Abstract
Purpose
We previously demonstrated a survival advantage for nodal metastasis of melanoma from an unknown primary (MUP) versus melanoma from a known primary (MKP). We hypothesized that this survival benefit would extend to MUP patients with distant (stage IV) metastasis.
Patients and Methods
We reviewed prospectively acquired data for 2,247 patients diagnosed with American Joint Committee on Cancer stage IV melanoma at our cancer center between 1971 and 2005. Cox regression analysis in a multivariate model identified prognostic factors significant for survival. MUP and MKP patients were then matched by significant covariates. Overall survival (OS) was estimated by Kaplan-Meier method and compared by log-rank analysis.
Results
There were 1,849 MKP and 398 MUP patients. Multivariate analysis of patients with complete data sets identified known/unknown primary (hazard ratio [HR], 1.141; P = .032) and five other significant covariates: age (HR, 1.148; P = .007), sex (HR, 1.17; P = .001), site of metastasis (HR, 1.336; P < .001), number of different metastatic sites (HR, 1.303; P < .001), and decade of diagnosis (HR, 0.713; P < .001). Prognostic matching yielded 392 MUP-MKP pairs. Median OS and 5-year OS rate were significantly greater (P < .001) for MUP patients than for all matched MKP patients or for MKP patients matched by M1 category (for M1b and M1c) or number of metastatic sites.
Conclusion
The survival advantage previously reported for patients with stage III MUP also applies to patients with stage IV MUP. The mechanism responsible for this improved survival may provide clues for more effective treatment of stage IV melanoma and therefore warrants further investigation. The improved results for MUP suggest that these patients deserve aggressive therapy.
INTRODUCTION
Of the more than 1.4 million new cases of cancer estimated in the United States this year, about 62,000 will be patients with melanoma.1 Approximately 1% to 4% of these patients will have metastatic disease to distant sites without an identifiable cutaneous, mucosal, or ocular primary.2–9 Among the several proposed etiologies for melanoma from an unknown primary (MUP), the most popular is immune-induced regression of the primary.2,4 Other explanations include malignant transformation of ectopic nevus cells, a missed primary on examination, and removal of the primary without histologic diagnosis.2,4,10
Although one of the earliest reports on MUP described an unfavorable outcome,11 the prognostic significance of MUP with distant metastasis has remained uncertain. Some studies report a guarded prognosis,9,12 while others demonstrate a more favorable survival for stage IV disease from MUP versus melanoma from a known primary (MKP; Table 1).4,6
Table 1.
Reported Rates of Survival for Stage IV Melanoma From MUP and MKP
| Reference | Year | MUP |
MKP |
P | ||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | Median Survival (months) | 5-Year OS (%) | No. of Patients | Median Survival (months) | 5-Year OS (%) | |||
| Lee et al (this study) | 2008 | 398 | 16 | 18 | 1,849 | 15 | 16 | .488 |
| 392* | 16 | 18 | 392* | 11 | 10 | < .001 | ||
| Katz et al6 | 2005 | 37 | 12 | 13.9 | NC | NC | NC | NC |
| Vijuk and Coates4 | 1998 | 146* | 7.7 | NR | 146* | 5.8 | NR | .024 |
| Chang et al9 | 1998 | 293 | NR | 15.8† | 823 | NR | 17.9† | NR‡ |
| Anbari et al10 | 1997 | 11 | 13.2 | NR | NC | NC | NC | NC |
| Schlagenhauff et al12 | 1997 | 8 | 6 | NR | NR | 5 | NR | .14 |
| Velez et al13 | 1991 | 30 | 7 | 10 | NC | NC | NC | NC |
| Reintgen et al14 | 1983 | 26* | 7.3 | NR | 26* | NR | NR | NR‡ |
| Chang and Knapper3 | 1982 | 91 | NR | 8 | NC | NC | NC | NC |
| Das Gupta et al7 | 1963 | 13 | 3§ | NR | NC | NC | NC | NC |
Abbreviations: MUP, melanoma of unknown primary; MKP, melanoma of known primary; OS, overall survival; NC, no MKP comparison or not complete; NR, not reported.
Matched.
Disease-specific survival, not matched.
Statistical comparison not reported but concluded as similar survival.
Average survival.
We previously reported a favorable outcome after regional lymphadenectomy for clinical stage III melanoma from MUP versus MKP.15 We hypothesized that if this survival advantage reflects an immunologic response to melanoma, then it should apply to patients with distant metastasis from MUP.
