Abstract
Cells in the eye have a limited capacity for regeneration and, as such, immune-mediated inflammation can lead to blindness. The eye is designed to quench immune-mediated inflammation – a condition known as immune privilege. An important component of immune privilege is the dynamic immunoregulatory process termed anterior chamber-associated immune deviation (ACAID), which is initiated when antigens enter the eye. ACAID suppresses the initiation of antigen-specific inflammation in the eye and the effector stages of immune reactions. Four organ systems are crucial for the induction of ACAID: the eye, thymus, spleen and sympathetic nervous system. Multiple cell populations contribute to ACAID, with natural killer T cells playing a crucial role in the thymic and splenic phases of ACAID. Interactions between natural killer T cells and multiple cell populations in the spleen culminate in the tight regulation of immune-mediated inflammation in the eye and the preservation of vision.
Keywords: anterior chamber, anterior chamber-associated immune deviation, corneal transplantation, eye, immune privilege, natural killer T cell, tolerance, T regulatory cell
Although the human eye is only a few centimeters in diameter, it contains a complex array of tissues, some of which are found nowhere else in the body (Figure 1). The eye is an extension of the brain and, as with some of the neurons in the brain, many cells in the eye cannot regenerate. In particular, cells of the retina and the cells that line the inner layer of the cornea are amitotic. Thus, injury to either of these ocular tissues can lead to blindness. Inflammation can be tolerated in many tissues, but in the eye it can have devastating consequences. Close regulation of ocular inflammatory responses – especially those that inflict a heavy burden of damage to innocent bystander cells – is of paramount importance. Fortunately, the eye is designed to minimize inflammation produced by either the innate or adaptive immune responses. This phenomenon is known as immune privilege and has been recognized for over 100 years.
Figure 1. Anatomy of the eye.
Image redrawn courtesy of National Eye Institute, NIH.
One of the first clues that certain forms of ocular inflammation were inhibited surfaced over a century ago, when the first successful corneal transplant was performed on a human [1]. It is noteworthy that this procedure was performed over half a century before the use of immunosuppressive drugs was commonplace, and prior to the application of HLA matching. Over the past 100 years, corneal transplantation has emerged as the most common and, arguably, the most successful form of organ transplantation [2].
Ocular immune privilege is not simply an anatomical accident in which the absence of lymphatics draining the eye isolates the interior of the eye from the immune apparatus, but rather is the sum total of a combination of anatomical, physiological and immunological properties that are unique to the eye [3,4]. A common misconception regarding ocular immune privilege is that it is universal and immutable. Ocular immune privilege can fail, which can culminate in corneal allograft rejection, immune-mediated microbial keratitis and uveitis [5–7].
Immune privilege and immune-mediated diseases of the eye involve elements of both the innate and adaptive immune systems. Emerging evidence suggests that natural killer (NK) T (NKT) cells participate in immune privilege and, on occasion, contribute to the events leading to loss of immune privilege and the development of immune-mediated diseases of the eye. Although the primary function of NK cells is to serve as effector cells of the innate immune system, they can indirectly affect the generation and expression of adaptive immune responses through their production of IFN-γ [8,9]. There is also evidence that NK cells can directly and independently mediate certain adaptive immune responses, such as contact hypersensitivity (CHS), in the absence of T and B cells [10]. NKT cells are a novel population of T cells that share characteristics with NK cells. The bulk of NKT cells share the αβ T-cell receptor (TCR) and express the NK cell marker NK 1.1. Most NKT cells express an invariant TCR, which is Vα14Jα182 in mice and Vα24JαQ in humans. Due to their expression of invariant T-cell markers, they are also called invariant NKT (iNKT) cells. NKT cells display properties of the innate immune system, such as their swift production of IL-4 and IFN-γ, which provides a jump-start for adaptive immune responses [11–14]. NKT cell development and function are dependent upon the MHC class I-like CD1d molecule, which presents glycolipids to the TCR on NKT cells. NKT cells are the ‘Janus-faced’ cells of the immune system, as they can act either as immune effectors against neoplasms or as integral players in immunoregulatory pathways [11,13–15]. NKT cells can also play a crucial role in the induction of adaptive immune responses (e.g., CHS) [16]. Campos et al. demonstrated that iNKT cells stimulated with the contact-sensitizing agent oxazalone (OX) produced IL-4, which activated OX-specific B-1 cells. B-1 cells, in turn, produced OX-specific IgM antibodies that were required for the recruitment of OX-specific T cells, which functioned as the end-stage effector cells in CHS. Interestingly, both NK cells and iNKT cells function in the generation of OX-specific CHS [10,16]. NK cells act as end-stage effector cells that directly mediate CHS, while iNKT cells initiate cellular interactions that culminate in the generation of antigen-specific T cells, which act as the end-stage effector cells in the expression of CHS. Interestingly, the NK cells and the iNKT cells in both models of CHS are derived from the liver [10,16]. In some conditions, NK and NKT cells can exacerbate the pathogenesis of immune-mediated diseases of the eye, while in other settings they can mitigate immune-based inflammation. Although there is considerable overlap in NK- and iNKT-cell functions both as cytolytic cells and in their shaping of adaptive immune responses, the author is aware of no published reports to date demonstrating a role for NK cells in the induction of Tregs.
