Abstract
Background
Clinical studies have demonstrated that 50% of individuals with chronic renal disease (CRD) die of cardiovascular causes, including advanced calcific arterial and valvular disease; however, the mechanisms of accelerated calcification in CRD remain obscure, and no therapies can prevent disease progression. We recently demonstrated in vivo that inflammation triggers cardiovascular calcification. In vitro evidence also indicates that elastin degradation products may promote osteogenesis. Here, we used genetically modified mice and molecular imaging to test the hypothesis in vivo that cathepsin S (catS), a potent elastolytic proteinase, accelerates calcification in atherosclerotic mice with CRD induced by 5/6 nephrectomy.
Methods and Results
Apolipoprotein-deficient (apoE−/−)/catS+/+ (n = 24) and apoE−/−/catS−/− (n = 24) mice were assigned to CRD and control groups. CRD mice had significantly higher serum phosphate, creatinine, and cystatin C levels than those without CRD. To visualize catS activity and osteogenesis in vivo, we coadministered catS-activatable and calcification-targeted molecular imaging agents 10 weeks after nephrectomy. Imaging coregistered increased catS and osteogenic activities in the CRD apoE−/−/catS+/+ cohort, whereas CRD apoE−/−/catS−/− mice exhibited less calcification. Quantitative histology demonstrated greater catS-associated elastin fragmentation and calcification in CRD apoE−/−/catS+/+ than CRD apoE−/−/catS−/− aortas and aortic valves. Notably, catS deletion did not cause compensatory increases in RNA levels of other elastolytic cathepsins or matrix metalloproteinases. Elastin peptide and recombinant catS significantly increased calcification in smooth muscle cells in vitro, a process further amplified in phosphate-enriched culture medium.
Conclusions
The present study provides direct in vivo evidence that catS-induced elastolysis accelerates arterial and aortic valve calcification in CRD, providing new insight into the pathophysiology of cardiovascular calcification.
Keywords: calcification; aortic valve; atherosclerosis; kidney failure, chronic; elastin
Westernized societies face a growing burden of cardiovascular calcification, a disease of disordered mineral metabolism.1–4 The interaction of prevalent epidemiological factors such as age, hypercholesterolemia, and renal insufficiency accelerates arterial and aortic valve calcification.5 Clinical studies have demonstrated that approximately 50% of individuals with chronic renal disease (CRD) die of a cardiovascular cause.6–8 In addition to the classic risk factors such as age and dyslipidemia, patients with CRD have hyperphosphatemia, which is considered an independent risk factor for cardiovascular death.9,10 CRD accelerates the development of atherosclerosis and excessive calcification in both the intima and media of atheromatous lesions.11,12 Although they have different causes, intimal and medial calcification both involve the activation of proinflammatory mechanisms and smooth muscle cell (SMC) proliferation, which likely share common calcification pathways.
Recent studies suggest that arterial and valvular calcification occurs through highly regulated molecular processes characterized by expression of osteogenic proteins and matrix-degrading proteinases. We have previously implicated proteolytic enzymes expressed by activated macrophages and myofibroblast-like cells in atherosclerotic plaque progression and aortic valve disease.13–15 During atherogenesis, macrophage-derived proteinases such as elastolytic cathepsins or metalloproteinases (MMP-2 and MMP-9) induce release of biologically active, soluble elastin-derived peptides that may promote osteogenic differentiation of myofibroblasts16 or SMCs.17 In addition, elastases degrade medial elastin, which favors calcification through an increase of elastin polarity that in turn enhances elastin affinity for calcium.18,19
Cardiovascular calcification commonly causes devastating complications, including plaque rupture20–24 and aortic valve stenosis, which currently have no suitable therapeutic alternative beyond valve replacement.25 In addition, a recent large-scale intervention trial showed that statin therapy did not prevent the progression of calcific aortic stenosis.26 This lack of therapeutic options demands the exploration of new pathways that contribute to cardiovascular calcification.1
Our recent studies used real-time molecular imaging approaches to establish in vivo that proatherogenic molecular and cellular processes induce cardiovascular calcification.24,25 The present study dissected the mechanisms underlying accelerated arterial and valvular calcification in CRD. Specifically, we tested the hypothesis that proinflammatory cathepsin S (catS), a highly potent elastase, contributes to arterial and valvular calcification in mice with atherosclerosis and CRD assessed by in vivo and ex vivo optical molecular imaging, correlative histopathology, and in vitro experiments. Our results provide new insights into the pathobiology of arterial and valvular calcification and aid the investigation of novel therapeutic strategies to reduce the onset of cardiovascular events, and thus mortality, in CRD.
Methods
An expanded Methods section is available as an online-only Data Supplement.
Animal Experiments
We studied osteogenic changes in arteries and aortic valves of 30-week-old apolipoprotein E (apoE)–deficient/catS wild-type mice (apoE−/−/catS+/+; n = 24) and apoE-deficient/catS-deficient (apoE−/−/catS−/−; n = 24) mice that consumed an atherogenic diet (Teklad TD 88137; Harlan, Indianapolis, Ind) from 10 weeks of age. At 20 weeks of age, mice in each group were randomized either to continue with the atherogenic diet or to a CRD group (12 mice per group). Age-matched wild-type C57/BL6 mice (Jackson Laboratory, Bar Harbor, Me) with normal renal function (n = 6) and CRD (n = 6) served as controls. We used a 2-step procedure to create CRD: Left heminephrectomy followed by right total nephrectomy 1 week later, procedures that are known to aggravate atherosclerosis.11,27 At 10 weeks after surgery, mice underwent intravital microscopy and were euthanized for ex vivo imaging and correlative histological analyses. The Subcommittee on Research Animal Care at Massachusetts General Hospital approved all procedures.
Blood Biochemical Analyses
Blood was obtained from the heart and spun in a refrigerated centrifuge, and serum was stored at −80°C. Serum levels of total cholesterol, cystatin C, creatinine, phosphate, and calcium were assessed (n = 5 per group).
