible explanation for some of the dominant negative effects of specific lamin A/C mutations. Functional loss of lamin A/C that results in reduced nuclear stiffness could contribute to increased cellular sensitivity to mechanical stress, which, along with additional defects in nuclear-cytoskeletal coupling, mechanotransduction signaling, tissue regeneration, cell proliferation, and cell differentiation such as myotubes fusion, could result in the progressive muscular phenotypes seen in some laminopathies. Lamin mutations that do not affect the overall stability of lamin A/C itself or its polymerization dynamics but can alter specific lamin functions (e.g. interaction with transcription factors) are likely responsible for some of the more specialized laminopathies such as familial partial lipodystrophy. Most of the mutations causing familial partial lipodystrophy are clustered together and alter the positive charge on the lamin tail Ig-fold92. Cells from HGPS patients have increased nuclear stiffness, changes in chromatin organization, and premature cell senescence, potentially altering stem cell maintenance and differentiation. Most recently, our group has demonstrated that increased cellular sensitivity to mechanical stress could also contribute to the development of arteriosclerosis in HGPS93. Thus, the laminopathies can be thought of a spectrum of diseases, with particular phenotypes resulting from which specific lamin functions are perturbed by a particular mutation.
Mechanical properties of the nucleus
The above examples illustrate how tissue-level diseases can arise from mutations in nuclear structural proteins; these diseases also correlate with changes in nuclear shape, structure, and stiffness. The transmission of mechanical forces to the nuclear interior and the induced nuclear deformations, which consequently could directly or indirectly modulate gene transcription, depend on the mechanical properties of the whole nucleus and its physical connection to the surrounding cytoskeleton. Here, we discuss the normal mechanical properties of the interphase nucleus and explain which nuclear components are the major determinants of nuclear stability. For more details on methods for the methodologies involved in measuring nuclear mechanics, please see the recent review by Lammerding, Dahl, et al.94
Although the exact values for measurements of nuclear stiffness vary over more than two orders of magnitude, ranging from as low as 0.1 kPa to 10 kPa95-98 depending on the cell type and experimental method chosen, most studies agree that the interphase nucleus is significantly stiffer than the surrounding cytoplasm. For example, parallel plate compression experiments revealed an effective elasticity of endothelial nuclei of 8 kPa compared to 0.5 kPa for the cytoplasm95. Micropipette aspiration studies of chondrocyte nuclei yielded static elastic moduli from 1 to 5 kPa, with data best fit by a three-parameter viscoelastic model96. Other studies of nuclear mechanics by micropipette aspiration have also found the nucleus of human HeLa cells99 and TC7 primate epithelial cells100 to be viscoelastic. In the former study, the HeLa nuclei behaved as viscoelastic solids; in the latter experiments, the nuclei were found to have a more complex viscoelastic rheology. These differences in mechanics may reflect differential nuclear organization, such as altered lamin A/C densities at the nuclear envelope or interior and/or changes in chromatin organization.
Our current understanding is that lamins provide a majority of the structural and mechanical support of the lamina and the overall nucleus. Lamin binding proteins can further stabilize the lamina and connect it to nuclear membrane and chromatin structures. The lamina has been shown to act as a stiff, load-bearing element necessary for the structural integrity of the nucleus34,100. Nuclei assembled in lamin-depleted Xenopus egg extracts are highly fragile101 and nuclei from mouse lmna-/- cells are mechanically weak7. In vitro rheology measurements of reconstituted lamin B1 filament solutions show these filaments to behave as stiff but elastic materials that display strain hardening and have mechanical strength comparable to that of other intermediate filaments102. Direct mechanical measurements of Xenopus oocyte nuclei also show the in situ, organized lamina to act as a stiff, elastic two-dimensional network34. While lamins and chromatin most likely both contribute to nuclear stiffness59, alteration of lamin concentration, particularly of A-type lamins, is suggested to modulate nuclear mechanics33. Our recent studies have shown that A-type lamins are the main contributors to nuclear stiffness, whereas loss of lamin B1 results in increased nuclear blebbing, but no changes in nuclear stiffness33. Similarly, only expression of ectopic lamin A, but not lamin B1, restored nuclear stiffness in lmna-/- mouse embryo fibroblasts33. These and other studies90,103 suggest that A- and B-type lamins may form distinct networks with specific structural differences.
In addition to the nuclear lamina, the nuclear interior also contributes to the mechanical behavior of the nucleus. Nuclear lamins, particularly A-type lamins, are also found in the nuclear interior and exchange with the nuclear lamina12. The presence of these internal lamins and lamin binding proteins such as LAP2α could provide structure and organization within the nucleoplasm. Chromatin itself is also thought to provide structure and mechanical stability to the nucleus100. Chromatin structures, which are highly entangled and interconnected, have a more viscous nature or “flow” more than the lamina network, which tends to stretch elastically59. Chromatin will also deform plastically, i.e. permanently, under high mechanical stress59. The role of chromatin organization (i.e. heterochromatin versus euchromatin) in nuclear mechanics has not yet been mechanistically studied, but alterations in chromatin by divalent salts100 or upregulation of heterochromatin proteins59,104 appear to both reduce chromatin movements inside the nucleus and stiffen the chromatin.
Proposed mechanisms of nuclear mechanotransduction
Knowledge of nuclear mechanical properties allows a quantitative assessment of nuclear deformation in response to a given force. With the stiffness of nuclear components roughly defined as in the sections above, the next step is to determine the physiological forces acting on the nucleus. Typically, these forces arise from forces acting on the extracellular matrix or from intracellular processes (e.g. actin-myosin interactions) and are thought to be transmitted to the nucleus via the cell’s cytoskeleton.
Transmission of forces to the nucleus: Cytoskeletal - nuclear connectivity
The organization of the cell cytoskeleton is known to actively participate in the ability of cells to sense and convert mechanical stresses to biological responses. In general, the cytoskeleton is composed of three distinct components: actin microfilaments, microtubules and intermediate filaments105-107. The actin cytoskeleton is thought to provide protrusive and contractile forces, and compressive bearing microtubules to form a polarized network allowing organelle and protein movement throughout the cell. Intermediate filaments provide added structure reinforcement. These structural features act together to provide cell shape, support and mechanical integrity108,109 and are necessary for cell motility and division110. The cytoskeleton has complex viscoelastic properties, reflective of its complex and heterogeneous composition and organization.
The cell is anchored to the extracellular matrix through focal adhesions, discrete complexes consisting of membrane spanning integrins and other proteins such as focal adhesion kinase (FAK), talin, and vinculin, which allow the cells to “communicate” with the extracellular matrix111,112. After the establishment of focal adhesions, interconnected actin fibers become stressed through the action of actin associated molecular motors. Cell adhesion1,113, shape1 (Discher et al. 2005), motility1,114 and differentiation76 can be mediated by the stiffness of the extracellular matrix and formation of focal adhesions. Thus, the properties of the extracellular matrix, including its mechanical character, are transmitted via focal adhesions to the cytoskeletal network of a cell.
