Synopsis
The circadian system in animals and humans, being near but not exactly 24-hours in cycle length, must be reset on a daily basis in order to remain in synchrony with external environmental time. This process of entrainment is achieved in most mammals through regular exposure to light and darkness. In this chapter, we review the results of studies conducted in our laboratory and others over the past 25 years in which the effects of light on the human circadian timing system were investigated. These studies have revealed, how the timing, intensity, duration, and wavelength of light affect the human biological clock. Our most recent studies also demonstrate that there is much yet to learn about the effects of light on the human circadian timing system.
Keywords: biological rhythm, core body temperature, illuminance, melatonin, phase response curve
Introduction
Circadian rhythms are variations in physiology and behavior that persist with a cycle length close to 24 hours even in the absence of periodic environmental stimuli. It is hypothesized that this system evolved in order to predict and therefore optimally time the behavior and physiology of the organism to the environmental periodicity associated with the earth’s rotation. Because the cycle length, or period, of this endogenous timing system is near, but, in most organisms, not exactly 24 hours, circadian rhythms must be synchronized or entrained to the 24-hour day on a regular basis. In most organisms, this process of entrainment occurs through regular exposure to light and darkness.
Early reports from studies of human circadian rhythms had suggested that humans were unlike other organisms, being relatively insensitive to light and more sensitive to social cues to entrain their circadian systems. However, subsequent studies, and re-analysis of results from those early studies, have found that the human circadian system is like that of other organisms in its organization and its response to light, and is as sensitive to light as other diurnal organisms. In this chapter we review the results of studies conducted in our laboratory and others over the past 25 years in which the effects of light on the human circadian timing system were investigated.
Neuroanatomy of the mammalian circadian system
Studies published in the early 1970’s established the suprachiasmatic nucleus of the hypothalamus as the central circadian pacemaker in mammals (1–5). This pacemaker is comprised of individual cells which, when isolated, can oscillate independently with a near-24-hour period (5;6). The SCN receives direct input from the retina (7–9), providing a mechanism by which entrainment to light-dark cycles occurs. Recently, a subset of retinal ganglion cells has been described that serve as photoreceptors for circadian and other non-image-forming responses (10–12). These specialized retinal ganglion cells are distributed throughout the retina, project to the SCN, are photosensitive, and contain melanopsin as their photopigment (13;14). While the photosensitive retinal ganglion cells can mediate circadian responses to light, there is also evidence that rod and cone photoreceptors can play a role in circadian responses to light (15;16). The relative contribution of different photoreceptors to circadian responses is not yet well understood, and this is an area of intense research currently. It is likely that the intensity, spectral distribution, and temporal pattern of light can all affect the relative contribution of different photoreceptors to circadian responses. The same neuroanatomical features of the circadian system described in mammals are also present in humans (17–24).
Phase-dependent response of the human circadian system to light
Studies of the effects of light on the circadian system of insects, plants, and animals conducted from the late 1950’s through the 1970’s had demonstrated that the timing of a light stimulus has an important influence on the direction and magnitude of response to that stimulus (25–28). Those studies indicated that the circadian system of both nocturnal and diurnal organisms is most sensitive to light during the biological night. Because humans sleep throughout most of their biological night, testing the influence of light on the human circadian system therefore required that in the sleep-wake cycle be shifted in order to deliver the light stimulus at the time of highest expected sensitivity. That manipulation of sleep-wake timing was a concern in the earliest human light studies, because of prior reports suggesting that social cues influenced human circadian rhythms (29;30).
For those reasons, we therefore conducted one of our earliest studies of the effect of light on the human circadian system on a subject whose circadian temperature rhythm had an unusual phase-relationship to her sleep-wake cycle (31). We identified a subject whose sleep-wake cycle timing was fairly normal, but whose circadian core body temperature rhythm was several hours earlier than normal, resulting in much of her biological night occurring prior to the time she went to bed. In the experiment we conducted, the subject was exposed to several hours of light every evening for a week, and the timing of her rhythms of core body temperature and plasma cortisol were assessed before and after that week of evening light exposure. Both rhythms were shifted by approximately 6 hours, and examination of temperature data collected throughout the experiment suggested that the shift had already occurred after only 2 days.
This finding that light could have this rapid and strong effect on the timing of human circadian rhythms led us to conduct a series of studies in normal young adults in which we applied a series of light stimuli over 2–3 days (32;33;33). In those studies carried out in the late 1980’s, we held the intensity, spectral distribution, and duration of the light stimulus constant, but varied the time at which the initial stimulus was applied. To do this, we had to shift the timing of the sleep-wake cycle so as to be able to present light stimuli across the entire 24-hour circadian cycle. In the course of doing these initial experiments, we were attempting to produce a phase response curve (PRC) (28).
Our results in some ways were not surprising, but in other ways were. We found that humans, like other organisms, are most sensitive to light stimuli during the biological night, and far less sensitive to light in the middle of the biological day (32;34). We also found that when humans are exposed to a light stimulus in the late biological day/early biological night, that stimulus produces a phase delay shift (a shift to a later hour), and light stimuli presented in the late biological night/early biological day produce phase advance shifts (shifts to an earlier hour).
What was somewhat unexpected in those studies was the large magnitude (up to 12 hours) of the phase shifts we were able to achieve, and the PRC that was developed from those 3-cycle light stimuli was a type 0 (strong) PRC. A type 0 PRC is characterized by large phase shifts of + 12 hours, with no “cross-over” point between maximal delays and maximal advances (35). Type 0 resetting also implied that the phase shift has been produced via changing the amplitude of oscillation of the underlying pacemaker (36–39). When we subsequently conducted additional experiments to test the phase shifts that could be achieved with a 2-cycle stimulus, we found that in some studies we could markedly reduce the amplitude of the core body temperature and cortisol rhythms (40). This finding suggested that the amplitude of the underlying pacemaker was affected by the stimulus, lending additional support to the idea that the phase shifts to the strong 3-cycle stimulus were type 0 (41).