Anatomical properties of the eye that contribute to ocular immune privilege
It was reported over half a century ago, and widely accepted until recently, that the anterior chamber (AC) of the eye lacked patent lymphatic drainage channels [3,4,17,18]. However, subsequent studies in mice revealed that antigens introduced into the AC induced clonal expansion of antigen-specific T cells in the sub-mandibular lymph node [19]. By contrast, trafficking of immune cells and macromolecules into the AC of the eye is limited by the tight junctional complexes in the vascular endothelial cells of the iris and retina.
MHC class I molecules are expressed on virtually all nucleated cells, with the exception of the neurons in the CNS and the corneal endothelium and retina [3,4,17,18]. Thus, the absence or low expression of conventional MHC class I molecules on the corneal endothelium and the neural retina renders them invisible to class I-restricted cytotoxic T lymphocytes and thus prevents them from being eliminated in the event of a viral infection. In addition to serving as crucial ‘docking stations’ for cytotoxic T lymphocytes, MHC class I molecules are important for regulating NK cell-mediated cytolysis. NK cells are programmed to kill any cell that lacks MHC class I molecules. However, engagement of MHC class I molecules on potential target cells with the killer inhibitor receptors on NK cells sends an ‘off’ signal to NK cells and prevents the expression of their cytolytic machinery [20]. However, the absence of MHC class I molecules places these cells at risk for NK cell-mediated attack. To compensate for this risk, corneal endothelial cells and retinal cells express nonclassical MHC class Ib molecules, such as HLA-G and HLA-E in humans and Qa-2 in mice, which can substitute for classical MHC class Ia molecules in transmitting an off signal to NK cells [21,22].
Soluble factors that contribute to ocular immune privilege
The aqueous humor (AH) that fills the AC of the eye contains a ‘pot pourri’ of soluble anti-inflammatory and immunosuppressive factors [23]. Delayed-type hypersensitivity (DTH) is a form of immune-mediated inflammation that can inflict extensive collateral damage to innocent bystander cells. Thus, it is noteworthy that at least four different factors in the AH inhibit DTH in the eye [23]:
TGF-β
α-melanocyte stimulating hormone
Vasoactive intestinal peptide
Calcitonin gene-related peptide
Corneal cells also produce an intracellular enzyme, indoleamine dioxygenase that catabolizes tryptophan – a key amino acid that is vital for T-cell survival [24,25]. Thus, inflammation involving elements of the adaptive immune response is closely regulated by soluble factors in the AH and cornea.
The AH also contains soluble factors that suppress the innate immune system. An AH-borne 10-kDa factor induces apoptosis of NK cells, macrophages and neutrophils [26]. The complement system can be activated by the alternate pathway and can thus claim membership in both the innate and adaptive immune systems. Activation of the complement cascade produces potent chemo-attractants that recruit and activate granulocytes. Granulocytes produce a variety of proteases and reactive oxygen species that can cause extensive injury to normal tissues. However, the AH is endowed with complement regulatory proteins that effectively inactivate the complement cascade and protect the eye from this form of inflammation [27–30].
As mentioned earlier, the expression of nonclassical HLA-G, HLA-E and Qa-2 molecules on the corneal endothelium and retina should protect corneal endothelial and retinal cells from NK cell-mediated attack. The AH also contains macrophage migration inhibitory factor (MIF) and TGF-β, which are potent inhibitors of NK cell-mediated cytolysis [31–33]. In vitro studies have confirmed that MIF and TGF-β – at concentrations found in the AH – inhibit NK cell-mediated cytolysis of corneal endothelial cells [31,32]. To date, no publications have reported the effect of MIF or TGF-β on NKT cell-mediated cytolytic activity. Thus, soluble factors in the eye closely regulate both adaptive and innate immune responses and protect amitotic tissues from immune-mediated injury (Table 1).
Table 1.
Aqueous humor-borne factors that inhibit innate and adaptive immune responses.
| Factor | Inhibitory effect |
|
|---|---|---|
| Innate immune response | Adaptive immune response | |
| TGF-β | Inhibits NK cell activity | Suppresses DTH |
| MIF | Inhibits NK cell activity | Unknown |
| 10-kDa factor | Induces apoptosis of NK cells, macrophages and neutrophils | Induces apoptosis of T cells |
| CRP | Inactivates the complement cascade | Inactivates the complement cascade |
| VIP | Unknown | Inhibits DTH |
| CGRP | Unknown | Inhibits DTH |
| α-MSH | Inhibits activation of macrophages and neutrophils | Inhibits DTH |
| sFasL | Inhibits activation of neutrophils | Induces apoptosis of T cells |
| IDO* | Unknown | Induces apoptosis of T cells by tryptophan deprivation |
IDO is not found in soluble form in the aqueous humor. It is an intracellular enzyme that depletes tryptophan from the surrounding environment.
MSH: α-melanocyte-stimulating hormone; AH: Aqueous humor; CGRP: Calcitonin gene-related peptide; CRP: Complement regulatory protein; DTH: Delayed-type hypersensitivity; IDO: Indoleamine dioxygenase; MIF: Macrophage migration inhibitory factor; NK: Natural killer; sFASL: Soluble Fas ligand; VIP: Vasoactive intestinal peptide.
