Summary
In Mycobacterium tuberculosis a rhamnosyl transferase (WbbL) catalyzes the transfer of an α-L-Rhap residue from dTDP-Rha to decaprenyldiphosphoryl-α-D-N-acetyl glucosamine (GlcNAc-P-P-DP) to form α-L-Rhap-(1→3)-α-D-GlcNAc-P-P-DP, which is then further elongated with Galf and Araf units and finally mycolylated and attached to the peptidoglycan. This enzyme is essential for M. tuberculosis viability and at the same time absent in eukaryotic cells, and therefore it is a good target for the development of new antituberculosis therapeutics. Here we report a microtiter plate based method for the assay for this enzyme using a crude membrane preparation from an E. coli strain overexpressing wbbL as an enzyme source, and the natural acceptor substrate GlcNAc-P-P-decaprenyl. Initial characterization of the enzyme included unequivocal identification of the product Rha-GlcNAc-P-P-DP by LC/MS and the facts that WbbL shows an absolute requirement for divalent cations and its activity is stimulated by β-mercaptoethanol. Its pH optimum and basic kinetic parameters were also determined and the kinetic analysis showed that WbbL uses a ternary complex mechanism reaction. The microtiter plate based assay for this enzyme was developed by taking advantage of the lipophilic nature of the product. This assay should be readily transferable to other glycosyl transferases which use lipid based acceptors and aid greatly in obtaining inhibitors of such glycosyl transferases for the purposes of new drug development.
Keywords: glycosyl transferase assay, rhamnosyl transferase, WbbL, tuberculosis, drug targeting, tuberculosis
Introduction
Many bacterial glycosyl transferases involved in the cell wall biosynthesis transfer a sugar from a sugar nucleotide to a lipid (often polyisoprene) containing acceptor. The N-acetylglucosamine (GlcNAc) transferase, MurG, is a key peptidoglycan synthetic enzyme that utilizes a lipid containing acceptor (Menginlecreulx et al., 1991). Another important example in gram positive bacteria is TagA (Zhang et al., 2006b) where a N-acetylmannosamine is attached to an undecaprenyl diphosphate linked GlcNAc. In the mycobacterial cell wall the biosynthesis of the polymer, arabinogalactan which connects the peptidoglycan and mycolic acids layers occurs on the lipid decaprenyl phosphate (DP) (Mikusova et al., 1996). Here a rhamnosyl residue is attached to a decaprenyl diphosphate linked GlcNAc by the rhamnosyl transferase WbbL (Mills et al., 2004). Convenient and inexpensive assays to search for inhibitors of such enzymes are lacking and using WbbL we describe the development of such an assay herein.
The wbbL gene is found in the genome of all mycobacteria. The protein sequence of this enzyme does not show homology to other rhamnosyl transferases in mycobacteria that synthesize various glycolipids. Previous studies with the temperature sensitive mutant of Mycobacterium smegmatis mc2155 (Mills et al., 2004) have shown that WbbL is essential for mycobacterial viability. The incubation of the temperature sensitive mutant at the non permissive temperature resulted in bacteria which could not be recovered at the lower permissive temperature suggesting that the inactivation of WbbL is a lethal defect (Mills et al., 2004). Therefore a facile assay for its activity is particularly important in the development of new TB drugs.
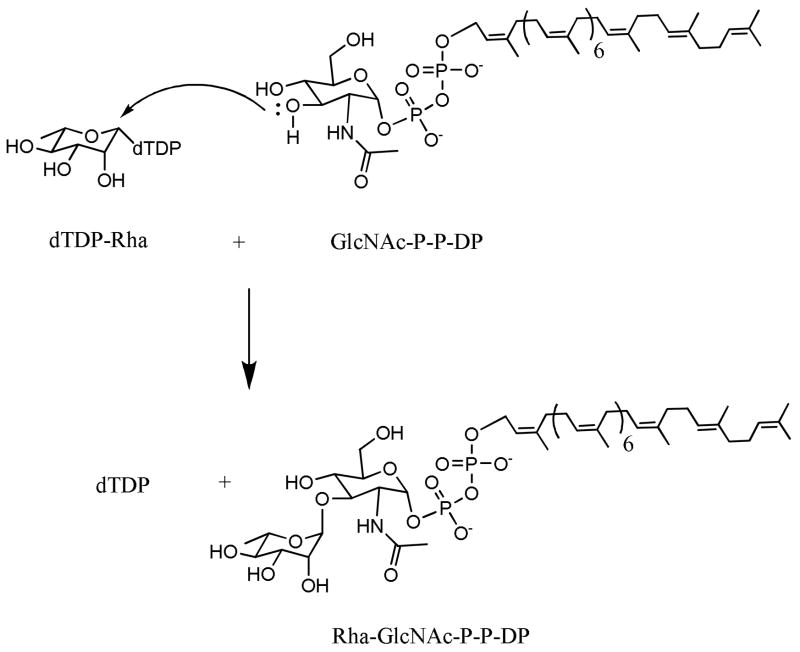
The substrates for WbbL are dTDP-Rha and decaprenyldiphosphoryl-D-N-acetyl glucosamine (GlcNAc-P-P-DP) (Fig. 1). GlcNAc-P-P-DP has hitherto not been available and all assays for WbbL have been done using crude membranes that contain the GlcNAc-1-P transferase, WecA, and endogenous DP which allows the in situ preparation of the GlcNAc-P-P-DP acceptor when UDP-GlcNAc is provided. Although such assays were used to identify the gene encoding WbbL (Mills et al., 2004) they are clearly inadequate to characterize the enzyme and to develop specific assays for its activity. Using WbbL substrates directly, we first describe the characterization of WbbL in terms of pH optimum, ion dependence, kinetics and then go on to describe the development of a microtiter plate based assay for its activity that takes advantage of the lipid nature of the enzyme product.