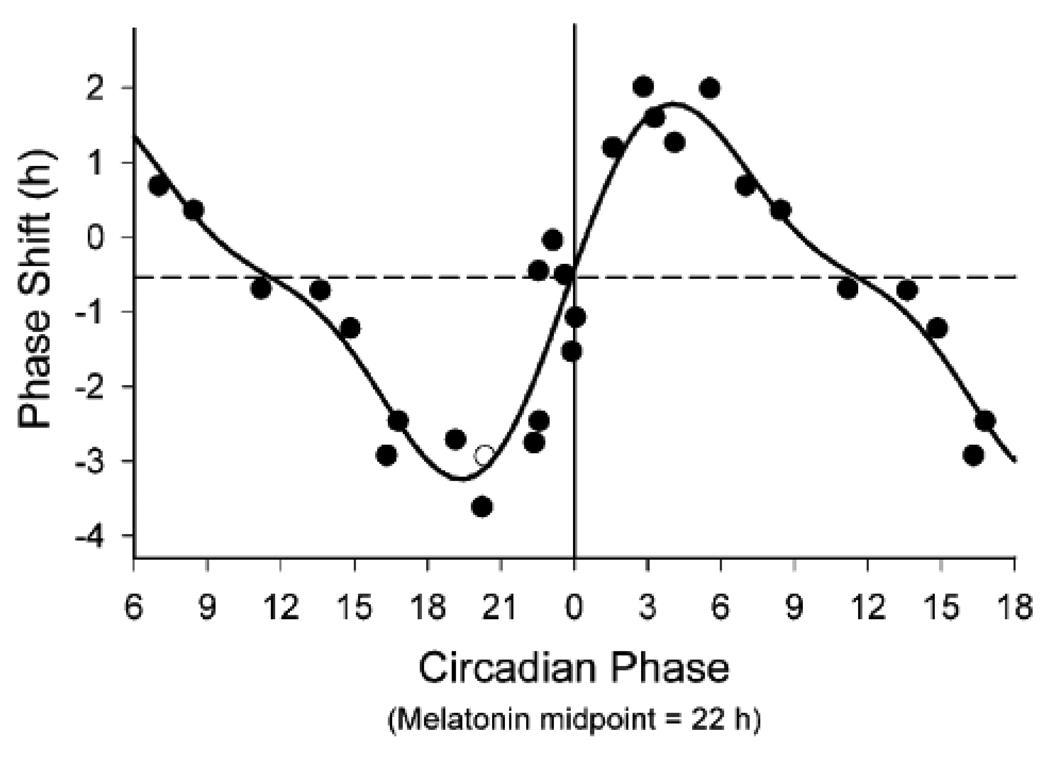
Since describing that PRC to a strong light stimulus, we and others have conducted PRCs to single light pulse stimuli (42;43;131;132). In the late 1990’s, we constructed a human PRC study to a single 6.7-hour light stimulus (42). Results from those studies indicated a type 1 PRC, with a shape and magnitude consistent with type 1 PRCs from other organisms. Type 1 PRCs are characterized by a lower amplitude than type 0 with smaller maximal phase shifts, as well as by a cross-over point between maximal delays and advances. In the human PRC to the 6.7-hour stimulus, the maximal phase shifts were 2–3 hours. There was a phase delay region in the late biological day/early biological night, a phase advance region in the early biological day, small phase shifts during the middle of the biological day, and a transition point towards the end of the biological night [see Figure 1, reproduced from (42)].
Figure 1.
Phase response curve to a single 6.5-hour episode of bright light in young adults. Phase shifts (in hours) of the plasma melatonin rhythm are plotted with respect to the circadian phase at which the center of the 6.5-hour light stimulus was presented. By convention, phase delay shifts (shifts to a later hour) are plotted as negative numbers, while phase advance shifts (shifts to an earlier hour) are plotted as positive numbers. Phase shift magnitude was determined by assessing phase before and after the light stimulus. Circadian phase of the light stimulus was defined relative to the midpoint of the plasma melatonin rhythm (defined as 22h) assessed just prior to the stimulus. Data from circadian phase 6–18 are double-plotted for better visualization. The open circle represents a subject whose phase shift was determined using salivary melatonin. The solid line represents a best-fit dual harmonic function to the data points. The dashed horizontal line represents the assumed 0.54-hour average phase delay drift of the human circadian pacemaker between the pre- and post-stimulus phase assessments. Figure reproduced with permission from Reference 42 [Khalsa SBS, Jewett ME, Cajochen C, and Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol (Lond) 2003; 549(Pt 3):945–952].
There is evidence from studies in insects that both type 0 and type 1 PRCs are possible in the same species, with the PRC type dependent on the strength of the light stimulus, and the organisms in which type 0 resetting has been demonstrated also show type 1 resetting to a weaker stimulus (35;38;39). In fact, the type 1 and type 0 PRCs of humans are remarkably similar to those from the mosquito (44) [see (45) for illustration of the human and mosquito PRCs].
Additional studies and analyses conducted in our laboratory have also revealed additional features of the human phase-dependent response to light. We have found that humans, like other diurnal species (46), are sensitive to light throughout the biological day (34), with little evidence of a so-called “dead zone” (a segment of the PRC during the biological day when no responses are observed). Our analyses have also found that phase shifts to light in humans appear to occur rapidly, with little evidence of transients (34;47). Together, these studies of the phase-dependent response of humans to light have reinforced the idea that the human circadian system is like that of other organisms (48).
Intensity-dependent response of the human circadian system to light
Reports from studies in non-human organisms indicated that the circadian system showed intensity-dependent responses to light stimuli (25;49–55), in addition to its phase-dependent responses to light. Investigation of the intensity-response relationship to light is typically done by applying light stimuli of the same duration and spectral composition at a fixed circadian phase, but varying the intensity of the light. Early reports from human studies had demonstrated that varying the intensity of a light stimulus would produce different amounts of suppression of the pineal hormone melatonin (56–59). Based on this animal and human evidence, we conducted a series of studies, beginning in the late 1980’s (60), to test the ability of different intensities of light to phase shift the human circadian system.
In the first series of studies, we applied a 3-cycle 5-hour light stimulus in the early biological day (the phase advance portion of the PRC), and studied groups of subjects at several different illuminance levels [0, 12, 180, 600, 1260, and 9,500 lux; see Figure 2, reproduced from (63)]. In those studies (61–63), we found that the groups exposed to light greater than room light level showed a significant phase advance shift, while the groups exposed to darkness or dim light (the 12 lux group) for the same 3-cycle 5-hour stimulus timing drifted to a later phase consistent with the longer than 24-hour period of the human circadian system (64). The 0-lux and 12-lux control groups also confirmed that the phase shifts produced by the light were mainly due to the light exposure itself, and not to the shift in the timing of the rest-activity schedule (65).
Figure 2.
Phase shifts (panel A) and melatonin suppression (Panel B) in response to a 3-cycle 5-hour light stimulus in young adults. The magnitude of the phase shift (in hours) is plotted with respect to the illuminance of the light stimulus (in lux). Symbols represent mean (± s.e.m.) responses from each group of 7–9 subjects. The solid line represents a 3-paramater logistic curve fit to the data, and the upper and lower 95% confidence intervals of this fit are shown in the dashed lines. Figure reproduced with permission from Reference 63 [Zeitzer JM, Khalsa SB, Boivin DB, Duffy JF, Shanahan TL, Kronauer RE, and Czeisler CA. Temporal dynamics of late-night photic stimulation of the human circadian timing system. Am J Physiol 2005; 289(3):R839–R844].
Subsequently, we conducted another study testing the effect that a single light exposure would have on the human circadian system when the illuminance was varied (66). In this study, we used a 6.5-hour light stimulus ranging from 3 to 9,100 lux, applied in the late biological day/early biological night, so as to produce phase delay shifts. We found that the resetting response and melatonin suppression was related in a non-linear way to illuminance, with minimal responses below 100 lux and saturating responses above ∼1,000 lux. The best model fit to the data was from a 4-parameter logistic model, which predicted a half-maximal response of ∼100 lux, in the range of normal indoor light (66). A similar study conducted in healthy older subjects found similar responses to low and high levels of illumination, with a suggestion of slightly less sensitivity in the older subjects compared with the younger adults (67).
In the 1-pulse study in young adults, we also examined the relationship between illuminance and measures of alertness, and found that brighter light had greater effects on subjective and objective measures of alertness (68).
Findings from all of these studies indicate that the human circadian system can be sensitive to rather dim levels of light, including candlelight (110). In fact, in the 1-pulse study by Zeitzer et al. (66), phase shifts of 50% magnitude of the maximal shift (obtained with a 9,100 lux stimulus) were obtained with stimuli of only ∼1% of that intensity (∼100 lux). However, we should note that the light stimuli in these studies were presented after exposure to many hours of very dim light and/or darkness, and as we discuss below, this prior exposure to dim light likely sensitized the system. Thus, while a ∼100 lux light pulse applied after several hours of very dim light does have a significant phase-shifting and melatonin-suppressing effect in humans, the same light pulse applied against a brighter background would likely produce a smaller effect.