Cell membrane factors that support ocular immune privilege
The cells lining the inside of the eye display cell membrane-bound molecules that inactivate the complement system and induce apoptosis of inflammatory cells. Fas ligand (FasL; CD95L) is expressed throughout the eye and induces apoptosis of inflammatory cells expressing its receptor (CD95) [34]. FasL is also important for promoting the survival of corneal allografts [35,36]. Programmed death ligand 1 (PD-L1) is another apoptosis-inducing molecule that is expressed on multiple cells in the mouse and human eye [37–39]. Engagement of PD-L1 on ocular cells with its receptor on T cells results in the inhibition of T-cell proliferation, induction of apoptosis, inhibition of proinflammatory cytokine production and enhancement of corneal allograft survival [37,38].
In addition to their presence in soluble forms in the AH, complement regulatory proteins are also expressed on the cell membranes of ocular cells and act as important buffers for controlling inflammation provoked by the complement cascade [30]. Thus, the eye employs both soluble and cell membrane-bound factors to control inflammation mediated by both the innate and adaptive immune systems (Table 2).
Table 2.
Cell membrane-bound factors that inhibit innate and adaptive immune responses.
| Factor | Inhibitory effect |
|
|---|---|---|
| Innate immune response | Adaptive immune response | |
| CRP | Inactivates the complement cascade | Inactivates the complement cascade |
| FasL | Induces apoptosis of activated neutrophils | Induces apoptosis of T cells |
| TRAIL | Induces apoptosis of macrophages and neutrophils | Induces apoptosis of T cells |
| MHC class Ib | Inhibits NK cells | Inhibits CTLs |
| PD-L1 | Unknown | Induces apoptosis of T cells and inhibits secretion of TNF-α by T cells |
CRP: Complement regulatory proteins; CTL: Cytotoxic T lymphocyte; FasL: Fas ligand; PD-L1: Programmed death ligand-1; NK: Natural killer; TRAIL: Tumor necrosis factor-related apoptosis-inducing ligand.
Role of ACAID in ocular immune privilege
The notion that dynamic immunoregulatory processes contribute to immune privilege in the eye was launched 30 years ago in seminal studies by Streilein et al. [3,4,18,40]. These studies demonstrated that antigens introduced into the AC elicited a systemic immune deviation that was characterized by antigen-specific suppression of DTH by CD4−CD8+ Tregs and the preferential production of non-complement-fixing antibodies. This anterior chamber-associated immune deviation (ACAID) was believed to be simply the preferential activation of a Th2-based immune response that crossregulated Th1 immunity. However, subsequent studies demonstrated that ACAID resulted in the inhibition of both Th1- and Th2-based immune-mediated inflammation [41]. It was also believed that the putative absence of lymphatic drainage of the AC resulted in antigens leaving the eye via the vascular route and that this was tantamount to an intravenous injection. Accordingly, it was thought by some that ACAID was simply a complicated form of intravenous-induced immune deviation. However, multiple studies have demonstrated numerous fundamental differences between ACAID and intravenous-induced immune tolerance [18], including the observation that antigens injected into the AC rapidly reach draining lymph nodes [19,42,43].
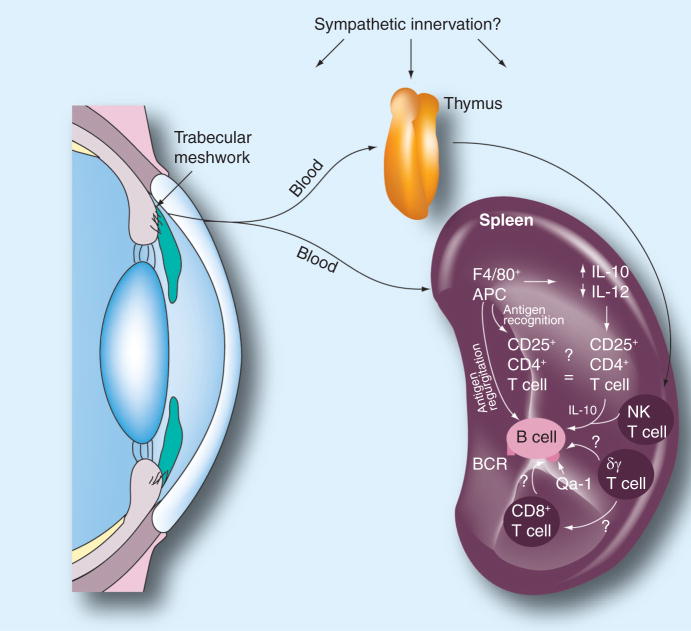
Anterior chamber-associated immune deviation is initiated either when antigens enter the eye or when corneal allografts are transplanted to the eye [44]. ACAID requires the participation of four organ systems: the eye, thymus, spleen and sympathetic nervous system (Figure 2). Removal of the eye, spleen or thymus within 3 days of AC antigen injection prevents the induction of ACAID and instead results in the development of a conventional immune response [45–47]. Chemical sympathectomy also prevents the induction of ACAID [48]. Although there is no evidence in the literature that NK cells play a role in the induction of ACAID, it is clear that NKT cells contribute to the induction of ACAID [49], both in the thymus [47,50,51] and in the spleen [52,53].
Figure 2. Organ systems involved in the induction of anterior chamber-associated immune deviation (ACAID).
