Abstract
We report an interesting buffer electric relaxation time tuning technique, coupled with a glutaraldehyde cross-linking cell fixation reaction, which allows for sensitive dielectrophoretic analysis and discrimination of bovine red blood cell (bRBC) starvation age. The buffer composition is selected such that two easily accessible dielectrophoretic crossover frequencies (cof) exist. Low concentration glutaraldehyde fixation was observed to produce a threefold decrease in the higher cof with a comparable increase in the lower cof also witnessed. More importantly, increased glutaraldehyde fixation concentration significantly increased the higher cof by a factor found to be sensitive to the bRBC starvation age.
INTRODUCTION
Dielectrophoresis (DEP) describes the field induced polarization and translational motion of a polarizable particle in a nonuniform ac electric field.1 In recent years much work has been done in attempt to utilize and integrate DEP into microfluidic devices for particle and cellular detection and manipulation.2, 3, 4 This has led to an increasing amount of research examining the dielectrophoretic consequences of both chemical modification of suspended cells and of buffer tweaking. For instance, Wang et al. utilized varied osmotic environments within electrorotation experiments to investigate the impact of DS19 Friend murine erythroleukemia cell membrane morphology.5 Markx et al. probed the relationship between suspending medium conductivity and dielectrophoretic response for E. coli and M. lysodeikticus cells with the aim of developing selective separation protocols.6, 7 Similarly, the ability to selectively release cells held under DEP force through variance of the suspending medium permittivity has been the subject of investigation.8 Dielectrophoresis has also been employed to explore chemically induced apoptosis with the intention of developing dielectrophoretic separation protocols.9, 10 Several additional examples of some form of dielectrophoretic optimization aimed at achieving a particular goal can be found within the literature.2, 11 The purpose of the work presented here is to further drive the concept of dielectrophoretic optimization through a novel combination of working electrolyte solution selection and suspending particle modification. In particular, the use of a low-conductivity zwitterion buffer was combined with a cell fixation reaction to dielectrophoretically distinguish bRBCs of varying starvation age, a parameter that is difficult to detect in an unoptimized system.
The concept of dielectrophoretic optimization is largely guided by traditional DEP models. Briefly, classical theory models the DEP force FDEP induced by an ac field E with frequency ω on a spherical particle of radius a as:
| (1) |
where εmedium is the real part of the suspending medium permittivity. The factor fcm is the frequency-dependent Clausius-Mossotti (cm) factor for a lossy dielectric homogeneous spheroid and is dependent upon the permittivity and conductivity of both the particle and the suspending medium. The sign associated with fcm, as shown in Fig. 1b dictates the sign given to FDEP, and thus leads to either positive DEP (pDEP, polarized particle attracted toward a local electric field maxima) or negative DEP (nDEP, particle attracted toward a local electric field minima). Furthermore, the precise frequency that produces a change from pDEP to nDEP is referred to as the crossover frequency (cof), and is of interest in devising microfluidic separation schemes as it typically differs from one cell type to another due to varying electrical properties.
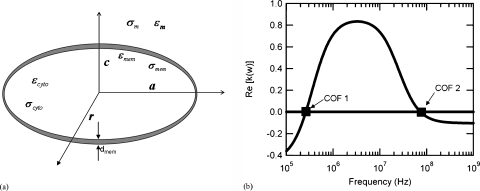
Figure 1.
The single shelled oblate DEP model for a red blood cell [schematic shown in (a)] predicts a low, 255 kHz, and high, 72 MHz, crossover frequency for the cells when suspended in a conventional electrolyte or biological buffer. The parameters employed within this model include; a RBC radius on the major axis of 3.1 μm (a=r) and on the minor axis of 1.5 μm (c), a cell wall thickness of 8 nm, a buffer conductivity of 12.4 mS∕cm, a cytoplasm conductivity of 0.312 S∕m, and a membrane dielectric constant of 60. A detailed derivation of the model used to produce (b) can be found in the literature (Ref. 12).