Fig. 1.
The reaction catalyzed by rhamnosyl transferase (WbbL).
Methods
PCR, Cloning, and expression
The DNA sequence of M. tuberculosis wbbL gene (906 bp) was acquired from M. tuberculosis genome database (http://genolist.pasteur.fr/TubercuList/). M. tuberculosis wbbL was amplified from M. tuberculosis H37Rv genomic DNA by Vent DNA polymerase (New England Biolabs) using CATATGGTAGCGGTGACCTAC and GGATCCTCAGTGCCGCCCTTC primers. NdeI and BamHI sites in primers facilitated cloning into pSTBlue 1 palsmid. The resulting pSTB-Mtb wbbL plasmid was digested by NdeI and BamHI and the wbbL gene was ligated into the NdeI and BamHI sites of plasmid pET16b, yielding an expression vector pET16b-Mtb wbbL. For expression, pET16b-Mtb wbbL was transformed into the following E.coli strains: BL21[DE3](Novagen), BL21[DE3]pLysS (Novagen), BL21[DE3] codon plus (Novagen), C41DE3(Miroux & Walker, 1996), ArcticExpressTMRP (Stratagene), ER2566 (New England Biolabs, Beverly, MA) and BL21[DE3] containing pKJE7 (TakaraMirus BioInc., Madison WI). Expression from pET16b in E. coli (ER2566) produced substantial WbbL in the membrane fraction and was used in further studies. The cells (E. coli, ER2566) were grown in LB medium containing 100 μg ml−1 of ampicillin at 37°C until the culture reached an OD600 0.6 and then induced with 0.5 mM IPTG for 4 hours at RT. The cells were harvested, then resuspended in 100 mM TAPS (3-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino-1-propanesulfonic acid) buffer pH 8.6 containing 5 mM β-mercaptoethanol and 10 mM MgCl2 (buffer 1) and broken by French press. The broken cells were centrifuged at 20,000 × g for 10 min and the obtained supernatant was ultracentrifuged at 100,000 × g for 2 h. The pellet containing membranes fractions was homogenized in buffer 1 and store at −80°C.
SDS-PAGE
SDS-PAGE was run using 12% gels and proteins were visualized with Coomassie brilliant blue and analyzed by Western blot using monoclonal anti-polyhistidine antibody (mouse IgG2a isotype; Sigma) as the first antibody and anti-mouse-IgG-alkaline phosphatase conjugate antibody (Sigma) as the second antibody.
