Abstract
The mucosal immune system in the upper female reproductive tract is uniquely prepared to maintain a balance between the presence of commensal bacteria, sexually transmitted bacterial and viral pathogens, allogeneic spermatozoa, and an immunologically distinct fetus. At the center of this dynamic system are the epithelial cells that line the Fallopian tubes, uterus, cervix and vagina. Epithelial cells provide a first line of defense that confers continuous protection, by providing a physical barrier as well as secretions containing bactericidal and virucidal agents. In addition to maintaining a state of ongoing protection, these cells have evolved to respond to pathogens, in part through Toll-like receptors (TLRs), to enhance innate immune protection and, when necessary, to contribute to the initiation of an adaptive immune response. Against this backdrop, epithelial cell innate and adaptive immune function is modulated to meet the constraints of procreation. The overall goal of this review is to focus on the dynamic role of epithelial cells in the upper reproductive tract, with special emphasis on the uterus, to define the unique properties of these cells as they maintain homeostasis in preparation for successful fertilization and pregnancy while at the same time confer protection against sexually transmitted infections, which threaten to compromise women’s reproductive health and survival. By understanding the nature of this protection and the ways in which innate and adaptive immunity are regulated by sex hormones, these studies provide the opportunity to contribute to the foundation of information essential for ensuring reproductive health.
Keywords: FRT, female reproductive tract; APC, antigen presenting cell; PPR, pattern recognition receptors; PAMPs, pathogen-associated microbial patterns; TLRs, Toll-like receptors; SLPI, Secretory Leukocyte Protease Inhibitor
I. INTRODUCTION
Among the life-threatening sexually transmitted diseases that have afflicted women, Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) is unique in human history in its rapid spread, its extent, and the depth of its impact. Approaching 25 million deaths worldwide with an additional 33.2 million (of which 15.4 million are women) estimated to be infected worldwide, HIV/AIDS will soon be the world’s worst pandemic [1]. Since the 1980s, HIV has shifted from a disease spread predominantly through needles and male-male contact to a sexually transmitted disease in which women worldwide are more likely to be infected than men. Each year brings an increase in the number of women infected with HIV. In particular, women and girls make up almost 57% of all people infected with HIV in Sub-Saharan Africa, where a striking 76% of young people (aged 15–24 years) living with HIV are female [1].
The failures of the Diaphragm trial, the Merck vaccine trial and the Microbicide gel trial [2–6], along with the recognition that for each person treated with anti-retroviral drugs, six people are newly infected with HIV [7], emphasizes the need to more fully understand the components of immune protection that exist within the reproductive tract of women and the ways in which they respond to changes in hormone balance. The mucosal immune system in the reproductive tract is the first line of defense against pathogenic organisms [8–10]. Unlike other mucosal surfaces throughout the body, the immune system in the female reproductive tract (FRT) has evolved to meet the unique requirements of balancing immune protection against sexually transmitted bacterial and viral pathogens, while being supportive to allogeneic spermatozoa and an immunologically distinct fetus [11,12]. In response to these requirements, the immune system in the FRT, which consists of both innate and adaptive immune components, is responsive to and precisely regulated by the female sex hormones, estradiol (E2) and progesterone (P), both of which are produced in a cyclic fashion by the ovary over the course of the menstrual cycle. In preparing the reproductive tract for fertilization and implantation, E2 and P simultaneously modulate the immune system in the Fallopian tubes, uterus, cervix and vagina to compliment the reproductive process (See [13] for review).
Recognizing that most but not all cells in the body, including those in the immune system, have E2 and P receptors, we have explored the mechanisms of hormone action and concluded that some effects of sex hormones are indirect and mediated through signals generated by one cell to another. As discussed in detail in the following sections, we and others have found that epithelial cells that line the lumen of the entire FRT are key players in immune protection and cell-cell communication. These cells are responsive to both the direct effects of sex hormones as well as the indirect effects. Through this dynamic balance, epithelial cells in the FRT protect by providing a physical barrier, secreting antimicrobials and transporting immunoglobulins (IgA and IgG) into FRT secretions, as well as by signaling the recruitment and activation of cells of the immune system. The effectiveness of this protection, which is initiated with the recognition of commensals and pathogens including HIV-1, has led to the recognition of epithelial cells as sentinels, the functions of which are now only being recognized (See [13] for review).
This chapter will review current knowledge regarding the sentinel role of epithelial cells in the Fallopian tubes, uterus and cervix with special emphasis on uterine cells, especially as it pertains to protection against genital tract pathogens, and highlight some of the unique responses of these cells to the direct and indirect actions of E2 and P.
II. EPITHELIAL CELL TIGHT JUNCTIONS
Epithelial cells throughout the FRT provide a physical barrier to protect against microbial infection and still permit the transport of sperm, ovum or conceptus. Stratified squamous epithelial cells lining both the vagina and the ectocervix are loosely connected. In contrast to the lower FRT, the upper FRT is lined with columnar epithelial cells that form tight junctions to maintain the integrity of the mucosal monolayer in the endocervix, uterus and Fallopian tubes. Tight junctions are intercellular connections between adjacent cells that restrict mixing of apical and basolateral compartments as well as control paracellular permeability across epithelial monolayers [14,15]. Tight junctions consist of transmembrane and peripheral membrane proteins interacting to form a complex adhesion network (See [16,17] for review). For example, the transmembrane claudins interact with the cellular occludin for structural integrity of the tight junction. These and other tight junction proteins are involved in other cell processes such as proliferation and differentiation. Aberrations in these proteins contribute to pathological conditions, including cancer.
Transepithelial resistance
In transwell-type cell culture system, epithelial cells generate an electrochemical gradient across a monolayer that reflects barrier function of the tight junctions [18], the integrity of the barrier can be measured as transepithelial resistance (TER) [19]. Achievement of high TER in primary cell cultures isolated from tissues indicates a confluent monolayer of columnar epithelial cells devoid of other cell types. We have isolated the epithelial cells from human, mouse and rat uteri and grown them to high TER in cell inserts [20–22]. A ten- to thirty-fold increase in TER above background is commonly achieved with primary cultures of uterine epithelial cells. Disruption of the tight junctions or damage to the epithelial layer can lead to infection, resulting in infertility and potential life-threatening illness. The tight junction barrier permits epithelial cells to respond to different stimuli and serve as a directional conduit for different factors. For example, the epithelial cell polymeric immunoglobulin receptor (pIgR) traverses the epithelial cell from the basolateral side to the apical side to release IgA into the lumen (See [23] for review). In other studies, uterine epithelial cells preferentially secrete cytokines such as Transforming Growth Factor beta (TGFβ̣) at the basolateral surface and Tumor Necrosis Factor alpha (TNFα) at the apical surface [22].
The epithelial selective semi-permeable barrier at mucosal surfaces is regulated by many factors including calcium, cytokines, growth factors, microorganisms and steroid hormones. Fluctuating concentrations of sex steroid hormones are a primary influence for the architectural and functional changes that occur during the menstrual cycle in preparation for the implantation of a conceptus. Estradiol is the dominant hormone during the proliferative phase, whereas during the secretory phase and pregnancy both E2 and P are elevated [24]. Nuclear and surface membrane receptors for estrogens have been identified. In the nucleus, both α- and β- receptors for estrogen are present in uterine epithelial cells. Physiological concentrations of E2 significantly reduce the TER of mouse epithelial cells [25,26]. With primary human uterine epithelial cells, concentrations of E2 as a 10% fetal bovine serum (FBS) supplement in media reduce TER by 10–20% relative to cells grown in media supplemented with stripped FBS that has been charcoal-dextran treated to remove sex steroid hormones (unpublished results). The FBS-induced reduction in TER was circumvented by pretreatment of the cells with the estrogen receptor antagonist, ICI 182,780, indicating that epithelial monolayer integrity is directly influenced by E2 via its receptor. Estradiol has also been shown to disrupt adheren junctions in endothelial cells [27] and to decrease TER in porcine vas deferens epithelia [28].
Modulation of tight junctions by microbes and TLR agonists
At mucosal surfaces epithelial cells are the first cells exposed to potentially pathogenic microorganisms. Human uterine and Fallopian tube epithelial cells express TLR 1 through 9 and therefore are prepared to respond to a range of pathogens that include viruses, bacteria and fungi [29,30]. Infection with one microorganism can often lead to secondary infection with another; if a microorganism can negatively affect TER, then it is more likely that infection may ensue for that microorganism as well as other pathogenic/opportunistic microorganisms. TLR agonists can have effects on epithelial cell TER. For example, Cario and associates showed that a TLR2 ligand enhances TER and barrier integrity of human intestinal epithelial cell lines [31]. On the other hand, lipopolysaccharide (LPS), a TLR4 ligand characteristic of gram negative bacterial pathogens, decreased TER and/or increased permeability in human corneal epithelial cells [32], rat retinal pigment epithelial cells [33], a human intestinal cell line [34], and rat small intestine epithelial cells [35]. Treatments with pathogenic microbes typically have deleterious effects on barrier integrity. Helicobacter pylori, a major cause of ulcers, decreased TER and barrier function in human intestinal epithelial cell lines [36,37]. Similar effects were observed with polarized epithelial cell monolayers treated with rotavirus [38], Salmonella enterica [39], and enteropathogenic Escherichia coli [40]. Some of these effects may be mediated by cytokines induced by a TLR agonist or pathogen. For example, TNF α caused an initial reduction in TER and/or barrier function of small intestine epithelial cells [41]. In mouse uterine epithelial cells, TNFα increased TER in a dose-dependent manner, while TGFβ suppressed TER [25]. In other studies, it was shown that commensals inhibit the reduction in TER caused by invasive pathogens [42,43].
Tight junctions with microbicides and vaccines
Topical microbicides to prevent HIV and herpes simplex virus (HSV) infection have considerable potential. However, the microbicides must be safe; that is, not toxic to the epithelial cells. The detergent Nonoxynol 9 is a topical microbicide that was initially thought to be effective in inhibiting HIV infection, but its toxicity to the epithelial cells resulted in breaches of the barrier and may have led to an increase in HIV infection by users [44]. Measuring epithelial barrier function by TER or permeability is one way to ascertain if a proposed topical microbicide is cytotoxic [45]. In addition, TER can be measured to test the potential cytotoxic effects of antimicrobial peptides on tight junctions prior to use in clinical situations [46]. Alternatively, increasing mucosal permeability may be an effective tool in vaccines. Marinaro and colleagues used zona occludens toxin to enhance delivery of antigen through mucosal immunization of mice [47]. The toxin reversibly disrupted tight junctions and demonstrated its effectiveness as a mucosal adjuvant in protecting mice from a systemic challenge of antigen. In summary, the tight junctions between columnar epithelial cells of the upper FRT help to prevent infection by microbial pathogens. Barrier protection is essential to the maintenance of proper physiological function in the uterus.
III. GROWTH FACTORS
In the uterus, proper endometrial function is dependent on stromal fibroblast-epithelial cell interactions. Both E2 and P regulate the environment of the uterus. These sex hormones can either act directly on epithelial cells or act indirectly through the underlying stromal fibroblast secretion of soluble factors [48]. For example, when vaginal epithelia from neonatal mice are grown in the presence of uterine stroma, the epithelia develop into a uterine-like structure [49]. Furthermore, it has been shown that in vivo E2 treatment of neonatal mice, which do not express the nuclear estrogen receptor-α (ERα), stimulated uterine epithelial cell proliferation [50]. This led to the hypothesis that E2 stimulates mitogenesis of uterine epithelial cells indirectly via the underlying stroma, which is ERα positive. To test this hypothesis, studies using ERα knockout and wild-type mice demonstrated that uterine epithelial cell mitogenesis was dependent on the indirect effect of E2 on stromal fibroblasts positive for ERα and that epithelial cell ERα was not necessary for E2-mediated epithelial cell proliferation [51].
Possible mediators of these indirect estrogenic effects on uterine epithelium are growth factors. Many growth factors have been identified within the FRT and are involved in normal cyclical changes that occur. Growth factors can induce rapid cell proliferation as well as rapid changes in cell morphology and function [48]. Several growth factor-growth factor receptor systems, including epidermal growth factor (EGF), insulin-like growth factor-I (IGF-I), hepatocyte growth factor (HGF), and keratinocyte growth factor (KGF) have been identified within the uterus and shown to be involved in stromal fibroblast-epithelial cell interactions [48,50,51]. Interactions between E2 and growth factors are complex and involve bidirectional cross-talk between ligands and receptors to influence the epithelial differentiation, growth and function.
Epidermal Growth Factor
EGF is among the most studied growth factors within the uterus and appears to be involved in stromal fibroblast-epithelial cell interactions as well as function as a mediator of E2 effects [48,52,53]. EGF elicits its effects by binding to the EGF receptor (EGFR) on the basolateral surface of epithelial cells [54]. Studies have shown that EGF expression fluctuates during the menstrual cycle with expression highest during the late proliferative and secretory phases [55,56]. In contrast, Ejskjaer et al. were unable to detect EGF in endometrial samples [57]. This discrepancy may be due to different collection times in that Ejskjaer et al. did not examine any samples during ovulation [55,57].