Removal of the eye, thymus or spleen within 72 h of anterior chamber injection of antigen prevents the induction of ACAID. Chemical sympathectomy within 4 days of anterior chamber injection of antigen abrogates ACAID. NK T cells are crucial in the thymic and splenic phases of ACAID. APC: Antigen-presenting cell; NK: Natural killer. Reproduced with permission from [3].
Ocular phase of ACAID
Antigens introduced into the AC initiate the induction of ACAID when they are captured by F4/80+ macrophages, which serve as ocular antigen-presenting cells (APCs). Under the influence of cytokines in the AH, most notably TGF-β, ocular APCs downregulate IL-12 and upregulate IL-10. After capturing antigen, ocular APCs also begin producing macrophage inflammatory protein-2 (MIP-2), which plays a crucial role in the subsequent stages of ACAID that occur in the spleen. It is believed that within 72 h of capturing antigen, the ocular APCs enter the bloodstream and migrate to the thymus and spleen. This conclusion is based on studies indicating that F4/80+ mononuclear cells can be isolated from the blood of mice injected in the AC with antigen, and that adoptive transfer of these cells to naive mice induces ACAID [54]. The blood-borne ocular APCs are remarkable in their capacity to produce immune tolerance; as few as 20 of these cells can induce ACAID when adoptively transferred to naive recipients [54]. However, Camelo et al. demonstrated that within 24 h of injection into the AC, the bulk of soluble antigens can be detected in multiple lymph nodes and the spleen in non-cell-associated forms [54]. However, given the infinitesimally small number of F4/80+ mononuclear cells that are capable of inducing ACAID, it is difficult to rule out a role for these cells in the induction of ACAID by simple morphological or conventional imaging techniques.
Thymic phase of ACAID
The induction of ACAID requires an intact thymus, which is an important source of NKT cells that are needed for the splenic phase of ACAID [51]. Within 72 h of leaving the AC, ocular APCs induce the generation of a unique population of CD4−CD8−NK1.1+ T cells [47]. The thymus-derived NKT cells are CD-1d-dependent and are only generated if the ocular APCs express CD-1d on their surface [51]. The thymic NKT cells emigrate from the thymus to the spleen within 4 days of the original AC injection of antigen [47]. The CD4−CD8−, NK1.1+ T cells that are recent thymic emigrants to the spleen do not become the splenic CD4+ NKT cells or the CD8+ ACAID Tregs [50]. The precise mechanism that the recent thymic emigrants employ in the generation of the end-stage CD8+ ACAID Tregs remains a mystery.
Splenic phase of ACAID
The population of ocular APCs that enters the spleen possesses unique properties [52,53,55–58]:
Expression of CD-1d
Elevated production of IL-10, IL-13 and MIP-2
Downregulation of IL-12
Activation of signal transducer and activator of transcription-6
Within the spleen, the ocular APCs produce MIP-2, which attracts CD4+ NKT cells that in turn produce RANTES. RANTES recruits additional cells into the marginal zone of the spleen where a complex dialog involving multiple cells ensues. This involves B cells, CD4+ NKT cells, CD4+ T cells, γδ T cells and CD8+ T cells, the latter of which differentiate to become end-stage ACAID Tregs. Two populations of APCs appear to function in the splenic phase of ACAID. F4/80+ ocular APCs must ligate the third component of complement (C3) via their C3b receptor in order for ACAID to be induced [59]. This, in turn, induces the ocular APCs to produce increased amounts of IL-10 and TGF-β, while simultaneously downregulating their production of IL-12. Under the influence of increased IL-10 and TGF- β, the ocular APCs process ocular antigens and release them into the marginal zone of the spleen, where they are captured by B cells. B cells capture and internalize the ocular antigenic peptides via their antigen-specific B-cell receptors and proliferate prior to presenting the reprocessed peptides to T cells [60,61]. CD4+ T cells are required for the generation of the CD8+ end-stage ACAID Tregs. Both the CD4+ and CD8+ T-cell populations are antigen specific and, thus, must encounter ocular antigens presented on MHC class II and MHC class I molecules, respectively. In vitro models of ACAID have provided evidence suggesting that ACAID B cells present both MHC class I-restricted and MHC class II-restricted antigens to CD8+ T cells and CD4+ T cells, respectively [62]. The MHC class I-like Qa-1 molecule is necessary for the induction of ACAID, as Qa-1-deficient mice fail to develop ACAID [63]. Moreover, Qa-1 compatibility between the ACAID B cells and ACAID T cells is required for the induction of ACAID, and suggests that antigen presentation by B cells to CD8+ T cells is via the Qa-1 molecule [64].
The induction of ACAID also requires the participation of splenic γδ T cells [65–68]. The precise role of γδ T cells in ACAID remains to be elucidated. Although it is clear that they must have the capacity to produce IL-10, they do not act as ancillary APCs or as end-stage Tregs [65].
The eye, spleen and thymus have dense sympathetic innervations and, coincidentally, are also necessary for the induction of ACAID. Although the eye has significant sympathetic nervous system innervation, the sympathetic nervous system does not appear to be necessary for the generation of ocular APCs [48]. However, chemical sympathectomy prevents the generation of CD4+ NKT cells and suggests that the sympathetic nervous system is required indirectly for ACAID for its putative role in the generation of the NKT-cell population that eventually enters the spleen and participates in the induction of CD8+ ACAID Tregs.