With regards to red blood cells, a shelled Maxwell-Wagner (MW) model describing them as approximate oblate spheroids of highly conducting cytoplasm [see Fig. 1a] surrounded by an insulating cell membrane has been developed.12 Each interface that separates the dielectric layers (two exist for a RBC: the inside and outside edge of the membrane) introduces a MW relaxation process, allowing for the possibility of two distinct cofs for RBCs [see Fig. 1b]. The induced dipole direction exhibited by a RBC after polarization is dependent on the conductivity and permittivity of the cell interior, membrane, and suspending medium, and thus these electrical properties dictate the two distinct cof values. Utilizing reported 0.85% saline suspended RBC electrical properties,13 cof1 and cof2, the low and high frequency cofs, are predicted to be 255 kHz and 72 MHz respectively. This large cof2 value, along with the significant difference between the high and low cof values, complicates a rapid measurement of these respective quantities. A reduction of cof2 is thus of interest, and a sensitivity study of the single-shell DEP model indicates that this can be achieved through a reduction in the electrolyte relaxation time, σcytoplasm∕εmedium (where σ represents conductivity).
In this work, cof2 reduction was accomplished through the use of a low-conductivity, zwitterion buffer (6-aminohexanoic acid, AHA). Zwitterions have been used in previous studies as an effective means to increase electrolyte permittivity.2 Resuspension of RBCs within this buffer results in osmotically driven water transport into the RBC, and thus a decrease in the cytoplasm conductivity. We have previously demonstrated the effectiveness of zwitterions in modifying DEP cof,14 and other work has shown that cells exhibit lasting stability with no signs of lysis in certain low-conductivity zwitterion buffers.2
MATERIALS AND METHODS
Bovine RBCs (Quad Five, Cat. #943) were stored within a 0.85% saline solution at 4°C for 4 weeks at a hematocrit of approximately 45%. At specific time points (1, 2, and 4 weeks), an aliquot of bRBCs was removed from the suspension, washed 3 times in phosphate-buffered saline (PBS, pH 7.4) and resuspended within fresh PBS. The bRBCs were then fixed through 30 min of reaction with aqueous glutaraldehyde (25%, Sigma Aldrich, Cat. #G6257). This reaction was initiated through the addition of an appropriate amount of glutaraldehyde to give solution concentrations of 0%, 0.06%, 0.1%, 0.3%, 0.6%, 1%, and 2.5% respectively. After fixation, the bRBCs were again washed 3 times in PBS to remove residual glutaraldehyde, a final time in 3M 6-aminohexanoic acid (AHA, pH 6.9, Sigma Aldrich, Cat. #A7824), and then resuspended in 3M AHA at a hematocrit of 2%.
After fixation, the bRBCs were immediately analyzed to determine their DEP response by placing a 75 μl aliquot suspension at the center of a quadrupole electrode array that was fabricated by patterning dual titanium (50 Å)-gold (2500 Å) layers onto precleaned 50×75 mm glass slides [see Fig. 2a]. The electrode patterns were defined onto the slides using the image reversal photoresist Shipley AZ-5214, and the arrays were designed as 4 triangular posts with an inner square side length of 40 μm and an electrode width of 125 μm. The array was connected to a function generator (Agilent, model #33220A) set to 10 Vpp via copper tape and wire leads that yielded the center region of the electrode array as an absolute field minimum and the wire edges as an absolute field maximum. The applied frequency was slowly increased from 50 kHz to 5 MHz to reveal the first cof, which was taken when the bRBCs migrated from the electrode center to the electrode edges [Fig. 2b], and cof2, which was taken when the bRBCs migrated back to the array center [Fig. 2c].
Figure 2.
A quadrupole electrode array (a) was utilized to probe for both positive DEP [shown in (b)], and negative DEP [shown in (c)] of bovine red blood cells. The quadrupole electrode array as shown produced an electric field absolute minimum at the center of the array and an absolute maximum along the edges of the electrodes.
