Summary
Background
Collagen biosynthesis and deposition is a complex, multistep process, which is tightly regulated to maintain proper tissue homeostasis. Sex steroid hormones have been implicated in regulating collagen synthesis; however the specific mechanisms regulating the process remain largely unknown.
Objective
To investigate the role of estrogens and androgens in the regulation of genes involved in collagen synthesis and fibrillogenesis using gonadectomized C57/B6 mice.
Methods
Collagen content was assessed by hydroxyproline measurement and acetic acid extraction of collagen with or without the addition of pepsin. The mRNA levels of fibrillar collagens and enzymes involved in fibrillogenesis were determined by QPCR analysis. The protein expression of decorin, lumican and fibromodulin was confirmed by immunostaining.
Results
We have shown that castration resulted in a markedly decreased skin thickness and collagen content without affecting collagen solubility. Furthermore, the mRNA levels of fibrillar collagen genes including types I, III, and V were decreased, suggesting that androgens positively regulate the rate of collagen gene transcription. Conversely, ovariectomy mainly affected collagen solubility. The absence of estrogens resulted in decreased expression levels of several of the small leucine-rich repeat proteins and proteoglycans (SLRPs) including decorin, fibromodulin and lumican.
Conclusions
Estrogens may not be directly involved in the regulation of collagen synthesis; however, they may play a critical role in regulating organization and stability of collagen fibrils. Androgens play a positive role in the regulation of collagen biosynthesis. In summary, our data demonstrate that androgens and estrogens regulate distinct aspects of collagen fibrillogenesis in mouse skin.
Keywords: Ovariectomy, Castration, Fibrillar collagens
1. Introduction
Collagen biosynthesis and deposition is a complex, multistep process. The synthesis of collagen fibrils starts with the nuclear transcription of the collagen genes, synthesis and assembly of procollagen molecules, followed by their secretion into the extracellular space, where the procollagen molecules are converted to collagen and arranged into stable, cross-linked collagen fibrils [1]. Fibril-forming collagens are the major structural components of the dermis responsible for its characteristic strength and resiliency. Biosynthesis of fibrillar collagen in the skin is tightly regulated to maintain proper tissue homeostasis; however the factors that regulate this process remain largely unknown. Previous studies have implicated sex steroid hormones in regulating various aspects of skin morphology and physiology, including hair growth, pigmentation, vascularity, water-holding capacity and elasticity [2, 3]. The influence of estrogens on the skin was primarily investigated in postmenopausal women in the context of hormone replacement therapy (HRT). Several studies have reported positive effects of HRT on collagen content and skin thickness [4, 5]; however, a contrary comprehensive study by Haapasaari et al.[6] demonstrated no effect of estrogen on collagen deposition after one year of continuous hormonal administration. Several in vitro and in vivo studies demonstrated that estrogen promotes cutaneous wound healing and that this process is associated with enhanced matrix deposition, rapid epithelialization, and a decrease of the inflammatory response[7, 8]. Furthermore, acute incisional wound repair was markedly delayed in ovariectomized rats [9]. Despite the general consensus of the importance of estrogens in regulating various skin functions the regulatory mechanisms involved in their action remain poorly understood.
Relatively little is known about the influence of testosterone on different skin functions. Testosterone and its more potent metabolite 5-α dihydrotestosterone (DHT) caused enlargement of sebaceous glands and also stimulated sebum production and secretion [10, 11]. In contrast to estrogen, testosterone inhibited the cutaneous wound healing in males and its effects were associated with an enhanced inflammatory response; moreover, castrated males demonstrated accelerated cutaneous wound healing [12]. The direct evidence for the involvement of androgens in regulating connective tissue synthesis came from the study of Markova et al.,[13]. It was shown that mice deficient in the androgen receptor had significantly reduced levels of collagen as compared to wild-type animals indicating a contribution of the androgen receptor pathway to collagen regulation in vivo. In contrast to these findings, no difference in dermal thickness was observed in castrated male mice [14].
Given the documented role of sex steroid hormones in regulating connective tissue in the skin and the lack of knowledge regarding specific mechanisms involved in this process, the goal of this study was to begin a systematic investigation of the role of estrogens and androgens in regulation of genes involved in collagen synthesis and fibrillogenesis using gonadectomized C57/B6 mice.
2. Materials and methods
2.1. Mice
Both sexes C57/B6 mice were used for the study. Mice were distributed into four groups of nine animals per group as follows: (1) Female controls (sham operated); (2) Females ovariectomized; (3) Male controls (sham operated); (4) Males castrated. Mice were sham operated or bilaterally gonadectomized under general anesthesia (Tribromoethanol [Avertin, Sigma]) at 6 weeks of age. All mice were sacrificed at 3 months of age. The Institutional Animal Care and Use Committee approved all animal procedures.