As discussed above, experimental evidence has demonstrated that lamin structures play pivotal roles as structural elements in the maintenance of normal nuclear mechanics and cell mechanotransduction7,32-34, where the role of A-type lamins seems to be more influential than B-type lamins33. Several experimental findings suggest that A-type lamin expression can also affect the mechanical properties of the cytoplasm and the organization of cytoskeletal elements. Myocytes isolated from lmna-/- mice have a considerable decrease of connectivity between desmin intermediate filaments and the nuclear surface, which is associated with dramatic alterations in the overall cell shape73. Lmna-/- cells have considerable perturbations in the organization of actin-, vimentin- and tubulin-based filaments115. Additionally, the cytoplasmic rheology of lmna-/- mouse embryonic fibroblasts is similar to that of wildtype cells in which actin and microtubules have been chemically disrupted85. These studies all suggest that there are substantial physical interactions between the nucleoskeleton and the actin, intermediate filament, and microtubule cytoskeletal components. Functionally, cytoskeletal alterations in lmna-/- cells result in mislocalized microtubule organizing centers (MTOC) and altered cell migration speed85.
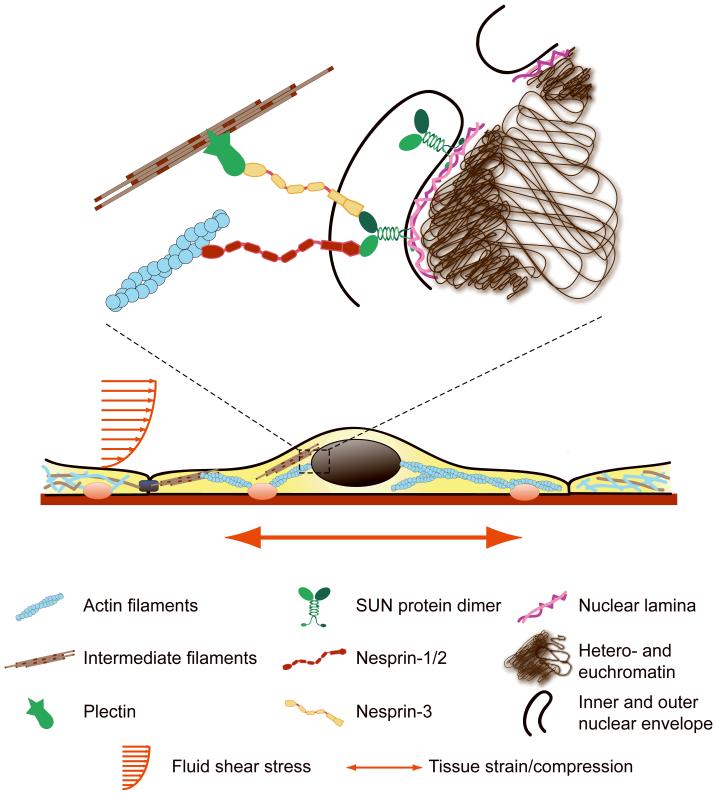
Physical connections between the cytoskeleton and the nuclear envelope provide a mechanism to transmit extracellular and cytoskeletal forces to the nucleus that is critical for nuclear mechanotransduction. Figure 1 provides an overview of our current understanding of nuclear-cytoskeletal coupling. SUN1116-118 and SUN2119 are inner nuclear membrane proteins that contain the Sad1-UNC homology domain (SUN) that is extended into the perinuclear space between the inner and outer nuclear membranes43. On the nucleoplasmic side, SUN proteins can interact with lamins, nuclear pore complexes, and other proteins, which are yet unknown. Nesprin proteins can bind to SUN proteins across the perinuclear space through a highly conserved C-terminal KASH domain (Klarsicht, Anc-1, Syne homology) consisting of a transmembrane domain and a luminal domain that interacts with SUN1/243. Recent findings suggest that mutations in the nuclear envelope proteins nesprin-1 and -2 could also contribute to Emery-Dreifuss muscular dystrophy120. While some smaller nesprin-1 and -2 isoforms are localized at the inner nuclear membrane and bind directly to lamin A120,121, many nesprin isoforms, including nesprin-3 and larger isoforms of nesprin-1 and -2, are outer nuclear membrane proteins122,123. The largest isoforms of nesprin-1 and -2 contain N-terminal actin binding domains, while nesprin-3 contains a site that binds to plectin, which stably associates to intermediate filaments117,123. This protein complex formed by the association of SUN proteins and nesprin proteins that allows a physical connection between the intermediate filament/actin cytoskeleton and the nucleoplasm via A-type lamins is aptly named the LINC (Linker of Nucleus and Cytoskeleton) complex117. Other lamin associated proteins such as the inner nuclear membrane protein emerin have been proposed to be an active component of the LINC complex35. Emerin stably interacts with lamins, chromatin, and inner nuclear membrane nesprins43,124. In emerin deficient cells, the nucleus is abnormally shaped and there are other deficiencies in cellular mechanotransduction125,126. Removal of other inner nuclear membrane lamin associated proteins such as LEM2 also result in severely altered nuclear morphology, but the mechanism has not been determined127. The above findings suggest that there are several, possibly redundant, protein complexes which can connect the cytoskeleton to the nucleoskeleton.
Figure 1. Pathways of force transmission from the extracellular matrix to the nucleus.
External forces can act on the cell through substrate strain or fluid shear stress. Integrins and other adhesion molecules physically couple the actin cytoskeleton to the extracellular matrix and can respond to extracellular ligands and intracellular signals. Cytoskeletal cross-linkers such as plectin can interconnect actin filaments, intermediate filaments, and microtubules. Plectin can also directly bind to nesprin-3 on the outer nuclear membrane, while the giant isoforms of nesprin-1 and -2 contain N-terminal actin binding domains. At the nuclear envelope, nesprins interact through their C-terminal KASH domain with SUN proteins, which cross the perinuclear space. At the inner nuclear membrane, SUN proteins can bind to lamins and other nuclear envelope proteins, which in turn can bind to DNA and chromatin, completing the physical link between the nucleus and the cytoskeleton. Cellular components are not drawn to scale.
Several lines of evidence also suggest direct connections between microtubules and the nuclear envelope. Microtubules directly interact with the nuclear envelope during nuclear envelope breakdown and may mechanically facilitate envelope rupture128, and cells treated with the microtubule depolymerizing drug nocodazole are deficient in the late stages of nuclear envelope breakdown129. Direct coupling of microtubules to the nuclear envelope is further supported by recent findings that in cells lacking either emerin or A-type lamins, the MTOC is often detached from the nucleus85. In addition, it was recently shown that emerin can directly interact with β-tubulin and thus serve as a docking element of the centrosome130. Other groups suggest that physical coupling between the nucleus and microtubules could be mediated by interactions of nesprins with kinesin motor proteins131. Microtubules are known to interact with actin and intermediate filaments via cross-linker and/or motor proteins109, so it is possible that observed changes in the localization of the MTOC in lmna-/- cells could be indirect consequences of alterations in the organization of actin and intermediate filaments.