Molecular Imaging Agents
Detection of Elastolytic Activity
A novel peptide-based catS-activatable agent consists of backbone of a catS-cleavable dipeptide substrate (Leu-Arg) and 2 polyethylene glycol chains that contain a fluorochrome in their dendritic arms.28 After enzymatic cleavage by catS, the fluorochromes separate, which results in signal amplification (excitation/emission 750/780 nm). In vitro validation studies indicated that catS, but not cathepsins B, K, or L, cleaves this probe.28
Detection of Osteogenic Activity
A bisphosphonate-conjugated imaging agent (OsteoSense680, VisEn Medical Inc, Woburn, Mass), which generates fluorescence evident through the near-infrared window (excitation/emission 650/680 nm), was used to detect osteogenic activity, as described previously.24,25,29
Detection of Inflammation
Cross-linked iron oxide (CLIO-gly) fluorescent nanoparticles, an imaging agent that produces fluorescence visible through the near-infrared window (excitation/emission 750/780 nm), was used to detect macrophages.
Intravital Dual-Channel Fluorescence Imaging
Mice (n = 12 per group) simultaneously received spectrally distinct imaging agents (activatable cathepsin S/750 and OsteoSense680 or CLIO-gly750 and OsteoSense680) or saline via intravenous injection 24 hours before imaging. We performed dual-channel fluorescence imaging using an Olympus IV 100 intravital laser scanning fluorescence microscope developed for imaging small experimental animals. 24,25 Excitation at 633 and 748 nm and image collection of the different channels were done serially to avoid cross talk between channels. Image stacks were processed and analyzed with ImageJ software (version 1.41, Bethesda, Md).
Macroscopic Fluorescence Reflectance Imaging
After mice were euthanized, aortas were perfused with saline, dissected, and imaged to map the macroscopic near-infrared osteogenic activity elaborated by the OsteoSense680 imaging agent with a fluorescence reflectance imaging system equipped with multichannel filter sets (Omega Optical, Brattleboro, Vt) as described previously (n = 12 per group).24
Histopathological Assessment
Morphological Characterization
Tissue samples were frozen in OCT compound, and 5-µm serial sections were cut and stained with hematoxylin and eosin for general morphology. Alkaline phosphatase activity (a marker of early osteogenic differentiation) was detected on cryosections according to the manufacturer’s instructions (alkaline phosphatase substrate kit, Vector Laboratories, Burlingame, Calif). Van Gieson stain was used to assess elastin. Von Kossa silver stain was used to histochemically image advanced calcification. Masson trichrome stain was used to evaluate glomerulosclerosis.
Immunohistochemistry
Immunohistochemistry for macrophages (anti-mouse Mac3, BD Biosciences, San Jose, Calif), catS (Santa Cruz Biotechnology, Santa Cruz, Calif), and cystatin C (Abcam, Cambridge, United Kingdom) was performed by the avidin-biotin peroxidase method. Fluorescence immunohistochemistry simultaneously visualized Texas Red–labeled catS and elastin. Images were captured and processed with an epifluorescence microscope (Eclipse 80i, Nikon Instruments, Melville, NY) with a cooled CCD camera (Cascade, Photometrics, Ariz).
Real-Time Reverse-Transcription Polymerase Chain Reaction
Total RNA was isolated from aorta with an RNeasy Mini Kit (Qiagen, Valencia, Calif) from all groups of mice (n = 5 per group). Polymerase chain reaction detection and quantification of cathepsins S, B, L, and K and MMP-2 and MMP-9 was done with a MyiQ single-color real-time PCR detection system and iQ SYBR Green Supermix (BioRad, Hercules, Calif). All measurements were duplicated, and the mean value was used. Data are expressed in arbitrary units that were normalized by β-actin.
Primary Human SMC Culture
Human vascular SMCs were treated with human aortic elastin peptides 100 mg/mL (Elastin Products Co, St Louis, Mo), either alone or in the presence of human recombinant catS 1 mg/mL (Calbiochem, San Diego, Calif); catS plus the cathepsin inhibitor cystatin C 1 mg/mL (Calbiochem); or cystatin C 1 mg/mL. Separate plates were treated with 2 mmol/L NaH2PO4 in addition to the above treatments. After 4 days of treatment, cells were stained for alkaline phosphatase activity. Cells were then fixed in 4% paraformaldehyde and restained for the presence of calcium phosphate crystals by the von Kossa method. Stained cells were imaged with a color CCD camera (Nikon DXM 1200-F, Nikon Inc, Melville, NY) and quantified as a percentage of total area with IPLab imaging software (version 3.9.3, Scanalytic Inc, Rockville, Md).
Statistical Analysis
Statistical analyses for comparisons of multiple groups used 1-way ANOVA followed by the Tukey post hoc test. Student t test was performed when 2 groups were compared (GraphPad Prism, version 4.0, GraphPad Software, San Diego, Calif). Data are presented as mean±SEM. P values <0.05 were considered significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Phosphate, Creatinine, and Cystatin C Levels Are Increased in CRD ApoE−/−/CatS+/+ Mice
Histopathological analysis 10 weeks after subtotal nephrectomy revealed that the kidneys of hypercholesterolemic CRD apoE−/−/catS+/+ and CRD apoE−/−/catS−/− mice had a 50% reduction in the number of nephrons and enlarged glomeruli and developed glomerulosclerosis (online-only Data Supplement Figure). Both CRD apoE−/−/catS+/+ and CRD apoE−/−/catS−/− groups had significantly increased serum phosphate and creatinine levels compared with apoE−/−/catS+/+ and apoE−/−/catS−/− mice with normal kidney function, whereas serum calcium levels remained unaffected (Table). Elevation of cystatin C, a marker of impaired glomerular filtration rate and a major endogenous inhibitor of catS, reflects a very early stage of chronic renal dysfunction.30 ApoE−/−/catS+/+ mice and especially CRD apoE−/−/catS+/+ mice had significantly increased cystatin C levels compared with apoE−/−/catS−/− and CRD apoE−/−/catS−/− mice lacking catS (Table).
Table.
Blood Biochemistry
| Genotype | Phosphate, mg/dL | Calcium, mg/dL | Creatinine, mg/dL | Cystatin C, mg/dL | Total Cholesterol, mg/dL |
|---|---|---|---|---|---|
| apoE+/+/catS+/+ | 4.70±0.37*† | 0.92±0.01 | 0.25±0.01*† | 0.25±0.01*† | 56.33±9.83*† |
| CRD apoE+/+/catS+/+ | 7.18±0.49* | 0.96±0.50 | 0.29±0.03*† | 0.27±0.01*† | 69.40±2.87*† |
| apoE−/−/catS+/+ | 6.28±0.40*† | 0.78±0.06 | 0.37±0.01*† | 0.56±0.13† | 467.2±51.11* |
| CRD apoE−/−/catS+/+ | 11.38±0.88 | 0.94±0.03 | 0.6±0.04 | 0.94±0.10† | 397.1±24.76† |
| apoE−/−/catS−/− | 7.18±0.50*† | 0.88±0.01 | 0.26±0.02*† | 0.17±0.01* | 842.0±58.55* |
| CRD apoE−/−/catS−/− | 10.28±1.40 | 0.78±0.21 | 0.45±0.05 | 0.21±0.03* | 659.6±114.20* |
Indicates significance between CRD apoE−/−/cat S+/+ and other groups.
