Abstract
In recent years, there has been a rapidly increasing number of studies focused on the relationship between dementia and metabolic disorders such as diabetes, obesity, hypertension and dyslipidemia. Etiological heterogeneity and co-morbidity pose challenges for determining relationships among metabolic disorders. The independent and interactive effects of brain vascular injury and classic pathological agents such as Aβ have also proved difficult to untangle in human patients, blurring the lines between Alzheimer's disease and vascular dementia. This review highlights recent work aimed at identifying convergent mechanisms such as insulin resistance that may underlie co-morbid metabolic disorders and thereby increase dementia risk. Identification of such convergent factors will not only provide important insights into the causes and interdependencies of late-life dementias, but will also inspire novel strategies for treating and preventing these disorders.
Introduction
This special issue highlights the relationship of disorders of metabolism to Alzheimer's disease (AD) and vascular dementia (VaD). Our review is not intended to be an exhaustive review of this burgeoning literature, but instead to highlight novel or integrative lines of inquiry. Substantiation of the link between metabolic disorders and dementia risk has raised new questions about specific mechanisms underlying these associations that have critical implications for the way we define and classify dementias. The independent and interactive effects of brain vascular injury and classic AD pathological agents such as Aβ have been nicely characterized in animal and in vitro models, but have proved difficult to untangle in human patients. Similar challenges exist in determining the independent and interactive effects of metabolic disorders, which are etiologically heterogeneous, have high degrees of co-morbidity, and whose expression may be distorted by treatment or time.
The lines between AD and VaD have also blurred. VaD is a heterogeneous construct with pathology that can range from multiple macroinfarcts to small vessel ischemic disease to microvascular injury. It is clear that for many patients, markers of vascular injury coexist with traditional AD hallmarks. In some cases, the AD hallmarks may conceivably be promoted by a specific form of vascular injury; for example, BBB dysfunction may affect Aβ transport between brain and periphery, and thereby contribute to parenchymal and neurovascular amyloid deposition. Conversely, AD pathology may cause vascular injury, as when Aβ-induced inflammation damages the endothelium. Or in yet other cases, isolated AD or vascular pathology may occur. Given such complexity, the temptation to retreat to reductionist models is understandable. Unifying themes are emerging, however, which may illuminate convergent mechanisms through which co-morbid metabolic disorders promote the development of AD and VaD. As we discuss below, one important theme is the concept that insulin resistance underlies several important metabolic disorders and their relationship to AD and VaD. Other unifying themes will undoubtedly emerge as the reader assimilates the thoughtful work presented in this issue.
Classification and Diagnosis of Metabolic Disorders
One source of confusion that impedes our comparison across studies concerns the terminology used to describe metabolic disorders.1 The insulin resistance syndrome occurs when tissues become unresponsive to the effects of insulin, and can selectively affect insulin's actions on muscle, liver, adipose tissue, endothelium, or brain. It is typically accompanied by compensatory hyperinsulinemia in the periphery, which has independent deleterious effects. Insulin resistance is thought to be the underlying cause of the metabolic syndrome, diabetes and vascular disorders such as hypertension and cardiovascular disease for the majority of patients (Table 1). It may thus be considered a core syndrome that increases risk of AD and VaD. Our ability to study the mechanistic relationship of insulin resistance to AD and VaD is hampered, however, by the complexity of its measurement. The hyperinsulinemic-euglycemic clamp, a method in which a standard dose of insulin is infused with dextrose, is the gold-standard measure,2 but is time and labor intensive. The integrated area under the curve for insulin during oral glucose tolerance testing has been proposed as an acceptable surrogate.3 The homeostasis model assessment for insulin resistance (HOMA-IR), which is based on fasting insulin and glucose values, correlates well with the hyperinsulinemic-euglycemic clamp,3 is relatively low-cost and is computationally straightforward.
Table 1.
