Abstract
Purpose
Africa is burdened by the AIDS epidemic and attendant increase in HIV/AIDS-related malignancies. Pragmatic approaches to therapeutic intervention could be of great value. Dose-modified oral chemotherapy for AIDS-related non-Hodgkin's lymphoma is one such approach.
Patients and Methods
The oral regimen consisted of lomustine 50 mg/m2 on day 1 (cycle 1 only), etoposide 100 mg/m2 on days 1 to 3, and cyclophosphamide/procarbazine 50 mg/m2 each on days 22 to 26 at 6-week intervals (one cycle) for two total cycles in HIV-infected patients with biopsy-proven non-Hodgkin's lymphoma.
Results
Forty-nine patients (21 in Uganda and 28 in Kenya) were treated. The majority of patients were female (59%) and had a poor performance status (63%); 69% of patients had advanced-stage disease; and 18 patients (37%) had access to antiretroviral therapy. In total, 79.5 cycles of therapy were administered. The regimen was well tolerated, had modest effects (decline) on CD4+ lymphocyte counts (P = .077), and had negligible effects on HIV-1 viral replication. Four febrile neutropenia episodes and three treatment-related deaths (6% mortality rate) occurred. The overall objective response rate was 78% (95% CI, 62% to 88%); median follow-up time was 8.2 months (range, 0.1 to 71 months); median event-free and overall survival times were 7.9 months (95% CI, 3.3 to 13.0 months) and 12.3 months (95% CI, 4.9 to 32.4 months), respectively; and 33% of patients survived 5 years.
Conclusion
Dose-modified oral chemotherapy is efficacious, has comparable outcome to that in the United States in the pre–highly active antiretroviral therapy setting, has an acceptable safety profile, and is pragmatic in sub-Saharan Africa. The international collaboration has been highly successful, and subsequent projects should focus on strategies to optimize combination antiretroviral therapy and chemotherapy and follow-up tissue correlative studies.
INTRODUCTION
As the AIDS pandemic advances, the burden of neoplastic disease is increasing in developing nations.1 In resource-constrained settings, intravenous chemotherapy and supportive care for patients with AIDS and cancer are challenging, and there is little published information on treatment outcomes.2–5 Systemic chemotherapy for AIDS and other virus-associated tumors in this setting report mortality rates ranging between 20% and 66% and a 15-week median survival duration for AIDS-related Burkitt's lymphoma.1,6–8
Resource-constrained settings need the development of simple, less myelotoxic therapeutic interventions for cancer.3–5 We hypothesized that dose-modified oral chemotherapy using a regimen that has had demonstrable activity in AIDS-related non-Hodgkin's lymphoma in the pre–highly active antiretroviral therapy (HAART) era in the United States would be efficacious and enhance the therapeutic index.9–11 Rationale for the four-drug combination (lomustine, etoposide, cyclophosphamide, and procarbazine) has been published.9 What is especially notable is the absence of anthracyclines and hence the avoidance of cardiotoxicity and the presence of agents that cross the blood-brain barrier (lomustine and procarbazine). Corticosteroids were also omitted because of additional immunosuppressive effects and potential tumor growth-promoting effects in patients with Kaposi's sarcoma (endemic and AIDS-related disease) in a region of the world with the highest incidence.12–16 Published studies confirmed that dose modification of chemotherapy lessened myelotoxicity without compromising efficacy in the pre-HAART era in the United States.17,18 On the basis of this rationale, we report our results of dose-modified oral chemotherapy for the treatment of AIDS-related lymphoma in East Africa.
PATIENTS AND METHODS
Patient Selection Criteria
All patients were evaluated and treated at the national referral centers in Uganda (Uganda Cancer Institute) or Kenya (Kenyatta National Hospital). Patients ≥ 18 years of age with biopsy-proven measurable or assessable non-Hodgkin's lymphoma, no prior therapy, and documented HIV-positive serology were eligible for participation. Patients were required to have Eastern Cooperative Oncology Group performance status of ≤ 3, an estimated life expectancy of more than 6 weeks, and acceptable end organ function (WBC ≥ 3,000/μL or granulocytes ≥ 1,500/μL, platelets ≥ 75,000/μL, creatinine < 3.0 mg/dL, and total bilirubin < 3.0 mg/dL). All patients underwent a thorough physical examination with assessment of involved sites of disease including tumor measurement, bone marrow aspiration, and CSF analysis to exclude leptomeningeal disease. On-study staging chest radiography and abdominal ultrasonography were discretionary, which is aligned with current practice in East Africa.
Treatment Plan and Patient Follow-Up
The chemotherapy regimen consisted of lomustine, etoposide, cyclophosphamide, and procarbazine. All drugs were administered orally according to the dose schedule (Table 1), with modifications as outlined in Table 2 and the Appendix (online only). A cycle of therapy comprised two 3-week treatment periods for a total of 12 weeks of therapy. At the end of two cycles, patients were evaluated for response, observed at 3-month intervals over the first year, and observed for survival thereafter.
Table 1.
Dosing Schedule
| Drug | Dose (mg/m2) |
|---|---|
| Lomustine | 50 day 1, cycle 1 only |
| Etoposide | 100 days 1-3, each cycle |
| Cyclophosphamide | 100 days 22-26, each cycle |
| Procarbazine | 100 days 22-26, each cycle |
NOTE. Protocol-prescribed oral chemotherapy was based on blood counts that guided dosing on days of chemotherapy administration (i.e., days 1 and 22) every cycle. A cycle of therapy was 6 weeks, consisting of two 3-week portions of chemotherapy. Two cycles were administered. Lomustine was omitted for cycle 2.
Table 2.
Dose Modifications
| WBC Count (/μL) | Platelet Count (/μL) | % Drug Dose (for each drug) |
|---|---|---|
| ≥ 3,000 | ≥ 100,000 | 100 |
| ≥ 1,500-2,999 | ≥ 50,000-99,999 | 50 |
| ≤ 1,499 | ≤ 49,999 | 0 |
NOTE. All patients received chemotherapy provided that WBC count was ≥ 1,500/μL and platelet count was ≥ 50,000/μL. Treatment was delayed until WBC ≥ 1,500/μL and platelets ≥ 50,000/μL or until 3 weeks elapsed, whichever occurred first. At that time, if counts still did not allow treatment, patients were taken off protocol. For patients experiencing febrile neutropenia, upon recovery, the next course of treatment was at 50% of the dose at which this event occurred, provided this was attributed to the chemotherapy.
Pathology Review
All patients had tissue pathology confirmation of lymphoma in East Africa at the time of enrollment. At study conclusion, tumor biopsies including hematoxylin and eosin–stained tissue sections and formalin-fixed paraffin-embedded tissue blocks were transferred and reviewed in Cleveland, Ohio. Tumors were graded according to the WHO classification scheme whenever possible.19 In some cases, the precise WHO classification was not possible as a result of inadequate tissue quality; therefore, all tumors were graded as low-, intermediate-, or high-grade lymphoma by Working Formulation criteria.20 The Mid-Region AIDS and Cancer Specimen Resource (Columbus, OH) provided lymphoma subtype using a tissue microarray (TMA) method described in the Appendix.21
Ethical Review
Signed informed consent was obtained from all patients in keeping with international, institutional, and US Food and Drug Administration guidelines and is fully described in the Appendix.22,23
Data Safety and Monitoring Plan, Toxicity, and Response Assessment
There was a project-specific Data Safety and Monitoring Plan (DSMP), which was fully integrated into the National Cancer Institute–approved institutional plan of the Case Comprehensive Cancer Center. On-site audits were conducted in 2003, 2004, 2005, and 2007. An independent review team at each audit had final authority to reconcile toxicity and response assessments including categories for unconfirmed complete response (CR) and unconfirmed partial response (PR).24–26 Response criteria and DSMP are described in the Appendix.
