Abstract
The incidence and RNA electropherotypes of rotavirus in stools or rectal swabs of children with diarrhea were studied for three rotavirus seasons (1981 through 1984) in Philadelphia, Pa. We used a simplified RNA analysis method involving polyacrylamide gel electrophoresis followed by silver staining. Phosphate-buffered saline suspensions of the stools and swab eluates were examined directly by polyacrylamide gel electrophoresis-silver staining analysis and enzyme-linked immunoadsorbent assay (Rotazyme; Abbott Laboratories); electron microscopy was performed on solid stool specimens. The RNA analysis results were compared with electron microscopy and enzyme-linked immunosorbent assay results and exhibited a sensitivity and specificity greater than or equal to that of electron microscopy or the enzyme-linked immunosorbent assay. Ten different electropherotypes were detected among the 68 rotavirus RNA-positive specimens examined over the 3-year study. The predominant electropherotype was different in each season. Our results indicate that the polyacrylamide gel electrophoresis-silver nitrate strain RNA analysis of simple unextracted stool suspensions is a uniquely useful diagnostic technique; it rapidly provides both a definitive positive result and immediate determination of the RNA electropherotype, which is of value for epidemiological study.
Full text
PDF
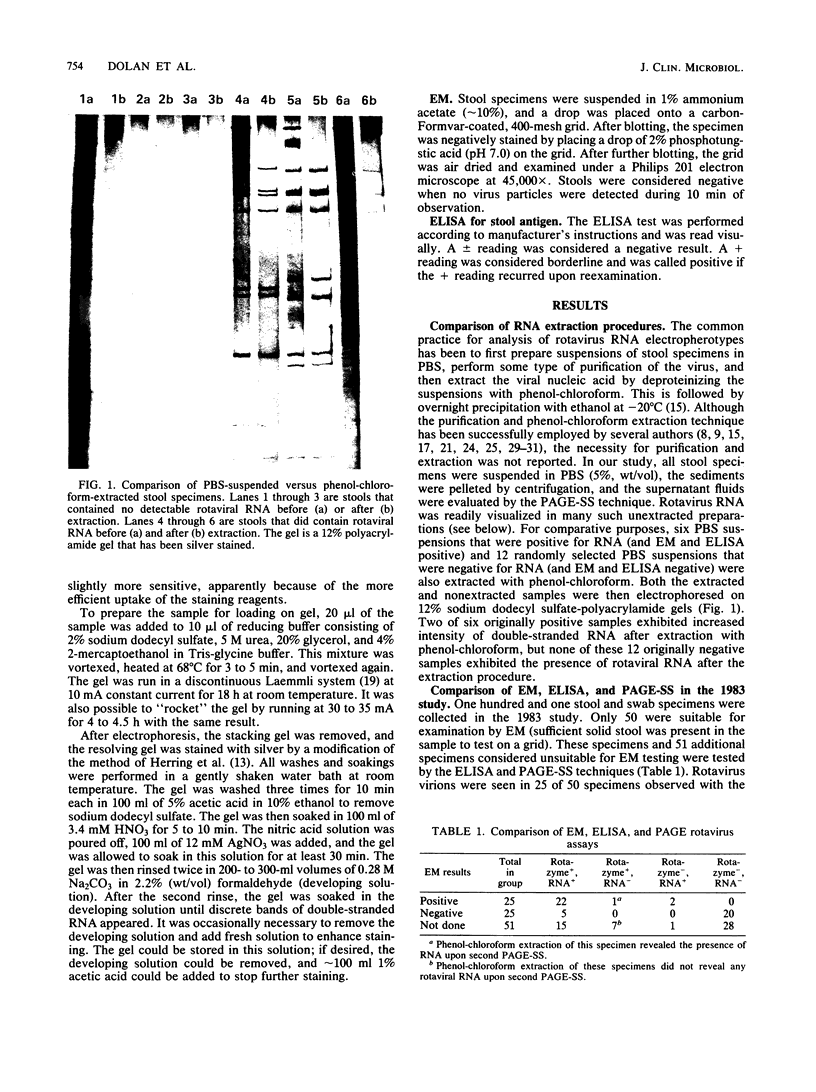

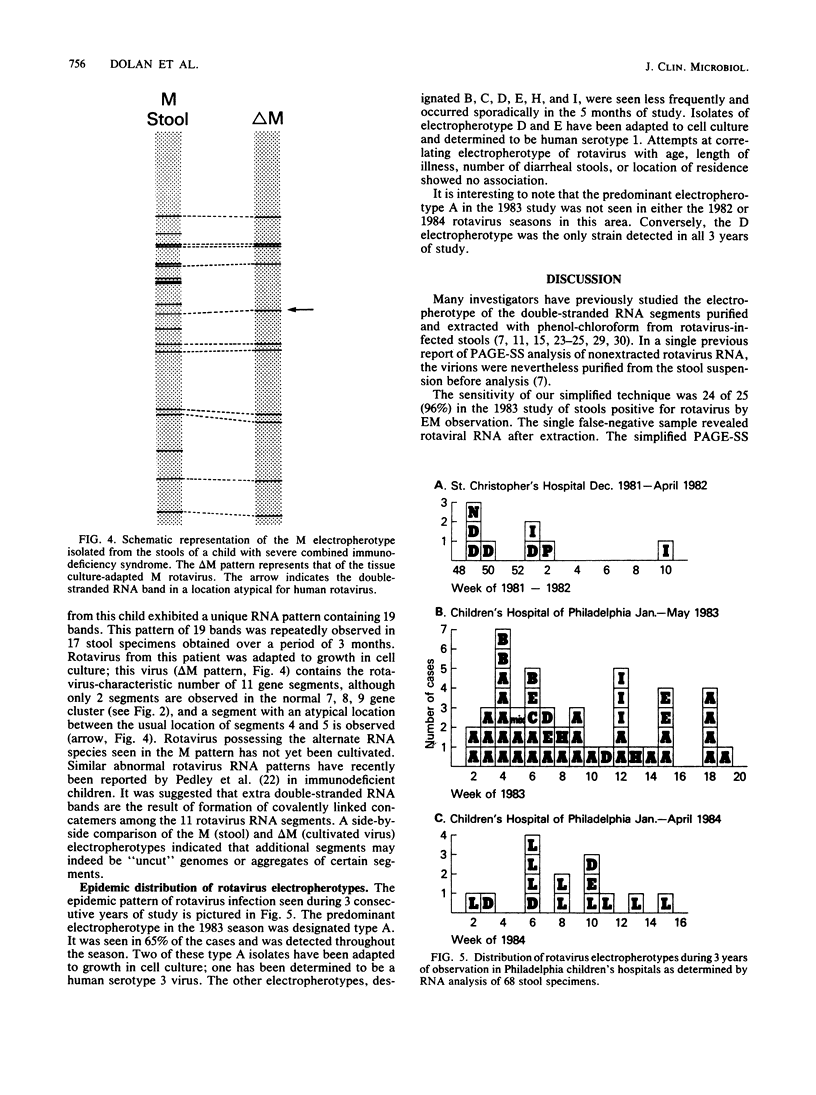


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Bishop R. F., Shann F. A. Epidemiology of rotavirus diarrhea in the Highlands of Papua, New Guinea, in 1979, as revealed by electrophoresis of genome RNA. J Clin Microbiol. 1983 Jan;17(1):162–164. doi: 10.1128/jcm.17.1.162-164.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M. J., Soenarto Y., Bishop R. F. Epidemiology of rotavirus diarrhea in Yogyakarta, Indonesia, as revealed by electrophoresis of genome RNA. J Clin Microbiol. 1982 Oct;16(4):731–733. doi: 10.1128/jcm.16.4.731-733.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Thomas L., Yolken R. H., Arrobio J. O., Kapikian A. Z., Parrott R. H., Chanock R. M. Comparison of direct electron microscopy, immune electron microscopy, and rotavirus enzyme-linked immunosorbent assay for detection of gastroenteritis viruses in children. J Clin Microbiol. 1981 May;13(5):976–981. doi: 10.1128/jcm.13.5.976-981.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung E. Y., Hnatko S. I., Gunning H., Wilson J. Comparison of Rotazyme and direct electron microscopy for detection of rotavirus in human stools. J Clin Microbiol. 1982 Sep;16(3):562–563. doi: 10.1128/jcm.16.3.562-563.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrystie I. L., Totterdell B. M., Banatvala J. E. False positive rotazyme tests on faecal samples from babies. Lancet. 1983 Oct 29;2(8357):1028–1028. doi: 10.1016/s0140-6736(83)91014-0. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. H., Estes M. K., Rangelova S. M., Shindarov L. M., Melnick J. L., Graham D. Y. Detection of antigenically distinct rotaviruses from infants. Infect Immun. 