Dementia-related Risk Factors Caused by Insulin Resistance
| Type 2 Diabetes Mellitus/Impaired Glucose Tolerance |
| Obesity/central adiposity |
| Dyslipidemia |
| Inflammation |
| Hypertension |
| Ischemia |
| Cardiovascular Disease |
In contrast, diabetes is solely a glucocentric diagnosis that is made when fasting glucose levels exceed 126 mg/dl, or when random/post-glucose tolerance testing levels exceed 200 mg/dl. As such, it is a heterogeneous disorder, with many potential etiologies, which complicates the determination of its relationship to late-life dementias. As noted, insulin resistance is a causal factor in the majority of patients with adult onset or Type 2 diabetes mellitus. Insulin resistance may be manifest only by mild glucose intolerance for many years prior to the onset of frank diabetes, as the pancreas is able to generate sufficient levels of insulin to maintain glucose levels beneath the diabetic threshold. Over time, the degree of insulin resistance increases as insulin secretion by pancreatic β cells is reduced, resulting in hyperglycemia of sufficient magnitude to warrant the diagnosis of T2DM. Of note, despite diminished insulin secretory capacity, patients with T2DM demonstrate hyperinsulinemia due to reduced insulin clearance.
The metabolic syndrome is defined by the co-occurrence of certain cardiovascular risk factors.1 The most commonly accepted definition requires three of the following conditions to be present: large waist circumference, hypertriglyceridemia, low HDL, elevated blood pressure, and fasting hyperglycemia. Of note, this definition does not include any reference to insulin resistance or hyperinsulinemia, despite clear evidence that these factors play a causal role in its occurrence in most patients.4
Mechanistic Links Between Insulin Resistance-Associated Conditions and AD/VaD
In the following section, we briefly review the role of insulin on normal brain function, and potential direct effects of insulin resistance on the brain and on AD pathophysiology.
Insulin and the brain
Recent reviews have provided comprehensive accounts of the role of insulin in normal brain function.5, 6 Insulin is readily transported into the CNS across the blood brain barrier (BBB) by a saturable, receptor-mediated process. Insulin receptors, located in astrocytes and neuronal synapses, are highly concentrated in the olfactory bulb, cerebral cortex, hippocampus, hypothalamus, amygdala, and septum. Localization of insulin receptors in hippocampus and medial temporal cortex is consistent with evidence that insulin influences memory. Likely memory-related mechanisms include modulation of synaptic structure and function, long-term potentiation and CNS levels of neurotransmitters such as acetylcholine and norepinephrine that are known to influence cognitive function.6 Specific regional effects of insulin on glucose metabolism via insulin-sensitive GLUT 4 and 8 glucose transporters may also impact brain function.
Direct effects of insulin and insulin resistance on Aβ and tau
Many of the important functions of insulin in the brain are disrupted in insulin resistant conditions. Interestingly, prolonged peripheral hyperinsulinemia associated with insulin resistance reduces insulin transport across the BBB, subsequently lowering insulin levels and activity in brain; this effect may have relevance for findings of reduced CSF insulin and brain insulin-signaling markers in AD.6, 7 Insulin resistance and hyperinsulinemia are implicated in a number of pathophysiological processes related to AD.5, 6 Reduced brain insulin signaling is associated with increased tau phosphorylation and Aβ levels in a streptozotocin mouse model of diabetes.8 Insulin also promotes release of intracellular Aβ in neuronal cultures and accelerates Aβ trafficking to the plasma membrane.9 In humans, raising plasma insulin through intravenous infusion increased cerebrospinal fluid (CSF) levels of the Aβ42 peptide, and this effect was exacerbated by age.10 Intravenous insulin infusion also raised plasma Aβ42 levels in AD patients, but not in normal adults, an effect that was exaggerated in AD patients with higher body mass index.11 This finding illustrated the close relationship between insulin resistance and obesity, a relationship that may have particular implications for AD and VaD pathogenesis as described below. Mechanisms regulating Aβ clearance (rather than production) may be of special importance in late-onset AD. Insulin may interfere with Aβ degradation via its regulation of the metalloprotease insulin degrading enzyme.12
Insulin Resistance-Related Metabolic Disorders and AD/VaD
Diabetes
The relationship between diabetes and increased AD and VaD risk has been discussed in several excellent recent reviews.13, 14 The weight of evidence suggests that diabetes increases risk of both AD and VaD, and that this risk occurs regardless of the age at which diabetes occurs.15 Risk-enhancing mechanisms include the effects of insulin resistance described above, hyperglycemia-related increased advanced glycation end-products and oxidative stress, inflammation, and macro- and micro-vascular injury.