How does force affect the nucleus?
Forces imposed on the cell surface, such as during flow, result in cell responses including the reorganization of cytoskeletal elements - actin microfilaments, intermediate filaments, microtubules132-134 and nuclear structures135 - away from the region of applied force. These observations suggest that mechanotransduction can be mediated by integrated elements of the cytoskeleton and may or may not be a localized phenomenon due to the complexity of percolated and interconnected cytoskeletal networks.
Even though the nucleus is the stiffest cellular organelle and is 2-10 times stiffer than the surrounding cytoskeleton95,96, extracellular forces and strain result in clearly detectable nuclear deformations96,135,136. In the case of cell monolayers exposed to fluid shear stress, the nucleus itself is exposed to significant amounts of force. Computational studies suggest that reordering of vascular endothelial cells in the direction of flow, as is seen in vitro and in vivo, could be explained by minimizing the force acting on the nucleus137. In addition to these observations of passive changes in nuclear shape and structure, there have also been studies showing the mechanical adaptation of nuclei to shear flow, suggesting that cells actively change nuclear structural elements in response to force. Micropipette aspiration of isolated nuclei show that nuclei exposed to shear stress have a reduced height and increased stiffness compared to non-sheared controls138. Atomic Force Microscopy (AFM) has also been used to investigate the elastic modulus of nuclei in whole cells and similarly found that nuclei in sheared cells are stiffer than control nuclei139. However, the molecular mechanism for this shear-induced stiffening of nuclear structure which persists after nuclear isolation remains unclear.
Nuclear shape and cell specialization
The cell nucleus is typically spheroidal or ellipsoid. However, due to changes in expression of structural and binding proteins some specialized cells undergo dramatic changes in nuclear shape during differentiation and maturation. For example, spermatids have extremely elongated nuclei140. Also, neutrophils develop extremely lobulated nuclei, which is associated with loss of lamin A/C141 and expression of lamin B receptor (LBR)142. Human embryonic stem cells have large, round nuclei, very mobile chromatin, and express no A-type lamins. As cells differentiate, changes in cell phenotype are correlated with reduction in chromatin movement as measured by histone mobility104, upregulation of A-type lamin components25 and changes in nuclear shape and stiffness59. Thus, as many cells specialize, one can observe concomitant changes in nuclear shape and structure as well as cellular function and phenotype. The functional changes may arise from modifications in chromatin structure that increase the accessibility of specialized genes necessary for differentiation, or, conversely, reduce accessibility of “unnecessary” genes to transcription factors. In many cases, one can also speculate that adaptations in nuclear shape and structure are directly related to the functionality of the cell: for example, more deformable lobulated nuclei in neutrophils allow increase intercellular translocation.
Studies focusing on nuclear shape and structure have revealed strong correlations between shape change and changes in cellular phenotype. By controlling the cellular environment with microfabricated patterning, Thomas and colleagues showed that collagen synthesis correlated more strongly with nuclear shape than with cell shape143. Studies on mammary epithelial cell tissue morphogenesis suggest that altering nuclear organization can modulate the cellular and tissue phenotype144. Compression-induced shapes changes in chondrocyte nuclei also correlate with changes in cartilage composition and density136. This correlative behavior becomes even more striking when pathological states are observed. Aberrations in gross nuclear morphology, such as increase in nuclear size, changes in nuclear shape, and loss of nuclear domains, are often used to identify cancerous tissue145. One study of breast cancer cells, which are affected by their mechanical and structural environment146, found a stronger correlation between a cancerous phenotype and nuclear morphology than cellular morphology and cancer147. Many cancers correlate with changes in nuclear structural proteins. For example, lamins A and C are overexpressed in ovarian cancers compared to control cells148, and increased levels of lamin B in prostate cancer strongly correlate with tumor differentiation87. Importantly, changes in nuclear stiffness can serve as indicator for increased mobility of tumor cells and metastasis potential149,150. As discussed earlier, decreased nuclear stiffness through the loss of lamin A/C and lobation may aid neutrophils and other cells to squeeze between endothelial cells during extravasation. These observed changes in nuclear shape may reflect changes in chromatin structure to modulate gene accessibility, differences in nuclear lamina composition that result in altered nuclear stiffness required for translocation, or both.
Conclusions and Outlook
The above examples clearly illustrate that nuclear shape, structure and/or stiffness strongly correlate with cellular function and phenotype in many physiological and pathological situations, particularly when force is involved. However, even with the wealth of information available on the connectivity of force-bearing elements in the cell and with the insight provided by laminopathies, transgenic and RNAi studies, there is still little or no mechanistic understanding of the direct role of force on nuclear mechanotransduction. The complexities of the biological systems, our limited knowledge of the function and organization of many nuclear structural proteins, as well as the intimate connection of these proteins with the DNA itself make it difficult to decouple mechanistic events. For example, in HGPS, both mutant and wildtype lamins accumulate at the nuclear envelope, causing a stiffer nuclear lamina32. However, the simultaneous reduction in lamin A in the nuclear interior leads to a loss of heterochromatin and other changes in chromatin organization74. As such, it will be challenging to decouple phenotypic cellular changes resulting from a stiffened nuclear envelope from epigenetic changes.
Searching for evidence: Can forces on the nucleus directly modulate gene transcription?
Currently there exist only limited and mostly anecdotal evidence that extracellular forces can directly affect gene transcription, e.g. by extracellular force transmitted to the nucleus acting directly on DNA elements. There are some compelling examples where physical connections have been seen which connect extracellular integrins to subnuclear elements135, and extracellular forces can be transmitted across the cytoskeleton to the nucleus, resulting in intranuclear deformations59. Inside the nucleus, these forces could result in conformation changes of the DNA double helix or higher order chromatin structure, which could then lead to changes in transcriptional activity. On the single molecule and macromolecular level, experiments examining mechanics of purified DNA, chromatin and chromosomes have shown that force can induce remodeling and disassembly, which may be required for transcription (see reviews on DNA, chromatin and chromosome mechanics151,152). Force induced conformational changes could further alter accessibility of chromatin and genes to transcription factors. Current imaging technology does not yet allow for direct visualization of force induced changes in DNA and chromatin organization in living cells, but advances in single molecule detection and imaging of transcription events in single cells may provide more direct evidence in the near future (see recent review of high resolution imaging in the nucleus153).