2.2. Histological measurements and cell count
Skin samples were removed from the dorsal side and stained with hematoxylin-eosin according to routine histologic method. The thickness of the skin was determined by measuring at least 5 randomly selected sections from the top of the granular layer to the junction between the dermis and subcutaneous fat on stained sections as previously described [15]. Fibroblast number in the skin was counted in each mouse in 5 randomly selected fields per slide.
2.3. Assessment of total collagen by hydroxyproline method
Measurement of hydroxyproline content in the skin was carried out as described by Bradshaw et al., [17]. Briefly, lyophilized 8 mm skin punches were placed in 6 N HCl in sealed tubes and were heated at 110°C for 3 hr. After incubation the hydrolysates were transferred to 50 ml conical tubes containing 10 ml of dH2O and 2ml of working buffer (0.1M anhydrous citric acid, 0.5M acetic acid, 0.7M sodium acetate, 0.4M sodium hydroxide, 1-propanol) and the solution were vortexed. Adjustments of pH 7–8 were made using 4N NaOH and 6N HCl. Next, chloramine T was added to each sample, and samples were incubated at RT for 20 min, followed by addition of p-dimethyl-amino-benzaldehyde and a further incubation at 60°C for 15 minutes. The absorbance of the samples was measured in a spectrophotometer at 558 nm. The amount of collagen in each sample was calculated by comparison to hydroxyproline standard curve and expressed as μg of hydroxyproline/ml.
2.4. Extraction of collagen from skin by acetic acid method with or without addition of pepsin
The acetic acid extraction of collagen was performed according to early described protocols [16, 18]. Briefly, 8mm skin punches were taken from the dorsa of each mouse. Next, skin pieces were minced and incubated in 10 volumes of phosphate-buffered saline overnight at 4°C with stirring. Tissue was harvested by centrifugation at 12,000 × g for 15 min and suspended in 10 volumes of cold 0.5M acetic acid with or without addition of pepsin (1:10 ratio of pepsin:tissue wet weight). Extraction was performed overnight at 4°C with stirring, and supernatant was dialyzed against 0.1M acetic acid. Next, the dialysates with addition of pepsin were treated with Pepstatin A (Sigma, Inc) followed by lyophilization. Lyophilized proteins were resuspended in cold 0.1M acetic acid and were tumbled-rotated ~ 20 hours. Equal aliquots from each sample were neutralized with 1M Tris-base, boiled in sample buffer with addition of 2-mercaptoethanol and resolved by 6% SDS-PAGE and stained with Coomassie blue. Collagen level was quantitated using NIH Image densitometry software. Appropriate collagen bands were scanned using Epson Perfection 4990 Photo Scanner. Band density expressed as an arbitrary units were recorded.
2.5. Quantitative real-time RT-PCR analysis
Total RNA was isolated using the guanidinium thiocyanate-phenol-chloroform method. 2 μg of RNA was reverse transcribed in a 10 μl reaction using random primers and Transcriptor First Strand synthesis kit (Roche, USA). Real-time PCR assays were performed using MyiQ™ Single-Color Real-Time PCR Detection System (Bio-Rad iCycler). Amplification mixture (10μl) contained 0.125μg of cDNA, 0.25μM of primers and 5μl of iQ™SYBR Green Supermix. Amplification was for 95°C for 3 min, followed by a 40 cycles of 95°C for 30s, 60°C for 1 min. All samples were analyzed in parallel for B2-mG expression as internal control. The fold change in the levels of genes of interest was determined by 2−ΔΔCT. To compare the different samples in an experiment, RNA expression in samples were compared to that of the control mB2MG in each experiment. The primers are listed in the Table 1.
Table 1.
Primers for real-time PCR
| Gene | Primers (5′-3′) | |
|---|---|---|
| Forward | Reverse | |
| mB2MG | TCGCTCGGTGACCCTAGTCTTT | ATGTTCGGCTTCCCATTCTCC |
| mCOL1A1 | GCCAAGAAGACATCCCTGAAG | TGTGGCAGATACAGATCAAGC |
| mCOL1A2 | CACCCCAGCGAAGAACTCATA | GCCACCATTGATAGTCTCTCC |
| mCOL3A1 | TTTGTGCAAGTGGAACCTG | TGGACTGCTGTGCCAAAATA |
| mCOL5A1 | GGACTAGTCCGCTTTCCCTGTCAACTTGTCCGATGG | GTGGTCACTGCGGCTGAGGAACTTC |
| mCOL5A2 | CAGAAGCCCAGACGTATCG | GGTGGTCAGGCACTTCAGAT |
| mDecorin | TGAGCTTCAACAGCATCACC | AAGTCATTTTGCCCAACTGC |
| mFibromodulin | CAATGTCTACACCGTCCCTGA | AGAAGGCTGCTGGAGTTGAAG |
| mLumican | AGATGCTTGATCTTGGAGTAAGA | CAATGAACTTGAAAAGTTTGATG |
| mPLOD2 | CAGTGCCAGTGGCAATTAATGGAA | TGAACCTGTGTCCATGAGTTTGGA |
2.6. Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue sections using a Vectastain ABC kit (Vector, Burlingame, CA) according to the manufacturer’s instructions. Five-μm-thick sections were mounted on APES-coated slides, deparaffinized with histoclear, and rehydrated through a graded series of ethanol. Endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide for 30 minutes. Sections were then heated at 90 degrees C for 45 minutes in Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA). To expose core proteins, sections were treated with appropriate enzymes (chondroitinase ABC for decorin, Peptide N-glycosidase for fibromodulin, beta-endogalactosidase for lumican). The sections were then incubated with antibodies against decorin, fibromodulin, or lumican (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:100 in blocking buffer (1% rabbit serum) overnight at room temperature, followed by the incubation for 1 hour with biotinylated rabbit anti-goat secondary antibody. The immunoreactivity was visualized with diaminobenzidine and the sections were counterstained with hematoxylin.