Outcome End Points and Statistical Analyses
Three patients were enrolled but not treated and thus excluded from analyses. The rate of confirmed non-Hodgkin's lymphoma diagnosis from pathology analysis and the overall rate of pathology analysis were estimated and their 95% CIs determined using Wilson's method.27 The survivor function was estimated using the Kaplan-Meier method28 and analyzed separately for numerous covariates including body mass index29 and the following prognostic scales: International Prognostic Index (IPI),30 HIV score,31 and the AIDS Clinical Trials Group 142 prognostic index.32 Differences between and among groups were examined using the log-rank test. To control the effect of prognostic factors on event-free survival (EFS) and overall survival (OS) simultaneously, multiple Cox regression model was used after checking proportional hazards assumption.33 For CR data, logistic regression was used to identify the factors that predict the outcome of response. The response rate and its 95% CI were estimated using Wilson's method.27 The profiles of lymphocyte counts and RNA levels were compared by partitioning the time period into three intervals and using the Kruskal-Wallis test followed by pairwise comparison without adjusting for multiple comparison. The effects of numerous covariates on log(CD4+ lymphocytes) and log(RNA levels) and their temporal pattern were further examined by generalized linear models.34,35 All tests were two-sided, and P ≤ .05 was considered statistically significant.
RESULTS
Patient Characteristics
From among 149 patients with confirmed lymphoma and positive HIV serology, 52 were enrolled (35% recruitment rate), and 49 received oral chemotherapy between May 2001 and August 2005 (Table 3). Patient recruitment was temporarily suspended between December 2002 and July 2003 to replenish an expired drug supply. The three patients who were enrolled and not treated included two patients in Uganda (one died within 4 days of signing consent during evaluation, and the other patient voluntarily withdrew consent after completing screening) and a single patient in Kenya (the patient and physician mutually decided that it was best not to treat after completing screening because he resided > 350 miles from Nairobi).
Table 3.
Patient Demographics and Clinical Characteristics, Clinical Staging Procedures, and Pathology Review and TMA Immunophenotype
| Patient Demographics and Clinical Characteristics | No. of Patients |
||
|---|---|---|---|
| Uganda | Kenya | Total | |
| Patients screened | 144 | 113 | 257 |
| Patients with confirmed lymphoma | 140 | 112 | 252 |
| Patients with confirmed HIV serology | 65 | 84 | 149 |
| Patients registered | 23 | 29 | 52 |
| Patients treated and analyzed | 21 | 28 | 49 |
| Sex | |||
| Male | 7 | 13 | 20 |
| Female | 14 | 15 | 29 |
| Age, years | |||
| Median | 40 | 36.5 | 39 |
| Range | 18-57 | 26-64 | 18-64 |
| Performance status | |||
| 0 | 5 | 0 | 5 |
| 1 | 6 | 7 | 13 |
| 2 | 6 | 7 | 13 |
| 3 | 4 | 14 | 18 |
| Clinical stage | |||
| I | 2 | 4 | 6 |
| II | 2 | 7 | 9 |
| III | 2 | 2 | 4 |
| IV | 15 | 15 | 30 |
| A | 5 | 1 | 6 |
| B | 16 | 27 | 43 |
| CD4 count, cells/μL | |||
| Median | 123 | 206.5 | 198 |
| Range | 5-1,364 | 21-409 | 5-1,364 |
| Plasma HIV viral load, copies/mL | |||
| Median | 338,864 | 55,000 | 99,741 |
| Range | 831-11,700,000 | Undetectable-> 750,000 | Undetectable-11,700,000 |
| Antiretroviral therapy | |||
| None | 12 | 19 | 31 |
| Yes | 9 | 9 | 18 |
| Prior AIDS | |||
| Yes | 13 | 20 | 33 |
| No | 8 | 8 | 16 |
| Extranodal sites | |||
| ≤ 1 | 14 | 21 | 35 |
| > 1 | 7 | 7 | 14 |
| LDH, U/L (n = 35) | |||
| Median | 670.5 | 520 | 550 |
| Range | 282-2,375 | 124-1,338 | 124-2,375 |
| Prior thrush | |||
| No | 15 | 4 | 19 |
| Yes | 6 | 24 | 30 |
| Prior opportunistic infection | |||
| No | 8 | 8 | 16 |
| Yes | 13 | 20 | 33 |
| Albumin, g/L | |||
| Median | 3.2 | 3.2 | 3.2 |
| Range | 1.4-5.0 | 2.1-4.4 | 1.4-5.0 |
| Hematocrit, % | |||
| Median | 29.3 | 33.6 | 31.5 |
| Range | 14.3-42.0 | 19.4-43.1 | 14.3-43.1 |
| Body mass index, kg/m2 | |||
| Median | 18.8 | 25.5 | 21.1 |
| Range | 12-29.3 | 16.6-33.2 | 12-33.2 |
| Second-line mCHOP | |||
| No | 14 | 24 | 38 |
| Yes | 7 | 4 | 11 |
| Clinical staging procedures at baseline | |||
| Physical examination and tumor measurement | 21 | 28 | 49 |
| Bone marrow aspiration biopsy | 20 | 28 | 48 |
| Lumbar puncture for CSF cytology | 19 | 27 | 46 |
| Chest radiograph | 21 | 28 | 49 |
| Abdominal sonography | 21 | 28 | 49 |
| Computed tomography (site specified) | 1 (abdomen in India) | 2 (both neck) | 3 |
| Pathology review in East Africa (based on hematoxylin and eosin staining) | |||
| Tumor grade (n = 49) by Working Formulation | |||
| High grade | 16 | 15 | 31 |
| Intermediate to high grade | 1 | 0 | 1 |
| Intermediate grade | 4 | 12 | 16 |
| Low grade | 0 | 1 | 1 |
| Total | 21 | 28 | 49 |
| Pathology review in United States (review of histopathology slides and TMA) | |||
| Tumor grade (n = 33) by Working Formulation | |||
| High grade | 8 | 8 | 16 |
| Intermediate to high grade | 1 | 3 | 4 |
| Intermediate grade | 5 | 5 | 10 |
| Low to intermediate grade | 0 | 2 | 2 |
| Hodgkin's lymphoma | 0 | 1 | 1 |
| Total | 14 | 19 | 33 |
| TMA immunophenotype (n = 28) | |||
| B-cell lymphoma (not otherwise specified) | 0 | 4* | 4 |
| Diffuse large-cell lymphoma | 1† | 6‡ | 7 |
| Lymphoblastic lymphoma | 0 | 3§ | 3 |
| Burkitt's lymphoma | 1‖ | 2¶ | 3 |
| Plasmacytoid/plasmablastic lymphoma | 2# | 1** | 3 |
| Hodgkin's lymphoma | 0 | 1 | 1 |
| Insufficient number of intact cells (necrosis) | 5 | 2 | 7 |
| Total | 9 | 19 | 28 |
Abbreviations: TMA, tissue microarray; LDH, lactate dehydrogenase; mCHOP, dose-modified cyclophosphamide, doxorubicin, vincristine, and prednisone.
CD20+.
c-myc positive and CD20+.
CD20+, n = 5; CD30+, n = 1.
TDT positive, n = 1; 2 CD79A+, n = 2.
EBER positive, c-myc positive, and CD20+.
EBER positive, n = 1; CD20+, n = 2.
EBER positive, c-myc positive/CD20+ (n = 1), CD30−, and CD79A−.
EBER positive, CD138+, MUM1 positive, TDT negative, and CD30–.
The majority of patients were female (59%) and had poor performance status (63%), advanced stage disease (69%), and prior AIDS diagnosis (67%); 37% of patients had access to antiretroviral therapy during the course of study, and 22% received subsequent second-line, dose-modified cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Thirty of the 33 reviewed patients had high-grade (n = 16), intermediate- to high-grade (n = 4), or intermediate-grade (n = 10) lymphoma.
Pathology Review
Thirty-three (67%) of 49 patients had tumor biopsy specimens submitted for pathology review and confirmation of lymphoma diagnosis; 28 samples were submitted for tumor immunophenotyping using TMA methodology (Table 3). The confirmed non-Hodgkin's lymphoma diagnosis rate was 31 (94%) of 33 patients (95% CI, 80% to 98%); of these 31 patients, 25 had TMA immunophenotyping performed. The overall proportion of pathology analyses was 32 (65%; including a patient with confirmed Hodgkin's lymphoma) of 49 patients (95% CI, 51% to 77%). c-myc gene rearrangements were confirmed in one patient with Burkitt's lymphoma (two patients had no probe signal), one patient with plasmacytoid, and one patient with diffuse large B-cell lymphoma. Six other samples had normal c-myc arrangements, and the remainder had no probe signal.
