Abstract
In most cases, pharmacologic strategies to treat genetic muscle disorders and certain acquired disorders, such as sporadic inclusion body myositis, have produced modest clinical benefits. In these conditions, inhibition of the myostatin pathway represents an alternative strategy to improve functional outcomes. Preclinical data that support this approach clearly demonstrate the potential for blocking the myostatin pathway. Follistatin has emerged as a powerful antagonist of myostatin that can increase muscle mass and strength. Follistatin was first isolated from the ovary and is known to suppress follicle-stimulating hormone. This raises concerns for potential adverse effects on the hypothalamic–pituitary–gonadal axis and possible reproductive capabilities. In this review we demonstrate a strategy to bypass off-target effects using an alternatively spliced cDNA of follistatin (FS344) delivered by adeno-associated virus (AAV) to muscle. The transgene product is a peptide of 315 amino acids that is secreted from the muscle and circulates in the serum, thus avoiding cell-surface binding sites. Using this approach our translational studies show increased muscle size and strength in species ranging from mice to monkeys. Adverse effects are avoided, and no organ system pathology or change in reproductive capabilities has been seen. These findings provide the impetus to move toward gene therapy clinical trials with delivery of AAV-FS344 to increase size and function of muscle in patients with neuromuscular disease.
Keywords: follistatin, myostatin inhibition, muscle disease, muscle enhancement
Strategies to increase muscle size and strength through inhibition of the myostatin pathway show promise for clinical application.34 Follistatin is a potent antagonist of myostatin that takes advantage of its ability to hinder access to signaling receptors on skeletal muscle. The muscle-building properties of follistatin are well demonstrated,36 but because it is a peptide with multiple functions, concerns have been raised regarding off-target effects when considering its appropriateness for treatment of muscle disease. The goal of this review is to thoroughly discuss these complex interactions and demonstrate a strategy that takes advantage of known follistatin properties that can be harnessed to promote efficacy to increase muscle mass and muscle strength in the absence of adverse clinical effects.
Emphasis on myostatin inhibition emerges because treating muscle disorders by most pharmacologic approaches has been disappointing. Androgen steroids, popular among athletes, pose long-term risks66 including: (1) endocrine (gonadal atrophy and sterility)28; (2) somatic (changes in blood lipid profiles and cardiac hypertrophy)3,30,37; and (3) neuropsychiatric (anxiety, depression, hostility, paranoia)57; and attempts to treat muscle disorders have been disappointing.5 Glucocorticosteroids, the only beneficial drug treatment for muscular dystrophy, are virtually entirely targeted toward the Duchenne muscular dystrophy (DMD) population.46,50 Even in this patient group the mechanism of benefit is poorly understood, and the evidence that muscle mass is increased is meager.46 For genetic muscle diseases, gene manipulation strategies are on the horizon, including gene replacement,12,19,20,62 exon skipping,1,44 and mutation suppression.7,23 Despite enthusiasm, experimental studies suggest that these approaches usually fall short of returning function to normal.40 Combinational approaches that include partial correction of the underlying defect (i.e., micro-dystrophin) combined with increasing muscle size and strength appear to offer more.2 For muscle diseases where correction of the underlying defect might not be an option, increasing muscle size and strength may be opportune for both genetic and acquired muscle diseases where treatment options are limited. Examples include some forms of muscular dystrophy where gene manipulation strategies are not yet applicable (e.g., facioscapulohumeral dystrophy, FSHD), acquired disorders such as sporadic inclusion body myositis, where pharmacologic treatment failures predominate, or cachectic disorders related to cancer or aging that may be ideally suited for a muscle-enhancing approach.
MYOSTATIN PATHWAY
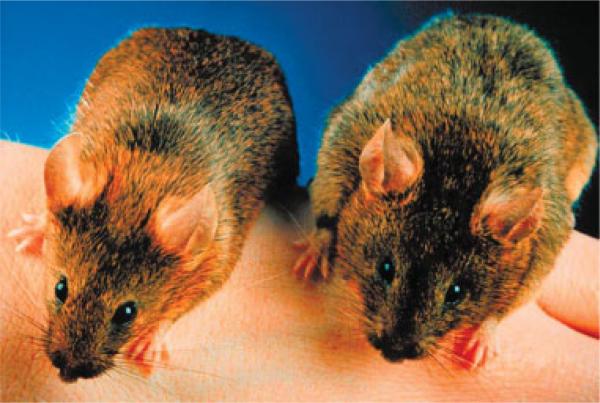
The potential for follistatin as a therapeutic agent for muscle disease cannot be fully understood without knowledge of the myostatin pathway. Myostatin is a member of the transforming growth factor-beta (TGF-β) superfamily of signal peptides. It is expressed specifically in developing and adult skeletal muscle.45 During development, myostatin expression limits the size of the muscle in concert with multiple factors that sculpt the limbs in relation to skeletal, vascular, and ectodermal patterns of growth.4 Myogenic cells respond to myostatin by downregulating the expression of Pax-3 and Myf-5, important transcriptional regulators of myogenic cell proliferation, and Myo-D, an early marker of muscle differentiation. In their sentinel report in 1997, McPherron et al.45 demonstrated the biological effect of targeted disruption of growth and differentiation factor-8 (GDF-8) gene in the mouse. GDF-8 null mice were significantly larger in size than wildtype animals, and there was widespread increase in skeletal muscle mass (Fig. 1). Individual muscles of mutant mice weighed 2−3 times more than those of wildtype animals. The increase in mass was the result of a combination of muscle hypertrophy and hyperplasia. These experiments established the GDF-8 peptide as a major player for inhibiting muscle growth, with the designated name “myostatin.”
FIGURE 1.
Myostatin null animals exhibit increased muscle mass. Adult myostatin null mice demonstrating increased size (right) as compared to wildtype (left) animals. Reprinted with permission from Lee SJ, McPherron AC. Curr Opin Genet Dev 1999:5:604−607.
MYOSTATIN SYNTHESIS
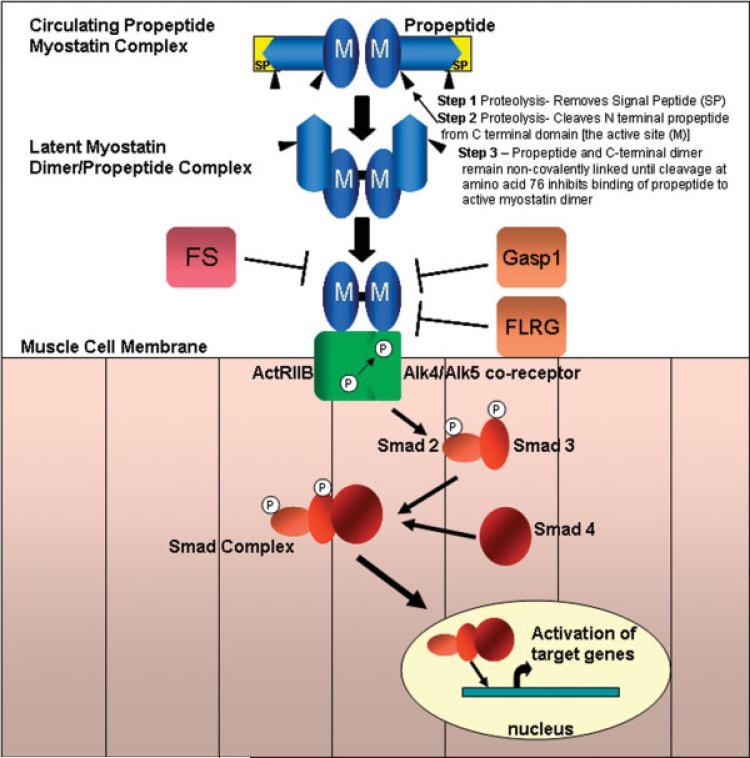
The human myostatin gene (MSTN) maps to chromosome 2q32.2.67 The gene contains three exons and three putative transcription start sites that encode a 376-amino acid precursor protein composed of a signal peptide, an N-terminal propeptide domain and a C-terminal domain that gives rise to the active peptide (Fig. 2). Myostatin activation requires stepwise proteolytic cleavages of the precursor protein. Initially, furin family enzymes remove the signal peptide (24-amino acid). A second cleavage event at amino acid sites 240−243 leaves two fragments: an N-terminal propeptide domain of 27,640 Da and C-terminal domain of 12,400 Da destined to become the active myostatin protein.34 Parallel fragments of the myostatin C-terminal are linked through a disulfide bond, referred to as the myostatin C-terminal dimer that remains noncovalently complexed to the N-terminal propeptide.33,71 This noncovalent complex circulates in the blood and maintains the myostatin C-terminal dimer in a latent, inactive state.25,33 A third cleavage at amino acid 76 is required for the myostatin C-terminal to become active.79 This occurs via a different enzyme group, a metalloproteinase that belongs to the bone morphogenic protein (BMP)-1/tolloid (TLD) family.