2.7. Statistical analysis
The student’s t-test analysis using GraphPad InStat Statistics Software (v 1.12) was performed to determine statistical significance. Values of less than or equal to 0.05 were considered statistically significant. *** indicate statistically significant values p<0.001; ** p<0.01; * p<0.05.
3. Results
3.1. Skin thickness is decreased in castrated males but not in ovariectomized females
To investigate the effect of gonadal hormones on skin thickness in male and female mice, skin samples were stained with hematoxylin-eosin. There were small variations in skin thickness within each group. In agreement with previous studies, the skin thickness in control male mice was about twice (376.6 ± 7.9μm, 164.3 ± 9.1μm, ***p<0.001) of that of female mice (Fig. 1A, B). In castrated males, the skin thickness decreased about 50% and was comparable to that of female mice (157.3 ± 7.2μm, 150 ± 13.6μm). The skin thickness did not change appreciably in ovariectomized females as compared to controls (Fig. 1A, B). To examine whether gonad removal affected fibroblast number in the skin, spindle-shaped cells were counted in five randomly selected fields per slide of each mouse (Fig. 1C). A slight increase in cell number was noticed in castrated males compared to intact mice (65 cells, 51 cells respectively). In ovariectomized females, fibroblast number was decreased as compared to controls (70 cells, 90 cells respectively). Number of fibroblast was higher in control female mice as compared to male controls (90 cells, 51 cells respectively).
Figure 1.
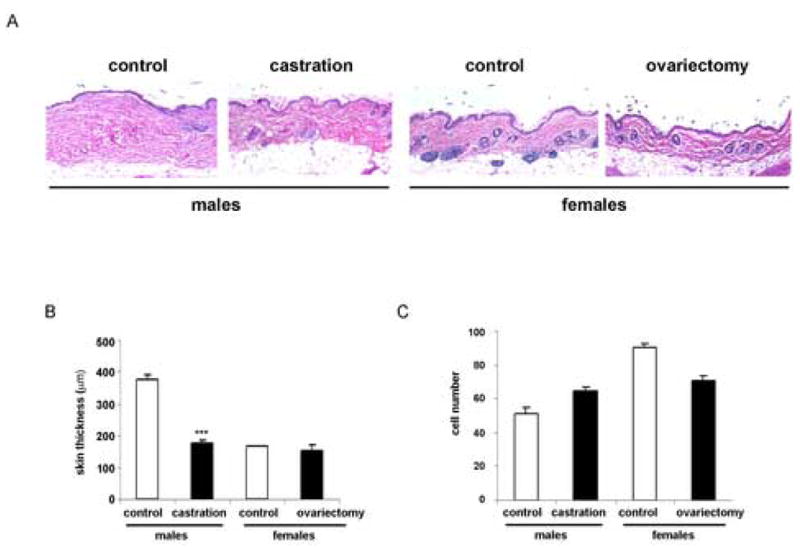
(A) Representative examples of skin sections stained with H/E. Skin samples were removed from the dorsal side and stained with hematoxylin-eosin according to routine histologic methods. (B) Graphical representation of skin thickness quantified using Spot Advanced Image software. Values are presented as means ± SD. (C) Graphical representation of fibroblast count in each experimental group.
3.2 Androgens positively regulate collagen deposition in the skin
To examine the influence of gonadal hormones on collagen accumulation in the skin, collagen content was assessed by the hydroxyproline assay. The comparison between control males and females showed significantly higher hydroxyproline content in male mice (33.8 ± 1.2, 19.80 ± 4.84 μg/ml, **p<0.01) (Fig. 2). There was also a significant decrease in hydroxyproline content in castrated males as compared to controls (33.8 ± 1.2, 22.9 ± 1.96 μg/ml, p<0.003). The hydroxyproline content in the skin of castrated males was comparable to that of the females (22.9 ± 1.96, 19.80 ± 4.8 μg/ml). The hydroxyproline content did not change in ovariectomized females (19.4 ± 0.91 μg/ml). These data indicate that collagen content in the skin is reduced in the absence of androgens.
Figure 2.