Treatment Course and Toxicity
A total of 79.5 cycles of therapy were administered; 32 patients (65%) completed the two protocol-prescribed courses; and therapy was well tolerated (Table 4). Only three patients (6%) developed CNS relapse. There were no difficulties with oral chemotherapy and protocol compliance. There was negligible nausea and vomiting and no cardiotoxicity. Dose modifications were required for hematologic toxicity but no other toxicity. No patients discontinued therapy as a result of nonresolution of intervening myelotoxicity. There were four episodes of grade 3 or 4 febrile neutropenia (5% of cycles) and four grade 3 infections. There were eight deaths during chemotherapy, and three were considered directly related to chemotherapy (6% treatment mortality rate; Table 4).
Table 4.
Treatment Course and Toxicity
| Treatment and Toxicity | Uganda (n = 21) | Kenya (n = 28) | Total (n = 49) |
|---|---|---|---|
| Total No. of cycles of therapy | 32.5 | 47 | 79.5 |
| Median per patient | 2 | 2 | 2 |
| Range per patient | 0.5-2.0 | 0.5-2.0 | 0.5-2.0 |
| Toxicity, No. of patients | |||
| Grade 3 diarrhea/dehydration | 1 | 2 | 3 |
| Grade 3 infection (OI)* | 4 | 0 | 4 |
| Grade 3 neutropenia | 6 | 2 | 8 |
| Grade 4 dehydration | 1 | 0 | 1 |
| Grade 3/4 febrile neutropenia | 3 | 1 | 4 |
| Grade 4 ANC/thrombocytopenia | 3 | 4 | 7 |
| Toxicity (grade 5, death), No. of patients | |||
| Treatment related | 2 | 1 | 3 |
| Not treatment related† | 1 | 4 | 5 |
Abbreviations: OI, opportunistic infection; ANC, absolute neutrophil count.
The four grade 3 infections included two cryptococcal meningitis, one Pneumocystis pneumonia, and one tuberculosis.
The five other causes of death on study that were not considered treatment-related included progressive lymphoma in two patients, progressive AIDS in two patients, and undetermined cause in one patient with no associated myelosuppression.
Impact of Chemotherapy on CD4+ Lymphocytes and HIV-1 Plasma RNA
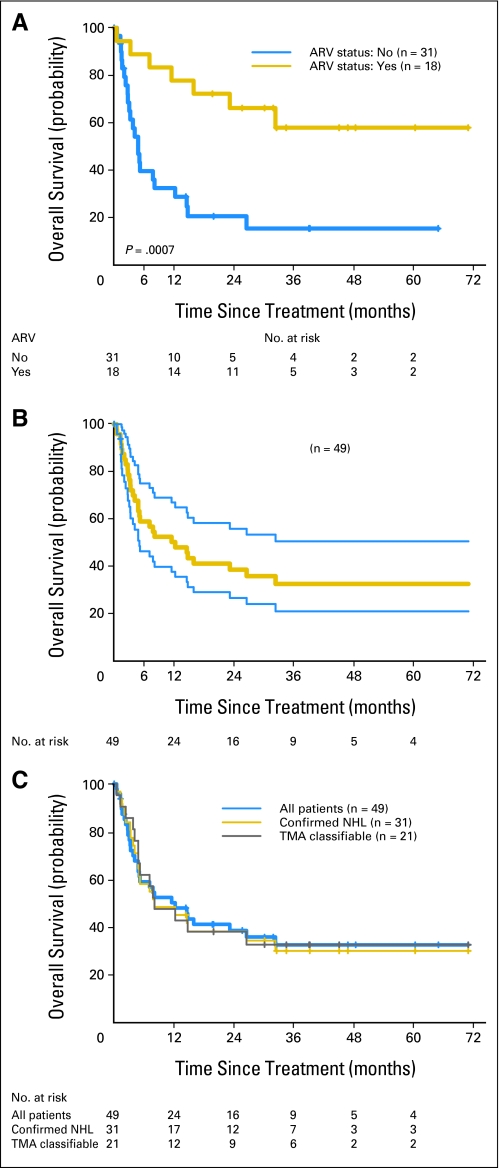
Eighteen patients (37%) received antiretroviral therapy at the start, during, or immediately on completion of chemotherapy. More patients were started on antiretroviral therapy during the later part of the study as access expanded in East Africa; seven patients received therapy in 2001 to 2003, and 11 received therapy in 2004 to 2005, corresponding to the roll out of national drug access programs. Patients with access to antiretroviral therapy over the course of the study had improved survival (P = .0007; Fig 1A). Oral chemotherapy had modest impact (decline) on CD4+ lymphocyte counts (P = .077) and no adverse effects on HIV-1 viral replication (Table 5). An increased incidence of opportunistic infections was not observed during chemotherapy. In separate multiple linear regression analyses, antiretroviral therapy (P = .013) and days after onset of chemotherapy (P < .0001) were predictors of CD4+ lymphocyte counts, and antiretroviral therapy (P = .018) was a significant predictor of HIV-1 RNA plasma levels.
Fig 1.
(A) Kaplan-Meier (KM) estimation of overall survival (OS) stratified by antiretroviral (ARV) status. (B) KM estimation of OS with 95% CIs. The median OS time was 12.3 months (95% CI, 4.9 to 32.4 months). The three cases of confirmed Burkitt's lymphoma survived 7.2, 12.3, and 14.8 months. (C) KM estimation of OS of all 49 treated patients superimposed on KM estimations from 31 confirmed non-Hodgkin's lymphoma (NHL) patients and from 21 subtyped (tissue microarray [TMA]) patients. (D) KM estimation of OS by intermediate and high tumor grade. Tumor grade was based on a composite of confirmed tumor grade and African grade for patients not reviewed or confirmed in United States.
Table 5.
Impact of Oral Chemotherapy on CD4+ Lymphocyte Counts and HIV-1 Plasma RNA
| Time Point | CD4+ Lymphocyte Count |
HIV-1 Plasma RNA |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | Median (cells/μL) | Range (cells/μL) | P* | No. of Patients | Median (copies/mL) | Range (copies/mL) | P* | |
| Baseline | 49 | 198 | 5-1,364 | — | 45 | 102,000 | 0-11,750,000 | — |
| 3-70 days | 32 | 150.5 | 4-586 | .077 (overall) | 30 | 119,455 | 325-1,810,000 | .904 (overall) |
| > 70 days | 21 | 88 | 5-304 | .027 (v baseline) | 19 | 78,683 | 200-8,200,000 | — |
P values were based on 21 patients with CD4+ lymphocyte count and 19 patients with HIV-1 plasma RNA who had complete data at the three time points.
Response and Survival
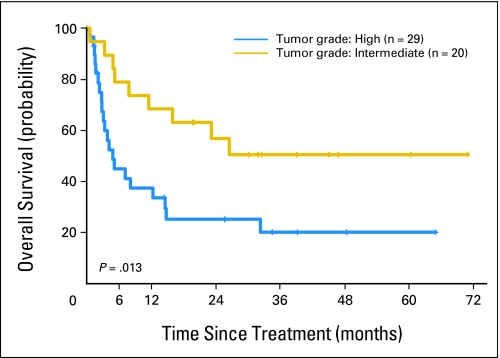
Forty (82%) of the 49 patients were assessable for response. The overall objective response rate (CR/unconfirmed CR + PR/unconfirmed PR) was 78% (95% CI, 62% to 88%), and the CR/unconfirmed CR response rate was 58% (95% CI, 42% to 71%; Appendix Table A1, online only). To identify factors that predict CR, a logistical regression was used. In univariate analysis, only antiretroviral therapy was significantly related to CR (P = .005). Comparing patients without antiretroviral therapy, the odds of having a CR for patients with antiretroviral therapy was increased less than 12-fold. The median OS time was 12.3 months (95% CI, 4.9 to 32.4 months; Fig 1B). The Kaplan-Meier estimations of survivor function for three cohorts (all 49 treated patients, 31 confirmed non-Hodgkin's lymphoma patients, and 21 TMA-classifiable patients) were essentially identical (Fig 1C). The Kaplan-Meier estimate of survivor function by tumor grade is shown in Appendix Fig A1 (online only). At time of last follow-up in July 2007, 33% of patients had survived 5 years, and 30 patients had died. Causes of death included treatment (n = 3), progressive AIDS (n = 11), progressive lymphoma (n = 7), both progressive lymphoma and AIDS (n = 6), and undetermined cause (n = 3). The median follow-up duration was 8.2 months (range, 0.1 to 71 months).