dTDP-Rha and dTDP-[14C]Rha preparation
dTDP-Rha was made from dTDP-Glc (Sigma-Aldrich) using recombinant RmlB (conversion to dTDP-4-keto-6-deoxy-D-xylohexulose), RmlC (di epimerization to form dTDP-4-keto-6-deoxyl-L-lyxo-hexulose) and RmlD (reduces the 4-keto group to form dTDP-Rha (Mills et al., 2004b). The standard 1 ml reaction mixture contained 1.2 mM dTDP-Glc, 1.8 mM NADPH, 20 mM MgCl2, 53 μg of RmlB from Salmonella typhimurium, 13 μg of RmlC (TB) and 5 μg of RmlD (TB) in 200 mM Tris-HCl pH 7.5. After 1 h incubation at 30°C the reaction mixture was centrifuged using Amicon Microcon with 10 kDa cut off membranes to remove protein. dTDP-Rha was purified by HPLC on Dionex column (CarboPacTm, PA-100). The HPLC flow rate was 2 ml min−1 and a gradient from 0–500 mM triethylammonium acetate over 30 min was applied. The fractions were monitored by reading absorbance at 260 nm and checked by analytical HPLC (isocratic gradient of 200 mM KH2PO4). The retention times for dTDP-Rha and dTDP-Glc were 5.74 and 6.52 respectively. The fractions containing dTDP-Rha were pooled and lyophilized. For quantification of dTDP-Rha, alditol acetates were prepared and analyzed by GC-MS. dTDP-[14C]Rha was made as previously described (Mills et al., 2004a). Briefly, [14C] sucrose (American Radiolabelled Chemicals) was converted to [14C]Glc-1-P by commercially available sucrose phosphorylase. Subsequently [14C]Glc-1-P and TTP were converted to dTDP-[14C] Glc by RmlA. The conversion of dTDP-[14C] Glc to dTDP-[14C]-Rha and purification was performed as described above for non-radioactive dTDP-Rha.
Enzymatic synthesis and purification GlcNAc-P-P-DP
Enzymatic synthesis of GlcNAc-P-P-DP was adopted from (Rush et al., 1997). GlcNAc-P-P-DP was synthesized from DP (Indofine) and UDP-GlcNAc using WecA enzyme. The membranes, overexpressing WecA enzyme, were prepared as previously described (Hyland & Anderson, 2003). The reaction mixture contained 0.5 mg of membrane protein, 50 mM Tris pH 8.0, 5 mM β-mercaptoethanol, 40 mM MgCl2,0.5% Chaps, 1 mM sodium orthovanadate, 4 mM UDP-GlcNAc and 2 mM DP in a total volume of 0.5 ml. After incubation for 1.5 h at 30°C the reaction was quenched by the addition of 7 ml of chloroform:methanol (2:1, v/v) and shaken at room temperature for 20 min. After addition of 500 μl of water, the organic layer was dried. The organic fraction was dissolved in 0.2 ml of 0.2 M NaOH in CH3OH and incubated for 20 min at 37°C (Mikusova, Mikus, Besra, Hancock, & Brennan, 1996). After neutralization of NaOH with 5 μl of CH3COOH the reaction mixture was dried, resuspended in 1.75 ml of chloroform:methanol:water (4:2:1, v/v/v). After extraction the organic layer was dried and applied to aluminum oxide column (0.5 × 5 cm). The column was developed with 6 column volumes of chloroform:methanol:ammonium hydroxide:water (6.5:2.5:0.2:0.2, v/v/v/v), followed by 8 volumes of chloroform: methanol:ammonium hydroxide:water (10:10:0.046:3, v/v/v/v). The fractions were checked by TLC (chloroform:methanol:ammonium hydroxide:water ; 65:25:0.5:3.5, v/v/v/v). The fractions containing GlcNAc-P-P-DP were pooled and dried. The yield, after quantification of GlcNAc-P-P-DP by preparation of alditol acetates followed by GC/MS analysis, was 0.25 mg providing enough substrate for 5 microtiter plates using the microtiter plate assay described below.
Deprotection of per-O-acetylated GlcNAc-P-P-DP
Per-O-acetylated GlcNAc-P-P-DP was provided as a gift by Drs. Michio Kurosu and Kai Li and prepared by them as described (Kurosu & Li, 2008) using acetylated GlcNAc-1-phosphate (Imperiali & Zimmerman, 1990) in the coupling reaction with DP. The O-acetylated product was purified by preparative TLC (RP-18F254s, Merck) using 0.05M NH4HCO3:THF (2:3, v/v). Deprotection was accomplished by treatment with 0.2 M anhydrous sodium methoxide in methanol for 30 min at room temperature. The mixture was neutralized with Dowex 50 (H+) and dried. The final product was stored at −80°C in chloroform:methanol:ammonium hydroxide mixture (2:1:0.003, v/v/v). The identity of product was verified by negative ion mass spectroscopy (ESI-MS). The mass spectrum was dominated by the M-1 ion m/z of 1060.640, which corresponds to calculated mass of 1060.641.