FIGURE 2.
Blocking the myostatin pathway. Myostatin (M) activation requires stepwise proteolytic cleavages of the precursor protein. After the signal peptide (SP) is removed, a second cleavage event leaves two fragments: an N-terminal propeptide domain of ≈28 kD and C-terminal domain of 12.5 kD destined to become the active myostatin protein. Parallel fragments of the myostatin C-terminal are linked through a disulfide bond, referred to as the myostatin C-terminal dimer that remains noncovalently complexed to the N-terminal propeptide. This noncovalent complex circulates in the blood maintaining the myostatin C-terminal dimer in a latent, inactive state. A third cleavage at amino acid 76 affects the ability of the propeptide to bind the active C terminal domain. Myostatin can be found in the serum or locally in an inactive state when bound to follistatin (FS), follistatin-related gene (FLRG), and growth and differentiation factor-associated serum protein-1 (GASP-1). These peptides block the activation of the myostatin pathway. If the pathway is not inhibited, the active myostatin dimer binds to the activin receptor type IIB (ActRIIB), which then recruits and activates by transphosphorylation the type I receptor (ALK4 or ALK5). Smad2 and Smad3 are subsequently activated and form aggregates with Smad4 and then are translocated to the nucleus, activating target gene transcription.
Myostatin signaling acts through the activin receptor type IIB (ActRIIB) on skeletal muscle by setting in motion an intracellular cascade of events. First, there is presumed recruitment of a type I co-receptor.34 Activin receptor-like kinases 4 and/or 5 (ALK-4, ALK-5) represent candidate coreceptors that are phosphorylated by ActRIIB.59 This in turn leads to phosphorylation of TGF-β specific Smads 2 and 3 that form a complex with Smad 4. The Smad 2/3/4 complex is translocated to the nucleus to regulate expression of targeted genes such as MyoD and myogenic regulatory factors (MRFs) (Fig. 2).32,33,42
Apart from an essential role in muscle growth, recent evidence indicates that myostatin has a regulatory role in skeletal muscle fibrosis. Li et al.38 revealed that myostatin and the ActRIIB receptor are expressed on muscle fibroblasts, thus inducing their proliferation and the production of extracellular matrix proteins. This proliferation leads to the induction of the canonical Smad signaling pathway in fibroblasts by Smad3 phosphorylation and downstream p38 MAPK and Akt pathways.38 This enhances the therapeutic potential for myostatin inhibition that could lead to muscle enlargement while at the same time decreasing muscle fibrosis. In many muscle disorders, active fibrosis leads to the irreversibility of the condition, be it inherited or acquired.
FOLLISTATIN SYNTHESIS, ISOFORMS, AND PHYSIOLOGIC ROLE
Follistatin, secreted as a glycoprotein, was originally identified in porcine ovarian follicular fluid and received its name because it suppresses synthesis and secretion of follicle-stimulating hormone (FSH) from the pituitary gland.56 It is highly conserved, with overall species homology of 83% and 95% in mammals. Two groups isolated and published their results in 1987. One coined the term follistatin,14 and the other named it FSH-suppressing protein (FSP).61 With time, follistatin became the popular designation, but the name hardly does justice to a peptide with functions that extend beyond FSH suppression.
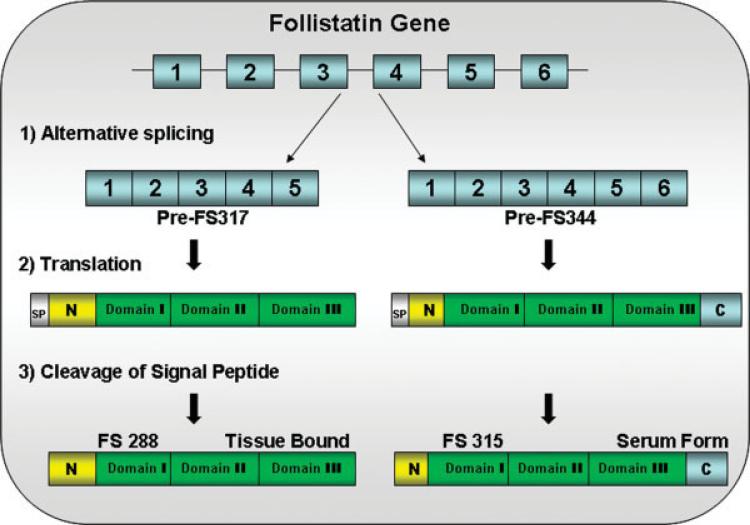
The follistatin gene localizes to chromosome 5q11.2. It is composed of a relatively small 6-kb genomic DNA consisting of six exons. There is an alternative splice site that generates two major species, a full-length version that encodes a 344-amino acid preprotein differing by a 27-amino acid sequence from its carboxy-shortened version of the 317-amino acid form missing exon 6 (Fig. 3).64,65 Prior to activation, follistatin, like myostatin, undergoes further posttranslational modification to lose another 29 amino acids by removal of the signal peptide that results in polypeptides of 315 (FS315), often referred to as the long isoform and 288 (FS288), called the short isoform. There is also evidence to suggest that FS315 can be proteolytically cleaved in vivo at the carboxy-terminal to give an intermediate isoform of 303 amino acids.69
FIGURE 3.
The follistatin gene consists of six exons. Alternative splicing generates two isoforms, FS317 and FS344. Alternative splicing occurs at the 3′ end of the gene between exon 5 and exon 6. Splicing out of intron 5 generates a stop codon immediately following the last amino acid of exon 5, and leads to the termination of the coding sequence for FS317. An alternative splice site results in the inclusion of exon 6 and generates FS344. After translation and prior to activation, follistatin undergoes further posttranslational modification by cleavage of the 29 amino acid signal peptide. This results in polypeptides FS315 (long-isoform from FS344) and FS288 (short-isoform from FS317).
At the time follistatin was first isolated, little was known of its mechanism of action. In a major breakthrough, follistatin was found to be an activin-binding protein.52 An important function of follistatin is its collaborative role in reproductive physiology with other TGF-β superfamily members, activin and inhibins. These TGF-β family peptides have overlapping autocrine/paracrine functions. All three were initially purified from gonadal fluids and characterized based on their ability to modulate FSH. In addition to gonadal sites of production (ovary/testes), these peptides are all produced by cells in the hypothalamic–pituitary axis (gonadatropes and folliculostellate cells). Follistatin binds activin and attenuates the release of FSH. Activin is secreted by the follicle of the ovary and serves to enhance FSH secretion. Inhibins, which are secreted in two forms (A and B), inhibit the release of FSH at the hypothalamic–pituitary level. In addition, it is well documented that follistatin can abrogate the effects of GnRH in stimulating FSH secretion.77,78 This is also due in part to the blocking of transcriptional activation of the GnRH receptor gene by activin.16
This complex interaction of follistatin in relation to pituitary and gonadal function has raised concerns about its potential use as a therapeutic agent in the clinic. However, potential recombinant products can take advantage of differences between the isoforms in their ability to bind heparin sulfate. A well-recognized follistatin heparin-binding site is present at residues 72−86, which is a region rich in basic amino acids.73 In contrast, the carboxy-terminal 27 amino acid sequence of FS-315, composed of 44% acidic amino acids, interferes with the heparin site.70 These considerations take on a novel perspective with regard to gene therapy considering potential transgene products. FS-288, the shorter alternatively spliced product has an ≈10-fold higher affinity to activin compared to FS-315.24,69,70 In addition, FS-288 targets heparin sulfate proteoglycan binding sites at cell surfaces, while FS-315 represents a soluble serum-based or circulating follistatin isoform.63 In developing a gene therapy product for clinical use, we have taken advantage of this property. In our preclinical research studies, adeno-associated (AAV) virus that carries cDNA FS-344 delivers a gene therapy product (FS-315) without interruption in reproductive capabilities in either males or females in species ranging from mice to monkeys. Our results support prior observations that impairment of activin binding is more closely allied with FS-288 and its cell surface-binding properties mediated by heparan sulfate proteoglycans. This strategy greatly enhances the margin of safety for clinical trials because the FS-315 isoform has a limited effect on activin modulation by protecting the pituitary–gonadal axis from unwanted alterations. The same can be said for avoiding off-target effects mediated by cell surface binding of follistatin, including functions related to cellular differentiation, repair, and apoptosis.5
The origin of follistatin under normal physiologic conditions is not entirely understood. Clearly, follistatin is produced locally in the pituitary gland and in gonads, ovaries, and testes. Overall, measurements of follistatin during the menstrual cycle show few changes.15,18,29 However, a notable exception is during pregnancy, when follistatin concentrations rise toward term in parallel with activin.15,76,79 Follistatin is widely distributed throughout multiple organs, and the majority of follistatin found in the circulation is likely secreted from the walls of blood vessels.