Prognostic Analyses of Outcome
Numerous variables and three prognostic scales were analyzed for effects on EFS and OS by univariate Cox model (Appendix Table A2, online only). In univariate analysis, serum lactate dehydrogenase (LDH; P = .0004) and hemoglobin (P = .002) were the only continuous covariates that were significantly related to EFS and OS (Table 6). Besides sex, those factors that were significant in univariate analysis were further included in the multiple Cox regression model after checking proportional hazard assumption (Table 6). The IPI was defined by age, stage, performance status, and extranodal sites of disease and excluded LDH. The IPI (P = .034) and access to antiretroviral therapy (P = .035) were significantly related to survival after controlling for the effects of other covariates. With every increase of the IPI index by 1, the hazard of dying was increased 1.75-fold (P = .034). Similarly, the hazard ratio of patients without antiretroviral therapy compared with those with access was 2.87 (P = .035).
Table 6.
Effect of Continuous Covariates on OS Estimated by Univariate Analysis and Effects of IPI and Other Covariates on OS Estimated by Multivariate Analysis
| Factor | Coefficient (β) | Hazard Ratio | P |
|---|---|---|---|
| Effect of continuous covariates on OS | |||
| Age, per year increase | −0.026 | 0.974 | .202 |
| LDH, per 100 U/L increase | 0.156 | 1.17 | .0004 |
| CD4+ count, per 100/μL decrease | −0.22 | 1.25 | .167 |
| HIV-1 RNA, per 107 copies/mL increase | 0.76 | 2.14 | .442 |
| Albumin, per 1 g/L decrease | −0.225 | 1.25 | .379 |
| Hemoglobin, per 1 g/dL decrease | −0.292 | 1.34 | .002 |
| Body mass index, per 1 point increase | 0.024 | 1.02 | .493 |
| Effect of IPI and other covariates on OS | |||
| Sex, female v male | −0.34 | 0.715 | .444 |
| Hemoglobin, 1 g/dL decrease | −0.154 | 1.17 | .184 |
| Antiretroviral therapy, no v yes | 1.05 | 2.87 | .035 |
| IPI, index increase by 1 | 0.557 | 1.75 | .034 |
| Tumor grade, high v intermediate | −0.147 | 1.16 | .766 |
Abbreviations: OS, overall survival; IPI, International Prognostic Index; LDH, lactate dehydrogenase.
DISCUSSION
To our knowledge, this is the first prospective study of the treatment of AIDS-related non-Hodgkin's lymphoma conducted in sub-Saharan Africa, a region of the world with a high burden of HIV disease.1,2,36,37 Several clinical observations from this study are noteworthy and provide a departure point to better understand the natural history of lymphoma in Africa. Foremost among these is that the study design was hypothesis driven, pragmatic, and fully aligned with current clinical practices. Oral chemotherapy is advantageous, with demonstrable efficacy and an acceptable safety profile. The 6% treatment mortality rate is a seminal observation and compares favorably to the 20% to 66% treatment mortality encountered in patients with advanced AIDS-related malignancies or endemic Burkitt's lymphoma.1,6,7 Demonstrable antitumor activity was also observed with a less myelosuppressive, non–dose-intense chemotherapy regimen in patients with high-grade disease including patients with AIDS-related Burkitt's lymphoma. Only three patients (6%) had leptomeningeal relapse, substantiating clinical benefit from inclusion of drugs in the oral regimen that are known to cross the blood-brain barrier.
Our histopathology review and lymphoma subtype analysis at study closure were instructive. Working Formulation criteria for lymphoma classification is the current standard of pathology in East Africa.20 The 33 patients with tissue blocks and original hematoxylin and eosin tissue sections presented challenges on review. Of the 16 patients excluded, the majority were patients referred from upcountry and regional hospitals in Uganda and Kenya to the national referral centers in our study. All of these patients had written pathology reports substantiating lymphoma diagnosis. Biopsy materials could not be retrieved from these remote locations, which were often hundreds of miles away. We undertook CI estimations of our pathology review—confidence in establishing a diagnosis of lymphoma in tissues that were available and overall confidence in pathologic diagnosis. There is acceptable confirmation of non-Hodgkin's lymphoma in patients reviewed for confirmation of lymphoma. In Figure 1C, all three Kaplan-Meier survival plots identify comparable outcomes irrespective of the assurance of diagnosis; thus, bias as a result of inappropriate diagnosis is less likely. Nonetheless, the TMA-based lymphoma subtype analyses identified a wide spectrum of lymphomas in the backdrop of HIV infection in sub-Saharan Africa. This diversity of lymphoma subtype was unexpected.38,39 It will be worthwhile for future capacity-building efforts to enable pathology contributions to focus on additional training and support for this vital endeavor to further our phenotypic and molecular characterization of treated cancers.
It is notable that we did not encounter profound immunosuppression in our East African patients (median CD4+ count, 198 cells/μL) compared with that reported in US trials in the pre-HAART era (CD4+ count range of 47 to 117 cells/μL at presentation).9,11,17,26,40,41 Patients with AIDS in Africa have been reported to present with slightly higher CD4+ counts with onset of opportunistic and neoplastic complications.42,43 Of interest is the apparent critical role that antiretroviral therapy plays in the management of patients with AIDS-related lymphoma in this setting. Oral chemotherapy had modest effects on CD4+ lymphocyte counts. There was no increase in opportunistic infection over the course of therapy, and there were negligible effects on underlying HIV-1 viral replication. Chemotherapy does not adversely affect HIV-1 viral replication in US adults and children with AIDS-related lymphoma, and this appears to be the case as well in Africa.18,44,45 Clearly, treatment of both disease processes (ie, HIV infection and neoplastic disease) is required for patient survival. Oral chemotherapy as prescribed in our study is compatible with contemporary antiretroviral usage in sub-Saharan Africa.
The IPI proved to be the most discriminating of the three prognostic scales for AIDS-related lymphoma that we analyzed, which has also been reported by others.46 On the basis of extensive physical examination and bone marrow and CSF examination, our results are remarkably consistent with published prognostic scales such as the IPI and AIDS Clinical Trials Group score. Admittedly, patients may be understaged as a result of lack of computed tomography and magnetic resonance imaging. It is important to point out, however, that careful scrutiny of essential findings on physical examination can be made in resource-constrained settings to identify the extent of extranodal involvement and to incorporate findings into various staging and prognostic classifications. Many of our patients had massively enlarged lymph nodes or extranodal masses that ulcerated the skin surface and extended to the musculature of the chest wall, abdominal wall, or groin and had massive organomegaly (hepatomegaly) for which it is entirely appropriate to assign extranodal involvement. Staging criteria allow for such assignment. This was performed and was captured in our staging evaluation in the absence of robust radiographic imaging capability. Additionally, patients underwent on-study bone marrow aspiration and CSF cytologic analysis, which are routinely performed in this setting.
As we proceed with future studies, our experience will help guide prognostic scales more adapted and suitable to sub-Saharan Africa. As an initial departure, we feel that these analyses are important to disseminate. Baseline hemoglobin and albumin may be incorporated or substituted for LDH as useful covariates to provide further independent prognostic information in subsequent trials because these are more readily available in East Africa. Access to antiretroviral therapy was also predictive of CR and prognostic of survival. All of these factors may guide therapeutic options in future trials in Africa.