PATIENTS AND METHODS
We computer-searched the clinical records for more than 13,000 melanoma patients registered into a prospective melanoma database from January 1, 1971, to December 31, 2005 (34-year period), to identify patients with American Joint Committee on Cancer (AJCC) stage IV metastatic melanoma.16 Our study group comprised a cohort of 2,247 patients who were diagnosed with stage IV melanoma within 3 months of presentation. Excluded were patients from a previously reported cohort initially diagnosed with stage III melanoma15 and patients presenting with solitary skin/soft tissue melanoma. The staging work-up to diagnose metastatic disease included brain and body imaging studies available at the time of diagnosis. In addition, patients with no apparent primary melanoma underwent cutaneous, ophthalmologic, and anogenital examination. MUP was defined as distant metastatic melanoma without evidence of a prior or synchronous cutaneous, mucosal, or ocular primary.
Each patient's age (< 60 or ≥ 60 years), sex, metastatic site category (M1a, M1b, M1c), number of different metastatic sites (1, 2, ≥ 3), decade of diagnosis (1971 to 1979, 1980 to 1989, 1990 to 1999, 2000 to 2005), status of primary (MUP or MKP), primary characteristics, and clinical outcomes were recorded.
Approval for this retrospective study was obtained from the John Wayne Cancer Institute-Saint John's Health Center (JWCI-SJHC) joint institutional review board.
Statistics
The prognostic significance of age, sex, metastatic site category, number of different metastatic sites, decade of diagnosis, and known versus unknown primary site was examined by multivariate analysis using Cox proportional hazards regression model. Excluded from this analysis were any patients with an incomplete data set. Significant variables (P < .05) identified by multivariate analysis were used for computer-based matching of MUP and MKP patients in a 1:1 ratio. Matched pairs were compared for overall survival (OS), defined as the time from diagnosis of metastatic disease to death due to any cause. Subjects alive at last follow-up were censored at that point. Survival estimates for matched pairs were derived by the nonparametric Kaplan-Meier method and compared with the log-rank test. All statistical analysis was performed using SAS software (SAS Institute Inc, Cary, NC).
RESULTS
Of the 2,247 study patients, 1,849 had MKP and 398 had MUP (Table 2). Distribution of age was similar for MKP (range, 1 to 95 years; median, 52 years) and MUP (range, 18 to 99 years; median, 50 years). Both groups had a greater proportion of younger patients and male patients. More than half (55% for MKP, 68% for MUP) of the patients in each group were in the M1c category, and more than half (57% for MKP, 57% for MUP) had more than one site involved with metastatic disease. General distribution of age, sex, number of metastatic sites, and decade of diagnosis was similar between MKP and MUP patients. However, the MKP group had more than twice the proportion of patients in the M1a category and a 13% lower percentage of patients in the M1c category as compared with the MUP group.
Table 2.
Characteristics of MUP and MKP Patients in Study
| Variable | Patients Before Matching |
Patients After Matching |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients (N = 2,247) | MKP (n = 1,849) | MUP (n = 398) | All Matched Patients (N = 784) | MKP (n = 392) | MUP (n = 392) | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||||||
| < 60 | 1,527 | 68.0 | 1,232 | 66.6 | 295 | 74.1 | 584 | 74.5 | 292 | 74.5 | 292 | 74.5 |
| ≥ 60 | 702 | 31.2 | 599 | 32.4 | 103 | 25.9 | 200 | 25.5 | 100 | 25.5 | 100 | 25.5 |
| Unknown | 18 | 0.8 | 18 | 1.0 | — | — | ||||||
| Sex | ||||||||||||
| Male | 1,413 | 62.9 | 1,139 | 61.6 | 274 | 68.8 | 542 | 69.1 | 271 | 69.1 | 271 | 69.1 |
| Female | 834 | 37.1 | 710 | 38.4 | 124 | 31.2 | 242 | 30.9 | 121 | 30.9 | 121 | 30.9 |