GENETICALLY ALTERED MICE OVER- AND UNDEREXPRESSING FOLLISTATIN
A component of understanding the functional role of follistatin can be gleaned from studies of genetically modified mice. Studies that evaluate the overexpression of the follistatin gene through genetically induced gain-of-function mutations are worth study to examine the potential for off-target effects. However, information derived from such models requires cautious interpretation because of species differences and influences of overexpression during development that are not clinically relevant. Despite caveats, the findings in a transgenic model in which the follistatin gene was introduced under control of a muscle-specific myosin light chain promoter are encouraging.33 Muscle mass increase was significantly greater than observed in the myostatin null mutant mouse. These results suggest that at least part of the effect of follistatin results from impact on another pathway independent of myostatin inhibition. This hypothesis is reinforced by additional studies in which mice that overexpress transgenic follistatin were crossed with myostatin null animals.36 The resulting phenotype appeared to be additive, with a quadrupling of muscle mass in follistatin+/myostatin−/− mice, outstripping the effects of either myostatin nulls or follistatin-overexpressing animals alone. These findings emphasize that other signaling pathways could be exploited to increase muscle size and strength.36
In another gain-of-function mutant mouse line, the metallothionein (MT)-1 promoter was placed upstream of the follistatin gene.21 Observations on gonadal changes in this model are thought-provoking, but they may not be directly related to clinical translational considerations. On a positive note, the MT-follistatin transgenic offspring were viable and developed to adults. In addition, no deleterious effects were seen in any organ system other than gonadal tissue. In males testes size was decreased, with variable Leydig cell hyperplasia, an arrest in spermatogenesis and seminiferous tubular degeneration leading to infertility. Females had thin uteri and small ovaries, and many became infertile. Interpreting these results in the context of what we would anticipate in a clinical trial of gene therapy requires caution. In the patient, our concern would be that high serum levels of follistatin would bind activin and result in reduced serum FSH levels leading to gonadal dysfunction. Instead, the follistatin-overexpressing mice had normal FSH levels. This implies that in the transgenic mouse model, follistatin overexpression exerted its effects through gene expression in the matrix of the end organ, disrupting local regulation. This would not parallel the gene therapy paradigm, where the transgene product, FS344, is nontissue bound, and has no effect on reproductive function.
On the opposite side of the spectrum, a loss-of-function mutant mouse was created by a targeted deletion of the follistatin gene.43 The mutant mice survived until birth but died within hours of delivery. Defects included growth-retardation and shiny, taut skin. There was poor whisker development, hyperkeratosis of skin, abnormal tooth development, defects in the hard palate, and reduced size of intercostal and diaphragm muscles. The central and peripheral nervous systems, however, were intact. At best, this short-lived model has little relevance to our goals of studying follistatin as a potential therapeutic agent, but it does reinforce that follistatin may mediate effects through pathways of the TGF-β family other than myostatin.
TRANSLATIONAL APPROACHES THAT INHIBIT MYOSTATIN IN MUSCULAR DYSTROPHY
Increasing muscle size and strength in animal models of muscle disease in experimental translational studies has important implications for neuromuscular patients. Table 1 summarizes the strategies that have been used to reinforce the potential for this approach. In the mdx mouse, a model for DMD, there was an increase in muscle size and strength using a monoclonal antibody that inhibited myostatin.8 In further support of myostatin inhibition in muscular dystrophy, the mdx mouse was crossed with the myostatin knockout, resulting in muscles of larger size associated with improved grip strength.74 An impressive outcome in this model was reduced fibrosis in the diaphragm muscle, a potential clinically meaningful result. Another approach to achieve myostatin blockade utilized the propeptide to keep the myostatin C-terminal dimer inactive, blocking access to the ActRIIB receptor. In one experimental paradigm, the propeptide was stabilized by fusion to IgG-Fc and systemically administered to the mdx mouse, which resulted in increased muscle size concomitant with treatment.9 In a second strategy, AAV was used to deliver a mutant myostatin propeptide in a mouse model of limb girdle muscular dystrophy (LGMD)2A that harbored a mutation of calpain-3. The mutation prevented cleavage of the propeptide and maintained the myostatin C-terminal domain in an inactive state. The calpain-3 deficient mice had increased muscle mass and improved force generation.6
Table 1.
Translational and clinical studies of myostatin inhibition.
| Preclinical study | MD model | Conclusions | Reference |
|---|---|---|---|
| Functional improvement of dystrophic muscle by myostatin blockade | mdx | Monoclonal antibody to myostatin resulted in increased muscle weight, size, and absolute strength in mdx mice | 8 |
| Loss of myostatin attenuates severity of muscular dystrophy in mdx mice | mdx;mstn −/− | Increased muscle mass was maintained over time in the Mstn −/− mdx mouse resulting in increased grip strength and reduced fibrosis in the diaphragm | 74 |
| Myostatin propeptide-mediated amelioration of dystrophic pathophysiology | mdx | Pharmacological blockade with a myostatin propeptide stabilized by fusion to IgG-Fc improved pathophysiology in mdx | 9 |
| Muscular atrophy of caveolin-3–deficient mice is rescued by myostatin inhibition | CAV3 Tg | CAV3 normally suppresses the myostatin-mediated signal, thereby preventing muscular atrophy. In CAV3 mutant, atrophy is prevented by overexpressing myostatin propeptide or by IP injection of ActRIIB receptor | 53 |
| AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not alpha-sarcoglycan deficiency | capn3 −/−sgca −/− | Delivery of mutated myostatin propeptide by AAV increased force generation, in capn3 −/−deficient mice. sgca −/− mice did not exhibit increased muscle mass or protection from Evans blue dye uptake | 6 |
| Myostatin blockade improves function but not histopathology in a murine model of limb-girdle muscular dystrophy 2C | sgcg −/− | Antibody mediated myostatin blockade led to increased fiber size, muscle mass, and absolute force with no improvement in muscle histopathology | 10 |
| Age-dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy | sgcd −/− | Early loss of myostatin achieved by monoclonal antibody administration or by gene deletion improved muscle mass regeneration in scgd −/−; late loss demonstrated no improvement | 54 |
| Elimination of myostatin does not combat muscular dystrophy in dy mice but increases postnatal lethality | dyW/dyW | dyW/dyW mice lacking myostatin showed increased muscle regeneration muscle mass, but demonstrated increased mortality | 39 |
| Long-term enhancement of skeletal muscle mass and strength by gene transfer of follistatin | mdx, wild-type | Gene transfer of myostatin inhibitory proteins increased muscle mass and strength >2 years | 22 |
| A phase I/II trial of MYO-029 in adult subjects with muscular dystrophy | BMD, FSHD, LGMD patients | Safety was demonstrated in clinical trial assessing neutralizing antibody to myostatin, MYO-029 in adult muscular dystrophy patients. Clinical efficacy was not found but bioactivity was demonstrated by DEXA and histology in a limited number of subjects | 75 |
BMD, Becker muscular dystrophy; capn3−/−, calpain3 deficient mouse; CAV3 Tg, caveolin-3 transgenic mouse deficient for caveolin-3; DEXA, dual-energy X-ray absorptiometry; dyW/dyW, laminin-alpha2 (merosin) deficient mouse; FSHD, facioscapulohumeral dystrophy; IP, intraperitoneal; LGMD, limb girdle muscular dystrophy; mdx−/−, Duchenne muscular dystrophy mouse model deficient for dystrophin; mstn−/−, myostatin knockout mouse; sgca −/−, alpha-sarcoglycan deficient mouse; sgcd −/−, delta-sarcoglycan deficient mouse; sgcg −/−, gamma-sarcoglycan deficient mouse.
