Abstract
Purpose
Primary CNS lymphoma (PCNSL) is confined to the CNS and/or the eyes at presentation and is usually initially treated with intravenous methotrexate-based chemotherapy and whole-brain radiotherapy (WBRT). However, the intact blood-brain barrier (BBB) can limit diffusion of methotrexate into brain and tumor. With BBB disruption (BBBD), enhanced drug delivery to the tumor can be achieved.
Patients and Methods
This report summarizes the multi-institutional experience of 149 newly diagnosed (with no prior WBRT) patients with PCNSL treated with osmotic BBBD and intra-arterial (IA) methotrexate at four institutions from 1982 to 2005. In this series, 47.6% of patients were age ≥ 60 years, and 42.3% had Karnofsky performance score (KPS) less than 70 at diagnosis.
Results
The overall response rate was 81.9% (57.8% complete; 24.2% partial). Median overall survival (OS) was 3.1 years (25% estimated survival at 8.5 years). Median progression-free survival (PFS) was 1.8 years, with 5-year PFS of 31% and 7-year PFS of 25%. In low-risk patients (age < 60 years and KPS ≥ 70), median OS was approximately 14 years, with a plateau after approximately 8 years. Procedures were generally well tolerated; focal seizures (9.2%) were the most frequent side effect and lacked long-term sequelae.
Conclusion
This large series of patients treated over a 23-year period demonstrates that BBBD/IA methotrexate-based chemotherapy results in successful and durable tumor control and outcomes that are comparable or superior to other PCNSL treatment regimens.
INTRODUCTION
The incidence of primary CNS lymphoma (PCNSL) in immunocompetent patients represents approximately 4% of intracranial neoplasms. This aggressive, extranodal non-Hodgkin's lymphoma is confined to the CNS and/or the eyes at presentation. Patients age ≥ 60 years account for more than 50% of the cases.1
Numerous studies suggest high-dose methotrexate (≥ 1 g/m2), despite its modest blood-brain barrier (BBB) permeability (approximately 5% of plasma levels),2,3 combined with brain irradiation results in improved patient response and prolonged progression-free survival (PFS) and overall survival (OS).4–8 Combined-modality therapy, however, is associated with a high incidence of delayed and often devastating neurocognitive toxicity in long-term survivors, especially those older than 60 years.4,9–20
We update our previously reported single-center results21,22 and present our large multi-institutional experience in the treatment of patients with PCNSL using BBB disruption (BBBD) in conjunction with intra-arterial (IA) methotrexate-based chemotherapy. We report for the first time the PFS in this large series, as well as OS and toxicity. This series is unique in that patients were treated over a 23-year period.
PATIENTS AND METHODS
Patients
Newly diagnosed immunocompetent patients with PCNSL (n = 131) were enrolled onto the study and prospectively treated by the BBBD programs at four institutions (Oregon Health & Science University, Portland, OR; Cleveland Clinic, Cleveland, OH; Hadassah Hebrew Medical Center, Jerusalem, Israel; and Ohio State University, Columbus, OH) between February 1982 and December 2005. Eligible patients had histologically confirmed PCNSL (by brain biopsy, CSF cytology, or vitrectomy), with no evidence of lymphoma elsewhere in the body and no HIV infection at diagnosis. Before treatment, patients were required to have an absolute granulocyte count more than 1,200/μL, platelet count more than 100,000/μL, and normal hepatic and renal function. Patients with uncontrolled pulmonary or cardiac complications were not eligible. Uniform protocols were approved by the local institutional review board (IRB) or ethics committee of each institution. Informed consent was obtained from the patients. Newly diagnosed patients (ie, those within 90 days of histologic diagnosis) and those who had not received prior whole-brain radiotherapy (WBRT) are included in this report.
In addition, each institution obtained IRB permission to include newly diagnosed patients with PCNSL (n = 18) treated off-protocol with methotrexate-based chemotherapy between the same dates and institutions who met the following criteria: no systemic lymphoma or HIV at diagnosis and no prior WBRT. Therefore, although the vast majority of the patients (131 of 149; 88%) were enrolled onto a prospective study, results from all patients treated with BBBD at the four institutions between 1982 and 2005 who met the above criteria are included in this report, whether treated on or off protocol.
Pretreatment studies included physical examination, Karnofsky performance score (KPS), cranial magnetic resonance imaging with and without contrast, and extraneural computed tomography staging (chest and abdomen). Before magnetic resonance imaging availability, cranial computed tomography scans were obtained. Bone marrow biopsy and ophthalmologic examination with slit lamp evaluation were performed as indicated, and CSF cytopathology was obtained if lumbar puncture could be safely performed.
Treatment
Chemotherapy protocols were uniform across the four BBBD consortium institutions. Comprehensive guidelines for anesthesia, transfemoral arterial cannulation, mannitol/chemotherapy infusion, pre- and post-BBBD patient care, and follow-up were centrally developed and followed by trained teams at all institutions.
Osmotic BBBD with IA infusion of methotrexate-based chemotherapy has been described previously.21–24 The BBBD and chemotherapy treatment details are summarized in Table 1. The treatment of intraocular lymphoma included intraocular methotrexate25 or ocular radiation. Leucovorin rescue (80 mg intravenously [IV]) was initiated 36 hours after the first dose of methotrexate and continued thereafter for 5 days (50 mg, orally or IV, every 6 hours).21,24,26 Patients received two BBBD treatments on 2 consecutive days every 4 weeks for up to 12 monthly courses unless there was evidence of disease progression.21,24
Table 1.
