Abstract
Ferritin, a major iron storage protein, is essential to iron homeostasis and is involved in a wide range of physiologic and pathologic processes. In clinical medicine, ferritin is predominantly utilized as a serum marker of total body iron stores. In cases of iron deficiency and overload, serum ferritin serves a critical role in both diagnosis and management. Elevated serum and tissue ferritin are linked to coronary artery disease, malignancy, and poor outcomes following stem cell transplantation. Ferritin is directly implicated in less common but potentially devastating human diseases including sideroblastic anemias, neurodegenerative disorders, and hemophagocytic syndrome. Additionally, recent research describes novel functions of ferritin independent of iron storage.
Keywords: ferritin, iron
Introduction
Ferritin, an iron storage protein, is the primary iron storage mechanism and is critical to iron homeostasis. Ferritin makes iron available for critical cellular processes while protecting lipids, DNA, and proteins from the potentially toxic effects of iron. Alterations in ferritin are seen commonly in clinical practice, often reflecting perturbations in iron homeostasis or metabolism. It is increasingly recognized that ferritin also plays a role in a multitude of other conditions, including inflammatory, neurodegenerative, and malignant diseases.
Ferritin in the context of iron homeostasis
In humans, the majority of iron is integrated within the globin proteins that facilitate the transport of oxygen throughout the body. Iron is also critical in converting oxygen into useable cellular energy by serving as a key component in the electron transfer chain. In addition to its’ role in respiration, iron is also utilized as an enzymatic co-factor in numerous other reactions. One such reaction is the conversion of ribose nucleotides to deoxyribose nucleotides, an iron-dependent process catalyzed by ribonucleotide reductase that is necessary for DNA replication and cell division. Despite iron’s integral role within the body, it also has the potential to be highly toxic by facilitating the formation of free radicals. Thus, carefully regulated mechanisms have evolved to transport iron across biological membranes, distribute it throughout the body, and store it in inert form until needed. As iron homeostasis has recently been reviewed elsewhere,1 our review will primarily focus on the central role of ferritin in this process.
Regulation of systemic iron balance occurs exclusively at the site of absorption, as there is no physiologic process present to excrete excess iron. The majority of iron absorption occurs via enterocytes in the proximal small intestine. Here, iron is transported across the cellular membrane by the divalent metal transporter, DMT1, a member of the N ramp family that transfers iron (and other divalent metals) across the apical membrane and into the cell through a proton-coupled process. Prior to transport, the iron must be in the ferrous state (Fe2+). The conversion of dietary inorganic non-heme iron to Fe2+ is facilitated by brush border ferrireductases. The absorption of heme iron is less well understood, although heme transporters have recently been discovered.1 DMT1 levels are upregulated in response to systemic iron deficiency, thereby increasing cellular uptake.1–4
Though some absorbed iron remains present within the enterocyte as ferritin, the majority is transported to other sites within the body. Ferroportin is a newly identified iron efflux pump that mediates the export of iron from the enterocyte.1 Prior to the transport of iron outside the cell, intracelluar iron must be converted to Fe3+. This is facilitated by either hephaestin or ceruloplasmin, both of which have ferroxidase activity (Fe2+ → Fe3+). Within the intestine, both of these proteins are active, whereas in the liver (a major storage site for iron) ceruloplasmin is the primary workhorse. Iron is then loaded onto transferrin, the primary transporter of iron in the circulation. When bound to transferrin, Fe3+ is soluble and non-reactive, thereby allowing it to enter the circulatory system.2,5
The primary consumer of iron is the bone marrow, where red cells require large amounts of iron to meet demand for the production of iron-containing hemoglobin. Within the bone marrow, erythroid precursors express transferrin receptors (TfRs) on their surfaces. Upon binding of the iron-saturated transferrin to its receptor, the complex is endocytosed. The acidic environment of the endosome prompts the release of iron from transferrin. The unbound iron is subsequently reduced to its ferric/ferrous form (Fe2+) by Steap 36 and transported out of the endosome into the cytoplasm by DMT1. The empty transferrin and transferrin receptors are returned to the cell’s surface where they dissociate at a neutral pH, and re-enter the circulation. Interestingly, the transport of iron into non-hematopoietic cells does not require the transferrin receptor. This is highlighted in mouse experiments, where a disrupted TFR gene leads to lethal anemia at an early stage of development, while other non-hematopoietic tissues contain normal amounts of intracellular iron.
The constant turnover of red cells demands recycling of the iron contained within hemoglobin. This recycling process is accomplished primarily within the macrophage, which is capable of phagocytosing erythrocytes, which are then lysed. The iron is liberated from the phagolysosome heme via hemoxygenase. Unstored iron within the macrophage is then exported in a process thought to be dependent on ferroportin.
The dynamics of the transport, distribution, and recycling of iron are tightly regulated within humans. While much remains to be discovered regarding the control of iron balance, hepcidin, a recently discovered 25 amino-acid protein, is believed to be critical to this process. Hepcidin serves as a negative regulator, and when elevated, results in reduced intestinal iron absorption and macrophage iron release. Hepcidin often increases in response to inflammation, a process that is thought to be responsible for much of the iron abnormalities that are a hallmark of anemia of chronic disease.7 The impact of inflammation on hepcidin has led some to theorize that hepcidin evolved as a method of host defense, decreasing available iron for invading pathogens and malignant cells to reproduce. Conversely, the deficiency of hepicidin, as seen in juvenile hemochromatosis, may lead to pronounced and toxic iron overload.8
Ferritin Structure
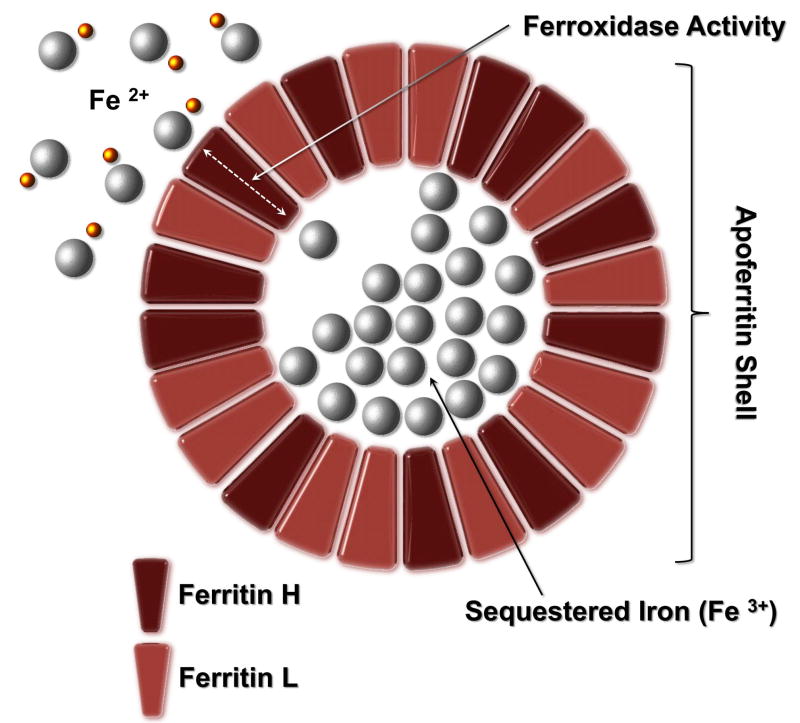
Ferritin is an iron-binding protein that exists in both intracellular and extracellular compartments (reviewed in 9.) Apoferritin forms a roughly spherical container within which ferric iron is stored as a ferrihydrite mineral, as shown in Figure 1. (Apoferritin refers to the iron-free form of the protein; the iron-containing form is termed holoferritin or simply ferritin). The apoferritin shell is composed of 24 subunits. The subunits are of two types, termed H and L. The ratio of these subunits varies widely depending on tissue type, and can be modified in inflammatory and infectious conditions. Tissue ferritins vary from H-subunit rich (found mostly in the heart and kidney) to L-subunit rich (found predominantly in liver and spleen). Each apo protein molecule is about 450,000 d. The L monomer contains 174 amino acids and has a molecular weight of 18,500 d; the H monomer has 182 amino acids with a molecular weight of 21,000 d.
Figure 1.