Other investigations have tempered enthusiasm and cautioned beneficial treatment strategies given the age and stage of dystrophy at treatment. For example, in a model of delta-sarcoglycan deficiency (scgd−/−) representing LGMD2F, loss of myostatin activity in very young mice using monoclonal antibody administration or gene deletion (mstn−/−; scgd−/−), increased muscle size, improved muscle regeneration, and reduced fibrosis.54 However, antibody-mediated myostatin inhibition at a late stage in the scgd−/− mouse failed to prevent progression of muscle pathology despite increasing muscle size. Another disappointing result was observed in the dyW/dyW mouse,39 a mouse model for merosin deficient congenital muscular dystrophy (MDC1A).47 The dyW/dyW mouse exhibits poor muscle regeneration31 that might be expected to show a favorable response to myostatin inhibition. However, attempts to increase muscle strength and prolong life-span by eliminating myostatin by crossing the dyW with the myostatin null mouse were disappointing. The homozygous dyW/dyW;mstn−/− improved muscle regeneration, but dystrophic features including necrosis, inflammation, and fibrosis were unaffected and the pre-weaning mortality was increased. The poor outcome was attributed to a reduction of brown fat in the neonatal period that was made worse by the additive effects of the underlying mutation combined with myostatin inhibition (dyW/dyW; mstn−/−). The gloomy prediction of this study should be balanced by findings that myostatin effects on adipocytes are influenced by age and gene targeting. For example, in adult mice the administration of myostatin has no effect on fat tissue.68 When myostatin overexpression is achieved with a muscle-specific promoter (MCK or MCK-3E) there are no effects on adipocytes.60
In another model of inherited muscle disease related to caveolins, the findings in translational studies set the stage for a possible favorable outcome for a myostatin inhibition clinical trial. Caveolins are integral membrane proteins and are the principal components of the sarcolemmal invaginations referred to as caveolae. These proteins play important roles in signal transduction and vesicular trafficking.17,55 Caveolin-3 (CAV3) gene mutations cause a spectrum of muscle disorders, including: autosomal dominant LGMD1C, rippling muscle disease (RMD), sporadic and familial forms of hyperCKemia, and distal myopathy.11 In a transgenic mouse mutant for P104L of CAV3, phosphorylated Smad2 was increased.53 These findings suggest that CAV3 normally suppresses the myostatin-mediated signal, thereby protecting the muscle from atrophy. Blocking myostatin by crossing CAV3 mutants with transgenics that overexpress the myostatin propeptide (CAV-3P104L/MSTNPro) or by intraperitoneal administration of the soluble ActRIIB receptor prevented muscle atrophy with evidence of suppression of phosphorylated-Smad2. These findings potentially bode well for myostatin inhibition in patients with CAV3 mutations.
The translational study cited above that looked at the potential efficacy of the neutralizing monoclonal antibody to myostatin (MYO-029) has been studied in a double-blind randomized clinical trial. One hundred sixteen muscular dystrophy subjects with varied illnesses including LGMD, Becker muscular dystrophy, and facioscapulohumeral dystrophy were included.75 Subjects were divided into sequential dose-escalation cohorts (Cohort 1 at 1 mg/kg; Cohort 2 at 3 mg/kg; Cohort 3 at 10 mg/kg; Cohort 4 at 30 mg/kg). MYO-029 showed good safety and tolerability, with the exception of a cutaneous hypersensitivity rash at the 10 and 30 mg/kg doses. There was no improvement in muscle strength or function, but the study was underpowered for efficacy.
Nevertheless, bioactivity of MYO-029 was supported by a trend in a limited number of subjects that demonstrated increased muscle size using dual-energy radiographic absorptiometry and muscle histology with a dose-dependent increase at 3 mg/kg and 10 mg/kg doses. This was the first clinical trial based on the hypothesis that systemic administration of myostatin inhibitors could be an appropriate strategy for patients with a variety of muscle disorders. Most important, there were no significant organ-related adverse events beyond the hypersensitivity skin reactions to the antibody. This study clearly paves the way for more potent myostatin inhibitors to be used for stimulating muscle growth in muscular dystrophy.
FOLLISTATIN EFFECTS ON MUSCLE ENHANCEMENT THROUGH GENE THERAPY
A gene therapy approach to myostatin inhibition represents an important consideration for patients with muscle disease. In contrast to pharmacologic administration of myostatin inhibitors such as neutralizing antibodies,8,69 or recombinant pharmacologic agents that have been used in translational studies (propeptide,9,58 soluble ActRIIB receptor,35 or drugs such as trichostatin A48), gene therapy offers the potential for a single administration of vector carrying the follistatin gene with persistent expression for many years, even perhaps throughout the lifetime of the individual. AAV is the vector of choice for muscle disease. It has a proven safety record in more than 40 clinical trials in a diverse group of diseases51 and demonstrated efficacy in Leber's congenital amaurosis.41 As muscle is the natural host for AAV, the advantage for treating a wide range of muscle disease is obvious. Several clinical trials that incorporate direct muscle injections are under way for alpha-1-anti-trypsin deficiency as well as Duchenne and LGMD, and no adverse effects have been encountered (unpubl. obs., Jerry Mendell, Barry Byrne, Terrence Flotte). While there is controversy over the immunogenicity of AAV,49 this is not an insurmountable obstacle, since immune suppression is being studied in viral gene transfer in the ongoing clinical trials.
An advantage of myostatin inhibition is the potential to treat both genetic and acquired diseases. Achieving clinically meaningful outcomes using current means of gene delivery to single muscle targets is also a realistic goal. An example of a potential recipient of this approach is the patient with quadriceps (knee extensor) muscle weakness who is disabled by frequent falls. These patients also suffer knee pain because of joint stress from genu recurvatum (back kneeing). Examples of muscle disorders that are predisposed to weakness of knee extensor muscles include myotonic muscular dystrophy type 1, Becker muscular dystrophy, and sporadic inclusion body myositis.
The therapeutic potential of AAV-mediated follistatin gene therapy has been extensively studied in our laboratories at the Center for Gene Therapy, Nationwide Children's Hospital (Columbus, Ohio), with demonstrable clinical implications.22 The follistatin gene used for these studies is the alternatively spliced cDNA FS-344 (Fig. 3). The final product of this transgene is the FS-315 circulating isoform. Because of its reduced affinity for heparin,70,69 cell surfaces of the gonadal–pituitary axis are much less likely to be affected, thus increasing the safety profile for this transgene product. This is reflected in our preclinical studies, as will be described.