The adoption of dose-modified oral chemotherapy, the use of targeted laboratory and limited radiographic assessments, and the capability to pursue tumor biopsy pathology confirmation during this clinical trial are aligned with current clinical practices in sub-Saharan Africa. Cost-effective therapeutic strategies for the management of HIV disease in Africa are clearly warranted.47 Even with the emerging availability of HAART, the safety of dose-modified oral chemotherapy is important new information. As in most developing countries, HAART is more available than chemotherapy safety monitoring. Over the course of this study, which was closely monitored, ethical considerations did not seem to present new hurdles. Our project-specific DSMP was well informed, and the ability to conduct on-site audits greatly complemented the conduct of this study.
Developing countries, which are generally overwhelmed by the burden of AIDS, have focused their limited resources more on public health and less on personal health. Limitations are reflected in lack of sophisticated radiology, clinical laboratory, blood transfusion, and histopathology.2,48–50 These limitations influenced the decision to prospectively evaluate a nonmyelosuppressive chemotherapy regimen for AIDS-related lymphoma and the choices made within this protocol. Although understaging of patients may have occurred as a result of lack of computed tomography/magnetic resonance imaging access, this is balanced by initial presentation at more advanced stages of disease than occurs in developed countries, allowing physical examination to be a reasonably reliable instrument of measure. Laboratory testing reflects use of available tests rather than those more commonly used in developed countries, which may limit comparability. This does not discount the usefulness of the tests used. Diagnostic pathology is not supported or organized in the same manner as in developed countries, and the limitations encountered with tissue studies was greater than anticipated. The prevalence and distribution of lymphoma subtypes throughout Africa, particularly among persons with AIDS, are currently not known. Although these limitations are immediate hurdles, there are several groups (eg, African Organisation for Research and Training in Cancer and National Institutes of Health) that are moving forward to mitigate these limitations that affect Africa. At the 50th Anniversary of the Discovery of Burkitt Lymphoma conference in 2008, these points were articulated and reported, as follows: “disease patterns have not been fully documented but diagnosis for the majority is still by morphology alone; there is a need to formulate and implement standards for lymphoma diagnosis and classification; and Africa is at the stage that the industrialized world was at in the 1960s and 1970s.”49 Our demonstration clearly informs about possible ways forward.
In summary, it is feasible to develop evidence-based, pragmatic therapeutic intervention to treat patients with advanced AIDS-associated malignancies in East Africa. This National Cancer Institute R01 project supported, to our knowledge, the first trial ever conducted on the African continent for AIDS-related non-Hodgkin's lymphoma. Its success provides a springboard for capacity building; further phenotypic and molecular characterization of lymphoma subtypes; and collaborative efforts to frame alternative, less myelotoxic therapeutic strategies suitable for evaluation in the resource-constrained setting. Successor projects should focus on strategies to optimize combination antiretroviral therapy and chemotherapy and follow-up correlative tissue and laboratory studies.
Acknowledgment
We thank the efforts of Ellen Feigal, MD (formerly Division Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD), whose support of this protocol was vital at the inception of this study; and Kishor Bhatia, PhD, Office of AIDS Malignancy Program, National Cancer Institute, for his support during the conduct of the study.
The independent audit reviewers who participated in the on-site audits in 2003, 2004, and 2005 in support of this study were Harriet Mayanja-Kizza, MB, ChB, Professor and Chair, Department of Medicine, Makerere University School of Medicine; Iga Matovu, MB, ChB, Senior Consultant, and Rosemary Byanyima, MB, ChB, Consultant, Department of Radiology, Makerere University School of Medicine, Kampala, Uganda; Geoffrey Ikundu, MB, ChB, Lecturer, Department of Diagnostic Radiology, University of Nairobi College of Health Sciences; Peter Odhiambo, MBBS, MMed, Professor of Surgery and former Dean, Faculty of Medicine, University of Nairobi College of Health Sciences; Frederick Antone Okoth, MB, ChB, Chief Research Officer, Centre for Virus Research, Kenya Medical Research Institute; Angeline Anyona Aywak, MB, ChB, Lecturer, Department of Diagnostic Radiology, University of Nairobi College of Health Sciences; Paresh Dave, MB, ChB, Staff Hematopathologist, M.P. Shah Hospital, Nairobi; and Francis Owilla, MB, ChB, MMed, Lecturer, Department of Surgery, Consultant Urologist, Kenyatta National Hospital, Nairobi, Kenya. Their collective efforts are acknowledged and greatly appreciated.
We also thank Brenda Cooper, MD, Chair, Data Safety and Toxicity Committee, Case Comprehensive Cancer Center; Janice Clayton for administrative support; and Rosemary Rochford, PhD, for her helpful suggestions in reviewing the manuscript. We wish to honor the memory of Rosemary Kigonya, MB, ChB, a hematologist at the Uganda Cancer Institute, who passed away in November 2007, for her support of the trial.
Appendix
Treatment Plan and Patient Follow-Up
Lomustine, etoposide, and cyclophosphamide were supplied by Bristol-Myers Squibb (Kenilworth, NJ), and procarbazine was supplied by Sigma Tau Pharmaceuticals (Rome, Italy). There was no interruption of therapy for any patients already on study during the conduct of the trial. Starting drug and modified drug doses were rounded to accommodate pill size. Lomustine was rounded to the nearest 10-mg increment (smallest pill size). All other drugs were rounded to the nearest 50-mg (pill size) increment (eg, for patients with a body-surface area of 1.75 m2, these drugs could be administered at 150 mg, alternating with 200 mg depending on 3 or 5 days of dosing per agent). Patients were seen approximately every 3 weeks during the first two cycles for safety assessment including determination of CBCs and serum chemistries. A cycle of chemotherapy was 6 weeks with two 3-week portions of treatment. CD4+ lymphocyte counts and HIV-1 RNA plasma levels were monitored before the first two cycles (baseline/day 1 and day 42), at the end of chemotherapy (day 84), and at one 3-month time point on discontinuing chemotherapy. Antiretroviral therapy and prophylactic antibiotics (other than isoniazid prophylaxis for patients latently infected with Mycobacterium tuberculosis) were not prescribed by the protocol (Whalen CC, Johnson JL, Okwera A, et al; N Engl J Med 337:801-808, 1997). No patients received concurrent antituberculous therapy and chemotherapy during the conduct of the trial. At study launch, national primary HIV care programs were nonexistent, and in all instances, efforts were undertaken to refer patients to health care providers or national health care programs as they became available for primary HIV care including access to antiretroviral therapy.
Immunohistochemistry and Tissue Microarray Phenotyping Pathology Methods
The Mid-Region AIDS and Cancer Specimen Resource (Columbus, OH) provided lymphoma subtype using a tissue microarray method.21 The study tissue microarray contained three 0.6-mm tissue cores per sample cored from the original block (Beecher Instruments, Sun Prairie, WI). Lymphoma subtyping included immunohistochemistry with monoclonal antibodies against CD3, CD15, CD20, CD30, CD34, CD43, CD45, CD45RO, CD68, CD79a, CD138, bcl-2, bcl-6, IgM, Ki67, MUM-1, TdT (DAKO, Carpinteria, CA), CD5, and CD10 (Novocastra, Bannockburn, IL); in situ hybridization for Epstein-Barr virus RNA (EBER) and kappa/lambda light chains (Ventana, Tucson, AZ); and fluorescent in situ hybridization for c-myc t(8;14) (Abbott/Vysis, Downer's Grove, IL). It is important to note that we did not incur any obstacles in transporting tissues for pathology analyses at the conclusion of our study.
Ethical Review
Signed informed consent was obtained from all patients in keeping with international, institutional, and US Food and Drug Administration guidelines (Declaration of Helsinki).22,23 The study protocol and informed consent document was initially submitted for review and approval to the University Hospitals Case Medical Center Institutional Review Board (IRB). On approval, the protocol and English version of the informed consent document were sent to collaborators in Uganda and Kenya, where the protocol was submitted for respective ethical committee reviews and back translation of the informed consent document into Luganda in Uganda and Kiswahili in Kenya. Informed consent documents were generated in English (legal language in each country) and the predominant tribal tongue. In Kampala, ethical review was required by the Uganda National Council for Science and Technology (Federal Wide Assurance [FWA] No. 00001293) and the AIDS Research Committee. In Kenya, ethical review was required by the Kenyatta National Hospital Ethical Review Committee (FWA No. 00002066). Once the protocol was approved in Uganda and Kenya, both IRB approval letters and informed consent documents (English and translated versions) were submitted to the IRB in Cleveland, OH, for final approval; the study then commenced.