RESULTS AND DISCUSSION
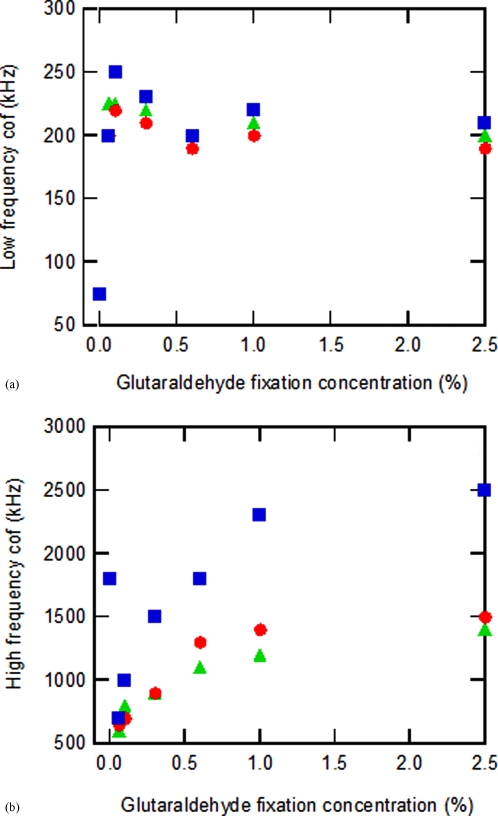
A dielectrophoretic investigation of normal (nonfixed) washed bRBCs resuspended in 3M AHA at a 2% hematocrit revealed a significant reduction in their cof2 value from 70 MHz to about 1.8 MHz, while cof1 was decreased from 1.3 MHz to 75 kHz. While the use of 3M AHA as the suspending medium allowed for a reduction in cof2, and thus also simplifying a rapid frequency scan, this alone did not permit dielectrophoretic differentiation of bovine RBCs that have been stored within a 0.85% saline solution for differing time periods (1, 2, and 4 weeks). To accomplish this aim, further dielectrophoretic optimization was achieved by glutaraldehyde-induced bRBC modification. The addition of glutaraldehyde to bRBCs eliminates positive amino groups from the surface of the cell,15, 16 and destroys the lipid membrane.17 This procedure cross-links both membrane and interior proteins, producing a network of polyelectrolytes within a particle that exhibits enhanced stability compared to normal bovine red blood cells,15, 17 and is expected to amplify distinguishing membrane and cell interior features. Figures 3a, 3b summarize crossover frequency measurements for both normal and glutaraldehyde fixed bovine RBCs after starvation age times of 1, 2, and 4 weeks. The normal bovine RBCs exhibited measured cof1 and cof2 values of 75 kHz and 1.8 MHz respectively, with no variance with respect to bRBC starvation age. Upon bRBC fixation at a low glutaraldehyde concentration of 0.06%, an increase in cof1 from 75 kHz to 225, 200, and 200 kHz was observed for starvation age groups of 1, 2, and 4 weeks respectively. The cof1 maximum for each bRBC age group occurred at a glutaraldehyde cross-linking concentration of 0.1%, and the measured values were 250, 225, and 220 kHz respectively. On the other hand, a decrease in cof2 from 1.8 MHz to 600 kHz was witnessed for all three age groups at a 0.06% glutaraldehyde fixation concentration. As the glutaraldehyde concentration was further increased from 0.1% to 2.5%, cof1 was found to slowly decrease to an asymptote of approximately 210 kHz for each bRBC suspension, while cof2 steadily increased until reaching an approximate asymptote of 2.3, 1.5, and 1.3 MHz for bRBC starvation ages of 1, 2, and 4 weeks respectively.
Figure 3.
This figure displays the observed crossover frequency measurements for different bovine red blood cell starvation ages and glutaraldehyde fixation concentrations. The first plot (a) shows the low-frequency cof1 values, while the second graph (b) depicts the high frequency cof2 measurements. The symbols employed in the plots correspond to: (◼) 1 week starvation age, (●) 2 week starvation age, and (▴) 4 week starvation age.