Within the uterus both EGF and EGFR are regulated by E2. For example, E2 not only increased the bioactivity of EGF, but also increased expression of EGFR [48]. Both EGF and E2 alone stimulate uterine epithelial cell proliferation and a relationship between EGFR signaling and ERα signaling has been shown [52,53]. Ovariectomized mice treated with EGF alone increased uterine epithelial cell proliferation resembling that seen with E2 alone [48]. It has also been demonstrated that blocking EGF activity inhibits E2-induced uterine epithelial cell proliferation [48]. Conversely, blocking of the ER attenuates the uterine response to EGF. Furthermore, EGF is unable to elicit a proliferative response in the uterus of mice lacking ERα [52,53]. Further evidence for EGFR and ERα cross-talk has been studied in breast cancer. Breast cancer cells treated with E2 have increased expression of EGFR, thus sensitizing the breast cancer cell to EGF activity [58].
Insulin-like Growth Factor-I
IGF-I is present in tissues throughout the body and is involved in many normal developmental processes. IGF-I can function in either an autocrine or paracrine manner via signaling through IGF-I receptor (IGF-IR) [59,60]. Bioactivity of IGF-I is dependent on insulin-like growth factor binding proteins (IGFBP). The availability of IGF-I to bind to its receptor is regulated by these IGFBPs, which can inhibit or augment IGF-I actions [61]. IGF-I seems to localize throughout the endometrial stromal fibroblast layer while IGF-IR is present within the endometrial epithelium [61]. There is evidence suggesting that IGF-I acts as a mediator of E2 within the uterus. Mice lacking IGF-I are infertile and have underdeveloped uteri similar to that of mice lacking ERα [62]. Similar to EGFR in breast cancer cells, increased IGF-IR has been demonstrated following E2 treatment, thus sensitizing the breast cancer cells to 11 IGF-I activity [58]. Tissue IGF-I levels peak during the late proliferative and early secretory phases of the menstrual cycle with lowest levels during mid- and late secretory phases as well as during early pregnancy [61]. Others have shown that ERα is essential for IGF-I-induced proliferation of mouse uterine epithelial cells. IGF-I levels and IGF-IR activity increase in response to E2 treatment [53], whereas blocking of ERα results in an inhibition of IGF-I mRNA expression [48]. Conversely, IGF-I signaling has been shown to increase ERα synthesis [52].
Hepatocyte Growth Factor
HGF is a pleiotropic stromal fibroblast-derived growth factor that has epithelial cell specific mitogenic and morphogenic properties [63–65]. HGF expression has been detected in uterine stromal fibroblasts and its effects are mediated by its receptor, HGFR, expressed by epithelial tissues [66–68]. The synthesis of HGF by stromal fibroblasts, together with its reported effects on epithelia, suggests a paracrine mode of action and a role in stromal fibroblast-epithelial interactions [69–71]. Negami et al. have demonstrated that during the normal menstrual cycle, HGF concentrations fluctuate, with peak levels detected during the mid- and late secretory phase [72]. During pregnancy, HGF concentrations increase in the late secretory phase and remain high until parturition [72]. Collectively, this suggests that HGF may influence endometrial repair and reconstruction after menstrual shedding and implantation. Interestingly, HGF has been identified as the stromal fibroblast mediator of E2-induced mammary epithelial cell proliferation [70]. Taken together, these results suggest that HGF may act as an E2 mediator within the endometrium. The role of growth factors in the innate immune system of the FRT is a relatively new field. Our laboratory has demonstrated that HGF increases TER in mouse uterine epithelial cells and that this effect is mediated via the HGFR. Furthermore, HGF inhibits TNFα secretion by mouse uterine epithelial cells [26]. These results suggest that HGF is a regulator of both cell integrity and cytokine release by uterine epithelial cells as well as having an important role in normal endometrial physiology.
Keratinocyte Growth Factor
KGF is a stromal fibroblast-derived secreted peptide. Though KGF is a member of the fibroblast growth factor family, it is unique in that it does not target fibroblasts, endothelial cells, or other non-epithelial cells that are the usual targets of fibroblast growth factors [73,74]. KGF induces the proliferation of uterine and vaginal epithelial cells [75]. The KGF receptor (KGFR) is expressed by epithelial cells [76,77]. Due to ligand and receptor expression patterns, it is thought that KGF functions as a paracrine mediator of stromal fibroblast-epithelial cell interactions [73,78,79]. Stage of the menstrual cycle influences the expression of KGF and KGFR in the human and primate endometria suggesting that KGF is under hormonal control [79–81]. KGF expression appears to be lowest during the proliferative phase and peaks during the late-secretory phase [80,81]. In contrast to KGF expression in the endometrium, mammary gland treatment with E2 increases KGF mRNA expression in a dose-dependent manner while progesterone is needed to stabilize KGFR mRNA [78,82]. In addition, Imagawa and Pedchenko have shown that E2 inhibits transcription of mammary gland KGFR mRNA expression [83]. Clinically, KGF and KGFR have been implicated in the progression of hormone-dependent endometrial cancer as well as in the progression of breast cancer [83–89]. KGF may also have a role in the innate immune system of the uterus. Recent data suggests that KGF increases the secretion of macrophage inflammatory protein 3α (MIP3α) (Haddad & Wira, unpublished results). This may be a very important finding since MIP3α is not only chemotactic for immature dendritic cells, but also functions as an antimicrobial molecule [90,91]. Taken together, KGF appears to be an essential mediator of stromal fiboblast-epithelial cell communication, which is critical for the endometrial structural and functional changes that occur throughout the menstrual cycle, implantation, and early pregnancy [92–94].
Significance
EGF, IGF-I, HGF, and KGF all have roles in normal endometrial physiology. These growth factors are essential mediators of stromal fibroblast-epithelial cell communication, which is critical for the endometrial structural and functional changes that occur throughout the menstrual cycle, implantation, and early pregnancy [48]. Successful reproduction is dependent on sex hormones and growth factors working together to regulate the innate immune system for proper fertilization and implantation to occur. Recent research has implicated HGF and KGF in the innate immune defense of the female reproductive tract while the roles of EGF and IGF-I in immune responses is relatively undefined. There has been some work examining the roles for EGF and its receptor as well as IGF-I in inflammatory responses, bacterial or viral infections and mucus secretion in other mucosal sites though not in the FRT [95–98]. Further research is needed to determine to what extent EGF, IGF-I, HGF and KGF affect the female reproductive tract innate immune function.
IV. ANTIGEN PRESENTATION
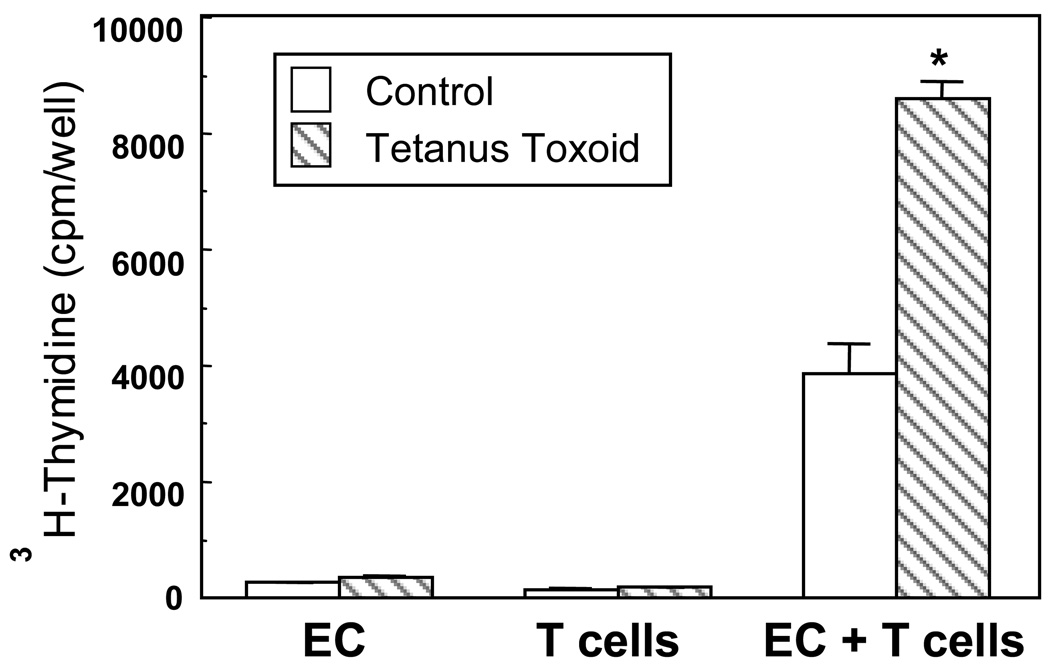
Studies conducted in animal models and with tissues from women indicate that the endometrium is an inductive site for antigen presentation owing to the presence of a number of antigen presenting cells (APC) [99,100]. As seen in Figure 1, highly purified uterine epithelial cells and APCs in the stroma (not shown) are capable of presenting antigen to autologous T cells [21,101–103]. However, the cellular events that underlie this process are still not well defined. Antigenic stimulation of T cells involves the following process: antigenic peptide bound to major histocompatibility complex (MHC) I or MHC II expressed on the surface of APC is recognized by T cell receptor (TCR) on T lymphocytes [104]. A sustained second signal provided via the interaction of co-stimulatory molecules expressed on APC with cognate ligands on T lymphocytes is crucial for activation and differentiation of effector T cells [105,106]. A number of co-stimulatory molecules have been described, with the majority being members of B7 family [107]. With regards to the FRT, MHC II expression has been demonstrated [108,109], but CD80 and CD86 have largely been undetectable [110,111]. However, in previous functional studies in a rat model, we found that antibodies to either CD80 or CD86 inhibit antigen presentation by uterine and vaginal cells suggesting that the antigen presentation was at least partly dependent on the two co-stimulatory molecules [112]. Nevertheless, it is possible that in addition to CD80 and CD86, other co-stimulatory molecules may be involved in this process. Recently it has been demonstrated that the pattern of expression of programmed death receptor ligand 1 (PDL-1) is critical for the establishment of fetal-maternal tolerance [113,114]. Whether PDL-1 or other related co-stimulatory molecules play a significant role in antigen presentation, particularly in the non-pregnant endometrium, remains to be determined. Furthermore, we have shown that uterine epithelial cells express CD1d and CD40, molecules that are integral for antigen presentation and T cell activation [101].
Figure 1.
Human uterine epithelial cells present antigen to autologous T cells. A highly purified suspension of irradiated endometrial uterine epithelial cells (EC) and purified T cells from a premenopausal (proliferative phase) patient were incubated alone or in combination and with or without (control) 10 mg/ml of tetanus toxoid for 5 days. Cultures were pulsed during the final 8 hours of culture with 3H-thymidine. Alone, neither T nor epithelial cells proliferated; however, when T and epithelial cells were combined they proliferated in the presence of tetanus toxoid. **Significantly greater than the control, P < 0.001. [101]
It is still unclear how antigen presentation by diverse APC in the FRT is regulated. In studies assessing the capacity of APC of the endometrium, cervix, vagina and Fallopian tube to present antigen, we showed that the process was independent of pre-or post-menstrual status [21,101]. A possible caveat in these studies is that antigen presentation in vivo may largely be determined by the cell-cell interactions within the FRT. Thus studying this process in vitro may not serve as an adequate system to defining the dynamics of antigen presentation in vivo. Evidence for hormonal regulation of antigen presentation has been obtained from our studies in animal models. For example, we have shown in a rat model that E2 enhances antigen presentation by uterine epithelial cells while concomitantly suppressing antigen presentation by uterine and vaginal APC [115–117]. Furthermore, the inhibitory effect of E2 on stromal APC is mediated by TGFβ that is secreted by uterine epithelial cells [9,117]. In addition, we have shown that antigen presentation by uterine epithelial cells is enhanced by stromal derived growth factors such as HGF or conditioned media from stromal fibroblast cells. These results imply that cell-cell interactions mediated by soluble factors released by underlying stromal fibroblasts are critical in the regulation of antigen presentation by epithelial cells of the FRT [103]. In contrast to the rat, antigen presentation to naïve and memory T cells by mouse uterine epithelial cells as well as APCs in the uterine and vaginal stroma is inhibited by E2 [103]. Collectively, these studies support a model in which antigen presentation in the FRT could be regulated by sex steroids acting either directly on respective APC or indirectly by influencing the production of soluble mediators by stromal fibroblasts including TGFβ and HGF.
Significance
There is ample evidence indicating that various cell types, including epithelial cells, within the FRT are capable of presenting antigens. Consequently, the FRT is now being recognized as an inductive site for generation of immune responses. It is also becoming apparent that the molecular mechanisms that underlie antigen presentation in the FRT may be fundamentally distinct, being influenced by the dynamics of cell-cell interactions within the mucosal site and by menstrual cycle-dependent fluctuations in sex hormones. A clear understanding of the mechanisms of antigen presentation in the FRT has direct implications for strategies aimed at the mucosal delivery of antigens that evoke specific immune responses to a plethora of mucosal pathogens including HIV.
V. IMMUNOGLOBULINS (Ig) and Ig TRANSPORT
A key component of immune protection at mucosal surfaces throughout the FRT is the presence of antibodies which protect against a spectrum of pathogens [118,119]. IgA and to a lesser extent IgG, are recognized as the main antibody isotypes in mucosal surfaces [118,120–122]. A number of studies have examined sources of immunoglobulins in FRT secretions and found cells producing IgA and IgG vary with the site analyzed in the FRT [123–126]. In women, whereas ovarian and uterine tissue had just detectable numbers of Ig producing cells, the cervix and vagina had significant numbers of cells with most secreting IgA (Reviewed in [120]). The FRT is unique in that humoral protection, which includes IgA and IgG as well as pIgR responsible for transporting IgA from tissues to secretions, is under hormonal control [119,127,128]. For example, when E2 was given to ovariectomized rats for 3 days, secretory component (SC), the cleaved portion of pIgR released into secretions, increased in uterine secretions and decreased in cervico-vaginal secretions [129]. Moreover, whereas IgA of blood origin enters uterine tissues within 2–4 hr after E2 treatment, IgA movement from tissue to lumen requires uterine epithelial cell production of pIgR [130,131]. In the human, the amount of IgA and IgG in the uterus varies with the stage of the menstrual cycle, as well as with anatomical location [132]. Uterine secretion of IgA appears to peak at around the time of ovulation [133].