Data Safety and Monitoring Plan, Toxicity, and Response Assessment
Because this project was primarily supported by a US Department of Health and Human Services/National Cancer Institute (NCI) research grant (No. CA83525), a project-specific data safety and monitoring plan (DSMP) was required. The project DSMP was fully integrated into the NCI-approved institutional plan of the Case Comprehensive Cancer Center (initially approved in July 2002 with revisions in January 2005 and August 2006). Essential features of the project DSMP are briefly described. Biweekly conference calls throughout the active phases of study recruitment and treatment were convened; conference calls focused on patient eligibility and safety (especially noting ≥ grade 3 toxicities, hospitalizations, and any deaths); minutes were recorded and distributed to all investigators, the Case Comprehensive Cancer Center Data Safety and Toxicity Committee, and National Institutes of Health program officers. On-site audits were conducted in March 2003, March 2004, September 2005, and May 2007 (final audit). A full report of regulatory, drug handling, good clinical practices, safety and response assessment, and data management was generated. Audit attestations were signed by all investigators and participants at the time of every audit. This also included independent review by investigators (including a radiologist at each site) not aligned or engaged in any manner with the clinical trial. The independent review team at each audit had final authority to reconcile toxicity and response assessments, which were also reflected in the audit report documents. These individuals are listed in the acknowledgments. Reports of these audits were submitted to the Data Safety and Toxicity Committee and National Institutes of Health program officers and incorporated into the annual IRB reporting of study progress to the University Hospitals Case Medical Center IRB. Annual IRB reporting, although not required in Africa (only end of study report), was undertaken in Cleveland, OH. Findings of these annual reviews were communicated to the East African ethical committees.
Toxicity assessment was graded using evolving NCI Common Toxicity Criteria over the course of the study.24 Traditional tumor response assessment allows evaluation of measurable disease on physical examination and requires that all studies performed on-study be repeated to document complete or partial response.25,26 All patients had undergone baseline on-study chest radiography and abdominal sonography for staging. These tests could not be repeated as a result of cost and were not a protocol requirement. Clinical response was manifest by marked improvement on physical examination or resolution of disease on repeat bone marrow aspiration, which was repeated in all cases when necessary to confirm response. In these instances, unconfirmed complete or partial response based on the available clinical data was allowed. Similarly, fine-needle aspiration or open biopsy confirmation was undertaken by African investigators when clinically indicated to reconcile equivocal disease status.
Outcome End Points and Statistical Analyses
Overall survival was measured from the date of onset of treatment to the date of death and censored at the date of last follow-up for survivors. Event-free survival was measured from the date of treatment to the date of relapse (disease progression) or death, whichever occurred first, and censored at the date of last follow-up for those still alive without relapse.
Patient Characteristics
Demographics of eligible patients who were enrolled and not enrolled were similar. Of the first 108 patients screened for study, the most common reasons that patients (often with multiple reasons) were excluded from participation included negative HIV serology in 54 patients; Eastern Cooperative Oncology Group performance status of four in 18 patients; positive CSF cytology in six patients; and refusal to provide informed consent in six patients.
Febrile Neutropenia
Although there is limited use of thermometers, patients would present to the clinic not feeling well and, on occasion, were found to be febrile and neutropenic, which would prompt hospitalization. We did incur shortages of antibiotics, which is not uncommon in this setting. Patients were treated with available antimicrobials suitable for the empiric management of febrile neutropenia. There was no consistent antibiotic regimen for the management of febrile neutropenia. These challenges have been discussed and published by our group.2,3,5 This is common in resource-constrained settings. Prior opportunistic infections did not predict for subsequent febrile neutropenic illness. The rate of grade 3 or higher febrile neutropenia was 6.3% (one of 16 patients) for patients without prior opportunistic infection compared with 9.1% (three of 33 patients) for those with prior opportunistic infection (P = .733).
Fig A1.
Kaplan-Meier estimation of overall survival by tumor grade—intermediate and high. Tumor grade was based on a composite of confirmed tumor grade and African grade for cases not reviewed in the United States.
Table A1.
ORR
| Response | Uganda (n = 21) |
Kenya (n = 28) |
Total (N = 49) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Assessable | 16 | 24 | 40 | |||
| CR/uCR | 7/3 | 63 | 8/5 | 54 | 15/8 | 58 |
| PR/uPR | 4/1 | 33 | 0/3 | 13 | 4/4 | 20 |
| Total ORR | 15 | 94 | 16 | 67 | 31 | 78 |
| Stable disease | 0 | 0 | 2 | 8 | 2 | 5 |
| Progressive disease | 1 | 6 | 6 | 25 | 7 | 18 |
NOTE. Unconfirmed complete and partial responses were assessed when radiographic studies at baseline were not repeated; the protocol did not mandate repeat radiography.
Abbreviations: ORR, objective response rate; CR, complete response; uCR, unconfirmed complete response; PR, partial response; uPR, unconfirmed partial response.
Table A2.
Effect of Prognostic Covariates on EFS and OS by Univariate Cox Model
| Variable | No. of Patients | No. of Deaths | EFS |
OS |
||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Whole group | 49 | 30 | 7.9 months (median) | 12.3 months (median) | ||
| Sex (male v female) | 1.04 | 0.54 to 2.03 | 0.98 | 0.47 to 2.05 | ||
| Female | 29 | 18 | ||||
| Male | 20 | 12 | ||||
| Stage (III, IV v I, II) | 1.35 | 0.66 to 2.75 | 1.19 | 0.55 to 2.54 | ||
| I, II | 15 | 10 | ||||
| III, IV | 34 | 20 | ||||
| Extranodal sites of disease (> 1 v ≤ 1) | 2.67* | 1.33 to 5.34 | 3.26* | 1.55 to 6.86 | ||
| ≤ 1 | 35 | 18 | ||||
| > 1 | 14 | 12 | ||||
| “B” symptoms (yes v no) | 0.82 | 0.32 to 2.12 | 2.8 | 0.66 to 11.78 | ||
| Yes | 43 | 28 | ||||
| No | 6 | 2 | ||||
| ECOG PS (2, 3 v 0, 1) | 2.38* | 1.16 to 4.88 | 4.57* | 1.85 to 11.28 | ||
| 0, 1 | 18 | 6 | ||||
| 2, 3 | 31 | 24 | ||||
| Tumor grade (high v intermediate)† | 1.64 | 0.83 to 3.23 | 2.63* | 1.19 to 5.79 | ||
| High | 29 | 21 | ||||
| Intermediate | 20 | 9 | ||||
| Prior thrush (yes v no) | 0.96 | 0.48 to 1.92 | 1.3 | 0.6 to 2.84 | ||
| Yes | 30 | 21 | ||||
| No | 19 | 9 | ||||
| Prior AIDS (yes v no) | 1.39 | 0.66 to 2.9 | 1.4 | 0.62 to 3.15 | ||
| Yes | 33 | 22 | ||||
| No | 16 | 8 | ||||
| Antiretroviral therapy (yes v no) | 0.34* | 0.16 to 0.17 | 0.25* | 0.1 to 0.59 | ||
| Yes | 18 | 7 | ||||
| No | 31 | 23 | ||||
| Hemoglobin (≤ 10.3 v > 10.3 g/dL) | 2.28* | 1.14 to 4.55 | 2.39* | 1.15 to 4.98 | ||
| > 10.3 g/dL | 24 | 13 | ||||
| ≤ 10.3 g/dL | 25 | 17 | ||||
| Serum LDH (n = 35) (> 270 v ≤ 270 U/L) | 1.67 | 0.5 to 5.55 | 2.22 | 0.52 to 9.49 | ||
| > 270 U/L | 31 | 22 | ||||
| ≤ 270 U/L | 4 | 2 | ||||
| IPI (age, stage, PS, ENS; 1 increase of IPI) | 1.75* | 1.22 to 2.51 | 2.1* | 1.4 to 3.16 | ||
| 0 | 6 | 3 | ||||
| 1 | 17 | 7 | ||||
| 2 | 15 | 10 | ||||
| 3 | 11 | 10 | ||||
| HIV score (1 increase of the score) | 1.26 | 0.84 to 1.91 | 1.38 | 0.87 to 2.19 | ||
| 0, 1 | 13 | 6 | ||||
| 2 | 22 | 13 | ||||
| 3 | 14 | 11 | ||||
| ACTG 142 prognostic index (1 increase of the index) | 1.49 | 0.92 to 2.43 | 1.89* | 1.11 to 3.2 | ||
| 0, 1 | 27 | 15 | ||||
| 2 | 15 | 10 | ||||
| 3 | 7 | 5 | ||||
| Second-line mCHOP (yes v no) | 1.29 | 0.62 to 2.69 | 0.46 | 0.18 to 1.21 | ||
| Yes | 11 | 5 | ||||
| No | 38 | 25 | ||||
NOTE. Analysis of the IPI in the table excluded LDH because this was not determined in 14 patients (29%). When analyzed by multivariate Cox model adjusting the effect of baseline LDH (n = 35), the IPI remains significantly associated with EFS (HR = 1.65, P = .012) and OS (HR = 1.72, P = .013).
