Introduction
The contributions of the renin-angiotensin-aldosterone system (RAAS) to the regulation of arterial pressure and the physiopathology of hypertension has long been recognised. Plasma renin is derived primarily from the juxtaglomerular cells on the afferent arterioles of the kidney,1 while angiotensinogen is primarily formed and constitutively secreted by liver cells into the circulation,2 thus allowing systemic formation of angiotensin I (Ang I) within the vascular compartment.2-4 It is important to recognise that the circulating concentrations of angiotensinogen are generally quite high, being more than 1000 times greater than the plasma Ang I and II concentrations.2,5,6 Figure 1 shows the representative plasma angiotensinogen concentrations measured in rats, expressed as pmol/ml while the Ang I and Ang II concentrations are expressed as fmol/ml, indicating that the active Ang II concentration in the plasma is a small fraction of the available Ang II in the form of angiotensinogen.5,6 Therefore, even small relative changes in the rates of Ang I and Ang II generation may make large absolute differences in the circulating concentrations. Because renin is synthesised and stored in the granules of juxtaglomerular cells, release mechanisms are rapid and can elicit large changes in plasma renin levels, leading to rapid changes in Ang I generation. However, the concentrations of angiotensinogen in the plasma are close to the Km value of the proteolytic activity of renin, so that changes in substrate concentration can also influence the Ang I generation rate. Nevertheless, changes in angiotensinogen synthesis and release occur slowly, so the effects of changes in angiotensinogen are not as dynamic as the effects of changes in plasma renin concentration.2,7 Once Ang I has been formed through the action of renin, it is rapidly converted to Ang II owing to the widespread localisation of angiotensin-converting enzyme (ACE) on the endothelial cells of many vascular beds, including the lung.8-10 Several angiotensinases and peptidases are then able to metabolise Ang II further.11 While other pathways for Ang II formation have been identified in certain tissues, the circulating levels of Ang II reflect primarily the consequences of the renin and ACE enzymatic cascade on angiotensinogen.5,12
Figure 1.
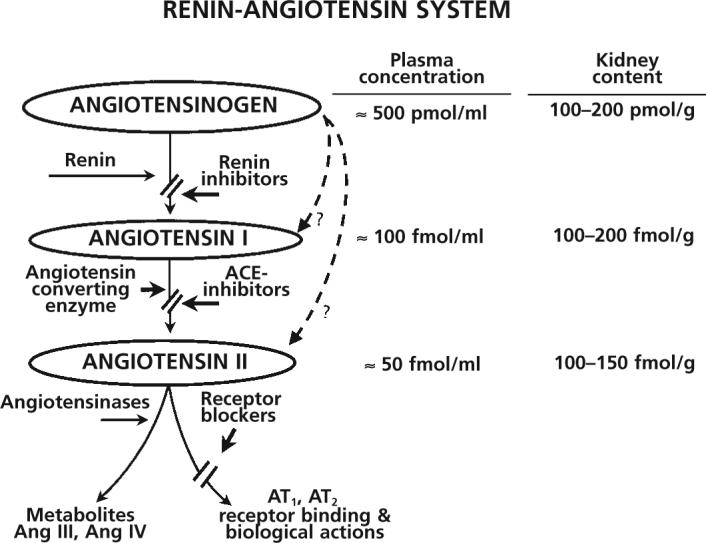
The renin-angiotensin system cascade with representative plasma concentrations and kidney contents for angiotensinogen, angiotensin I and angiotensin II. The dashed arrows represent alternative pathways for formation of angiotensin I and II.
Recent attention has been focused on findings from several laboratories that local tissue Ang II levels are differentially regulated and cannot be explained on the basis of circulating concentrations. This has led to a greater interest in Ang II acting as a paracrine factor.5,13-15 In particular, the Ang II contents of renal and adrenal tissues are much higher than can be explained on the basis of equilibration with the circulating concentrations.5,6,16,17 Furthermore, the demonstration of much higher concentrations of Ang II in specific regions and compartments within the kidney indicates selective local regulation of Ang II levels within the kidney.18,19 Thus, it is now apparent that the mechanisms regulating intrarenal Ang II levels are distinct from those responsible for regulating circulating Ang II concentrations.
Intrarenal levels of angiotensin II
Ang II is a dynamically-regulated peptide that is formed and degraded rapidly, thus making it difficult to obtain accurate indices of intrarenal Ang II activity that appropriately reflect the levels existing in vivo. Total tissue contents have been assessed by rapidly harvesting and processing tissue samples in solutions that arrest further formation and metabolism.20-22 While this approach provides a value for overall tissue content that is reproducible, it does not allow assessment of the concentrations within specific compartments or domains in the kidney. Other approaches include measurements of concentrations in samples taken from the renal vein, the interstitial fluid, the tubular fluid or the urine, but each has advantages and disadvantages.17-19
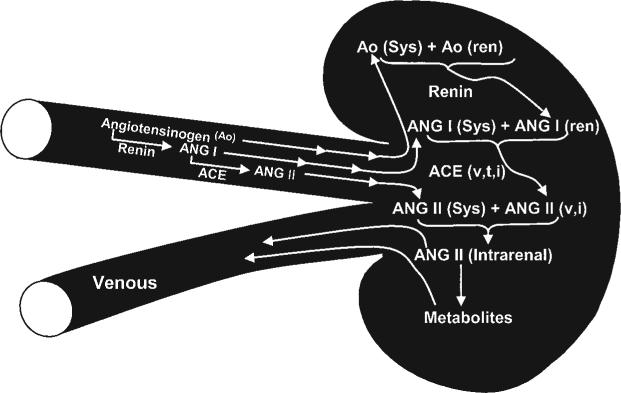
The limitations of total content measurements not withstanding, this approach provides a useful method to determine the effects of various dietary and experimental procedures on overall intrarenal Ang II levels.5,21,23 When the absolute levels, expressed per gram of tissue, are compared with the corresponding plasma levels, the Ang II tissue contents are higher than can be explained on the basis of non-specific equilibration between plasma and the intrarenal extracellular fluid.5,21,24 The differences between plasma concentrations and tissue contents are maintained during variations in dietary NaCl intake. For each level of NaCl intake, the total kidney contents, expressed as fmol/g kidney weight (fm/g) are higher than the plasma concentrations expressed as fmol/ml.5,21,23,25-27 Campbell et al.5 used HPLC separation techniques and found that kidney Ang II content was about 340 fmol/g, compared with 50 fmol/ml for plasma concentrations. Likewise, kidney Ang I concentrations were 2−3 times higher than the plasma concentrations. Several experimental models of hypertension, such as the 2 kidney 1 clip (2K-1C) Goldblatt hypertension and Ang II-induced hypertension, also have intrarenal Ang II levels that can be dissociated from the circulating Ang II concentrations.6,26,27 Collectively, the data show that the intrarenal levels of Ang II are regulated in a complex manner such that there is accumulation of Ang II from the amount delivered to the kidney and/or augmentation of intrarenally-formed Ang II. The kidney also metabolises and/or degrades most of the Ang II delivered in the arterial blood, such that the net intrarenal Ang II content is the consequence of several mechanisms.17,28 Figure 2 depicts the multiple formative processes and metabolic pathways that are involved in the regulation of intrarenal Ang II content. In addition to intrarenal formation of Ang II there is also intrarenal conversion from systemically delivered Ang I and angiotensinogen and from intrarenally-formed Ang I, originating from either circulating or locally-formed angiotensinogen. There are species variations in the conversion rates by the kidney. Previous studies in dogs indicate that about 20% of the arterially-delivered Ang I is converted to Ang II.28 In contrast, Danser et al.29 infused 125I-Ang I into the renal artery in humans and found less than 10% net conversion rate.