Ferritin Structure: Apoferritin forms a roughly spherical container within which ferric iron is stored as a ferrihydrite mineral. Apoferritin refers to the iron-free form of the protein; the iron-containing form is termed holoferritin or simply ferritin. The apoferritin shell is composed of 24 subunits of two types, termed H and L, the ratio of which varies widely depending on tissue type and inflammation. Iron is toxic in cellular systems because of its capacity to generate reactive species (shown as yellow spheres) which can directly damage DNA and proteins.
Ferritin is also present extracellularly within the serum, where it serves as an important clinical marker of iron status. Despite its regular use in clinical medicine, the precise source of serum ferritin has yet to be determined. It appears that the preponderance of serum ferritin is immunologically related to ferritin L. In support of a link between ferritin L and serum ferritin is the genetic disease hyperferritinemia, in which an abnormality of the ferritin L gene results in dramatically increased levels of serum ferritin. Increases in serum levels of H-type ferritins have also been reported in some pathophysiologic conditions, including malignant disease, which are reviewed in a later section.
Ferritin Function
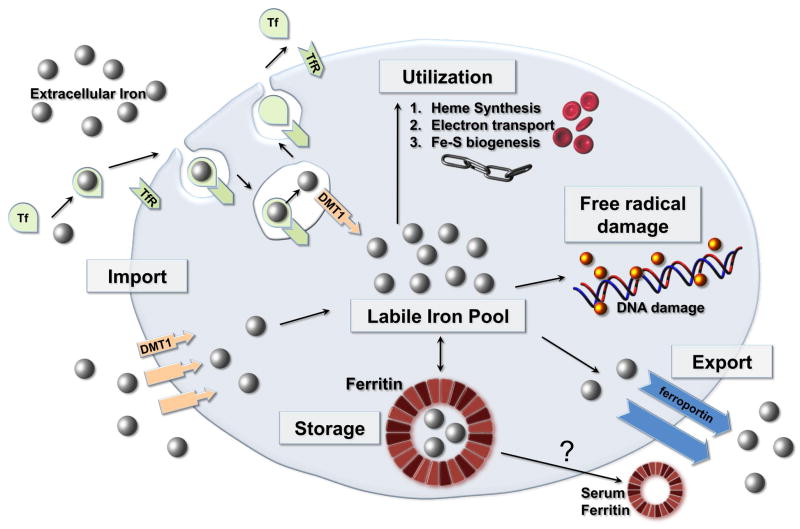
Ferritin serves as a critical component of iron homeostasis, as shown in Figure 2. Its’ primary role is in iron sequestration in which it functions as a ferroxidase, converting Fe(II) to Fe(III) as iron is internalized and sequestered in the ferritin mineral core.
Figure 2.
Intracellular Iron Homeostasis: Ferritin functions as a ferroxidase, converting Fe2+ to Fe3+ as iron is internalized and sequestered in the ferritin mineral core. Reactive species (shown as yellow spheres) can directly damage DNA and proteins. DMT1 = divalent metal ion transporter 1, Tf = Transferrin, TfR = Transferrin receptor.
Iron is toxic in cellular systems because of its capacity to generate reactive species which can directly damage DNA and proteins. Ferritin captures and buffers the intracellular iron pool, and thus is a key component in organism survival. Homozygous murine knock outs of ferritin H are lethal.
Common Clinical Applications
Ferritin in Iron Deficiency
Clinically, serum ferritin is most commonly obtained in combination with other iron parameters to gauge the iron status of a specific patient. Of the various laboratory values within an iron panel, the serum ferritin is the most useful in diagnosing iron deficiency. Though bone marrow biopsy with iron staining remains the gold standard, a low serum ferritin (< 12 ug/L) is highly specific for iron depletion. Only two conditions other than iron deficiency are known to lower serum ferritin; hypothyroidism and ascorbate deficiency. Even when present, they rarely confound the clinical interpretation of ferritin.10 In clinical practice, the use of a higher cutoff value for ferritin is recommended when screening for iron deficiency. Specifically, a cut-off of 40 ug/L improves diagnostic sensitivity in patients whose conditions are not complicated by infections or inflammation. In one study, 25% of women with absent stainable bone marrow iron had serum ferritin levels greater than 15 ug/L.11
Serum ferritin is most frequently collected as part of the work-up for unexplained anemia, often in an attempt to distinguish between iron deficiency and anemia of chronic disease. As detailed above, the test can be nearly diagnostic of iron deficiency if the value is very low. Unfortunately, in a majority of cases, ferritin is less than diagnostic, and further work-up is often indicated, as the inflammatory response modifies iron regulation.12 Measuring the amount of soluble transferrin receptors (sTfR) within the serum may be helpful in these cases. These receptors are shed by “iron-hungry” eythropoietic cells, reflecting the fact that transferrin receptors are up-regulated in response to iron deficiency. Therefore, sTfR is expected to be increased in the setting of iron deficiency anemia, helping to differentiate it from anemia of chronic disease. However, sTfR may also be upregulated in the setting of increased or ineffective erythropoiesis, complicating its’ interpretation. Use of the transferrin receptor-ferritin index (ratio of sTfR to the logarithm of serum ferritin) has been proposed to address this complexity. A value greater than 2 suggests the presence of iron deficiency, while a value less than 1.0 is consistent with anemia of chronic disease. 13
Another clinical scenario in which iron status is evaluated is end-stage kidney disease. However, while ferritin is useful in the evaluation of iron-deficiency anemia, it is much less reliable in the setting of end-stage kidney disease.14 Even with ferritins greater than 200 ug/L, the anemia seen in hemodialysis patients will often respond favorably to iron administration. In these circumstances, it is theorized that the erythroid iron demand is not adequately met. This lack of bioavailable iron in the face of plentiful storage iron is termed “functional iron deficiency”, and can be estimated by the transferrin saturation (TSAT). The TSAT is calculated by dividing the serum iron by the total iron-binding capacity, and is useful in predicting the response to iron therapy. Among patients with end-stage kidney disease, multiple studies have established the value of using a TSAT of less than 20% to determine which patients have the highest likelihood of responding to intravenous iron infusion.15 Using both the TSAT and serum ferritin, the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines propose initiating iron replacement in dialysis patients when the TSAT is <20% or the serum ferritin is <100 ug/L, both of which correlate with depletion of bone marrow iron.16 An increase in serum ferritin to a level of 800 ug/L usually represents adequate iron repletion. Another test being explored in end-stage kidney disease is the reticulocyte hemoglobin content. It evaluates the amount of hemoglobin only within the newly created red blood cells known as reticulocytes, and therefore represents a “snapshot” of iron status within the prior 48 hours. However, this test is not in widespread clinical use.
In most circumstances, serum ferritin is regarded as the most sensitive and specific of the various blood tests available to diagnose iron deficiency. Red cell zinc protoporphyrin levels are elevated in both anemia of chronic inflammation and iron deficiency anemia, as this moiety accumulates in place of red cell iron protoporphyrin if iron is not available. Ferritin outperforms transferrin saturation, mean cell volume, and red cell zinc protoporphyrin levels in terms of sensitivity and specificity at any level.17
Iron Overload Conditions
Ferritin is also clinically useful in the identification and treatment of iron overload. Because iron is primarily regulated at the site of absorption and there is no physiologic process to excrete excess iron, most cases of iron overload occur as a result of abnormal iron absorption or excess iron administration (usually the result of repeated red cell transfusions). Excess iron collects within the liver and heart where it causes chronic free-radical induced injury. Over time, this tissue injury can lead to progressive heart and liver failure, eventually resulting in significant morbidity and early mortality. Other clinical manifestations associated with iron deposition include arthropathy, particularly of the second and third metacarpophalangeal joints, skin changes, and endocrine dysfunction resulting from iron deposition. The phenotype of advanced iron overload has been termed “bronze diabetes”, describing the triad of skin hyperpigmentation, diabetes resulting from pancreatic endocrine dysfunction, and cirrhosis.
Hereditary Hemochromatoses
The classic example of iron overload is hereditary hemochromatosis, an autosomal recessive disorder affecting the absorption of iron. The most common genetic abnormality resulting in the hemochromatosis phenotype is the result of a homozygous C282Y allele. This specific abnormality accounts for 90% of primary hemochromatosis cases and is the most common monoallelic inherited condition among Caucasians. Homozygotes for the C282Y gene express altered HFE protein, which is a major-histocompatibility-complex class I-like protein which forms a heterodimer with beta2-microglobulin. This mutation alters the conformation of the HFE protein on the surface of cells including the duodenal crypt cells and macrophages and interferes with functioning, ultimately leading to increased iron absorption. H63D is an alternate mutation of the HFE gene that can also result in hemochromatosis in combination with a second abnormal allele, most frequently C282Y. Heterozygosity of either of these abnormalities has also been associated with the iron overload phenotype, most commonly in those with other evidence of hepatic insult such as hepatitis or chronic alcohol abuse.