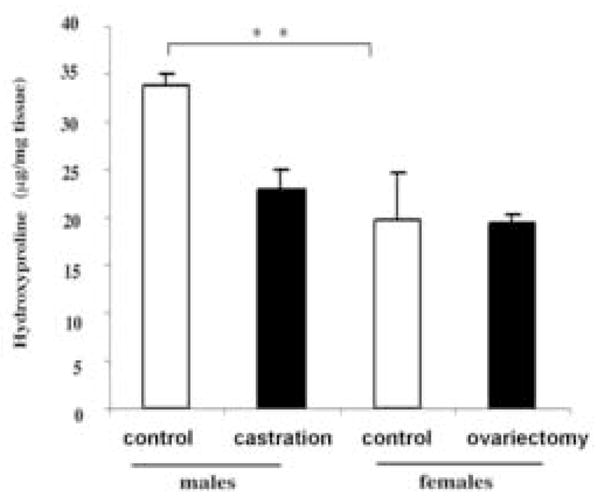
Androgens modulate hydroxyproline content in mouse skin. Hydroxyproline levels were measured as described in Methods. The amount of hydroxyproline in each sample was calculated by comparison to a hydroxyproline standard curve and expressed as μg of hydroxyproline/ml. Values are presented as means ± SD.
To further investigate the effects of gonadal hormones on the collagen species in the skin, collagen was extracted with 0.5 M acetic acid with the addition of pepsin [18]. For the extraction, 8 mm punches from the dorsa of each mouse were used. Equal aliquots from each sample were analyzed by SDS-PAGE. The pattern of collagen bands was similar in all samples, suggesting no qualitative differences in collagen composition (Fig. 3). Consistent with the hydroxyproline content results, significantly more collagen was extracted from the skin of male mice as compared to females (3.2 ± 0.3 fold, p<0.01). Similarly, more collagen was extracted from the skin of control versus castrated males (1.8 ± 0.3 fold, p<0.04), whereas there was no difference between control and ovariectomized females (Fig. 3B).
Figure 3.
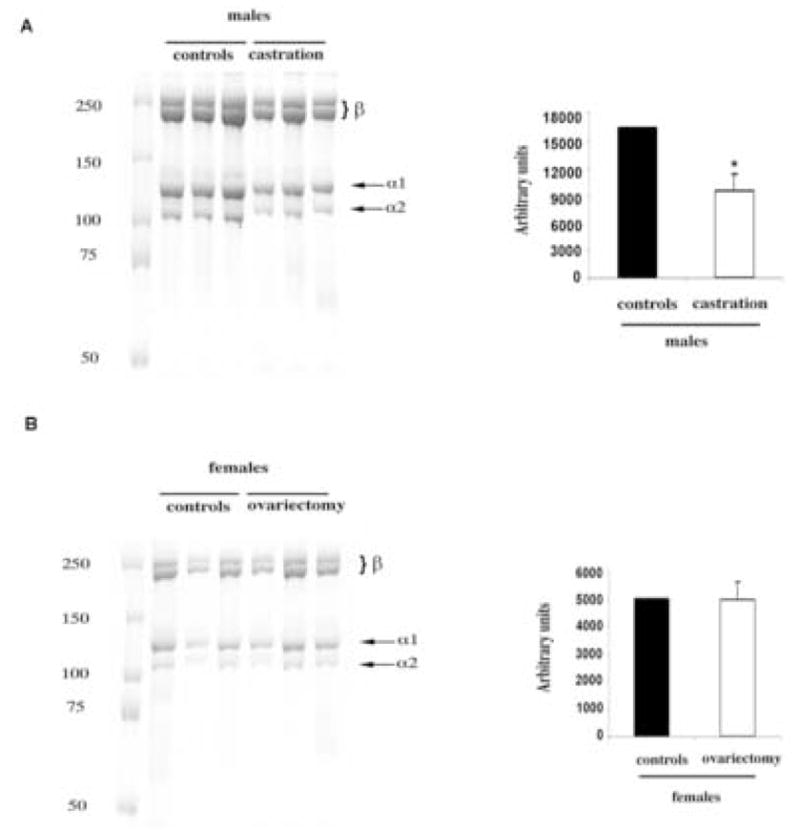
Pepsin-soluble collagen content is decreased in castrated males. (A) The acetic extraction of collagen was performed with addition of pepsin (see Methods). Arrows indicate collagen α1(I) and α2(I) subunits. Slower migrating bands represent cross-linked α-chain dimers (also termed β-components [32]). Molecular weight marker for proteins (kd) is shown on the left. (B). Graphical representation of collagen level quantified using NIH Image densitometry software. Values are presented as means ± SD.
3.3 The amount of acetic acid solubilized collagen is increased in ovariectomized females
To assess the amounts of the freshly synthesized (non-cross-linked) collagens that are not yet incorporated into large fibrils, collagen was extracted by 0.5 M acetic acid [16, 18] without the addition of pepsin. 8 mm skin punches were used for extraction and equal aliquots from each sample were analyzed by SDS-PAGE. In contrast to hydroxyproline and pepsin-soluble collagen content, there were no quantitative differences in the amount of extracted collagen between controls and castrated males. However, there was a 2.6 ± 0.3 fold of increase in acetic acid soluble collagen in ovariectomized females as compared to controls, suggesting either increased synthesis or increased collagen solubility in ovariectomized female mice (Fig. 4).