| Primary site | ||||||||||||
| Head/neck | 338 | 18.3 | 338 | 18.3 | NA | 80 | 20.4 | 80 | 20.4 | NA | ||
| Trunk | 768 | 41.5 | 768 | 41.5 | NA | 197 | 50.3 | 197 | 50.3 | NA | ||
| Extremities | 534 | 28.9 | 534 | 28.9 | NA | 115 | 29.3 | 115 | 29.3 | NA | ||
| Other | 209 | 11.3 | 209 | 11.3 | NA | |||||||
| Ulceration | ||||||||||||
| Yes | 232 | 12.6 | 232 | 12.6 | NA | 47 | 12.0 | 47 | 12.0 | NA | ||
| No | 513 | 27.7 | 513 | 27.7 | NA | 119 | 30.4 | 119 | 30.4 | NA | ||
| Unknown | 1,104 | 59.7 | 1,104 | 59.7 | NA | 226 | 57.6 | 226 | 57.6 | NA | ||
| Metastatic site category | ||||||||||||
| M1a | 321 | 14.3 | 294 | 15.9 | 27 | 6.8 | 52 | 6.6 | 26 | 6.6 | 26 | 6.6 |
| M1b | 633 | 28.2 | 533 | 28.8 | 100 | 25.1 | 190 | 24.2 | 95 | 24.2 | 95 | 24.2 |
| M1c | 1,293 | 57.5 | 1,022 | 55.3 | 271 | 68.1 | 542 | 69.2 | 271 | 69.2 | 271 | 69.2 |
| No. of metastatic sites | ||||||||||||
| 1 | 965 | 42.9 | 795 | 43.0 | 170 | 42.7 | 340 | 43.4 | 170 | 43.4 | 170 | 43.4 |
| 2 | 550 | 24.5 | 473 | 25.6 | 77 | 19.4 | 146 | 18.6 | 73 | 18.6 | 73 | 18.6 |
| ≥ 3 | 732 | 32.6 | 581 | 31.4 | 151 | 37.9 | 298 | 38.0 | 149 | 38.0 | 149 | 38.0 |
| Decade of diagnosis | ||||||||||||
| 1971-1979 | 321 | 14.3 | 272 | 14.7 | 49 | 12.3 | 92 | 11.7 | 46 | 11.7 | 46 | 11.7 |
| 1980-1989 | 448 | 19.9 | 361 | 19.5 | 87 | 21.9 | 174 | 22.2 | 87 | 22.2 | 87 | 22.2 |
| 1990-1999 | 1,158 | 51.5 | 956 | 51.7 | 202 | 50.7 | 400 | 51.0 | 200 | 51.0 | 200 | 51.0 |
| 2000-2005 | 320 | 14.3 | 260 | 14.1 | 60 | 15.1 | 118 | 15.1 | 59 | 15.1 | 59 | 15.1 |
Abbreviations: MUP, melanoma of unknown primary; MKP, melanoma of known primary; NA, not applicable.
At a median and mean follow-up of 14 and 31 months, respectively (range, 0.2 to 416 months), for all 2,247 patients, median OS and 5-year rate of OS were 16 months and 18% ± 2%, respectively, for 398 patients with MUP, as compared with 15 months and 16% ± 1%, respectively, for 1,849 patients with MKP, an insignificant difference (P = .488). The higher than expected survival for MKP patients was influenced by the greater proportion of M1a category patients.
Multivariate analysis of the 2,247 study patients identified six significant factors: age, sex, M1 category, number of sites, decade of diagnosis, and status of primary (MUP or MKP; Table 3). With each successive decade of diagnosis, there was an improvement in survival (median OS of 10.2 months for 1970 to 1979 and 1980 to 1989, 17.0 months for 1990 to 1999, and 21.3 months for 2000 to 2005). Less risk was associated with age younger than 60 years, female sex, lower M1 category, fewer involved metastatic sites, and MUP status. The significance in the multivariate variables was primarily driven by the larger MKP group.
Table 3.
Multivariate Analysis of Prognostic Factors
| Variable | P | Hazard Ratio | 95% CI |
|---|---|---|---|
| Sex, female v male* | .001 | 1.170 | 1.064 to 1.287 |
| Age, < 60 v ≥ 60 years* | .007 | 1.148 | 1.038 to 1.268 |
| Metastatic site category, M1a v M1b v M1c† | < .001 | 1.336 | 1.252 to 1.425 |
| No. of sites, 1 v 2 v ≥ 3† | < .001 | 1.303 | 1.235 to 1.375 |
| Decade of diagnosis, 1970-1979 v 1980-1989 v 1990-1999 v 2000-2005† | < .001 | 0.713 | 0.676 to 0.752 |
| MUP v MKP* | .032 | 1.141 | 1.012 to 1.287 |
Abbreviations: MUP, melanoma of unknown primary; MKP, melanoma of known primary.
Dichotomous.
Categorical.
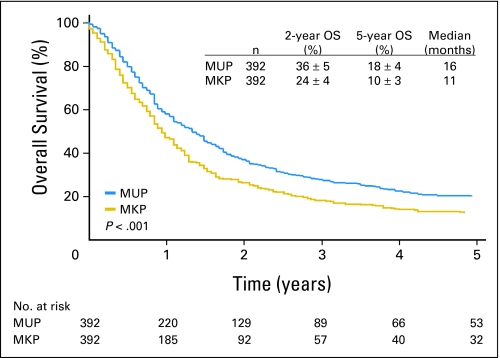
All significant factors excluding primary status were used for prognostic matching of MUP and MKP patients. Computer matching identified 392 pairs of MUP and MKP patients (Table 2). A significantly better survival was observed for MUP compared to MKP (P < .001). Median OS and 5-year rate of OS were 16 months and 18% ± 4%, respectively, for patients with MUP versus 11 months and 10% ± 3%, respectively, for patients with MKP (Fig 1).