1983 Aug;41(2):523–526. doi: 10.1128/iai.41.2.523-526.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Avendaño L. F., Muñoz O., Romero P., Eternod J. G., Lopez S., Moncaya J. Comparison of human rotaviruses isolated in Mexico City and in Santiago, Chile, by electrophoretic migration of their double-stranded ribonucleic acid genome segments. Infect Immun. 1980 Nov;30(2):342–348. doi: 10.1128/iai.30.2.342-348.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Muñz O., Serafin F., Romero P. Shift in the prevalent human rotavirus detected by ribonucleic acid segment differences. Infect Immun. 1980 Feb;27(2):351–354. doi: 10.1128/iai.27.2.351-354.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett E. A., Sanders R. C., Beards G. M., Hundley F., Desselberger U. Molecular epidemiology of human rotaviruses. Analysis of outbreaks of acute gastroenteritis in Glasgow and the west of Scotland 1981/82 and 1982/83. J Hyg (Lond) 1984 Apr;92(2):209–222. doi: 10.1017/s0022172400064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Ahluwalia G. S., Klisko B., Hazelton P. R. Human rotavirus detection by counterimmunoelectrophoresis versus enzyme immunoassay and electron microscopy after direct ultracentrifugation. J Clin Microbiol. 1984 Mar;19(3):439–441. doi: 10.1128/jcm.19.3.439-441.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes I. H. Viral gastroenteritis. Prog Med Virol. 1979;25:1–36. [PubMed] [Google Scholar]
- Kalica A. R., Garon C. F., Wyatt R. G., Mebus C. A., van Kirk D. H., Chanock R. M., Kapikian A. Z. Differentiation of human and calf reoviruslike agents associated with diarrhea using polyacrylamide gel electrophoresis of RNA. Virology. 1976 Oct 1;74(1):86–92. doi: 10.1016/0042-6822(76)90131-8. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Greenberg H. B., Kalica A. R., Kim H. W., Brandt C. D., Rodriguez W. J., Parrott R. H., Chanock R. M. Approaches to immunization of infants and young children against gastroenteritis due to rotaviruses. Rev Infect Dis. 1980 May-Jun;2(3):459–469. doi: 10.1093/clinids/2.3.459. [DOI] [PubMed] [Google Scholar]
- Konno T., Sato T., Suzuki H., Kitaoka S., Katsushima N., Sakamoto M., Yazaki N., Ishida N. Changing RNA patterns in rotaviruses of human origin: demonstration of a single dominant pattern at the start of an epidemic and various patterns thereafter. J Infect Dis. 1984 May;149(5):683–687. doi: 10.1093/infdis/149.5.683. [DOI] [PubMed] [Google Scholar]
- Krause P. J., Hyams J. S., Middleton P. J., Herson V. C., Flores J. Unreliability of Rotazyme ELISA test in neonates. J Pediatr. 1983 Aug;103(2):259–262. doi: 10.1016/s0022-3476(83)80361-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nicolas J. C., Pothier P., Cohen J., Lourenco M. H., Thompson R., Guimbaud P., Chenon A., Dauvergne M., Bricout F. Survey of human rotavirus propagation as studied by electrophoresis of genomic RNA. J Infect Dis. 1984 May;149(5):688–693. doi: 10.1093/infdis/149.5.688. [DOI] [PubMed] [Google Scholar]