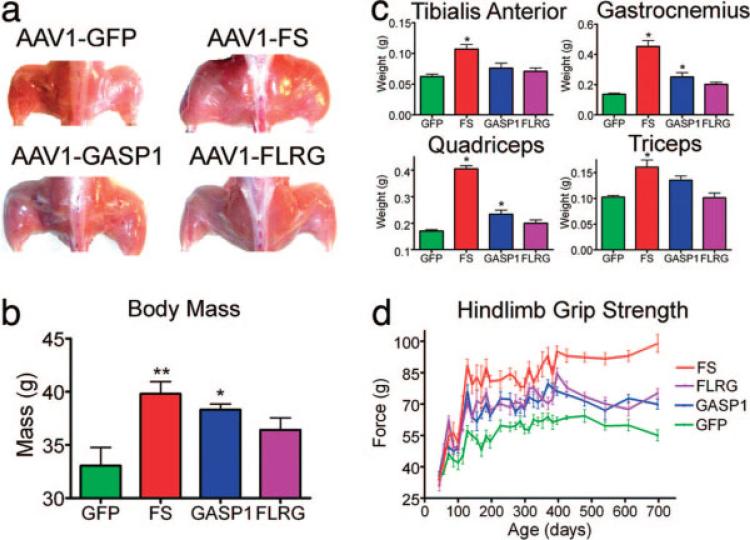
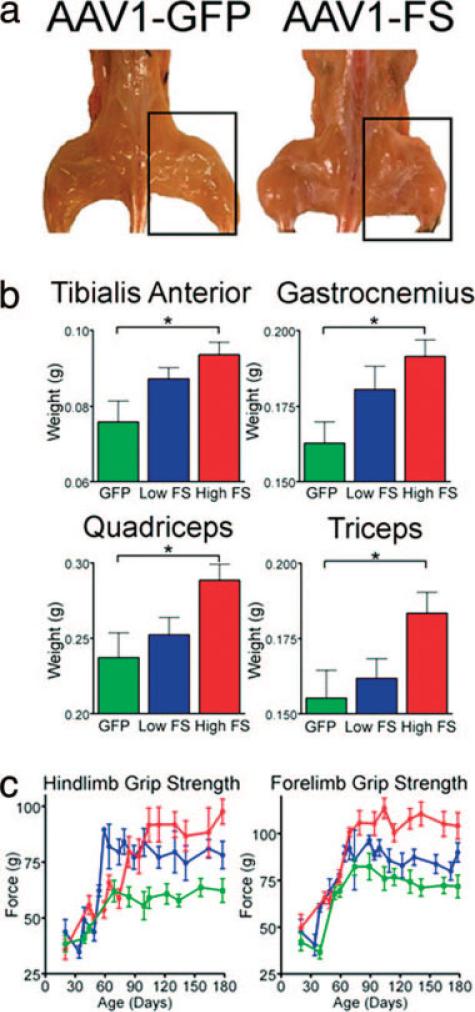
To determine the optimal gene product for clinical advancement, follistatin was directly compared with other myostatin-inhibitory genes, including growth and differentiation factor-associated serum protein-1(GASP-1)26 and the follistatin-related gene (FLRG).72 The quadriceps and tibialis anterior muscles of wildtype mice were injected with AAV sero-type1 that encodes FS-344, FLRG, and GASP-1 under the control of a strong cytomegalovirus (CMV)-based promoter. Increased body mass and muscle enlargement were observed with all three transgenes tested; however, the greatest increase occurred in FS-344-treated animals (Fig. 4a,b). The increased muscle mass was found in the injected hindlimb muscles and at sites remote to the injection, including upper extremity muscles such as the triceps (Fig. 4c). Thus, these inhibitors were secreted into the circulation from the site of muscle injection in the hindlimbs. The enlarged muscle mass was accompanied by functional improvement, demonstrated by an increase in hindlimb grip strength (Fig. 4d). There was no effect on heart size or histological appearance of cardiomyocytes, indicating that myostatin inhibition was selective to skeletal muscle tissue. We found no change in reproductive capacity in mice treated with our AAV1 carrying the FS-344 transgene (AAV1-FS, Table 2). Furthermore, we found no histological/pathological alterations in the gonadal tissue, heart, liver, or kidneys of FS-344-treated mice compared with controls, indicating that this treatment appeared to be safe and well tolerated.
FIGURE 4.
Myostatin inhibitor proteins increase muscle mass and strength in wildtype C57Bl/6 mice. (a) Gross hindlimb muscle mass is increased in all myostatin-inhibitor-protein-treated mice at 725 days of age compared with AAV1-GFP-injected controls. (b) Total body mass is significantly increased in AAV1-FS344-injected (**P < 0.01) and AAV1-GASP-1-injected (*P < 0.05) mice compared with AAV1-GFP controls at 725 days of age (n = 10). (c) The mass of individual hindlimb and forelimb muscles is increased in mice injected with AAV expressing myostatin inhibitory proteins (n = 10). *P < 0.05. (d) Hindlimb grip strength improves >2 years in all treated mice with the greatest differences in AAV1-FS344 treated animals compared with AAV1-GFP controls (n = 10). Error bars represent standard error.
Table 2.
Normal reproduction in C57BL/6 and mdx mice treated with AAV1.FS
| Group | Treatment | Mean litter size (SD) |
|---|---|---|
| C57BL/6 | AAV1.FS treated normal male mated with normal female (n=4) | 9.0 (2.582) (n=4) |
| AAV1.FS treated normal female mated with normal male (n=4) | 9.25 (1.708) (n=4) | |
| Untreated normal male mated with untreated normal female (n=4) | 9.0 (2.160) (n=4) | |
| mdx−/− | AAV1.FS treated mdx−/− male mated with mdx−/− female (n=3) | 4.5 (0.707) (n=3) |
| AAV1.FS treated mdx−/− female mated with mdx−/− male (n=2) | 2.0 (0) (n=2) | |
| Untreated mdx−/− male mated with untreated mdx−/− female (n=6) | 3.83 (1.169) (n=6) |
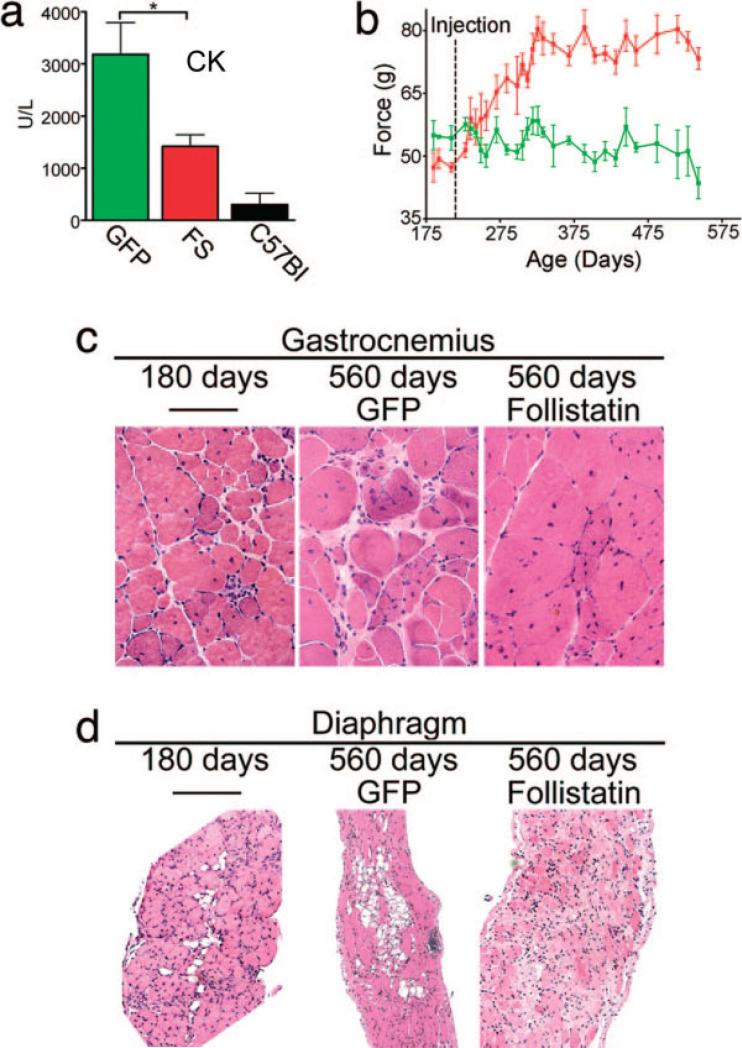
Subsequently, we tested the potential for AAV1-FS-344 to increase muscle mass and strength along with delaying muscle deterioration in the mdx mouse. The quadriceps and tibialis anterior muscles were injected at 3 weeks of age and were followed for 5 months before necropsy. A 15-fold increase in serum follistatin was seen in mice that received high dose (1.5 × 1012 vg/kg) and a 6-fold increase was detected at low dose (1.5 × 1011 vg/kg). Treated mice displayed a significant increase in muscle size (Fig. 5a), with the greatest individual muscle weight increase in high-dose follistatin-treated animals (Fig. 5b). As shown in previous studies in wildtype mice, effects could be seen at sites remote to the viral injection (e.g., triceps; Fig. 5b). Increased muscle mass translated to a dose-dependent improvement in muscle strength in the hindlimbs and forelimbs of treated animals compared with controls (Fig. 5c). AAV-FS-344-treated mice also demonstrated a significant reduction in serum creatine kinase (Fig. 6a). This is of interest, given that follistatin was protective despite its lack of correction of the underlying dystrophin deficiency. One might speculate that increasing the strength of individual fibers makes them more resistant to damage from the stress of normal activities.