Figure 2.
Mechanisms of intrarenal formation of angiotensin II. See text for detailed explanation.
The intrarenal content of Ang II is not distributed in a homogeneous manner but is compartmentalised.30 There is a marked difference in the distribution of Ang II content in medulla vs. cortex.25,31 Within the cortex,there is distribution of Ang II among the interstitial fluid, tubular fluid and the intracellular compartments.The recognition that much of the Ang II that binds to receptors is internalised via receptor-mediated endocytosis has suggested that some of the internalised Ang II remains intact and contributes to the total Ang II content measured in tissue homogenates.23,32-36
Ang II in the renal medulla is of interest because various studies have suggested that Ang II influences medullary haemodynamics to a greater extent than cortical haemodynamics.14,37-42 In addition, receptor binding studies have demonstrated that the Ang II receptor density is much greater in the medulla than in the cortex.43,44 Recent studies have shown that medullary Ang II levels are higher than cortical levels in both normal and Ang II-infused hypertensive rats.25,31 The combination of high Ang II levels in the medulla, coupled with the high density of Ang II receptors, suggest that Ang II does indeed exert a powerful role in regulating haemodynamics and tubular function in the medulla. However, the total mass of the medulla is less than 1/10 that of the cortex so that the overall contribution of medullary Ang II levels to the total intrarenal Ang II content is relatively small. Nevertheless, these data suggest that there may be specialised Ang II-forming pathways or accumulation mechanisms in the medulla that are subject to localised regulation.
The interstitial compartment also contributes to the increased total Ang II levels. Earlier measurements of renal lymph, considered a reflection of renal interstitial fluid, suggested that interstitial fluid Ang II concentrations were much higher than plasma concentrations.45-47 These results were consistent with the notion that Ang II is formed locally and added to the interstitial compartment. However, it was not clear how much of renal lymph Ang II concentrations were due to continued Ang II formation within the lymphatic compartment, since lymph also contains ample quantities of renin and renin substrate.46,47 More recent studies have assessed interstitial fluid concentrations of Ang II using microdialysis probes implanted in the renal cortex.48 The microdialysis tubing had a molecular weight cut-off of 5000,thus allowing equilibration of smaller molecules such as the angiotensin peptides. However, renin, angiotensinogen and various enzymes with higher molecular weights do not cross the membrane and thus there should be limited formation and/or metabolism of Ang II after it crosses the dialysis membrane. Using this procedure, Siragy et al.48 found that interstitial fluid Ang II concentrations in the dog are in the range of 10−20 pmoles/ml, much higher than the plasma levels. These data indicate that interstitial fluid concentrations are substantially higher than plasma levels and contribute to the elevated intrarenal Ang II content.
Tubular compartmentalisation of angiotensin II
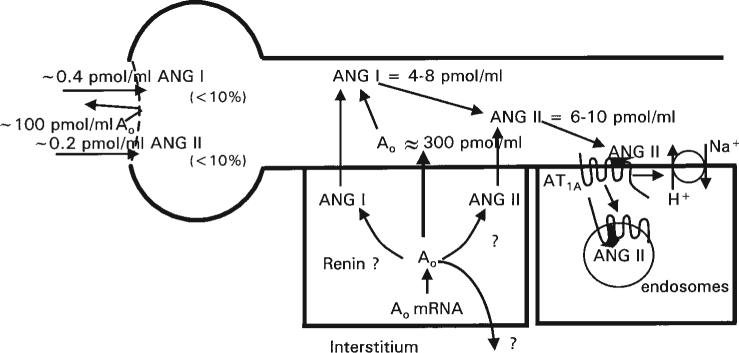
Micropuncture studies directly evaluating the proximal tubule fluid concentrations of Ang I and Ang II have demonstrated that these peptides are also present at concentrations much greater than the plasma concentrations.Seikaly et al.18 reported that Ang II concentrations in the proximal tubule fluid of rats were in the nanomolar range (30−40 nM) and about 1000 times higher than the plasma concentrations. Braam et al.19 also found nanomolar concentrations of Ang II in proximal tubule fluid and demonstrated further that similar concentrations were present in tubular fluid of microperfused proximal tubule segments. Fluid samples collected from downstream segments had Ang II concentrations similar to those measured from free-flow collections from non-perfused tubules. These findings provided definitive evidence that the tubular fluid Ang II concentrations are not derived from the filtrate, indicating that the proximal tubule secretes Ang II or a precursor into the proximal tubule fluid. Braam et al. 19 and Boer et al.49 have also demonstrated a dissociation between circulating and intratubular Ang II concentrations, in that extracellular fluid volume expansion reduced plasma Ang II concentrations but did not reduce intratubular Ang II concentrations. Navar et al.50 demonstrated that Ang I, as well as Ang II, was present at nanomolar concentrations in proximal tubular fluid, thus indicating that the Ang II could be secreted preformed or formed by the action of brush border ACE on Ang I. Figure 3 depicts the approximate values for intratubular Ang I and Ang II concentrations.
Figure 3.
Concentrations and sources of proximal tubule angiotensinogen, angiotensin I and angiotensin II and AT1-receptor mediated uptake of angiotensin II into endosomal compartments. Angiotensinogen and peptides may be secreted only into the tubular lumen or possibly also into the interstitium.
Origins of intrarenal angiotensin II
The evidence suggesting that renal interstitial fluid Ang II concentrations are much higher than the plasma levels has accrued for many years and it is well recognised that all of the components needed for Ang II generation are present within the renal interstitium.3,51 Most of the renin is thought to be secreted by the juxtaglomerular cells into the interstitial compartment, where it acts on angiotensinogen that was thought to originate primarily by diffusion across the peritubular capillaries. The presence of abundant ACE and Ang II receptors on tubular basolateral membranes and vascular smooth muscle of arterioles provided ample support for the importance of interstitial Ang II in regulating both haemodynamic and transport function.3,13,51,52 While these concepts remain valid, the findings that proximal tubule cells produce angiotensinogen has added substantial complexity to the mechanisms regulating intrarenal angiotensinogen levels.