Expert commentary & five-year view
The presence of multiple overlapping mechanisms for regulating immune-mediated inflammation in the eye seems to be by design, rather than being an immunological accident. On first consideration, one might think that disabling ocular immune responses would unwittingly create an ‘immunological blind spot’ and render the eye vulnerable to a variety of infectious diseases; however, the eye is an extension of the brain and cannot tolerate immune-mediated inflammation. In particular, ACAID limits the immunological options that are available for ridding the eye of infectious agents. This seems counterintuitive for the wellbeing of the host. Although ACAID prevents the generation of DTH responses, which are notorious for inflicting extensive injury to innocent bystander cells, the eye compensates by sparing the production of neutralizing, noncomplement-fixing antibodies and preserving elements of the innate immune response, which can be highly effective in controlling at least some of the infectious agents that enter the eye [69]. However, ACAID is neither immutable nor universal, as certain microbial agents and highly immunogenic tumors can circumvent ACAID and provoke robust DTH responses [70–72]. Over 20 years ago, Streilein noted that the eye and the immune system are engaged in a ‘dangerous compromise’, in which the eye is protected from immune-mediated inflammation at the risk of uncontrolled ocular infections [40]. The exquisite acuity of the mammalian visual system is a testament to the success of this compromise.
Regulatory T cells that are virtually identical to ACAID Tregs can be generated in vitro. These in vitro-generated ACAID Tregs have been used to enhance corneal allograft survival and to mitigate Th2-mediated pulmonary inflammation in animal models. Moreover, the induction of ACAID with retinal autoantigens, such as the interphotoreceptor retinoid-binding protein, mitigates experimental autoimmune uveitis in rodents. Although there are many logistical obstacles that must be negotiated before this strategy can be applied clinically, it holds promise as a novel therapeutic modality for managing a variety of diseases. The next 5 years should bring even greater insights into the mechanisms and potential applications of ocular immune privilege.
Key issues
Immune privilege is the condition that prevents immune-mediated injury to ocular tissues that cannot regenerate.
Immune privilege is the sum total of multiple physiological, anatomical and immunoregulatory features of the eye that reduce the likelihood of inflammation within the eye.
Soluble factors in the aqueous humor suppress both the innate and adaptive immune responses in the eye.
Cell membrane-bound factors induce apoptosis of inflammatory cells entering the eye.
Anterior chamber-associated immune deviation (ACAID) is a dynamic immunoregulatory process that is induced when antigens enter the eye or when a corneal transplant is grafted onto the eye.
ACAID suppresses antigen-specific immune-mediated inflammation.
Natural killer T cells participate in multiple stages of ACAID.
Footnotes
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Zirm E. A successful total keratoplasty. Albrecht von Graefes Arch Ophthalmol. 1906;64:580–593. [Google Scholar]
- 2.Niederkorn JY. The immune privilege of corneal grafts. J Leukoc Biol. 2003;74(2):167–171. doi: 10.1189/jlb.1102543. [DOI] [PubMed] [Google Scholar]
- 3.Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7(4):354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 4.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3(11):879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 5.Caspi RR. Ocular autoimmunity: the price of privilege? Immunol Rev. 2006;213:23–35. doi: 10.1111/j.1600-065X.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 6.Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007;32(12):1005–1016. doi: 10.1080/02713680701767884. [DOI] [PubMed] [Google Scholar]
- 7.Streilein JW, Dana MR, Ksander BR. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today. 1997;18(9):443–449. doi: 10.1016/s0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- 8.Bouley DM, Kanangat S, Rouse BT. The role of the innate immune system in the reconstituted SCID mouse model of herpetic stromal keratitis. Clin Immunol Immunopathol. 1996;80(1):23–30. doi: 10.1006/clin.1996.0090. [DOI] [PubMed] [Google Scholar]
- 9.Lighvani S, Huang X, Trivedi PP, Swanborg RH, Hazlett LD. Substance P regulates natural killer cell interferon-γ production and resistance to Pseudomonas aeruginosa infection. Eur J Immunol. 2005;35(5):1567–1575. doi: 10.1002/eji.200425902. [DOI] [PubMed] [Google Scholar]