Until recently, it was assumed that homozygosity for the C282Y mutation would ultimately confer the clinical manifestations of iron overload. However, a long-term population study of over 31,000 persons in Melbourne, Australia evaluated the incidence of iron-overload-related disease in C282Y homozygotes as compared to that of matched controls showed that clinical manifestations of fatigue, use of arthritis medicine, and a history of liver disease were more likely to reported by male C282Y homozygotes with a serum ferritin level of 1000 micrograms per liter or more compared to men with the wild-type genotype. Documented iron-overload-related disease (defined as documented iron overload and one of more of the following conditions: cirrhosis, liver fibrosis, hepatocellular carcinoma, elevated aminotransferase levels, physician-diagnosed symptomatic hemochromatosis, and arthropathy of the second and third metacarpophalangeal joints) in C282Y homozygotes was 28.4% for men and 1.2% for women. Only one non-C282Y homozygote (a compound heterozygote) had documented iron-overload-related disease. 18,19 The lower prevalence of iron-overload-related disease in women was always presumed to result from menstrual iron loss; however sex-related disease-modifier genes may contribute.
Because of the prevalence and unpredictable penetrance of hereditary hemochromatosis, as well as it considerable potential for morbidity, routine screening is recommended in first degree relatives of patients with confirmed hereditary hemochromatosis. Recommendations for screening of asymptomatic Caucasian male populations vary. The goal of screening is to identify affected individuals prior to significant iron loading within the organs. Screening allows for early detection via a relatively safe method (blood testing) that is acceptable to patients and clinicians, and potential for timely effective treatment. When therapeutic phlebotomy is used to normalize iron stores early in the course of disease, survival is comparable to age-matched controls without hemachromatosis. The most cost effective strategy for screening involves the use of standard iron markers, particularly the serum ferritin and transferrin saturation. Current recommendations suggest that an elevated ferritin in combination with a TSAT > 45% should prompt genetic confirmation.20 However, a recent population based hemochromatosis screening study of nearly 30,000 white subjects showed that use of the single marker ferritin was a useful strategy; a serum ferritin of more than 1000 ug/L detected only those who were at the highest risk for serious clinical manifestations (ie, cirrhosis).21 Following a diagnosis of hereditary hemochromatosis, liver biopsy is necessary in some patients to asses the degree of end-organ damage. In those with evidence of prolonged (age > 40 at diagnosis) or severe iron overload (ferritin > 1000 ug/L), biopsy of the liver is recommended to obtain direct evidence of the degree of iron overload and liver damage.
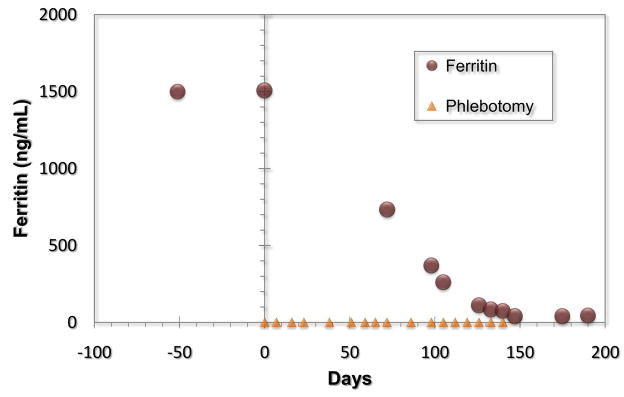
The serum ferritin is also critical in the successful management of hemochromatosis. Hereditary hemochromatosis and other iron overload conditions are treated with therapeutic phlebotomy, typically one unit (consisting of approximately 250 mg of iron) weekly or twice weekly. The patient is phlebotomized to the point of hypoferritinemia with careful monitoring of the hemoglobin to avoid induction of iron deficiency anemia. Thereafter, hypoferritinemia with a goal ferritin of less than 50 ug/L is maintained with periodic phlebotomy typically four to six times yearly in men and one to two times yearly in post menopausal women. Unfortunately, blood withdrawn for phlebotomy cannot be used for blood donation in the United States. Figure 3 shows the decline in ferritin over time after initiation of therapeutic phlebotomy in a patient with hereditary hemochromatosis.
Figure 3.
Decline of ferritin with therapeutic phlebotomy in a patient with hereditary hemochromatosis.
African iron overload was originally thought to be entirely attributable to dietary iron overload from home-brewed beer, as increased dietary iron was associated with an increased transferrin saturation. In the index subjects, increased iron was present in both hepatocytes and cells of the mononuclear-phagocyte system, and livers were enlarged.22 However, further studies showed evidence of a genetic mutation distinct from any HLA-linked gene. A common polymorphism in the ferroportin 1 gene was subsequently shown to be associated with a trend to higher serum ferritin concentration, and lower hemoglobin concentrations. 23 Other genetic lesions leading to iron overload/hemochromatosis include mutations in transferrin receptor 2, hepcidin and hemojuvelin.1
Transfusional Iron Overload
Chronic transfusion therapy is a mainstay of therapy for children and adults with thalassemia major. As a result of the body’s inability to excrete excess iron, repeated transfusion can rapidly result in iron overload, as each unit of packed red blood cells contains 200 to 250 mg of elemental iron. Transfused iron is deposited first within the reticuloendothelial cells prior to parenchymal iron loading within the heart and liver. However, as in primary iron overload, the majority of morbidity and mortality ultimately results from progressive heart and liver failure. It has also been hypothesized that excess iron contributes to ineffective erythropoiesis, thereby further complicating many marrow failure diseases that require chronic transfusions. This possibility is supported by the observation that treatment of iron overload improves cell counts in patients with myelodysplastic syndrome (MDS).
Treatment with phlebotomy is not possible in patients with transfusional iron overload, as they are dependent on chronic transfusions. In these patients, chelation therapy is the preferred choice for treatment.24 Deferoxamine is a very effective iron chelator that is administered either subcutaneously or intravenously. There is over three decades of experience with its use, and until recently it represented the only iron chelator available in the United States. Recommended treatment is at least five nights per week subcutaneously delivered via a pump for 8–12 hours. Understandably, adherence to this strict regimen is difficult, and noncompliance is common. Neurotoxicity, ocular toxicity, oto-toxicity, and poor growth have been reported with the overuse of deferoxamine.25
In order to overcome these limitations, there has been an active search for oral iron chelators. Deferiprone is an iron chelator first identified in the 1980s and subsequently approved for clinical use in Canada and Europe in patients in whom deferoxamine is contraindicted. It has a short half-life of only 1.5 hours, and thus requires three times daily dosing. Unfortunately, it does not control liver iron as well as deferoxamine, even after years of continued treatment.26 In contrast, a recent study in patients with thalassemia showed better myocardial function in those receiving deferiprone.27 The high risk of agranulocytosis necessitates weekly blood monitoring.
A novel oral iron chelator, deferasirox (Exjade®, Novartis) was approved by the US Food and Drug Administration in 2005 and represents a significant advancement in the treatment of iron overload. It is a tridentate oral iron chelator which is lipid soluble but highly protein bound. It has a plasma half life of about 12 hours and thus is ideal for once daily dosing. It binds iron in a 2:1 ratio. It is excreted by the hepatobiliary system and the chelated iron is excreted via the feces. The effective dose is between 20–40 mg/kg. It is generally well tolerated by patients, although some dose modifications may be necessary for diarrhea. Phase III trials demonstrated that deferasirox at 20–30 mg/kg per day led to the maintenance or reduction of iron burden as measured by liver iron content in chronically transfused patients, including those with thalassemia and sickle cell syndrome as well as rare anemias and myelodysplastic syndromes. Reductions in liver iron content and serum ferritin were similar to those found in the subcutaneous use of deferoxamine. 25, 28
Other measures of Iron Stores
The monitoring of iron overload was recently reviewed.26, 29, 30 New approaches include biomagnetic susceptometry and magnetic resonance imaging, which offer a noninvasive measure of iron deposition in the heart and liver.