Fig 1.
Overall survival (OS) for 392 matched pairs of patients with melanoma from an unknown primary (MUP) and melanoma from a known primary (MKP) patients.
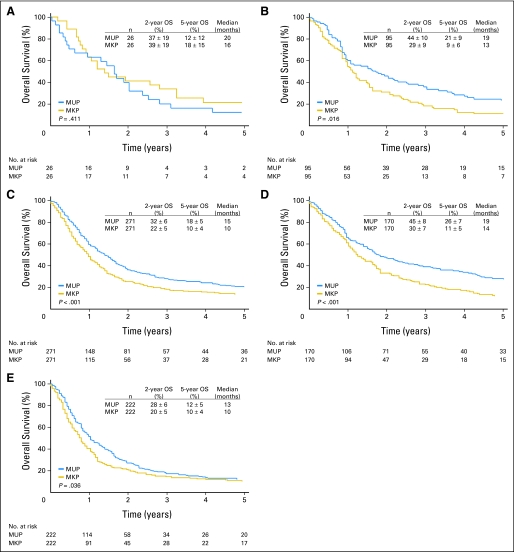
When the 392 matched pairs were stratified by M1 category, OS was significantly greater for MUP patients with M1b and M1c disease but not for the smaller group of MUP patients with M1a disease. Respective median OS and 5-year rate of OS for MUP versus MKP were 20 months and 12% ± 12% versus 16 months and 18% ± 15% for M1a category (P = .411), 19 months and 21% ± 9% versus 13 months and 9% ± 6% for M1b category (P = .016), and 15 months and 18% ± 5% versus 10 months and 10% ± 4% for M1c category (P < .001; Figs 2A, 2B, and 2C). Survivals observed for MKP patients were similar to reported survivals for the respective M1 categories.16
Fig 2.
Overall survival (OS) for 392 matched pairs of patients with melanoma from an unknown primary (MUP) and melanoma from a known primary (MKP) patients stratified by (A) M1a category, (B) M1b category, (C) M1c category, (D) one metastatic site, and (E) more than one metastatic site.
When the 392 matched pairs were further stratified by number of different metastatic sites, OS was higher for MUP patients. With one metastatic site, median OS and 5-year rate of OS were 19 months and 26% ± 7%, respectively, for MUP patients versus 14 months and 11% ± 5%, respectively, for MKP patients (P < .001; Fig 2D). With more than one metastatic site, median OS and 5-year rate of OS were 13 months and 12% ± 5%, respectively, for MUP patients versus 10 months and 10% ± 4%, respectively, for MKP patients (P = .036; Fig 2E).
The outcome for the matched 392 pairs was evaluated by the first treatment for stage IV melanoma. A total of 319 patients (81%) with MKP and 322 patients (82%) with MUP received treatment. The distribution of patients was similar, although there were more patients who underwent surgery in the MUP group and more who received immunotherapy (primarily cell-based vaccine formulations or nonspecific immunomodulants such as bacille Calmette-Guerin) as the initial treatment in the MKP group (Table 4). The outcome for 319 MKP patients treated for stage IV disease was significantly better than the outcome for 73 untreated patients (median and 5-year OS of 13 months and 11% ± 4% versus 6 months and 6% ± 3%, respectively; P < .001). Similarly, the outcome for 322 MUP patients treated for stage IV disease was significantly better than the outcome for 70 untreated patients (median and 5-year OS of 19 months and 21% ± 5% versus 7 months and 7% ± 7%, respectively; P < .001). When survival of patients shown in Table 4 was recalculated without the surgical group, median and 5-year OS was 10 months and 7% ± 3%, respectively, for MKP patients, as compared with 12 months and 9% ± 4%, respectively, for MUP patients (P < .015).
Table 4.
Initial Treatment of Matched MUP and MKP Patients
| Treatment | MKP Patients |
MUP Patients |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Surgery | 90 | 23 | 133 | 34 |
| Immunotherapy* | 75 | 19 | 43 | 11 |
| Biochemotherapy/chemotherapy | 74 | 19 | 66 | 17 |
| Radiation therapy | 35 | 9 | 38 | 10 |
| Other | 45 | 11 | 42 | 11 |
| No therapy/supportive care | 73 | 19 | 70 | 18 |
Abbreviations: MUP, melanoma of unknown primary; MKP, melanoma of known primary.