The sources of intrarenally-formed Ang II have been explored in several ways. Studies utilising immunohistochemical techniques demonstrated that Ang I and Ang II are co-localised with renin in JGA cells and vascular smooth muscle cells of the afferent arteriole.53-56 These studies suggested that Ang II could be formed or internalised into the smooth muscle cells.32 Because there is no direct evidence for intracellular angiotensinogen in vascular smooth muscle cells, it is more likely that Ang I and/or Ang II are internalised via receptor-mediated endocytosis in smooth muscle cells.32,33,53 In contrast to vascular smooth muscle cells, proximal tubular cells contain angiotensinogen mRNA and protein.57-60 Immunohistochemical studies using antibodies to angiotensinogen have clearly localised angiotensinogen to the proximal tubule segment.56,61 These results indicate that much of the intrarenally-produced angiotensinogen is derived from proximal tubule cells and suggest it is one source of the intratubular concentrations of Ang I and Ang II. It has been noted that the preponderance of immunoreactive angiotensinogen in proximal tubule cells is located along the luminal membrane of the proximal tubule cells thus supporting the notion that angiotensinogen could be secreted into the tubular lumen and/or producing its metabolites intracellularly and secreting them directly into the tubule lumen.56,61,62
To test for the presence of angiotensinogen in proximal tubule fluid, proximal tubule fluid samples were collected and incubated with excess renin, in order to determine total available Ang II from angiotensinogen or related precursors secreted into the lumen.63 As shown in Figure 3, we found that the proximal tubule fluid angiotensinogen concentrations in rats were over 300 nM and greatly exceeded the free Ang I and Ang II tubular fluid concentrations. Because angiotensinogen is a 55−60 kD glycoprotein, it seems unlikely that much of the plasma angiotensinogen would filter across the glomerular membrane.64,65 These data provide further support to the scheme, depicted in Figure 3, that proximal tubule cells secrete angiotensinogen directly into the tubule, as recently suggested.31,61,62,65,66 Thus, Ang I could be formed either within the proximal cells or the tubular lumen if renin or other renin-like enzymes are present in the tubular fluid, on the brush border or inside the cells.57,67,68 Several studies have reported that cultured proximal tubule cells do indeed produce renin and contain renin mRNA, thus suggesting that a low level of constitutive renin secretion may occur in proximal tubule cells.57,67,69 In addition, Leyssac70 reported low but measurable renin concentrations in proximal tubule fluid. It has also been shown that there are abundant amounts of ACE and its mRNA associated with the proximal tubule brush border.71 ACE has also been measured in proximal and distal tubular fluid, but is more abundant in proximal tubular fluid.72 It seems clear that Ang II would have to be continuously produced or added to the proximal tubule fluid in view of the abundant ACE and angiotensinases found in the brush border.10,11,73,74 At present, however, it is not known how much of the peptides are formed intracellularly and how much are formed in the tubular fluid. It is also not clear if the angiotensinogen produced in proximal tubule cells is secreted both at luminal and basolateral sites or primarily into the tubular lumen, as suggested by others.62,66
The Ang II concentration in tubular fluid from the other segments of the nephron remains unknown,owing to the problems of collecting sufficient volumes to detect the anticipated concentrations. However, there are several studies supporting an important role for Ang II in regulating reabsorptive function in the distal nephron and collecting duct segments as well as in proximal tubule segments, and Ang II receptors have been identified on the luminal border of distal nephron segments.60,75-79 There have been efforts to use urinary Ang II concentrations or excretion rates as indices of intrarenal levels.80-82 However, the reported urinary concentrations of Ang II have been as low as 1 fmol/ml to about 35 fmol/ml,81,82 much lower than the proximal or interstitial concentrations reported. Recent data from our laboratory have indicated that urine Ang II concentrations in anaesthetised rats may be somewhat higher, in the range of about 0.8 pmol/ml.83 If similar concentrations exist in the distal tubule or collecting duct fluid, they would be sufficient to exert an influence on transport function. Wang and Giebisch75 found that Ang II concentrations as low as 10−11 M (10 fmol/ml) stimulated distal tubular sodium transport.Whatever the Ang II concentration in distal tubular fluid, it appears that it is not derived from plasma Ang II, since systemically-infused, tritiated Ang II could not be recovered in the urine.82
Angiotensinogen is also present in urine, suggesting that continued Ang I generation may occur throughout the tubule.62,66,80,84 Recent studies have localised renin to the luminal side of cells from the connecting tubule of the distal nephron, suggesting that renin may also be secreted into the distal tubular fluid.62 When coupled with the findings of angiotensinogen in urine, it now seems highly likely that intraluminal Ang II formation continues throughout the length of the nephron, with residual angiotensinogen appearing in the urine.62,66,80 Ding et al.66 demonstrated, in transgenic mice harbouring the gene for human angiotensinogen fused to the kidney androgen-protein promoter, that human angiotensinogen was localised primarily in proximal tubule cells. They found abundant human angiotensinogen in the urine but only slight traces in the systemic circulation. It was suggested that most of the angiotensinogen formed in proximal tubular cells is destined for secretion into the lumen. Rohrwasser et al.62 emphasised the luminal localisation of angiotensinogen in proximal tubular cells in vivo and showed in monolayer proximal tubule cell cultures that most of the angiotensinogen was detected in the apical compartment. They also reported that angiotensinogen was detected at low (nanomolar) concentrations in urine from mice and human volunteers. Mice placed on low dietary salt intake showed increased urinary angiotensinogen levels. Thus, it seems possible that urinary angiotensinogen concentrations or excretion rates may be a useful marker of intrarenal angiotensinogen production.80
Intrarenal angiotensin II formation in hypertensive states
Several studies have demonstrated that intrarenal Ang II levels can be dissociated from circulating renin and Ang II concentrations in several forms of angiotensin-dependent hypertension.6,25,85-87 Progressive increases in intrarenal Ang II content occur during the development of hypertension, leading to renal Ang II contents that are greater than can be explained from equilibration with the circulating concentrations. Interestingly, in 2K-1C Goldblatt hypertension, Ang II-induced hypertension and the TGR Ren2 rat model of hypertension, the increases in renal Ang II content occur even in kidneys that become renin-depleted owing to the elevations in arterial pressure.6,85-87 In 2K-1C Goldblatt hypertensive rats, intrarenal Ang II increased not only in the clipped kidney but also in the non-clipped kidney, which had an elevated or sustained intrarenal Ang II content even in the face of elevated arterial pressures and renal renin depletion.26,27
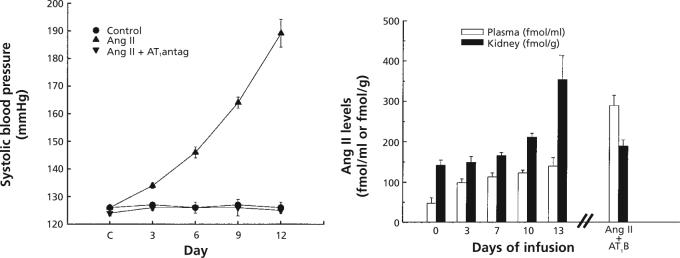
To evaluate mechanisms responsible for the augmented intrarenal Ang II in renin-depleted kidneys, further experiments were performed using the Ang II-infused hypertension model that leads to marked suppression of both circulating and tissue renin activity.6,26,88,89 Instead of renal arterial stenosis, an osmotic minipump containing Ang II was implanted, in order to raise the plasma Ang II concentrations to levels similar to those observed in 2K-1C rats. As shown in Figure 4, this procedure leads to a slowly-developing hypertension that mimics the development of hypertension in the 2K-1C model. The renal renin content and renin mRNA and the plasma renin activity are all markedly suppressed in this model.88,90 Renal Ang II content increased significantly after 8−10 days of Ang II infusion to levels substantially greater than could be explained by the circulating Ang II concentrations.6,26 While some of the intrarenal Ang II could be due to accumulation of circulating Ang II, continued intrarenal formation of Ang II was also likely, since both the renal angiotensinogen activity and kidney Ang I contents were not significantly reduced from control levels.6
Figure 4.