Response of the human circadian system to intermittent bright light exposure
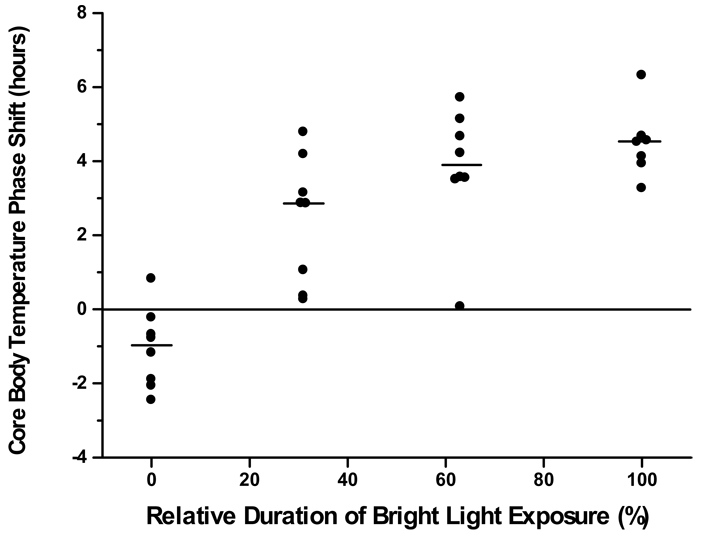
Studies of light effects in mammals had demonstrated that brief pulses of light could affect the circadian system, and that the system appeared to integrate brief light pulses applied in sequence (44;53;69). We conducted experiments to explore whether the human circadian system is responsive to short duration stimuli, and if the human circadian system is capable of integrating short light stimuli (70;71). In the first such experiment (70), we used a 3-cycle light stimulus applied in the phase-advance region (late biological night/early biological day) of the PRC, and tested two different light stimulus patterns. The first pattern used four ∼46-minute light stimuli interspersed with ∼44-minute episodes of darkness, such that the entire pattern took 5 hours. The second light stimulus pattern used briefer stimuli, with 13 5.3-minute light stimuli interspersed with 19.7-minute episodes of darkness. We compared the results of these two intermittent light patterns with results from two groups in which we used a continuous 5-hour bright light stimulus or a continuous 5-hour darkness stimulus (65). Even thought the duration of bright light in the two intermittent light groups was only 63% or 31% of the continuous light group, respectively, we still observed significant phase advance shifts [see Figure 3, reproduced from (70)]. The intermittent light group that received 63% of the light duration showed phase shifts that were not significantly different from the continuous bright light group, with a response approximately 88% of that of the continuous light group. The intermittent light group with the shorter light duration (31%) showed phase advances that were approximately 70% of the magnitude of the continuous light group. These findings demonstrated that humans were responsive to shorter durations of bright light exposure than had been previously recognized, and that the magnitude of the response was related in a non-linear way to the duration of light contained within the stimulus (72;73).
Figure 3.
Phase resetting responses to a 3-cycle 5-hour stimulus in young adults. The magnitude of the phase shift (in hours) is plotted with respect to the relative duration of the bright light exposure. The 0% group was exposed to complete darkness throughout the 5-hour stimulus and the 100% group was exposed to continuous bright light throughout the stimulus. Two groups received intermittent bright light exposure during the 5-hour stimulus: 5.3 minutes of bright light interspersed with 19.7 minutes of darkness (31% relative duration group); or 46 minutes of bright light interspersed with 44 minutes of darkness (63% relative duration group). Each point represents the response from 1 subject, and the horizontal bars represent the median phase shift for the group. Figure reproduced with permission from Reference 70 [Rimmer DW, Boivin DB, Shanahan TL, Kronauer RE, Duffy JF, and Czeisler CA. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol 2000; 279(5):R1574–R1579]
We also conducted an experiment testing the effects of an intermittent light stimulus using a 1-cycle light stimulus (71). In that study, we used a 6.5-hour stimulus presented in the phase delay region (early biological night), and compared subjects exposed to continuous bright light, continuous very dim light, and intermittent light. The intermittent light pattern consisted of six 15-minute bright light pulses separated by 60 minutes of very dim light, and therefore contained 23% of the duration of the continuous bright light stimulus. We found that both groups exposed to light phase delay shifted by a significant amount, and that the magnitude of the phase delay was not significantly different between the two groups, with approximately 75% of the resetting response achieved with 23% of the bright light duration. When suppression of melatonin by the intermittent light stimuli were examined, we found that melatonin was suppressed within 5 minutes of the start of each light stimulus, that each subsequent light pulse suppressed melatonin by a similar percentage, and that melatonin levels began to increase within 10 minutes after each light pulse was ended (74).
The finding from these studies, that the human circadian system is responsive to very short pulses of light, has many practical implications. It suggests that light treatments can be shortened or interrupted without reducing their effectiveness, and it also suggests that entrainment to the 24-hour day may be greatly influenced by brief exposures to bright light (34;75). In fact, studies of natural light exposure in humans living in a number of different cities have found that most people get relatively little bright light exposure (76–80). How such patterns of brief and intermittent exposure to outdoor levels of light influence phase angle of entrainment in modern humans is currently not well-understood, but available evidence suggests that prolonged exposure to outdoor light each day can have a significant influence on both sleep timing and the timing of hormonal secretion (81–83).
Intermittent bright light stimuli have been tested as a method to adapt the circadian rhythms of shift workers to a night work / day sleep schedule. Reports from such studies have indicated that intermittent bright light during night work can aid in adjusting the circadian system to a night work schedule (133;134), although the bright light groups in those studies were also required to be in darkness at specified daytime sleep times. Given that the scheduling of daytime darkness/sleep can itself aid in adaptation to a night work schedule (127;128), it is not clear whether it was the intermittent bright light, or the combination of intermittent light and scheduled sleep/darkness that produced greater adjustment to the night work schedule.
Wavelength-sensitivity of the human circadian system
Photic resetting of the circadian system is part of a larger class of non-image-forming responses (NIF) to retinal light exposure which have been observed in both humans and in other mammals. After studies in animals had suggested a role for a non-rod, non-cone photoreceptor in circadian responses to light, melanopsin was identified as the photopigment present in those specialized photoreceptors (10–13;23;84–87). Studies of light suppression of melatonin secretion in humans had identified a short wavelength peak in spectral sensitivity of that response (88;89), suggesting that human NIF responses were also mediated by a melanopsin-like photopigment. In fact, several years earlier, we had reported that some visually blind humans could show NIF responses to light, retaining an ability to show melatonin suppression in response to ocular light exposure at night (90), and that light could phase-shift the circadian rhythms in some blind individuals (91).
To explore whether human phase-shifting to light would show a short-wavelength sensitivity, a study was conducted in our laboratory in which a 6.5-hour exposure to monochromatic light was applied in the phase delay region in sighted human subjects (92). Responses to monochromatic light of 460nm and 555nm of equal photon density were compared, and we observed that both phase-shifting and melatonin suppression were significantly greater in the subjects exposed to 460nm light than to those who received 555nm light. We also found that during the 6.5-hour light exposure, subjects exposed to the 460nm light rated themselves as significantly more alert, showed faster reaction times and fewer lapses of attention, and showed less EEG delta power and more EEG high alpha power than subjects exposed to 555nm light, consistent with a greater alerting effect of the short wavelength light (93). More recently, a study conducted in our laboratory has reported that non-image-forming responses to light in two visually-blind individuals was short-wavelength sensitive (24).