In addition to direct effects of force on DNA structure, force-induced changes in nuclear shape could also result in large scale reorganization of genes within the nucleus. The shape and mechanics of the nucleus is known to adapt and reorder when cells are exposed to force138,139. However, it remains unclear how the genes within the nucleus are subsequently reordered or if pockets of heterochromatin are altered by force or by lamin reorganization. Lamins are not only found at the nuclear periphery, but also form intranuclear structures and can modulate chromatin organization154,155. Several LMNA mutations are associated with loss of heterochromatin74, and loss of lamin B1 can affect positioning of chromosome territories within the nucleus41. Recent work suggests that a lamin B1-dependent nucleoskeleton is required for RNA synthesis in human cells156. Lamins may play a significant role as epigenetic modifiers of nuclear structure and organization since recruitment of certain genes to the nuclear envelope (and conversion from euchromatin to heterochromatin) is generally considered a cellular mechanism of transcriptional regulation and gene silencing11. Therefore, changes in nuclear stiffness measured in lamin-deficient and LMNA mutant cells could, in addition to changes in nuclear lamina organization, also reflect changes in intranuclear matrix and chromatin organization. Recent experiments confirm that gross epigenetic modifications during differentiation can be detected as changes in the mechanical properties of the nucleus, clearly demonstrating a relationship between chromatin structure, gene regulation, and nuclear structure and stiffness59. In these cases, altered gene regulation does not necessarily arise from changes in nuclear stiffness, but rather nuclear stiffness reflects changes in intranuclear organization and structure.
The challenge thus lies in the fact that nuclear stiffness is governed by both the lamina and the chromatin, which are inherently biologically and mechanically coupled. Also, even if we could conclude that lamins can directly affect force-induced gene expression, determining the underlying mechanism will prove difficult. Do disease causing mutations in lamins primarily allow changes in large-scale nuclear deformations by altering nuclear stiffness that causes increased conformational changes in DNA and chromatin organization, or can lamins molecularly modulate chromatin organization through their interaction with DNA and DNA processing proteins? We suggest that the answer will be a combination of these mechanisms based on the studies presented in this review. We are optimistic that cell based, top-down approaches and bottom-up in vitro experiments on force induced changes in DNA structure and function will converge to better elucidate the role in which force can directly modulate transcription of regions of DNA in the nucleus. The nuclear envelope, participating in cytoskeletal-nuclear force transmission and directly involved in chromatin organization, presents an important interface of the mechanical and biological domains.
In the post-genomic era we look to the regulation and expression of the genome. With this comes the recognition that in addition to decoding the meaning of linear DNA sequences, three-dimensional structure and organization of chromatin are critical components of nuclear gene regulation. As extracellular forces are transmitted to the nucleus, where they can cause substantial deformations, it should be no surprise if these forces could directly or indirectly contribute to changes in chromatin structure and transcriptional activity. To date, the fact that mechanical force and extracellular mechanical environment are additional, and essential, criteria for regulating cell response has been recognized in many other aspects of cell biology. Hopefully, with further study we will be able to better describe the direct mechanisms by which force interacts with the genome and how nuclear shape relates to mechanotransduction.
Acknowledgements
The authors would like to thank Valerie LRM Verstraeten for helpful discussions and feedback.
Sources of Funding
This work was supported by National Institutes of Health grants HL082792 and NS059348, the American Heart Association grant 0635359N, the Progeria Research Foundation.
Footnotes
Disclosures: None.
References
- 1.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CP, Tang HY, Carag C, Speicher DW, Discher DE. Forced unfolding of proteins within cells. Science. 2007;317:663–666. doi: 10.1126/science.1139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 4.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 5.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259:381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 6.Bengtsson L. What MAN1 does to the Smads. TGFbeta/BMP signaling and the nuclear envelope. FEBS J. 2007;274:1374–1382. doi: 10.1111/j.1742-4658.2007.05696.x. [DOI] [PubMed] [Google Scholar]
- 7.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozaki T, Saijo M, Murakami K, Enomoto H, Taya Y, Sakiyama S. Complex formation between lamin A and the retinoblastoma gene product: identification of the domain on lamin A required for its interaction. Oncogene. 1994;9:2649–2653. [PubMed] [Google Scholar]
- 9.Markiewicz E, Dechat T, Foisner R, Quinlan RA, Hutchison CJ. Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein. Mol Biol Cell. 2002;13:4401–4413. doi: 10.1091/mbc.E02-07-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivorra C, Kubicek M, González JM, Sanz-González SM, Alvarez-Barrientos A, O’Connor JE, Burke B, Andrés V. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev. 2006;20:307–320. doi: 10.1101/gad.349506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007;128:787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Broers JLV, Kuijpers HJH, Ostlund C, Worman HJ, Endert J, Ramaekers FCS. Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization. Exp Cell Res. 2005;304:582–592. doi: 10.1016/j.yexcr.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Pederson T, Aebi U. Nuclear actin extends, with no contraction in sight. Mol Biol Cell. 2005;16:5055–5060. doi: 10.1091/mbc.E05-07-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann WA, Johnson T, Klapczynski M, Fan JL, Lanerolle P. From transcription to transport: emerging roles for nuclear myosin I. Biochem Cell Biol. 2006;84:418–426. doi: 10.1139/o06-069. [DOI] [PubMed] [Google Scholar]
- 15.Young KG, Kothary R. Spectrin repeat proteins in the nucleus. Bioessays. 2005;27:144–152. doi: 10.1002/bies.20177. [DOI] [PubMed] [Google Scholar]
- 16.Pederson T. Half a century of “the nuclear matrix”. Mol Biol Cell. 2000;11:799–805. doi: 10.1091/mbc.11.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meaburn KJ, Misteli T. Cell biology: chromosome territories. Nature. 2007;445:379–381. doi: 10.1038/445379a. [DOI] [PubMed] [Google Scholar]