Indicates significance between CRD apoE−/−/cat S−/− and other groups.
Calcification Is Increased in the Aortas of CRD ApoE−/−/CatS+/+ Mice Compared With CatS-Deficient Mice
Macroscopic fluorescence reflectance imaging was used to map osteogenic activity in mouse aortas (Figure 1). A bisphosphonate-derivatized near-infrared fluorescent imaging agent (OsteoSense680) administered via tail-vein injection 24 hours before imaging enabled monitoring of osteogenesis as gauged by detection of nanomolar concentrations of hydroxyapatite crystals. The plaques had a target-to-background ratio of 5.4±0.5 versus 1.7±0.2 in uninjected controls (P<0.01). Fluorescence reflectance imaging in CRD apoE−/−/catS+/+ mice yielded strong osteogenic fluorescent signals in the entire aorta compared with aortas of mice with normal renal function (Figure 1A). In contrast, osteogenic activity decreased in CRD apoE−/−/catS−/− mouse aortas (Figure 1B). Quantitative histological assessment further demonstrated less calcification in CRD apoE−/−/catS−/− mouse aortas than in CRD apoE−/−/catS+/+ mice detected by von Kossa staining (P<0.01; Figure 1B and 1C). We then examined whether catS deficiency suppressed calcification due to retarded plaque development. Histological analysis showed that catS deletion did not reduce plaque size (Figure 1D) or macrophage accumulation in CRD mice (Figure 1E).
Figure 1.
Calcification increased in aortas of CRD apoE−/−/catS+/+ mice and decreased with catS deletion. A, Macroscopic fluorescence reflectance imaging of calcification in CRD apoE−/−/catS+/+ mice yielded strong osteogenic signals in the entire aorta compared with aortas of CRD apoE−/−/catS−/− mice and mice with normal renal function. Bar = 1 cm. Histological analysis (B; von Kossa; bar = 200 µm) and quantitative assessment (C through E) showed that only CRD apoE−/−/catS+/+ mice had increased calcification, whereas no significant difference in plaque size and macrophage (Mac-3) accumulation was observed between CRD apoE−/−/catS+/+ and CRD apoE−/−/catS−/− mice.
Augmented Osteogenic and Elastolytic Activity in CRD ApoE−/−/CatS+/+ Mice Compared With CatS-Deficient Mice
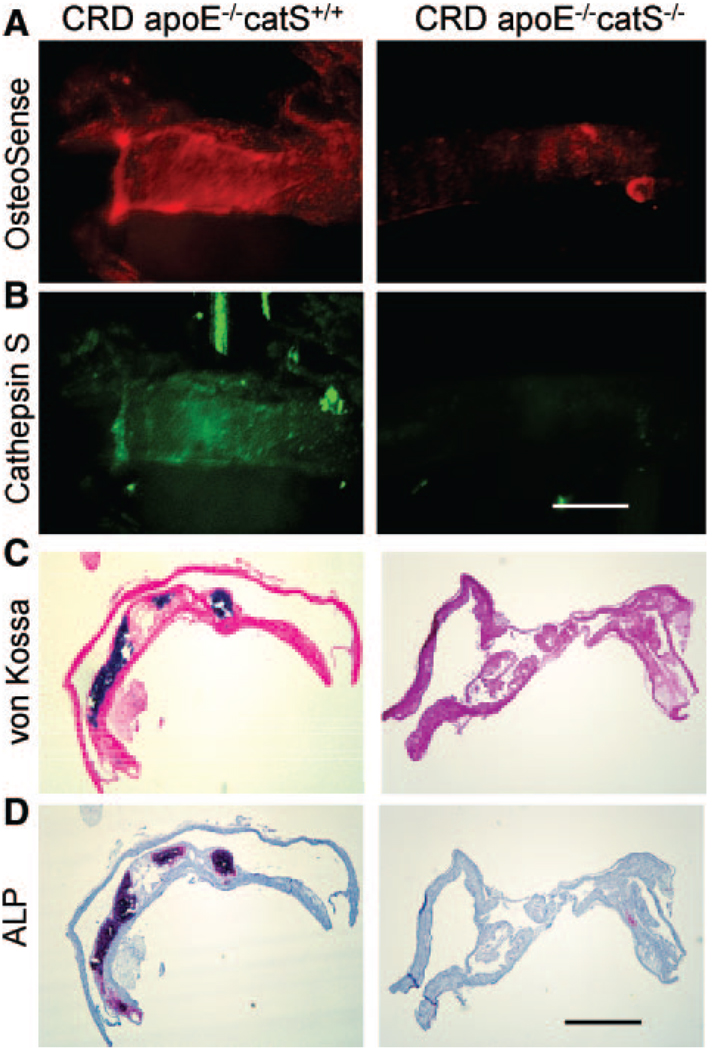
Intravital multichannel, high-resolution laser scanning fluorescence microscopy visualized in vivo real-time osteogenic and elastolytic activities in the atherosclerotic carotid artery plaques.24 Intravital microscopy coregistered osteogenic and elastolytic signals in CRD apoE−/−/catS+/+ mice (Figure 2A). In contrast, CRD apoE−/−/catS−/− mice contained less osteogenic signal in the carotid plaque, where catS elastolytic activity remained undetectable (Figure 2B). Correlative histological analysis showed advanced calcification in CRD apoE−/−/catS+/+ mouse arteries detected by von Kossa staining (Figure 2C) and alkaline phosphatase activity (Figure 2D), whereas CRD apoE−/−/catS−/− mice showed negligible calcification and alkaline phosphatase activity, which corroborated the molecular imaging results.
Figure 2.
Increased osteogenic action and catS activity in carotid atherosclerotic plaques of CRD apoE−/−/catS+/+ mice. A and B, Intravital microscopy revealed increased osteogenic and catS signal intensity in CRD apoE−/−/catS+/+ mice vs lower osteogenic signal and negligible elastolytic catS activity in CRD apoE−/−/catS−/−. Bar = 500 µm. C and D, Correlative histological analysis showed advanced calcification in CRD apoE−/−/catS+/+ mouse artery (von Kossa and alkaline phosphatase activity [ALP]), whereas CRD apoE−/−/catS−/− mice exhibited no calcification. Bar = 200 µm.