Non-microtiter plate enzyme assay
The reaction mixture contained 100 mM TAPS pH 8.6, 5 mM β-mercaptoethanol, 10 mM MgCl2, 30 μM GlcNAc-P-P-DP, 30 μM dTDP-Rha, 1.2 μM dTDP-[14C]Rha (20,000 cpm),0.1% n-octyl- β-glucopyranoside and 5 μg of membrane proteins in a total volume of 25 μl. GlcNAc-P-P-DP was dried in a test tube and resuspended in 1% n-octyl-β-glucopyranoside by ultrasounds (ultrasound bath, 1 min) prior to the addition of other compounds. The reaction was initiated by adding membrane proteins. After incubation at 30°C for 30 min the reaction was quenched by the addition of 1 ml of chloroform:methanol (2:1, v/v) and 141 μl of water. After separation of the organic layer from the aqueous layer a portion of the organic layer was subjected to scintillation counting. For TLC, the organic layer was dissolved in chloroform:methanol (2:1, v/v) and TLC plates were run in chloroform:methanol:ammonium hydroxide:water (65:25:0.5:3.5, v/v/v/v) solvent system. Autoradiography was carried using a phosphoimager system.
LC-MS of Rha-GlcNAc-P-P-DP
LC-MS was performed using Agilent 6220 TOF spectrometer. A C-18 X-bridge (Waters) HPLC column was used at a flow rate was 0.32 ml min−1. A gradient was run starting with 5mM ammonium acetate in methanol and progressing linearly to 5 mM ammonium acetate in 20% hexanes in n-propanol over 45 minutes. The column temperature was 45°C. The instrument was operated in an electrospray negative ionization mode (ESI−) and scanned from 180 to 3200 amu. The sample for analysis was prepared as described above with a minor modification. The total volume of the reaction mixture was increased to 100 μl and only 2 μg of membrane protein was used in the reaction. The incubation time was extended to 1 h. The negative control sample was exactly the same as the positive sample except that GlcNAc-P-P-DP was omitted.
Kinetic studies
For the determination of apparent Km, substrates were added at various concentrations and protein concentration was reduced to 2 μg. Incubation times were chosen to obtain initial rate data with conversion to product less than 20%. The apparent Km’s were obtained by nonlinear regression method using the program Grafit 5.0. To determine if WbbL follows a ternary complex reaction mechanism or a double displacement (ping-pong), the reaction velocity over a range of dTDP-Rha or GlcNAc-P-P-DP concentrations at several fixed concentration of both substrates was measured.
Microtiter plate based assay
The assay for rhamnosyl transferase was performed in 96-well scintillation plates (MicroBeta 1450). The reaction mixture contained 100 mM TAPS pH 8.6, 5 mM β-mercaptoethanol, 10 mM MgCl2, 18 μM GlcNAc-P-P-DP, 35 μM dTDP-Rha, dTDP-[14C]Rha (6,000 to 25,000 cpm depending on the experiment), 0.1% n-octyl-β-glucopyranoside and 1 μg of membrane proteins in a total volume of 25 μl. GlcNAc-P-P-DP was dried in a test tube and resuspended in 1% n-octyl-β-glucopyranoside by ultrasound (ultrasound bath, 10 min) prior to the addition of 100 mM TAPS pH 8.6, 5 mM β-mercaptoethanol, 10 mM MgCl2 and 1 μg of membrane protein. This mixture (15 μl) was applied to the wells of the microtiter plate. The reaction was initiated by adding the mixture of dTDP-Rha and dTDP-[14C]Rha in buffer (10 μl) and was performed for 15 min at 30°C. The reaction was stopped by adding 100 μl of water saturated butanol and thoroughly mixed 20 times using a multichannel pipette. Then 75 μl of “Organic Ready” scintillation cocktail was added.
The plates were counted using Perkin-Elmer Life Sciences Microbeta Trilux scintillation system.