Neuropathological studies of patients with diabetes and clinically-diagnosed AD and VaD have faced challenges similar to those experienced in early epidemiologic studies. Few autopsy cohorts have captured quality data regarding metabolic and cognitive status to allow reliable diagnosis of both diabetes and dementia subtype. Instead, studies often rely on self-reported diabetes, which underestimates prevalence by 50%, or on medical records whose completeness can not be verified. Thus control samples may include many undiagnosed diabetics, obscuring differences between groups. Historic diagnostic biases are also problematic, as patients with diabetes were often presumed to have dementia of vascular origin. Perhaps the greatest challenge has been to determine the effects of medication. Insulin and oral hypoglycemics are the most common treatments for T2DM, and have been demonstrated to affect AD markers, as well as vascular integrity. However, important details regarding length and type of treatment, as well as dose, are often poorly documented.
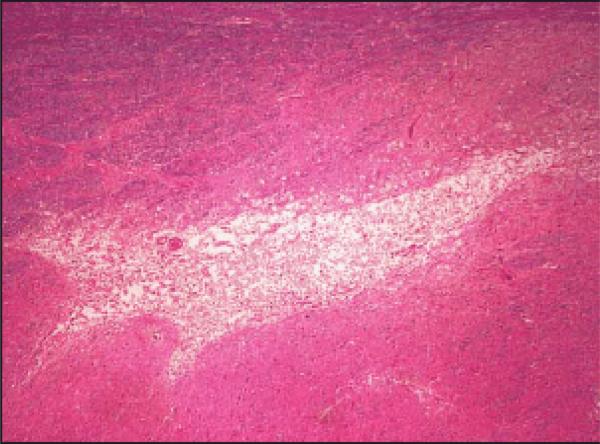
Despite these challenges, an interesting pattern has emerged in recent neuropathologic studies, in which patients with treated diabetes demonstrate a reduced amyloid load compared to non-diabetics with similar levels of dementia.16 As described in this issue, patients with treated diabetes and dementia had Aβ plaque loads similar to non-demented cases, and instead had increased microvascular infarcts and IL-6.17 In contrast, untreated diabetics with dementia had plaque loads that were similar to non-diabetic dementia cases. One intriguing interpretation of these results is that diabetic treatment affected amyloid load, but not degree of dementia. If true, this finding would raise questions about the role of amyloid plaques in dementia symptoms. This interpretation must be considered speculative, however, given the small number of cases and the fact that treated diabetics typically have more severe diabetes. Similarly, the finding of increased microvascular injury in treated diabetics with dementia, but not in similarly affected treated diabetics without dementia raises the question of which diabetic factors are associated with microvascular infarcts? Clearly not all treated diabetics developed dementia, only those cases with concomitant microvascular infarcts. The nature of this microvascular injury is also an important consideration. Given their small volume (Fig 1), it is unlikely that microvascular infarcts directly cause dementia, but rather serve as a marker for more extensive microvascular dysfunction. These findings illustrate the importance of careful assessment of treatment and metabolic status in future neuropathologic studies.
Figure 1.
Arteriolar microinfarct in patient with dementia and diabetes.
Obesity
Obesity has reached epidemic proportions in many Western societies, and is a primary cause of insulin resistance; eighty percent of all obese individuals are insulin resistant.1 Evidence concerning obesity as a risk factor for AD and VaD has been mixed.13 In general, mid-life obesity is consistently found to be a risk factor for later dementia, whereas more variability in risk is observed for adiposity in older age.18 Free fatty acids (FFAs) are a critical mechanistic link between obesity and insulin resistance.1 In normal metabolism, insulin inhibits adipocyte hormone-sensitive lipase activity, thereby decreasing FFA release from adipose tissue. This process is disrupted in obesity and insulin resistant conditions, leading to persistent FFA elevations. Normalizing FFA levels results in a 50% increase in insulin sensitivity in obese adults.19 The link between FFA elevations and development of T2DM is supported by findings that normoglycemic individuals with a family history of diabetes show high fasting FFA levels,20 and that elevated plasma FFAs predict progression to diabetes.2, 4
Obesity-associated FFA elevations may affect AD pathogenesis. FFAs inhibit IDE, the metalloprotease that plays a key role in clearance of Aβ, and which is also essential for normal insulin signaling to occur.21 FFAs stimulate the assembly of amyloid and tau filaments in vitro.22, 23 They also induce inflammation, particularly through interactions with TNFα. TNFα is over-expressed in adipose tissue of obese insulin resistant rodents and humans, and neutralization of TNFα increases insulin sensitivity and decreases plasma FFA levels.24 TNFα is a cytokine that has received increasing attention in theories of AD pathogenesis. TNFα is elevated in the brains and CSF of patients with AD and in adults with mild cognitive impairment (MCI),.25, 26 and nhibits Aβtransport from brain to periphery.27 Thus TNFα elevations associated with obesity, insulin resistance and hyperinsulinemia may result in increased brain accumulation of Aβ.