To examine the influence of the menstrual cycle on pIgR synthesis by epithelial cells in the human, uterine washings were obtained prior to gynecological surgery. Samples were collected under sterile conditions using the Gravlee jet wash device [134,135]. Levels of SC in human uterine secretions varied considerably with the stage of the menstrual cycle [131]. When expressed as the percentage of total wash protein, uterine SC levels were highest during the secretory phase, significantly reduced during the proliferative phase and lowest during menstruation. Total SC was also greatest during the secretory phase, averaging approximately 2-fold higher than SC in proliferative and menstrual samples. IgG levels in secretions from the uterine mucosa were highest during the peri-ovulatory phase, whereas levels in the Fallopian tube were lowest at that time. In contrast, IgA and IgG levels in cervical secretions were lowest at the midcycle stage (ovulation) of the menstrual cycle [133]. Suppression of IgA and IgG in cervical secretions throughout the menstrual cycle was observed when women were treated with oral contraceptives. These studies demonstrate that sex hormones regulate IgA, IgG and the IgA transporter pIgR in both the human and rodent female reproductive tract. These changes occur normally, as part of the adaptive immune system that protects the reproductive tract from potential pathogens.
Significance
As with other mucosal surfaces, FRT secretions from women contain significant levels of immunoglobulins which, when analyzed for specificity are directed against microorganisms including E. Coli, Trichomonas vaginalis, Candida albicans, and herpes simplex virus (HSV) [120,136]. As to the role of IgA in the FRT, the most important are the prevention of attachment and/or neutralization of pathogenic bacteria and viruses, prior to colonization and/or infection of the genital mucosa [118,137]. Prevention of attachment of microorganisms to epithelial cells is accomplished by non-neutralizing IgA and IgG antibodies [138,139]. In addition to non-neutralizing antibodies, poly-reactive antibodies with broad specificities bathe the mucosal epithelium. In other studies, IgA has been shown to neutralize viruses within epithelial cells [140,141]. During the transport of dimeric IgA through the epithelial cell by pIgR, IgA can intercept and neutralize pathogens such as HIV-1 in epithelial cells [142]. These studies suggest that secretory IgA antibodies can be effective in blocking entry of HIV-1 into the epithelium and thereby prevent virus from productively infecting target cells. The extent to which these mechanisms play a role genital mucosal protection remains to be elucidated. As mentioned above, both IgA and IgG in the FRT are under hormonal control and vary with stage of the menstrual cycle. Nardelli-Haefliger et al. demonstrated that anti-human papillomavirus 16 virus-like particle (VLP) IgG titers in cervical secretions dropped approximately 9-fold at midcycle during ovulatory cycles [143]. An implication of these findings as it pertains to the use of vaccines is that despite a highly effective response, cyclic changes, with midcycle declines, might contribute to the risk in individuals in which humoral antibody responses were induced. Conversely, without an awareness of cyclic changes in IgA and IgG in FRT secretions, underestimations of vaccine responses might result.
VI. CHEMOKINES AND CYTOKINES
In the human endometrium, progressive tissue growth and remodeling occurs during each menstrual cycle, including the sloughing off of the outer two-thirds of the uterine mucosa during menses. Growth in preparation for fertilization, implantation, and successful pregnancy is mediated by a changing pattern of chemokine, cytokine and adhesion molecule expression that is regulated by the sex steroid hormones [144–146]. Kaysili and associates reported on the importance of IL-8 and Macrophage Chemotactic Factor (MCP-1) in normal uterine physiology, particularly in proliferation, angiogenesis, menstruation, implantation, cervical ripening and parturition [147]. Chemokines and cytokines regulate production of themselves and other chemokines/cytokines by autocrine and paracrine mechanisms, as well as by sex hormones. For example, P withdrawal results in up-regulation of MCP-1 and IL-8, leading to chemotaxis and activation of monocytes and neutrophils, which results in the release and activation of matrix metalloproteinases for initiation of menstruation [148].
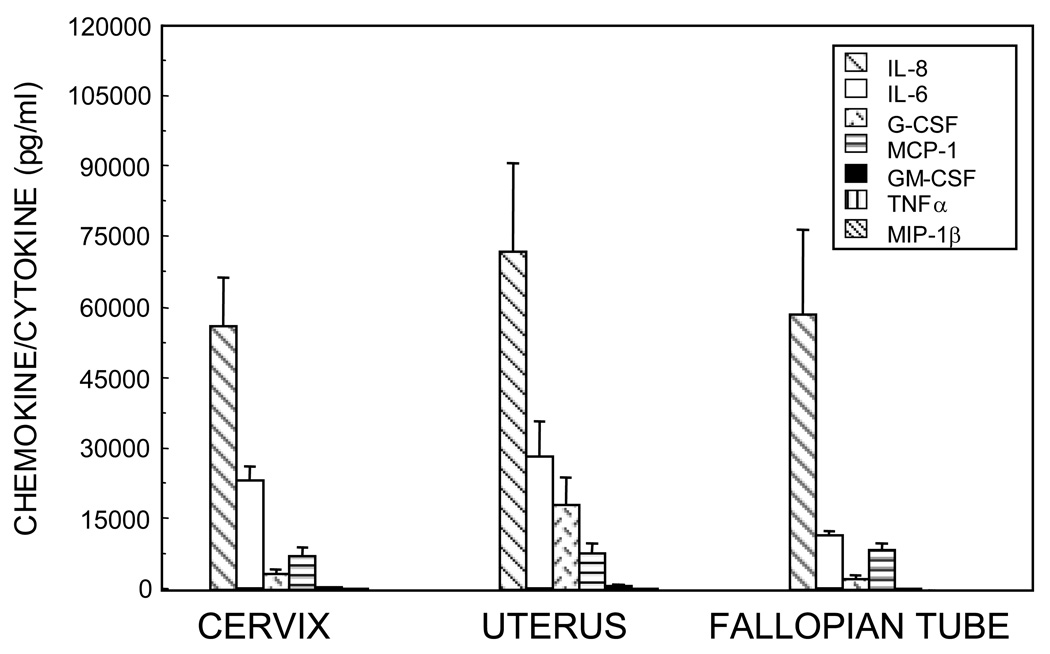
We have demonstrated the constitutive secretion of chemokines and cytokines by primary epithelial cells from the cervix, uterus and Fallopian tubes (Figure 2). Most of these inflammatory mediators were preferentially secreted into the apical compartment of inserts, even after accounting for volume differences between the apical and basolateral compartments. The presence of epithelial secreted chemokines and cytokines in the lumen that are not microbe-induced provide a level of immune awareness to allow for immediate responsiveness to pathogenic microbes, both as microbicides and activators of immune cells should a breech of the epithelial barrier occur. The chemokines contribute to the resident and temporary populations of immune cells in the sub-epithelial layers of the endometrium, and could account for the influx of lymphocytes and other leukocytes that form lymphoid aggregates during the secretory phase [149] as well as the sampling of luminal fluids for pathogenic microbials by leukocytes [150]. Thus, the preferential secretions to the luminal side contribute to innate immune surveillance.
Figure 2.
Total secretion (apical + basolateral) of IL-8, IL-6, G-CSF, MCP-1, GM-CSF, TNFα, and MIP-1β over 48 hours from polarized epithelial cells isolated from the cervix, uterus and Fallopian tube. Media from a minimum of four inserts of epithelial cells were separately collected and analyzed by ELISA. The average of mean values for each set of epithelial cells from each patients’ tissues is shown +/−SEM for uterus and Fallopian tube or the range for cervix. [171]
Secreted pro-inflammatory mediators attract and stimulate the immune cells, often provoking further inflammation. For example, the chemokines IL-8, MCP-1 and Macrophage Inflammatory Protein–1beta (MIP-1β) are chemoattractants for neutrophils, monocytes and T cells, respectively, all of which can be involved in inflammation. The cytokines TNFα, IL-6, Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF), and Granulocyte-Colony Stimulating Factor (G-CSF) induce differentiation of leukocytes to more functionally active pro-inflammatory cells. Some FRT epithelial secreted factors, particularly chemokines, have multiple effects in both innate and adaptive immunity.
Production of chemokines and cytokines by FRT epithelial cells may contribute to pathological conditions during pregnancy. A correlation between elevated concentrations of IL-6, IL-8 and MCP-1 and amniotic microbial infection have been found in cervicovaginal fluids and amniotic fluids from patients in preterm labor and premature rupture of membranes [151–153]. Although some of these factors participate in normal parturition [154], levels were excessive in women with infection. In contrast, low cervical fluid concentrations of IL-1β, IL-8 and IL-6 correlated with clinical chorioamnionitis in early pregnancy [155]. These studies concluded that low concentrations of cytokines indicated a broad immune hypo-responsiveness that could create a permissive environment for ascending infection. Romero and colleagues reported on the association between bacterial vaginosis and pre-term birth and provided compelling evidence that elevated levels of TNFα at preterm parturition was associated with infection [156]. Although the issues on quantities of specific chemokines and cytokines and infection are complex [156], most investigators recommend monitoring the levels of specific chemokine(s) and/or cytokine(s) in the FRT during pregnancy where there is a high risk of preterm birth.
IL-8 and MCP-1 are potent chemokines for neutrophils [157] and monocytes [158], respectively. We have demonstrated that uterine epithelial cell secretions contain concentrations of IL-8 and MCP-1 sufficient to induce chemotaxis comparable to that seen with recombinant chemokine [159,160]. In addition, chemotactic activity for neutrophils or monocytes was effectively removed by pre-incubation of the epithelial secretions with specific neutralizing anti IL-8 antibodies. In addition to chemotaxis, IL-8 has been associated with proliferation and angiogenesis during early to mid-secretory phase, as well as with apoptosis during menstruation [147], a process in which neutrophils contribute prominently. Since production of chemokines and cytokines are often up-regulated in an autocrine and/or paracrine manner, pro-inflammatory peptides may be produced by the FRT epithelial cells to marshal neutrophil, monocyte/macrophage, dendritic cell, T cell and B cell forces against potential pathogens. In addition to direct effects, chemokines and cytokines can also modulate pro-inflammatory responses by regulating the number of chemokine and cytokine receptors expressed on leukocytes [161].
Significance
Many chemokines have been shown to have microbicidal effects, and some microbicides have chemotactic properties. For example, the microbicide human beta-defensin 2 (HBD2), which is produced by human primary polarized uterine epithelial cells in culture [30], acts also as a chemokine for the recruitment of memory T cells, immature dendritic cells [162] and neutrophils [163]. The chemokine MIP-3α, which is secreted by uterine epithelial cells in response to LPS [20,164], has also been shown to have anti-bacterial activity [165].
Cytokines can be included in vaccine formulations to enhance recruitment and activation of APC at sites of immunization [166]. Mucosal (intranasal) vaccination of an IL-15 plasmid with a HSV DNA vaccine increased primary and memory CD8+ T cell responses as well as higher HSV-antigen IgA titers in the vaginal tract [167]. Increased vaginal IgA, cytotoxic T lymphocyte responses and protection against challenge with HSV-1 were also observed with co-immunization of HSV-antigen and plasmids encoding CCL19 (MIP-3β) and CCL21 (6Ckine) [168,169]. Researchers have used Th1 or Th2 cytokines as adjuvants to enhance immune responses to vaccines. For example, IL-12 has been shown to increase cytotoxic T cell (CTL) mediated immune responses against a DNA vaccine encoding HIV antigen [170]. FRT epithelial cells, through their chemokine, cytokine and microbicidal secretions, contribute to the maintenance of homeostasis and a state of preparedness against microbial pathogens. As gatekeepers of innate immune protection in the FRT, epithelial cells protect against sexually transmitted infections (STI), and use their secretions to enhance innate and adaptive immunity in response to pathogenic challenge [20,171,172]. Female sex steroid hormones regulate this protection to optimize the best reproductive scenario.
VII. PATHOGEN RECOGNITION RECEPTORS (PRR)
Toll-like Receptors (TLRs) as PRR
In contrast to the adaptive arm of the immune system, recognition of microbial pathogens by the innate immune arm is based on the germline-encoded PRR [173]. PRR recognize highly conserved microbial components and are referred to as pathogen associated molecular patterns (PAMPs) [173]. A number of PRR and the associated PAMPs have been described allowing innate immune recognition of both intracellular and extracellular pathogens [173]. Among the PRR, TLRs are highly conserved type I transmembrane receptor proteins [174,175]. At least eleven TLRs designated as TLR1-11 have been described and shown to be expressed either on the cell membrane (TLR1,2,4,5,6) or in endosomes (TLR3,7,8,9) [174,175] in a cell-specific manner. TLRs interact with specific PAMPs [174,175]. The resultant intracellular signaling cascade leads to secretion of pro-inflammatory cytokines and accompanying cellular activation (Reviewed in [176]).