Results
Cloning and expression of M. tuberculosis Rv3265c
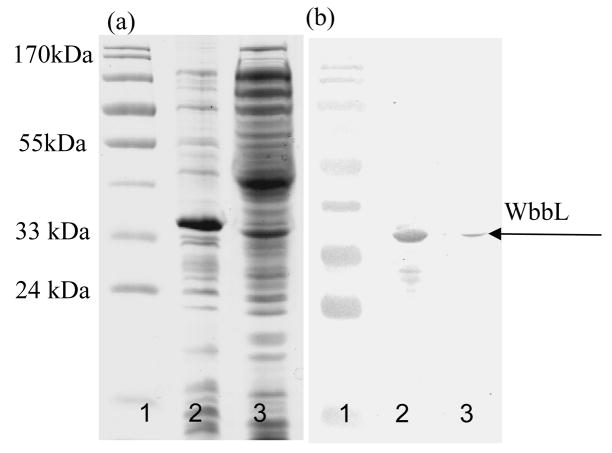
The analysis of the sequence of Rv3265c by Sosui (http://bp.nuap.nagoyau.ac.jp/sosui/sosui_submit.html) indicated that WbbL is a soluble protein but our data suggested that it is a very tightly associated peripheral membrane protein. After investigation of several systems, expression from pET16b in E. coli (ER2566) produced substantial WbbL in the membrane fraction (Fig. 2). The recombinant protein showed molecular weight consistent with that predicted for Rv3265c-Histag (~34 kDA). A small amount of enzyme was also expressed in a soluble form (Fig. 2). Soluble and membrane bound enzyme was assayed using dTDP-[14C]-Rha and GlcNAc-P-P-DP in the non-microtiter plate assay as described in methods (the synthetic form of GlcNAc-P-P-DP was used in most experiments given its greater yield). Both soluble and membrane bound enzyme were active but the soluble fraction could not be purified via a Ni column and was not a practical enzyme source. There was no enhancement of soluble enzyme after expression in chaperones-producing strains like ArcticExpressTMRP or BL21[DE3]:pKJE7. Cloning Rv3265c using GST-gene fusion system did not improve solubility in a significant way nor did solubilization from membranes using detergents and/or salts. Thus the enzyme was used directly in its membrane associated form.
Fig. 2.
SDS-PAGE analysis of subcellular fractions from E. coli expressing Rv 3265c (WbbL) visualized by (a) Coomassie brilliant blue and (b) by Western blot. For both (a) and (b) the lanes are: molecular weight markers (1); membranes (2); cytosol (3).
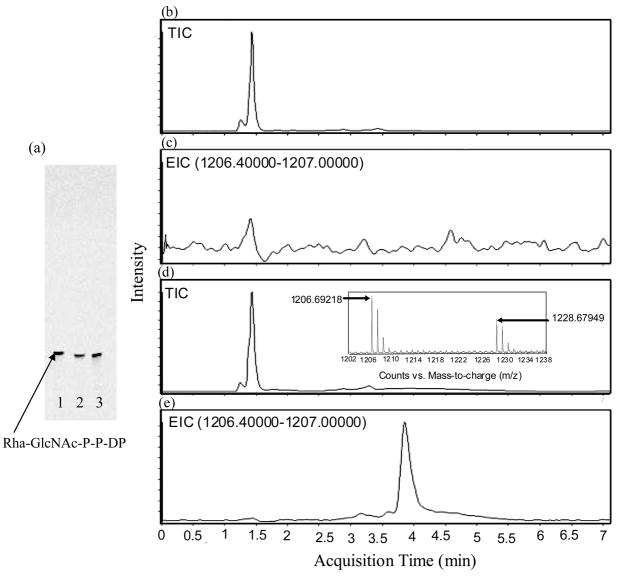
The identity of the lipid product produced by the membrane associated enzyme was checked by TLC. A single band migrating the same as a standard of Rha-GlcNAc-P-P-DP was present (Fig. 3a). Analysis of WbbL lipid product was performed by negative ion mass spectrometry (ESI-MS). The standard reaction sample showed the presence of a M-1 ion at m/z 1206.6921, which corresponds to the calculated Rha-GlcNAc-P-P-DP (minus a proton) mass of 1206.6986 (Fig. 3d and Fig. 3e). There was no presence of this ion in a control sample lacking the acceptor substrate GlcNAc-P-P-DP (Fig. 3b and Fig. 3c).
Fig. 3.
TLC and LC-MS-EIS analysis of WbbL lipid product.
(a) TLC analysis of organic soluble material obtained after incubation with WbbL expressed in (1) membranes or, (2) cytosol of E. coli. A standard of Rha-GlcNAc-P-P-DP prepared using M. smegmatis membranes (Mikusova, Mikus, Besra, Hancock, & Brennan, 1996) is also shown (3). Organic soluble material obtained after incubation with membranes was analyzed by HPLC-MS (electrospray negative mode). (b) The total ion chromatogram and (c) the m/z 1206 ion chromatogram obtained after injection of the control reaction (no GlcNAc-P-P-DP). (d) The total ion chromatogram and (e) the m/z 1206 ion chromatogram obtained after injection of the complete reaction mixture. The mass spectral region between m/z 1202 and 1238 is shown as an insert in (d); the ion at m/z 1228 represent the M-2H+Na ion.
Determining pH and temperature optima and divalent cations requirements
Preliminary experiments were carried out to determine suitable conditions for studying the kinetics and developing a microtiter plate based assay for WbbL. The enzyme showed optimum activity at pH 8.6. and was absolutely dependent on the presence of divalent cation. The addition of 5 mM EDTA abolished the enzyme activity almost completely. The enzyme activity was restored after adding to buffer containing 5mM EDTA, 20 mM Mg++ or 20 mM Mn++, but not 20 mM Ca++. The optimum temperature for the activity was determined to be 30°C. It was also found that β-mercaptoethanol is required for the enzyme activity (data not presented).