Hepatic Iron Index
Though ferritin continues to be the mainstay for the initial clinical evaluation of iron overload, liver biopsy is the gold standard for quantifying iron. Hepatic iron concentration exceeding 80 micromoles/gram of liver dry weight is consistent with iron overload, as is a hepatic index of greater than 1.9 mmol per kilogram per year (the hepatic iron index is the ratio of hepatic iron concentration to the age of the patient in years).2 Total body iron can be accurately measured by assessment of liver iron content (LIC) obtained from liver biopsy. However, the need for a relatively large volume of tissue (4mg net weight) as well as risk of this invasive procedure (hemorrhage occurs in about 0.5% of cases) has made this less appealing to most clinicians and patients.26
Imaging Modalities for Assessment of Iron Overload
SQUID (Superconducting Quantum Interference Device) assesses total body iron using biomagnetic susceptometry. Ferritin and hemosiderin are the only paramagnetic materials in the human body, thus the magnitude is directly related to the amount of iron in a certain volume of tissue. It shows excellent correlation between liver iron as measured by biopsy, but unfortunately is very expensive and available at only a few centers worldwide.26
MRI is becoming increasingly important in the evaluation of iron status because it is non-invasive and rapidly becoming more widely available. It has the additional benefit of identifying relatively early iron overload within organs, prior to the onset of dysfunction. With the use of microwaves, MRI is able to detect the extent of magnetic disruption due to iron deposition. In iron-overloaded tissues, this disruption results in a magnetic gradient echo referred to as T2* (pronounced T2 star). MRI has been used to measure iron deposition within the heart, liver, and pituitary. At this time, it does not appear that a single organ gives the full picture of total body iron overload. In fact, patients can accumulate cardiac iron, despite apparently normal hepatic iron levels, and thus be at risk for arrhythmia or congestive heart failure. The discordance of values in these two tissues can be resolved with the use of MRI to detect cardiac iron. Studies by Wood et al show that abnormal cardiac T2* represents a preclinical degree of cardiac iron overloading.31,32, 33 The authors present a clinical model which classifies patients into groups based on cardiac T2* values. Patients with the shortest T2* values represent those with sufficiently increased risk of cardiac decomposition to require immediate review and intensification of chelation therapy.
Non-invasive measurement of liver iron concentration has also been achieved using a magnetic resonance technique based on proton transverse relaxation rates within the liver. The technique can be implemented on most clinical 1.5-T MRI instruments, making it readily available to the clinical community. 34, 35 On average, this method involves a 20 minute data acquisition. This technique resulted in a high specificity and sensitivity over a greater range of liver iron concentrations than any other MRI-based method of liver iron concentration assesment.35 A study comparing MRI and biomagnetic liver susceptometry showed a significant linear correlation between the liver iron content as measured by both methods.36
While these imaging studies are sophisticated and provide valuable information regarding the often variable degree of iron deposition in end-organs, serum ferritin offers an easy, inexpensive surrogate for total body iron overload. It is particularly useful both as an indicator for initiation of chelation therapy in iron overload syndromes, and as a marker for treatment progress.
Ferritin and Human Disease
Still’s disease
Adult onset Still’s disease is a systemic inflammatory disorder characterized by fever, arthritis, and rash that typically affects young women.37,38 Elevated serum ferritin levels were seen in 89% of these patients in a recent series, nearly half of whom had levels greater than five times normal, though this did not correlate with time to disease remission or the presence of chronic or deforming arthritis.38 Another recent case control study established that an exaggerated ferritin response with levels greater than fives times the upper limit of normal and high ferritin to C-reactive protein ratios were useful in distinguishing between adult Still’s disease and rheumatoid arthritis, and that ferritin levels greater than five times the upper limit of normal were associated with a chronic disease course.39 Whether the disproportionate ferritin response is a pathogenic mechanism or is merely a by-product of inflammation remains unknown. Another confounder is that elevated ferritin in patients with Still’s disease may represent hemophagocytic syndrome, which is frequently seen in this population as well as in other autoimmune disorders.
Hemophagocytic Syndrome
Hemophagocytic syndrome (also known as macrophage activation syndrome or lymphohistiocytic syndrome) is a heterogeneous group of disorders with a final common pathway consisting of hypertriglyceridemia, hyperferritinemia, pancytopenia, and multiple organ failure which is highly fatal.40 The syndrome is strongly associated with autoimmune disorders, particularly systemic lupus erythematosis and Still’s disease, and viral infections, particularly Epstein-Barr virus. Ingestion of red blood cells and red blood cells precursors by macrophages or histiocytes in the bone marrow is the hallmark characteristic of this disorder. Demonstration of hemophagocytosis on biopsy of the bone marrow, spleen, liver or lymph nodes is considered the gold standard for diagnosis of this syndrome; however, serial biopsies may be necessary to confirm the diagnosis.
Loss of natural killer cell activity or decreased numbers of natural killer cells is thought to be the causative mechanism in most cases. Hemophagocytic syndrome should be considered in any critically ill patient with evidence of systemic inflammation or multiple organ involvement with associated cytopenias. Serum ferritin is classically elevated above 10,000 ug/L. However, increased diagnostic sensitivity may be obtained with a lower cutoff of ferritin if combined with the assessment of other specific markers of this disease, such as soluble CD163, which is exclusively expressed on cells of monocyte/macrophage lineage.41
Of note, in a prospective study of adult hemophagocytic syndrome patients, the percentage of glycosylated ferritin was lower in patients with HPS suggesting that low glycosylated ferritin could be a marker of severe HPS.42 The elevated ferritin is hypothesized to be due to passive release from cell damage in the liver and spleen, increased secretion by macrophages or hepatocytes, or decreased clearance due to lower glycosylation or down regulation of ferritin receptors. It is known that the H chain exerts immunomodulatory activity. Another possibility is that the massively increased ferritin expression may exceed the glycosylation capacity of the endoplasmic reticulum in HPS.
Sideroblastic anemias
Sideroblastic anemias represent a heterogeneous group of hematopoietic disorders with a common manifestation of ineffective erythropoiesis and associated iron overload. Deranged heme synthesis in developing red cells leads to misshapen erythrocytes. As iron delivery is not reduced, the excess iron that has been delivered to the developing erythroblasts is deposited within the mitochondria. Enhanced intestinal absorption of iron is also a feature of sideroblastic anemias, although the etiology remains undefined. In sideroblastic anemias, in contrast to genetic hemochromatosis, the iron deposition occurs in bone marrow macrophages, although there is also deposition in parenchymal tissues. The end organ manifestations, however, are similar and irreversible organ damage to the liver, the heart, and endocrine system occur. In addition, these patients may acquire iron overload from the transfusions given to treat their anemia.
Sideroblastic anemias can be hereditary but are more commonly acquired. Refractory anemia with ringed sideroblasts comprises one subset of myelodysplastic syndromes, a heterogeneous group of acquired bone marrow failure disorders. Bone marrow morphology is characterized by the presence of erythroid precursors in which a ring of ten or more siderotic granules encircle one third or more of the nucleus. Hemosiderin-laden macrophages are often abundant. Mitochondrial ferritin, an unusual and newly described form of ferritin, has been observed in ringed sideroblasts43, and has been proposed as a sensitive marker for classification of myelodysplastic syndromes44. Anti-tuberculosis drugs and alcoholism can cause ringed sideroblasts, as can copper deficiency and/or associated zinc toxicity.45 Heme synthesis is decreased presumably secondary to low activity of the copper containing enzyme cytochrome oxidase, leading to a slower rate of electron flow and ATP production, and ultimately diminished hemoglobin synthesis.46 Zinc toxicity leads to clinical copper deficiency.
Ferritin in Selected Neurologic Disorders
Several iron disorders that affect movement and other neurologic functions have been well characterized, and have been the subject of recent comprehensive reviews.47,48 We present those which are most common (Parkinson’s disease and restless legs syndrome) and those whose pathophysiology is most directly linked to abnormalities in ferritin.
Parkinson’s Disease
The role of iron in neurologic disease is the focus of intense study, based on the observation that brain iron content is increased in patients with Parkinson’s disease and other neurodegenerative disorders. Although many of the proteins involved in systemic iron homeostasis are also found in brain tissue, genetic disorders resulting in the loss of function of these proteins rarely result in pathologic iron deficits or excess. Specific regions of the brain vary widely in iron content. In the basal ganglia, the concentration is greatest and equivalent to that found in the liver. Iron is also particularly abundant in astrocytes, supporting the idea that these cells and other types of glia function in iron storage and regulation. It is thought that brain iron homeostasis is separated from systemic iron homeostasis by the blood brain barrier, which will allow minimal effects on central nervous system by alterations in systemic iron homeostasis.49
The brains of parkinsonian patients have been shown to contain increased levels of iron; however, the most striking increases are seen in the substantia nigra pars compacta. The severity of the disease in the living patient can be assessed by MRI. Post mortem studies of brains from normal subjects at different ages suggests a relationship between substantia nigra echogenicity with higher levels of iron, L-ferritin, and H-ferritin, and a reduced neuromelanin concentration.50,51 This model may describe a toxic cellular milieu that promotes the generation of oxyradicals and cell damage.