A more rigorous quantitative analysis of the cof spectra is outside the scope of this paper, however preliminary results derived from the oblate shell model indicate that glutaraldehyde fixation increases the conductivity of the bRBC cytoplasm while concurrently decreasing the membrane dielectric constant. Both of these effects along with a more quantitative physical analysis as to how glutaraldehyde modifies the bRBC are currently being studied in detail and will be the subject of a follow up paper.
Based on the presented measurements, bRBC treatment by glutaraldehyde appears to be responsible for the observed starvation age dependent electrical property changes of the cell that enabled effective discrimination through DEP. Additionally, the use of an appropriate suspending buffer, 3M aminohexanoic acid, allowed for a reduction in the higher crossover frequency value from 70 MHz to 1.8 MHz. This reduction simplified a rapid frequency scan that accurately revealed both bovine RBC crossover frequencies.
ACKNOWLEDGMENTS
We would like to thank Shramik Sengupata for very useful discussions about the underlying mechanisms involved in glutaraldehyde cross-linking of bovine RBCs and the resulting impact on their dielectrophoretic behavior.
References
- Pohl H. A., Dielectrophoresis (Cambridge University Press, Cambridge, 1978). [Google Scholar]
- Arnold W. M., IEEE Trans. Ind. Appl. 10.1109/28.952523 37, 1468 (2001). [DOI] [Google Scholar]
- Green N. G., Morgan H., and Milner J. J., J. Biochem. Biophys. Methods 10.1016/S0165-022X(97)00033-X 35, 89 (1997). [DOI] [PubMed] [Google Scholar]
- Green N. G. and Morgan H., J. Phys. D 10.1088/0022-3727/30/11/001 30, L41 (1997). [DOI] [Google Scholar]
- Wang X. B., Huang Y., Gascoyne P. R., Becker F. F., Hölzel R., and Pethig R., Biochim. Biophys. Acta Biomembranes 1193, 330 (1994). [DOI] [PubMed] [Google Scholar]
- Markx G. H., Zhou X. F., and Pethig R., in Electrostatics 1995: Proceedings of the 9th International Conference, York, 2–5 April 1995, IOP Conference Proceedings No. 143, edited by Cunningham S. (IOPP, Bristol, UK, 1995), p. 145.
- Markx G. H., Dyda P. A., and Pethig R., J. Biotechnol. 10.1016/0168-1656(96)01617-3 51, 175 (1996). [DOI] [PubMed] [Google Scholar]
- Lee R. S., Arnold W. M., and Pethig R., in 2004 Annual Report Conference on Electrical Insulation and Dielectric Phenomena (IEEE, New York, 2004), p. 352. [Google Scholar]
- Wang X. J., Becker F. F., and Gascoyne P. R. C., Biochim. Biophys. Acta Biomembranes 1564, 412 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethig R. and Talary M. S., IET Nanobiotechnology 1, 2 (2007). [DOI] [PubMed] [Google Scholar]
- Gascoyne P. R. C., Pethig R., Burt J. P. H., and Becker F. F., Biochim. Biophys. Acta 1149, 119 (1993). [DOI] [PubMed] [Google Scholar]
- Jones T. B., Electromechanics of Particles (Cambridge University Press, Cambridge, 1995). [Google Scholar]
- Gascoyne P., Satayavivad J., and Ruchirawat M., Acta Trop. 89, 357 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon Z. and Chang H. C., Electrophoresis 10.1002/elps.200500129 26, 3725 (2005). [DOI] [PubMed] [Google Scholar]
- Georgieva R., Neu B., Shilov V. M., Knippel E., Budde A., Latza R., Donath E., Kiesewetter H., and Baumler H., Biophys. J. 74, 2114 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath E., Budde A., Knippel E., and Bäumler H., Langmuir 12, 4832 (1996). [Google Scholar]
- Heard D. H. and Seaman G. V. F., Biochim. Biophys. Acta 53, 366 (1961). [DOI] [PubMed] [Google Scholar]