While these studies provide additional evidence that the human circadian system includes a short-wavelength-sensitive photoreceptor as in other mammals, they do not rule out the role of visual photoreceptors in mediating circadian responses to light in humans (15;16;94;95). The relative contribution of different photoreceptors to circadian light responses is not yet well-understood, and may depend on the intensity and duration of exposure.
Adaptation of the human circadian system to prior light-dark exposure
Studies in humans and animals have provided evidence that the response of the circadian system to light is influenced by prior exposure to light and darkness (96–102). We have conducted several recent studies in order to examine systematically how the duration and relative intensity of prior light exposure affect the subsequent response to a light pulse (103;104). In a study we conducted recently, we exposed subjects to a 6.5-hour 200 lux light stimulus during the biological nighttime, and measured the degree of melatonin suppression. Before the light stimulus, subjects were in a background light that was very dim (∼0.5 lux) or of room intensity (200 lux, the same intensity as the light stimulus) for 15 hours. Exposure to the dim background resulted in significantly greater melatonin suppression in response to the 200-lux light stimulus than did exposure to 200-lux background light (103). The design of that study did not allow for an estimate of phase-shifting response, but a subsequent study conducted in our laboratory using a modified design has examined both melatonin suppression and phase-shifting responses to a light stimulus following a dim light or a room light background. Preliminary results from that recent study (104) show phase shifting results consistent with the melatonin suppression findings from our earlier report (103).
Studies in circadian photoreceptors suggest a mechanism by which the response observed in human studies may occur. Those studies have demonstrated that the response of those photoreceptors are influenced by prior light history, demonstrating larger responses to light stimuli after dim light exposure, and reduced responsiveness to light stimuli after bright background light exposure (102).
Together, these findings suggest that the overall 24-hour pattern of light and darkness to which humans are exposed plays a role in subsequent sensitivity to light exposure, and thus to entrainment. These findings also suggest that the circadian system of individuals who get little bright light exposure may become more sensitive to moderate levels of light (97). Given that most studies show that modern humans get relatively little bright light exposure and instead spend most of their waking day in light of indoor intensity (76–80), these findings may have very important practical relevance for most humans.
Entrainment of the human circadian system by light
As we outlined above, regular exposure to light and darkness is the primary synchronizer of the human circadian system to the solar day. On average, the period of the human circadian system is longer than 24 hours (64;75;105–112). This means that in order that the circadian system remains in synchrony with the external environment, in most people it must be reset by a small phase advance shift each day. For individuals whose circadian period is shorter than 24 hours, entrainment is achieved through a phase delay shift (136).
Entrainment theory states that the range of entrainment is related to the strength of the synchronizing signal, meaning that a weak synchronizer will be able to entrain individuals whose periods are very close to 24 hours, but a stronger synchronizer is required to entrain those individuals whose periods are further away from 24 hours (35;113). Furthermore, this theory holds that the phase angle of entrainment is related to the strength of the synchronizing signal, and evidence for this had been obtained in animals (113–115).
By the late 1990’s, studies conducted by our group and others had demonstrated that humans show a range of circadian periods close to, but on average slightly longer than 24 hours (64;105–109), and also that humans show differences in phase angle of entrainment (116–118). We had also reported that in young humans there is a relationship between circadian period and phase angle of entrainment (119), in accordance with entrainment theory. We therefore embarked on several studies to explore entrainment in humans.
In the first such study, we examined whether humans could entrain to a very weak synchronizer (light of ∼1.5 lux in the angle of gaze), and tested that ability using three different day lengths (110). We found that most (five of six) subjects tested could entrain to a 24.0–hour day in this very weak synchronizer, but that subjects studied on a 23.5- or 24.5-hour day length did not remain entrained.
We also conducted two other studies in which we examined how phase angle of entrainment in humans is related to circadian period, and how this relationship is affected by light intensity (75;112). In the first of these studies, phase angle was assessed following variety of routines, including a normal routine at home with uncontrolled lighting, following exposure to a very strong synchronizer throughout the waking day for five days, and after 24 hours in very dim (∼1.5 lux in the angle of gaze) light. We found, as in our prior study (119), that phase angle of entrainment is significantly associated with circadian period, such that individuals with shorter periods have a longer interval between evening melatonin onset and usual bedtime than those individuals with longer periods (112). We also found that when a very strong synchronizer was applied, the range of phase angles was reduced, but the relationship between phase angle and period was still present.
In a subsequent entrainment study, we examined the ability of synchronizers of different strengths to entrain human circadian rhythms to a longer than 24-hour day (75). In this study, we first assessed the period of each subject, and then randomized them to one of three different synchronizer strength groups. The synchronizers were then applied during a month when the each subject was scheduled to a day length one hour longer than his circadian period, so that the entrainment challenge was the same for all subjects. We found that most (three of four) subjects living in 25 lux of light were unable to entrain to the imposed day that was one hour longer than their circadian period, but all subjects living under 100 lux of light were able to entrain. As would be predicted by entrainment theory, the subjects who entrained to the longer day showed a different phase angle than they had at the beginning of the study.
Together, these entrainment studies demonstrated that the human circadian system is very much like that of other organisms, and that phase angle of entrainment in humans is strongly influenced by light. This information has implications for understanding and developing treatments for circadian rhythm sleep disorders (120;121).
Summary
As we have outlined above, over the past three decades studies conducted in our laboratory and others have revealed a wealth of information about how the human circadian system is affected by light. The knowledge from these studies has improved our understanding of entrainment of human circadian rhythms to the 24-hour environment, has revealed important insights into circadian rhythm sleep disorders, and has allowed for the design of light treatment regimens for night workers, jet travelers, and patients with circadian rhythm sleep disorders (122–129).
Additional laboratory-based and field studies are still necessary to better understand some features of the human circadian response to light. We are only beginning to understand how prior exposure to light affects the subsequent response to a light stimulus, and our understanding of how light exposure can affect the period of the human circadian system is also limited (135). In addition, very little is known currently about individual differences in circadian sensitivity to light, nor do we understand how polymorphisms in so-called “clock genes” (or other genes) affect sensitivity to light. Furthermore, while some of our knowledge has been translated into light treatment regimens for circadian rhythm disorders, many of the current treatments are impractical, and development and testing of lighting devices and treatment plans that optimize outcomes with shorter and more effective exposures are required. Such studies are time consuming and expensive to conduct in human subjects, but additional well-controlled laboratory-based studies where light response phenotyping and genotyping are conducted in tandem are still necessary to fully understand the effects of light on the human circadian system and therefore translate this knowledge into optimized light treatments.
Acknowledgments
The authors wish to thank the many subjects who participated in the studies reviewed here; the dedicated subject recruitment, technical, and administrative staff of our laboratory whose efforts have made this work possible; the Brigham and Women’s Hospital General Clinical Research Center, where many of the studies were conducted; J.M. Ronda and E.N. Brown; and the many current and former members of the Division of Sleep Medicine who contributed to the work reviewed here, including: J.S. Allan, D.B. Boivin, C. Cajochen, A.M. Chang, D.J. Dijk, J.J. Gooley, C. Gronfier, M.E. Jewett, S.B.S. Khalsa, E.B. Klerman, S.W. Lockley, D.W. Rimmer, M.W. Schoen, N. Santhi, T.L. Shanahan, K.A. Smith, K.P. Wright, Jr., and J.M. Zeitzer. We also wish to thank Professor R.E. Kronauer for his many important contributions to this work, which cannot be overstated.