Kinetic studies
The kinetic parameters of Rv3265c were investigated using different concentration of substrates. The apparent Km for GlcNAc-P-P-DP was determined to be 18 μM at 35 μM dTDP-Rha and the apparent Km for dTDP-Rha was found to be 35 μM at 18 μM GlcNAc-P-P-DP.
To determine if WbbL follows a ternary complex reaction mechanism or a double displacement (ping-pong), the reaction velocity over a range of dTDP-Rha or GlcNAc-P-P-DP concentrations at several fixed concentrations of the other substrate were measured (Table 1a and Table 1b). The Km value of both substrates remained almost the same over a wide range of the other substrate concentration, indicating that the enzyme utilizes a ternary complex mechanism in which the two substrates must bind together to the enzyme. Since the substrate inhibition was observed above 60 μM for both substrates, kinetic studies were limited to lower concentrations.
Table 1.
| (a). Apparent Km of GlcNAc-P-P-DP at indicated concentrations of dTDP-Rha.
| |
|---|---|
| dTDP-Rha (μM)* | Km of GlcNAc-P-P-Dec |
|
| |
| 20 | 17 ± 9 |
| 35 | 18 ± 4 |
| 45 | 16 ± 3 |
| 50 | 15 ± 2 |
| 64 | 15 ± 4 |
| 72 | 16 ± 3 |
|
Table 1 (b). Apparent Km of dTDP-Rha at indicated concentrations of GlcNAc-P-P-DP | |
| GlcNAc-P-P-Dec (μM)* | Km of dTDP-Rha (μM) |
|
| |
| 10 | 35 ± 10 |
| 18 | 35 ± 7 |
| 35 | 36 ± 7 |
| 40 | 33 ± 17 |
| 50 | 36 ± 5 |
| 60 | 34 ± 3 |
| 72 | 33 ±12 |
| 80 | 32 ± 3 |
| 120 | 36 ± 12 |
Lower and higher concentrations were repeated at least two times
Development of a microtiter plate based assay for rhamnosyl transferase
We have taken advantage of the organic nature of product to determine enzymatic activity by measuring the incorporation of radioactive product [14C]Rha-GlcNAc-P-P-DP into a scintillation cocktail (Ready Organic) that is immiscible with aqueous material. We found that by adding butanol to the microtiter plate wells followed by Ready Organic scintillation cocktail we could get reasonable (~ 67%) counting efficiency of the Rha-GlcNAc-P-P-decaprenyl product as determined by comparing the Ready Organic/butanol microtiter plate counting with counting the product prepared by the standard extraction protocol. The efficiency of counting a known amount of radioactivity in dTDP-[14C]-Rha (determined by standard liquid scintillation counting) in the Ready Organic scintillation cocktail mixed with butanol was very low at only ~ 1.5%.
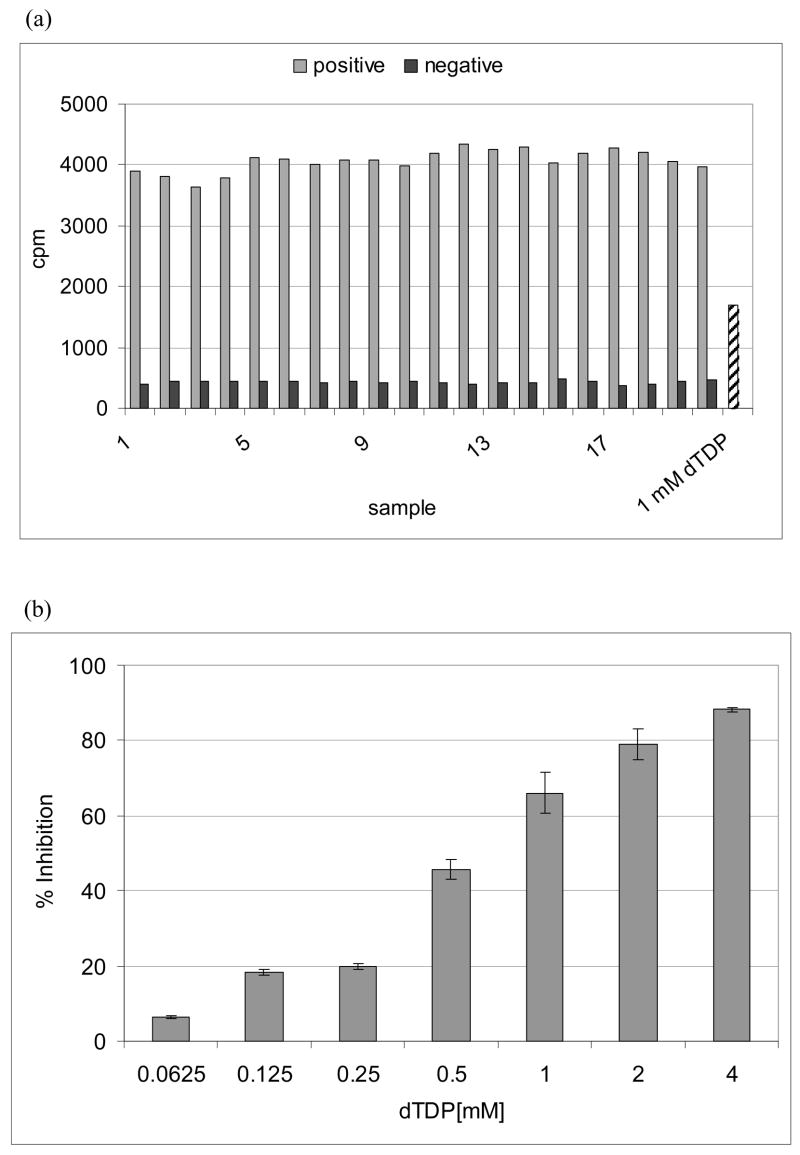