Eleven MKP and 13 MUP patients received whole-cell melanoma vaccines; most of the remaining patients received nonspecific immunomodulators.
DISCUSSION
The staff at the John Wayne Cancer Institute has treated more than 3,500 patients with AJCC stage IV melanoma, and to our knowledge, this study evaluating the survival characteristics and prognostic factors represents the largest study for MUP with distant metastatic disease. This large comparative analysis of patients with metastatic melanoma found a survival advantage for MUP patients as compared with MKP patients matched for factors significant on multivariate analysis. A significant survival difference was not evident before matching because the greater proportion of patients with M1a disease drove the survival higher for MKP patients.
Vijuk and Coates4 matched 146 MUP and 146 MKP patients by age, sex, and dominant site and reported a significant survival difference favoring MUP patients. However, the median survivals were still fairly dismal: 7.7 months for MUP and 5.8 months for MKP. An important observation by the authors included the possibility of lead-time bias for MKP patients, whose known primaries likely placed them under closer surveillance from the outset. Such bias could favor a survival benefit for MKP, but this was not the case for their study or our current analysis. In fact, it is likely that the observed survival benefit for MUP would increase if MUP patients were under similar surveillance before detection of metastatic disease.
A few other studies compare the survival of patients with stage IV MUP versus MKP, but these studies do not control for prognostic factors.9,12,14 The National Cancer Data Base study on melanoma reported a 5-year survival rate of 17.9% versus 15.8% for 823 MKP patients versus 293 MUP patients, but did not include a statistical comparison of survival.9 Reintgen et al14 compared 26 MUP and 26 MKP patients; survival rates were similar and survival curves were superimposable. It is uncertain by their report if patients were matched by prognostic factors or compared by loosely defined criteria. Similarly, Schlagenhauff et al12 reported no difference in outcome for 8 MUP patients versus an unknown number of MKP patients.
Most reports on MUP with metastatic melanoma do not have adequate comparison MKP groups3,6,7,10,13 and report guarded prognoses.3,7,13 However, some6,10 seem to indicate higher survivals for MUP patients compared to reported survivals from larger stage IV studies.16–18
When we stratified our prognostically matched pairs by M1 category, OS associated with M1b and M1c metastases was significantly better for MUP versus MKP; there was no difference among patients with M1a metastases. In their study of a contemporary cohort of patients with stage IV melanoma, Neuman et al19 demonstrated the prognostic significance of lactate dehydrogenase (LDH) level for M1b and M1c disease, but not for M1a disease. Because LDH is a relatively recent addition to the AJCC staging guidelines for distant melanoma, it was not included in our analysis. We speculate that the more favorable survival of our MUP patients with M1b or M1c disease might reflect lower LDH levels. The lack of significance for M1a pairs in our study might simply be due to small sample size—or it might reflect an intriguing biologic similarity for MUP and MKP patients with M1a disease. Further studies are warranted to clarify the interaction between LDH, other known prognostic factors for stage IV disease, and biologic factors that might be clinically relevant.
Importantly, survival rates for MKP patients in our study were similar to those reported by Balch et al.16 Their study, which was based on prospectively accumulated data from the AJCC Melanoma Database of 30,450 patients, reported 2-year and 5-year survival rates of 37% and 19% for 179 M1a, 23% and 7% for 186 M1b, and 24% and 10% for 793 M1c patients, respectively. Katz et al6 also evaluated survival characteristics of 37 MUP patients by M1 category, but they did not have a cohort of MKP patients for comparison. In their study, the rate of 5-year survival was 24.9% for 12 M1a patients and 20.0% for 5 M1b patients (both rates higher than historical controls), but 6.1% for 20 M1c patients (rate lower than historical controls).
A significant survival difference was also noted when the matched pairs were stratified by the number of different metastatic sites. As expected, more than one disease-involved site was associated with poorer prognosis. Although no other study on MUP evaluated this stratification, there have been studies on metastatic melanoma from known primaries that have similarly reported worse survivals when more sites are involved with tumor.17–20
Consistent with our prior analysis,15 the decade of diagnosis was found to be statistically significant in the multivariate model for stage IV disease. The significance in this study was due to the improvement in survival over the decades, but the reason for this improvement is unclear. Several other studies demonstrated survival improvement during the most recent decade of diagnosis, but not specifically for stage IV melanoma.21,22 Although newer treatment modalities have been introduced over time, no single treatment has had a consistent and overwhelming impact on melanoma survival in the metastatic setting. Changing biology of melanoma is a possible explanation; even more likely is that the advent of computed tomography, positron emission tomography, and magnetic resonance imaging improved the detection of subclinical distant disease in the latter half of the study, thereby allowing earlier therapeutic interventions. Inclusion of the decade of diagnosis as a matching variable was clearly essential to account for temporal differences in melanoma detection, staging, treatment, and perhaps even melanoma biology.