- 18.Parnaik VK, Manju K. Laminopathies: multiple disorders arising from defects in nuclear architecture. J Biosci. 2006;31:405–421. doi: 10.1007/BF02704113. [DOI] [PubMed] [Google Scholar]
- 19.Scaffidi P, Misteli T. Lamin A-Dependent Nuclear Defects in Human Aging. Science. 2006 May 19;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krohne G, Benavente R. The nuclear lamins. A multigene family of proteins in evolution and differentiation. Exp Cell Res. 1986;162:1–10. doi: 10.1016/0014-4827(86)90421-0. [DOI] [PubMed] [Google Scholar]
- 21.Tilli CM, Ramaekers FC, Broers JL, Hutchison CJ, Neumann HA. Lamin expression in normal human skin, actinic keratosis, squamous cell carcinoma and basal cell carcinoma. Br J Dermatol. 2003;148:102–109. doi: 10.1046/j.1365-2133.2003.05026.x. [DOI] [PubMed] [Google Scholar]
- 22.Takamori Y, Tamura Y, Kataoka Y, Cui Y, Seo S, Kanazawa T, Kurokawa K, Yamada H. Differential expression of nuclear lamin, the major component of nuclear lamina, during neurogenesis in two germinal regions of adult rat brain. Eur J Neurosci. 2007;25:1653–1662. doi: 10.1111/j.1460-9568.2007.05450.x. [DOI] [PubMed] [Google Scholar]
- 23.Hutchison CJ, Worman HJ. A-type lamins: guardians of the soma? Nat Cell Biol. 2004;6:1062–1067. doi: 10.1038/ncb1104-1062. [DOI] [PubMed] [Google Scholar]
- 24.Lin F, Worman HJ. Expression of nuclear lamins in human tissues and cancer cell lines and transcription from the promoters of the lamin A/C and B1 genes. Exp Cell Res. 1997;236:378–384. doi: 10.1006/excr.1997.3735. [DOI] [PubMed] [Google Scholar]
- 25.Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 2006;24:177–185. doi: 10.1634/stemcells.2004-0159. [DOI] [PubMed] [Google Scholar]
- 26.Harboth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–4565. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broers JL, Bronnenberg NM, Kuijpers HJ, Schutte B, Hutchison CJ, Ramaekers FC. Partial cleavage of A-type lamins concurs with their total disintegration from the nuclear lamina during apoptosis. Eur J Cell Biol. 2002;81:677–691. doi: 10.1078/0171-9335-00282. [DOI] [PubMed] [Google Scholar]
- 29.Moir RD, Yoon M, Khuon S, Goldman RD. Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J Cell Biol. 2000;151:1155–1168. doi: 10.1083/jcb.151.6.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman-Roblick R, Roblick UJ, Hellman U, Conrotto P, Liu T, Becker S, Hirschberg D, Jörnvall H, Auer G, Wiman KG. p53 targets identified by protein expression profiling. Proc Natl Acad Sci U S A. 2007;104:5401–5406. doi: 10.1073/pnas.0700794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pekovic V, Harborth J, Broers JL, Ramaekers FC, van Engelen B, Lammens M, von Zglinicki T, Foisner R, Hutchison C, Markiewicz E. Nucleoplasmic LAP2alpha-lamin A complexes are required to maintain a proliferative state in human fibroblasts. 2007 doi: 10.1083/jcb.200606139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006 July 5;103:10271–10276. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, Lee RT. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem. 2006 September 1;281:25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 34.Dahl KN, Kahn SM, Wilson KL, Discher DE. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J Cell Sci. 2004 September 15;117:4779–4786. doi: 10.1242/jcs.01357. [DOI] [PubMed] [Google Scholar]
- 35.Stewart CL, Roux KJ, Burke B. Blurring the boundary: the nuclear envelope extends its reach. Science. 2007;318:1408–1412. doi: 10.1126/science.1142034. [DOI] [PubMed] [Google Scholar]
- 36.Yang SH, Meta M, Qiao X, Frost D, Bauch J, Coffinier C, Majumdar S, Bergo MO, Young SG, Fong LG. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J Clin Invest. 2006;116:2115–2121. doi: 10.1172/JCI28968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worman HJ, Courvalin JC. The inner nuclear membrane. J Membr Biol. 2000;177:1–11. doi: 10.1007/s002320001096. [DOI] [PubMed] [Google Scholar]
- 38.Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, Koeppen A, Hogan K, Ptácek LJ, Fu YH. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38:1114–1123. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Rolef Ben-Shahar T, Riemer D, Treinin M, Spann P, Weber K, Fire A, Gruenbaum Y. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell. 2000;11:3937–3947. doi: 10.1091/mbc.11.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vergnes L, Péterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci U S A. 2004;101:10428–10433. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malhas A, Lee CF, Sanders R, Saunders NJ, Vaux DJ. Defects in lamin B1 expression or processing affect interphase chromosome position and gene expression. J Cell Biol. 2007;176:593–603. doi: 10.1083/jcb.200607054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holaska JM, Wilson KL, Mansharamani M. The nuclear envelope, lamins and nuclear assembly. Curr Opin Cell Biol. 2002;14:357–364. doi: 10.1016/s0955-0674(02)00329-0. [DOI] [PubMed] [Google Scholar]
- 43.Schirmer EC, Foisner R. Proteins that associate with lamins: many faces, many functions. Exp Cell Res. 2007;313:2167–2179. doi: 10.1016/j.yexcr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Holaska JM, Wilson KL. Multiple roles for emerin: implications for Emery-Dreifuss muscular dystrophy. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:676–680. doi: 10.1002/ar.a.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stierlé V, Couprie J, Ostlund C, Krimm I, Zinn-Justin S, Hossenlopp P, Worman HJ, Courvalin JC, Duband-Goulet I. The carboxyl-terminal region common to lamins A and C contains a DNA binding domain. Biochemistry. 2003;42:4819–4828. doi: 10.1021/bi020704g. [DOI] [PubMed] [Google Scholar]
- 46.Segura-Totten M, Kowalski AK, Craigie R, Wilson KL. Barrier-to-autointegration factor: major roles in chromatin decondensation and nuclear assembly. J Cell Biol. 2002;158:475–485. doi: 10.1083/jcb.200202019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pederson T. As functional nuclear actin comes into view, is it globular, filamentous, or both? J Cell Biol. 2008;180:1061–1064. doi: 10.1083/jcb.200709082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoenenberger CA, Buchmeier S, Boerries M, Sütterlin R, Aebi U, Jockusch BM. Conformation-specific antibodies reveal distinct actin structures in the nucleus and the cytoplasm. J Struct Biol. 2005;152:157–168. doi: 10.1016/j.jsb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Jockusch BM, Schoenenberger CA, Stetefeld J, Aebi U. Tracking down the different forms of nuclear actin. Trends Cell Biol. 2006;16:391–396. doi: 10.1016/j.tcb.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Percipalle P, Visa N. Molecular functions of nuclear actin in transcription. J Cell Biol. 2006;172:967–971. doi: 10.1083/jcb.200512083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson KL, Holaska JM, Montes de Oca R, Tifft K, Zastrow M, Segura-Totten M, Mansharamani M, Bengtsson L. Nuclear membrane protein emerin: roles in gene regulation, actin dynamics and human disease. Novartis Found Symp. 2005;264:51–58. [PubMed] [Google Scholar]