Figure 4.
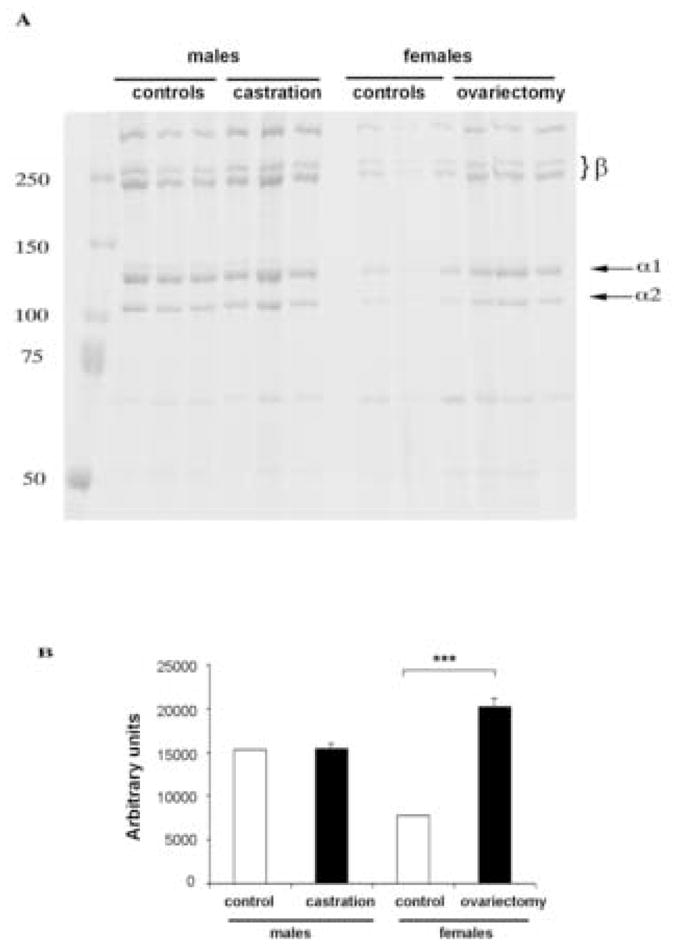
Soluble collagen content is increased in ovariectomized female mice. (A) Acetic acid extraction of collagen was performed as described in Methods section. Arrows indicate collagen α1 (I) and α2(I) subunits. β-components represent cross-linked α-chain dimers. Molecular weight marker for proteins (kd) is shown on the left (B) Graphical representation of collagen level quantified using NIH Image densitometry software. Values are presented as means ± SD.
3.4 Pattern of mRNA expression of matrix-related genes differs in castrated male and ovariectomized female mice
To determine the effect of gonadal hormones on expression of genes involved in collagen fibrillogenesis, total RNA was isolated from tissue punches taken from the dorsa of the female and male mice. Quantitative Real-time PCR was employed to measure mRNA levels of procollagen type I, III, and V. In castrated males the mRNA levels of various collagen chains were significantly decreased (Fig. 5A). The level of pro-Col1a1 decreased 1.86 ± 0.2 fold (p<0.01), pro-Col1a2 3.6 ± 0.1 fold (p<0.01), pro-Col3a1 2.31 ± 0.1 fold (p<0.01), pro-Col5a1 2.94 ± 0.2 fold (p<0.01) and pro-col5a2 1.51 ±0.1 fold (p<0.05). In ovariectomized females the expression levels of pro-Col1a1, pro-Col1a2 and pro-Col3a1 were not affected, however there was a marked decrease of pro-Col5a1 (8.3 ±0.4 fold p<0.02), as well as a significant decrease of pro-Col5a2 chain (2.86 ± 0.2 fold p<0.01). We next determined the expression level of several enzymes involved in the process of fibrillogenesis (Fig. 5B). The mRNA level of lumican (Lum), fibromodulin (Fmod), decorin (Dcn) and lysyl hydroxylase 2 (Plod2) were measured by Q Real-time PCR. In castrated males, only two of these mRNAs were significantly changed: lumican was elevated (2.2 ± 0.2 fold, p<0.01), while Plod2 was decreased (2.53 ± 0.2 fold, p<0.03). In ovariectomized females, a significant decrease of all of the mRNA levels was observed: lumican (2.36 ± 0.2 fold, p<0.03), fibromodulin (5.16 ± 0.3 fold, p<0.01), decorin (2.32 ± 0.3 fold, p<0.01), Plod2 (5.85 ± 0.2 fold, p<0.03).
Figure 5.