In the brain, ferritin is expressed in oligodendrocytes, astrocytes, and microglial cells, but not in neurons. In Parkinson’s disease, ferritin cores in the substantial nigra are denser and contain more iron than those in the substantial nigra of normal subjects. The ferritin-rich microglial cells are located in close proximity to melanin-containing or degeneration neurons. Neuronal iron is predominantly bound to neuromelanin. It is unclear whether neuromelanin has a cytoprotective role in binding transition metals, or whether it triggers cytotoxicity if iron is released within neurons. Neurons that degenerate in PD contain neuromelanin and high amounts of iron, while neurons that survive in PD are free of neuromelanin, contain low amounts of iron, and are better protected against oxidative stress 50,52
Friedreich’s Ataxia
Friedreich’s Ataxia is an autosomal recessive disorder characterized by sensory, cerebellar, and cardiomyocyte degeneration. An expansion of a GAA repeats produces a dysfunctional frataxin gene, which in turn decreases the assembly of iron-sulphur clusters within the mitochondria. The lack of effective cluster formation leads to oxidative damage and neurodegeneration as a result of labile iron accumulation. The iron accumulation is specific to certain tissues and often occurs without evidence of systemic iron overload, such as an elevated serum ferritin. The focal nature of the iron accumulation is evident in Friederich’s ataxia patients, where MRI studies can detect excess iron within the dentate nuclei of the central nervous system.53 Recent efforts to reverse this iron accumulation with the membrane-permeable chelator, deferiprone, have shown promise. Use of deferiprone in a small group of adolescent Friedereich’s ataxia patients reduced the amount of iron within the dentate nuclei as detected by R2* MRI. More impressively, the neurologic symptoms associated with Friedereich’s ataxia showed early signs of improvement.54 These studies further define the importance of iron homeostasis in the pathogenesis and treatment of neurologic disease.
Neuroferritinopathy
Neuroferritinopathy, also known as dominant adult-onset basal ganglia disease, is an autosomal dominant extrapyramidal disease resulting from mutations in the light chain of ferritin. Symptoms include choreoathetosis, dystonia, spasticity, dysarthria. It is thought that the mutations impair ferritin assembly, leading to loss of iron storage capacity within brain cells, and thus subsequent iron-mediated cell injury.55 All patients with neuroferritinopathy have dystonia in association with basal ganglia iron accumulation. Interestingly, the ferritin light chain mutation in patients with neuroferritinopathy is present in all cells, but with the exception of decreased serum ferritin, there are no abnormalities of systemic iron homeostasis. It is uncertain whether this represents a greater sensitivity of brain cells to this iron-dependent injury, or in fact whether there is a unique role for the ferritin light chain in these brain cell types. The similar pathology of neuroferritinopathy and aceruloplasinemia suggest a common iron-dependent mechanism for the neurodegeneration that is found in both diseases.
Restless Legs Syndrome
Restless Legs Syndrome is a neurologic disorder characterized by unpleasant sensations in the legs that appear mostly at night upon retiring, including an irrepressible urge to move the limbs. Of note, in an Italian study, 26% of pregnant women had symptoms of restless leg syndrome, of which only 10% had symptoms prior to the pregnancy. In a study by Kotagal and Silber56, serum ferritin levels were decreased in 83% of 24 patients with restless legs syndrome, suggesting that iron deficiency is characteristic of the disorder. However, in a large Italian study, ferritin concentrations were similar in individuals with and without restless legs syndrome, although those that had restless legs syndrome had higher serum concentrations of soluble transferrin receptor, possibly indicating early iron deficiency 57
There has been some suggestion that patients with restless legs syndrome have significantly lower spinal fluid ferritin levels compared to controls; although serum ferritin and transferrin levels can be similar in both groups, suggesting a central etiology to the disorder.58 Connor showed a dramatic decrease in iron and ferritin heavy chains and an increase in transferrin staining in the substantia nigra of restless legs syndrome brains compared to controls.59 Thus, restless legs syndrome may be a functional disorder resulting from impaired iron acquisition by the neuromelanin cells. These patients are typically managed by dopaminergic drugs. It is reasonable to check iron profiles in patients who present with RLS and to replete iron if indeed there is evidence of iron deficiency on serum markers.
Ferritin cataract syndrome- ferritin L hyperferritienemia
An autosomal dominant syndrome was reported in two Italian families which resulted in a combination of congenital nuclear cataracts and elevated serum ferritin in the presence of a normal serum iron and transferrin saturation. The syndrome was attributed to a mutation in a regulatory element of the L ferritin mRNA in this family.60 Consistent with this etiology, studies using monoclonal antibodies showed elevation of ferritin L subunit but no ferritin H subunit in affected individuals. However, it is unclear how the perturbation in ferritin L leads to lens opacity61,62.
Future Directions/Evolving Applications
Coronary Artery Disease
Coronary artery disease is a leading cause of death in developed countries. The relationship between iron overload and increased risk for developing cardiovascular disease was recently reviewed.63 Epidemiologic studies have shown a correlation between elevated serum ferritin and an increased risk of coronary artery disease and myocardial infarction.64 This association was first reported by Salonen et al. in the Finnish Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD) of greater than 1,900 middle aged men who were followed for an average of three years.65 There was a 2.2 higher risk of myocardial infarction in men with a serum ferritin ≥ 200 ug/L. The association was stronger in men with higher LDL concentrations. In another study, Klipstein-Grobusch et al. showed a positive correlation between serum ferritin and the risk of myocardial infarction, particularly in current or former smokers.66
Conversely, iron depletion has been shown to reduce the risk of myocardial infarction in other cardiac disease events. There was a significant decrease in HDL, LDL, triglycerides, fibrinogen and blood pressure in 31 patients who were phlebotomized to achieve an iron depletion state. A prospective 5 year follow up study of a cohort of over 2,500 Finnish men showed that myocardial infarction was decreased 86% in blood donors as compared to non-donors.67
Nonetheless, there are many epidemiologic studies that do not confirm the presence of a direct association between iron status and the risk of developing cardiovascular disease. The issue of whether an elevated ferritin level is a contributor or causative factor for coronary artery disease or simply a byproduct of the disease process will be addressed by atherosclerotic animal models, in which transgenic over expression of ferritin L or ferritin H can be created.
Ferritin as an iron concentrator and chelator target
Recently a new property of ferritin was discovered: gated pores. These are highly conserved in ferritins of humans and bacteria. The pore gates can be manipulated by mutation, temperature and physiologic concentrations of urea to selectively open, thus increasing chelator access. Future chelators may be targeted to ferritin protein pores. Thus, the potential exists for a class of more effective ferritin-specific iron chelators with low toxicity.68
Ferritin in malignancy
Breast cancer
The idea that excess iron can potentiate carcinogenesis is an intriguing one. Free iron can induce oxidative stress and DNA damage. Animal models have shown that excess free iron is carcinogenic. Kabat and Rohan recently reviewed the evidence for the role of excess iron in breast carcinogenesis.69 Oxidative stress is induced by a reactive oxygen species, the formation of which is mediated by free iron. Ferric iron (Fe3+) released from ferritin and hemosiderin is reduced to ferrous iron (Fe2+) which, in the presence of super oxide and hydrogen peroxide (H2O2), can catalyze the formation of the hydroxyl radical (*OH). The hydroxyl radical is a powerful oxidizing agent which can promote lipid peroxidation, mutagenesis, DNA strand breaks, activation of oncogenes, and tumor suppressor gene inhibition. Conflicting evidence exists regarding the contribution of lipid peroxidation products in breast cancer.
It is known that excess iron alters the distribution of T-lymphocyte subsets and suppresses the action of helper T (CD4) cells, as well as the tumoricidal action of macrophages and monocytes. In hereditary hemochromatosis patients, iron overload increases the numbers and activities of suppressor T (CD8) cells and decreases the numbers and activities of CD4 cells resulting in increased CD8:CD4 ratios. Thus, it is thought that the excess iron may impair surveillance for cancer cells by these mechanisms.