Similar to our previous analysis for stage III melanoma, MUP with stage IV metastatic melanoma is more common in males than females and more common in patients younger than 60 years old, although it involves the spectrum of ages. However, few studies4 have compared the sex or age differences for MUP versus MKP with stage IV disease. Although male sex would suggest poorer outcomes, the higher proportion of males versus females with MUP is a puzzling contradiction to the clear survival advantage for MUP versus MKP. Younger patients with melanoma have a more favorable outcome than older patients according to population-based studies23,24; we speculate that this might reflect a stronger endogenous immune system. This favorable outcome was also demonstrated in a multivariate model for patients with stage IV MUP.4 The greater proportion of younger than older patients with MUP in our analysis may reflect a greater proportion of male patients, and hence contribute to the survival advantage.
Our data also suggest that distant metastasis of MUP is more likely to involve more than one metastatic site and more likely to be categorized as M1c. This might simply reflect the previously documented direct correlation between sample size and higher M1 category.16 Alternatively, this may be a reflection of the guarded nature of stage IV melanoma. Certainly, patients with more than one metastatic site plus M1c disease have the worst prognosis. Interestingly, outcomes by M1 category are better for MUP patients than MKP patients after controlling for important prognostic factors. The matching process should indirectly control for treatment-related factors that may affect survival differences, since prognostically matched patients likely received similar treatment. However, unidentified biologic factors have not been controlled for and would require further investigation.
The various theories proposed to explain MUP include: failure to recognize the primary melanoma on physical evaluation; removal of the primary lesion without pathologic diagnosis; de novo transformation of an ectopic nevus; and immune-induced spontaneous regression of the primary. Das Gupta et al7 originally suggested that either the primary lesion was removed or the primary lesion spontaneously regressed. In their study, the possibility of a missed primary melanoma on physical examination was discredited because some patients who were treated for nodal metastasis did not continue to have further metastases or growth of a primary that should have been physically detectable.
It may be plausible that the primary may have been removed by either trauma or surgical excision without a pathologic diagnosis; however, this theory is unsupported because most patients with MUP are unlikely to have such prior histories.
Shenoy et al25 reported a case of malignant melanoma developing in an axillary node from a precursor nevus cell. However, ectopic nevus cells are too rare (< 1%)26 to support malignant transformation as a plausible explanation for all cases of MUP. Further investigations are needed to determine the occurrence of nevus cells in pathologic specimens of metastatic sites. Lopez et al27 suggested that melanocytes which migrate to ectopic, noncutaneous sites during embryogenesis may have undergone malignant transformation, giving rise to melanoma in metastatic sites. However, it is unclear if the ectopic melanocytes would have migrated to more than one site or to a single organ site and what embryologic processes would be involved in determining the organ-specific migration and timing of transformation. This is difficult to prove and we can only speculate how this process would affect survival outcomes.
A more commonly accepted theory is spontaneous regression of the primary through an endogenous immune mechanism that destroys the tumor.15,28 Histologic evidence of complete regression without the presence of melanoma tumor has been reported, although rarely documented.29 Humoral antimelanoma factors and cellular immune recognition of melanoma-associated antigens have been implicated in this immune-mediated regression.30–33 Unlike other theories, immunologic processes have been associated with survival outcomes in patients with melanoma.34,35 Immune-induced regression might apply to metastatic as well as primary lesions, particularly if the immunosuppressive effect of a large tumor burden has been reduced by cytoreductive surgery and/or effective systemic therapy.36 This could explain the more favorable survival we observed for stage IV patients who received treatment versus those who did not. The nature and clinical significance of an endogenous immune response in stage IV MUP remain speculative but merit further investigations of the biology of MUP versus MKP.
In conclusion, although outcomes have been dismal for patients with distant metastasis from MKP, comparable patients treated for distant metastasis from MUP do relatively better. This difference suggests that the survival advantage previously documented for patients with stage III palpable nodal metastasis from MUP versus MKP is the result of an innate process that applies to all stages of disease. If patients with stage IV melanoma associated with MUP have an innate survival advantage, then they may be excellent candidates for aggressive management of disease.