Blood-Brain Barrier Disruption Treatment
| Day | Treatment |
|---|---|
| Day 1, admission | History and physical examination |
| Routine labs | |
| Neuroimaging | |
| Days 2 and 3 | BBBD treatment |
| General anesthesia | |
| Antiepileptic drugs | |
| Transfemoral catheterization of carotid or vertebral artery | |
| 25% warmed mannitol IA infusion over 30 seconds | |
| IV imaging agent | |
| Chemotherapy* | |
| IA methotrexate | |
| Brain imaging to document disruption | |
| Day 4 | Leucovorin rescue |
| Discharge | |
| Day 5 | Granulocyte colony-stimulating factor |
Abbreviations: BBBD, blood-brain barrier disruption; IA, intra-arterial; IV, intravenous.
Between 1982 and 1993, chemotherapy used in combination with methotrexate included etoposide (150 mg/m2 IV days 1 and 2) or cyclophosphamide (15 mg/kg IV days 1 and 2) and procarbazine (100 mg orally days 3 through 16; 44 patients). Between 1994 and 2005, etoposide or etoposide phosphate (150 mg/m2 IV days 1 and 2) and cyclophosphamide (500 mg/m2 IV days 1 and 2) were used (105 patients). Granulocyte colony-stimulating factor was added in 1994. At that time, etoposide or etoposide phosphate replaced oral procarbazine.
Response Evaluation and Follow-Up
Follow-up studies included complete neurologic examinations, CBCs, ophthalmologic examination, and CSF cytopathology. Neuroimaging studies were obtained before each monthly treatment course, after the final treatment course, and thereafter every 3 months for 1 year, every 6 months for 2 years, and then annually. Standard parameters were used to determine treatment response.27 For instance, a complete response (CR) was documented if there was complete resolution of enhancing abnormalities on imaging, no evidence of active ocular lymphoma, negative CSF cytology with absence of leptomeningeal disease-related symptoms, and no corticosteroids.
Standard data collection forms and BBBD chemotherapy records were sent to the BBBD coordinating center (Oregon Health & Science University) for review and database entry. Data were reviewed for correctness by each institution and centrally. Data was collected through December 2005. Protocol adherence was overseen by the IRB, and quality assurance audits began in 1996 with the establishment of a cancer center at the BBBD coordinating center.
Statistical Methods
Demographic, baseline, and treatment characteristics were summarized using descriptive statistics. Complications were summarized as both proportion of patients with each complication, the total number of episodes, and the rate of episodes per number of procedures. OS and PFS were measured from the first BBBD treatment date to date of first relapse (for PFS), death, or last follow-up. OS and PFS were estimated using Kaplan-Meier estimates. Potential predictors for OS and PFS were assessed by fitting stratified univariate analyses and comparing strata using the log-rank test or the generalized Wilcoxon test (the latter test used when visual comparison of the stratified curves was not consistent with the proportional hazards assumption). Potential predictors of OS and PFS were age (< 60 v ≥ 60 years), KPS (≥ 70 v < 70), Memorial Sloan-Kettering Cancer Center (MSKCC) risk classes (class 1, age < 50 years; class 2, age ≥ 50 years and KPS ≥ 70; class 3, age ≥ 50 years and KPS < 70),28 sex, CSF cytology (negative, positive, or atypical/suspicious), whether or not the patient discontinued treatment as a result of an adverse event, whether or not the patient had intraocular lymphoma, and whether or not surgical resection was performed. The MSKCC risk scores are effectively a statistical interaction term (the interaction of age and KPS) that uses age more than and less than 50 years. In our previous report, we used age more than and less than 60 years. Both of these age cut-points were assessed in this report. Multivariable Cox proportional hazards models were also fit using these potential predictors. Variable selection was made using a modified forward-variable selection approach evaluating both changes in the Akaike's information criterion and P values.
RESULTS
Patient Characteristics
One hundred forty-nine patients were treated with BBBD followed by IA methotrexate-based chemotherapy (2,079 procedures). Seventy-four of these patients were included in previous reports.21,22 Patient characteristics are summarized in Table 2. Seventy-eight women and 71 men were included. The mean age (±SD) at diagnosis was 54.5 years (±15.5 years); 47.6% were age 60 years or older. Mean baseline KPS was 69.4 (±20.3). Sixty-one patients had surgical resection as part of their diagnostic regimen, and 27 patients had started chemotherapy at outside centers and then were referred to a BBB consortium facility for treatment within 90 days of diagnosis. Twenty-five patients had positive CSF for lymphoma cells or atypical cells. The number of patients treated at each center ranged from eight to 104.
Table 2.
Patient Demographics and Clinical Characteristics at PCNSL Diagnosis
| Characteristic | No. of Patients | % |
|---|---|---|
| No. of patients | 149 | |
| Sex | ||
| Male | 71 | 47.6 |
| Female | 78 | 52.4 |
| Age, years | ||
| Mean | 54.5 | |
| SD | 15.5 | |
| < 60 | 78 | 52.4 |
| ≥ 60 | 71 | 47.6 |
| KPS | ||
| Mean | 69.4 | |
| SD | 20.3 | |
| ≥ 70 | 86 | 57.7 |
| < 70 | 63 | 42.3 |
| Disease site | ||
| CNS | 131 | 87.9 |
| CNS and intraocular | 15 | 10.1 |
| Intraocular only | 3 | 2.0 |
| CSF cytology | ||
| Negative | 96 | 64.4 |
| Atypical/suspicious | 14 | 9.4 |
| Positive | 11 | 7.4 |
| Unknown | 28 | 18.8 |
| Surgical resection | ||
| No | 88 | 59.1 |
| Yes | 61 | 40.9 |
| Vitrectomy | ||
| No | 138 | 92.6 |
| Yes | 11 | 7.4 |
Abbreviations: PCNSL, primary CNS lymphoma; KPS, Karnofsky performance score; SD, standard deviation.