Increases in systolic arterial pressure (left), and plasma and intrarenal angiotensin II levels (right) in rats infused with angiotensin II for two weeks. Treatment with AT1-receptor blockers (AT1B) prevented the increases in arterial pressure and intrarenal angiotensin II even though plasma angiotensin II concentrations increased further.
These initial results led to the possibility that the Ang II infusions initiated an active accumulation process that could be mediated by activation of Ang II receptors and internalisation via the Ang II-receptor complex, such as has been shown in several cell systems.32,35,36,91,92 As also shown in Figure 4, chronic treatment with the AT1-receptor blocker, losartan, not only prevented the development of hypertension but also prevented the increases in intrarenal Ang II content caused by two weeks of Ang II infusion.6,93 This finding indicates that AT1-receptor activation either stimulates intrarenal Ang II formation or leads to internalisation and intracellular accumulation of the circulating Ang II into an intracellular compartment that protects the peptide from degradation.34 This conclusion has received further support from recent studies using mice that have deletion of the AT1A receptor.94 Cervenka et al.94 found that, although the circulating Ang II concentrations in AT1A knockout mice are much higher than in the wild type controls, the kidney Ang II contents are lower in the AT1A knockout mice.These findings suggest a role for the AT1A-receptor in mediating the increases in intrarenal Ang II content during conditions of elevated plasma Ang II concentrations.
To determine the extent of intracellular accumulation of circulating Ang II, Zou et al.89 infused Val5-Ang II, which has essentially the same immunoreactivity and biological activity in the rat as the endogenous Ang II that has isoleucine in the five position. By separating the Ang II peptides using HPLC, it was possible to determine the amounts of each peptide present in tissues after two weeks of infusion. Val5-Ang II elicited the slowly developing hypertension previously observed and increases in total intrarenal Ang II similar to those found with Ile5-Ang II.89 Analysis of the renal Ang II contents revealed levels of Val5-Ang II that were much higher than the circulating levels, indicating that Val5-Ang II had accumulated in the kidney. About two thirds of the intrarenal Figure 4, chronic treatment with the AT1-receptor blocker, losartan, not only prevented the development of hypertension but also prevented the increases in intrarenal Ang II content caused by two weeks of Ang II infusion.6,93 This finding indicates that AT1-receptor activation either stimulates intrarenal Ang II formation or leads to internalisation and intracellular accumulation of the circulating Ang II into an intracellular compartment that protects the peptide from degradation.34 This conclusion has received further support from recent studies using mice that have deletion of the AT1A receptor.94 Cervenka et al.94 found that, although the circulating Ang II concentrations in AT1A knockout mice are much higher than in the wild type controls, the kidney Ang II contents are lower in the AT1A knockout mice.These findings suggest a role for the AT1A-receptor in mediating the increases in intrarenal Ang II content during conditions of elevated plasma Ang II concentrations.
To determine the extent of intracellular accumulation of circulating Ang II, Zou et al.89 infused Val5-Ang II, which has essentially the same immunoreactivity and biological activity in the rat as the endogenous Ang II that has isoleucine in the five position. By separating the Ang II peptides using HPLC, it was possible to determine the amounts of each peptide present in tissues after two weeks of infusion. Val5-Ang II elicited the slowly developing hypertension previously observed and increases in total intrarenal Ang II similar to those found with Ile5-Ang II.89 Analysis of the renal Ang II contents revealed levels of Val5-Ang II that were much higher than the circulating levels, indicating that Val5-Ang II had accumulated in the kidney. About two thirds of the intrarenal Ang II content was Val5-Ang II; however, the native form of Ang II (Ile5-Ang II) content was not reduced from that seen in control rats. In agreement with the previous study,6 chronic treatment with losartan markedly reduced the renal content of Val5-Ang II, demonstrating that the accumulation process involves AT1-receptor activation.93 Collectively, these results indicate that the augmentation of intrarenal Ang II is due, in part, to receptor-mediated accumulation of circulating Ang II.
It is also important to recognise that, in the Ang II infused model of hypertension, there is sustained production of endogenous Ang II. Endogenous production of Ang II can be due, in part, to Ang II-stimulated angiotensinogen production. In vivo and in vitro studies have shown that Ang II stimulates angiotensinogen mRNA levels in rats and in a murine proximal tubule cell line.95-97 Kobori et al.98 was also able to demonstrate that there were significant increases in intrarenal angiotensinogen protein as well as angiotensinogen mRNA levels in response to two weeks of Ang II infusion. This positive feedback system may be responsible for sustained or enhanced generation of angiotensinogen, leading to continued intrarenal production of Ang II under conditions of elevated circulating concentrations. The intrarenally-produced Ang II would be additive with the Ang II that is internalised by the AT1-receptors, leading to the overall increased intrarenal Ang II content. It remains unclear whether the Ang II is recycled and secreted in order to exert further biological function by binding to Ang II receptors on the cell membrane.Ang II may also migrate to the nucleus to exert genomic effects.32,36 Recently, Chen et al.36 transfected Chinese hamster ovary cells with an AT1a-receptor fused with green fluorescent protein (GFP),which allowed visualisation of trafficking of the internalised ligand-receptor complex. Ang II increased co-localisation of GFP fluorescence with nuclear markers, suggesting migration of the receptor complex to the nucleus.36
Until recently there was no direct evidence that Ang II was present in substantive concentrations in intracellular organelles. Endocytosis of the Ang II-AT1-receptor complex has been demonstrated to be required for the full expression of functional responses.99-102 In the proximal tubule, binding of Ang II to the AT1-receptor and endocytosis of the AT1-receptor-Ang II complex is coupled to the activation of signal transduction pathways and enhanced sodium transport.103 Recent studies evaluated the presence of angiotensin peptides, ACE and Ang II receptors in renal endosomes.23 It was found that renal intermicrovillar clefts and endosomes contain both Ang I and Ang II, with the Ang II content being greater than that of Ang I. In addition, both AT1-receptors and ACE were found in these structures. ACE activity was important for the maintenance of Ang II content in the endosomes and microvillar clefts, as they were markedly reduced by acute ACE inhibition. These results demonstrate that Ang II is either formed or trafficked through intracellular endosomal compartments. In a recent preliminary report, the effects of Ang II infusions on Ang II content in intrarenal endosomes were evaluated.104 Renal cortical endosomes were harvested after two weeks of Ang II infusion. As previously reported, overall kidney Ang II levels were increased. Ang II levels in both light and heavy endosomes were significantly increased. In agreement with previous studies showing the effects of AT1-receptor blockade to prevent the increases in renal Ang II content, concurrent treatment with candesartan prevented the increases in endosomal Ang II levels. These data provide additional support to the concept that there is increased uptake and trafficking of Ang II into renal endosomes, which is mediated by AT1-receptors. The prevention of these increases in intracellular levels of Ang II may be of importance, not only in reducing the hypertensive effects of Ang II but also in minimising the renal injury that occurs in Ang II-dependent hypertension.