CatS-Associated Elastin Breaks Are More Common in CRD ApoE−/−/CatS+/+ Plaques Than in Those in CatS-Deficient Mice
Fluorescence immunohistochemistry detected abundant immunoreactive catS expression in CRD apoE−/−/catS+/+ plaque associated with increased fragmentation of elastin (Figure 3A). apoE−/−/catS−/− and CRD apoE−/−/catS−/− mice had undetectable levels of catS and fewer elastin fragments (Figure 3A), which was verified by quantitative histological assessment (P<0.001; Figure 3B).
Figure 3.
Increased catS-associated elastin fragmentation in CRD apoE−/−/catS+/+ mouse aorta. A, Fluorescence immunohistochemistry detected abundant catS expression (red) in CRD apoE−/−/catS+/+ plaque associated with increased fragmentation of elastin (green). apoE−/−/catS−/− and CRD apoE−/−/catS−/− mice had negligible catS expression and a reduced number of elastin fragments, confirmed by quantitative histological assessment (B). L indicates lumen. Dotted line depicts the plaque margin. Bar = 50 µm.
Intimal and Medial Calcification Is Associated With CatS Expression and Elastin Degradation in CRD ApoE−/−/CatS+/+ Mouse Aortas
Advanced calcification in the intima and media was associated with elastin fragmentation in the aortas of CRD apoE−/−/catS+/+ mice, whereas aortas of CRD apoE−/−/catS−/− mice had preserved elastin and negligible calcification (Figure 4A). Further histopathological analysis of CRD apoE−/−/catS+/+ plaques suggested a link between catS expression, reduced elastin (van Gieson staining), and calcifying cells in the media and intima (alkaline phosphatase activity and von Kossa staining; Figure 4B). Notably, CRD apoE−/−/catS+/+ mice had low cystatin C tissue expression, which suggests imbalance that would favor elastin degradation in the arterial wall (Figure 4B). Correlative fluorescence imaging further colocalized immunoreactive catS with osteogenic nearinfrared signal detected in CRD apoE−/−/catS+/+ mice injected with OsteoSense680 (Figure 4C).
Figure 4.
Intimal and medial calcification was associated with catS expression and elastin degradation in CRD apoE−/−/catS+/+ mouse aortas. A, Histological analysis revealed distinct phenotypic changes in the atheroma of CRD apoE−/−/catS+/+ and CRD apoE−/−/catS−/− mice: Advanced calcification in the intima and scarce in the media (von Kossa; arrows) of CRD apoE−/−/catS+/+ mouse aorta was associated with elastin loss (van Gieson; arrowheads), whereas CRD apoE−/−/catS−/− mice had preserved elastin and undetectable calcification. B, Adjacent sections stained with hematoxylin and eosin (HE) showed an association of catS expression with calcifying cells in the media and intima (ALP [alkaline phosphatase] and von Kossa; arrows) and insufficient elastin (van Gieson; arrowheads) in CRD apoE−/−/catS+/+ plaque. Notably, cystatin C tissue expression was considerably low. C, Fluorescence imaging colocalized immunoreactive catS (green) with osteogenic near-infrared signal (OsteoSense680; red). Calcifying cells (yellow) surrounded by fragmented elastin fibers (arrows) were evident on the merged image. Bars = 50 µm.
Aortic Valves of CRD ApoE−/−/CatS+/+ Mice Exhibit Features of Calcific Valve Disease
Gross morphology and dual-channel fluorescence microscopy (Figure 5A) demonstrated advanced calcification (OsteoSense) in inflamed aortic valves (CLIO-gly). Individual mapping showed that signal intensities peaked at the areas of leaflet attachment to the aortic wall (Figure 5A). Histological analysis further demonstrated that aortic leaflets obtained from CRD apoE−/−/catS+/+ mice contained abundant macrophages that expressed catS in association with undetectable elastin (van Gieson negative), whereas CRD apoE−/−/catS−/− mice had preserved valvular elastin and no macrophage-derived catS (Figure 5B). In addition, valves from CRD apoE−/−/catS+/+ mice showed substantial calcification (von Kossa), whereas no calcification occurred in CRD apoE−/−/catS+/+ valves (Figure 5B). Quantitative analysis showed no significant difference between macrophage accumulations in the 2 CRD groups (Figure 5C).
Figure 5.
Inflamed aortic valves of CRD apoE−/−/catS+/+ mice had characteristics of early calcific disease. A, Gross morphology and ex vivo fluorescence microscopy (image stacks) of calcified aortic valves (top) visualized osteogenic activity (OsteoSense, red) in the areas of leaflet attachment to the aortic wall in inflamed valves (CLIO-gly, green). Individual mapping (bottom) showed maximum signal intensities at the areas of high mechanical stress (arrows). The data represent 3 mice that showed similar results. B, Aortic leaflets from CRD apoE−/−/catS+/+ mice contained abundant macrophages (Mac 3) that expressed catS in association with undetectable elastin (van Gieson negative) and showed evidence of calcification (von Kossa), the characteristics of calcific aortic valve disease (left). In contrast, inflamed leaflets of CRD apoE−/−/catS−/− mice exhibited negligible catS expression, preserved elastin, and no evidence of calcification (right). Bar = 100 µm. C, Quantitative assessment showed no significant difference in macrophage accumulation between CRD apoE−/−/catS+/+ and CRD apoE−/−/catS−/− mice. Ao indicates aorta.