Dyslipidemia
In a recent meta-analysis of 18 prospective studies examining the relationship of total cholesterol and risk for AD and VaD, mid-life total cholesterol levels were consistently associated with an increased risk of AD and all dementia, whereas no increased risk was observed for late-life total cholesterol.28 Interestingly, no relationship between total cholesterol and VaD was observed at any age. Dyslipidemia is an important component of the insulin resistance syndrome; insulin is a primary regulator of lipid metabolism, stimulating lipogenesis and reducing lipolysis. As noted previously, in adipocytes, insulin resistance leads to accelerated lipolysis and increased FFA levels. In turn, excess FFA influx into liver inhibits insulin suppression of hepatic VLDL secretion, an essential process for preventing post-prandial hyperlipidemia. Acute inhibitory effects of insulin on lipid production are essential for rapid hepatic adaptation to metabolic shifts between fasting and refeeding in order to maintain plasma lipids within an optimal physiologic range.29 Thus, insulin resistant adults have higher and more prolonged post-prandial excursions of VLDL and other deleterious lipids.
This tendency has important implications for AD pathophysiology. Interactions among lipids, lipoproteins and Aβ play a critical role in Aβ production and clearance. In rodents, increased peripheral VLDL secretion precedes Aβ deposition in brain,30 and high fat feeding increases brain amyloid burden.31 In humans, elevated mid-life cholesterol levels increase AD risk 2 to 3-fold,32 and are associated with increased plasma Aβ40 levels.33 Increased post-prandial chylomicron and LDL levels, with normal fasting levels, have been associated with AD.32 The specific mechanisms through which lipids and lipoproteins affect Aβ production and clearance are a subject of in intense inquiry. Aβ40, in contrast to Aβ42, is rapidly cleared across the BBB by lipoprotein receptor related protein-2 (LRP2). Apolipoproteins E and J (apoE and apoJ) mediate Aβ transport between brain and periphery. Binding of Aβ to apoE reduces efflux, whereas binding of Aβ42 to apoJ increases LRP2-mediated efflux. The lipidation status of apoE affects its interaction with Aβ. Highly lipidated apoE increases Aβ brain efflux thereby reducing Aβ deposition, and poorly lipidated apoE increases amyloid burden.34 The lipidation status of peripheral Aβ may also affect its clearance.35 Obstructing Aβ clearance in the peripheral “sink” may increase brain accumulation of Aβ. The specific mediators of peripheral Aβ clearance are controversial, but may include apoE and apoJ, whereas LRP1 may mediate both hepatic uptake and clearance of Aβ.36
Soluble LRP (sLRP) may be a key mediator of peripheral Aβ clearance. LRP N terminal cleavage by β-secretase releases sLRP into plasma where it binds to Aβ. Peripherally administered recombinant LRP blocked Aβ transport across the BBB in mice, and reduced brain Aβ40 and 42 levels, brain vascular Aβ levels, and amyloid burden by 90%.37 AD patients had a 30% decrease in sLRP, a 280% increase in proportion of oxidized sLRP (which has a lower affinity for Aβ), and large increases in the percentage of free plasma Aβ40 and 42. Notably, only a small fraction of plasma Aβ was bound to apoE or apoJ, in contrast to other reports. Thus, investigations of sLRP and its modulators may provide important evidence for this promising therapeutic target. Insulin modulates LRP expression and translocation to the plasma membrane where it may more readily encounter β-secretase and produce sLRP. In rats, insulin rapidly increased both hepatic expression of LRP-1 in the plasma membrane fraction and hepatic uptake of Aβ40.38 Insulin resistance may interfere with this translocation, reducing levels of sLRP, or increasing sLRP oxidation.