- 52.Kiseleva E, Drummond SP, Goldberg MW, Rutherford SA, Allen TD, Wilson KL. Actin- and protein-4.1-containing filaments link nuclear pore complexes to subnuclear organelles in Xenopus oocyte nuclei. J Cell Sci. 2004;117:2481–2490. doi: 10.1242/jcs.01098. [DOI] [PubMed] [Google Scholar]
- 53.Krauss SW, Chen C, Penman S, Heald R. Nuclear actin and protein 4.1: essential interactions during nuclear assembly in vitro. Proc Natl Acad Sci U S A. 2003;100:10752–10757. doi: 10.1073/pnas.1934680100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mislow JM, Holaska JM, Kim MS, Lee KK, Segura-Totten M, Wilson KL, McNally EM. Nesprin-1alpha self-associates and binds directly to emerin and lamin A in vitro. FEBS Lett. 2002;525:135–140. doi: 10.1016/s0014-5793(02)03105-8. [DOI] [PubMed] [Google Scholar]
- 55.Sridharan D, Brown M, Lambert WC, McMahon L, Lambert MW. Nonerythroid alphaII spectrin is required for recruitment of FANCA and XPF to nuclear foci induced by DNA interstrand cross-links. J Cell Sci. 2003;116:823–835. doi: 10.1242/jcs.00294. [DOI] [PubMed] [Google Scholar]
- 56.Lodén M, van Steensel B. Whole-genome views of chromatin structure. Chromosome Res. 2005;13:289–298. doi: 10.1007/s10577-005-2166-z. [DOI] [PubMed] [Google Scholar]
- 57.Woodcock CL. Chromatin architecture. Curr Opin Struct Biol. 2006;16:213–220. doi: 10.1016/j.sbi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Sarma K, Reinberg D. Histone variants meet their match. Nat Rev Mol Cell Biol. 2005;6:139–149. doi: 10.1038/nrm1567. [DOI] [PubMed] [Google Scholar]
- 59.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci U S A. 2007;104:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raska I, Shaw PJ, Cmarko D. Structure and function of the nucleolus in the spotlight. Curr Opin Cell Biol. 2006;18:325–334. doi: 10.1016/j.ceb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 61.Raska I, Koberna K, Malínský J, Fidlerová H, Masata M. The nucleolus and transcription of ribosomal genes. Biol Cell. 2004;96:579–594. doi: 10.1016/j.biolcel.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 62.Melling M, Hochmeister S, Blumer R, Schilcher K, Mostler S, Behnam M, Wilde J, Karimian-Teherani D. Atomic force microscopy imaging of the human trigeminal ganglion. Neuroimage. 2001;14:1348–1352. doi: 10.1006/nimg.2001.0924. [DOI] [PubMed] [Google Scholar]
- 63.Maniotis AJ, Bojanowski K, Ingber DE. Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. J Cell Biochem. 1997;65:114–130. [PubMed] [Google Scholar]
- 64.Deryusheva S, Gall JG. Dynamics of coilin in Cajal bodies of the Xenopus germinal vesicle. Proc Natl Acad Sci U S A. 2004;101:4810–4814. doi: 10.1073/pnas.0401106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimber A, Nguyen QD, Gespach C. Nuclear bodies and compartments: functional roles and cellular signalling in health and disease. Cell Signal. 2004;16:1085–2104. doi: 10.1016/j.cellsig.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 66.Platani M, Goldberg I, Swedlow JR, Lamond AI. In vivo analysis of Cajal body movement, separation, and joining in live human cells. J Cell Biol. 2000;151:1561–1574. doi: 10.1083/jcb.151.7.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J, Shiels C, Sasieni P, Wu PJ, Islam SA, Freemont PS, Sheer D. Promyelocytic leukemia nuclear bodies associate with transcriptionally active genomic regions. J Cell Biol. 2004;164:515–526. doi: 10.1083/jcb.200305142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stuurman N, de Graaf A, Floore A, Josso A, Humbel B, de Jong L, van Driel R. A monoclonal antibody recognizing nuclear matrix-associated nuclear bodies. J Cell Sci. 1992;101:773–784. doi: 10.1242/jcs.101.4.773. [DOI] [PubMed] [Google Scholar]
- 69.Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8:1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- 70.Worman HJ, Bonne G. “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fidziańska A, Hausmanowa-Petrusewicz I. Architectural abnormalities in muscle nuclei. Ultrastructural differences between X-linked and autosomal dominant forms of EDMD. J Neurol Sci. 2003;210:47–51. doi: 10.1016/s0022-510x(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 72.Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, Gruenbaum Y, Khuon S, Mendez M, Varga R, Collins FS. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nikolova V, Leimena C, McMahon AC, Tan JC, Chandar S, Jogia D, Kesteven SH, Michalicek J, Otway R, Verheyen F, Rainer S, Stewart CL, Martin D, Feneley MP, Fatkin D. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J Clin Invest. 2004;113:357–369. doi: 10.1172/JCI19448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, Erdos MR, Eriksson M, Goldman AE, Khuon S, Collins FS, Jenuwein T, Goldman RD. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci U S A. 2006;103:8703–8708. doi: 10.1073/pnas.0602569103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Rusinol A, Sinensky M, Wang Y, Zou Y. DNA damage responses in progeroid syndromes arise from defective maturation of prelamin A. J Cell Sci. 2006;119:4644–4649. doi: 10.1242/jcs.03263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 77.Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 2006;20:486–500. doi: 10.1101/gad.1364906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cenni V, Sabatelli P, Mattioli E, Marmiroli S, Capanni C, Ognibene A, Squarzoni S, Maraldi NM, Bonne G, Columbaro M, Merlini L, Lattanzi G. Lamin A N-terminal phosphorylation is associated with myoblast activation: impairment in Emery-Dreifuss muscular dystrophy. J Med Genet. 2005;42:214–220. doi: 10.1136/jmg.2004.026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, Vidaillet HJ, Spudich S, De Girolami U, Seidman JG, Seidman C, Muntoni F, Müehle G, Johnson W, McDonough B. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 80.Goldfarb LG, Vicart P, Goebel HH, Dalakas MC. Desmin myopathy. Brain. 2004;127:723–734. doi: 10.1093/brain/awh033. [DOI] [PubMed] [Google Scholar]
- 81.Sjöberg G, Saavedra-Matiz CA, Rosen DR, Wijsman EM, Borg K, Horowitz SH, Sejersen T. A missense mutation in the desmin rod domain is associated with autosomal dominant distal myopathy, and exerts a dominant negative effect on filament formation. Hum Mol Genet. 1999;8:2191–2198. doi: 10.1093/hmg/8.12.2191. [DOI] [PubMed] [Google Scholar]
- 82.Grattan MJ, Kondo C, Thurston J, Alakija P, Burke BJ, Stewart C, Syme D, Giles WR. Skeletal and cardiac muscle defects in a murine model of Emery-Dreifuss muscular dystrophy. Novartis Found Symp. 2005;264:118–133. doi: 10.1002/0470093765.ch9. [DOI] [PubMed] [Google Scholar]
- 83.Muchir A, Pavlidis P, Bonne G, Hayashi YK, Worman HJ. Activation of MAPK in hearts of EMD null mice: similarities between mouse models of X-linked and autosomal dominant Emery Dreifuss muscular dystrophy. Hum Mol Genet. 2007;16:1884–1895. doi: 10.1093/hmg/ddm137. [DOI] [PubMed] [Google Scholar]