Other Intracellular PRR
In addition to TLRs, recent studies have identified nucleotide-binding oligomerization domain (NOD)-like receptors (NLRS) and retinoic acid-inducible gene-1 (RIG-1) as additional receptors for recognition of intracellular pathogens [177,178]. The RIG-1 receptors that include RIG-1 and melanoma differentiation-associated gene 5 (MDA 5) are primarily involved in the recognition of intracellular viruses and the subsequent signaling that induces innate antiviral responses [177,178]. On the other hand, NLRs such as NOD-1 and NOD-2 are utilized in the initiation of antibacterial responses [177]. More recent evidence indicates that there is potential for the interactions between TLRs and the other intracellular innate receptors (RIG-1 and NLRs) in either a synergistic or antagonistic manner [179].
Expression of TLR in the FRT
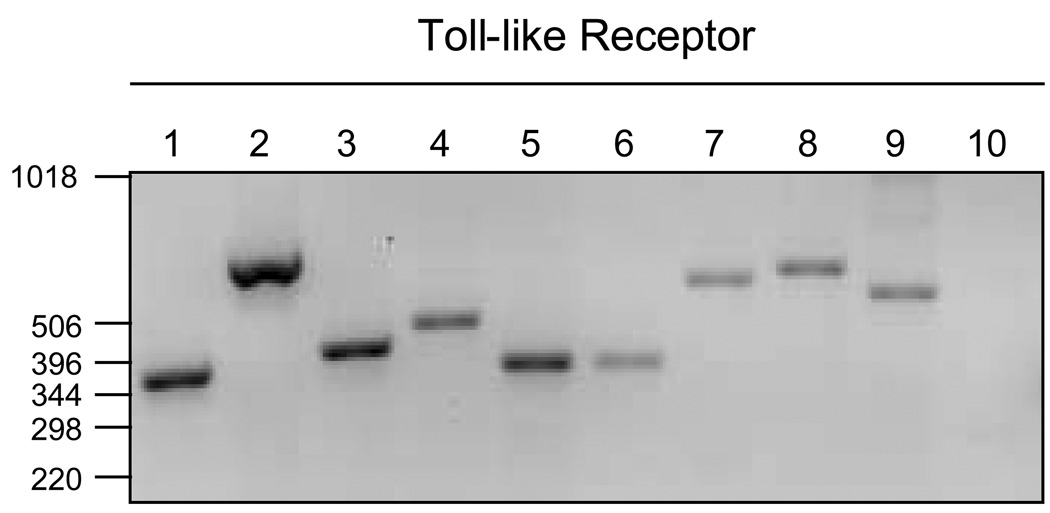
TLRs have been shown by our group and others to be expressed in the human FRT [180–183]. These studies demonstrated a site-specific pattern of expression of TLRs in the FRT. For example, TLR2 and TLR4 are predominantly expressed in the upper tract (endometrium and Fallopian tubes) and are rarely observed in the lower tract (ectocervix and vagina) [180,182,184]. This pattern of expression of TLRs may minimize the generation of immune responses against commensal bacteria abundant in the lower reproductive tract [185]. In other studies (Figure 3), we found that isolated primary uterine and Fallopian tube (not shown) epithelial cells express TLR 1–9 [29,186,187]. We have extended these findings to show that TLRs expressed by uterine epithelial cells are functional, as indicated by the secretion of cytokines and chemokines upon ligation with specific ligands [29,187]. These studies suggest that TLR ligation is the first step in innate protection within the endometrial mucosa.
Figure 3.
Toll-like receptor (TLR) expression in uterine epithelial cells by RT-PCR. Lanes 1–10 correspond to TLR1–10, respectively. Total RNA isolated from purified primary uterine epithelial cells and examined by RT-PCR for TLR mRNA expression. [30]
The pattern of expression of TLRs varies with immune cell subset in the FRT. In the Fallopian tube, for example, TLR4 is reportedly expressed exclusively in stromal fibroblasts [182]. In contrast, in the endometrium both stromal fibroblasts and epithelial cells express TLR4, with the receptor only functional in the latter cell type [184]. We have found that TLR4 is also expressed by the epithelial cells from the Fallopian tube [187]. Additional studies conducted from our group have also shown that endometrial Natural Killer (NK) cells express TLR2, TLR3 and TLR4 and respond to peptidoglycan or poly(I:C), the ligands for TLR2 and TLR3 respectively, by producing interferon gamma (IFN-γ) [188]. Altogether, these findings suggest that there is a characteristic pattern of expression of TLRs at distinct anatomical sites of the FRT by different cell subsets, and that this may represent a functional evolution that facilitates discrimination against pathogens versus commensal microorganisms within these sites.
Little is known regarding regulation of TLRs and other innate immune receptors in the FRT. It is possible that the cytokine milieu in different sites of the FRT results in compartmentalization in the profile of expression of these receptors. Recent studies by our group have suggested a role for TGFβ in modulating TLR-mediated immune responses of NK cells and neutrophils [188,189]. In addition, recent findings also support the role of sex hormones in the expression of TLRs in the endometrium in that TLR 2–6, 9 and 10 were higher in the endometrium during the secretory phase of the menstrual cycle relative to that seen at other times [190,191]. Similarly, in a murine model, the expressions of TLR1-10 were significantly higher in the vaginal epithelial cells during diestrus or with Depo-Provera (progesterone only contraceptive) [192]. However, it remains to be determined if the molecular mechanisms by which menstrual cycle-associated changes in sex hormones influence the expression of TLRs in the FRT. This would have important implications for pathogen recognition and innate immune protection during the various phases of the menstrual cycle.
Significance
Along with commensal microorganisms, the FRT is periodically exposed to a variety of pathogens. The mechanisms that facilitate discrimination of commensal versus pathogens may be largely determined by the pattern and functions of expression of the innate immune receptors at different sites throughout the FRT. Thus, characterization of the pattern of expression of TLRs and other innate immune receptors is critical in defining the nature of innate immune responses in the FRT. As a result, TLR targeting may be an effective strategy to augment both humoral and cellular immune responses to both peptide and DNA antigens [193,194]. The mechanisms that underlie this process are not clearly defined, but are likely to include modulation of antigen processing and presentation and enhanced cellular activation [195–197]. Nevertheless, conjugation of candidate vaccine antigens with TLR ligands for cognate TLRs expressed in the FRT may represent a novel strategy for generating optimal protective immunity to a number of mucosal pathogens including STI.
VIII. ENDOGENOUS MICROBICIDES
Endogenous microbicides are molecules produced by the cells of the FRT that provide a natural protection against bacterial, viral, and fungal pathogens. Endogenous microbicides can be secretory or intracellular and mediate their functions either directly or by inducing cytokines/chemokines resulting in the recruitment of effector cells. An innate immune response by the epithelial cells can result in recruitment of FRT effector cells such as macrophages, neutrophils, NK cells, and dendritic cells.
Secretory Microbicides
Secretory microbicides are soluble factors produced by the cells of the FRT that exhibit antimicrobial activity. FRT epithelial cells constitutively secrete factors that exhibit potent antimicrobial activity including human α-defensin-5 (HD5), β-defensins 1–4 (HBD1-4), and secretory leukocyte protease inhibitor (SLPI) [9,30,198]. Several of these molecules have at least two distinct functions. For example, defensins and chemokines such as MIP-3α are both antimicrobial and chemotactic [90,199]. SLPI is a protease inhibitor as well as a potent microbicide [200]. In addition to constitutive production, cells can be induced to increase production/secretion of antimicrobials in the presence of pathogens or pathogen-derived products. For example, oral epithelial cells have been shown to secrete SLPI, HBD2 and HBD3 following HIV-1 infection or exposure to HIV-1 proteins and this is thought to partly explain the paucity of HIV-1 infection via the oral route [201,202]. The production of type I interferons (IFNs) can also be induced by viral glycoproteins, including HIV gp120 [203]. In addition, the envelope glycoprotein gp120 from X4/T-Tropic HIV-1 induces the mRNA expression of HBD2, SLPI, and IL-8 by uterine and Fallopian tube epithelial cells (Ghosh et al, unpublished observations).
Several of the antimicrobials described above are effective in killing gram-positive and gram-negative bacteria, fungi, and viruses including N. gonorrhoeae, C. trachomatis, C. albicans, herpes simplex virus-2 (HSV-2), and HIV-1, all of which are important reproductive tract pathogens [202,204–207]. Other factors found in FRT secretions exhibiting antiviral activity, notably against HIV-1, are the chemokines MIP-1α, MIP-1β, regulated upon activation, normal T-cell expressed and secreted (RANTES), and stromal derived factor 1 alpha (SDF-1α) which inhibit HIV-1 infection of target host cells by interfering with the ability of HIV-1 to bind co-receptors CCR5 and CXCR4 found on host cells [208]. The fact that secretions from isolated epithelial cells from the FRT contain numerous antimicrobial factors is directly correlated with findings that whole secretions from the FRT are able to directly kill pathogens. Our laboratory has shown that conditioned media (secretions) from primary uterine or Fallopian tube epithelial cells in culture directly inhibits N. gonorrhoea, C. albicans, and HIV-1, all major pathogens of the FRT (Fahey and Ghosh, unpublished observations). In other studies, John et al. have tested cervico-vaginal lavage (CVL) from healthy women and found that they have an intrinsic ability to inhibit HSV-2. Anti-HSV-2 activity correlated with the concentrations of α-defensins in CVL [207]. CVL has also been shown to contain antimicrobials SLPI, lactoferrin and gp340 that are inhibitory to HIV replication in vitro and therefore might potentially inhibit viral entry in vivo [209].
A number of other endogenous microbicides have been described in humans. Some have been found in the FRT (as well as other mucosal sites) and have known microbicidal functions, but have yet to be evaluated for anti-HIV activity. These include Surfactant protein D (SP-D), lipocalins, and lipophilin C [210–214]. SLPI related protein Elafin also falls within this category as it has bactericidal activity [215]. Other factors like thrombospondin (TSP-1) have been characterized at other mucosal sites such as the oral mucosa in terms of its anti-HIV activity [216]. Still others such as cathelicidin (LL37) have been described in the FRT and shown to have anti-HIV activity [217].
Intracellular Microbicides
Another level of innate immune defense is mediated by intracellular molecules that interfere with potential intracellular pathogens. These factors are responsible for protection against intracellular pathogens such as viruses. Some are by-products of type I interferon (IFN) production by infected cells, a crucial step for limiting early viral replication [218]. Type I IFNs exert their activity through the IFN-α/β receptor and include IFN-α, -β, -δ, -τ, -κ and -ω. While all Type I IFNs are important for an effective antiviral response, IFN-β is crucial to this process, since its absence results in the host being highly susceptible to viral infection [219]. Type I IFNs induce the expression of intracellular virucidal factors, including 2’,5’-oligoadenlylate synthetase (2’,5’-OAS), RNA-dependent protein kinase R (PKR), and myxovirus resistance gene A (MxA) [218]. Once activated, 2’,5’-OAS polymerizes ATP into 2’,5’-linked oligoadenylates that are specific activators of a latent endoribonuclease, RNAase L. RNase L degrades viral and cellular RNAs and results in the inhibition of protein synthesis [220]. PKR exerts its anti-viral activity by phosphorylating the alpha subunit of translation initiation factor 2, which results in the shut-down of protein synthesis [221]. MxA proteins are interferon-induced GTPases that appear to detect viral infection by sensing the presence of nucleocapsid-like structures and sequestering them so that assembly of new virus particles is inhibited [220]. All three exhibit broad-spectrum inhibition of multiple virus families [220,221]. Another intracellular virucidal factor is APOBEC3G (apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G). The APOBEC family of proteins consists of at least 10 members in humans with diverse physiological functions. Of these, APOBEC3G has been the protein most characterized for its anti-HIV activity [222]. APOBEC3G can inhibit the replication of a broad range of retroviruses including HIV-1. APOBEC3G cytidine deaminases incorporate into retroviral cores where they lethally hyper-mutate nascent DNA reverse transcripts [222]. Previous work from our laboratory has demonstrated that IFN-β, 2’,5’-OAS and MxA are constitutively expressed by both primary uterine and Fallopian tube epithelial cells [30,187].
Sex Hormones and Endogenous Microbicides
An additional level of complexity arises when hormonal influences on the expression/secretion of antimicrobial factors are considered. It has been shown that stage of the menstrual cycle influences the expression of several FRT antimicrobial factors. Levels of HBD1, 2, and 5 peak during the secretory phase, HBD4 during the proliferative phase, HBD2 during menstruation, and SLPI during the mid-late secretory phase of the menstrual cycle [198]. Our group has demonstrated that SLPI secretion by primary polarized uterine epithelial cells is significantly reduced in post-menopausal women when compared to pre-menopausal women [9]. Recently, Keller and colleagues found a midcycle suppression of endogenous antimicrobials in CVL [223]. Analysis of cytokines, chemokines and antimicrobials in CVL indicated that SLPI, HBD2, HNP1-3, and lactoferrin dropped significantly at midcycle (day 13) and remained depressed for 7–10 days prior to returning to proliferative phase levels just prior to menstruation. In contrast, protein levels remained unchanged over the course of the menstrual cycle. This suggests that stage of the menstrual cycle can have a significant impact on FRT susceptibility to sexually transmitted pathogens. The influences of menstrual cycle on susceptibility to STI are more clearly delineated in the case of HIV-1 infection. Previously, we found that HIV-1 receptors, CD4, CCR5, CXCR4, and GalCer levels change during the menstrual cycle, with highest levels observed at midcycle [149]. These findings suggest that women may be more or less susceptible to HIV-1 infection based on their menstrual status. Others have shown that women with low circulating levels of estrogen are more likely to become infected with HIV and conversely, that treatment of SIV macaques with estrogen protects against SIV infection [224,225]. Therefore, the influence of sex hormones on the antimicrobial immune responses in the FRT is likely a determinant to whether the host is susceptible or resistant to infection by STI.