The studies reviewed here were supported by NIH grants MH45130, AG06072, AG09975, HL077453, HL08978, AT002571; by NASA grants NAG9-524, NAGW-4033, NAG5-3952; by NASA Cooperative Agreement NCC9-58 with the National Space Biomedical Research Institute; and by Air Force Office of Scientific Research grant F49620-94. Many of these studies were conducted in the General Clinical Research Center at Brigham and Women’s Hospital, supported by NIH grant RR02635.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure
Dr. Duffy reports no conflicts of interest. Dr. Czeisler has received consulting fees from or served as a paid member of scientific advisory boards for: Actelion, Ltd.; Avera Pharmaceuticals, Inc.; Axon Labs, Inc.; Cephalon, Inc.; Delta Airlines; Eli Lilly and Co.; Fedex Kinko’s; Garda Inspectorate, Republic of Ireland, Fusion Medical Education, LLC, Hypnion, Inc.; Morgan Stanley; Sanofi-Aventis, Inc.; the Portland Train Blazers; Sleep Multimedia, Inc.; Sleep Research Society (for which Dr. Czeisler served as president); Respironics, Inc.; Koninklijke Philips Electronics, N.V.; Sepracor, Inc.; Somnus Therapeutics, Inc.; Takeda Pharmaceuticals; Vanda Pharmaceuticals, Inc., Vital Issues in Medicine and Warburg-Pincus. Dr. Czeisler also owns an equity interest in Axon Labs, Inc.; Lifetrac, Inc.; Somnus Therapeutics, Inc.; and Vanda Pharmaceuticals, Inc.
Dr. Czeisler has received lecture fees from the Accreditation Council of Graduate Medical Education; Alfresa; Cephalon, Inc.; Clinical Excellence Commission (Australia); Dalhousie University; Duke University Medical Center; Institute of Sleep Health Promotion (NPO); London Deanery; Morehouse School of Medicine; Sanofi-Aventis, Inc.; Takeda; Tanabe Seiyaku Co., Ltd.; Tokyo Electric Power Company (TEPCO).
Dr. Czeisler has also received clinical trial research contracts from Cephalon, Inc., Merck & Co., Inc., and Pfizer, Inc.; an investigator-initiated research grant from Cephalon, Inc.; and his research laboratory at the Brigham and Women’s Hospital has received unrestricted research and education funds and/or support for research expenses from Cephalon, Inc., Koninklijke Philips Electronics, N.V., ResMed, and the Brigham and Women’s Hospital.
The Harvard Medical School Division of Sleep Medicine (HMS/DSM), which Dr. Czeisler directs, has received unrestricted research and educational gifts and endowment funds from: Boehringer Ingelheim Pharmaceuticals, Inc., Cephalon, Inc., George H. Kidder, Esq., Gerald McGinnis, GlaxoSmithKline, Herbert Lee, Hypnion, Jazz Pharmaceuticals, Jordan’s Furniture, Merck & Co., Inc., Peter C. Farrell, Ph.D., Pfizer, ResMed, Respironics, Inc., Sanofi-Aventis, Inc., Sealy, Inc., Sepracor, Inc., Simmons, Sleep Health Centers LLC, Spring Aire, Takeda Pharmaceuticals, Tempur-Pedic, Aetna US Healthcare, Alertness Solutions, Inc., Axon Sleep Research Laboratories, Inc., Boehringer Ingelheim Pharmaceuticals, Inc., Bristol-Myers Squibb, Catalyst Group, Cephalon, Inc., Clarus Ventures, Comfortaire Corporation, Committee for Interns and Residents, Farrell Family Foundation, George H. Kidder, Esq., GlaxoSmithKline, Hypnion, Inc., Innovative Brands Goup, Nature’s Rest, Jordan’s Furniture, King Koil Sleep Products, King Koil, Division of Blue Bell Mattress, Land and Sky, Merck Research Laboratories, MPM Capital, Neurocrine Biosciences, Inc., Orphan Medical/Jazz Pharmaceuticals, Park Place Corporation, Pfizer Global Pharmaceuticals, Pfizer Healthcare Division, Pfizer, Inc., Pfizer/Neurocrine Biosciences, Inc., Purdue Pharma L. P., ResMed, Inc., Respironics, Inc., Sanofi-Aventis, Inc., Sanofi-Synthelabo, Sealy Mattress Company, Sealy, Inc., Sepracor, Inc., Simmons Co., Sleep Health Centers LLC, Spring Air Mattress Co., Takeda Pharmaceuticals, Tempur-Pedic Medical Division, Total Sleep Holdings, Vanda Pharmaceuticals, Inc., and the Zeno Group, together with gifts from many individuals and organizations through an annual benefit dinner.
The HMS/DSM Sleep and Health Education Program has received Educational Grant funding from Cephalon, Inc., Takeda Pharmaceuticals, Sanofi-Aventis, Inc. and Sepracor, Inc.
Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms.
References
- 1.Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 2.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 4.Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic nucleus: the mind's clock. New York: Oxford University Press; 1991. [Google Scholar]
- 5.Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 1998;13(2):100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 6.Welsh DK, Logothetis DE, Meister M, et al. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 7.Moore RY. Retinohypothalamic projection in mammals: a comparative study. Brain Res. 1973;49:403–409. doi: 10.1016/0006-8993(73)90431-9. [DOI] [PubMed] [Google Scholar]
- 8.Sadun AA, Schaechter JD, Smith LEH. A retinohypothalamic pathway in man: light mediation of circadian rhythms. Brain Res. 1984;302:371–377. doi: 10.1016/0006-8993(84)90252-x. [DOI] [PubMed] [Google Scholar]
- 9.Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J Comp Neurol. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- 10.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 11.Hattar S, Liao H-W, Takao M, et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gooley JJ, Lu J, Chou TC, et al. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4(12):1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 14.Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflügers Arch. 2007;454(5):849–855. doi: 10.1007/s00424-007-0242-2. [DOI] [PubMed] [Google Scholar]
- 15.Revell VL, Skene DJ. Light-induced melatonin suppression in humans with polychromatic and monochromatic light. Chronobiol Int. 2007;24(6):1125–1137. doi: 10.1080/07420520701800652. [DOI] [PubMed] [Google Scholar]
- 16.Güler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lydic R, Schoene WC, Czeisler CA, et al. Suprachiasmatic region of the human hypothalamus: homolog to the primate circadian pacemaker? Sleep. 1980;2(3):355–361. doi: 10.1093/sleep/2.3.355. [DOI] [PubMed] [Google Scholar]
- 18.Stopa EG, King JC, Lydic R, et al. Human brain contains vasopressin and vasoactive intestinal polypeptide neuronal subpopulations in the suprachiasmatic region. Brain Res. 1984;297:159–163. doi: 10.1016/0006-8993(84)90553-5. [DOI] [PubMed] [Google Scholar]
- 19.Friedman DI, Johnson JK, Chorsky RL, et al. Labeling of human retinohypothalamic tract with the carbocyanine dye, DiI. Brain Res. 1991;560:297–302. doi: 10.1016/0006-8993(91)91246-w. [DOI] [PubMed] [Google Scholar]
- 20.Moore RY, Speh JC. A putative retinohypothalamic projection containing substance P in the human. Brain Res. 1994;659:249–253. doi: 10.1016/0006-8993(94)90887-7. [DOI] [PubMed] [Google Scholar]