Vascular dysfunction and hypertension
Insulin resistance has many negative effects on vascular function which are directly related to impaired insulin action, as well as caused by insulin resistance-induced dyslipidemia and inflammation. Insulin directly affects vasoreactivity and hemodynamic functions, such as capillary recruitment, vasodilation and regional blood flow. Hemodynamic and metabolic effects working in concert enhance energy substrate delivery.39 Insulin normally increases NO-mediated vasodilation and regulates vasoconstriction via endothelin-1. Conversely, insulin resistance decreases NO and increases endothelin-1 activity, favoring vasoconstriction and reducing capillary recruitment. In turn, endothelial dysfunction reduces insulin transport, ultimately reducing capillary recruitment and microvascular blood flow. This exacerbates glucose and lipid abnormalities, and establishes a negative feedback loop between progressive endothelial dysfunction and increasing insulin resistance.39 In brain, vasoconstriction and reduced capillary recruitment may interfere with functions of the neurovascular unit, the coordinated interaction of astrocyte, neuron, and endothelium which couples neural activity with increased blood flow.
Hypertension affects 25% of the adult population, and is diagnosed when systolic blood pressure exceeds 140 mm Hg, or diastolic pressure exceeds 90 mm Hg.40 Although heterogeneous in etiology, 50% of hypertensive patients are insulin resistant, and manifest insulin resistance-induced endothelial dysfunction due to previously described direct effects on vasoreactivity and microvascular blood flow, as well as indirect effects of related dyslipidemia and inflammation. Hypertension impairs functional hyperemia, the process by which brain activity and blood flow are coordinated. This impairment is induced by dysregulation of vasoactive mediators such as NO and endothelin-1, by oxidative stress, by structural alteration of the blood vessels, and by inadequate cerebral autoregulation.40 All of these processes have been linked to insulin resistance. As with obesity, mid-life hypertension is a risk factor for AD and VaD in epidemiologic studies, whereas patients with AD appear to show reduced blood pressure at disease onset. Recent reviews have summarized evidence from animal models, in which deposition of Aβ is increased with hypertension, and in turn induces vascular dysfunction that impairs functional hyperemia.40
Co-morbidity of metabolic disorders
We have noted the challenges of determining the independent and interactive roles of metabolic disorders. Kloppenborg et al. reviewed epidemiologic results from studies of T2DM, hypertension, and dyslipidemia, and noted the paucity of work that adequately examines interactive effects.15 They also note the potential value of a heuristic approach focusing on the underlying construct of insulin resistance rather than individual metabolic conditions. Unfortunately, few studies have directly characterized insulin resistance, and instead have focused on related conditions such as obesity and T2DM. Support for the additive effects of metabolic conditions has been observed in studies documenting that mid-life obesity, hypercholesterolemia, and high systolic blood pressure increase dementia risk additively.3, 41
Challenges for Future Research
As the preceding review indicates, considerable progress has been made in establishing relationships among metabolic disorders and late life dementing illnesses. A number of challenges must be addressed as we move forward to determine key mechanisms underlying these associations. A consistent nosology of brain vascular injury, and delineation of the interactions between subtypes of vascular injury and AD pathology, will be critical in defining the interface between AD and VaD. Similarly, elucidation of the interactions among various metabolic disorders, and identification of convergent pathophysiology underlying co-morbidities will likely provide important clues to dementia-related mechanisms. Careful attention to the measurement and characterization of the insulin resistance syndrome may further this effort.