Outcomes
The 149 patients received a median of 16 IA/BBBD treatments (eight monthly courses). This resulted in clinical and radiographic CR in 86 patients and partial response in 36 patients (overall response rate, 81.9%). Stable disease was documented in 8.1% and progressive disease in 3.4%. Ten patients (6.7%) were not assessable/unknown. Stratified by age, the response rate was 83.3% for patients younger than 60 years and 80.3% for patients ≥ 60 years. Patients with baseline KPS ≥ 70 had a response rate of 82.6% and patients with KPS less than 70 had a response rate of 81.0%.
Fifty-six patients (37.6%) had no evidence of disease progression at last follow-up, whereas 93 patients (62.4%) experienced disease progression. Median PFS was 1.8 years (95% CI, 1.3 to 2.8 years); 5-year PFS was 31% and 7-year PFS was 25% (Fig 1). KPS ≥ 70 (P = .0007, Wilcoxon test) and MSKCC risk class (P < .0001, Wilcoxon test) were significant predictors of PFS. Age younger than 60 years (P = .32, Wilcoxon test), sex (P = .64, Wilcoxon test), CSF cytology (P = .28, Wilcoxon test), discontinuation owing to complications (P = .24, Wilcoxon test), intraocular disease (P = .90, Wilcoxon test), and surgical resection (P = .78, Wilcoxon test) were not significant predictors.
Fig 1.
Progression-free survival (yellow line; median, 1.8 years; 95% CI, 1.3 to 2.8 years) from date of first intra-arterial/blood-brain barrier disruption treatment (149 patients, 93 have experienced disease progression) with 95% CI (blue lines). Symbols on lines indicate censored observations.
Ninety-three patients experienced relapse, with the longest after 9.7 years. The site of first relapse was CNS only (73 patients, 49.0%), CNS and intraocular (nine patients, 6.0%), and intraocular only (two patients, 1.3%). Nine patients (6.0%) experienced relapse systemically.
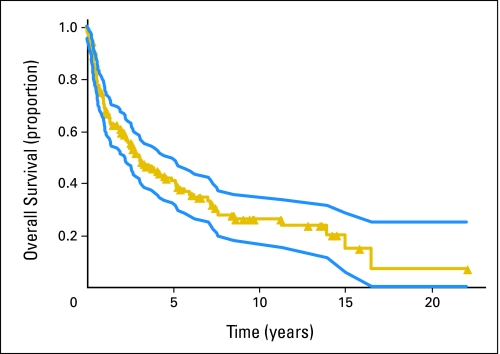
Ninety-six patients (64.4%) have died. Median OS was 3.1 years (95% CI, 2.2 to 5.0 years), with 41% estimated 5-year survival and 25% estimated 8.5-year survival (Fig 2). Age younger than 60 years (P = .0019, log-rank test), KPS ≥ 70 (P < .0001, log-rank), MSKCC risk classes (P < .0001, log-rank test), and sex (P = 0.039, Wilcoxon test) were significant predictors for OS. CSF cytology (P = .93, Wilcoxon test), discontinuation owing to complications (P = .92, Wilcoxon test), intraocular disease (P = .76, Wilcoxon test), and surgical resection (P = .72, Wilcoxon test) were not significant predictors. Patients younger than 60 years (n = 78) had a median OS of 5.2 years and a 5-year survival rate of 52%; patients ≥ 60 years of age had median OS of 2.2 years and a 5-year survival rate of 30% (P < .0019). A plateau in OS was seen at 8.5 years in patients younger than 60 years, suggesting that some of these 13 patients may have achieved a “cure.”
Fig 2.
Overall survival (yellow line; median, 3.1 years; 95% CI, 2.2 to 5.0 years) from date of first intra-arterial/blood-brain barrier disruption treatment (149 patients, 96 deaths) with 95% CI (blue lines). Symbols on lines indicate censored observations.