CatS Deficiency Does Not Alter Expression Levels of Other Major Cathepsins
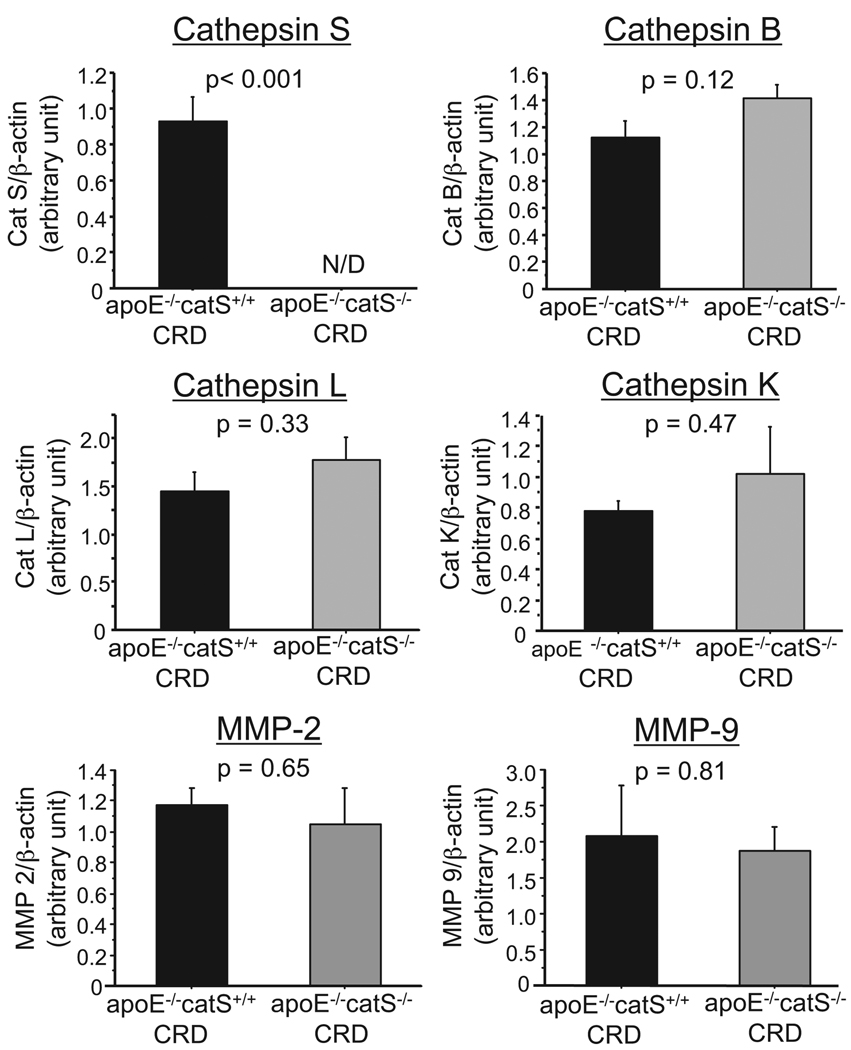
Germline manipulation of a gene in mice could cause “compensatory” changes in other genes that share similar functions with the targeted gene. Therefore, further experiments were conducted to examine the expression of other elastolytic enzymes. Quantitative real-time reverse-transcription polymerase chain reaction analysis demonstrated β-actin–adjusted levels of RNAs encoding cathepsins S, B, L, and K and MMP-2 and MMP-9 in the aortas of CRD apoE−/−/catS+/+ or CRD apoE−/−/catS−/− mice (Figure 6). As expected, CRD apoE−/−/catS−/− mice did not express catS. In addition, catS deletion did not cause statistically significant changes in expression of cathepsins B, L, or K and MMP-2 and MMP-9.
Figure 6.
CatS deletion did not alter expression levels of major elastolytic enzymes. Quantitative real-time reverse-transcription polymerase chain reaction demonstrated β-actin–adjusted levels of RNAs encoding cathepsins S, B, L, and K and MMP-2 and MMP-9 in the aortas of CRD apoE−/−/catS+/+ or CRD apoE−/−/catS−/− mice. CRD apoE−/−/catS−/− mouse aortas did not express detectable levels of catS. CatS deletion did not cause statistically significant changes in expression of elastolytic cathepsins and MMPs.
SMCs Treated With Aortic Elastin Peptide and Recombinant CatS in Phosphate-Enriched Culture Medium Display Greater Osteogenic Potential
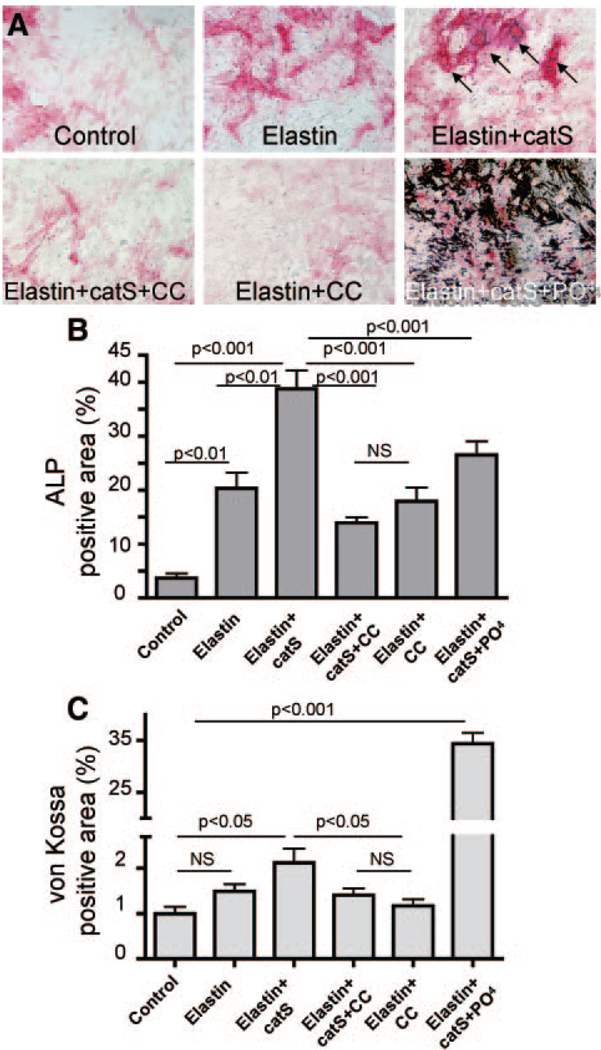
To explore the mechanisms by which catS deletion decreased calcification, we tested in vitro the ability of elastin peptides to promote calcification of primary cultures of human vascular SMCs (Figure 7). Treatment of SMCs in osteogenic medium with elastin peptides significantly increased alkaline phosphatase activity relative to control levels (P<0.01). The addition of catS further increased alkaline phosphatase activity up to 2-fold relative to elastin peptides (P<0.001; Figure 7A and 7B), which suggests that elastin fragments cleaved by catS promote osteogenic activity more potently than randomly cleaved elastin fragments. CatS inhibition by simultaneous addition of cystatin C, an endogenous inhibitor of cathepsins, attenuated the increase in osteogenesis that resulted from cathepsin activity (Figure 7A through 7C). High serum phosphate levels contribute to cardiovascular calcification; therefore, we also tested the effect of increased phosphate on calcification of vascular SMCs. The addition of monosodium phosphate moderately increased calcification with or without cleaved elastin (data not shown); however, with catS, monosodium phosphate promoted calcification more strikingly (35-fold, P<0.001; Figure 7A and 7C). Of note, alkaline phosphatase activity decreases in more prominently calcifying cells.