- 10.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7(5):507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114(10):1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4(3):231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 13.Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J Exp Med. 2005;202(12):1623–1626. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28(11):491–496. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Nowak M, Stein-Streilein J. Invariant NKT cells and tolerance. Int Rev Immunol. 2007;26(1–2):95–119. doi: 10.1080/08830180601070195. [DOI] [PubMed] [Google Scholar]
- 16.Campos RA, Szczepanik M, Lisbonne M, et al. Invariant NKT cells rapidly activated via immunization with diverse contact antigens collaborate in vitro with B-1 cells to initiate contact sensitivity. J Immunol. 2006;177(6):3686–3694. doi: 10.4049/jimmunol.177.6.3686. [DOI] [PubMed] [Google Scholar]
- 17.Niederkorn JY. Immune privilege and immune regulation in the eye. Adv Immunol. 1990;48:191–226. doi: 10.1016/s0065-2776(08)60755-5. [DOI] [PubMed] [Google Scholar]
- 18.Niederkorn JY. Immune privilege in the anterior chamber of the eye. Crit Rev Immunol. 2002;22:13–46. [PubMed] [Google Scholar]
- 19.Egan RM, Yorkey C, Black R, et al. Peptide-specific T cell clonal expansion in vivo following immunization in the eye, an immune-privileged site. J Immunol. 1996;157(6):2262–2271. [PubMed] [Google Scholar]
- 20.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 21.Le Discorde M, Moreau P, Sabatier P, Legeais JM, Carosella ED. Expression of HLA-G in human cornea, an immune-privileged tissue. Hum Immunol. 2003;64(11):1039–1044. doi: 10.1016/j.humimm.2003.08.346. [DOI] [PubMed] [Google Scholar]
- 22.Niederkorn JY, Chiang EY, Ungchusri T, Stroynowski I. Expression of a nonclassical MHC class Ib molecule in the eye. Transplantation. 1999;68(11):1790–1799. doi: 10.1097/00007890-199912150-00025. [DOI] [PubMed] [Google Scholar]
- 23.Taylor AW. Ocular immunosuppressive microenvironment. Chem Immunol. 2007;92:71–85. doi: 10.1159/000099255. [DOI] [PubMed] [Google Scholar]
- 24••.Beutelspacher SC, Pillai R, Watson MP, et al. Function of indoleamine 2,3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-expression. Eur J Immunol. 2006;36(3):690–700. doi: 10.1002/eji.200535238. Demonstrates that corneal cells produce indoleamine dioxygenase (IDO), which drastically limits the survival of T cells that enter the eye and, as a result, IDO promotes corneal allograft survival. It is yet one more example of how intraocular factors create a milieu that limits the survival and function of potentially dangerous T lymphocytes. [DOI] [PubMed] [Google Scholar]
- 25.Ryu YH, Kim JC. Expression of indoleamine 2,3-dioxygenase in human corneal cells as a local immunosuppressive factor. Invest Ophthalmol Vis Sci. 2007;48(9):4148–4152. doi: 10.1167/iovs.05-1336. [DOI] [PubMed] [Google Scholar]
- 26.D’Orazio TJ, DeMarco BM, Mayhew ES, Niederkorn JY. Effect of aqueous humor on apoptosis of inflammatory cell types. Invest Ophthalmol Vis Sci. 1999;40(7):1418–1426. [PubMed] [Google Scholar]
- 27.Goslings WR, Blom DJ, de Waard-Siebinga I, et al. Membrane-bound regulators of complement activation in uveal melanomas. CD46, CD55, and CD59 in uveal melanomas. Invest Ophthalmol Vis Sci. 1996;37(9):1884–1891. [PubMed] [Google Scholar]
- 28.Lass JH, Walter EI, Burris TE, et al. Expression of two molecular forms of the complement decay-accelerating factor in the eye and lacrimal gland. Invest Ophthalmol Vis Sci. 1990;31(6):1136–1148. [PubMed] [Google Scholar]
- 29.Sohn JH, Kaplan HJ, Suk HJ, Bora PS, Bora NS. Complement regulatory activity of normal human intraocular fluid is mediated by MCP, DAF, and CD59. Invest Ophthalmol Vis Sci. 2000;41(13):4195–4202. [PMC free article] [PubMed] [Google Scholar]
- 30.Sohn JH, Kaplan HJ, Suk HJ, Bora PS, Bora NS. Chronic low level complement activation within the eye is controlled by intraocular complement regulatory proteins. Invest Ophthalmol Vis Sci. 2000;41(11):3492–3502. [PMC free article] [PubMed] [Google Scholar]
- 31.Apte RS, Niederkorn JY. Isolation and characterization of a unique natural killer cell inhibitory factor present in the anterior chamber of the eye. J Immunol. 1996;156(8):2667–2673. [PubMed] [Google Scholar]
- 32.Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. 1998;160(12):5693–5696. [PubMed] [Google Scholar]
- 33.Rook AH, Kehrl JH, Wakefield LM, et al. Effects of transforming growth factor β on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986;136(10):3916–3920. [PubMed] [Google Scholar]
- 34.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270(5239):1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 35.Stuart PM, Griffith TS, Usui N, et al. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J Clin Invest. 1997;99(3):396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamagami S, Kawashima H, Tsuru T, et al. Role of Fas–Fas ligand interactions in the immunorejection of allogeneic mouse corneal transplants. Transplantation. 1997;64(8):1107–1111. doi: 10.1097/00007890-199710270-00004. [DOI] [PubMed] [Google Scholar]