- 84.Stewart CL, Kozlov S, Fong LG, Young SG. Mouse models of the laminopathies. Exp Cell Res. 2007;313:2144–2156. doi: 10.1016/j.yexcr.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, Tseng Y, Stewart CL, Hodzic D, Wirtz D. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys J. 2007;93:2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolf CM, Wang L, Alcalai R, Pizard A, Burgon PG, Ahmad F, Sherwood M, Branco DM, Wakimoto H, Fishman GI, See V, Stewart CL, Conner DA, Berul CI, Seidman CE, Seidman JG. Lamin A/C haploinsufficiency causes dilated cardiomyopathy and apoptosis-triggered cardiac conduction system disease. J Mol Cell Cardiol. 2008;44:293–303. doi: 10.1016/j.yjmcc.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coradeghini R, Barboro P, Rubagotti A, Boccardo F, Parodi S, Carmignani G, D’Arrigo C, Patrone E, Balbi C. Differential expression of nuclear lamins in normal and cancerous prostate tissues. Oncol Rep. 2006 March;15:609–613. [PubMed] [Google Scholar]
- 88.Yang SH, Bergo MO, Toth JI, Qiao X, Hu Y, Sandoval S, Meta M, Bendale P, Gelb MH, Young SG, Fong LG. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc Natl Acad Sci U S A. 2005;102:10291–10296. doi: 10.1073/pnas.0504641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang SH, Qiao X, Farber E, Chang SY, Fong LG, Young SG. Eliminating the synthesis of mature lamin a reduces disease phenotypes in mice carrying a hutchinson-gilford progeria syndrome allele. J Biol Chem. 2008;283:7094–7099. doi: 10.1074/jbc.M708138200. [DOI] [PubMed] [Google Scholar]
- 90.Delbarre E, Tramier M, Coppey-Moisan M, Gaillard C, Courvalin JC, Buendia B. The truncated prelamin A in Hutchinson-Gilford progeria syndrome alters segregation of A-type and B-type lamin homopolymers. Hum Mol Genet. 2006;15:1113–1122. doi: 10.1093/hmg/ddl026. [DOI] [PubMed] [Google Scholar]
- 91.Gilchrist S, Gilbert N, Perry P, Ostlund C, Worman HJ, Bickmore WA. Altered protein dynamics of disease-associated lamin A mutants. BMC Cell Biol. 2004;5:46. doi: 10.1186/1471-2121-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lloyd DJ, Trembath RC, Shackleton S. A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum Mol Genet. 2002;11:769–777. doi: 10.1093/hmg/11.7.769. [DOI] [PubMed] [Google Scholar]
- 93.Verstraeten VL, Ji JY, Cummings KS, Lee RT, Lammerding J. Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: Effects of farnesyltransferase inhibitors. Aging Cell. 2008 doi: 10.1111/j.1474-9726.2008.00382.x. Accepted online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lammerding J, Dahl KN, Discher DE, Kamm RD. Nuclear mechanics and methods. Methods Cell Biol. 2007;83:269–294. doi: 10.1016/S0091-679X(07)83011-1. [DOI] [PubMed] [Google Scholar]
- 95.Caille N, Thoumine O, Tardy Y, Meister JJ. Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech. 2002;35:177–187. doi: 10.1016/s0021-9290(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 96.Guilak F, Tedrow JR, Burgkart R. Viscoelastic properties of the cell nucleus. Biochem Biophys Res Commun. 2000;269:781–786. doi: 10.1006/bbrc.2000.2360. [DOI] [PubMed] [Google Scholar]
- 97.Vaziri A, Mofrad MR. Mechanics and deformation of the nucleus in micropipette aspiration experiment. J Biomech. 2007;40:2053–2062. doi: 10.1016/j.jbiomech.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 98.Kha HN, Chen BK, Clark GM, Jones R. Stiffness properties for Nucleus standard straight and contour electrode arrays. Med Eng Phys. 2004;26:677–685. doi: 10.1016/j.medengphy.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 99.Rowat AC, Foster LJ, Nielsen MM, Weiss M, Ipsen JH. Characterization of the elastic properties of the nuclear envelope. J R Soc Interface. 2005;2:63–69. doi: 10.1098/rsif.2004.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-Law Rheology of Isolated Nuclei with Deformation Mapping of Nuclear Substructures. Biophys J. 2005 October;89:2855–2864. doi: 10.1529/biophysj.105.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Newport JW, Wilson KL, Dunphy WG. A lamin-independent pathway for nuclear envelope assembly. J Cell Biol. 1990;111:2247–2259. doi: 10.1083/jcb.111.6.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Panorchan P, Schafer BW, Wirtz D, Tseng Y. Nuclear envelope breakdown requires overcoming the mechanical integrity of the nuclear lamina. J Biol Chem. 2004;279:43462–43467. doi: 10.1074/jbc.M402474200. [DOI] [PubMed] [Google Scholar]
- 103.Goldberg MW, Huttenlauch I, Hutchison CJ, Stick R. Filaments made from A- and B-type lamins differ in structure and organization. J Cell Sci. 2008;121:215–225. doi: 10.1242/jcs.022020. [DOI] [PubMed] [Google Scholar]
- 104.Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell. 2006;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 106.Wang N. Mechanical interactions among cytoskeletal filaments. Hypertension. 1998;32:162–165. doi: 10.1161/01.hyp.32.1.162. [DOI] [PubMed] [Google Scholar]
- 107.Bao G, Suresh S. Cell and molecular mechanics of biological materials. Nat Mater. 2003;2:715. doi: 10.1038/nmat1001. [DOI] [PubMed] [Google Scholar]
- 108.Helfand BT, Chang L, Goldman RD. Intermediate filaments are dynamic and motile elements of cellular architecture. J Cell Sci. 2004;117:133–141. doi: 10.1242/jcs.00936. [DOI] [PubMed] [Google Scholar]
- 109.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 110.Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic. 2004;5:470–477. doi: 10.1111/j.1600-0854.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 111.Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2:392–396. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- 112.Wiesner S, Legate KR, Fässler R. Integrin-actin interactions. Cell Mol Life Sci. 2005;62:1081–1099. doi: 10.1007/s00018-005-4522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang G, Huang AH, Cai Y, Tanase M, Sheetz MP. Rigidity sensing at the leading edge through alphavbeta3 integrins and RPTPalpha. Biophys J. 2006;90:1804–1809. doi: 10.1529/biophysj.105.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Broers JL, Peeters EA, Kuijpers HJ, Endert J, Bouten CV, Oomens CW, Baaijens FP, Ramaekers FC. Decreased mechanical stiffness in LMNA-/- cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet. 2004;13:2567–2580. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- 116.Padmakumar VC, Libotte T, Lu W, Zaim H, Abraham S, Noegel AA, Gotzmann J, Foisner R, Karakesisoglou I. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J Cell Sci. 2005;118:3419–3430. doi: 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- 117.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, Trembath RC, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hodzic DM, Yeater DB, Bengtsson L, Otto H, Stahl PD. Sun2 is a novel mammalian inner nuclear membrane protein. J Biol Chem. 2004;279:25805–25812. doi: 10.1074/jbc.M313157200. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Q, D RC, Skepper JN, Worth NF, Warren DT, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J Cell Sci. 2005;118:673–687. doi: 10.1242/jcs.01642. [DOI] [PubMed] [Google Scholar]