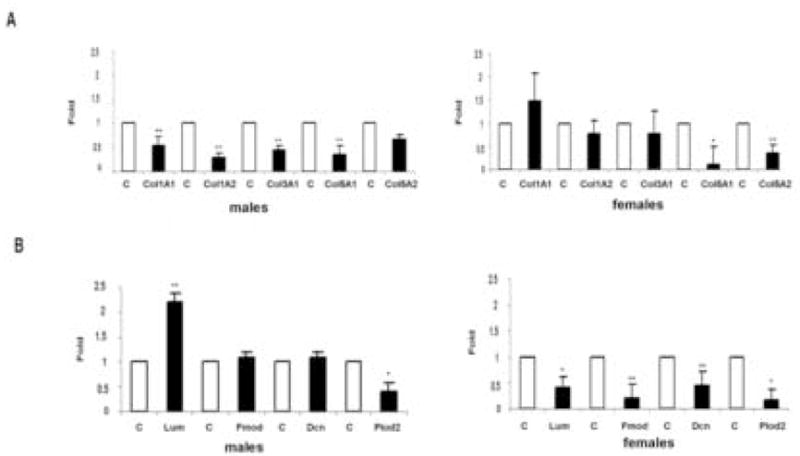
Quantitative real-time PCR analysis (qPCR) analyses of matrix-related genes in control and gonadectomized mice. (A) qPCR analysis of gene expression of fibrillar collagens in skin isolated from female and male control (white bars) and gonadectomized (black bars) mice. The expression level of each gene in control mice was set at 1. Values are presented as means ± SD. (B) qPCR analysis of gene expression of decorin (Dcn), fibromodulin (Fmod), lumican (Lum), and Plod2 in female and male control (white bars) and gonadectomized (black bars) mice. The expression level of each gene in control mice was set at 1. Values are presented as means ± SD.
3.5 The protein expression of decorin, lumican and fibromodulin is decreased in the skin of ovariectomized females
To further investigate the expression of decorin, lumican, and fibromodulin, immunostaining of skin section from wild type and ovariectomized female mice was performed. The skin sections were pretreated with the appropriate enzymes to remove glycosaminoglycan chains from core proteins. High expression level of decorin, fibromodulin, and lumican were observed in control skin fibroblasts and in association with collagen fiber bundles in the ECM (Fig. 6). Consistent with mRNA data the amounts of decorin were markedly diminished in the skin of ovariectomized mice. Likewise, the levels of lumican and fibromodulin were decreased in the dermis of ovariectomized mice compared to control skin sections.
Figure 6.
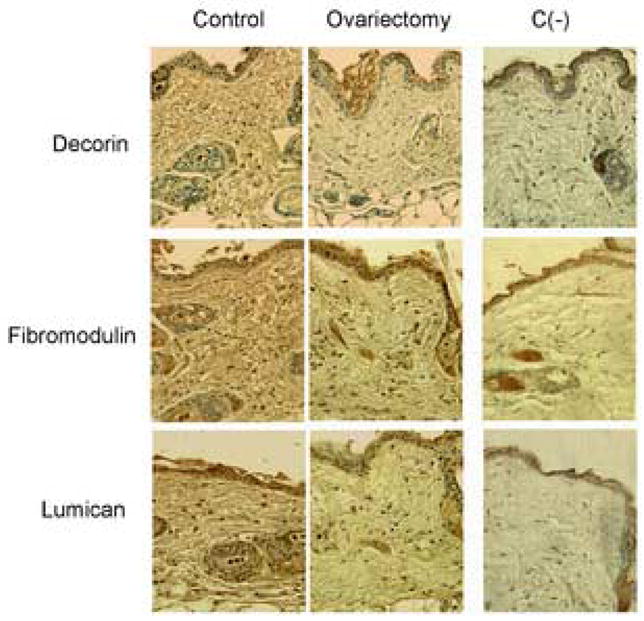
Expression of lumican, fibromodulin and decorin proteins are decreased in ovariectomized female mice. Immunochemistry was performed on paraffin embedded, formalin fixed skin tissue from control and ovariectomized female mice (see Methods). To expose core proteins, sections were treated with appropriate enzymes, as described in Methods. C (−) represents negative control sections – incubation with isotype matched preimmune serum without antigen retrieval by proper enzymes.
4. Discussion
This study demonstrates that male and female sex hormones regulate distinct aspects of collagen fibrillogenesis in mouse skin. Previous reports have shown that the level of testosterone in castrated males is very low [19]. The results of our study are consistent with the positive role of androgens in regulating collagen biosynthetic pathway in male mice. Specifically, we have shown that castration resulted in markedly decreased skin thickness and collagen content. We have established that proliferation of fibroblasts did not contribute to the changes of skin thickness. The mRNA levels of fibrillar collagen genes including types I, III, and V were also decreased, suggesting that androgens positively regulate the rate of collagen gene transcription. Interestingly, the level of Plod2, an enzyme involved in formation of collagen cross-links was also significantly decreased suggesting a decreased stability of collagen fibrils. The findings of this study are consistent with the work by Markova et al., [13] which described similar skin changes in mice lacking androgen receptor. While, in contrast to our study, only slight differences in dermal thickness were observed in a previous study using gonadectomized male mice [14], the reason for this discrepancy may be related to the shorter duration of their experiments (3 weeks versus 6 weeks after gonadectomy).