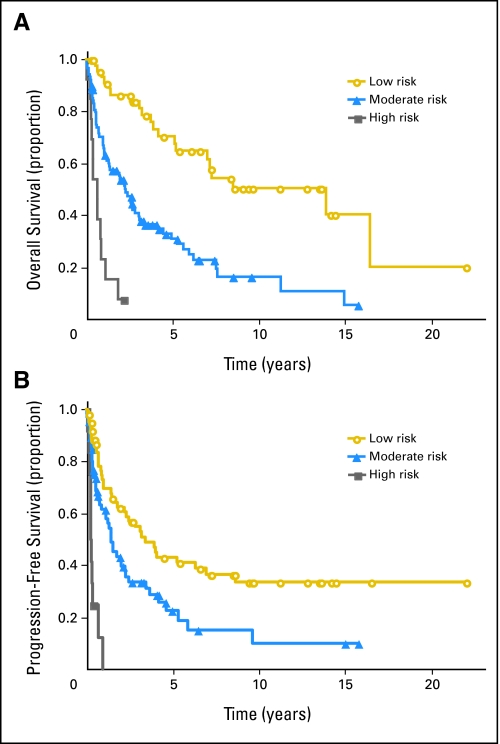
The best predictive model for OS included MSKCC class 3 (age ≥ 50 years and KPS < 70; hazard ratio = 9.08; P < .0001), age ≥ 60 years with KPS less than 70 (hazard ratio = 0.150; P < .0001), and age older than 60 years (hazard ratio = 2.39; P = .0008). To understand these interactions of age with KPS, all combinations of age younger than 50, age 50 to 60, and age ≥ 60 years and KPS less than 70 and ≥ 70 were fit in one model. The difference among these six combinations was highly significant (P < .0001). Visual inspection of the survival curves and improvement in model fit (based on Akaike's information criterion) suggested three risk groups: low risk (age < 60 years and KPS ≥ 70), moderate risk (age < 50 years with KPS < 70 or age ≥ 60 years), and high risk (age 50 to 60 years and KPS < 70; Fig 3A). Estimated hazard ratios were 3.24 (95% CI, 1.92 to 5.47) and 11.63 (95% CI, 5.33 to 25.37) for the moderate- and high-risk groups, respectively. Median OS for these three risk groups were 13.9 years (95% CI, 5.2 years to not reached) for the low risk group, 2.3 years (95% CI, 1.3 to 3.1 years) for the moderate risk group, and 0.6 years (95% CI, 0.3 to 0.9 years) for the high-risk group. Similar analyses for PFS also identified three risk categories (albeit with different groups): low (age 50 to 60 years with KPS ≥ 70 or age < 50 years), moderate (age ≥ 60 years), and high (age 50 to 60 years with KPS < 70; Fig 3B). Estimated hazard ratios were 1.91 (95% CI, 1.19 to 3.06) and 10.01 (95% CI, 4.61 to 21.75) for moderate and high risk, respectively.
Fig 3.
(A) Overall survival according to proposed risk groups. Survival from date of first intra-arterial/blood-brain barrier disruption treatment stratified by age and Karnofsky performance score (KPS). Low risk, age younger than 60 years with KPS ≥ 70 (47 patients); moderate risk, age older than 60 years with any KPS or age younger than 50 years with KPS less than 70 (89 patients); and high risk, age 50 to less than 60 years with KPS less than 70 (13 patients). Symbols on lines indicate censored observations. (B) Progression-free survival according to proposed risk groups. Progression-free survival from date of first intra-arterial/blood-brain barrier disruption treatment stratified by age and KPS. Low risk, age 50 to less than 60 years with KPS greater than 70, or age less than 50 years with any KPS (65 patients); moderate risk, age ≥ 60 years with any KPS (71 patients); and high risk, age 50 to less than 60 years with KPS less than 70 (13 patients). Symbols on lines indicate censored observations.
When separately analyzed, the off-protocol patients (n = 18) had lower KPS and were older than the on-protocol patients (n = 131). The 18 off-protocol patients had higher mean MSKCC risk scores, shorter median OS (1.02 years, 95% CI, 0.37 to 3.75; v 3.24 years, 95% CI, 2.28 to 5.29), shorter median PFS (0.62 years, 95% CI, 0.18 to 1.99; v 2.23 years, 95% CI, 1.43 to 3.45), and a lower rate of CR than the on-protocol patients.
Eighty-seven (90.6%) of the 96 deaths occurred more than 30 days after BBBD/IA. Forty-six patients (47.9%) died as a result of CNS lymphoma progression. Systemic recurrence was the cause of death in nine patients (9.4%). Three patients (3.1%) died as a result of other toxicity such as respiratory complications, five patients (5.2%) died as a result of CNS toxicity, seven patients (7.3%) died as a result of other causes such as cardiac disease, and in 17 patients (17.7%), the cause of death was unknown.
Nine (9.4%) of the 96 deaths occurred less than 30 days after BBBD/IA. One patient died within 48 hours; autopsy showed a pulmonary embolism. Eight patients died 3 to 30 days after BBBD/IA. Four of these patients died of infection, and four patients died from complications related to carotid dissection, myocardial infarction, heart failure, and unknown cause (one each).
Treatment-Related Complications
A total of 697 complications were identified during 2,079 BBBD procedures (33.5%). The complications are summarized in Table 3. The most frequent complication was periprocedural focal seizures occurring in 50 patients (33.6%; 9.2% of procedures). The majority of these occurred during barrier disruption and chemotherapy infusion, were aborted with IV barbiturates, and resulted in no permanent neurologic dysfunction or uncontrolled seizures. Patients who experienced seizures had an increased likelihood to seize during subsequent procedures (191 seizures occurred in 50 patients; median of three seizures per patient). The overall rate of procedural morbidity is 327 events (15.7% of procedures). However, if focal seizures are excluded,29 there were 136 (6.5%) of 2,079 events. Clinical strokes occurred in 11 patients (7.4%); four patients were left with permanent neurologic deficits. The estimated risk of permanent neurologic deficit is 0.2% per IA/BBBD procedure.
Table 3.