The microtiter plate based assay was run using substrates at their Km concentrations. To stay in the linear range it was decided to perform a microtiter plate based assay at 20% conversion of dTDP-[14C]-Rha. We then determined that DMSO had no negative effect up to at least 2% v/v and in fact increased the counting efficiency to 80% (data not presented). We next determined the Z’ value (Zhang et al., 1999) for the assay using positive (all components including 1% DMSO) and background (same as positive except lacking enzyme). This data (Fig. 4a) yielded a Z’ value of be 0.83 indicating an excellent assay. The assay was further validated by showing inhibition of WbbL activity in a dose dependent manner by dTDP (Fig. 4b).
Fig. 4.
A validation of a microtiter plate based assay for WbbL
(a) Z’ factor determination for microtiter plate based assay was done with 20 positive and 20 negative controls (no enzyme). (b) Titration of dTDP.
The assay was performed using 18 μM GlcNAc-P-P-DP, 35 μM dTDP-Rha, 1.5 μM dTDP-[14C]Rha (25,000 cpm) and 1 μg of membrane proteins.
Discussion
WbbL has been classified into family 2 of the GTs with a characteristic fold of superfamily GT-A (http://cazy.org/tools/search.html). GT-2 enzymes use inverting mechanism, as does WbbL which utilizes dTDP-β-rhamnose as a substrate and generates an α-rhamnosyl product. Given its inverting mechanism it would be expected to catalyze the attachment of the rhamnosyl residue in an SN2 like fashion. Our kinetic evidence is consistent with this mechanism as it clearly shows that WbbL forms a ternary complex with both substrates being bound. However detailed kinetic studies were hampered by substrate inhibition. A similar phenomenon was observed by (Zhang et al., 2006a) in working with Bacillus subtilis TagA, a glycosyltransferase that catalyzes a very similar reaction, i.e. the formation of a β-glycosidic bond between N-acetylmannosamine and undecaprenyldiphosphoryl-D-N-acetyl glucosamine (also inversion—in this case an αsugar nucleotide to a β-linkage).
An earlier assay for enzymes with lipid linked acceptors uses a solid-liquid bead-based separation system to selectively adsorb the highly hydrophobic products of the reaction (Hyland & Anderson, 2003). In contrast, the microtiter plate based assay herein is based on the organic solubility of the WbbL product, Rha-GlcNAc-P-P-DP in distinction to the aqueous solubility of the dTDP-Rha donor and, importantly, no scintillation beads are required. The key component to transferring from an extraction method to direct and selective counting in an organic scintillation cocktail was the addition of butanol to the reaction mixtures; otherwise little radioactivity was detected even when radioactive product was present. The microtiter plate assay shows good reproducibility and should aid dramatically in the effort to detect WbbL inhibitors in new TB drug discovery efforts. Furthermore this methodology is readily applicable to other enzymes such as TagA that transfer a glycosyl residue from a sugar nucleotide to a lipid linked substrate.