Significance
Mechanisms that regulate the mucosal immune system in the FRT have received relatively little attention. This is due in part to the complexities of the immune and endocrine systems, and also due to the difficulties of conducting experiments at the interface of these two disciplines. That women have natural protection in the form of endogenous microbicides and these might be enhanced to confer protection against pathogens is a relatively new area of research priority. In the context of HIV research such approaches have been tested recently. Lederman et al. demonstrated that topical application of a modified analog of the chemokine RANTES, a co-receptor antagonist for R5/Macrophage tropic HIV-1, protected against vaginal challenge of SHIV in rhesus macaques [226]. In conclusion, endogenous microbicides in the FRT are a woman’s natural protection against pathogens. The multitude of microbicidal factors in the FRT shows possibilities of being exploited to develop novel interventions. Developing methods to enhance the production of these endogenous secreted microbicides within the FRT represents a promising and exciting avenue in the field of reproductive immunology.
IX. CONCLUSIONS
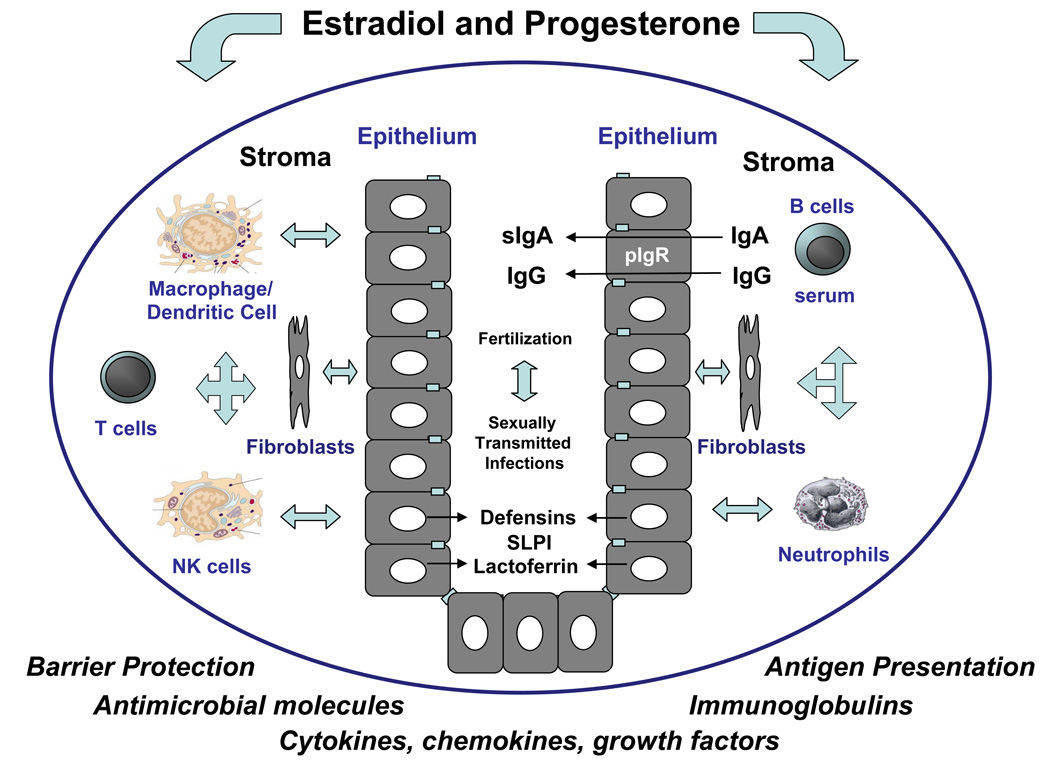
The studies presented in this review indicate that epithelial cells that line the human female reproductive tract provide multiple levels of immune protection (Figure 4). In addition to acting as a physical barrier, these cells function as sentinels of the innate and adaptive immune systems present throughout the FRT. Understanding how epithelial cells in the FRT maintain homeostasis and protect against bacterial and viral challenges requires that we understand the unique characteristics of the immune system and the cell-cell interactions that occur in the Fallopian tube, uterus, cervix and vagina that lead to either enhanced and/or suppressed immune responses at particular times in a woman's life. As presented in this review, epithelial cells are precisely regulated by the female sex hormones that alter epithelial permeability, microbicidal activity, cytokine/chemokine secretion as well as immune cell recruitment and activation to ensure maternal and fetal protection. Limited success in dealing with fertility problems and protecting against STI demonstrates the need to more fully understand the complex role sex hormones in regulating immune protection throughout the FRT. The studies presented suggest that by understanding the ways in which sex hormones regulate epithelial cell function, new avenues may be identified both to protect against potential pathogens and to enhance the quality of women’s reproductive health.
Figure 4.
Schematic of the multiple functions carried out by epithelial cells throughout the upper female reproductive tract as sentinels of immune protection. Acting as the first line of defense, epithelial cells provide host protection against sexually transmitted diseases, while supporting the process of fertilization, in a number of ways that includes providing a mechanical barrier, producing and/or secreting antimicrobial molecules, transporting IgA, processing and presenting antigen and communicating with underlying immune cells including macrophages, dendritic cells, T cells, NK cells and neutrophils as well as by secreting cytokines, chemokines and growth factors that are important in autocrine and paracrine signaling, growth and differentiation. To accomplish this, epithelial cells communicate with underlying stromal cells and vise-versa via soluble paracrine signals. Sex hormone (estradiol and progesterone) regulation of epithelial cell function is both direct and indirect. In some cases hormone effects are mediated through estradiol receptors (ER) in epithelial cells. Others are indirect and mediated through ER located in the underlying stromal cells (fibroblasts) as well as immune cells.
Footnotes
This work was supported by a National Institutes of Health Grants AI-51877, AI-13541 and AI-071761 (CRW).
References
- 1.UNAIDS 2007. Geneva, Switzerland: AIDS epidemic update JUNPoHAUaWHOW. 2007
- 2.Padian N, van der Straten A, Ramjee G, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet. 2007;370:251–261. doi: 10.1016/S0140-6736(07)60950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen J. AIDS research. Promising AIDS vaccine's failure leaves field reeling. Science. 2007;318:28–29. doi: 10.1126/science.318.5847.28. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. Promising prevention interventions perform poorly in trials. Science. 2007;317:440. doi: 10.1126/science.317.5837.440. [DOI] [PubMed] [Google Scholar]
- 5.Editorial team. Phase III anti-HIV microbicide trial in Africa and India stopped as preliminary results show gel may increase risk of infection. Euro Surveill. 2007;12:E070208.6. doi: 10.2807/esw.12.06.03137-en. [DOI] [PubMed] [Google Scholar]
- 6.Honey K. Microbicide trial screeches to a halt. J Clin Invest. 2007;117:1116. doi: 10.1172/JCI32291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piot P. UNAIDS Executive Director; May 9, 2007; San Francisco Chronicle. 2007. [Google Scholar]
- 8.Ogra P, Yamanaka T, Losonsky GA. Local immunologic defenses in the genital tract. In: Fleicher N, editor. Reproductive Immunology. New York: Alan R. Liss, Inc.; 1981. pp. 381–394. [PubMed] [Google Scholar]
- 9.Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185:1606–1613. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- 10.Wira CR, Fahey JV, Schaefer TM, et al. Innate and adaptive immunity in the human female reproductive tract: influence of the menstrual cycle and menopause on the mucosal immune system in the uterus. In: Glasser S, Aplin J, Giudice L, Tabibzadeh S, editors. The Endometrium. 2007. pp. 491–521. [Google Scholar]
- 11.Wira CR, Richardson J, Prabhala R. Endocrine regulation of mucosal immunity: Effect of sex hormones and cytokines on the afferent and efferent arms of the immune system in the female reproductive tract. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGhee JR, et al., editors. Handbook of Mucosal Immunology. New York: Academic Press; 1994. pp. 705–718. [Google Scholar]
- 12.Kutteh WH, Mestecky J, Wira CR. Mucosal immune system in the human female reproductive tract. In: Mestecky JLM, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. 3rd Edition ed. Burlington, MA: Elselvier Academic Press; 2005. pp. 1631–1646. [Google Scholar]
- 13.Wira CR, Crane-Godreau MA, Grant-Tschudy KS. Endocrine regulation of mucosal immune system in the female reproductive tract. In: Mestecky J, Lamm M, Strober W, Bienenstock J, McGhee J, et al., editors. Mucosal Immunology. Burlington, MA: Elselvier Academic Press; 2005. pp. 1661–1678. [Google Scholar]
- 14.Godfrey RWA. Human airway epithelial tight junctions. Microscopy Res Tech. 1997;38:488–499. doi: 10.1002/(SICI)1097-0029(19970901)38:5<488::AID-JEMT5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 15.Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- 16.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 17.Matter K, Aijaz S, Tsapara A, et al. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Planchon S, Fiocchi C, Takafuji V, et al. Transforming growth factor-beta1 preserves epithelial barrier function: identification of receptors, biochemical intermediates and cytokine antagonists. J Cell Physiol. 1999;181:55–66. doi: 10.1002/(SICI)1097-4652(199910)181:1<55::AID-JCP6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 20.Crane-Godreau MA, Wira CR. CCL20/macrophage inflammatory protein 3alpha and tumor necrosis factor alpha production by primary uterine epithelial cells in response to treatment with lipopolysaccharide or Pam3Cys. Infect Immun. 2005;73:476–484. doi: 10.1128/IAI.73.1.476-484.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahey JV, Prabhala RH, Guyre PM, et al. Antigen-presenting cells in the human female reproductive tract: analysis of antigen presentation in pre- and post-menopausal women. Am J Reprod Immunol. 1999;42:49–57. doi: 10.1111/j.1600-0897.1999.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 22.Grant KS, Wira CR. Effect of mouse uterine stromal cells on epithelial cell transepithelial resistance (TER) and TNFalpha and TGFbeta release in culture. Biol Reprod. 2003;69:1091–1098. doi: 10.1095/biolreprod.103.015495. [DOI] [PubMed] [Google Scholar]
- 23.Kaushic C, Wira CR. IgA and Reproductive Tract Immunity. In: Kaetzel C, editor. Mucosal Immune Defense: Immunoglobulin A. New York: Kluwer Academic/Plenum Publisher; 2008. pp. 291–320. [Google Scholar]
- 24.Gruber CJ, Tschugguel W, Schneeberger C, et al. Production and actions of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 25.Grant-Tschudy KS, Wira CR. Paracrine mediators of mouse uterine epithelial cell transepithelial resistance in culture. J Reprod Immunol. 2005;67:1–12. doi: 10.1016/j.jri.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Grant-Tschudy KS, Wira CR. Hepatocyte Growth Factor Regulation of Uterine Epithelial Cell Transepithelial Resistance and Tumor Necrosis Factor {alpha} Release In Culture. Biol Reprod. 2004;72:814–821. doi: 10.1095/biolreprod.104.035618. [DOI] [PubMed] [Google Scholar]
- 27.Groten T, Pierce AA, Huen AC, et al. 17 beta-estradiol transiently disrupts adherens junctions in endothelial cells. FASEB J. 2005;19:1368–1370. doi: 10.1096/fj.04-2558fje. [DOI] [PubMed] [Google Scholar]
- 28.Phillips ML, Schultz BD. Steroids modulate transepithelial resistance and Na+ absortion across cultured porcine vas deferens epithelia. Biol Reprod. 2002;66:1016–1023. doi: 10.1095/biolreprod66.4.1016. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer TM, Desouza K, Fahey JV, et al. Toll-like receptor (TLR) expression and TLRmediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428–436. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer TM, Fahey JV, Wright JA, et al. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 31.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–238. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Yi X, Wang Y, Yu FS. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest Ophthalmol Vis Sci. 2000;41:4093–4100. [PubMed] [Google Scholar]
- 33.Zech JC, Pouvreau I, Cotinet A, et al. Effect of cytokines and nitric oxide on tight junctions in cultured rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1998;39:1600–1608. [PubMed] [Google Scholar]
- 34.Chen J, Tsang LL, Ho LS, et al. Modulation of human enteric epithelial barrier and ion transport function by Peyer's patch lymphocytes. World J Gastroenterol. 2004;10:1594–1599. doi: 10.3748/wjg.v10.i11.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorenzo-Zuniga V, Rodriguez-Ortigosa CM, Bartoli R, et al. Insulin-like growth factor-I improves intestinal barrier function in cirrhotic rats. Gut. 2006;55:1306–1312. doi: 10.1136/gut.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lytton SD, Fischer W, Nagel W, et al. Production of ammonium by Helicobacter pylori mediates occludin processing and disruption of tight junctions in Caco-2 cells. Microbiology. 2005;151:3267–3276. doi: 10.1099/mic.0.28049-0. [DOI] [PubMed] [Google Scholar]
- 37.Terres AM, Pajares JM, Hopkins AM, et al. Helicobacter pylori disrupts epithelial barrier function in a process inhibited by protein kinase C activators. Infect Immun. 1998;66:2943–2950. doi: 10.1128/iai.66.6.2943-2950.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciarlet M, Crawford SE, Estes MK. Differential infection of polarized epithelial cell lines by sialic acid-dependent and sialic acid-independent rotavirus strains. J Virol. 2001;75:11834–11850. doi: 10.1128/JVI.75.23.11834-11850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tafazoli F, Magnusson KE, Zheng L. Disruption of epithelial barrier integrity by Salmonella enterica serovar typhimurium requires geranylgeranylated proteins. Infect Immun. 2003;71:872–881. doi: 10.1128/IAI.71.2.872-881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muza-Moons MM, Koutsouris A, Hecht G. Disruption of cell polarity by enteropathogenic Escherichia coli enables basolateral membrane proteins to migrate apically and to potentiate physiological consequences. Infect Immun. 2003;71:7069–7078. doi: 10.1128/IAI.71.12.7069-7078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakravortty D, Kumar KS. Modulation of barrier function of small intestinal epithelial cells by lamina propria fibroblasts in response to lipopolysaccharide: possible role in TNFalpha in inducing barrier dysfunction. Microbiol Immunol. 1999;43:527–533. doi: 10.1111/j.1348-0421.1999.tb02438.x. [DOI] [PubMed] [Google Scholar]