An additional question with important implications concerns the timing of risk: why are the associations between many metabolic disorders and dementia strongest at mid-life? A commonly cited reason is that dementia onset may be associated with counter-regulatory factors which alter the presentation of the risk-initiating metabolic condition. This phenomenon is likely contributory, but begs the question of what pathogenetic mechanisms are induced by these disorders at midlife? Identification of a clear risk profile or risk-related pattern of biomarker-s in mid-life may guide strategies for early diagnosis and prevention. It is also possible that long-term treatments for these disorders obscure or change the nature of their association at the time of dementia onset. Ascertaining the effects of treatment on dementia-related pathology is a daunting but necessary task, complicated by the fact that that the treatments for one class of disorders affect the expression of other disorders; for example, some anti-hypertensives reduce the risk of diabetes, and some anti-diabetic agents improve blood pressure. These interactive effects may provide clues to shared etiologies among metabolic disorders. Notably, the same inter-relatedness that has complicated efforts to derive simple, linear models of association may benefit therapeutic efforts, as targeting one metabolic disorder may improve related conditions. Candidate therapies currently under study include statins, anti-hypertensive therapies, and insulin-sensitizing drugs. Considerable interest has also arisen regarding the effects of lifestyle interventions such as exercise and dietary/nutriceutical manipulations. Future research aimed at identifying mechanisms that underlie co-morbid associations will not only provide important insights into the causes and interdependencies of late-life dementias, but will also inspire novel strategies for treating and preventing these disorders.
References
- 1.Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008 Dec;29(7):777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 3.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008 Jan;294(1):E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 4.Reaven G. Why a cluster is truly a cluster: insulin resistance and cardiovascular disease. Clin Chem. 2008 May;54(5):785–787. doi: 10.1373/clinchem.2008.105254. [DOI] [PubMed] [Google Scholar]
- 5.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004 Mar;3(3):169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer's disease. Biochim Biophys Acta. 2008 Nov 5; doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D. Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease - Relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998 Jan;50(1):164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 8.Jolivalt CG, Lee CA, Beiswenger KK, et al. Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: parallels with Alzheimer's disease and correction by insulin. J Neurosci Res. 2008 Nov 15;86(15):3265–3274. doi: 10.1002/jnr.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gasparini L, Gouras GK, Wang R, et al. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci. 2001 Apr 15;21(8):2561–2570. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson GS, Peskind ER, Asthana S, et al. Insulin increases CSF Abeta42 levels in normal older adults. Neurology. 2003 Jun 24;60(12):1899–1903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- 11.Kulstad JJ, Green PS, Cook DG, et al. Differential modulation of plasma beta-amyloid by insulin in patients with Alzheimer disease. Neurology. 2006 May;66(10):1506–1510. doi: 10.1212/01.wnl.0000216274.58185.09. [DOI] [PubMed] [Google Scholar]
- 12.Qiu WQ, Ye Z, Kholodenko D, Seubert P, Selkoe DJ. Degradation of amyloid beta-protein by a metalloprotease secreted by microglia and other neural and non-neural cells. J Biol Chem. 1997 Mar 7;272(10):6641–6646. doi: 10.1074/jbc.272.10.6641. [DOI] [PubMed] [Google Scholar]
- 13.Luchsinger JA. Adiposity, hyperinsulinemia, diabetes and Alzheimer's disease: an epidemiological perspective. Eur J Pharmacol. 2008 May 6;585(1):119–129. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strachan MW, Reynolds RM, Frier BM, Mitchell RJ, Price JF. The relationship between type 2 diabetes and dementia. Br Med Bull. 2008;88(1):131–146. doi: 10.1093/bmb/ldn042. [DOI] [PubMed] [Google Scholar]
- 15.Kloppenborg RP, van den Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol. 2008 May 6;585(1):97–108. doi: 10.1016/j.ejphar.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 16.Beeri MS, Schmeidler J, Silverman JM, et al. Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology. Neurology. 2008 Sep 2;71(10):750–757. doi: 10.1212/01.wnl.0000324925.95210.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. doi: 10.1001/archneurol.2008.579. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008 Sep 30;71(14):1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 19.Kivipelto M, Solomon A. Alzheimer's disease - the ways of prevention. J Nutr Health Aging. 2008 Jan;12(1):89S–94S. doi: 10.1007/BF02982595. [DOI] [PubMed] [Google Scholar]