Abbreviations: EFS, event-free survival; OS, overall survival; IPI, International Prognostic Index; HR, hazard ratio; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PS, performance status; ENS, extranodal sites of disease; ACTG, AIDS Clinical Trials Group; mCHOP, dose-modified cyclophosphamide, doxorubicin, vincristine, and prednisone.
Statistically significant (P < .05).
Tumor grade was based on a composite of confirmed tumor grade and African grade for patients not reviewed or confirmed in the United States.
Footnotes
Supported in part by National Institutes of Health Grants No. CA83528, AI36219, CA70081, CA43703, CA066531, and TW00011. Bristol-Myers Squibb (Kenilworth, NJ) and Sigma Tau Pharmaceuticals (Rome, Italy) provided the chemotherapy drugs for this trial.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
Clinical trial information can be found for the following: NCT00049439.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Walter O. Mwanda, Jackson Orem, Pingfu Fu, Jodi Black, Edward Katongole-Mbidde, Scot C. Remick
Financial support: Christopher C. Whalen, Michael M. Lederman, Leona W. Ayers, Scot C. Remick
Administrative support: Walter O. Mwanda, Jackson Orem, Cecilia Banura, Caren Auma Onyango, Anne Ness, Sherrie Reynolds, John L. Johnson, Jodi Black, Edward Katongole-Mbidde, Scot C. Remick
Provision of study materials or patients: Walter O. Mwanda, Jackson Orem, Cecilia Banura, Joweria Kakembo, Caren Auma Onyango, Henry Wabinga, Fatuma K. Abdallah, Edward Katongole-Mbidde
Collection and assembly of data: Walter O. Mwanda, Jackson Orem, Pingfu Fu, Joweria Kakembo, Caren Auma Onyango, Anne Ness, Vivek Subbiah, Jacob Bako, Leona W. Ayers, Scot C. Remick
Data analysis and interpretation: Walter O. Mwanda, Jackson Orem, Pingfu Fu, Anne Ness, Sherrie Reynolds, John L. Johnson, Vivek Subbiah, Jacob Bako, Michael M. Lederman, Leona W. Ayers, Edward Katongole-Mbidde, Scot C. Remick
Manuscript writing: Walter O. Mwanda, Jackson Orem, Pingfu Fu, Anne Ness, Sherrie Reynolds, John L. Johnson, Vivek Subbiah, Jacob Bako, Henry Wabinga, Howard J. Meyerson, Michael M. Lederman, Jodi Black, Leona W. Ayers, Edward Katongole-Mbidde, Scot C. Remick
Final approval of manuscript: Walter O. Mwanda, Jackson Orem, Pingfu Fu, Cecilia Banura, Joweria Kakembo, Caren Auma Onyango, Anne Ness, Sherrie Reynolds, John L. Johnson, Vivek Subbiah, Jacob Bako, Henry Wabinga, Fatuma K. Abdallah, Howard J. Meyerson, Christopher C. Whalen, Michael M. Lederman, Jodi Black, Leona W. Ayers, Edward Katongole-Mbidde, Scot C. Remick
REFERENCES
- 1.Orem J, Mwanda OW, Remick SC. AIDS-associated cancer in developing nations. Curr Opin Oncol. 2004;16:468–476. doi: 10.1097/00001622-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Otieno MW, Banura C, Katongole-Mbidde E, et al. Therapeutic challenges of AIDS-related non-Hodgkin's lymphoma in the United States and East Africa. J Natl Cancer Inst. 2002;94:718–732. doi: 10.1093/jnci/94.10.718. [DOI] [PubMed] [Google Scholar]
- 3.Orem J, Mwanda OW, Banura C, et al. Capacity building for the clinical investigation of AIDS malignancies. Cancer Detect Prevent. 2005;29:133–145. doi: 10.1016/j.cdp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Rochford R, Feuer G, Orem J, et al. Strategies to overcome myelotoxic therapy for the treatment of Burkitt's and AIDS-related non-Hodgkin's lymphoma. East Afr Med J. 2005;82(suppl 9):S157–S162. doi: 10.4314/eamj.v82i9.9388. [DOI] [PubMed] [Google Scholar]
- 5.Orem J, Mwanda OW, Remick SC. Challenges and opportunities for treatment and research of AIDS-related malignancies in Africa. Curr Opin Oncol. 2006;18:479–486. doi: 10.1097/01.cco.0000239887.90665.10. [DOI] [PubMed] [Google Scholar]
- 6.Hesseling PB, Broadhead R, Molyneux E, et al. Malawi pilot study of Burkitt lymphoma treatment. Med Pediatr Oncol. 2003;41:532–540. doi: 10.1002/mpo.10322. [DOI] [PubMed] [Google Scholar]
- 7.Hesseling P, Broadhead R, Mansvelt E, et al. The 2000 Burkitt lymphoma trial in Malawi. Pediatr Blood Cancer. 2005;44:245–250. doi: 10.1002/pbc.20254. [DOI] [PubMed] [Google Scholar]
- 8.Mwanda OW, Remick SC, Whalen C. Adult Burkitt's lymphoma in patients with and without human immunodeficiency virus infection in Kenya. Int J Cancer. 2001;92:687–691. doi: 10.1002/1097-0215(20010601)92:5<687::aid-ijc1246>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Remick SC, McSharry JJ, Wolf BC, et al. Novel oral combination chemotherapy in the treatment of intermediate-grade and high-grade AIDS-related non-Hodgkin lymphoma. J Clin Oncol. 1993;11:1691–1702. doi: 10.1200/JCO.1993.11.9.1691. [DOI] [PubMed] [Google Scholar]
- 10.Remick SC, Sedransk N, Haase R, et al. Oral combination chemotherapy in the management of AIDS-related lymphoproliferative malignancies. Drugs. 1999;58(suppl 3):99–107. doi: 10.2165/00003495-199958003-00014. [DOI] [PubMed] [Google Scholar]
- 11.Remick SC, Sedransk N, Haase RF, et al. Oral combination chemotherapy in conjunction with filgrastim (G-CSF) in the treatment of AIDS-related non-Hodgkin's lymphoma: Evaluation of the role of G-CSF; quality of life analysis; and long-term follow-up. Am J Hematol. 2001;66:178–188. doi: 10.1002/1096-8652(200103)66:3<178::aid-ajh1042>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticoids. Rev Infect Dis. 1989;11:954–963. doi: 10.1093/clinids/11.6.954. [DOI] [PubMed] [Google Scholar]
- 13.Gill PS, Loureiro C, Bernstein-Singer M, et al. Clinical effect of glucocorticoids on Kaposi's sarcoma related to the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1989;110:937–940. doi: 10.7326/0003-4819-110-11-937. [DOI] [PubMed] [Google Scholar]
- 14.Real FX, Krown SE, Koziner B. Steroid-induced development of Kaposi's sarcoma in a homosexual man with Burkitt's lymphoma. Am J Med. 1986;80:119–122. doi: 10.1016/0002-9343(86)90060-4. [DOI] [PubMed] [Google Scholar]
- 15.Wabinga HR, Parkin DM, Wabmire-Mangen F, et al. Trends in cancer incidence in Kyadondo County, Uganda, 1960-1997. Br J Cancer. 2000;82:1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wabinga HR, Parkin DM, Wabwire-Mangen F, et al. Cancer in Kampala, Uganda in 1989-91: Changes in incidence in the era of AIDS. Int J Cancer. 1993;54:26–36. doi: 10.1002/ijc.2910540106. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan LD, Straus DJ, Testa MA, et al. Low-dose compared with standard-dose m-BACOD chemotherapy for non-Hodgkin's lymphoma associated with human immunodeficiency virus infection. N Engl J Med. 1997;336:1641–1648. doi: 10.1056/NEJM199706053362304. [DOI] [PubMed] [Google Scholar]