Complications Summary
| Event | No. of Patients | % | No. of Episodes | Incidence (% of BBBD procedures)* |
|---|---|---|---|---|
| Total | 149 | 697 | 33.5 | |
| BBBD procedure–related | ||||
| Periprocedural seizure† | 50 | 33.6 | 191 | 9.2 |
| Temporary neurologic deficit | 27 | 18.1 | 39 | 1.9 |
| Ophthalmologic | 25 | 16.8 | 25 | 1.2 |
| Carotid or vertebral artery injury‡ | 16 | 10.7 | 22 | 1.1 |
| Obtundation > 24 hours§ | 15 | 10.1 | 22 | 1.1 |
| Periprocedural dysrhythmia requiring intervention | 12 | 8.0 | 25 | 1.2 |
| Clinical stroke‖ | 11 | 7.4 | 16 | 0.8 |
| Permanent neurologic deficit | 4 | 2.7 | 4 | 0.2 |
| Urethral tear with bleeding | 2 | 1.3 | 2 | 0.1 |
| Pneumothorax | 2 | 1.3 | 2 | 0.1 |
| Respiratory arrest | 1 | 0.7 | 1 | 0.0 |
| Femoral arterial thrombosis | 1 | 0.7 | 2 | 0.1 |
| Death within 48 hours | 1 | 0.7 | 1 | 0.0 |
| Chemotherapy/malignancy-related | ||||
| RBC transfusion required | 43 | 28.9 | 75 | 3.6 |
| Granulocytopenic fever | 40 | 26.8 | 59 | 2.8 |
| Deep vein thrombosis | 34 | 22.8 | 43 | 2.1 |
| Septicemia with neutropenia | 33 | 22.2 | 42 | 2.0 |
| Septicemia, nongranulocytopenic | 31 | 20.8 | 41 | 2.0 |
| Pneumonia | 27 | 18.1 | 32 | 1.5 |
| Pulmonary embolus | 11 | 7.4 | 11 | 0.5 |
| Platelet transfusion required | 10 | 6.7 | 13 | 0.6 |
| Delayed orthopedic | 10 | 6.7 | 10 | 0.5 |
| Death within 30 days | 8 | 5.4 | 8 | 0.4 |
| Gastrointestinal bleeding | 7 | 4.7 | 8 | 0.4 |
| Dementia | 2 | 1.3 | 2 | 0.1 |
| Acute myelogenous leukemia | 1 | 0.7 | 1 | 0.0 |
Abbreviation: BBBD, blood-brain barrier disruption.
Total number of BBBD procedures is 2,079.
Primarily focal motor seizures without clinical sequelae (text for details).
The most common arterial injury that occurs during BBBD is a subintimal tear. This complication is usually asymptomatic and is noticed during fluoroscopy of the carotid and vertebral arteries.
Approximately 10% of patients have decreased level of consciousness after BBBD. When this complication occurs, the patients are treated with dexamethasone and usually return to baseline neurologic status within 48 hours.
Eleven patients had a clinical stroke. Four of these patients (2.7%) had a residual permanent neurologic deficit.
Chemotherapy toxicity and underlying malignant condition-related complications accounted for 370 events (17.8%). Hematologic abnormalities were the most common presentation: RBC transfusions (3.6% of procedures) and granulocytopenic fever (2.8%). These patients had a hypercoagulable tendency with a 2.6% incidence of deep vein thrombosis or pulmonary embolism periprocedurally. Twenty-four patients (16.1%) discontinued the 12-month regimen because of complications; 11 patients discontinued treatment because of secondary infections.
DISCUSSION
Data from three multicenter PCNSL trials that used chemotherapy (high-dose IV methotrexate) alone (New Approaches to Brain Tumor Therapy,30 German Cancer Society Neuro-Oncology Working Group (NOA) 03,31 and European Organisation for Research and Treatment of Cancer [EORTC] 26952,32) are summarized in Table 4. Our results with IA methotrexate/BBBD demonstrated comparable if not superior outcomes to high-dose IV methotrexate used in the other three multicenter trials.
Table 4.
Multicenter Studies of Newly Diagnosed PCNSL
| Study | No. of Patients | RR (CR + PR; %) | PFS (months) |
OS (months) |
||
|---|---|---|---|---|---|---|
| Median | 95% CI | Median | 95% CI | |||
| NABTT 96-07,30 high-dose methotrexate | 25 | 74 | 12.8 | Not reached (> 23) | ||
| NOA-03,31 high-dose methotrexate | 37 | 35 | 10 | 25 | ||
| EORTC 26952,32 high-dose methotrexate | 50 (> 60 years old) | 48 | 6.8 | 3.4 to 10.6 | 14.3 | 6.2 to 42 |
| RTOG 93-10,33 high-dose methotrexate plus WBRT | 102 | 94 | 24 | 36.9 | ||
| EORTC 20962,34 high-dose methotrexate plus WBRT | 52 (< 65 years old) | 81 | — | 46 | ||
| BBBD consortium, current study | ||||||
| Total patients | 149 | 81.9 | 21.3 | 15.1 to 34.0 | 37.1 | 27.0 to 59.5 |
| Age < 60 years | 78 | 83.3 | 29.8 | 12.2 to 49.1 | 61.9 | 34.2 to 167.3 |
| Age ≥ 60 years | 71 | 80.3 | 17.6 | 10.6 to 27.6 | 26.6 | 14.5 to 37.1 |
Abbreviations: PCNSL, primary CNS lymphoma; RR, response rate; CR, complete response; PR, partial response; PFS, progression-free survival; OS, overall survival; NABTT, New Approaches to Brain Tumor Therapy; NOA, German Cancer Society Neuro-Oncology Working Group; EORTC, European Organisation for Research and Treatment of Cancer; RTOG, Radiation Therapy Oncology Group; WBRT, whole-brain radiation; BBBD, blood-brain barrier disruption.