- 21.Dai J, Swaab DF, Buijs RM. Distribution of vasopressin and vasoactive intestinal polypeptide (VIP) fibers in human hypothalamus with special emphasis on suprachiasmatic nucleus efferent projections. J Comp Neurol. 1997;383:397–414. doi: 10.1002/(sici)1096-9861(19970714)383:4<397::aid-cne1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 22.Dai J, van der Vliet J, Swaab DF, et al. Human retinohypothalamic tract as revealed by in vitro postmortem tracing. J Comp Neurol. 1998;397:357–370. [PubMed] [Google Scholar]
- 23.Provencio I, Rodriguez IR, Jiang G, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20(2):600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaidi FH, Hull JT, Peirson SN, et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17(24):2122–2128. doi: 10.1016/j.cub.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hastings JW, Sweeney BM. A persistent diurnal rhythm of luminescence in Gonyaulax polyedra. Biol Bull. 1958;115:440–458. [Google Scholar]
- 26.DeCoursey PJ. Daily light sensitivity rhythm in a rodent. Science. 1960;131:33–35. doi: 10.1126/science.131.3392.33. [DOI] [PubMed] [Google Scholar]
- 27.Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol [A] 1976;106:253–266. [Google Scholar]
- 29.Wever R. Zur Zeitgeber-Stärke eines Licht-Dunkel-Wechsels für die circadiane Periodik des Menschen. Pfluegers Arch. 1970;321:133–142. doi: 10.1007/BF00586368. [DOI] [PubMed] [Google Scholar]
- 30.Aschoff J, Fatranská M, Giedke H, et al. Human circadian rhythms in continuous darkness: entrainment by social cues. Science. 1971;171:213–215. doi: 10.1126/science.171.3967.213. [DOI] [PubMed] [Google Scholar]
- 31.Czeisler CA, Allan JS, Strogatz SH, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 32.Czeisler CA, Kronauer RE, Allan JS, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 33.Allan JS, Czeisler CA. Persistence of the circadian thyrotropin rhythm under constant conditions and after light-induced shifts of circadian phase. J Clin Endocrinol Metab. 1994;79:508–512. doi: 10.1210/jcem.79.2.8045970. [DOI] [PubMed] [Google Scholar]
- 34.Jewett ME, Rimmer DW, Duffy JF, et al. Human circadian pacemaker is sensitive to light throughout subjective day without evidence of transients. Am J Physiol. 1997;273:R1800–R1809. doi: 10.1152/ajpregu.1997.273.5.r1800. [DOI] [PubMed] [Google Scholar]
- 35.Johnson CH. Phase response curves: what can they tell us about circadian clocks? In: Hiroshige T, Honma K, editors. Circadian clocks from cell to human. Sapporo: Hokkaido Univ. Press; 1992. pp. 209–249. [Google Scholar]
- 36.Winfree AT. Integrated view of resetting a circadian clock. J Theor Biol. 1970;28:327–374. doi: 10.1016/0022-5193(70)90075-5. [DOI] [PubMed] [Google Scholar]
- 37.Winfree AT. Resetting the amplitude of Drosophila's circadian chronometer. J Comp Physiol. 1973;85:105–140. [Google Scholar]
- 38.Winfree AT. New York: Springer-Verlag; 1980. The geometry of biological time. [Google Scholar]
- 39.Kronauer RE, Jewett ME, Czeisler CA. Commentary: The human circadian response to light - Strong and weak resetting. J Biol Rhythms. 1993;8:351–360. doi: 10.1177/074873049300800409. [DOI] [PubMed] [Google Scholar]
- 40.Jewett ME, Kronauer RE, Czeisler CA. Light-induced suppression of endogenous circadian amplitude in humans. Nature. 1991;350:59–62. doi: 10.1038/350059a0. [DOI] [PubMed] [Google Scholar]
- 41.Lakin-Thomas PL. Commentary: Strong or weak phase resetting by light pulses in humans? J Biol Rhythms. 1993;8(4):348–350. doi: 10.1177/074873049300800408. [DOI] [PubMed] [Google Scholar]
- 42.Khalsa SBS, Jewett ME, Cajochen C, et al. A phase response curve to single bright light pulses in human subjects. J Physiol (Lond) 2003;549(Pt 3):945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lockley SW, Gooley JJ, Kronauer RE, et al. Phase response curve to single one-hour pulses of 10,000 lux bright white light in humans. Abstracts, Soc Res Biol Rhythms. 2006 [Google Scholar]
- 44.Peterson EL. A limit cycle interpretation of a mosquito circadian oscillator. J Theor Biol. 1980;84:281–310. doi: 10.1016/s0022-5193(80)80008-7. [DOI] [PubMed] [Google Scholar]
- 45.Czeisler CA, Wright KP., Jr . Influence of light on circadian rhythmicity in humans. In: Turek FW, Zee PC, editors. Neurobiology of Sleep and Circadian Rhythms. New York: Marcel Dekker, Inc; 1999. pp. 149–180. [Google Scholar]
- 46.Pohl H. Characteristics and variability in entrainment of circadian rhythms to light in diurnal rodents. In: Aschoff J, Daan S, Groos GA, editors. Vertebrate circadian systems: Structure and physiology. Berlin: Springer-Verlag; 1982. pp. 339–346. [Google Scholar]
- 47.Khalsa SBS, Jewett ME, Duffy JF, et al. The timing of the human circadian clock is accurately represented by the core body temperature rhythm following phase shifts to a three-cycle light stimulus near the critical zone. J Biol Rhythms. 2000;15(6):524–530. doi: 10.1177/074873040001500609. [DOI] [PubMed] [Google Scholar]
- 48.Johnson CH. Nashville, TN: Department of Biology, Vanderbilt University; 1990. An atlas of phase response curves for circadian and circatidal rhythms. [Google Scholar]
- 49.Brainard GC, Richardson BA, King TS, et al. The suppression of pineal melatonin content and N-acetyltransferase activity by different light irradiances in the Syrian hamster: a dose-response relationship. Endocrinology. 1983;113(1):293–296. doi: 10.1210/endo-113-1-293. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi JS, DeCoursey PJ, Bauman L, et al. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- 51.Joshi D, Chandrashekaran MK. Light flashes of different durations (0.063–3.33 msec) phase shift the circadian flight activity of a bat. J Exp Zool. 1985;233:187–192. doi: 10.1002/jez.1402330204. [DOI] [PubMed] [Google Scholar]
- 52.Nelson DE, Takahashi JS. Comparison of visual sensitivity for suppression of pineal melatonin and circadian phase-shifting in the golden hamster. Brain Res. 1991;554:272–277. doi: 10.1016/0006-8993(91)90200-f. [DOI] [PubMed] [Google Scholar]
- 53.Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) J Physiol. 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bauer MS. Irradiance responsivity and unequivocal type-1 phase responsivity of rat circadian activity rhythms. Am J Physiol. 1992;263:R1110–R1114. doi: 10.1152/ajpregu.1992.263.5.R1110. [DOI] [PubMed] [Google Scholar]
- 55.Sharma VK, Chandrashekaran MK, Singaravel M, et al. Relationship between light intensity and phase resetting in a mammalian circadian system. Journal of Experimental Zoology. 1999;283:181–185. doi: 10.1002/(sici)1097-010x(19990201)283:2<181::aid-jez8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 56.Lewy AJ, Wehr TA, Goodwin FK, et al. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 57.Bojkowski CJ, Aldhous ME, English J, et al. Suppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in man. Horm Metab Res. 1987;19:437–440. doi: 10.1055/s-2007-1011846. [DOI] [PubMed] [Google Scholar]
- 58.Brainard GC, Lewy AJ, Menaker M, et al. Dose-response relationship between light irradiance and the suppression of plasma melatonin in human volunteers. Brain Res. 1988;454:212–218. doi: 10.1016/0006-8993(88)90820-7. [DOI] [PubMed] [Google Scholar]