FIGURE 5.
Single injection of AAV1.FS-344 increases muscle mass and strength in young mdx mice. (a) Gross hindlimb muscle mass is increased in AAV1.FS-344-injected mdx animals at 180 days of age compared with AAV1.GFP-injected controls. (b) The mass of individual hindlimb and forelimb muscles is increased at 180 days of age in mice injected at 3 weeks of age with AAV1.FS-344 paired with AAV1.GFP controls (n = 15). *P ≤ 0.05. (c) Grip strength is improved in a dose-dependent manner in young mdx mice injected at 3 weeks of age with AAV1-FS-344 followed for 180 days (n = 15). Red, high-dose AAV1.FS; blue, low-dose AAV1.FS-344; green, AAV1.GFP controls. Error bars represent standard errors.
FIGURE 6.
Effects of AAV1.FS344 on muscle enzymes, strength, and morphology in older mice. (a) Serum creatine kinase levels (units/liter) are decreased at 3 months after injection with AAV1.FS-344 compared with AAV1.GFP-injected controls. (*P < 0.05; n = 10.) Error bars represent standard errors. Aged mdx mice treated with AAV1.FS-344. (b) Hindlimb grip strength is significantly increased (P < 0.05) at 275 days and beyond in aged mdx mice treated with AAV1.FS-344 at 210 days of age (n = 15). Red, high-dose AAV1.FS; green, AAV1.GFP controls. (c) Hematoxylin and eosin (H&E) stain of aged gastrocnemius (pre-treatment 180 days) demonstrates reduced pathology at 560 days.
We next evaluated the potential for AAV1-FS-344 to increase muscle strength in mdx animals treated at an older age. Mice injected in the quadriceps and tibialis anterior muscles at 210 days of age demonstrated increased muscle strength measured by hindlimb grip strength for more than 60 days after administration. After reaching a plateau, increased strength persisted long-term, throughout the 560 days evaluated in this study (Fig. 6b,c). Reduced inflammation and endomysial connective tissue in the diaphragm supported previous findings related to follistatin, broadening its potential for clinical application. In the diaphragm of the AAV1-FS-344-treated mdx mouse, the muscle that most closely recapitulates the dystrophic process in humans,74 we found a reduction in endomysial connective tissue (Fig. 6d). In addition, mdx muscle examined at 560 days of age demonstrated fewer focal groups of necrotic muscle fibers and mononuclear cell infiltrates. This antiinflammatory effect of follistatin has been studied in other translational paradigms. In models of experimental (trinitrobenzene sulfonic acid, oral dextran sulfate sodium) and spontaneous colitis (interleukin-10 gene-deficiency), follistatin pretreatment increased survival, decreased plasma levels of inflammatory cytokines, and reduced tissue inflammation.13 Follistatin has also been shown to reduce proinflammatory cytokines induced by lipopolysaccharide stimulation.27
Given the positive results in mice, we also extended our follistatin gene therapy studies to nonhuman primates to establish evidence that direct muscle injections are a viable means of increasing strength in a larger model that more closely simulates the clinical setting. All too often, studies in mice fail to translate to the clinic; however, demonstrating efficacy and safety using the same means of viral transfer and the same cassette carrying the gene of interest in a larger animal species provides reasonable assurance of success for patients. Our studies in the cynamologous macaque have been remarkably successful (first presented at the American Society for Gene Therapy Meeting 2008; in preparation). We have studied AAV1-FS-344 under control of a specific (muscle creatine kinase) and a nonspecific (CMV) promoter. We have seen a remarkable increase in muscle size and strength with both promoters. Extensive observations on these monkeys with regard to reproductive organ function and postmortem tissue analysis have not revealed any organ system pathology, including organs of the pituitary–gonadal axis.
Our studies therefore indicate that AAV-mediated follistatin gene therapy has potential for treatment of muscular dystrophy, and it is well positioned to benefit certain acquired diseases. We have also observed that combinational treatment, meaning replacement of the defective gene, in combination with follistatin gene therapy may offer more than either alone. For example, ongoing studies in our laboratory have demonstrated that combining AAV-mediated micro-dystrophin gene therapy with AAV-delivered follistatin increases force generation of the muscle and protection against eccentric contraction to a greater degree than either can achieve alone. The antiinflammatory effects of follistatin that accompany AAV-mediated follistatin gene therapy are also of potential benefit to certain patient groups such as those with sporadic inclusion body myositis. The dual effects of increased muscle strength and reduced mononuclear cell infiltration provide a promising combination for treatment of an otherwise refractory muscle disease.
In conclusion, gene therapy using follistatin to inhibit myostatin holds promise for the treatment of muscle disease. Our findings that demonstrate increased muscle size and strength with reduced fibrosis in the mdx mouse, as well as our success in translating the findings to nonhuman primates, sets the stage for human clinical trials. We have encountered no adverse effects, no effect on reproductive capacity, and no immunogenicity of the follistatin transgene or its product. Therefore, the antiinflammatory effects of follistatin will facilitate efforts in a variety of diseases, particularly those like sporadic inclusion body myositis, where medical management has left many patients disabled and without treatment options.
Abbreviations
- AAV
adeno-associated virus
- ActRIIB
activin receptor type IIB
- ALK-4
activin receptor like kinase 4, 5
- BMP
bone morphogenic protein
- capn3−/−
calpain3 deficient mouse
- CAV-3 Tg
caveolin-3 transgenic mouse deficient for caveolin-3
- DMD
Duchenne muscular dystrophy
- dyW/dyW
laminin-alpha2 (merosin) deficient mouse
- FLRG
follistatin related gene
- FS
follistatin
- FSH
follicle stimulating hormone
- FSHD
facioscapulohumeral dystrophy
- FSP
FSH suppressing protein
- GASP-1
growth and differentiation factor-associated serum protein-1
- GDF-8
growth and differentiation factor 8
- LGMD
limb girdle muscular dystrophy
- mdx−/−
Duchenne dystrophy mouse model deficient for dystrophin
- MSTN
myostatin
- mstn−/−
myostatin deficient mouse
- sgca−/−
alpha-sarcoglycan deficient mouse
- sgcd−/−
delta-sarcoglycan deficient mouse
- TGF-β
transforming growth factor beta
- TLD
tolloid
Footnotes
Supported by the Myositis Association, Muscular Dystrophy Association, Department of Defense Grant W81XWH-OS-1-D616, Jesse's Journey.