Similarly, the results of Radiation Therapy Oncology Group 93-1033 and EORTC 2096234 are summarized in Table 4. Both of these trials used combination chemotherapy and brain irradiation. IA methotrexate-based BBBD achieved similar results without the neurocognitive sequelae associated with brain irradiation.35 Caution must be exercised, as definitive conclusions cannot be drawn when comparing our results with those of other multicenter nonrandomized clinical trials of newly diagnosed patients with PCNSL. Nonetheless, we feel that summarizing results obtained from the other multicenter studies, as we have done in Table 4, may be of interest to clinicians. Given the rarity of PCNSL and the limited number of institutions currently trained in BBBD, a randomized PCNSL trial using BBBD as one arm is currently not possible.
Cognitive outcomes were not available for all patients in our series; however, a subset of these patients have been evaluated as part of other published series.11,21,22,35–39 Neuropsychologic assessments include a comprehensive test battery conducted by neuropsychologists. Results revealed cognitive improvement or preservation in the majority of patients relative to pretreatment status at follow-up, between 1 to 7 years after achieving CR.11,21,22,35–39 Further evaluation of neurocognitive outcomes in long-term survivors is underway (L. Maron, personal communication, July 2008).
Toxicity in BBBD-treated patients is generally manageable. However, the treatment delivery regimen is complex and should be undertaken only by trained teams at centers where neuro-oncology, interventional neurosurgery/neuroradiology, neuroanesthesia, and experienced oncology nursing are available.
Potential predictors of OS and PFS are combinations of age and KPS. Other potential predictors were not statistically significant when added to these models, and the hazard ratios for the moderate and high-risk groups were not changed when these other potential predictors were added. We also assessed MSKCC risk classes, but not the International Extranodal Lymphoma Study Group score (serum lactate dehydrogenase and CSF protein were not regularly available).40 Comparisons of multivariable models suggested that, in this case series, neither MSKCC risk categories nor age younger than 60 or ≥ 60 years and KPS less than 70 or ≥ 70 were adequate predictors. Six age-KPS combinations were fit and then grouped into three risk categories. The high-risk group (age 50 to 60 years and KPS < 70) was the likely reason for this interaction. This high-risk group included only 13 patients and may reflect random, small subgroup variation (albeit a group with markedly poor OS and PFS). Many of these 13 patients had numerous premorbid conditions or concomitant complications unrelated to treatment (eg, one patient died secondary to spinal cord injury complications after a fall). Drawing any specific conclusions about this small subset of patients is risky and may be unnecessary. These risk groups are not intended to represent general risk categories (such as MSKCC or International Extranodal Lymphoma Study Group) but merely describe outcomes in this series.
Another potential confounder in this series is the small number of patients treated off-protocol at participating institutions. If we restrict the series to only on-protocol patients, the median OS increases slightly from the full series (from 3.1 to 3.2 years) and the median PFS increases (from 1.8 to 2.2 years). Even though the inclusion of off-protocol patients negatively impacts the efficacy reported in this series, we feel that their inclusion is important and more accurately reflects the overall therapeutic results using BBBD in patients with PCNSL.
Currently, systemic diffuse large B-cell lymphoma is treated with chemotherapy in conjunction with rituximab; however, this monoclonal antibody does not routinely cross the BBB and reach malignant lymphoma cells in the CNS.41–44 A reasonable approach might be to use a technique such as osmotic BBBD to deliver effective monoclonal antibody/chemotherapy or other novel agents.
In conclusion, we report response rates and OS from a large, multicenter series of newly diagnosed patients with PCNSL treated with IA methotrexate-based chemotherapy with osmotic BBBD, which confirm our previously reported single-center results.21,22 This treatment option resulted in durable tumor control, manageable toxicity, and the potential for deferral of radiation and its associated cognitive compromise.11,22,45 BBBD results in enhanced delivery not simply to areas with overtly leaky BBB associated with enhancing tumor, but also to brain and CSF globally by as much as 50- to 100- fold.46 The long patient follow-up suggests this is an effective first-line treatment option with a meaningful impact on OS and PFS, as well as neurocognitive status.35
Footnotes
Supported by a Veterans Administration Merit Review Grant and National Institutes of Health Grants No. CA137488, NS34608, and NS44687 from the National Cancer Institute and the National Institute of Neurological Disorders and Stroke to E.A.N.