- 42.Czerucka D, Dahan S, Mograbi B, et al. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect Immun. 2000;68:5998–6004. doi: 10.1128/iai.68.10.5998-6004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130:731–746. doi: 10.1053/j.gastro.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Fichorova RN, Zhou F, Ratnam V, et al. Anti-human immunodeficiency virus type 1 microbicide cellulose acetate 1,2-benzenedicarboxylate in a human in vitro model of vaginal inflammation. Antimicrob Agents Chemother. 2005;49:323–335. doi: 10.1128/AAC.49.1.323-335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dezzutti CS, James VN, Ramos A, et al. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob Agents Chemother. 2004;48:3834–3844. doi: 10.1128/AAC.48.10.3834-3844.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maher S, McClean S. Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem Pharmacol. 2006;71:1289–1298. doi: 10.1016/j.bcp.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Marinaro M, Fasano A, De Magistris MT. Zonula occludens toxin acts as an adjuvant through different mucosal routes and induces protective immune responses. Infect Immun. 2003;71:1897–1902. doi: 10.1128/IAI.71.4.1897-1902.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooke PS, Buchanan DL, Kurita T. Role of Stromal-Epithelial Interactions in Hormonal Responses of the Uterus. In: Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S, editors. The Endometrium. New York: Taylor & Francis; 2002. pp. 151–166. [Google Scholar]
- 49.Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool. 1976;196:361–370. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- 50.Cunha GR, Young P. Role of stroma in oestrogen-induced epithelial proliferation. Epithelial Cell Biol. 1992;1:18–31. [PubMed] [Google Scholar]
- 51.Cooke PS, Buchanan DL, Young P, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17:309–317. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]
- 53.Shupnik MA. Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene. 2004;23:7979–7989. doi: 10.1038/sj.onc.1208076. [DOI] [PubMed] [Google Scholar]
- 54.Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- 55.Imai T, Kurachi H, Adachi K, et al. Changes in epidermal growth factor receptor and the levels of its ligands during menstrual cycle in human endometrium. Biol Reprod. 1995;52:928–938. doi: 10.1095/biolreprod52.4.928. [DOI] [PubMed] [Google Scholar]
- 56.Moller B, Rasmussen C, Lindblom B, et al. Expression of the angiogenic growth factors VEGF, FGF-2, EGF and their receptors in normal human endometrium during the menstrual cycle. Mol Hum Reprod. 2001;7:65–72. doi: 10.1093/molehr/7.1.65. [DOI] [PubMed] [Google Scholar]
- 57.Ejskjaer K, Sorensen BS, Poulsen SS, et al. Expression of the epidermal growth factor system in human endometrium during the menstrual cycle. Mol Hum Reprod. 2005;11:543–551. doi: 10.1093/molehr/gah207. [DOI] [PubMed] [Google Scholar]
- 58.Lee AV, Cui X, Oesterreich S. Cross-talk among estrogen receptor, epidermal growth factor, and insulin-like growth factor signaling in breast cancer. Clin Cancer Res. 2001;7:4429s–4435s. discussion 11s–12s. [PubMed] [Google Scholar]
- 59.Murphy LJ, Ghahary A. Uterine insulin-like growth factor-1: regulation of expression and its role in estrogen-induced uterine proliferation. Endocr Rev. 1990;11:443–453. doi: 10.1210/edrv-11-3-443. [DOI] [PubMed] [Google Scholar]
- 60.Woelfle J, Chia DJ, Massart-Schlesinger MB, et al. Molecular physiology, pathology, and regulation of the growth hormone/insulin-like growth factor-I system. Pediatr Nephrol. 2005;20:295–302. doi: 10.1007/s00467-004-1602-1. [DOI] [PubMed] [Google Scholar]
- 61.Rutanen EM. Insulin-like growth factors in endometrial function. Gynecol Endocrinol. 1998;12:399–406. doi: 10.3109/09513599809012842. [DOI] [PubMed] [Google Scholar]
- 62.Moyano P, Rotwein P. Mini-review: estrogen action in the uterus and insulin-like growth factor-I. Growth Horm IGF Res. 2004;14:431–435. doi: 10.1016/j.ghir.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Pollack AL, Runyan RB, Mostov KE. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev Biol. 1998;204:64–79. doi: 10.1006/dbio.1998.9091. [DOI] [PubMed] [Google Scholar]
- 64.Murakami S, Miyamoto Y, Fujiwara C, et al. Expression and action of hepatocyte growth factor in bovine endometrial stromal and epithelial cells in vitro. Mol Reprod Dev. 2001;60:472–480. doi: 10.1002/mrd.1112. [DOI] [PubMed] [Google Scholar]
- 65.Pollack AL, Apodaca G, Mostov KE. Hepatocyte growth factor induces MDCK cell morphogenesis without causing loss of tight junction functional integrity. Am J Physiol Cell Physiol. 2004;286:C482–C494. doi: 10.1152/ajpcell.00377.2003. [DOI] [PubMed] [Google Scholar]
- 66.Nasu K, Sugano T, Matsui N, et al. Expression of hepatocyte growth factor in cultured human endometrial stromal cells is induced through a protein kinase C-dependent pathway. Biol Reprod. 1999;60:1183–1187. doi: 10.1095/biolreprod60.5.1183. [DOI] [PubMed] [Google Scholar]
- 67.Yang XM, Park M. Expression of the hepatocyte growth factor/scatter factor receptor tyrosine kinase is localized to epithelia in the adult mouse. Lab Invest. 1995;73:483–491. [PubMed] [Google Scholar]
- 68.Chen C, Spencer TE, Bazer FW. Expression of hepatocyte growth factor and its receptor c-met in the ovine uterus. Biol Reprod. 2000;62:1844–1850. doi: 10.1095/biolreprod62.6.1844. [DOI] [PubMed] [Google Scholar]
- 69.Khan KN, Masuzaki H, Fujishita A, et al. Immunoexpression of hepatocyte growth factor and c-Met receptor in the eutopic endometrium predicts the activity of ectopic endometrium. Fertil Steril. 2003;79:173–181. doi: 10.1016/s0015-0282(02)04535-1. [DOI] [PubMed] [Google Scholar]
- 70.Zhang HZ, Bennett JM, Smith KT, et al. Estrogen mediates mammary epithelial cell proliferation in serum-free culture indirectly via mammary stroma-derived hepatocyte growth factor. Endocrinology. 2002;143:3427–3434. doi: 10.1210/en.2002-220007. [DOI] [PubMed] [Google Scholar]
- 71.Imagawa W, Pedchenko VK, Helber J, et al. Hormone/growth factor interactions mediating epithelial/stromal communication in mammary gland development and carcinogenesis. J Steroid Biochem Mol Biol. 2002;80:213–230. doi: 10.1016/s0960-0760(01)00188-1. [DOI] [PubMed] [Google Scholar]
- 72.Negami AI, Sasaki H, Kawakami Y, et al. Serum human hepatocyte growth factor in human menstrual cycle and pregnancy: a novel serum marker of regeneration and reconstruction of human endometrium. Horm Res. 1995;44 Suppl 2:42–46. doi: 10.1159/000184660. [DOI] [PubMed] [Google Scholar]
- 73.Rubin JS, Osada H, Finch PW, et al. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Finch PW, Rubin JS, Miki T, et al. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245:752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- 75.Hom YK, Young P, Thomson AA, et al. Keratinocyte growth factor injected into female mouse neonates stimulates uterine and vaginal epithelial growth. Endocrinology. 1998;139:3772–3779. doi: 10.1210/endo.139.9.6182. [DOI] [PubMed] [Google Scholar]
- 76.Miki T, Bottaro DP, Fleming TP, et al. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci U S A. 1992;89:246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bottaro DP, Rubin JS, Ron D, et al. Characterization of the receptor for keratinocyte growth factor. Evidence for multiple fibroblast growth factor receptors. J Biol Chem. 1990;265:12767–12770. [PubMed] [Google Scholar]
- 78.Pedchenko VK, Imagawa W. Estrogen treatment in vivo increases keratinocyte growth factor expression in the mammary gland. J Endocrinol. 2000;165:39–49. doi: 10.1677/joe.0.1650039. [DOI] [PubMed] [Google Scholar]
- 79.Pekonen F, Nyman T, Rutanen EM. Differential expression of keratinocyte growth factor and its receptor in the human uterus. Mol Cell Endocrinol. 1993;95:43–49. doi: 10.1016/0303-7207(93)90027-h. [DOI] [PubMed] [Google Scholar]
- 80.Siegfried S, Pekonen F, Nyman T, et al. Expression of mRNA for keratinocyte growth factor and its receptor in human endometrium. Acta Obstet Gynecol Scand. 1995;74:410–414. doi: 10.3109/00016349509024400. [DOI] [PubMed] [Google Scholar]
- 81.Koji T, Chedid M, Rubin JS, et al. Progesterone-dependent expression of keratinocyte growth factor mRNA in stromal cells of the primate endometrium: keratinocyte growth factor as a progestomedin. J Cell Biol. 1994;125:393–401. doi: 10.1083/jcb.125.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pedchenko VK, Imagawa WT. Mammogenic hormones differentially modulate keratinocyte growth factor (KGF)-induced proliferation and KGF receptor expression in cultured mouse mammary gland epithelium. Endocrinology. 1998;139:2519–2526. doi: 10.1210/endo.139.5.6007. [DOI] [PubMed] [Google Scholar]
- 83.Imagawa W, Pedchenko VK. In vivo inhibition of keratinocyte growth factor receptor expression by estrogen and antagonism by progesterone in the mouse mammary gland. J Endocrinol. 2001;171:319–327. doi: 10.1677/joe.0.1710319. [DOI] [PubMed] [Google Scholar]
- 84.Li Y, Rinehart CA. Regulation of keratinocyte growth factor expression in human endometrium: implications for hormonal carcinogenesis. Mol Carcinog. 1998;23:217–225. doi: 10.1002/(sici)1098-2744(199812)23:4<217::aid-mc4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 85.Siegfried S, Pekonen F, Nyman T, et al. Distinct patterns of expression of keratinocyte growth factor and its receptor in endometrial carcinoma. Cancer. 1997;79:1166–1171. doi: 10.1002/(sici)1097-0142(19970315)79:6<1166::aid-cncr15>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 86.Taniguchi F, Harada T, Sakamoto Y, et al. Activation of mitogen-activated protein kinase pathway by keratinocyte growth factor or fibroblast growth factor-10 promotes cell proliferation in human endometrial carcinoma cells. J Clin Endocrinol Metab. 2003;88:773–780. doi: 10.1210/jc.2002-021062. [DOI] [PubMed] [Google Scholar]
- 87.Zang XP, Pento JT. Keratinocyte growth factor-induced motility of breast cancer cells. Clin Exp Metastasis. 2001;18:573–580. doi: 10.1023/a:1011997317994. [DOI] [PubMed] [Google Scholar]
- 88.Tamaru N, Hishikawa Y, Ejima K, et al. Estrogen receptor-associated expression of keratinocyte growth factor and its possible role in the inhibition of apoptosis in human breast cancer. Lab Invest. 2004;84:1460–1471. doi: 10.1038/labinvest.3700166. [DOI] [PubMed] [Google Scholar]
- 89.Hishikawa Y, Tamaru N, Ejima K, et al. Expression of keratinocyte growth factor and its receptor in human breast cancer: its inhibitory role in the induction of apoptosis possibly through the overexpression of Bcl-2. Arch Histol Cytol. 2004;67:455–464. doi: 10.1679/aohc.67.455. [DOI] [PubMed] [Google Scholar]
- 90.Yang D, Chen Q, Hoover DM, et al. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74:448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 91.Perez-Canadillas JM, Zaballos A, Gutierrez J, et al. NMR solution structure of murine CCL20/MIP-3alpha, a chemokine that specifically chemoattracts immature dendritic cells and lymphocytes through its highly specific interaction with the beta-chemokine receptor CCR6. J Biol Chem. 2001;276:28372–28379. doi: 10.1074/jbc.M103121200. [DOI] [PubMed] [Google Scholar]
- 92.Taniguchi F, Harada T, Yoshida S, et al. Paracrine effects of bFGF and KGF on the process of mouse blastocyst implantation. Mol Reprod Dev. 1998;50:54–62. doi: 10.1002/(SICI)1098-2795(199805)50:1<54::AID-MRD7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 93.Slayden OD, Rubin JS, Lacey DL, et al. Effects of keratinocyte growth factor in the endometrium of rhesus macaques during the luteal-follicular transition. J Clin Endocrinol Metab. 2000;85:275–285. doi: 10.1210/jcem.85.1.6251. [DOI] [PubMed] [Google Scholar]
- 94.Matsui H, Taga M, Kurogi K, et al. Gene expressions of keratinocyte growth factor and its receptor in the human endometrium/decidua and chorionic villi. Endocr J. 1997;44:867–871. doi: 10.1507/endocrj.44.867. [DOI] [PubMed] [Google Scholar]
- 95.Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303–312. doi: 10.1016/S0002-9440(10)63654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mikami F, Gu H, Jono H, et al. Epidermal growth factor receptor acts as a negative regulator for bacterium nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via an Src-dependent p38 mitogen-activated protein kinase signaling pathway. J Biol Chem. 2005;280:36185–36194. doi: 10.1074/jbc.M503941200. [DOI] [PubMed] [Google Scholar]