REFERENCES
- 1.Aartsma-Rus A, Kaman WE, Weij R, den Dunnen JT, van Ommen GJ, van Deutekom JC. Exploring the frontiers of therapeutic exon skipping for Duchenne muscular dystrophy by double targeting within one or multiple exons. Mol Ther. 2006;14:401–407. doi: 10.1016/j.ymthe.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Abmayr S, Gregorevic P, Allen JM, Chamberlain JS. Phenotypic improvement of dystrophic muscles by rAAV/microdystrophin vectors is augmented by Igf1 codelivery. Mol Ther. 2005;12:441–450. doi: 10.1016/j.ymthe.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Alen M, Rahkila P. Reduced high-density lipoprotein-cholesterol in power athletes: use of male sex hormone derivates, an atherogenic factor. Int J Sports Med. 1984;5:341–342. doi: 10.1055/s-2008-1025929. [DOI] [PubMed] [Google Scholar]
- 4.Amthor H, Huang R, McKinnell I, Christ B, Kambadur R, Sharma M, et al. The regulation and action of myostatin as a negative regulator of muscle development during avian embryogenesis. Dev Biol. 2002;251:241–257. doi: 10.1006/dbio.2002.0812. [DOI] [PubMed] [Google Scholar]
- 5.Balagopal P, Olney R, Darmaun D, Mougey E, Dokler M, Sieck G, et al. Oxandrolone enhances skeletal muscle myosin synthesis and alters global gene expression profile in Duchenne muscular dystrophy. Am J Physiol Endocrinol Metab. 2006;290:E530–539. doi: 10.1152/ajpendo.00412.2005. [DOI] [PubMed] [Google Scholar]
- 6.Bartoli M, Poupiot J, Vulin A, Fougerousse F, Arandel L, Daniele N, et al. AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not alpha-sarcoglycan deficiency. Gene Ther. 2007;14:733–740. doi: 10.1038/sj.gt.3302928. [DOI] [PubMed] [Google Scholar]
- 7.Barton-Davis ER, Cordier L, Shoturma DI, Leland SE, Sweeney HL. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Invest. 1999;104:375–381. doi: 10.1172/JCI7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 9.Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J. 2005;19:543–549. doi: 10.1096/fj.04-2796com. [DOI] [PubMed] [Google Scholar]
- 10.Bogdanovich S, McNally EM, Khurana TS. Myostatin blockade improves function but not histopathology in a murine model of limb-girdle muscular dystrophy 2C. Muscle Nerve. 2008;37:308–316. doi: 10.1002/mus.20920. [DOI] [PubMed] [Google Scholar]
- 11.Cagliani R, Bresolin N, Prelle A, Gallanti A, Fortunato F, Sironi M, et al. A CAV3 microdeletion differentially affects skeletal muscle and myocardium. Neurology. 2003;61:1513–1519. doi: 10.1212/01.wnl.0000097320.35982.03. [DOI] [PubMed] [Google Scholar]
- 12.Cerletti M, Negri T, Cozzi F, Colpo R, Andreetta F, Croci D, et al. Dystrophic phenotype of canine X-linked muscular dystrophy is mitigated by adenovirus-mediated utrophin gene transfer. Gene Ther. 2003;10:750–757. doi: 10.1038/sj.gt.3301941. [DOI] [PubMed] [Google Scholar]
- 13.Dohi T, Ejima C, Kato R, Kawamura YI, Kawashima R, Mizutani N, et al. Therapeutic potential of follistatin for colonic inflammation in mice. Gastroenterology. 2005;128:411–423. doi: 10.1053/j.gastro.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 14.Esch FS, Shimasaki S, Mercado M, Cooksey K, Ling N, Ying S, et al. Structural characterization of follistatin: a novel follicle-stimulating hormone release-inhibiting polypeptide from the gonad. Mol Endocrinol. 1987;1:849–855. doi: 10.1210/mend-1-11-849. [DOI] [PubMed] [Google Scholar]
- 15.Evans LW, Muttukrishna S, Groome NP. Development, validation and application of an ultra-sensitive two-site enzyme immunoassay for human follistatin. J Endocrinol. 1998;156:275–282. doi: 10.1677/joe.0.1560275. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Vazquez G, Kaiser UB, Albarracin CT, Chin WW. Transcriptional activation of the gonadotropin-releasing hormone receptor gene by activin A. Mol Endocrinol. 1996;10:356–366. doi: 10.1210/mend.10.4.8721981. [DOI] [PubMed] [Google Scholar]
- 17.Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 18.Gilfillan CP, Robertson DM. Development and validation of a radioimmunoassay for follistatin in human serum. Clin Endocrinol (Oxf) 1994;41:453–461. doi: 10.1111/j.1365-2265.1994.tb02576.x. [DOI] [PubMed] [Google Scholar]
- 19.Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12:787–789. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Q, Kumar TR, Woodruff T, Hadsell LA, DeMayo FJ, Matzuk MM. Overexpression of mouse follistatin causes reproductive defects in transgenic mice. Mol Endocrinol. 1998;12:96–106. doi: 10.1210/mend.12.1.0053. [DOI] [PubMed] [Google Scholar]
- 22.Haidet AM, Rizo L, Handy C, Umapathi P, Eagle A, Shilling C, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci U S A. 2008;105:4318–4322. doi: 10.1073/pnas.0709144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamed SA. Drug evaluation: PTC-124—a potential treatment of cystic fibrosis and Duchenne muscular dystrophy. IDrugs. 2006;9:783–789. [PubMed] [Google Scholar]
- 24.Hashimoto O, Nakamura T, Shoji H, Shimasaki S, Hayashi Y, Sugino H. A novel role of follistatin, an activin-binding protein, in the inhibition of activin action in rat pituitary cells. Endocytotic degradation of activin and its acceleration by follistatin associated with cell-surface heparan sulfate. J Biol Chem. 1997;272:13835–13842. doi: 10.1074/jbc.272.21.13835. [DOI] [PubMed] [Google Scholar]
- 25.Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolf-man NM, et al. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem. 2002;277:40735–40741. doi: 10.1074/jbc.M206379200. [DOI] [PubMed] [Google Scholar]
- 26.Hill JJ, Qiu Y, Hewick RM, Wolfman NM. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains. Mol Endocrinol. 2003;17:1144–1154. doi: 10.1210/me.2002-0366. [DOI] [PubMed] [Google Scholar]
- 27.Jones KL, Mansell A, Patella S, Scott BJ, Hedger MP, de Kretser DM, et al. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc Natl Acad Sci U S A. 2007;104:16239–16244. doi: 10.1073/pnas.0705971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keller ET, Ershler WB, Chang C. The androgen receptor: a mediator of diverse responses. Front Biosci. 1996;1:d59–71. doi: 10.2741/a116. [DOI] [PubMed] [Google Scholar]
- 29.Khoury RH, Wang QF, Crowley WF, Jr, Hall JE, Schneyer AL, Toth T, et al. Serum follistatin levels in women: evidence against an endocrine function of ovarian follistatin. J Clin Endocrinol Metab. 1995;80:1361–1368. doi: 10.1210/jcem.80.4.7714112. [DOI] [PubMed] [Google Scholar]
- 30.Kindermann W, Urhausen A. Left ventricular dimensions and function in strength athletes. Re: Hartgens F, Cheriex EC, Kuipers H. Prospective echocardiographic assessment of androgenic-anabolic steroids effects on cardiac structure and function in strength athletes. Int J Sports Med. 2003;24:344–351. doi: 10.1055/s-2003-40705. Int J Sports Med 2004;25:241−242; author reply 243−244. [DOI] [PubMed] [Google Scholar]
- 31.Kuang W, Xu H, Vilquin JT, Engvall E. Activation of the lama2 gene in muscle regeneration: abortive regeneration in laminin alpha2-deficiency. Lab Invest. 1999;79:1601–1613. [PubMed] [Google Scholar]
- 32.Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 2002;277:49831–49840. doi: 10.1074/jbc.M204291200. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- 35.Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci U S A. 2005;102:18117–18122. doi: 10.1073/pnas.0505996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS ONE. 2007;2:e789. doi: 10.1371/journal.pone.0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenders JW, Demacker PN, Vos JA, Jansen PL, Hoitsma AJ, van 't Laar A, et al. Deleterious effects of anabolic steroids on serum lipoproteins, blood pressure, and liver function in amateur body builders. Int J Sports Med. 1988;9:19–23. doi: 10.1055/s-2007-1024972. [DOI] [PubMed] [Google Scholar]