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 3, 2007, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Lilyana Angelov, Nancy D. Doolittle, Dale F. Kraemer, Tali Siegal, Gene H. Barnett, David M. Peereboom, Glen Stevens, John McGregor, Kristoph Jahnke, Susan Bell, Edward A. Neuwelt
Financial support: Edward A. Neuwelt
Administrative support: Lilyana Angelov, Edward A. Neuwelt
Provision of study materials or patients: Lilyana Angelov, Tali Siegal, Gene H. Barnett, David M. Peereboom, Glen Stevens, John McGregor, Kristoph Jahnke, Cynthia A. Lacy, Edna Shalom, Sandra Ference, Susan Bell, Lisa Sorenson, Rose Marie Tyson, Marianne Haluska, Edward A. Neuwelt
Collection and assembly of data: Lilyana Angelov, Nancy D. Doolittle, Kristoph Jahnke, Cynthia A. Lacy, Nancy A. Hedrick, Edna Shalom, Sandra Ference, Susan Bell, Lisa Sorenson, Rose Marie Tyson, Marianne Haluska
Data analysis and interpretation: Lilyana Angelov, Nancy D. Doolittle, Dale F. Kraemer, Cynthia A. Lacy, Edward A. Neuwelt
Manuscript writing: Lilyana Angelov, Nancy D. Doolittle, Dale F. Kraemer, Edward A. Neuwelt
Final approval of manuscript: Lilyana Angelov, Nancy D. Doolittle, Dale F. Kraemer, Tali Siegal, Gene H. Barnett, David M. Peereboom, Glen Stevens, John McGregor, Kristoph Jahnke, Cynthia A. Lacy, Nancy A. Hedrick, Edna Shalom, Sandra Ference, Susan Bell, Lisa Sorenson, Rose Marie Tyson, Marianne Haluska, Edward A. Neuwelt
REFERENCES
- 1.Panageas KS, Elkin EB, DeAngelis LM, et al. Trends in survival from primary central nervous system lymphoma, 1975-1999: A population-based analysis. Cancer. 2005;104:2466–2472. doi: 10.1002/cncr.21481. [DOI] [PubMed] [Google Scholar]
- 2.Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: A reality check. J Clin Oncol. 2007;25:2295–2305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 3.Neuwelt E, Abbott NJ, Abrey L, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- 4.Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16:859–863. doi: 10.1200/JCO.1998.16.3.859. [DOI] [PubMed] [Google Scholar]
- 5.Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: The next step. J Clin Oncol. 2000;18:3144–3150. doi: 10.1200/JCO.2000.18.17.3144. [DOI] [PubMed] [Google Scholar]
- 6.DeAngelis LM, Yahalom J, Thaler HT, et al. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992;10:635–643. doi: 10.1200/JCO.1992.10.4.635. [DOI] [PubMed] [Google Scholar]
- 7.Glass J, Gruber ML, Cher L, et al. Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: Long-term outcome. J Neurosurg. 1994;81:188–195. doi: 10.3171/jns.1994.81.2.0188. [DOI] [PubMed] [Google Scholar]
- 8.Ferreri AJ, Abrey LE, Blay JY, et al. Summary statement on primary central nervous system lymphomas from the Eighth International Conference on Malignant Lymphoma, Lugano, Switzerland, June 12 to 15, 2002. J Clin Oncol. 2003;21:2407–2414. doi: 10.1200/JCO.2003.01.135. [DOI] [PubMed] [Google Scholar]
- 9.Blay JY, Conroy T, Chevreau C, et al. High-dose methotrexate for the treatment of primary cerebral lymphomas: Analysis of survival and late neurologic toxicity in a retrospective series. J Clin Oncol. 1998;16:864–871. doi: 10.1200/JCO.1998.16.3.864. [DOI] [PubMed] [Google Scholar]
- 10.Correa DD, DeAngelis LM, Shi W, et al. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62:548–555. doi: 10.1212/01.wnl.0000109673.75316.d8. [DOI] [PubMed] [Google Scholar]
- 11.Dahlborg SA, Henner WD, Crossen JR, et al. Non-AIDS primary CNS lymphoma: First example of a durable response in a primary brain tumor using enhanced chemotherapy delivery without cognitive loss and without radiotherapy. Cancer J Sci Am. 1996;2:166–174. [PubMed] [Google Scholar]
- 12.Harder H, Holtel H, Bromberg JE, et al. Cognitive status and quality of life after treatment for primary CNS lymphoma. Neurology. 2004;62:544–547. doi: 10.1212/wnl.62.4.544. [DOI] [PubMed] [Google Scholar]
- 13.Lai R, Abrey LE, Rosenblum MK, et al. Treatment-induced leukoencephalopathy in primary CNS lymphoma: A clinical and autopsy study. Neurology. 2004;62:451–456. doi: 10.1212/01.wnl.0000106941.51340.a2. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien P, Roos D, Pratt G, et al. Phase II multicenter study of brief single-agent methotrexate followed by irradiation in primary CNS lymphoma. J Clin Oncol. 2000;18:519–526. doi: 10.1200/JCO.2000.18.3.519. [DOI] [PubMed] [Google Scholar]
- 15.Omuro AM, Ben-Porat LS, Panageas KS, et al. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol. 2005;62:1595–1600. doi: 10.1001/archneur.62.10.1595. [DOI] [PubMed] [Google Scholar]
- 16.Omuro AM, DeAngelis LM, Yahalom J, et al. Chemoradiotherapy for primary CNS lymphoma: An intent-to-treat analysis with complete follow-up. Neurology. 2005;64:69–74. doi: 10.1212/01.WNL.0000148641.98241.5E. [DOI] [PubMed] [Google Scholar]
- 17.Pels H, Schmidt-Wolf IG, Glasmacher A, et al. Primary central nervous system lymphoma: Results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21:4489–4495. doi: 10.1200/JCO.2003.04.056. [DOI] [PubMed] [Google Scholar]
- 18.Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25:4730–4735. doi: 10.1200/JCO.2007.12.5062. [DOI] [PubMed] [Google Scholar]
- 19.Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol. 2006;24:1281–1288. doi: 10.1200/JCO.2005.04.8819. [DOI] [PubMed] [Google Scholar]