- 59.McIntyre IM, Norman TR, Burrows GD, et al. Human melatonin suppression by light is intensity dependent. J Pineal Res. 1989;6:149–156. doi: 10.1111/j.1600-079x.1989.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 60.Allan JS, Czeisler CA, Duffy JF, et al. Non-linear dose response of the human circadian pacemaker to light. 154th Annual AAAS Meeting, AAAS Publication No.897-30, 101.1988. [Google Scholar]
- 61.Boivin DB, Duffy JF, Kronauer RE, et al. Sensitivity of the human circadian pacemaker to moderately bright light. J Biol Rhythms. 1994;9(3–4):315–331. doi: 10.1177/074873049400900311. [DOI] [PubMed] [Google Scholar]
- 62.Boivin DB, Duffy JF, Kronauer RE, et al. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 63.Zeitzer JM, Khalsa SB, Boivin DB, et al. Temporal dynamics of late-night photic stimulation of the human circadian timing system. Am J Physiol. 2005;289(3):R839–R844. doi: 10.1152/ajpregu.00232.2005. [DOI] [PubMed] [Google Scholar]
- 64.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 65.Duffy JF, Kronauer RE, Czeisler CA. Phase-shifting human circadian rhythms: influence of sleep timing, social contact and light exposure. J Physiol (Lond) 1996;495(1):289–297. doi: 10.1113/jphysiol.1996.sp021593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeitzer JM, Dijk DJ, Kronauer RE, et al. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol (Lond) 2000;526(3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28:799–807. doi: 10.1016/j.neurobiolaging.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cajochen C, Zeitzer JM, Czeisler CA, et al. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115(1):75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 69.van den Pol AN, Cao V, Heller HC. Circadian system of mice integrates brief light stimuli. Am J Physiol. 1998;275:R654–R657. doi: 10.1152/ajpregu.1998.275.2.R654. [DOI] [PubMed] [Google Scholar]
- 70.Rimmer DW, Boivin DB, Shanahan TL, et al. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol. 2000;279(5):R1574–R1579. doi: 10.1152/ajpregu.2000.279.5.R1574. [DOI] [PubMed] [Google Scholar]
- 71.Gronfier C, Wright KP, Jr, Kronauer RE, et al. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol. 2004;287:E174–E181. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kronauer RE, Forger DB, Jewett ME. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the photopic range. J Biol Rhythms. 1999;14(6):500–515. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- 73.Kronauer RE, Forger DB, Jewett ME. Errata: Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the photopic range. J Biol Rhythms. 2000;15(2):184–186. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- 74.Gronfier C, Wright KP, Jr, Czeisler CA. Time course of melatonin suppression in response to intermittent bright light exposure in humans. J Sleep Res. 2002;11(S1):86–87. [Google Scholar]
- 75.Gronfier C, Wright KP, Jr, Kronauer RE, et al. Entrainment of the human circadian pacemaker to longer-than-24h days. Proc Natl Acad Sci USA. 2007;104(21):9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cole RJ, Kripke DF, Wisbey J, et al. Seasonal variation in human illumination exposure at two different latitudes. J Biol Rhythms. 1995;10(4):324–334. doi: 10.1177/074873049501000406. [DOI] [PubMed] [Google Scholar]
- 77.Hébert M, Dumont M, Paquet J. Seasonal and diurnal patterns of human illumination under natural conditions. Chronobiol Int. 1998;15(1):59–70. doi: 10.3109/07420529808998670. [DOI] [PubMed] [Google Scholar]
- 78.Laffan AM, Duffy JF. Light exposure patterns in healthy young and older adults. Sleep. 2002;25:A307–A308. doi: 10.1177/0748730410361916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawinska A, Dumont M, Selmaoui B, et al. Are modifications of melatonin circadian rhythm in the middle years of life related to habitual patterns of light exposure? J Biol Rhythms. 2005;20(5):451–460. doi: 10.1177/0748730405280248. [DOI] [PubMed] [Google Scholar]
- 80.Scheuermaier K, Laffan AM, Duffy JF. Light exposure patterns in healthy older people living in New England, USA. J Sleep Res. 2006;15(S1):94. [Google Scholar]
- 81.Illnerová H, Buresová M, Nedvídková J, et al. Maintenance of a circadian phase adjustment of the human melatonin rhythm following artificial long days. Brain Res. 1993;626:322–326. doi: 10.1016/0006-8993(93)90595-e. [DOI] [PubMed] [Google Scholar]
- 82.Vondrasová D, Hájek I, Illnerová H. Exposure to long summer days affects the human melatonin and cortisol rhythms. Brain Res. 1997;759:166–170. doi: 10.1016/s0006-8993(97)00358-2. [DOI] [PubMed] [Google Scholar]
- 83.Louzada F, Inacio AM, Souza FHM, et al. Exposure to light versus way of life: effects on sleep patterns of a teenager-case report. Chronobiol Int. 2004;21(3):497–499. doi: 10.1081/cbi-120038743. [DOI] [PubMed] [Google Scholar]
- 84.Foster RG, Provencio I, Hudson D, et al. Circadian photoreception in the retinally degenerate mouse (rd/rd) J Comp Physiol [A] 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- 85.Freedman MS, Lucas RJ, Soni B, et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 86.Lucas RJ, Freedman MS, Muñoz M, et al. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 87.Lucas RJ, Foster RG. Neither functional rod photoreceptors nor rod or cone outer segments are required for the photic inhibition of pineal melatonin. Endocrinology. 1999;140(4):1520–1524. doi: 10.1210/endo.140.4.6672. [DOI] [PubMed] [Google Scholar]
- 88.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535(1):261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Czeisler CA, Shanahan TL, Klerman EB, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332(1):6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 91.Klerman EB, Shanahan TL, Brotman DJ, et al. Photic resetting of the human circadian pacemaker in the absence of conscious vision. J Biol Rhythms. 2002;17:548–555. doi: 10.1177/0748730402238237. [DOI] [PubMed] [Google Scholar]
- 92.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88(9):4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 93.Lockley SW, Evans EE, Scheer FAJL, et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29(2):161–168. [PubMed] [Google Scholar]
- 94.Zeitzer JM, Kronauer RE, Czeisler CA. Photopic transduction implicated in human circadian entrainment. Neurosci Lett. 1997;232:135–138. doi: 10.1016/s0304-3940(97)00599-5. [DOI] [PubMed] [Google Scholar]
- 95.Czeisler CA, Gooley JJ. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol. 2007;72:579–597. doi: 10.1101/sqb.2007.72.064. [DOI] [PubMed] [Google Scholar]
- 96.Meijer JH, Rusak B, Ganshirt G. The relation between light-induced discharge in the suprachiasmatic nucleus and phase shifts of hamster circadian rhythms. Brain Res. 1992;598:257–263. doi: 10.1016/0006-8993(92)90191-b. [DOI] [PubMed] [Google Scholar]
- 97.Owen J, Arendt J. Melatonin suppression in human subjects by bright and dim light in Antarctica: time and season-dependent effects. Neurosci Lett. 1992;137:181–184. doi: 10.1016/0304-3940(92)90399-r. [DOI] [PubMed] [Google Scholar]