Figure 7.
Elastin degradation and high phosphate levels promote vascular SMC calcification. A, Human primary vascular SMCs were treated with elastin peptides (elastin), alone or in the presence of catS, CatS plus cathepsin inhibitor cystatin C (CC), or catS plus sodium monophosphate (PO4), as shown. After treatment, cells were stained for alkaline phosphatase (ALP) activity and then fixed and restained for the presence of calcium phosphate crystals with von Kossa. B and C, ALP activity (B; red reaction product) and (C) hydroxyapatite crystals stained with von Kossa (black) were quantified as percentage of total area.
Discussion
The present study provides evidence that catS promotes arterial and valvular calcification in hypercholesterolemic mice with CRD. Key findings documented here (1) show the suppression of arterial and aortic valve calcification in catS-deficient mice; (2) demonstrate in vivo that arterial calcification is associated with catS activity as detected by real-time visualization of spectrally distinct near-infrared signals amplified by catS-activatable and calcification-targeted molecular imaging agents; (3) correlate catS expression with calcifying cells and elastolysis in atheroma and inflamed aortic valves; and (4) provide mechanistic in vitro evidence that human vascular SMCs treated with elastin fragmented by catS undergo osteogenic changes, a process augmented in phosphate-enriched culture medium. Clinical and preclinical studies suggest that calcification of arteries or valves can cause devastating complications, including plaque rupture and aortic valve stenosis. These results support the hypothesis that elastolysis promotes osteogenesis. The present study observations suggest that antiinflammatory therapies and/or maintenance of elastin integrity by limitation of elastolytic activity might prevent cardiovascular calcification and its complications when introduced early.
The present study extends our previous work on the pathogenesis of cardiovascular calcification, which demonstrated that atherosclerotic calcification and calcific aortic valve disease share similar risk factors and proinflammatory molecular and cellular processes, as determined by advanced molecular imaging approaches.24,25,31,32 Notably, the present study used molecular imaging of early calcification and elastolytic activity to address new mechanisms underlying accelerated calcification in patients with CRD and suggests that calcification is a multifactorial process induced by proinflammatory stimuli33 that promote accumulation of elastolytic macrophages. Because mature aortic valves have an elastin-rich, multilayered structure14 and can develop inflammatory lesions that recapitulate features of atherosclerotic plaques,24,25 we propose that similar mechanisms of catS-associated elastin degradation contribute to the development of calcific aortic valve disease. Aortic valve calcification impairs the movement of aortic valve leaflets, which affects cardiac function, and can only be alleviated through costly and invasive procedures. Therefore, early diagnosis of and interference with aortic valve calcification could provide enormous clinical benefits.
CatS, one of the most potent mammalian elastases abundantly expressed by macrophages and SMCs in atheroma, contributes significantly to proteolysis of elastic laminae.34 Previous studies suggested that elastic fibers resist degradation due to numerous cross-links and the highly hydrophobic characteristics of tropoelastin chains.35,36 However, during progression of atherosclerosis, when disruption of the balance between cathepsins and their inhibitor cystatin C occurs, powerful proteases such as catS may degrade elastin, generating soluble peptides.18,37 These elastin-derived peptides serve as biologically active molecules (so-called matrikines or elastokines) that are highly chemotactic for macrophages and can regulate cell migration and proliferation, as well as release of bone-regulating proteins from SMCs and valvular myofibroblasts, which leads to plaque growth and calcification. 16,17,38–40 In addition, human genetic studies link mutations in the elastin gene and calcific valvular aortic stenosis.17,41
Previous studies established the contribution of catS to the pathogenesis of atherosclerosis34,42 and aortic stenosis.43 The present study used genetically modified mice and molecular imaging to seek direct in vivo evidence for the role of catS in calcification. We showed in vivo that lack of catS elastolytic activity is associated with decreased calcification in atherosclerotic plaques and aortic valves. Histopathological validation studies also showed that calcification decreased significantly in catS-deficient mice with CRD.
The present study further demonstrated an in vitro preparation that permits exploration of the mechanisms of vascular cell calcification, which suggests that treatment of primary human SMCs with human aortic elastin peptide and recombinant catS induces calcification of vascular cells. Moreover, the amplified osteogenic responses in phosphate-enriched culture medium suggest that catS-associated elastin degradation promotes arterial calcification in the hyperphosphatemic milieu of chronic renal impairment. These in vitro results and in vivo findings in hyperphosphatemic mice with CRD coincide with clinical studies that imply that an imbalance of the mineral metabolism causes vascular calcification in patients with renal failure.44 Clinical reports further suggest that elevated serum phosphate concentrations are associated with a substantially greater risk of end-stage CRD, and that risk increases up to 5-fold for each 1.0-mg/dL increment in the mean serum phosphate concentration.45
We used cystatin C as an established marker of the glomerular filtration rate to validate our model of CRD. The present study also demonstrated that apoE deficiency increased cystatin C, which agrees with clinical evidence that patients with documented atherosclerosis have elevated levels of this inhibitor.46 Although circulating cystatin C increased in CRD apoE−/− mice, local expression in atherosclerotic lesions appears to decrease, in agreement with our previous report34; however, the mechanism of this paradox remains unknown. Because a wide variety of tissues produce cystatin C, systemic cystatin C production, rather than local changes in cystatin C expression in atherosclerotic plaques, may determine its elevated serum concentrations.30
Collectively, our findings suggest the potential multistep mechanism that accelerates cardiovascular calcification in CRD (Figure 8). In atherosclerotic lesions and inflamed valves, macrophage-derived catS and other elastolytic enzymes participate in degradation of elastin matrix. On disruption of the elastic lamina, mesenchymal cells (arterial SMCs or valvular myofibroblast-like cells) proliferate, which causes lesion formation and growth. In addition, elastolysis-triggered release of biologically active elastin-degradation products (matrikines) and various uremia-specific proinflammatory factors27 may attract more macrophages, which in turn produce more proteolytic enzymes, thus forming an amplification loop and promoting further expansion of the lesion. Mesenchymal cells, activated by elastin-degradation products, undergo osteogenic differentiation. The systemic mineral imbalance, such as hyperphosphatemia in patients with CRD, further accelerates calcification of vascular SMCs or valvular myofibroblasts through phosphate-induced release of matrix vesicles and apoptosis.31,47
Figure 8.