Perspective
Collectively, the results of experiments evaluating Ang II-dependent hypertension have shown that elevated intrarenal Ang II levels can occur even when plasma renin levels and intrarenal renin content are reduced. The elevated intrarenal Ang II levels contribute to hypertension via multiple effects on the vasculature and the tubules, leading to sodium retention, vasoconstriction and long-term proliferative actions. Studies in Ang II-infused rats have demonstrated that intrarenal accumulation of Ang II is due, in part, to uptake of circulating Ang II via an AT1-receptor-mediated mechanism; however, endogenous production of Ang II is sustained. Some of the internalised Ang II appears to be protected from degradation and therefore potentially available for intracellular actions. Circulating Ang II also exerts a positive feedback action by which the Ang II infusions lead to augmented intrarenal levels of angiotensinogen mRNA and protein, which contribute to the overall enhancement of intrarenal Ang II in hypertension. In addition, renal AT1-receptor protein and mRNA levels are maintained, allowing increased Ang II levels to elicit progressive effects.While the systemic vascular effects of Ang II are important in maintaining elevated peripheral vascular resistance, it is the antinatriuretic consequences caused by the synergistic actions of the augmented intrarenal Ang II levels that are responsible for maintaining a chronic state of hypertension. As long as the renal effects are sustained, the hypertension can be maintained even after the circulating Ang II concentrations return to near normal levels.105,106
Acknowledgements
We acknowledge the assistance provided by Debbie Olavarrieta in preparing the manuscript and figures. Research performed in the authors’ laboratories was supported by NHLBI grant HL26371.
References
- 1.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, Physiology, and Molecular Biology of Renin Secretion. Physiol Rev. 1990;70:1067–116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 2.Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27(part 2):465–75. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell KD, Navar LG. The renin-angiotensin-aldosterone system in volume control. In: Baylis PH, editor. Bailliere's Clinical Endocrinology and Metabolism. Bailliere Tindall; London: 1989. pp. 393–430. [DOI] [PubMed] [Google Scholar]
- 4.Navar LG, Rosivall L. Contribution of the renin angiotensin system to the control of intrarenal hemodynamics. Kidney Int. 1984;25:857–68. doi: 10.1038/ki.1984.102. [DOI] [PubMed] [Google Scholar]
- 5.Campbell DJ, Lawrence AC, Towrie A, Kladis A, Valentijn AJ. Differential regulation of angiotensin peptide levels in plasma and kidney of the rat. Hypertension. 1991;18:763–73. doi: 10.1161/01.hyp.18.6.763. [DOI] [PubMed] [Google Scholar]
- 6.Zou L, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal Ang II augmentation in Ang II-infused rats. Hypertension. 1996;28:669–77. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]
- 7.Deschepper CF. Angiotensinogen: Hormonal regulation and relative importance in the generation of angiotensin II. Kidney Int. 1994;46:1561–3. doi: 10.1038/ki.1994.446. [DOI] [PubMed] [Google Scholar]
- 8.Erdos EG. Angiotensin I converting enzyme and the changes in our concepts through the years. Hypertension. 1990;16:363–70. doi: 10.1161/01.hyp.16.4.363. [DOI] [PubMed] [Google Scholar]
- 9.Johnston CI. Tissue angiotensin converting enzyme in cardiac and vascular hypertrophy, repair, and remodeling. Hypertension. 1994;23:258–68. doi: 10.1161/01.hyp.23.2.258. [DOI] [PubMed] [Google Scholar]
- 10.Schulz WW, Hagler HK, Buja LM, Erdos EG. Ultrastructural Localisation of Angiotensin I-Converting Enzyme (EC 3.4.15.1) and Neutral Metalloendopeptidase (EC 3.4.24.11) in the Proximal Tubule of the Human Kidney. Lab Invest. 1988;59:789–97. [PubMed] [Google Scholar]
- 11.Goodfriend TL. Angiotensinases. In: Robertson JIS, Nichols MG, editors. Renin-Angiotensin System. Gower Med Pub; London: 1993. pp. 1–5. [Google Scholar]
- 12.Urata H, Nishimura H, Ganten D. Chymase-dependent angiotensin II forming system in humans. Am J Hypertens. 1996;9:277–84. doi: 10.1016/0895-7061(95)00349-5. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell KD, Navar LG. Intrarenal actions of angiotensin II in the pathogenesis of experimental hypertension. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. Raven Press, Ltd.; New York: 1995. pp. 1437–50. [Google Scholar]
- 14.Navar LG, Inscho EW, Majid DSA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 15.Müller DN, Bohlender J, Hilgers KF, et al. Vascular angiotensin-converting enzyme expression regulates local angiotensin II. Hypertension. 1997;29:98–104. doi: 10.1161/01.hyp.29.1.98. [DOI] [PubMed] [Google Scholar]
- 16.Mendelsohn FAO. Angiotensin II: Evidence for its role as an intrarenal hormone. Kidney Int. 1982;22(Suppl 12):S-78–S-81. [PubMed] [Google Scholar]
- 17.Rosivall L, Narkates AJ, Oparil S, Navar LG. De novo intrarenal formation of angiotensin II during control and enhanced renin secretion. Am J Physiol. 1987;252:F1118–F1123. doi: 10.1152/ajprenal.1987.252.6.F1118. [DOI] [PubMed] [Google Scholar]
- 18.Seikaly MG, Arant BS, Jr., Seney FD., Jr. Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest. 1990;86:1352–7. doi: 10.1172/JCI114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braam B, Mitchell KD, Fox J, Navar LG. Proximal tubular secretion of angiotensin II in rats. Am J Physiol-Renal Physiol. 1993;264:F891–F898. doi: 10.1152/ajprenal.1993.264.5.F891. [DOI] [PubMed] [Google Scholar]
- 20.Mendelsohn FAO. A method for measurement of angiotensin II in tissues and its application to rat kidney. Clin Sci Mol Med. 1976;51:111–25. doi: 10.1042/cs0510111. [DOI] [PubMed] [Google Scholar]
- 21.Fox J, Guan S, Hymel AA, Navar LG. Dietary Na and ACE inhibition effects on renal tissue angiotensin I and II and ACE activity in rats. Am J Physiol-Renal Physiol. 1992;262:F902–F909. doi: 10.1152/ajprenal.1992.262.5.F902. [DOI] [PubMed] [Google Scholar]