- 20.Gavrilovic IT, Hormigo A, Yahalom J, et al. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24:4570–4574. doi: 10.1200/JCO.2006.06.6910. [DOI] [PubMed] [Google Scholar]
- 21.McAllister LD, Doolittle ND, Guastadisegni PE, et al. Cognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphoma. Neurosurgery. 2000;46:51–60. discussion 60-61. [PubMed] [Google Scholar]
- 22.Neuwelt EA, Goldman DL, Dahlborg SA, et al. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: Prolonged survival and preservation of cognitive function. J Clin Oncol. 1991;9:1580–1590. doi: 10.1200/JCO.1991.9.9.1580. [DOI] [PubMed] [Google Scholar]
- 23.Doolittle ND, Miner ME, Hall WA, et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 2000;88:637–647. doi: 10.1002/(sici)1097-0142(20000201)88:3<637::aid-cncr22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.Jahnke K, Doolittle ND, Muldoon LL, et al. Implications of the blood-brain barrier in primary central nervous system lymphoma. Neurosurg Focus. 2006;21:E11. doi: 10.3171/foc.2006.21.5.12. [DOI] [PubMed] [Google Scholar]
- 25.Smith JR, Rosenbaum JT, Wilson DJ, et al. Role of intravitreal methotrexate in the management of primary central nervous system lymphoma with ocular involvement. Ophthalmology. 2002;109:1709–1716. doi: 10.1016/s0161-6420(02)01125-9. [DOI] [PubMed] [Google Scholar]
- 26.Cohen IJ. Defining the appropriate dosage of folinic acid after high-dose methotrexate for childhood acute lymphatic leukemia that will prevent neurotoxicity without rescuing malignant cells in the central nervous system. J Pediatr Hematol Oncol. 2004;26:156–163. doi: 10.1097/00043426-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 28.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 29.Marchi N, Angelov L, Masaryk T, et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batchelor T, Carson K, O'Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: A report of NABTT 96-07. J Clin Oncol. 2003;21:1044–1049. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 31.Herrlinger U, Schabet M, Brugger W, et al. German Cancer Society Neuro-Oncology Working Group NOA-03 multicenter trial of single-agent high-dose methotrexate for primary central nervous system lymphoma. Ann Neurol. 2002;51:247–252. doi: 10.1002/ana.10102. [DOI] [PubMed] [Google Scholar]
- 32.Hoang-Xuan K, Taillandier L, Chinot O, et al. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: A multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol. 2003;21:2726–2731. doi: 10.1200/JCO.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 33.DeAngelis LM, Seiferheld W, Schold SC, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003;21:4483–4488. doi: 10.1200/JCO.2003.03.108. [DOI] [PubMed] [Google Scholar]
- 35.Correa DD, Maron L, Harder H, et al. Cognitive functions in primary central nervous system lymphoma: Literature review and assessment guidelines. Ann Oncol. 2007;18:1145–1151. doi: 10.1093/annonc/mdl464. [DOI] [PubMed] [Google Scholar]
- 36.Crossen JR, Garwood D, Glatstein E, et al. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 37.Crossen JR, Goldman DL, Dahlborg SA, et al. Neuropsychological assessment outcomes of nonacquired immunodeficiency syndrome patients with primary central nervous system lymphoma before and after blood-brain barrier disruption chemotherapy. Neurosurgery. 1992;30:23–29. doi: 10.1227/00006123-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Neuwelt EA, Guastadisegni PE, Varallyay P, et al. Imaging changes and cognitive outcome in primary CNS lymphoma after enhanced chemotherapy delivery. AJNR Am J Neuroradiol. 2005;26:258–265. [PMC free article] [PubMed] [Google Scholar]
- 39.Roman-Goldstein S, Mitchell P, Crossen JR, et al. MR and cognitive testing of patients undergoing osmotic blood-brain barrier disruption with intraarterial chemotherapy. AJNR Am J Neuroradiol. 1995;16:543–553. [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 41.Rubenstein JL, Combs D, Rosenberg J, et al. Rituximab therapy for CNS lymphomas: Targeting the leptomeningeal compartment. Blood. 2003;101:466–468. doi: 10.1182/blood-2002-06-1636. [DOI] [PubMed] [Google Scholar]
- 42.Feugier P, Virion JM, Tilly H, et al. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: Influence of rituximab. Ann Oncol. 2004;15:129–133. doi: 10.1093/annonc/mdh013. [DOI] [PubMed] [Google Scholar]
- 43.Doolittle ND, Abrey LE, Shenkier TN, et al. Brain parenchyma involvement as isolated central nervous system relapse of systemic non-Hodgkin lymphoma: An International Primary CNS Lymphoma Collaborative Group report. Blood. 2008;111:1085–1093. doi: 10.1182/blood-2007-07-101402. [DOI] [PubMed] [Google Scholar]
- 44.Doolittle ND, Jahnke K, Belanger R, et al. Potential of chemo-immunotherapy and radioimmunotherapy in relapsed primary central nervous system (CNS) lymphoma. Leuk Lymphoma. 2007;48:1712–1720. doi: 10.1080/10428190701493902. [DOI] [PubMed] [Google Scholar]
- 45.Tyson RM, Siegal T, Doolittle ND, et al. Current status and future of relapsed primary central nervous system lymphoma (PCNSL) Leuk Lymphoma. 2003;44:627–633. doi: 10.1080/1042819021000055057. [DOI] [PubMed] [Google Scholar]
- 46.Neuwelt EA, Diehl JT, Vu LH, et al. Monitoring of methotrexate delivery in patients with malignant brain tumors after osmotic blood-brain barrier disruption. Ann Intern Med. 1981;94:449–454. doi: 10.7326/0003-4819-94-4-449. [DOI] [PubMed] [Google Scholar]