Our study suggests that estrogens may not be directly involved in regulation of collagen synthesis; however, they appear to play a critical role in regulating organization and stability of collagen fibrils. In the course of our 6-week study, there were no changes in total collagen content. Likewise, the level of mRNA expression of collagen type I and III were similar. On the other hand, the levels of collagen type V chains were significantly decreased. Type V collagen was recently shown to play a critical role in initiation of collagen fibrillogenesis. The Col5a1−/− mice die at embryonic day 10, while heterozygotes are viable and show defective collagen fibril formation, decreased fibrils number and dermal collagen content [21–23]. Since we did not observe reduced collagen content in our model, it is possible that longer time periods are required for the manifestation of this effect.
Previous studies have shown that the levels of estrogen is undetectable in ovariectomized female mice [24]. In addition, it was shown that the level of testosterone is also very low in control females [20]. In our study, ovariectomy resulted in a decreased expression levels of several of the small leucine-rich repeat proteins and proteoglycans (SLRPs) including decorin, fibromodulin and lumican. While the specific roles of these proteins remain to be elucidated, they have been shown to play important roles in collagen fibrillogenesis [25, 26]. Interestingly, structural alterations in collagen fibrils have been previously described in ovariectomized rats [27]. Collagen fibrils with reduced diameter were observed at 6 weeks and the differences were more pronounced at 6 and 12 months after ovariectomy. Our study suggests that the dysregulated expression of SLRPs may directly contribute to these effects. Consistent with this notion, a recent in vitro study has demonstrated that decorin, fibromodulin or lumican may protect collagen fibrils from degradation by collagenases [28]. We have also observed diminished levels of cross-linking enzyme Plod2 suggesting that collagen fibril stability may be compromised in ovariectomized animals. These changes are consistent with increased solubility of collagen observed in our study.
Decreased skin thickness and collagen content is closely associated with postmenopausal skin ageing [29]. Whereas the majority of studies support the model that estrogen treatment can reverse these effects, little is known about the specific mechanisms of its action. Our results suggest that absence of estrogen may primarily affect the final steps of collagen fibrillogenesis resulting in collagen fibrils, which are more susceptible to degradation. This, combined with the known increases of collagen-degrading matrix metalloproteinases observed in photodamaged [30] or aged skin [31] may explain overall decreased collagen content in postmenopausal women and the positive effects of estrogen therapy.
Acknowledgments
This work was supported in part by grants from National Institutes of Health PO1-CA78582 (DKW, MT) and Scleroderma Foundation (MM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gelse K, Poschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Brincat MP, Baron YM, Galea R. Estrogens and the skin. Climacteric. 2005;8:110–123. doi: 10.1080/13697130500118100. [DOI] [PubMed] [Google Scholar]
- 3.Verdier-Sevrain S, Bonte F, Gilchrest B. Biology of estrogens in skin: implications for skin aging. Exp Dermatol. 2006;15:83–94. doi: 10.1111/j.1600-0625.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 4.Callens A, Vaillant L, Lecomte P, Berson M, Gall Y, Lorette G. Does hormonal skin aging exist? A study of the influence of different hormone therapy regimens on the skin of postmenopausal women using non-invasive measurement techniques. Dermatology. 1996;193:289–294. doi: 10.1159/000246272. [DOI] [PubMed] [Google Scholar]
- 5.Maheux R, Naud F, Rioux M, Grenier R, Lemay A, Guy J, Langevin M. A randomized, double-blind, placebo-controlled study on the effect of conjugated estrogens on skin thickness. Am J Obstet Gynecol. 1994;170:642–649. doi: 10.1016/s0002-9378(94)70242-x. [DOI] [PubMed] [Google Scholar]
- 6.Haapasaari KM, Raudaskoski T, Kallioinen M, Suvanto-Luukkonen E, Kauppila A, Laara E, Risteli J, Oikarinen A. Systemic therapy with estrogen or estrogen with progestin has no effect on skin collagen in postmenopausal women. Maturitas. 1997;27:153–162. doi: 10.1016/s0378-5122(97)01128-6. [DOI] [PubMed] [Google Scholar]
- 7.Ashcroft GS, Dodsworth J, van Boxtel E, Tarnuzzer RW, Horan MA, Schultz GS, Ferguson MW. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat Med. 1997;3:1209–1215. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- 8.Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155:1137–1146. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvin M, Dyson M, Rymer J, Young SR. The effects of ovarian hormone deficiency on wound contraction in a rat model. Br J Obstet Gynaecol. 1998;105:223–227. doi: 10.1111/j.1471-0528.1998.tb10057.x. [DOI] [PubMed] [Google Scholar]