- 20.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005 Jan 25;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 21.Bravata DM, Wells CK, Concato J, Kernan WN, Brass LM, Gulanski BI. Two measures of insulin sensitivity provided similar information in a U.S. population. J Clin Epidemiol. 2004 Nov;57(11):1214–1217. doi: 10.1016/j.jclinepi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Axen KV, Dikeakos A, Sclafani A. High dietary fat promotes syndrome X in nonobese rats. J Nutr. 2003 Jul;133(7):2244–2249. doi: 10.1093/jn/133.7.2244. [DOI] [PubMed] [Google Scholar]
- 23.Bray GA, Lovejoy JC, Smith SR, et al. The influence of different fats and fatty acids on obesity, insulin resistance and inflammation. J Nutr. 2002 Sep;132(9):2488–2491. doi: 10.1093/jn/132.9.2488. [DOI] [PubMed] [Google Scholar]
- 24.Piers LS, Walker KZ, Stoney RM, Soares MJ, O'Dea K. The influence of the type of dietary fat on postprandial fat oxidation rates: monounsaturated (olive oil) vs saturated fat (cream) Int J Obes Relat Metab Disord. 2002 Jun;26(6):814–821. doi: 10.1038/sj.ijo.0801993. [DOI] [PubMed] [Google Scholar]
- 25.Proietto J, Filippis A, Nakhla C, Clark S. Nutrient-induced insulin resistance. Mol Cell Endocrinol. 25 1999 May;151(12):143–149. doi: 10.1016/s0303-7207(99)00050-7. [DOI] [PubMed] [Google Scholar]
- 26.Vessby B, Unsitupa M, Hermansen K, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia. 2001 Mar;44(3):312–319. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- 27.López SBB, Pacheco YM, Villar J, Abia R, Muriana FJ. Distinctive postprandial modulation of beta cell function and insulin sensitivity by dietary fats: monounsaturated compared with saturated fatty acids. American Journal of Clinical Nutrition. 2008;88(3):638–644. doi: 10.1093/ajcn/88.3.638. [DOI] [PubMed] [Google Scholar]
- 28.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008 May;16(5):343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 29.Kamagate A, Qu S, Perdomo G, et al. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008 Jun;118(6):2347–2364. doi: 10.1172/JCI32914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess BL, McIsaac SA, Naus KE, et al. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer's disease mouse models with abundant A beta in plasma. Neurobiol Dis. 2006 Oct;24(1):114–127. doi: 10.1016/j.nbd.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Sechi LA. Mechanisms of insulin resistance in rat models of hypertension and their relationships with salt sensitivity. J Hypertens. 1999 Sep;17(9):1229–1237. doi: 10.1097/00004872-199917090-00001. [DOI] [PubMed] [Google Scholar]
- 32.Mamo JC, Jian L, James AP, Flicker L, Esselmann H, Wiltfang J. Plasma lipoprotein beta-amyloid in subjects with Alzheimer's disease or mild cognitive impairment. Ann Clin Biochem. 2008 Jul;45(Pt 4):395–403. doi: 10.1258/acb.2008.007214. [DOI] [PubMed] [Google Scholar]
- 33.Smith CC, Betteridge DJ. Plasma beta-amyloid (A beta) 40 concentration, lipid status and age in humans. Neurosci Lett. 2004 Aug 26;367(1):48–50. doi: 10.1016/j.neulet.2004.05.081. [DOI] [PubMed] [Google Scholar]
- 34.Wahrle SE, Jiang H, Parsadanian M, et al. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest. 2008 Feb;118(2):671–682. doi: 10.1172/JCI33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghiso J, Shayo M, Calero M, et al. Systemic catabolism of Alzheimer's Abeta40 and Abeta42. J Biol Chem. 2004 Oct 29;279(44):45897–45908. doi: 10.1074/jbc.M407668200. [DOI] [PubMed] [Google Scholar]
- 36.Jaeger S, Pietrzik CU. Functional role of lipoprotein receptors in Alzheimer's disease. Curr Alzheimer Res. 2008 Feb;5(1):15–25. doi: 10.2174/156720508783884675. [DOI] [PubMed] [Google Scholar]
- 37.Sagare A, Deane R, Bell RD, et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007 Sep;13(9):1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamaki C, Ohtsuki S, Terasaki T. Insulin facilitates the hepatic clearance of plasma amyloid beta-peptide (1 40) by intracellular translocation of low-density lipoprotein receptor-related protein 1 (LRP-1) to the plasma membrane in hepatocytes. Mol Pharmacol. 2007 Oct;72(4):850–855. doi: 10.1124/mol.107.036913. [DOI] [PubMed] [Google Scholar]
- 39.Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev. 2006 Nov-Dec;22(6):423–436. doi: 10.1002/dmrr.634. [DOI] [PubMed] [Google Scholar]
- 40.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008 Jun;7(6):476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]