Schematic of the proposed multistep mechanism of accelerated atherosclerotic and valvular calcification in CRD. In atherosclerotic artery and inflamed aortic valve, macrophage-derived catS degrades elastin. Biologically active elastin-degradation products initiate calcification of mesenchymal cells (eg, vascular SMCs or valvular myofibroblast-like cells). Furthermore, CRD-associated hyperphosphatemia accelerates calcification of mesenchymal cells through phosphate-induced release of matrix vesicles and apoptosis, which results in more advanced calcification. PO4 indicates sodium monophosphate.
The increased risk of mortality and morbidity associated with cardiovascular calcification drives the development of new therapeutic strategies to prevent and potentially reverse this process.1 Future therapeutic interventions in calcification-prone individuals may target inflammation and phosphate balance. New therapeutic options may also include selective inhibition of catS. More importantly, identification of high-risk patients requires new imaging modalities or biomarkers that target calcification. Clinical molecular imaging of osteogenic activity may identify calcifying atherosclerotic plaques and aortic valve lesions while the disease remains silent or reversible.24 Although calcification in atherosclerosis typically involves the tunica intima, CRD calcification occurs primarily in the media. Therefore, development of high-resolution imaging of calcification in different arterial layers may distinguish the origin of calcification and provide a powerful tool in personalized preventive cardiovascular medicine. A better understanding of the molecular and cellular mechanisms of cardiovascular calcification will lead to improved therapies for unstable coronary plaques and calcific aortic valves. Ongoing efforts that combine rigorous assessment of arterial and valvular pathobiology and further development of imaging technologies will provide novel insights into optimum therapeutic strategies for treatment of calcific vasculopathy/valvulopathy, particularly in patients with CRD.
CLINICAL PERSPECTIVE
Aging societies face a growing burden of vascular and valvular calcification. Half of all individuals with chronic renal disease succumb to cardiovascular complications. Despite its vast clinical significance, the mechanisms of accelerated cardiovascular calcification, particularly in chronic renal disease, remain obscure, and we currently lack therapies to prevent its progression. Recent clinical trials showed no benefit of statin therapy on progression of calcific aortic valve stenosis, a growing clinical challenge without alternatives to costly invasive treatments. Hence, the need to discover new pathways that contribute to cardiovascular calcification and to develop new therapeutic strategies to prevent or reverse calcification has driven our recent research investigations. The present study provides support for the hypothesis that elastin breakdown products produced by cathepsin S, a proteinase regulated by inflammation and overexpressed in diseased arteries and valves, can trigger osteogenesis. These results indicate that antiinflammatory therapies and preservation of elastin integrity might prevent cardiovascular calcification and its complications when introduced early. Therapeutic interventions in calcification-prone patients with chronic renal disease may target inflammation and phosphate balance. New therapeutic options may also include inhibition of elastolytic cathepsins, particularly cathepsin S. Although calcification in atherosclerosis typically occurs in the intima, chronic renal disease calcification primarily involves the media. Therefore, development of high-resolution imaging of calcification in different arterial layers may distinguish the cause of calcification and provide a powerful tool in personalized medicine. More importantly, identification of high-risk patients requires new imaging modalities to detect calcifying atherosclerotic plaques and aortic valve lesions at stages during which the disease remains silent or reversible.
Supplementary Material
Acknowledgments
We thank Drs Ching-Hsuan Tung, Amit K. Galande, and Nikolay Sergeyev, Center for Molecular Imaging Research, Massachusetts General Hospital, for the development and synthesis of the imaging agents. We acknowledge Drs Robert Padera, Brigham and Women’s Hospital, and Joan Krepinsky and Alistair Ingram, Division of Nephrology, McMaster University, for helpful discussions. The authors thank Joan Perry for editorial expertise.
Sources of Funding
This study was supported in part by the Donald W. Reynolds Foundation (Drs Libby and Weissleder), the Translational Program of Excellence in Nanotechnology (Drs Weissleder and Aikawa), the Leducq Foundation (07CVD04; Dr Aikawa), and the American Heart Association (0835460N; Dr Aikawa).
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.108.827972/DC1.
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
Drs Weissleder and Jaffer are shareholders of VisEn Medical, Woburn, Mass. The remaining authors report no conflicts.
References
- 1.Otto CM. Calcific aortic stenosis: time to look more closely at the valve. N Engl J Med. 2008;359:1395–1398. doi: 10.1056/NEJMe0807001. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 3.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Towler DA. Vascular calcification: a perspective on an imminent disease epidemic. IBMS BoneKEy. 2008;5:41–58. [Google Scholar]
- 5.Fox CS, Larson MG, Vasan RS, Guo CY, Parise H, Levy D, Leip EP, O’Donnell CJ, D’Agostino RB, Sr, Benjamin EJ. Cross-sectional association of kidney function with valvular and annular calcification: the Framingham Heart Study. J Am Soc Nephrol. 2006;17:521–527. doi: 10.1681/ASN.2005060627. [DOI] [PubMed] [Google Scholar]
- 6.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 7.Amann K, Tyralla K, Gross ML, Eifert T, Adamczak M, Ritz E. Special characteristics of atherosclerosis in chronic renal failure. Clin Nephrol. 2003;60:S13–S21. [PubMed] [Google Scholar]
- 8.Campean V, Neureiter D, Varga I, Runk F, Reiman A, Garlichs C, Achenbach S, Nonnast-Daniel B, Amann K. Atherosclerosis and vascular calcification in chronic renal failure. Kidney Blood Press Res. 2005;28:280–289. doi: 10.1159/000090182. [DOI] [PubMed] [Google Scholar]
- 9.Jono S, Shioi A, Ikari Y, Nishizawa Y. Vascular calcification in chronic kidney disease. J Bone Miner Metab. 2006;24:176–181. doi: 10.1007/s00774-005-0668-6. [DOI] [PubMed] [Google Scholar]