Acknowledgments
We gratefully acknowledge the gift of synthetic GlcNAc-P-P-decaprenyl from Kai Li and Michio Kurosu. We also would like to thank Dr. Matt Anderson for providing pET 11a-WecA plasmid, Dr. Vara Vissa for help in constructing truncated WbbL and Donald Dick for running LCMS. This work was supported by U.S. Public Health Service Grant NIH-NIAID AI 33706.
Abbreviations
- AG
arabinogalactan
- dTDP-Rha
dTDP-L rhamnose
- ESI
electrospray ionization
- GC-MS
gas chromatography-mass spectrometry
- GlcNAc-P-P-DP
decaprenyldiphosphoryl-α-D-N-acetyl glucosamine
- GT
glycosyltransferase
- IPTG
isopropyl-β-D-thiogalactopyranose
- LC
liquid chromatography
- TAPS
3-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino-1-propanesulfonic acid
- TB
tuberculosis
Footnotes
This is an author manuscript that has been accepted for publication in Microbiology, copyright Society for General Microbiology, but has not been copy-edited, formatted or proofed. Cite this article as appearing in Microbiology. This version of the manuscript may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 17, Title 17, US Code), without permission from the copyright owner, Society for General Microbiology. The Society for General Microbiology disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by any other parties. The final copy-edited, published article, which is the version of record, can be found at http://mic.sgmjournals.org, and is freely available without a subscription.
Contents category: Biochemistry and Molecular Biology
Reference List
- Hyland SA, Anderson MS. A high-throughput solid-phase extraction assay capable of measuring diverse polyprenyl transferases as exemplified by the phosphate: sugar-1-phosphate WecA, MraY, and MurG proteins. Analytical Biochemistry. 2003;317:156–164. doi: 10.1016/s0003-2697(03)00088-5. [DOI] [PubMed] [Google Scholar]
- Imperiali B, Zimmerman JW. Synthesis of Dolichypyrophosphate-linked Oligosaccharides. Tetrahedron Lett. 1990;31:6485–6488. [Google Scholar]
- Kurosu M, Li K. Synthetic studies on Mycobacterium tuberculosis specific fluorescent Park’s nucleotide probe. Heterocycles. 2008;76 In Press. [Google Scholar]
- Menginlecreulx D, Texier L, Rousseau M, Vanheijenoort J. The Murg Gene of Escherichia-Coli Codes for the Udp-N-Acetylglucosamine - N-Acetylmuramyl-(Pentapeptide) Pyrophosphoryl-Undecaprenol N-Acetylglucosamine Transferase Involved in the Membrane Steps of Peptidoglycan Synthesis. Journal of Bacteriology. 1991;173:4625–4636. doi: 10.1128/jb.173.15.4625-4636.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikusova K, Mikus M, Besra G, Hancock I, Brennan PJ. Biosynthesis of the linkage region of the mycobacterial cell wall. J Biol Chem. 1996;271:7820–7828. doi: 10.1074/jbc.271.13.7820. [DOI] [PubMed] [Google Scholar]
- Mills JA, Motichka K, Jucker M, et al. Inactivation of the mycobacterial rhamnosyltransferase, which is needed for the formation of the arabinogalactan-peptidoglycan linker, leads to irreversible loss of viability. J Biol Chem. 2004b;279:43540–43546. doi: 10.1074/jbc.M407782200. [DOI] [PubMed] [Google Scholar]
- Mills JA, Motichka K, Jucker M, et al. Inactivation of the mycobacterial rhamnosyltransferase, which is needed for the formation of the arabinogalactan-peptidoglycan linker, leads to irreversible loss of viability. J Biol Chem. 2004a;279:43540–43546. doi: 10.1074/jbc.M407782200. [DOI] [PubMed] [Google Scholar]
- Miroux B, Walker JE. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol. 1996;260:289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- Rush JS, Rick PD, Waechter CJ. Polyisoprenyl phosphate specificity of UDP-GlcNAc:undecaprenyl phosphate N-acetylglucosaminyl 1-P transferase from E.coli. Glycobiology. 1997;7:315–322. doi: 10.1093/glycob/7.2.315. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Chung TDY, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. Journal of Biomolecular Screening. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Ginsberg C, Yuan YQ, Walker S. Acceptor substrate selectivity and kinetic mechanism of Bacillus subtilis TagA. Biochemistry. 2006a;45:10895–10904. doi: 10.1021/bi060872z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Ginsberg C, Yuan YQ, Walker S. Acceptor substrate selectivity and kinetic mechanism of Bacillus subtilis TagA. Biochemistry. 2006b;45:10895–10904. doi: 10.1021/bi060872z. [DOI] [PMC free article] [PubMed] [Google Scholar]