In addition, the fact that iron levels accumulate with age, particularly in post menopausal women, may contribute to the age-associated risk of breast cancer. However, it is not known whether iron levels in breast tissue increase with age.
Tissue ferritin levels have been shown to be six-fold higher in breast cancer tissue compared to normal or benign breast cancer tissue. 61, 70, 71 Levels of transferrin and transferrin receptor proteins have also been shown to be higher in breast cancer tissue.72–74 In addition, compared to women without breast cancer in whom serum ferritin was normal, 41% of women with preoperative breast cancer had elevated serum ferritin levels. In addition, iron levels in breast cancer biopsy tissue were five times higher than levels in breast tissue from women without breast cancer.75 However, no studies to date have demonstrated that ferritin is the etiology for the cancer rather than merely a serum maker for the presence of cancer. It is postulated that iron interacts with known agents in breast carcinogenesis, particularly estradiol, ethanol and ionizing radiation. Iron overload favors the production of reactive oxygen species, lipid peroxidation, and DNA damage. If indeed future studies show that excess body iron levels contribute to the development of breast cancer, it may be feasible to reduce this risk by the use of chelating agents.76
Bone marrow transplant patients
Serum ferritin has recently been identified as a prognostic marker in patients undergoing allogeneic stem cell transplant. In a recent study by Armand et al, the outcomes of 590 patients with pre-transplant ferritin levels were reported.77 An elevated ferritin was independently associated with increased mortality in acute myeloid leukemia and myelodsyplastic syndrome patients. A similar effect on survival was seen in transplanted thalassemia patients with suspected iron overload.78 It is likely that iron overload, with serum ferritin serving as a surrogate measure, is mechanistically responsible for the impact on transplant survival. This is supported by our own data showing that the use of multiple measures of iron overload is more closely associated with survival than serum ferritin alone.79 Other non-invasive studies measuring iron overload, such as T2* MRI, have yet to be examined in the transplant setting.
Iron overload appears to increase treatment-related mortality (TRM), rather than increasing the aggressiveness or relapse rate of the underlying disease.80, 81 Exactly how iron overload increases TRM is unclear but may be related to increased rates of infection. There is pre-clinical data suggesting that an increase in available iron may result in the proliferation of infectious organisms, such as bacteria and fungi.82 In transplant patients with iron overload at autopsy, rates of documented invasive aspergillosis were significantly higher.83 Perhaps of most clinical relevance is the observation that lethal infections occur at a disproportionately higher rate in those with iron overload,79 further supporting the link between iron overload and infection.
Prospective studies to further define the impact of iron overload in the transplant setting are ongoing.84 The use of chelation therapy has been associated with improved outcomes in thalassemia 85, suggesting that early treatment of iron overload may improve transplant survival. It is likely that ferritin, given its prognostic utility and easy availability, will play a critical role in the diagnosis and management of iron overload in the transplant setting.
Ferritin Binding Proteins/Kininogen
Ferritin binding partners in plasma include alpha 2 macroglobulin86, apolipoprotein B through hemin87, and high molecular weight kininogen (HK)88. Ferritin/HK binding is one of the best studied of these interactions. HK is a co-factor in the intrinsic coagulation cascade, and is cleaved by the serine protease kallikrein at cell surfaces to release bradykinin (BK) and two-chain high molecular weight kininogen (HKa)89. HK and its cleavage products have been implicated in the progression of various inflammatory diseases including asthma90, inflammatory bowel disease91 and cancer92. Ferritin, through binding to HK, inhibits kallikrein-mediated HK cleavage93. Ferritin also inhibits the cleavage of HK by the inflammatory proteases neutrophil elastase and mast cell tryptase. This effect is independent of iron, as both apoferritin (iron free ferritin) and holoferritin (iron rich ferritin) exhibit this property94. Through decreasing HK cleavage, ferritin reduces the levels of BK and HKa and may serve to dampen the inflammatory response. Indeed, ferritin, elastase and HK have been shown to co-localize in alveolar macrophages in a murine model of lung inflammation94. The regulation of HK may represent a novel function for ferritin independent of iron homeostasis.
PRACTICE POINTS
Low serum ferritin is highly specific for iron deficiency anemia in otherwise healthy patients, as virtually no other clinically significant conditions will result in very low levels. In patients with underlying inflammation or infection, the use of ferritin with other markers of iron deficiency, especially soluble transferrin receptor, allows the clinician to distinguish between anemia of inflammation and iron deficiency anemia.
Ferritin levels are not particularly useful in end stage renal disease to predict bioavailable iron. However, high quality evidence-based guidelines exist to guide appropriate iron therapy in these patients.
Magnetic resonance imaging is a widely available technique that shows excellent promise in measuring iron content in specific organs, especially the liver and heart.
Though hepatic biopsy is the gold standard, ferritin is a useful non-invasive surrogate marker to assess degree of end-organ iron overload in hereditary hemochromatosis.
Acknowledgments
Supported in part by grants from the National Institutes of Health (R01DK71892, SVT; R37 DK42421, FMT) and a predoctoral fellowship from the American Heart Association (LGC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69(69–85):69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 2.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341(26):1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 3.Andrews NC. Understanding heme transport. N Engl J Med. 2005;353(23):2508–2509. doi: 10.1056/NEJMcibr053987. [DOI] [PubMed] [Google Scholar]
- 4.Anderson GJ, Frazer DM. Hepatic iron metabolism. Semin Liver Dis. 2005;25(4):420–432. doi: 10.1055/s-2005-923314. [DOI] [PubMed] [Google Scholar]
- 5.Heeney MM, Andrews NC. Iron homeostasis and inherited iron overload disorders: an overview. Hematol Oncol Clin North Am. 2004;18(6):1379–403. ix. doi: 10.1016/j.hoc.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108(4):1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005;12(2):107–111. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Taketani S. Aquisition, mobilization and utilization of cellular iron and heme: endless findings and growing evidence of tight regulation. Tohoku J Exp Med. 2005;205(4):297–318. doi: 10.1620/tjem.205.297. [DOI] [PubMed] [Google Scholar]
- 9.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99(10):3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 10.Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145(5):657–663. [PMC free article] [PubMed] [Google Scholar]
- 11.Hallberg L, Bengtsson C, Lapidus L, Lindstedt G, Lundberg PA, Hulten L. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. Br J Haematol. 1993;85(4):787–798. doi: 10.1111/j.1365-2141.1993.tb03225.x. [DOI] [PubMed] [Google Scholar]
- 12.Weiss G. Modification of iron regulation by the inflammatory response. Best Pract Res Clin Haematol. 2005;18(2):183–201. doi: 10.1016/j.beha.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89(3):1052–1057. [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Kalantar-Zadeh K, Lee GH. The fascinating but deceptive ferritin: to measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol. 2006;1(Suppl 1):S9–18. S9–18. doi: 10.2215/CJN.01390406. [DOI] [PubMed] [Google Scholar]