- 97.Huang W, Rothe MJ, Grant-Kels JM. The burning mouth syndrome. J Am Acad Dermatol. 1996;34:91–98. doi: 10.1016/s0190-9622(96)90840-3. [DOI] [PubMed] [Google Scholar]
- 98.Congote LF. Monitoring insulin-like growth factors in HIV infection and AIDS. Clin Chim Acta. 2005;361:30–53. doi: 10.1016/j.cccn.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 99.Wira CR, Rossoll RM. Antigen-presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84:505–508. [PMC free article] [PubMed] [Google Scholar]
- 100.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 101.Fahey JV, Wallace PK, Johnson K, et al. Antigen presentation by human uterine epithelial cells to autologous T cells. Am J Reprod Immunol. 2006;55:1–11. doi: 10.1111/j.1600-0897.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 102.Wallace PK, Yeaman GR, Johnson K, et al. MHC class II expression and antigen presentation by human endometrial cells. J Steroid Biochem Mol Biol. 2001;76:203–211. doi: 10.1016/s0960-0760(00)00149-7. [DOI] [PubMed] [Google Scholar]
- 103.Wira CR, Rossoll RM, Young RC. Polarized uterine epithelial cells preferentially present antigen at the basolateral surface: Role of stromal cells in regulating Class II mediated epithelial cell antigen presentation. J Immunol. 2005;175:1795–1804. doi: 10.4049/jimmunol.175.3.1795. [DOI] [PubMed] [Google Scholar]
- 104.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 105.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 106.Iezzi G, Scotet E, Scheidegger D, et al. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur J Immunol. 1999;29:4092–4101. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 107.Kroczek RA, Mages HW, Hutloff A. Emerging paradigms of T-cell co-stimulation. Curr Opin Immunol. 2004;16:321–327. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 108.Edelstam GA, Lundkvist OE, Klareskog L, et al. Cyclic variation of major histocompatibility complex class II antigen expression in the human fallopian tube epithelium. Fertil Steril. 1992;57:1225–1229. [PubMed] [Google Scholar]
- 109.Bjercke S, Brandtzaeg P. Glandualr distribution of immunolglobulins, J chain, secretory component and HLA-DR in the human endometrium throughout the menstrual cycle. Hum Reprod. 1993;8:1420–1425. doi: 10.1093/oxfordjournals.humrep.a138271. [DOI] [PubMed] [Google Scholar]
- 110.Ljunggren G, Anderson DJ. Cytokine induced modulation of MHC class I and class II molecules on human cervical epithelial cells. J Reprod Immunol. 1998;38:123–138. doi: 10.1016/s0165-0378(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 111.Sallinen K, Verajankorva E, Pollanen P. Expression of antigens involved in the presentation of lipid antigens and induction of clonal anergy in the female reproductive tract. J Reprod Immunol. 2000;46:91–101. doi: 10.1016/s0165-0378(99)00061-3. [DOI] [PubMed] [Google Scholar]
- 112.Wira CR, Rossoll RM, Kaushic C. Antigen-presenting cells in the female reproductive tract: influence of estradiol on antigen presentation by vaginal cells. Endocrinology. 2000;141:2877–2885. doi: 10.1210/endo.141.8.7594. [DOI] [PubMed] [Google Scholar]
- 113.Petroff MG, Chen L, Phillips TA, et al. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod. 2003;68:1496–1504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]
- 114.Petroff MG, Chen L, Phillips TA, et al. B7 family molecules: novel immunomodulators at the maternal-fetal interface. Placenta. 2002;23 Suppl A:S95–S101. doi: 10.1053/plac.2002.0813. [DOI] [PubMed] [Google Scholar]
- 115.Prabhala RH, Wira CR. Sex hormone and IL-6 regulation of antigen presentation in the female reproductive tract mucosal tissues. J Immunol. 1995;155:5566–5573. [PubMed] [Google Scholar]
- 116.Wira CR, Fahey JV, White HD, et al. The Mucosal Immune System in the Human Female Reproductive Tract: Influence of Stage of the Menstrual Cycle and Menopause on Mucosal Immunity in the Uterus. In: Glasser S, Aplin J, Giudice L, Tabibzadeh S, editors. The Endometrium. London: Taylor & Francis; 2002. pp. 371–404. [Google Scholar]
- 117.Wira CR, Fahey JV, Abrahams VM, et al. Influence of stage of the reproductive cycle and estradiol on thymus cell antigen presentation. J Steroid Biochem Mol Biol. 2003;84:79–87. doi: 10.1016/s0960-0760(03)00002-5. [DOI] [PubMed] [Google Scholar]
- 118.Woof JM, Mestecky J. Mucosal immunoglobulins. Immunol Rev. 2005;206:64–82. doi: 10.1111/j.0105-2896.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 119.Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4:667–677. doi: 10.1016/s1286-4579(02)01585-x. [DOI] [PubMed] [Google Scholar]
- 120.Parr MB, Parr EL. Immunoglobulins in the female genital tract. In: Bronson RD, Alexander NJ, Anderson DJ, Branch DW, Kuteh WH, editors. Reproductive Immunology. Cambridge: Massachusetts: Blackwell Science; 1996. pp. 275–308. [Google Scholar]
- 121.Mestecky J, Moro I, Kerr MA, et al. Mucosal Immunoglobulins. In: Mestecky JLM, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. Burlington, MA: Elselvier Academic Press; 2005. pp. 153–181. [Google Scholar]
- 122.Brandtzaeg P. Role of secretory antibodies in defence against infections. Int J Med Microbiol. 2003;293:3–15. doi: 10.1078/1438-4221-00241. [DOI] [PubMed] [Google Scholar]
- 123.Tourville DR, Ogra S, Lippes J, et al. The human female reproductive tract: immunohistochemical localization of gA, gG, gM, secretory piece and lactoferrin. Am J Obstet Gynecol. 1970;108:1102–1108. doi: 10.1016/0002-9378(70)90460-6. [DOI] [PubMed] [Google Scholar]
- 124.Rebello R, Green F, Fox H. A study of secretory immune system of the female reproductive tract. Br J Obstet Gynecol. 1975;82:812–816. [PubMed] [Google Scholar]
- 125.Kutteh WH, Hatch KD, Blackwell RE, et al. Secretory immune system of the female reproductive tract: I. Immunoglobulin and secretory component-containing cells. Obstet Gynecol. 1988;71:56–60. [PubMed] [Google Scholar]
- 126.Vaerman J, Ferin J. Local immunological response in vagina, cervix and endometrium. Acta Endocrinol Suppl (Copenh) 1975;194:281–305. [PubMed] [Google Scholar]
- 127.Brandtzaeg P. Mucosal immunity in the female genital tract. J Reprod Immunol. 1997;36:23–50. doi: 10.1016/s0165-0378(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 128.Mestecky J, Moldoveanu Z, Russell MW. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am J Reprod Immunol. 2005;53:208–214. doi: 10.1111/j.1600-0897.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 129.Wira CR, Sullivan DA. Estradiol and progesterone regulation of IgA, IgG and secretory component in cervico-vaginal secretions of the rat. Biol Reprod. 1985;32:90–95. doi: 10.1095/biolreprod32.1.90. [DOI] [PubMed] [Google Scholar]
- 130.Sullivan DA, Underdown BJ, Wira CR. Steroid hormone regulation of free secretory component in the rat uterus. Immunology. 1983;49:379–386. [PMC free article] [PubMed] [Google Scholar]
- 131.Sullivan DA, Richardson GS, MacLaughlin DT, et al. Variations in the levels of secretory component in human uterine fluid during the menstrual cycle. J Steroid Biochem. 1984;20:509–513. doi: 10.1016/0022-4731(84)90263-2. [DOI] [PubMed] [Google Scholar]
- 132.Tauber PF, Wettich W, Nohlen M, et al. Diffusible proteins of the mucosa of the human cervix, uterus, and fallopian tubes: Distribution and variations during the menstrual cycle. Am J Obstet Gynecol. 1985;15:1115–1125. doi: 10.1016/0002-9378(85)90394-1. [DOI] [PubMed] [Google Scholar]
- 133.Schumacher GFB. Humoral immune factors in the female reproductive tract and their changes during the cycle. In: Dinsda D, Schumacher G, editors. Immunological Aspects of Infertility and Fertility Control. North Holland, New York: Elsevier; 1980. pp. 93–141. [Google Scholar]
- 134.Gravlee LC. Jet irrigation method for the diagnosis of endometrial carcinoma. Obstet Gynecol. 1969;34:168–172. [PubMed] [Google Scholar]
- 135.Sylvan PE, MacLaughlin DT, Richardson GS, et al. Human uterine fluid proteins associated with secretory phase endometrium: progesterone-induced proteins? Biol Reprod. 1981;24:423–429. doi: 10.1095/biolreprod24.2.423. [DOI] [PubMed] [Google Scholar]
- 136.Waldmann R, Cruz J, Rowe D. Immunoglobulin levels to Candida albicans in human cervicovaginal secretions. Clin Exp Immunol. 1971;9:427–434. [PMC free article] [PubMed] [Google Scholar]
- 137.Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006;208:270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- 138.Burns JW, Siadat-Pajouh M, Krishnaney AA, et al. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 139.Michetti P, Mahan MJ, Slauch JM, et al. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mazanec MB, Kaetzel CS, Lamm ME, et al. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci U S A. 1992;89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mazanec MB, Kaetzel CS, Lamm ME, et al. Intracellular neutralization of Sendai and influenza viruses by IgA monoclonal antibodies. Adv Exp Med Biol. 1995;371A:651–654. doi: 10.1007/978-1-4615-1941-6_137. [DOI] [PubMed] [Google Scholar]
- 142.Huang YT, Wright A, Gao X, et al. Intraepithelial cell neutralization of HIV-1 replication by IgA. J Immunol. 2005;174:4828–4835. doi: 10.4049/jimmunol.174.8.4828. [DOI] [PubMed] [Google Scholar]
- 143.Nardelli-Haefliger DJ, Wirthner D, Schiller J, et al. Specific Antibody Levels at the Cervix During the Menstrual Cycle of Women Vaccinated With Human Papillomavirus 16 Virus–Like Particles. J Nat Cancer Inst. 2003;95:1128–1137. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- 144.Dominguez F, Pellicer A, Simon C. Paracrine dialogue in implantation. Mol Cell Endocrinol. 2002;186:175–181. doi: 10.1016/s0303-7207(01)00659-1. [DOI] [PubMed] [Google Scholar]
- 145.Tabibzadeh S. The signals and molecular pathways involved in human menstruation, a unique process of tissue destruction and remodelling. Mol Hum Reprod. 1996;2:77–92. doi: 10.1093/molehr/2.2.77. [DOI] [PubMed] [Google Scholar]
- 146.Tabibzadeh S. Evidence of T-cell activation and potential cytokine action in human endometrium. J Clin Endocrinol Metab. 1990;71:645–649. doi: 10.1210/jcem-71-3-645. [DOI] [PubMed] [Google Scholar]
- 147.Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol. 2002;47:213–221. doi: 10.1034/j.1600-0897.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 148.Critchley HO, Kelly RW, Brenner RM, et al. The endocrinology of menstruation--a role for the immune system. Clin Endocrinol (Oxf) 2001;55:701–710. doi: 10.1046/j.1365-2265.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- 149.Yeaman GR, Howell AL, Weldon S, et al. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology. 2003;109:137–146. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 151.Goepfert AR, Goldenberg RL, Andrews WW, et al. The Preterm Prediction Study: association between cervical interleukin 6 concentration and spontaneous preterm birth. Am J Obstet Gynecol. 2001;184:483–488. doi: 10.1067/mob.2001.109653. [DOI] [PubMed] [Google Scholar]
- 152.Jacobsson B, Holst RM, Wennerholm UB, et al. Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol. 2003;189:1161–1167. doi: 10.1067/s0002-9378(03)00594-5. [DOI] [PubMed] [Google Scholar]
- 153.Matsuda Y, Kouno S, Nakano H. Effects of antibiotic treatment on the concentrations of interleukin-6 and interleukin-8 in cervicovaginal fluid. Fetal Diagn Ther. 2002;17:228–232. doi: 10.1159/000059374. [DOI] [PubMed] [Google Scholar]
- 154.Tanaka Y, Narahara H, Takai N, et al. Interleukin-1beta and interleukin-8 in cervicovaginal fluid during pregnancy. Am J Obstet Gynecol. 1998;179:644–649. doi: 10.1016/s0002-9378(98)70058-4. [DOI] [PubMed] [Google Scholar]
- 155.Simhan HN, Caritis SN, Krohn MA, et al. Decreased cervical proinflammatory cytokines permit subsequent upper genital tract infection during pregnancy. Am J Obstet Gynecol. 2003;189:560–567. doi: 10.1067/s0002-9378(03)00518-0. [DOI] [PubMed] [Google Scholar]
- 156.Romero R, Chaiworapongsa T, Kuivaniemi H, et al. Bacterial vaginosis, the inflammatory response and the risk of preterm birth: a role for genetic epidemiology in the prevention of preterm birth. Am J Obstet Gynecol. 2004;190:1509–1519. doi: 10.1016/j.ajog.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 157.Baggiolini M. Activation and recruitment of neutrophil leukocytes. Clin Exp Immunol. 1995;101 Suppl 1:5–6. doi: 10.1111/j.1365-2249.1995.tb06151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yoshimura T, Leonard EJ. Human monocyte chemoattractant protein-1: structure and function. Cytokines. 1992;4:131–152. [PubMed] [Google Scholar]