- Pedley S., Hundley F., Chrystie I., McCrae M. A., Desselberger U. The genomes of rotaviruses isolated from chronically infected immunodeficient children. J Gen Virol. 1984 Jul;65(Pt 7):1141–1150. doi: 10.1099/0022-1317-65-7-1141. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Bishop R. F., Birch C., McLean B., Holmes I. H. Molecular epidemiology of human rotaviruses in Melbourne, Australia, from 1973 to 1979, as determined by electrophoresis of genome ribonucleic acid. J Clin Microbiol. 1981 Feb;13(2):272–278. doi: 10.1128/jcm.13.2.272-278.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Holmes I. H. Comparison of the genomes of simian, bovine, and human rotaviruses by gel electrophoresis and detection of genomic variation among bovine isolates. J Virol. 1979 Jun;30(3):839–846. doi: 10.1128/jvi.30.3.839-846.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. S., Miller M. F. Comparison of an enzyme immunoassay with electron microscopic procedures for detecting rotavirus. J Clin Microbiol. 1982 May;15(5):938–944. doi: 10.1128/jcm.15.5.938-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Inaba Y., Miura Y., Tokuhisa S., Matumoto M. Antigenic relationships between rotaviruses from different species as studied by neutralization and immunofluorescence. Arch Virol. 1982;73(1):45–50. doi: 10.1007/BF01341726. [DOI] [PubMed] [Google Scholar]
- Schnagl R. D., Rodger S. M., Holmes I. H. Variation in human rotavirus electropherotypes occurring between rotavirus gastroenteritis epidemics in central Australia. Infect Immun. 1981 Jul;33(1):17–21. doi: 10.1128/iai.33.1.17-21.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Tzipori S. Gel electrophoresis of rotavirus RNA derived from six different animal species. Aust J Exp Biol Med Sci. 1979 Dec;57(6):583–585. doi: 10.1038/icb.1979.61. [DOI] [PubMed] [Google Scholar]
- Theil K. W., McCloskey C. M., Saif L. J., Redman D. R., Bohl E. H., Hancock D. D., Kohler E. M., Moorhead P. D. Rapid, simple method of preparing rotaviral double-stranded ribonucleic acid for analysis by polyacrylamide gel electrophoresis. J Clin Microbiol. 1981 Sep;14(3):273–280. doi: 10.1128/jcm.14.3.273-280.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima H., Kim B., Tajima T., Araki K., Yoshino K., Shinozaki T., Fujii R. Epidemiology of rotavirus infection in Tokyo during two winter seasons, as revealed by analyses of recovered viral RNA. Eur J Pediatr. 1984 Apr;142(1):71–72. doi: 10.1007/BF00442598. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., Kapikian A. Z., Mebus C. A. Induction of cross-reactive serum neutralizing antibody to human rotavirus in calves after in utero administration of bovine rotavirus. J Clin Microbiol. 1983 Sep;18(3):505–508. doi: 10.1128/jcm.18.3.505-508.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Leister F. J. Evaluation of enzyme immunoassays for the detection of human rotavirus. J Infect Dis. 1981 Oct;144(4):379–379. doi: 10.1093/infdis/144.4.379. [DOI] [PubMed] [Google Scholar]