- 37•.Hori J, Wang M, Miyashita M, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177(9):5928–5935. doi: 10.4049/jimmunol.177.9.5928. First report indicating that programmed death-ligand-1 (PD-L1) is expressed in the eye and is capable of inducing apoptosis of T cells. This is another example of how inflammatory cells are purged from the eye as a means of preserving ocular immune privilege. [DOI] [PubMed] [Google Scholar]
- 38•.Shen L, Jin Y, Freeman GJ, Sharpe AH, Dana MR. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J Immunol. 2007;179(6):3672–3679. doi: 10.4049/jimmunol.179.6.3672. Published shortly after reference 35 and reported identical findings indicating the importance of apoptosis-inducing molecules in supporting the immune privilege of corneal allografts. [DOI] [PubMed] [Google Scholar]
- 39.Yang W, Li H, Chen PW, et al. PD-L1 expression on human ocular cells and its possible role in regulating immune-mediated ocular inflammation. Invest Ophthalmol Vis Sci. 2009;50(1):273–280. doi: 10.1167/iovs.08-2397. [DOI] [PubMed] [Google Scholar]
- 40.Streilein JW. Immune regulation and the eye: a dangerous compromise. FASEB J. 1987;1(3):199–208. [PubMed] [Google Scholar]
- 41••.Katagiri K, Zhang-Hoover J, Mo JS, Stein-Streilein J, Streilein JW. Using tolerance induced via the anterior chamber of the eye to inhibit Th2-dependent pulmonary pathology. J Immunol. 2002;169(1):84–89. doi: 10.4049/jimmunol.169.1.84. Demonstrated for the first time that anterior chamber-associated immune deviation (ACAID) also affects Th2-based inflammation and has potential as a therapeutic strategy for managing immune-mediated diseases in organs other than the eye. [DOI] [PubMed] [Google Scholar]
- 42.Camelo S, Kezic J, Shanley A, Rigby P, McMenamin PG. Antigen from the anterior chamber of the eye travels in a soluble form to secondary lymphoid organs via lymphatic and vascular routes. Invest Ophthalmol Vis Sci. 2006;47(3):1039–1046. doi: 10.1167/iovs.05-1041. [DOI] [PubMed] [Google Scholar]
- 43.Camelo S, Shanley A, Voon AS, McMenamin PG. The distribution of antigen in lymphoid tissues following its injection into the anterior chamber of the rat eye. J Immunol. 2004;172(9):5388–5395. doi: 10.4049/jimmunol.172.9.5388. [DOI] [PubMed] [Google Scholar]
- 44.Niederkorn JY. Anterior chamber-associated immune deviation and its impact on corneal allograft survival. Curr Opin Organ Transplant. 2006;11:360–365. [Google Scholar]
- 45.Niederkorn JY, Streilein JW. Induction of anterior chamber-associated immune deviation (ACAID) by allogeneic intraocular tumors does not require splenic metastases. J Immunol. 1982;128(6):2470–2474. [PubMed] [Google Scholar]
- 46.Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981;153(5):1058–1067. doi: 10.1084/jem.153.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Goldschneider I, Foss D, et al. Direct thymic involvement in anterior chamber-associated immune deviation: evidence for a nondeletional mechanism of centrally induced tolerance to extrathymic antigens in adult mice. J Immunol. 1997;158(5):2150–2155. [PubMed] [Google Scholar]
- 48 ••.Li X, Taylor S, Zegarelli B, et al. The induction of splenic suppressor T cells through an immune-privileged site requires an intact sympathetic nervous system. J Neuroimmunol. 2004;153(1–2):40–49. doi: 10.1016/j.jneuroim.2004.04.008. The first report documenting that the sympathetic nervous system is essential for the induction of ACAID and ocular immune privilege. It demonstrates the complexity of the dynamic processes that sustain ocular immune privilege and it also shows that multiple organs participate in dampening inflammation in the eye. [DOI] [PubMed] [Google Scholar]
- 49.Sonoda KH, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190(9):1215–1226. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Wang Y, Urso D, O’Rourke J, Cone RE. Thymocytes induced by antigen injection into the anterior chamber activate splenic CD8+ suppressor cells and enhance the antigen-induced production of immunoglobulin G1 antibodies. Immunology. 2004;113(1):44–56. doi: 10.1111/j.1365-2567.2004.01928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Goldschneider I, O’Rourke J, Cone RE. Blood mononuclear cells induce regulatory NK T thymocytes in anterior chamber-associated immune deviation. J Leukoc Biol. 2001;69(5):741–746. [PubMed] [Google Scholar]
- 52.Faunce DE, Sonoda KH, Stein-Streilein J. MIP-2 recruits NKT cells to the spleen during tolerance induction. J Immunol. 2001;166(1):313–321. doi: 10.4049/jimmunol.166.1.313. [DOI] [PubMed] [Google Scholar]
- 53.Faunce DE, Stein-Streilein J. NKT cell-derived RANTES recruits APCs and CD8+ T cells to the spleen during the generation of regulatory T cells in tolerance. J Immunol. 2002;169(1):31–38. doi: 10.4049/jimmunol.169.1.31. [DOI] [PubMed] [Google Scholar]
- 54.Wilbanks GA, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID). 1 Evidence that an antigen-specific, ACAID-inducing, cell-associated signal exists in the peripheral blood. J Immunol. 1991;146(8):2610–2617. [PubMed] [Google Scholar]