- 159.Shen L, Fahey JV, Hussey SB, et al. Synergy between IL-8 and GM-CSF in reproductive tract epithelial cell secretions promotes enhanced neutrophil chemotaxis. Cell Immunol. 2004;230:23–32. doi: 10.1016/j.cellimm.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 160.Meter RA, Wira CR, Fahey JV. Secretion of monocyte chemotactic protein-1 by human uterine epithelium directs monocyte migration in culture. Fertil Steril. 2005;84:191–201. doi: 10.1016/j.fertnstert.2005.01.104. [DOI] [PubMed] [Google Scholar]
- 161.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 162.Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 163.Niyonsaba F, Ogawa H, Nagaoka I. Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology. 2004;111:273–281. doi: 10.1111/j.0019-2805.2004.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. AJRI. 2005;53:65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 165.Hoover DM, Boulegue C, Yang D, et al. The structure of human macrophage inflammatory protein-3alpha /CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human beta-defensins. J Biol Chem. 2002;277:37647–37654. doi: 10.1074/jbc.M203907200. [DOI] [PubMed] [Google Scholar]
- 166.Chabalgoity JA, Baz A, Rial A, et al. The relevance of cytokines for development of protective immunity and rational design of vaccines. Cytokine Growth Factor Rev. 2007;18:195–207. doi: 10.1016/j.cytogfr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 167.Toka FN, Rouse BT. Mucosal application of plasmid-encoded IL-15 sustains a highly protective anti-Herpes simplex virus immunity. J Leukoc Biol. 2005;78:178–186. doi: 10.1189/jlb.1004621. [DOI] [PubMed] [Google Scholar]
- 168.Toka FN, Gierynska M, Rouse BT. Codelivery of CCR7 ligands as molecular adjuvants enhances the protective immune response against herpes simplex virus type 1. J Virol. 2003;77:12742–12752. doi: 10.1128/JVI.77.23.12742-12752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Toka FN, Pack CD, Rouse BT. Molecular adjuvants for mucosal immunity. Immunol Rev. 2004;199:100–112. doi: 10.1111/j.0105-2896.2004.0147.x. [DOI] [PubMed] [Google Scholar]
- 170.Tsuji T, Hamajima K, Fukushima J, et al. Enhancement of cell-mediated immunity against HIV-1 induced by coinnoculation of plasmid-encoded HIV-1 antigen with plasmid expressing IL-12. J Immunol. 1997;158:4008–4013. [PubMed] [Google Scholar]
- 171.Fahey JV, Schaefer TM, Channon JY, et al. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–1446. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 172.Wira CR, Fahey JV. The innate immune system: gatekeeper to the female reproductive tract. Immunology. 2004;111:13–15. doi: 10.1111/j.1365-2567.2003.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 174.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 175.Underhill DM. Toll-like receptors and microbes take aim at each other. Curr Opin Immunol. 2004;16:483–487. doi: 10.1016/j.coi.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 176.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 177.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 178.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like Receptors in Host Defense and Disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 179.Underhill DM. Collaboration between the innate immune receptors dectin-1, TLRs, and Nods. Immunol Rev. 2007;219:75–87. doi: 10.1111/j.1600-065X.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 180.Pioli PA, Amiel E, Schaefer TM, et al. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun. 2004;72:5799–5806. doi: 10.1128/IAI.72.10.5799-5806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fazeli A, Bruce C, Anumba DO. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum Reprod. 2005;20:1372–1378. doi: 10.1093/humrep/deh775. [DOI] [PubMed] [Google Scholar]
- 182.Itoh H, Nasu K, Nishida M, et al. Human oviductal stromal fibroblasts, but not oviductal epithelial cells, express Toll-like receptor 4: the site-specific mucosal immunity of the human fallopian tube against bacterial infection. Am J Reprod Immunol. 2006;56:91–101. doi: 10.1111/j.1600-0897.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 183.Nasu K, Itoh H, Yuge A, et al. Human oviductal epithelial cells express Toll-like receptor 3 and respond to double-stranded RNA: Fallopian tube-specific mucosal immunity against viral infection. Hum Reprod. 2007;22:356–361. doi: 10.1093/humrep/del385. [DOI] [PubMed] [Google Scholar]
- 184.Hirata T, Osuga Y, Hirota Y, et al. Evidence for the presence of toll-like receptor 4 system in the human endometrium. J Clin Endocrinol Metab. 2005;90:548–556. doi: 10.1210/jc.2004-0241. [DOI] [PubMed] [Google Scholar]
- 185.Larsen B, Monif GR. Understanding the bacterial flora of the female genital tract. Clin Infect Dis. 2001;32:e69–e77. doi: 10.1086/318710. [DOI] [PubMed] [Google Scholar]
- 186.Young SL, Lyddon TD, Jorgenson RL, et al. Expression of Toll-like receptors in human endometrial epithelial cells and cell lines. Am J Reprod Immunol. 2004;52:67–73. doi: 10.1111/j.1600-0897.2004.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ghosh M, Schaefer TM, Fahey JV, et al. Antiviral responses of human Fallopian tube epithelial cells to toll-like receptor 3 agonist poly(I:C) Fertil Steril. 2007 doi: 10.1016/j.fertnstert.2007.05.023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Eriksson M, Meadows SK, Basu S, et al. TLRs mediate IFN-gamma production by human uterine NK cells in endometrium. J Immunol. 2006;176:6219–6224. doi: 10.4049/jimmunol.176.10.6219. [DOI] [PubMed] [Google Scholar]
- 189.Shen L, Smith JM, Shen Z, et al. Inhibition of human neutrophil degranulation by transforming growth factor-beta1. Clin Exp Immunol. 2007;149:155–161. doi: 10.1111/j.1365-2249.2007.03376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Aflatoonian R, Tuckerman E, Elliott SL, et al. Menstrual cycle-dependent changes of Toll-like receptors in endometrium. Hum Reprod. 2007;22:586–593. doi: 10.1093/humrep/del388. [DOI] [PubMed] [Google Scholar]
- 191.Hirata T, Osuga Y, Hamasaki K, et al. Expression of toll-like receptors 2, 3, 4, and 9 genes in the human endometrium during the menstrual cycle. J Reprod Immunol. 2007;74:53–60. doi: 10.1016/j.jri.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 192.Yao XD, Fernandez S, Kelly MM, et al. Expression of Toll-like receptors in murine vaginal epithelium is affected by the estrous cycle and stromal cells. J Reprod Immunol. 2007;75:106–119. doi: 10.1016/j.jri.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 193.Ishii KJ, Akira S. Toll or toll-free adjuvant path toward the optimal vaccine development. J Clin Immunol. 2007;27:363–371. doi: 10.1007/s10875-007-9087-x. [DOI] [PubMed] [Google Scholar]
- 194.Mata-Haro V, Cekic C, Martin M, et al. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 195.West MA, Wallin RP, Matthews SP, et al. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 196.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 197.Blander JM. Coupling Toll-like receptor signaling with phagocytosis: potentiation of antigen presentation. Trends Immunol. 2007;28:19–25. doi: 10.1016/j.it.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 198.King AE, Critchley HO, Kelly RW. Innate immune defenses in the human endometrium. Reprod Biol Endocrinol. 2003;1:116–123. doi: 10.1186/1477-7827-1-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang D, Chertov O, Oppenheim JJ. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37) J Leukoc Biol. 2001;69:691–697. [PubMed] [Google Scholar]
- 200.Williams SE, Brown TI, Roghanian A, et al. SLPI and elafin: one glove, many fingers. Clin Sci (Lond) 2006;110:21–35. doi: 10.1042/CS20050115. [DOI] [PubMed] [Google Scholar]
- 201.Weinberg A, Quinones-Mateu ME, Lederman MM. Role of human beta-defensins in HIV infection. Adv Dent Res. 2006;19:42–48. doi: 10.1177/154407370601900109. [DOI] [PubMed] [Google Scholar]
- 202.Shugars DC. Endogenous mucosal antiviral factors of the oral cavity. J Infect Dis. 1999;179 Suppl 3:S431–S435. doi: 10.1086/314799. [DOI] [PubMed] [Google Scholar]
- 203.Capobianchi MR. Induction of lymphomonocyte activation by HIV-1 glycoprotein gp120. Possible role in AIDS pathogenesis. J Biol Regul Homeost Agents. 1996;10:83–91. [PubMed] [Google Scholar]
- 204.Garzino-Demo A. Chemokines and defensins as HIV suppressive factors: an evolving story. Curr Pharm Des. 2007;13:163–172. doi: 10.2174/138161207779313696. [DOI] [PubMed] [Google Scholar]
- 205.Porter E, Yang H, Yavagal S, et al. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect Immun. 2005;73:4823–4833. doi: 10.1128/IAI.73.8.4823-4833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Feng Z, Jiang B, Chandra J, et al. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans. J Dent Res. 2005;84:445–450. doi: 10.1177/154405910508400509. [DOI] [PubMed] [Google Scholar]
- 207.John M, Keller MJ, Fam EH, et al. Cervicovaginal secretions contribute to innate resistance to herpes simplex virus infection. J Infect Dis. 2005;192:1731–1740. doi: 10.1086/497168. [DOI] [PubMed] [Google Scholar]
- 208.Garzino-Demo A, DeVico AL, Gallo RC. Chemokine receptors and chemokines in HIV infection. J Clin Immunol. 1998;18:243–255. doi: 10.1023/A:1027329721892. [DOI] [PubMed] [Google Scholar]
- 209.Cummins JE, Christensen L, Lennox JL, et al. Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res Hum Retroviruses. 2006;22:788–795. doi: 10.1089/aid.2006.22.788. [DOI] [PubMed] [Google Scholar]
- 210.Oberley RE, Goss KL, Ault KA, et al. Surfactant protein D is present in the human female reproductive tract and inhibits Chlamydia trachomatis infection. Mol Hum Reprod. 2004;10:861–870. doi: 10.1093/molehr/gah117. [DOI] [PubMed] [Google Scholar]
- 211.Logdberg L, Wester L. Immunocalins: a lipocalin subfamily that modulates immune and inflammatory responses. Biochim Biophys Acta. 2000;1482:284–297. doi: 10.1016/s0167-4838(00)00164-3. [DOI] [PubMed] [Google Scholar]
- 212.Ganz T. Antimicrobial polypeptides. J Leukoc Biol. 2004;75:34–38. doi: 10.1189/jlb.0403150. [DOI] [PubMed] [Google Scholar]
- 213.Cole AM, Liao HI, Stuchlik O, et al. Cationic polypeptides are required for antibacterial activity of human airway fluid. J Immunol. 2002;169:6985–6991. doi: 10.4049/jimmunol.169.12.6985. [DOI] [PubMed] [Google Scholar]
- 214.Fluckinger M, Haas H, Merschak P, et al. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother. 2004;48:3367–3372. doi: 10.1128/AAC.48.9.3367-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.King AE, Critchley HO, Sallenave JM, et al. Elafin in human endometrium: an antiprotease and antimicrobial molecule expressed during menstruation. J Clin Endocrinol Metab. 2003;88:4426–4431. doi: 10.1210/jc.2003-030239. [DOI] [PubMed] [Google Scholar]
- 216.Moutsopoulos NM, Greenwell-Wild T, Wahl SM. Differential mucosal susceptibility in HIV-1 transmission and infection. Adv Dent Res. 2006;19:52–56. doi: 10.1177/154407370601900111. [DOI] [PubMed] [Google Scholar]
- 217.Steinstraesser L, Tippler B, Mertens J, et al. Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides. Retrovirology. 2005;2:2. doi: 10.1186/1742-4690-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14:432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- 219.Deonarain R, Alcami A, Alexiou M, et al. Impaired antiviral response and alpha/beta interferon induction in mice lacking beta interferon. J Virol. 2000;74:3404–3409. doi: 10.1128/jvi.74.7.3404-3409.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Haller O, Kochs G, Weber F. Interferon, Mx, and viral countermeasures. Cytokine Growth Factor Rev. 2007;18:425–433. doi: 10.1016/j.cytogfr.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Garcia MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 222.Holmes RK, Malim MH, Bishop KN. APOBEC-mediated viral restriction: not simply editing? Trends Biochem Sci. 2007;32:118–128. doi: 10.1016/j.tibs.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 223.Keller M, Guzman E, Hazrati E, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21:467–476. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 224.Martin HL, Jr, Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178:1053–1059. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 225.Smith SM, Mefford M, Sodora D, et al. Topical estrogen protects against SIV vaginal transmission without evidence of systemic effect. Aids. 2004;18:1637–1643. doi: 10.1097/01.aids.0000131393.76221.cc. [DOI] [PubMed] [Google Scholar]
- 226.Lederman MM, Veazey RS, Offord R, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–487. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]