- 15.Coyne D. Iron indices: what do they really mean? Kidney Int Suppl. 2006;(101):S4–S8. doi: 10.1038/sj.ki.5000404. [DOI] [PubMed] [Google Scholar]
- 16.Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1 (Suppl 1):S4–S8. doi: 10.2215/CJN.01490506. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7(2):145–153. doi: 10.1007/BF02598003. [DOI] [PubMed] [Google Scholar]
- 18.Allen KJ, Gurrin LC, Constantine CC, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358(3):221–230. doi: 10.1056/NEJMoa073286. [DOI] [PubMed] [Google Scholar]
- 19.Bacon BR, Britton RS. Clinical penetrance of hereditary hemochromatosis. N Engl J Med. 2008;358(3):291–292. doi: 10.1056/NEJMe078215. [DOI] [PubMed] [Google Scholar]
- 20.Qaseem A, Aronson M, Fitterman N, Snow V, Weiss KB, Owens DK. Screening for hereditary hemochromatosis: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005;143(7):517–521. doi: 10.7326/0003-4819-143-7-200510040-00010. [DOI] [PubMed] [Google Scholar]
- 21.Waalen J, Felitti VJ, Gelbart T, Beutler E. Screening for hemochromatosis by measuring ferritin levels: a more effective approach. Blood. 2008;111(7):3373–3376. doi: 10.1182/blood-2007-07-102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordeuk V, Mukiibi J, Hasstedt SJ, et al. Iron overload in Africa. Interaction between a gene and dietary iron content. N Engl J Med. 1992;326(2):95–100. doi: 10.1056/NEJM199201093260204. [DOI] [PubMed] [Google Scholar]
- 23.Gordeuk VR, Caleffi A, Corradini E, et al. Iron overload in Africans and African-Americans and a common mutation in the SCL40A1 (ferroportin 1) gene. Blood Cells Mol Dis. 2003;31(3):299–304. doi: 10.1016/s1079-9796(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 24.Maggio A. Light and shadows in the iron chelation treatment of haematological diseases. Br J Haematol. 2007;138(4):407–421. doi: 10.1111/j.1365-2141.2007.06666.x. [DOI] [PubMed] [Google Scholar]
- 25.Barton JC. Optimal management strategies for chronic iron overload. Drugs. 2007;67(5):685–700. doi: 10.2165/00003495-200767050-00004. [DOI] [PubMed] [Google Scholar]
- 26.Porter JB. Monitoring and treatment of iron overload: state of the art and new approaches. Semin Hematol. 2005;42(2 Suppl 1):S14–S18. doi: 10.1053/j.seminhematol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Magri D, Sciomer S, Fedele F, et al. Early impairment of myocardial function in young patients with beta-thalassemia major. Eur J Haematol. 2008;80(6):515–522. doi: 10.1111/j.1600-0609.2008.01054.x. [DOI] [PubMed] [Google Scholar]
- 28.Stumpf JL. Deferasirox. Am J Health Syst Pharm. 2007;64(6):606–616. doi: 10.2146/ajhp060405. [DOI] [PubMed] [Google Scholar]
- 29.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 30.Sheth S, Tang H, Jensen JH, et al. Methods for noninvasive measurement of tissue iron in Cooley’s anemia. Ann N Y Acad Sci. 2005;1054:358–372. doi: 10.1196/annals.1345.044. [DOI] [PubMed] [Google Scholar]
- 31.Wood JC. Magnetic resonance imaging measurement of iron overload. Curr Opin Hematol. 2007;14(3):183–190. doi: 10.1097/MOH.0b013e3280d2b76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood JC, Enriquez C, Ghugre N, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(4):1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22(23):2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 34.St Pierre TG, Clark PR, Chua-Anusorn W. Measurement and mapping of liver iron concentrations using magnetic resonance imaging. Ann N Y Acad Sci. 2005;1054:379–385. doi: 10.1196/annals.1345.046. [DOI] [PubMed] [Google Scholar]
- 35.St Pierre TG, Clark PR, Chua-Anusorn W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105(2):855–861. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 36.Carneiro AA, Fernandes JP, de Araujo DB, et al. Liver iron concentration evaluated by two magnetic methods: magnetic resonance imaging and magnetic susceptometry. Magn Reson Med. 2005;54(1):122–128. doi: 10.1002/mrm.20510. [DOI] [PubMed] [Google Scholar]
- 37.Zandman-Goddard G, Shoenfeld Y. Ferritin in autoimmune diseases. Autoimmun Rev. 2007;6(7):457–463. doi: 10.1016/j.autrev.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Uppal SS, Al-Mutairi M, Hayat S, Abraham M, Malaviya A. Ten years of clinical experience with adult onset Still’s disease: is the outcome improving? Clin Rheumatol. 2007;26(7):1055–1060. doi: 10.1007/s10067-006-0440-x. [DOI] [PubMed] [Google Scholar]
- 39.Evensen KJ, Swaak TJ, Nossent JC. Increased ferritin response in adult Still’s disease: specificity and relationship to outcome. Scand J Rheumatol. 2007;36(2):107–110. doi: 10.1080/03009740600958504. [DOI] [PubMed] [Google Scholar]
- 40.Sekigawa I, Suzuki J, Nawata M, et al. Hemophagocytosis in autoimmune disease. Clin Exp Rheumatol. 2001;19(3):333–338. [PubMed] [Google Scholar]
- 41.Emmenegger U, Schaer DJ, Larroche C, Neftel KA. Haemophagocytic syndromes in adults: current concepts and challenges ahead. Swiss Med Wkly. 2005;135(21–22):299–314. doi: 10.4414/smw.2005.10976. [DOI] [PubMed] [Google Scholar]
- 42.Emmenegger U, Frey U, Reimers A, et al. Hyperferritinemia as indicator for intravenous immunoglobulin treatment in reactive macrophage activation syndromes. Am J Hematol. 2001;68(1):4–10. doi: 10.1002/ajh.1141. [DOI] [PubMed] [Google Scholar]
- 43.Cazzola M, Invernizzi R, Bergamaschi G, et al. Mitochondrial ferritin expression in erythroid cells from patients with sideroblastic anemia. Blood. 2003;101(5):1996–2000. doi: 10.1182/blood-2002-07-2006. [DOI] [PubMed] [Google Scholar]
- 44.la Porta MG, Malcovati L, Invernizzi R, et al. Flow cytometry evaluation of erythroid dysplasia in patients with myelodysplastic syndrome. Leukemia. 2006;20(4):549–555. doi: 10.1038/sj.leu.2404142. [DOI] [PubMed] [Google Scholar]
- 45.Jaffe ES. World Health Organization. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press ; Oxford : Oxford University Press (distributor); 1943. 2001. [Google Scholar]
- 46.Haddad AS, Subbiah V, Lichtin AE, Theil KS, Maciejewski JP. Hypocupremia and bone marrow failure. Haematologica. 2008;93(1):e1–e5. doi: 10.3324/haematol.12121. [DOI] [PubMed] [Google Scholar]
- 47.Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- 48.Thomas M, Jankovic J. Neurodegenerative disease and iron storage in the brain. Curr Opin Neurol. 2004;17(4):437–442. doi: 10.1097/01.wco.0000137534.61244.d1. [DOI] [PubMed] [Google Scholar]
- 49.Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- 50.Gotz ME, Double K, Gerlach M, Youdim MB, Riederer P. The relevance of iron in the pathogenesis of Parkinson’s disease. Ann N Y Acad Sci. 2004;1012:193–208. doi: 10.1196/annals.1306.017. [DOI] [PubMed] [Google Scholar]
- 51.Zecca L, Berg D, Arzberger T, et al. In vivo detection of iron and neuromelanin by transcranial sonography: a new approach for early detection of substantia nigra damage. Mov Disord. 2005;20(10):1278–1285. doi: 10.1002/mds.20550. [DOI] [PubMed] [Google Scholar]
- 52.Hirsch EC. Biochemistry of Parkinson’s disease with special reference to the dopaminergic systems. Mol Neurobiol. 1994;9(1–3):135–142. doi: 10.1007/BF02816113. [DOI] [PubMed] [Google Scholar]
- 53.Waldvogel D, van GP, Hallett M. Increased iron in the dentate nucleus of patients with Friedrich’s ataxia. Ann Neurol. 1999;46(1):123–125. doi: 10.1002/1531-8249(199907)46:1<123::aid-ana19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 54.Boddaert N, Le Quan Sang KH, Rotig A, et al. Selective iron chelation in Friedreich ataxia: biologic and clinical implications. Blood. 2007;110(1):401–408. doi: 10.1182/blood-2006-12-065433. [DOI] [PubMed] [Google Scholar]
- 55.Levi S, Cozzi A, Arosio P. Neuroferritinopathy: a neurodegenerative disorder associated with L-ferritin mutation. Best Pract Res Clin Haematol. 2005;18(2):265–276. doi: 10.1016/j.beha.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 56.Kotagal S, Silber MH. Childhood-onset restless legs syndrome. Ann Neurol. 2004;56(6):803–807. doi: 10.1002/ana.20292. [DOI] [PubMed] [Google Scholar]