- 22.Campbell DJ. Tissue renin-angiotensin system: sites of angiotensin formation. J Cardiovasc Pharmacol. 1987;10(Suppl 7):S1–S8. doi: 10.1097/00005344-198706107-00002. [DOI] [PubMed] [Google Scholar]
- 23.Imig JD, Navar GL, Zou LX, et al. Renal endosomes contain angiotensin peptides, converting enzyme, and AT1A receptors. Am J Physiol-Renal Physiol. 1999;277:F303–F311. doi: 10.1152/ajprenal.1999.277.2.F303. [DOI] [PubMed] [Google Scholar]
- 24.Reams G, Villarreal D, Wu Z, Bauer JH. Renal tissue angiotensin II: Response to infusions of angiotensin I and an angiotensin-converting enzyme inhibitor. Am J Kidney Diseases. 1993;22:851–7. doi: 10.1016/s0272-6386(12)70345-1. [DOI] [PubMed] [Google Scholar]
- 25.Navar LG, Harrison-Bernard LM, Imig JD, Mitchell KD. Renal actions of angiotensin II at AT1 receptor blockers. In: Epstein M, Brunner HR, editors. Angiotensin II Receptor Antagonists. Hanley & Belfus, Inc.; Philadelphia: 2000. pp. 189–214. [Google Scholar]
- 26.Von Thun AM, Vari RC, El-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol-Renal Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 27.Guan S, Fox J, Mitchell KD, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension. 1992;20:763–7. doi: 10.1161/01.hyp.20.6.763. [DOI] [PubMed] [Google Scholar]
- 28.Rosivall L, Rinder DF, Champion J, Khosla MC, Navar LG, Oparil S. Intrarenal angiotensin I conversion at normal and reduced renal blood flow in the dog. Am J Physiol. 1983;245:F408–F415. doi: 10.1152/ajprenal.1983.245.3.F408. [DOI] [PubMed] [Google Scholar]
- 29.Danser AH, Admiraal PJJ, Derkx FH, Schalekamp MA. Angiotensin I-to-II conversion in the human renal vascular bed. J Hypertens. 1988;16:2051–6. doi: 10.1097/00004872-199816121-00029. [DOI] [PubMed] [Google Scholar]
- 30.Navar LG, Harrison-Bernard LM, Imig JD. Compartmentalization of intrarenal angiotensin II. In: Ulfendahl HR, Aurell M, editors. Renin-Angiotensin. Portland Press; London: 1998. pp. 193–208. [Google Scholar]
- 31.Navar LG, Imig JD, Zou L, Wang C-T. Intrarenal production of angiotensin II. Sem Nephrol. 1997;17:412–22. [PubMed] [Google Scholar]
- 32.Anderson KM, Peach MJ. Receptor binding and internalization of a unique biologically active angiotensin II-colloidal gold conjugate: Morphological analysis of angiotensin II processing in isolated vascular strips. J Vasc Res. 1994;31:10–7. doi: 10.1159/000159026. [DOI] [PubMed] [Google Scholar]
- 33.Thomas WG, Thekkumkara TJ, Baker KM. Molecular mechanisms of angiotensin II (AT1A) receptor endocytosis. Clin Exp Pharmacol Physiol. 1996;23:S74–S80. doi: 10.1111/j.1440-1681.1996.tb02817.x. [DOI] [PubMed] [Google Scholar]
- 34.Van Kats JP, de Lannoy LM, Danser AHJ, van Meegen JR, Verdouw PD, Schalekamp MAH. Angiotensin II type 1 (AT1) receptor-mediated accumulation of angiotensin II in tissues and its intracellular half-life in vivo. Hypertension. 1997;30(part 1):42–9. doi: 10.1161/01.hyp.30.1.42. [DOI] [PubMed] [Google Scholar]
- 35.Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT1 receptors: Evidence for selective sorting of receptor and ligand. Mol Endocrinol. 1997;11:1266–77. doi: 10.1210/mend.11.9.9975. [DOI] [PubMed] [Google Scholar]
- 36.Chen R, Mukhin YV, Garnovskaya MN, et al. A functional angiotensin II receptor-GFP fusion protein: evidence for agonist-dependent nuclear translocation. Am J Physiol Renal Physiol. 2000;279:F440–F448. doi: 10.1152/ajprenal.2000.279.3.F440. [DOI] [PubMed] [Google Scholar]
- 37.Chou S-Y, Porush JG, Faubert PF. Renal medullary circulation: Hormonal control. Kidney Int. 1990;37:1–13. doi: 10.1038/ki.1990.1. [DOI] [PubMed] [Google Scholar]
- 38.Chou S-Y, Spitalewitz S, Faubert PF, Park IY, Porush JG. Inner medullary hemodynamics in chronic salt-depleted dogs. Am J Physiol-Renal Physiol. 1984;246:F146–F154. doi: 10.1152/ajprenal.1984.246.2.F146. [DOI] [PubMed] [Google Scholar]
- 39.Faubert PF, Chou S-Y, Porush JG. Regulation of papillary plasma flow by angiotensin II. Kidney Int. 1987;32:472–8. doi: 10.1038/ki.1987.234. [DOI] [PubMed] [Google Scholar]
- 40.Pallone TL. Vasoconstriction of outer medullary vasa recta by angiotensin II is modulated by prostaglandin E2. Am J Physiol-Renal Physiol. 1994;266:F850–F857. doi: 10.1152/ajprenal.1994.266.6.F850. [DOI] [PubMed] [Google Scholar]
- 41.Pallone TL, Robertson CR, Jamison RL. Renal Medullary Microcirculation. Physiol Rev. 1990;70:885–920. doi: 10.1152/physrev.1990.70.3.885. [DOI] [PubMed] [Google Scholar]
- 42.Zimmerhackl BL, Robertson CR, Jamison RL. The medullary microcirculation. Kidney Int. 1987;31:641–7. doi: 10.1038/ki.1987.46. [DOI] [PubMed] [Google Scholar]
- 43.Mendelsohn FAO, Dunbar M, Allen A, et al. Angiotensin II receptors in the kidney. Federation Proc. 1986;45:1420–5. [PubMed] [Google Scholar]
- 44.Zhuo J, Alcorn D, Allen AM, Mendelsohn FAO. High resolution localisation of angiotensin II receptors in rat renal medulla. Kidney Int. 1992;42:1372–80. doi: 10.1038/ki.1992.429. [DOI] [PubMed] [Google Scholar]
- 45.Bailie MD, Rector FC, Jr, Seldin DW. Angiotensin II in arterial and renal venous plasma and renal lymph in the dog. J Clin Invest. 1971;50:119–26. doi: 10.1172/JCI106465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilcox CS, Peart WS. Release of renin and angiotensin II into plasma and lymph during hyperchloremia. Am J Physiol-Renal Physiol. 1987;253:F734–F741. doi: 10.1152/ajprenal.1987.253.4.F734. [DOI] [PubMed] [Google Scholar]
- 47.Wilcox CS, Dzau VJ. Effect of captopril on the release of the components of the renin-angiotensin system into plasma and lymph. J Am Soc Nephrol. 1992;2:1241–50. doi: 10.1681/ASN.V271241. [DOI] [PubMed] [Google Scholar]
- 48.Siragy HM, Howell NL, Ragsdale NV, Carey RM. Renal inter-stitial fluid angiotensin:Modulation by anesthesia,epinephrine, sodium depletion and renin inhibition. Hypertension. 1995;25:1021–4. doi: 10.1161/01.hyp.25.5.1021. [DOI] [PubMed] [Google Scholar]