- 10.Goodman WG. Vascular calcification in chronic renal failure. Lancet. 2001;358:1115–1116. doi: 10.1016/S0140-6736(01)06299-7. [DOI] [PubMed] [Google Scholar]
- 11.Massy ZA, Ivanovski O, Nguyen-Khoa T, Angulo J, Szumilak D, Mothu N, Phan O, Daudon M, Lacour B, Drueke TB, Muntzel MS. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J Am Soc Nephrol. 2005;16:109–116. doi: 10.1681/ASN.2004060495. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz U, Buzello M, Ritz E, Stein G, Raabe G, Wiest G, Mall G, Amann K. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant. 2000;15:218–223. doi: 10.1093/ndt/15.2.218. [DOI] [PubMed] [Google Scholar]
- 13.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 14.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 15.Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinckerhoff CE, Sukhova GK, Libby P. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilization. Circulation. 1998;97:2433–2444. doi: 10.1161/01.cir.97.24.2433. [DOI] [PubMed] [Google Scholar]
- 16.Simionescu A, Simionescu DT, Vyavahare NR. Osteogenic responses in fibroblasts activated by elastin degradation products and transforming growth factor-beta1: role of myofibroblasts in vascular calcification. Am J Pathol. 2007;171:116–123. doi: 10.2353/ajpath.2007.060930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 18.Qin X, Corriere MA, Matrisian LM, Guzman RJ. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:1510–1516. doi: 10.1161/01.ATV.0000225807.76419.a7. [DOI] [PubMed] [Google Scholar]
- 19.Bouvet C, Moreau S, Blanchette J, de Blois D, Moreau P. Sequential activation of matrix metalloproteinase 9 and transforming growth factor beta in arterial elastocalcinosis. Arterioscler Thromb Vasc Biol. 2008;28:856–862. doi: 10.1161/ATVBAHA.107.153056. [DOI] [PubMed] [Google Scholar]
- 20.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 21.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 22.Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. doi: 10.1161/01.cir.103.8.1051. [DOI] [PubMed] [Google Scholar]
- 23.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006;103:14678–14683. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 25.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 26.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 27.Bro S, Bentzon JF, Falk E, Andersen CB, Olgaard K, Nielsen LB. Chronic renal failure accelerates atherogenesis in apolipoprotein E-deficient mice. J Am Soc Nephrol. 2003;14:2466–2474. doi: 10.1097/01.asn.0000088024.72216.2e. [DOI] [PubMed] [Google Scholar]
- 28.Galande AK, Hilderbrand SA, Weissleder R, Tung CH. Enzyme-targeted fluorescent imaging probes on a multiple antigenic peptide core. J Med Chem. 2006;49:4715–4720. doi: 10.1021/jm051001a. [DOI] [PubMed] [Google Scholar]
- 29.Zaheer A, Lenkinski RE, Mahmood A, Jones AG, Cantley LC, Frangioni JV. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat Biotechnol. 2001;19:1148–1154. doi: 10.1038/nbt1201-1148. [DOI] [PubMed] [Google Scholar]
- 30.Bokenkamp A, Herget-Rosenthal S, Bokenkamp R, Cystatin C. kidney function and cardiovascular disease. Pediatr Nephrol. 2006;21:1223–1230. doi: 10.1007/s00467-006-0192-5. [DOI] [PubMed] [Google Scholar]
- 31.Shanahan CM. Inflammation ushers in calcification: a cycle of damage and protection? Circulation. 2007;116:2782–2785. doi: 10.1161/CIRCULATIONAHA.107.749655. [DOI] [PubMed] [Google Scholar]
- 32.Towler DA. Imaging aortic matrix metabolism: Mirabile visu! Circulation. 2007;115:297–299. doi: 10.1161/CIRCULATIONAHA.106.675397. [DOI] [PubMed] [Google Scholar]
- 33.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 34.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 36.Mecham RP. Methods in elastic tissue biology: elastin isolation and purification. Methods. 2008;45:32–41. doi: 10.1016/j.ymeth.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brassart B, Fuchs P, Huet E, Alix AJ, Wallach J, Tamburro AM, Delacoux F, Haye B, Emonard H, Hornebeck W, Debelle L. Conformational dependence of collagenase (matrix metalloproteinase-1) up-regulation by elastin peptides in cultured fibroblasts. J Biol Chem. 2001;276:5222–5227. doi: 10.1074/jbc.M003642200. [DOI] [PubMed] [Google Scholar]
- 38.Jacob MP, Fulop T, Jr, Foris G, Robert L. Effect of elastin peptides on ion fluxes in mononuclear cells, fibroblasts, and smooth muscle cells. Proc Natl Acad Sci U S A. 1987;84:995–999. doi: 10.1073/pnas.84.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinek A, Rabinovitch M, Keeley F, Okamura-Oho Y, Callahan J. The 67-kD elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J Clin Invest. 1993;91:1198–1205. doi: 10.1172/JCI116280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duca L, Floquet N, Alix AJ, Haye B, Debelle L. Elastin as a matrikine. Crit Rev Oncol Hematol. 2004;49:235–244. doi: 10.1016/j.critrevonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Curran ME, Atkinson DL, Ewart AK, Morris CA, Leppert MF, Keating MT. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell. 1993;73:159–168. doi: 10.1016/0092-8674(93)90168-p. [DOI] [PubMed] [Google Scholar]
- 42.Rodgers KJ, Watkins DJ, Miller AL, Chan PY, Karanam S, Brissette WH, Long CJ, Jackson CL. Destabilizing role of cathepsin S in murine atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006;26:851–856. doi: 10.1161/01.ATV.0000203526.75772.4b. [DOI] [PubMed] [Google Scholar]
- 43.Helske S, Syvaranta S, Lindstedt KA, Lappalainen J, Oorni K, Mayranpaa MI, Lommi J, Turto H, Werkkala K, Kupari M, Kovanen PT. Increased expression of elastolytic cathepsins S, K, and V and their inhibitor cystatin C in stenotic aortic valves. Arterioscler Thromb Vasc Biol. 2006;26:1791–1798. doi: 10.1161/01.ATV.0000228824.01604.63. [DOI] [PubMed] [Google Scholar]
- 44.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronaryartery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 45.Mazhar AR, Johnson RJ, Gillen D, Stivelman JC, Ryan MJ, Davis CL, Stehman-Breen CO. Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int. 2001;60:324–332. doi: 10.1046/j.1523-1755.2001.00803.x. [DOI] [PubMed] [Google Scholar]
- 46.Muntner P, Mann D, Winston J, Bansilal S, Farkouh ME. Serum cystatin C and increased coronary heart disease prevalence in US adults without chronic kidney disease. Am J Cardiol. 2008;102:54–57. doi: 10.1016/j.amjcard.2008.02.098. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.