- 55.Nakamura T, Sonoda KH, Faunce DE, et al. CD4+ NKT cells, but not conventional CD4+ T cells, are required to generate efferent CD8+ T regulatory cells following antigen inoculation in an immune-privileged site. J Immunol. 2003;171(3):1266–1271. doi: 10.4049/jimmunol.171.3.1266. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura T, Terajewicz A, Stein-Streilein J. Mechanisms of peripheral tolerance following intracameral inoculation are independent of IL-13 or STAT6. J Immunol. 2005;175(4):2643–2646. doi: 10.4049/jimmunol.175.4.2643. [DOI] [PubMed] [Google Scholar]
- 57.Sonoda KH, Faunce DE, Taniguchi M, et al. NK T cell-derived IL-10 is essential for the differentiation of antigen- specific T regulatory cells in systemic tolerance. J Immunol. 2001;166(1):42–50. doi: 10.4049/jimmunol.166.1.42. [DOI] [PubMed] [Google Scholar]
- 58.Sonoda KH, Taniguchi M, Stein-Streilein J. Long-term survival of corneal allografts is dependent on intact CD1d-reactive NKT cells. J Immunol. 2002;168(4):2028–2034. doi: 10.4049/jimmunol.168.4.2028. [DOI] [PubMed] [Google Scholar]
- 59.Sohn JH, Bora PS, Suk HJ, et al. Tolerance is dependent on complement C3 fragment iC3b binding to antigen-presenting cells. Nat Med. 2003;9(2):206–212. doi: 10.1038/nm814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashour HM, Niederkorn JY. Expansion of B cells is necessary for the induction of T-cell tolerance elicited through the anterior chamber of the eye. Int Arch Allergy Immunol. 2007;144(4):343–346. doi: 10.1159/000106461. [DOI] [PubMed] [Google Scholar]
- 61.Skelsey ME, Mayhew E, Niederkorn JY. Splenic B cells act as antigen presenting cells for the induction of anterior chamber-associated immune deviation. Invest Ophthalmol Vis Sci. 2003;44(12):5242–5251. doi: 10.1167/iovs.03-0768. [DOI] [PubMed] [Google Scholar]
- 62••.Ashour HM, Niederkorn JY. Peripheral tolerance via the anterior chamber of the eye: role of B cells in MHC class I and II antigen presentation. J Immunol. 2006;176(10):5950–5957. doi: 10.4049/jimmunol.176.10.5950. Induction of ACAID involves two categories of antigen-presenting cell (APC) and the generation of CD4+ regulatory T cells (Tregs) and CD8+ Tregs. This study demonstrates that B cells act as ACAID APCs and present antigens to MHC class I-restricted CD8+ T cells and MHC class II-restriced CD4+ T cells. [DOI] [PubMed] [Google Scholar]
- 63.Chattopadhyay S, O’Rourke J, Cone RE. Implication for the CD94/NKG2A-Qa-1 system in the generation and function of ocular-induced splenic CD8+ regulatory T cells. Int Immunol. 2008;20(4):509–516. doi: 10.1093/intimm/dxn008. [DOI] [PubMed] [Google Scholar]
- 64.D’Orazio TJ, Mayhew E, Niederkorn JY. Ocular immune privilege promoted by the presentation of peptide on tolerogenic B cells in the spleen. II Evidence for presentation by Qa-1. J Immunol. 2001;166(1):26–32. doi: 10.4049/jimmunol.166.1.26. [DOI] [PubMed] [Google Scholar]
- 65•.Ashour HM, Niederkorn JY. γδ T cells promote anterior chamber-associated immune deviation and immune privilege through their production of IL-10. J Immunol. 2006;177(12):8331–8337. doi: 10.4049/jimmunol.177.12.8331. In vivo and in vitro experiments demonstrate that γδ T cells are required for the induction of ACAID. γδ T cells must produce IL-10 for the induction of ACAID. However, γδ T cells do not act as Tregs nor do they act as APCs in the induction of ACAID. [DOI] [PubMed] [Google Scholar]
- 66.Skelsey ME, Mellon J, Niederkorn JY. γδ T cells are needed for ocular immune privilege and corneal graft survival. J Immunol. 2001;166(7):4327–4333. doi: 10.4049/jimmunol.166.7.4327. [DOI] [PubMed] [Google Scholar]
- 67.Xu Y, Kapp JA. γδ T cells are critical for the induction of anterior chamber-associated immune deviation. Immunology. 2001;104(2):142–148. doi: 10.1046/j.0019-2805.2001.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu Y, Kapp JA. γδ T cells in anterior chamber-induced tolerance in CD8+ CTL responses. Invest Ophthalmol Vis Sci. 2002;43(11):3473–3479. [PubMed] [Google Scholar]
- 69.Clarke DW, Alizadeh H, Niederkorn JY. Failure of Acanthamoeba castellanii to produce intraocular infections. Invest Ophthalmol Vis Sci. 2005;46(7):2472–2478. doi: 10.1167/iovs.05-0140. [DOI] [PubMed] [Google Scholar]
- 70.Kielty D, Cousins SW, Atherton SS. HSV-1 retinitis and delayed hypersensitivity in DBA/2 and C57BL/6 mice. Invest Ophthalmol Vis Sci. 1987;28(12):1994–1999. [PubMed] [Google Scholar]
- 71.Li XY, Niederkorn JY. Immune privilege in the anterior chamber of the eye is not extended to intraocular Listeria monocytogenes. Ocular Immunol Inflamm. 1997;5(4):245–257. doi: 10.3109/09273949709085065. [DOI] [PubMed] [Google Scholar]
- 72.Niederkorn JY. The immunopathology of intraocular tumour rejection. Eye. 1991;5(Pt 2):186–192. doi: 10.1038/eye.1991.33. [DOI] [PubMed] [Google Scholar]