- 121.Libotte T, Zaim H, Abraham S, Padmakumar VC, Schneider M, Lu W, Munck M, Hutchison C, Wehnert M, Fahrenkrog B, Sauder U, Aebi U, Noegel AA, Karakesisoglou I. Lamin A/C-dependent localization of Nesprin-2, a giant scaffolder at the nuclear envelope. Mol Biol Cell. 2005;16:3411–3424. doi: 10.1091/mbc.E04-11-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Starr DA, Fischer JA. KASH ’n Karry: the KASH domain family of cargo-specific cytoskeletal adaptor proteins. Bioessays. 2005;27:1136–1146. doi: 10.1002/bies.20312. [DOI] [PubMed] [Google Scholar]
- 123.Wilhelmsen K, Ketema M, Truong H, Sonnenberg A. KASH-domain proteins in nuclear migration, anchorage and other processes. J Cell Sci. 2006;119:5021–5029. doi: 10.1242/jcs.03295. [DOI] [PubMed] [Google Scholar]
- 124.Vlcek S, Foisner R. Lamins and lamin-associated proteins in aging and disease. Curr Opin Cell Biol. 2007;19:298–304. doi: 10.1016/j.ceb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 125.Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, T LR. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170:781–791. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rowat AC, Lammerding J, Ipsen JH. Mechanical properties of the cell nucleus and the effect of emerin deficiency. Biophys J. 2006 December 15;91:4649–4664. doi: 10.1529/biophysj.106.086454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ulbert S, Antonin W, Platani M, Mattaj IW. The inner nuclear membrane protein Lem2 is critical for normal nuclear envelope morphology. FEBS Lett. 2006;580:6435–6441. doi: 10.1016/j.febslet.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 128.Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 2002;108:83–96. doi: 10.1016/s0092-8674(01)00627-4. [DOI] [PubMed] [Google Scholar]
- 129.Mühlhäusser P, Kutay U. An in vitro nuclear disassembly system reveals a role for the RanGTPase system and microtubule-dependent steps in nuclear envelope breakdown. J Cell Biol. 2007;178:595–610. doi: 10.1083/jcb.200703002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Salpingidou G, Smertenko A, Hausmanowa-Petrucewicz I, J HP, Hutchison CJ. A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J Cell Biol. 2007;178:897–904. doi: 10.1083/jcb.200702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fan J, Beck KA. A role for the spectrin superfamily member Syne-1 and kinesin II in cytokinesis. J Cell Sci. 2004;117:619–629. doi: 10.1242/jcs.00892. [DOI] [PubMed] [Google Scholar]
- 132.Helmke BP, Davies PF. The cytoskeleton under external fluid mechanical forces: hemodynamic forces acting on the endothelium. Ann Biomed Eng. 2002;30:284–296. doi: 10.1114/1.1467926. [DOI] [PubMed] [Google Scholar]
- 133.Helmke BP, Rosen AB, Davies PF. Mapping mechanical strain of an endogenous cytoskeletal network in living endothelial cells. Biophys J. 2003;84:2691–2699. doi: 10.1016/S0006-3495(03)75074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, Salanga M, Mazur E, Ingber DE. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J. 2006;90:3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guilak F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech. 1995;28:1529–1541. doi: 10.1016/0021-9290(95)00100-x. [DOI] [PubMed] [Google Scholar]
- 137.Hazel AL, Pedley TJ. Vascular endothelial cells minimize the total force on their nuclei. Biophys J. 2000;78:47–54. doi: 10.1016/S0006-3495(00)76571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Deguchi S, Maeda K, Ohashi T, Sato M. Flow-induced hardening of endothelial nucleus as an intracellular stress-bearing organelle. J Biomech. 2005;38:1751–1759. doi: 10.1016/j.jbiomech.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 139.Mathur AB, Reichert WM, Truskey GA. Flow and high affinity binding affect the elastic modulus of the nucleus, cell body and the stress fibers of endothelial cells. Ann Biomed Eng. 2007;35:1120–1130. doi: 10.1007/s10439-007-9288-8. [DOI] [PubMed] [Google Scholar]
- 140.Dadoune JP. Expression of mammalian spermatozoal nucleoproteins. Microsc Res Tech. 2003;61:56–75. doi: 10.1002/jemt.10317. [DOI] [PubMed] [Google Scholar]
- 141.Yabuki M, Miyake T, Doi Y, Fujiwara T, Hamazaki K, Yoshioka T, Horton AA, Utsumi K. Role of nuclear lamins in nuclear segmentation of human neutrophils. Physiol Chem Phys Med NMR. 1999;31:77–84. [PubMed] [Google Scholar]
- 142.Hoffmann K, Sperling K, Olins AL, Olins DE. The granulocyte nucleus and lamin B receptor: avoiding the ovoid. Chromosoma. 2007;116:227–235. doi: 10.1007/s00412-007-0094-8. [DOI] [PubMed] [Google Scholar]
- 143.Thomas CH, Collier JH, Sfeir CS, Healy KE. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci U S A. 2002;99:1972–1977. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lelièvre SA, Weaver VM, Nickerson JA, Larabell CA, Bhaumik A, Petersen OW, Bissell MJ. Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc Natl Acad Sci U S A. 1998;95:14711–14716. doi: 10.1073/pnas.95.25.14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004 September;4:677–687. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 146.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 147.Bissell MJ, Weaver VM, Lelièvre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59:1757–1763. [PubMed] [Google Scholar]
- 148.Hudson ME, Pozdnyakova I, Haines K, Mor G, Snyder M. Identification of differentially expressed proteins in ovarian cancer using high-density protein microarrays. Proc Natl Acad Sci U S A. 2007;104:17494–17499. doi: 10.1073/pnas.0708572104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wolf K, Friedl P. Molecular mechanisms of cancer cell invasion and plasticity. Br J Dermatol. 2006;154:11–15. doi: 10.1111/j.1365-2133.2006.07231.x. [DOI] [PubMed] [Google Scholar]
- 150.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 151.Zlatanova J, Leuba SH. Stretching and imaging single DNA molecules and chromatin. J Muscle Res Cell Motil. 2002;23:377–395. doi: 10.1023/a:1023498120458. [DOI] [PubMed] [Google Scholar]
- 152.Marko JF, Poirier MG. Micromechanics of chromatin and chromosomes. Biochem Cell Biol. 2003;81:209–220. doi: 10.1139/o03-047. [DOI] [PubMed] [Google Scholar]
- 153.Trinkle-Mulcahy L, Lamond AI. Toward a high-resolution view of nuclear dynamics. Science. 2007;318:1402–1407. doi: 10.1126/science.1142033. [DOI] [PubMed] [Google Scholar]
- 154.Stuurman N, Heins S, Aebi U. Nuclear Lamins: Their Structure, Assembly, and Interactions. J Struct Biol. 1998;122:42–66. doi: 10.1006/jsbi.1998.3987. [DOI] [PubMed] [Google Scholar]
- 155.Mattout-Drubezki A, Gruenbaum Y. Dynamic interactions of nuclear lamina proteins with chromatin and transcriptional machinery. Cell Mol Life Sci. 2003;60:2053–2063. doi: 10.1007/s00018-003-3038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tang CW, Maya-Mendoza A, Martin C, Zeng K, Chen S, Feret D, Wilson SA, Jackson DA. The integrity of a lamin-B1-dependent nucleoskeleton is a fundamental determinant of RNA synthesis in human cells. J Cell Sci. 2008;121:1014–1024. doi: 10.1242/jcs.020982. [DOI] [PubMed] [Google Scholar]