- 38.Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem. 2008;283:19371–19378. doi: 10.1074/jbc.M802585200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li ZF, Shelton GD, Engvall E. Elimination of myostatin does not combat muscular dystrophy in dy mice but increases postnatal lethality. Am J Pathol. 2005;166:491–497. doi: 10.1016/S0002-9440(10)62271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu M, Yue Y, Harper SQ, Grange RW, Chamberlain JS, Duan D. Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol Ther. 2005;11:245–256. doi: 10.1016/j.ymthe.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374:360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]
- 44.McClorey G, Moulton HM, Iversen PL, Fletcher S, Wilton SD. Antisense oligonucleotide-induced exon skipping restores dystrophin expression in vitro in a canine model of DMD. Gene Ther. 2006;13:1373–1381. doi: 10.1038/sj.gt.3302800. [DOI] [PubMed] [Google Scholar]
- 45.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 46.Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, et al. Randomized, double-blind six-month trial of prednisone in Duchenne's muscular dystrophy. N Engl J Med. 1989;320:1592–1597. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- 47.Mendell JR, Boue DR, Martin PT. The congenital muscular dystrophies: recent advances and molecular insights. Pediatr Dev Pathol. 2006;9:427–443. doi: 10.2350/06-07-0127.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minetti GC, Colussi C, Adami R, Serra C, Mozzetta C, Parente V, et al. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med. 2006;12:1147–1150. doi: 10.1038/nm1479. [DOI] [PubMed] [Google Scholar]
- 49.Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- 50.Moxley RT, 3rd, Ashwal S, Pandya S, Connolly A, Florence J, Mathews K, et al. Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2005;64:13–20. doi: 10.1212/01.WNL.0000148485.00049.B7. [DOI] [PubMed] [Google Scholar]
- 51.Mueller C, Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura T, Takio K, Eto Y, Shibai H, Titani K, Sugino H. Activin-binding protein from rat ovary is follistatin. Science. 1990;247:836–838. doi: 10.1126/science.2106159. [DOI] [PubMed] [Google Scholar]
- 53.Ohsawa Y, Hagiwara H, Nakatani M, Yasue A, Moriyama K, Murakami T, et al. Muscular atrophy of caveolin-3-deficient mice is rescued by myostatin inhibition. J Clin Invest. 2006;116:2924–2934. doi: 10.1172/JCI28520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parsons SA, Millay DP, Sargent MA, McNally EM, Molkentin JD. Age-dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy. Am J Pathol. 2006;168:1975–1985. doi: 10.2353/ajpath.2006.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parton RG. Caveolae—from ultrastructure to molecular mechanisms. Nat Rev Mol Cell Biol. 2003;4:162–167. doi: 10.1038/nrm1017. [DOI] [PubMed] [Google Scholar]
- 56.Phillips DJ, de Kretser DM. Follistatin: a multifunctional regulatory protein. Front Neuroendocrinol. 1998;19:287–322. doi: 10.1006/frne.1998.0169. [DOI] [PubMed] [Google Scholar]
- 57.Pope HG, Jr, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiatry. 1994;51:375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- 58.Qiao C, Li J, Jiang J, Zhu X, Wang B, Li J, et al. Myostatin propeptide gene delivery by adeno-associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice. Hum Gene Ther. 2008;19:241–254. doi: 10.1089/hum.2007.159. [DOI] [PubMed] [Google Scholar]
- 59.Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230–7242. doi: 10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, et al. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab. 2003;285:E876–888. doi: 10.1152/ajpendo.00107.2003. [DOI] [PubMed] [Google Scholar]
- 61.Robertson DM, Klein R, de Vos FL, McLachlan RI, Wettenhall RE, Hearn MT, et al. The isolation of polypeptides with FSH suppressing activity from bovine follicular fluid which are structurally different to inhibin. Biochem Biophys Res Commun. 1987;149:744–749. doi: 10.1016/0006-291x(87)90430-x. [DOI] [PubMed] [Google Scholar]
- 62.Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 63.Schneyer AL, Wang Q, Sidis Y, Sluss PM. Differential distribution of follistatin isoforms: application of a new FS315-specific immunoassay. J Clin Endocrinol Metab. 2004;89:5067–5075. doi: 10.1210/jc.2004-0162. [DOI] [PubMed] [Google Scholar]
- 64.Shimasaki S, Koga M, Esch F, Cooksey K, Mercado M, Koba A, et al. Primary structure of the human follistatin precursor and its genomic organization. Proc Natl Acad Sci U S A. 1988;85:4218–4222. doi: 10.1073/pnas.85.12.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimasaki S, Koga M, Esch F, Mercado M, Cooksey K, Koba A, et al. Porcine follistatin gene structure supports two forms of mature follistatin produced by alternative splicing. Biochem Biophys Res Commun. 1988;152:717–723. doi: 10.1016/s0006-291x(88)80097-4. [DOI] [PubMed] [Google Scholar]
- 66.Sjoqvist F, Garle M, Rane A. Use of doping agents, particularly anabolic steroids, in sports and society. Lancet. 2008;371:1872–1882. doi: 10.1016/S0140-6736(08)60801-6. [DOI] [PubMed] [Google Scholar]
- 67.Solinas-Toldo S, Lengauer C, Fries R. Comparative genome map of human and cattle. Genomics. 1995;27:489–496. doi: 10.1006/geno.1995.1081. [DOI] [PubMed] [Google Scholar]
- 68.Stolz LE, Li D, Qadri A, Jalenak M, Klaman LD, Tobin JF. Administration of myostatin does not alter fat mass in adult mice. Diabetes Obes Metab. 2008;10:135–142. doi: 10.1111/j.1463-1326.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 69.Sugino K, Kurosawa N, Nakamura T, Takio K, Shimasaki S, Ling N, et al. Molecular heterogeneity of follistatin, an activin-binding protein. Higher affinity of the carboxyl-terminal truncated forms for heparan sulfate proteoglycans on the ovarian granulosa cell. J Biol Chem. 1993;268:15579–15587. [PubMed] [Google Scholar]
- 70.Sumitomo S, Inouye S, Liu XJ, Ling N, Shimasaki S. The heparin binding site of follistatin is involved in its interaction with activin. Biochem Biophys Res Commun. 1995;208:1–9. doi: 10.1006/bbrc.1995.1297. [DOI] [PubMed] [Google Scholar]
- 71.Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, et al. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- 72.Tsuchida K, Arai KY, Kuramoto Y, Yamakawa N, Hasegawa Y, Sugino H. Identification and characterization of a novel follistatin-like protein as a binding protein for the TGF-beta family. J Biol Chem. 2000;275:40788–40796. doi: 10.1074/jbc.M006114200. [DOI] [PubMed] [Google Scholar]
- 73.Ueno N, Ling N, Ying SY, Esch F, Shimasaki S, Guillemin R. Isolation and partial characterization of follistatin: a single-chain Mr 35,000 monomeric protein that inhibits the release of follicle-stimulating hormone. Proc Natl Acad Sci U S A. 1987;84:8282–8286. doi: 10.1073/pnas.84.23.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol. 2002;52:832–836. doi: 10.1002/ana.10385. [DOI] [PubMed] [Google Scholar]
- 75.Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 76.Wakatsuki M, Shintani Y, Abe M, Liu ZH, Shitsukawa K, Saito S. Immunoradiometric assay for follistatin: serum immunoreactive follistatin levels in normal adults and pregnant women. J Clin Endocrinol Metab. 1996;81:630–634. doi: 10.1210/jcem.81.2.8636280. [DOI] [PubMed] [Google Scholar]
- 77.Wang QF, Farnworth PG, Burger HG, Findlay JK. Acute inhibitory effect of follicle-stimulating hormone-suppressing protein (FSP) on gonadotropin-releasing hormone-stimulated gonadotropin secretion in cultured rat anterior pituitary cells. Mol Cell Endocrinol. 1990;72:33–42. doi: 10.1016/0303-7207(90)90237-3. [DOI] [PubMed] [Google Scholar]
- 78.Wang QF, Farnworth PG, Findlay JK, Burger HG. Chronic inhibitory effect of follicle-stimulating hormone (FSH)-suppressing protein (FSP) or follistatin on activin- and gonadotropin-releasing hormone-stimulated FSH synthesis and secretion in cultured rat anterior pituitary cells. Endocrinology. 1990;127:1385–1393. doi: 10.1210/endo-127-3-1385. [DOI] [PubMed] [Google Scholar]
- 79.Woodruff TK, Sluss P, Wang E, Janssen I, Mersol-Barg MS. Activin A and follistatin are dynamically regulated during human pregnancy. J Endocrinol. 1997;152:167–174. doi: 10.1677/joe.0.1520167. [DOI] [PubMed] [Google Scholar]