- 49.Boer WH, Braam B, Fransen R, Boer P, Koomans HA. Effects of reduced renal perfusion pressure and acute volume expansion on proximal tubule and whole kidney angiotensin II content in the rat. Kidney Int. 1997;51:44–9. doi: 10.1038/ki.1997.6. [DOI] [PubMed] [Google Scholar]
- 50.Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins I and II in anesthetized rats. J Am Soc Nephrol. 1994;5:1153–8. doi: 10.1681/ASN.V541153. [DOI] [PubMed] [Google Scholar]
- 51.Thurau K. Angiotensin (Handbook of Experimental Pharmacology, 37) Springer-Verlag; New York: 1974. Intrarenal action of angiotensin. pp. 475–89. [Google Scholar]
- 52.Mitchell KD, Navar LG. Influence of intrarenally generated angiotensin II on renal hemodynamics and tubular reabsorption. Renal Physiol Biochem. 1991;14:155–63. doi: 10.1159/000173401. [DOI] [PubMed] [Google Scholar]
- 53.Taugner R, Marin-Grez M, Keilbach R, Hackenthal E, Nobiling R. Immunoreactive renin and angiotensin II in the afferent glomerular arterioles of rats with hypertension due to unilateral renal artery constriction. Histochemistry. 1982;76:61–9. doi: 10.1007/BF00493286. [DOI] [PubMed] [Google Scholar]
- 54.Celio MR, Inagami T. Angiotensin II immunoreactivity coexists with renin in the juxtaglomerular granular cells of the kidney. Proc Natl Acad Sci USA. 1981;78:3897–900. doi: 10.1073/pnas.78.6.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taugner R, Mannek E, Nobiling R, et al. Coexistence of renin and angiotensin II in epitheloid cell secretory granules of rat kidney. Histochemistry. 1984;81:39–45. doi: 10.1007/BF00495399. [DOI] [PubMed] [Google Scholar]
- 56.Hunt MK, Ramos SP, Geary KM, et al. Colocalization and release of angiotensin and renin in renal cortical cells. Am J Physiol-Renal Physiol. 1992;263:F363–F373. doi: 10.1152/ajprenal.1992.263.3.F363. [DOI] [PubMed] [Google Scholar]
- 57.Yanagawa N, Capparelli AW, Jo OD, Friedal A, Barrett JD, Eggena P. Production of angiotensinogen and renin-like activity by rabbit proximal tubular cells in culture. Kidney Int. 1991;39:938–41. doi: 10.1038/ki.1991.117. [DOI] [PubMed] [Google Scholar]
- 58.Gomez RA, Lynch KR, Chevalier RL, et al. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol-Renal Physiol. 1988;254:F900–F906. doi: 10.1152/ajprenal.1988.254.6.F900. [DOI] [PubMed] [Google Scholar]
- 59.Ingelfinger J, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. J Clin Invest. 1990;85:417–23. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localisation of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993;43:1251–9. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- 61.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 62.Rohrwasser A, Morgan T, Dillon HF, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–74. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 63.Navar LG, Lewis L, Hymel A, Mitchell KD. Proximal tubular fluid levels of angiotensinogen in anesthetized rats. FASEB J. 1996;10:A22. [Google Scholar]
- 64.Lynch KR, Peach MJ. Molecular Biology of Angiotensinogen. Hypertension. 1991;17:263–9. doi: 10.1161/01.hyp.17.3.263. [DOI] [PubMed] [Google Scholar]
- 65.Jeunemaitre X, Ménard J, Clauser E, Corvol P. Angiotensinogen: molecular biology and genetics. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. Raven Press, Ltd.; New York: 2000. pp. 1653–65. [Google Scholar]
- 66.Ding Y, Davisson RL, Hardy DO, et al. The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J Biol Chem. 1997;272:28142–8. doi: 10.1074/jbc.272.44.28142. [DOI] [PubMed] [Google Scholar]
- 67.Moe OW, Ujiie K, Star RA, et al. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–9. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taugner R, Hackenthal E, Rix E, Nobiling R, Poulsen K. Immunocytochemistry of the renin-angiotensin system: Renin, angiotensinogen, angiotensin I, angiotensin II, and converting enzyme in the kidneys of mice, rats, and tree shrews. Kidney Int. 1982;22(Suppl 12):33–43. [PubMed] [Google Scholar]
- 69.Henrich WL, McAllister EA, Eskue A, Miller T, Moe OW. Renin regulation in cultured proximal tubular cells. Hypertension. 1996;27:1337–40. doi: 10.1161/01.hyp.27.6.1337. [DOI] [PubMed] [Google Scholar]
- 70.Leyssac PP. Changes in single nephron renin release are mediated by tubular fluid flow rate. Kidney Int. 1986;30:332–9. doi: 10.1038/ki.1986.189. [DOI] [PubMed] [Google Scholar]
- 71.Sibony M, Gasc J-M, Soubrier F, Alhenc-Gelas F, Corvol P. Gene expression and tissue localisation of the two isoforms of angiotensin I converting enzyme. Hypertension. 1993;21:827–35. doi: 10.1161/01.hyp.21.6.827. [DOI] [PubMed] [Google Scholar]
- 72.Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol-Renal Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 73.Ikemoto F, Ito S, Song G, et al. Contribution of renal angiotensin converting enzyme (ACE) to blood pressure regulation: Possible role of brush border ace. Clin and Exper Theory and Practice. 1987;9:441–7. doi: 10.3109/10641968709164211. [DOI] [PubMed] [Google Scholar]
- 74.Ward PE, Erdös EG, Gedney CD, Dowben RM, Reynolds RC. Isolation of membrane-bound renal enzymes that metabolize kinins and angiotensins. Biochem J. 1976;157:643–50. doi: 10.1042/bj1570643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol-Renal Physiol. 1996;271:F143–F149. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 76.Weiner ID, New AR, Milton AE, Tisher CC. Regulation of luminal alkalinization and acidification in the cortical collecting duct by angiotensin II. Am J Physiol-Renal Physiol. 1995;269:F730–F738. doi: 10.1152/ajprenal.1995.269.5.F730. [DOI] [PubMed] [Google Scholar]
- 77.Levine DZ, Iacovitti M, Buckman S, Burns KD. Role of angiotensin II in dietary modulation of rat late distal tubule bicarbonate flux in vivo. J Clin Invest. 1996;97:120–5. doi: 10.1172/JCI118378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barreto-Chaves MLM, Mello-Aires M. Effect of luminal angiotensin II and ANP on early and late cortical distal tubule HCO3- reabsorption. Am J Physiol-Renal Physiol. 1996;271:F977–F984. doi: 10.1152/ajprenal.1996.271.5.F977. [DOI] [PubMed] [Google Scholar]