- 98.Nelson DE, Takahashi JS. Integration and saturation within the circadian photic entrainment pathway of hamsters. Am J Physiol. 1999;277(46):R1351–R1361. doi: 10.1152/ajpregu.1999.277.5.R1351. [DOI] [PubMed] [Google Scholar]
- 99.Aggelopoulos NC, Meissl H. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J Physiol. 2000;523(1):211–222. doi: 10.1111/j.1469-7793.2000.t01-1-00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Refinetti R. Dark adaptation in the circadian system of the mouse. Physiol Behav. 2001;74:101–107. doi: 10.1016/s0031-9384(01)00546-7. [DOI] [PubMed] [Google Scholar]
- 101.Hébert M, Martin SK, Lee C, et al. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48(6):1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 103.Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- 104.Chang A-M, Scheer FA, Czeisler CA. Adaptation of the human circadian system by prior light history. Sleep. 2008;31(S):A45–A46. [Google Scholar]
- 105.Wyatt JK, Ritz-De Cecco A, Czeisler CA, et al. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 106.Waterhouse J, Minors D, Folkard S, et al. Light of domestic intensity produces phase shifts of the circadian oscillator in humans. Neurosci Lett. 1998;245:97–100. doi: 10.1016/s0304-3940(98)00174-8. [DOI] [PubMed] [Google Scholar]
- 107.Kelly TL, Neri DF, Grill JT, et al. Nonentrained circadian rhythms of melatonin in submariners scheduled to an 18-hour day. J Biol Rhythms. 1999;14(3):190–196. doi: 10.1177/074873099129000597. [DOI] [PubMed] [Google Scholar]
- 108.Carskadon MA, Labyak SE, Acebo C, et al. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci Lett. 1999;260:129–132. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- 109.Middleton B, Arendt J, Stone BM. Human circadian rhythms in constant dim light (8 lux) with knowledge of clock time. J Sleep Res. 1996;5:69–76. doi: 10.1046/j.1365-2869.1996.d01-67.x. [DOI] [PubMed] [Google Scholar]
- 110.Wright KP, Jr, Hughes RJ, Kronauer RE, et al. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci USA. 2001;98(24):14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wyatt JK, Cajochen C, Ritz-De Cecco A, et al. Low-dose, repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27(3):374–381. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- 112.Wright KP, Jr, Gronfier C, Duffy JF, et al. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20(2):168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pittendrigh CS, Dann S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as clock. J Comp Physiol [A] 1976;106:291–331. [Google Scholar]
- 114.Hoffmann K. Zur beziehung zwischen phasenlage und spontanfrequenz bei der endogenen tagesperiodik. Z Naturforsch. 1963;18:154–157. [Google Scholar]
- 115.Sharma VK, Chandrashekaran MK, Singaravel M. Relationship between period and phase angle differences in Mus booduga under abrupt versus gradual light-dark transitions. Naturwissenschaften. 1998;85:183–186. doi: 10.1007/s001140050481. [DOI] [PubMed] [Google Scholar]
- 116.Duffy JF, Dijk DJ, Klerman EB, et al. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275:R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 117.Duffy JF, Dijk DJ, Hall EF, et al. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- 118.Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- 119.Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115(4):895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- 120.Jones CR, Campbell SS, Zone SE, et al. Familial advanced sleep-phase syndrome: a short-period circadian rhythm variant in humans. Nat Med. 1999;5(9):1062–1065. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 121.Wyatt JK, Stepanski EJ, Kirkby J. Circadian phase in delayed sleep phase syndrome: predictors and temporal stability across multiple assessments. Sleep. 2006;29(8):1075–1080. doi: 10.1093/sleep/29.8.1075. [DOI] [PubMed] [Google Scholar]
- 122.Czeisler CA, Johnson MP, Duffy JF, et al. Exposure to bright light and darkness to treat physiologic maladaptation to night work. N Engl J Med. 1990;322:1253–1259. doi: 10.1056/NEJM199005033221801. [DOI] [PubMed] [Google Scholar]
- 123.Terman M, Lewy AJ, Dijk DJ, et al. Light treatment for sleep disorders: consensus report.IV. Sleep phase and duration disturbances. J Biol Rhythms. 1995;10(2):135–147. doi: 10.1177/074873049501000206. [DOI] [PubMed] [Google Scholar]
- 124.Boulos Z, Campbell SS, Lewy AJ, et al. Light treatment for sleep disorders: consensus report. VII. Jet lag. J Biol Rhythms. 1995;10(2):167–176. doi: 10.1177/074873049501000209. [DOI] [PubMed] [Google Scholar]
- 125.Eastman CI, Boulos Z, Terman M, Light treatment for sleep disorders: consensus reportVI, et al. Shift Work. J Biol Rhythms. 1995;10(2):157–164. doi: 10.1177/074873049501000208. [DOI] [PubMed] [Google Scholar]
- 126.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007;30(11):1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Horowitz TS, Cade BEWolfeJM, Wolfe JM, et al. Efficacy of bright light and sleep/darkness scheduling in alleviating circadian maladaptation to night work. Am J Physiol. 2001;281:E384–E391. doi: 10.1152/ajpendo.2001.281.2.E384. [DOI] [PubMed] [Google Scholar]
- 128.Santhi N, Duffy JF, Horowitz TS, et al. Scheduling of sleep/darkness affects the circadian phase of night shift workers. Neurosci Lett. 2005;384(3):316–320. doi: 10.1016/j.neulet.2005.04.094. [DOI] [PubMed] [Google Scholar]
- 129.Santhi N, Duffy JF, Horowitz TS, et al. Erratum to "Scheduling of sleep/darkness affects the circadian phase of night shift workers". Neurosci Lett. 2005;390:187. doi: 10.1016/j.neulet.2005.04.094. [DOI] [PubMed] [Google Scholar]
- 130.Cain SW, Rimmer DW, Duffy JF, et al. Exercise distributed across day and night does not alter circadian period in humans. J Biol Rhythms. 2007;22(6):534–541. doi: 10.1177/0748730407306884. [DOI] [PubMed] [Google Scholar]
- 131.Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- 132.Honma K, Honma S. A human phase response curve for bright light pulses. Jpn J Psychiatry Neurol. 1988;42(1):167–168. [Google Scholar]
- 133.Baehr EK, Fogg LF, Eastman CI. Intermittent bright light and exercise to entrain human circadian rhythms to night work. Am J Physiol. 1999;277(6 Pt 2):R1598–R1604. doi: 10.1152/ajpregu.1999.277.6.R1598. [DOI] [PubMed] [Google Scholar]
- 134.Smith MR, Cullnan EE, Eastman CI. Shaping the light/dark pattern for circadian adaptation to night shift work. Physiol Behav. 2008;95(3):449–456. doi: 10.1016/j.physbeh.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 135.Scheer FA, Wright KP, Jr, Kronauer RE, et al. Plasticity of the intrinsic period of the human circadian timing system. PLoS ONE. 2007;2(8):e721. doi: 10.1371/journal.pone.0000721. doi:10.1371/journal.pone. 0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duffy JF, Wright KP., Jr Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20(4):326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]