- 57.Hogl B, Kiechl S, Willeit J, et al. Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology. 2005;64(11):1920–1924. doi: 10.1212/01.WNL.0000163996.64461.A3. [DOI] [PubMed] [Google Scholar]
- 58.Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54(8):1698–1700. doi: 10.1212/wnl.54.8.1698. [DOI] [PubMed] [Google Scholar]
- 59.Connor JR, Boyer PJ, Menzies SL, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61(3):304–309. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- 60.Bonneau D, Winter-Fuseau I, Loiseau MN, et al. Bilateral cataract and high serum ferritin: a new dominant genetic disorder? J Med Genet. 1995;32(10):778–779. doi: 10.1136/jmg.32.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Girelli D, Corrocher R, Bisceglia L, et al. Molecular basis for the recently described hereditary hyperferritinemia-cataract syndrome: a mutation in the iron-responsive element of ferritin L-subunit gene (the “Verona mutation”) Blood. 1995;86(11):4050–4053. [PubMed] [Google Scholar]
- 62.Bonneau D, Winter-Fuseau I, Loiseau MN, et al. Bilateral cataract and high serum ferritin: a new dominant genetic disorder? J Med Genet. 1995;32(10):778–779. doi: 10.1136/jmg.32.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.You SA, Wang Q. Ferritin in atherosclerosis. Clin Chim Acta. 2005;357(1):1–16. doi: 10.1016/j.cccn.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 64.de Godoy MF, Takakura IT, Machado RD, Grassi LV, Nogueira PR. Serum ferritin and obstructive coronary artery disease: angiographic correlation. Arq Bras Cardiol. 2007;88(4):430–433. doi: 10.1590/s0066-782x2007000400011. [DOI] [PubMed] [Google Scholar]
- 65.Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86(3):803–811. doi: 10.1161/01.cir.86.3.803. [DOI] [PubMed] [Google Scholar]
- 66.Klipstein-Grobusch K, Koster JF, Grobbee DE, et al. Serum ferritin and risk of myocardial infarction in the elderly: the Rotterdam Study. Am J Clin Nutr. 1999;69(6):1231–1236. doi: 10.1093/ajcn/69.6.1231. [DOI] [PubMed] [Google Scholar]
- 67.Tuomainen TP, Salonen R, Nyyssonen K, Salonen JT. Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland. BMJ. 1997;314(7083):793–794. doi: 10.1136/bmj.314.7083.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu X, Theil EC. Ferritin as an iron concentrator and chelator target. Ann N Y Acad Sci. 2005;1054:136–140. doi: 10.1196/annals.1345.016. [DOI] [PubMed] [Google Scholar]
- 69.Kabat GC, Rohan TE. Does excess iron play a role in breast carcinogenesis? an unresolved hypothesis. Cancer Causes Control. 2007;18(10):1047–1053. doi: 10.1007/s10552-007-9058-9. [DOI] [PubMed] [Google Scholar]
- 70.Elliott RL, Elliott MC, Wang F, Head JF. Breast carcinoma and the role of iron metabolism. A cytochemical, tissue culture, and ultrastructural study. Ann N Y Acad Sci. 1993;698:159–166. doi: 10.1111/j.1749-6632.1993.tb17204.x. [DOI] [PubMed] [Google Scholar]
- 71.Weinstein RE, Bond BH, Silberberg BK. Tissue ferritin concentration in carcinoma of the breast. Cancer. 1982;50(11):2406–2409. doi: 10.1002/1097-0142(19821201)50:11<2406::aid-cncr2820501127>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 72.Faulk WP, Hsi BL, Stevens PJ. Transferrin and transferrin receptors in carcinoma of the breast. Lancet. 1980;2(8191):390–392. doi: 10.1016/s0140-6736(80)90440-7. [DOI] [PubMed] [Google Scholar]
- 73.Marcus DM, Zinberg N. Measurement of serum ferritin by radioimmunoassay: results in normal individuals and patients with breast cancer. J Natl Cancer Inst. 1975;55(4):791–795. doi: 10.1093/jnci/55.4.791. [DOI] [PubMed] [Google Scholar]
- 74.Rossiello R, Carriero MV, Giordano GG. Distribution of ferritin, transferrin and lactoferrin in breast carcinoma tissue. J Clin Pathol. 1984;37(1):51–55. doi: 10.1136/jcp.37.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ionescu JG, Novotny J, Stejskal V, Latsch A, Blaurock-Busch E, Eisenmann-Klein M. Increased levels of transition metals in breast cancer tissue. Neuro Endocrinol Lett. 2006;27(Suppl 1):36–39. [PubMed] [Google Scholar]
- 76.Buss JL, Torti FM, Torti SV. The role of iron chelation in cancer therapy. Curr Med Chem. 2003;10(12):1021–1034. doi: 10.2174/0929867033457638. [DOI] [PubMed] [Google Scholar]
- 77.Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109(10):4586–4588. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lucarelli G, Galimberti M, Polchi P, et al. Bone marrow transplantation in patients with thalassemia. N Engl J Med. 1990;322(7):417–421. doi: 10.1056/NEJM199002153220701. [DOI] [PubMed] [Google Scholar]
- 79.Storey JA, Connor R, Lewis ZT, et al. The transfusion iron overload score is a potential predictor of survival in stem cell transplant patients. Blood. 2007;110(11):336A. [Google Scholar]
- 80.Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109(10):4586–4588. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Altes A, Remacha AF, Sureda A, et al. Iron overload might increase transplant-related mortality in haematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29(12):987–989. doi: 10.1038/sj.bmt.1703570. [DOI] [PubMed] [Google Scholar]
- 82.Bullen JJ, Rogers HJ, Spalding PB, Ward CG. Natural resistance, iron and infection: a challenge for clinical medicine. J Med Microbiol. 2006;55(Pt 3):251–258. doi: 10.1099/jmm.0.46386-0. [DOI] [PubMed] [Google Scholar]
- 83.Altes A, Remacha AF, Sarda P, et al. Frequent severe liver iron overload after stem cell transplantation and its possible association with invasive aspergillosis. Bone Marrow Transplant. 2004;34(6):505–509. doi: 10.1038/sj.bmt.1704628. [DOI] [PubMed] [Google Scholar]
- 84.Altes A, Remacha AF, Sarda P, et al. Early clinical impact of iron overload in stem cell transplantation. A prospective study. Ann Hematol. 2007;86(6):443–447. doi: 10.1007/s00277-007-0266-x. [DOI] [PubMed] [Google Scholar]
- 85.Lucarelli G, Galimberti M, Polchi P, et al. Marrow transplantation in patients with thalassemia responsive to iron chelation therapy. N Engl J Med. 1993;329(12):840–844. doi: 10.1056/NEJM199309163291204. [DOI] [PubMed] [Google Scholar]
- 86.Massover WH. Alpha 2-macroglobulin: a ferritin-binding protein. Ann N Y Acad Sci. 1994;737:468–471. doi: 10.1111/j.1749-6632.1994.tb44342.x. [DOI] [PubMed] [Google Scholar]
- 87.Seki T, Kunichika T, Watanabe K, Orino K. Apolipoprotein B binds ferritin by hemin-mediated binding: evidence of direct binding of apolipoprotein B and ferritin to hemin. Biometals. 2008;21(1):61–69. doi: 10.1007/s10534-007-9093-8. [DOI] [PubMed] [Google Scholar]
- 88.Torti SV, Torti FM. Human H-kininogen is a ferritin-binding protein. J Biol Chem. 1998;273(22):13630–13635. doi: 10.1074/jbc.273.22.13630. [DOI] [PubMed] [Google Scholar]
- 89.Schmaier AH, McCrae KR. The plasma kallikrein-kinin system: its evolution from contact activation. J Thromb Haemost. 2007;5(12):2323–2329. doi: 10.1111/j.1538-7836.2007.02770.x. [DOI] [PubMed] [Google Scholar]
- 90.Barnes PJ. Effect of bradykinin on airway function. Agents Actions Suppl. 1992;38(Pt 3):432–438. [PubMed] [Google Scholar]
- 91.Sainz IM, Pixley RA, Colman RW. Fifty years of research on the plasma kallikrein-kinin system: from protein structure and function to cell biology and in-vivo pathophysiology. Thromb Haemost. 2007;98(1):77–83. [PubMed] [Google Scholar]
- 92.Stewart JM, Gera L, Chan DC, et al. Combination cancer chemotherapy with one compound: pluripotent bradykinin antagonists. Peptides. 2005;26(8):1288–1291. doi: 10.1016/j.peptides.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 93.Parthasarathy N, Torti SV, Torti FM. Ferritin binds to light chain of human H-kininogen and inhibits kallikrein-mediated bradykinin release. Biochem J. 2002;365(Pt 1):279–286. doi: 10.1042/BJ20011637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coffman LG, Brown JC, Johnson DA, et al. Cleavage of high-molecular-weight kininogen by elastase and tryptase is inhibited by ferritin. Am J Physiol Lung Cell Mol Physiol. 2008;294(3):L505–L515. doi: 10.1152/ajplung.00347.2007. [DOI] [PubMed] [Google Scholar]