- 79.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, El-Dahr SS. Immunohistochemical localisation of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol-Renal Physiol. 1997;273:F170–F177. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 80.Wang E, Yayama K, Takano M, Okamoto H. Sexual dimorphism of urine angiotensinogen excretion in the rat. Japan J Pharmacol. 1994;64:243–50. doi: 10.1254/jjp.64.243. [DOI] [PubMed] [Google Scholar]
- 81.Vos PF, Boer P, Braam B, Koomans HA. The origin of urinary angiotensins in humans. J Am Soc Nephrol. 1994;5:215–23. doi: 10.1681/ASN.V52215. [DOI] [PubMed] [Google Scholar]
- 82.Reams G, Villarreal D, Wu Z, Bauer JH. Urinary angiotensin II: a marker of renal tissue activity? Nephron. 1994;67:450–8. doi: 10.1159/000188215. [DOI] [PubMed] [Google Scholar]
- 83.Wang C-T, Mitchell KD, Navar LG. Proximal tubular fluid angiotensin II levels in angiotensin II-infused hypertensive rats. J Am Soc Nephrol. 1997;8:1428. [Google Scholar]
- 84.Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiological Genomics. 1999;1:3–9. doi: 10.1152/physiolgenomics.1999.1.1.3. [DOI] [PubMed] [Google Scholar]
- 85.Navar LG, Harrison-Bernard LM. Intrarenal angiotensin II augmentation in angiotensin II dependent hypertension. Hypertens Res. 2000;23:291–301. doi: 10.1291/hypres.23.291. [DOI] [PubMed] [Google Scholar]
- 86.Cervenka L, Wang C-T, Mitchell KD, Navar LG. Proximal tubular angiotensin II levels and renal functional responses to AT1 receptor blockade in nonclipped kidneys of Goldblatt hypertensive rats. Hypertension. 1999;33:102–7. doi: 10.1161/01.hyp.33.1.102. [DOI] [PubMed] [Google Scholar]
- 87.Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol-Renal Physiol. 1997;273:F246–F253. doi: 10.1152/ajprenal.1997.273.2.F246. [DOI] [PubMed] [Google Scholar]
- 88.Von Thun AM, El-Dahr SS, Vari RC, Navar LG. Modulation of renin-angiotensin and kallikrein gene expression in experimental hypertension. Hypertension. 1994;23(suppl I):I-131–I-136. doi: 10.1161/01.hyp.23.1_suppl.i131. [DOI] [PubMed] [Google Scholar]
- 89.Zou L, Hymel A, Imig JD, Navar LG. Renal accumulation of circulating angiotensin II in angiotensin II-infused rats. Hypertension. 1996;27(part 2):658–62. doi: 10.1161/01.hyp.27.3.658. [DOI] [PubMed] [Google Scholar]
- 90.El-Dahr SS, Dipp S, Guan S, Navar LG. Renin, angiotensinogen, and kallikrein gene expression in two-kidney Goldblatt hypertensive rats. Am J Hypertens. 1993;6:914–9. doi: 10.1093/ajh/6.11.914. [DOI] [PubMed] [Google Scholar]
- 91.Becker BN, Cheng HF, Burns KD, Harris RC. Polarized rabbit type 1 angiotensin II receptors manifest differential rates of endocytosis and recycling. Am J Physiol. 1995;269:C1048–C1056. doi: 10.1152/ajpcell.1995.269.4.C1048. [DOI] [PubMed] [Google Scholar]
- 92.Ullian ME, Linas SL. Role of receptor cycling in the regulation of angiotensin II surface receptor number and angiotensin II uptake in rat vascular smooth muscle cells. J Clin Invest. 2001;84:840–6. doi: 10.1172/JCI114244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zou L, Imig JD, Hymel A, Navar LG. Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens. 1998;11:570–8. doi: 10.1016/s0895-7061(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 94.Cervenka L, Mitchell KD, Oliverio MI, Coffman TM, Navar LG. Renal function in the AT1A receptor knockout mouse during normal and volume-expanded conditions. Kidney Int. 1999;56:1855–62. doi: 10.1046/j.1523-1755.1999.00757.x. [DOI] [PubMed] [Google Scholar]
- 95.Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol-Endocrinol Metab. 1992;263:E863–E869. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- 96.Ingelfinger JR, Jung F, Diamant D, et al. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol-Renal Physiol. 1999;276:F218–F227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 97.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–9. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001 doi: 10.1161/01.hyp.37.5.1329. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schelling JR, Linas SL. Angiotensin II-dependent proximal tubule sodium transport requires receptor-mediated endocytosis. Am J Physiol-Cell Physiol. 1994;266:C669–C675. doi: 10.1152/ajpcell.1994.266.3.C669. [DOI] [PubMed] [Google Scholar]
- 100.Schelling JR, Hanson As, Marzec R, Linas SL. Cytoskeleton-dependent endocytosis is required for apical type 1 angiotensin II receptor-mediated phospholipase C activation in cultured rat proximal tubule cells. J Clin Invest. 1992;90:2472–80. doi: 10.1172/JCI116139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Becker BN, Harris RC. A potential mechanism for proximal tubule angiotensin II-mediated sodium flux associated with receptor-mediated endocytosis and arachidonic acid release. Kidney Int. 1996;50(Suppl 57):S-66–S-72. [PubMed] [Google Scholar]
- 102.Linas SL. Role of receptor mediated endocytosis in proximal tubule epithelial function. Kidney Int. 1997;52(Suppl 61):S-18–S-21. [PubMed] [Google Scholar]
- 103.Becker BN, Cheng H-F, Harris RC. Apical ANG II-stimulated PLA2 activity and Na+ flux: a potential role for Ca2+-independent PLA2. Am J Physiol-Renal Physiol. 1997;273:F554–F562. doi: 10.1152/ajprenal.1997.273.4.F554. [DOI] [PubMed] [Google Scholar]
- 104.Zhuo JL, Imig JD, Raibstein SR, Benes E, Hammon TG, Navar LG. Intra-renal trafficking of angiotensin II through renal cortical endosomes is prevented by candesartan cilexetil during angiotensin II-induced hypertension. Proceedings of the International Symposium on Ang II Receptor Blockade. JRAAS. 2001;2(Suppl 1):244. (abstract) [Google Scholar]
- 105.Navar LG. The kidney in blood pressure regulation and development of hypertension. Medical Clinics of North America. 1997;81:1165–98. doi: 10.1016/s0025-7125(05)70573-3. [DOI] [PubMed] [Google Scholar]
- 106.Guyton AC. Blood pressure control - special role of the kidneys and body fluids. Science. 1991;252:1813–6. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]