Footnotes
Supported by Grants No. CA29605 and CA12582 from the National Cancer Institute, the Wayne and Gladys Valley Foundation (Oakland, CA), the Harold J. McAlister Charitable Foundation (Los Angeles, CA), the Family of Robert Novick (Los Angeles, CA), the Weil Family Fund (Los Angeles, CA), the Wrather Family Foundation (Los Alamos, CA), the Amyx Foundation Inc (Boise, ID), Berton M. Kirshner (Los Angeles, CA), Todd Kirshner (Los Angeles, CA), Mr. and Mrs. Louis Johnson, (Stanfield, AZ), Heather and Jim Murren (Las Vegas, NV), Mrs. Marianne Reis (Lake Forest, CA), and the Wallis Foundation (Los Angeles, CA).
Presented at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30 to June 3, 2008.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Chris C. Lee, Mark B. Faries, Donald L. Morton
Financial support: Donald L. Morton
Administrative support: Donald L. Morton
Collection and assembly of data: Chris C. Lee, Leslie A. Wanek
Data analysis and interpretation: Chris C. Lee, Mark B. Faries, Leslie A. Wanek, Donald L. Morton
Manuscript writing: Chris C. Lee, Mark B. Faries, Donald L. Morton
Final approval of manuscript: Chris C. Lee, Mark B. Faries, Leslie A. Wanek, Donald L. Morton
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano AE, Moseley HS, Morton DL. Clinical aspects of unknown primary melanoma. Ann Surg. 1980;191:98–104. doi: 10.1097/00000658-198001000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang P, Knapper WH. Metastatic melanoma of unknown primary. Cancer. 1982;49:1106–1111. doi: 10.1002/1097-0142(19820315)49:6<1106::aid-cncr2820490607>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Vijuk G, Coates AS. Survival of patients with visceral metastatic melanoma from an occult primary lesion: A retrospective matched cohort study. Ann Oncol. 1998;9:419–422. doi: 10.1023/a:1008201931959. [DOI] [PubMed] [Google Scholar]
- 5.Milton GW, Shaw HM, McCarthy WH. Occult primary malignant melanoma: Factors influencing survival. Br J Surg. 1977;64:805–808. doi: 10.1002/bjs.1800641114. [DOI] [PubMed] [Google Scholar]
- 6.Katz KA, Jonasch E, Hodi FS, et al. Melanoma of unknown primary: Experience at Massachusetts General Hospital and Dana-Farber Cancer Institute. Melanoma Res. 2005;15:77–82. doi: 10.1097/00008390-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Das Gupta T, Bowden L, Berg JW. Malignant melanoma of unknown primary origin. Surg Gynecol Obstet. 1963;117:341–345. [PubMed] [Google Scholar]
- 8.Baab GH, McBride CM. Malignant melanoma: The patient with an unknown site of primary origin. Arch Surg. 1975;110:896–900. doi: 10.1001/archsurg.1975.01360140040008. [DOI] [PubMed] [Google Scholar]
- 9.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade: The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Anbari KK, Schuchter LM, Bucky LP, et al. Melanoma of unknown primary site: Presentation, treatment, and prognosis–a single institution study: University of Pennsylvania Pigmented Lesion Study Group. Cancer. 1997;79:1816–1821. doi: 10.1002/(sici)1097-0142(19970501)79:9<1816::aid-cncr26>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Pack GT, Gerber DM, Scharnagel IM. End results in the treatment of malignant melanoma: A report of 1190 cases. Ann Surg. 1952;136:905–911. doi: 10.1097/00000658-195212000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlagenhauff B, Stroebel W, Ellwanger U, et al. Metastatic melanoma of unknown primary origin shows prognostic similarities to regional metastatic melanoma: Recommendations for initial staging examinations. Cancer. 1997;80:60–65. doi: 10.1002/(sici)1097-0142(19970701)80:1<60::aid-cncr8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Velez A, Walsh D, Karakousis CP. Treatment of unknown primary melanoma. Cancer. 1991;68:2579–2581. doi: 10.1002/1097-0142(19911215)68:12<2579::aid-cncr2820681209>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Reintgen DS, McCarty KS, Woodard B, et al. Metastatic malignant melanoma with an unknown primary. Surg Gynecol Obstet. 1983;156:335–340. [PubMed] [Google Scholar]
- 15.Lee CC, Faries MB, Wanek LA, et al. Improved survival after lymphadenectomy for nodal metastasis from an unknown primary melanoma. J Clin Oncol. 2008;26:535–541. doi: 10.1200/JCO.2007.14.0285. [DOI] [PubMed] [Google Scholar]