- 10.Hsia SL, Voigt W. Inhibition of dihydrotestosterone formation: an effective means of blocking androgen action in hamster sebaceous gland. J Invest Dermatol. 1974;62:224–227. doi: 10.1111/1523-1747.ep12676791. [DOI] [PubMed] [Google Scholar]
- 11.Pochi PE, Strauss JS. Endocrinologic control of the development and activity of the human sebaceous gland. J Invest Dermatol. 1974;62:191–201. doi: 10.1111/1523-1747.ep12676783. [DOI] [PubMed] [Google Scholar]
- 12.Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J Clin Invest. 2002;110:615–624. doi: 10.1172/JCI15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markova MS, Zeskand J, McEntee B, Rothstein J, Jimenez SA, Siracusa LD. A role for the androgen receptor in collagen content of the skin. J Invest Dermatol. 2004;123:1052–1056. doi: 10.1111/j.0022-202X.2004.23494.x. [DOI] [PubMed] [Google Scholar]
- 14.Azzi L, El-Alfy M, Martel C, Labrie F. Gender differences in mouse skin morphology and specific effects of sex steroids and dehydroepiandrosterone. J Invest Dermatol. 2005;124:22–27. doi: 10.1111/j.0022-202X.2004.23545.x. [DOI] [PubMed] [Google Scholar]
- 15.McGaha T, Saito S, Phelps RG, Gordon R, Noben-Trauth N, Paul WE, Bona C. Lack of skin fibrosis in tight skin (TSK) mice with targeted mutation in the interleukin-4R alpha and transforming growth factor-beta genes. J Invest Dermatol. 2001;116:136–143. doi: 10.1046/j.1523-1747.2001.00217.x. [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Helene Sage E. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol. 2003;120:949–955. doi: 10.1046/j.1523-1747.2003.12241.x. [DOI] [PubMed] [Google Scholar]
- 17.Bradshaw AD, Reed MJ, Sage EH. SPARC-null mice exhibit accelerated cutaneous wound closure. J Histochem Cytochem. 2002;50:1–10. doi: 10.1177/002215540205000101. [DOI] [PubMed] [Google Scholar]
- 18.Miller EJ, Rhodes RK. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82(Pt A):33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- 19.Laubach VE, Foley PL, Shockey KS, Tribble CG, Kron IL. Protective roles of nitric oxide and testosterone in endotoxemia: evidence from NOS-2-deficient mice. Am J Physiol. 1998;275:H2211–2218. doi: 10.1152/ajpheart.1998.275.6.H2211. [DOI] [PubMed] [Google Scholar]
- 20.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 21.Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 22.Wenstrup RJ, Florer JB, Cole WG, Willing MC, Birk DE. Reduced type I collagen utilization: a pathogenic mechanism in COL5A1 haplo-insufficient Ehlers-Danlos syndrome. J Cell Biochem. 2004;92:113–124. doi: 10.1002/jcb.20024. [DOI] [PubMed] [Google Scholar]
- 23.Wenstrup RJ, Florer JB, Davidson JM, Phillips CL, Pfeiffer BJ, Menezes DW, Chervoneva I, Birk DE. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem. 2006;281:12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- 24.McAsey ME, Cady C, Jackson LM, Li M, Randall S, Nathan BP, Struble RG. Time course of response to estradiol replacement in ovariectomized mice: brain apolipoprotein E and synaptophysin transiently increase and glial fibrillary acidic protein is suppressed. Exp Neurol. 2006;197:197–205. doi: 10.1016/j.expneurol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj J. 2002;19:287–293. doi: 10.1023/A:1025348417078. [DOI] [PubMed] [Google Scholar]
- 26.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 27.Kafantari H, Kounadi E, Fatouros M, Milonakis M, Tzaphlidou M. Structural alterations in rat skin and bone collagen fibrils induced by ovariectomy. Bone. 2000;26:349–353. doi: 10.1016/S8756-3282(99)00279-3. [DOI] [PubMed] [Google Scholar]
- 28.Brinckmann J, Kim S, Wu J, Reinhardt DP, Batmunkh C, Metzen E, Notbohm H, Bank RA, Krieg T, Hunzelmann N. Interleukin 4 and prolonged hypoxia induce a higher gene expression of lysyl hydroxylase 2 and an altered cross-link pattern: important pathogenetic steps in early and late stage of systemic scleroderma? Matrix Biol. 2005;24:459–468. doi: 10.1016/j.matbio.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Hall GK, Phillips TJ. Skin and hormone therapy. Clin Obstet Gynecol. 2004;47:437–449. doi: 10.1097/00003081-200406000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 31.Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 32.Chan D, Lamande SR, Cole WG, Bateman JF. Regulation of procollagen synthesis and processing during ascorbate-induced extracellular matrix accumulation in vitro. Biochem J. 1990;269:175–181. doi: 10.1042/bj2690175. [DOI] [PMC free article] [PubMed] [Google Scholar]