- 18.Ratner L, Lee J, Tang S, et al. Chemotherapy for human immunodeficiency virus-associated non-Hodgkin's lymphoma in combination with highly active antiretroviral therapy. J Clin Oncol. 2001;19:2171–2178. doi: 10.1200/JCO.2001.19.8.2171. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe E, Harris N, Stein H, et al., editors. World Health Organization Classification of Tumors. Lyon, France: IARC Press; 2001. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. [Google Scholar]
- 20.Non-Hodgkin's Lymphoma Pathologic Classification Project. NCI sponsored study of classification of non-Hodgkin's lymphoma: Summary and description of a working formulation for clinical usage. Cancer. 1982;49:2112–2135. doi: 10.1002/1097-0142(19820515)49:10<2112::aid-cncr2820491024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 22.World Medical Association Declaration of Helsinki: Revised Edition. Hong Kong, China: 41st World Medical Assembly; 1989. [Google Scholar]
- 23.Council for International Organizations of Medical Sciences. International ethical guidelines for biomedical research involving human subjects. Geneva, Switzerland: Council for International Organizations of Medical Sciences; 1993. [PubMed] [Google Scholar]
- 24.National Cancer Institute. National Cancer Institute Common Terminology Criteria of Adverse Events, Version 3.0. Bethesda, MD: National Cancer Institute; 2003. [Google Scholar]
- 25.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 26.Sparano JA, Wiernik PH, Hu X, et al. Pilot trial of infusional cyclophosphamide, doxorubicin, and etoposide plus didanosine and filgrastim in patients with human immunodeficiency virus-associated non-Hodgkin's lymphoma. J Clin Oncol. 1996;14:3026–3035. doi: 10.1200/JCO.1996.14.11.3026. [DOI] [PubMed] [Google Scholar]
- 27.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16:101–133. [Google Scholar]
- 28.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 29.Malvy D, Thiebaut R, Marimoutou C, et al. Weight loss and body mass index as predictors of HIV disease progression to AIDS in adults: Aquitane Cohort, France, 1985-1997. J Am Coll Nutr. 2001;20:609–615. doi: 10.1080/07315724.2001.10719065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The International Non-Hodgkin's Lymphoma Prognostic Index Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 31.Mounier N, Spina M, Gabarre J, et al. AIDS-related non-Hodgkin lymphoma: Final analysis of 485 patients treated with risk-adapted intensive chemotherapy. Blood. 2006;107:3832–3840. doi: 10.1182/blood-2005-09-3600. [DOI] [PubMed] [Google Scholar]
- 32.Straus DJ, Huang J, Testa MA, et al. Prognostic factors in the treatment of human immunodeficiency virus-associated non-Hodgkin's lymphoma: Analysis of AIDS Clinical Trials Group Protocol 142—Low-dose vs. standard-dose m-BACOD plus granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 1998;16:3601–3606. doi: 10.1200/JCO.1998.16.11.3601. [DOI] [PubMed] [Google Scholar]
- 33.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 34.Hughes JP. Mixed effects models with censored data with application to HIV RNA levels. Biometrics. 1999;55:625–629. doi: 10.1111/j.0006-341x.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 35.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford, United Kingdom: Clarendon Press; 1994. [Google Scholar]
- 36.Mbulaiteye SM, Katabira ET, Wabinga H, et al. Spectrum of cancers among HIV-infected persons in Africa: The Uganda AIDS-Cancer Registry Match Study. Int J Cancer. 2006;118:985–990. doi: 10.1002/ijc.21443. [DOI] [PubMed] [Google Scholar]
- 37.Mbulaiteye SM, Biggar RJ, Goedert JJ, et al. Immune deficiency and risk for malignancy among persons with AIDS. J Acquir Immune Defic Syndr. 2003;32:527–533. doi: 10.1097/00126334-200304150-00010. [DOI] [PubMed] [Google Scholar]
- 38.Ziegler JL, Beckstead JA, Volberding PA, et al. Non-Hodgkin's lymphoma in 90 homosexual men: Relation to generalized lymphadenopathy and the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:565–570. doi: 10.1056/NEJM198408303110904. [DOI] [PubMed] [Google Scholar]
- 39.Carbone A. Emerging pathways in the development of AIDS-related lymphoma. Lancet Oncol. 2003;4:22–29. doi: 10.1016/s1470-2045(03)00957-4. [DOI] [PubMed] [Google Scholar]
- 40.Sparano JA, Wiernik PH, Strack M, et al. Infusional cyclophosphamide, doxorubicin, and etoposide in human immunodeficiency virus– and human T-cell leukemia virus type I–related non-Hodgkin's lymphoma: A highly active regimen. Blood. 1993;81:2810–2815. [PubMed] [Google Scholar]
- 41.Sparano JA, Wiernik PH, Strack M, et al. Infusional cyclophosphamide, doxorubicin and etoposide in HIV-related non-Hodgkin's lymphoma: A follow-up report of a highly active regimen. Leuk Lymphoma. 1994;14:263–271. doi: 10.3109/10428199409049677. [DOI] [PubMed] [Google Scholar]
- 42.Boerma JT, Nunn AJ, Whitworth JA. Mortality impact of AIDS epidemic: Evidence from community sites in less developed countries. AIDS. 1998;12(suppl):S3–S14. [PubMed] [Google Scholar]
- 43.Mbulaiteye SM, Parkin DM, Rabkin CS. Epidemiology of AIDS-related malignancies: An international perspective. Hematol Oncol Clin North Am. 2003;17:673–696. doi: 10.1016/s0889-8588(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 44.Little RF, Pittaluga S, Grant N, et al. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: Impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101:4653–4659. doi: 10.1182/blood-2002-11-3589. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez CE, Adde M, Taylor P, et al. Impact of chemotherapy for AIDS-related malignancies in pediatric HIV disease. Ann NY Acad Sci. 2000;918:362–366. doi: 10.1111/j.1749-6632.2000.tb05507.x. [DOI] [PubMed] [Google Scholar]
- 46.Navarro J-T, Ribera J-M, Oriol A, et al. International Prognostic Index is the best prognostic factor for survival in patients with AIDS-related non-Hodgkin's lymphoma treated with CHOP: A multivariate study of 46 patients. Haematologica. 1998;83:508–513. [PubMed] [Google Scholar]
- 47.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings: The case of Cote d'Ivoire. N Engl J Med. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 48.N'Galy B, Bertozzi S, Ryder RW. Obstacles to the optimal management of HIV infection/AIDS in Africa. J Acquir Immune Defic Syndr. 1990;3:430–437. [PubMed] [Google Scholar]
- 49.Wakabi W. Kenya and Uganda grapple with Burkitt lymphoma. Lancet Oncol. 2008;9:319. doi: 10.1016/s1470-2045(08)70088-3. [DOI] [PubMed] [Google Scholar]
- 50.Lingwood RJ, Boyle P, Milburn A, et al. The challenge of cancer control in Africa. Nat Rev Cancer. 2008;8:398–403. doi: 10.1038/nrc2372. [DOI] [PubMed] [Google Scholar]