- 16.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 17.Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181:193–201. [PubMed] [Google Scholar]
- 18.Brand CU, Ellwanger U, Stroebel W, et al. Prolonged survival of 2 years or longer for patients with disseminated melanoma: An analysis of related prognostic factors. Cancer. 1997;79:2345–2353. [PubMed] [Google Scholar]
- 19.Neuman HB, Patel A, Ishill N, et al. A single-institution validation of the AJCC staging system for stage IV melanoma. Ann Surg Oncol. 2008;15:2034–2041. doi: 10.1245/s10434-008-9915-0. [DOI] [PubMed] [Google Scholar]
- 20.Balch CM. Cutaneous melanoma: Prognosis and treatment results worldwide. Semin Surg Oncol. 1992;8:400–414. doi: 10.1002/ssu.2980080611. [DOI] [PubMed] [Google Scholar]
- 21.Peric B, Zgajnar J, Besic N, et al. Changing biology of cutaneous melanoma. Melanoma Res. 2008;18:225–229. doi: 10.1097/CMR.0b013e3282f94651. [DOI] [PubMed] [Google Scholar]
- 22.Lasithiotakis KG, Leiter U, Eigentler T, et al. Improvement of overall survival of patients with cutaneous melanoma in Germany, 1976-2001: Which factors contributed? Cancer. 2007;109:1174–1182. doi: 10.1002/cncr.22511. [DOI] [PubMed] [Google Scholar]
- 23.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: Validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 24.Lasithiotakis K, Leiter U, Meier F, et al. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer. 2008;112:1795–1804. doi: 10.1002/cncr.23359. [DOI] [PubMed] [Google Scholar]
- 25.Shenoy BV, Fort L, III, Benjamin SP. Malignant melanoma primary in lymph node: The case of the missing link. Am J Surg Pathol. 1987;11:140–146. doi: 10.1097/00000478-198702000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Ridolfi RL, Rosen PP, Thaler H. Nevus cell aggregates associated with lymph nodes: Estimated frequency and clinical significance. Cancer. 1977;39:164–171. doi: 10.1002/1097-0142(197701)39:1<164::aid-cncr2820390127>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 27.Lopez R, Holyoke ED, Moore RH, et al. Malignant melanoma with unknown primary site. J Surg Oncol. 1982;19:151–154. doi: 10.1002/jso.2930190308. [DOI] [PubMed] [Google Scholar]
- 28.Giuliano AE, Cochran AJ, Morton DL. Melanoma from unknown primary site and amelanotic melanoma. Semin Oncol. 1982;9:442–447. [PubMed] [Google Scholar]
- 29.Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: Recognition, prevalence and significance. Histopathology. 1992;20:315–322. doi: 10.1111/j.1365-2559.1992.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 30.Maurer H, McIntyre OR, Rueckert F. Spontaneous regression of malignant melanoma: Pathologic and immunologic study in a ten year survivor. Am J Surg. 1974;127:397–403. doi: 10.1016/0002-9610(74)90286-4. [DOI] [PubMed] [Google Scholar]
- 31.Giuliano AE, Moseley HS, Irie RF, et al. Immunologic aspects of unknown primary melanoma. Surgery. 1980;87:101–105. [PubMed] [Google Scholar]
- 32.Saleh FH, Crotty KA, Hersey P, et al. Primary melanoma tumour regression associated with an immune response to the tumour-associated antigen melan-A/MART-1. Int J Cancer. 2001;94:551–557. doi: 10.1002/ijc.1491. [DOI] [PubMed] [Google Scholar]
- 33.Saleh FH, Crotty KA, Hersey P, et al. Autonomous histopathological regression of primary tumours associated with specific immune responses to cancer antigens. J Pathol. 2003;200:383–395. doi: 10.1002/path.1369. [DOI] [PubMed] [Google Scholar]
- 34.Hsueh EC, Gupta RK, Yee R, et al. Does endogenous immune response determine the outcome of surgical therapy for metastatic melanoma? Ann Surg Oncol. 2000;7:232–238. doi: 10.1007/BF02523659. [DOI] [PubMed] [Google Scholar]
- 35.Torisu-Itakura H, Lee JH, Huynh Y, et al. Monocyte-derived IL-10 expression predicts prognosis of stage IV melanoma patients. J Immunother. 2007;30:831–838. doi: 10.1097/CJI.0b013e318158795b. [DOI] [PubMed] [Google Scholar]
- 36.Morton DL, Ollila DW, Hsueh EC, et al. Cytoreductive surgery and adjuvant immunotherapy: A new management paradigm for metastatic melanoma. CA Cancer J Clin. 1999;49:101–116. doi: 10.3322/canjclin.49.2.101. [DOI] [PubMed] [Google Scholar]