Abstract
In the seminiferous epithelium, Eps8 is localized to actin-based cell junctions at the blood-testis barrier (BTB) and the apical ectoplasmic specialization (ES) in stage V–VI tubules but is considerably diminished in stage VIII tubules. Eps8 down-regulation coincides with the time of BTB restructuring and apical ES disassembly, implicating the role of Eps8 in cell adhesion. Its involvement in Sertoli-germ cell adhesion was substantiated in studies using an in vivo animal model by treating rats with 1-(2,4-dichlorobenzy)-1H-indazole-3-carbohydrazide (adjudin) to induce anchoring junction restructuring, during which Eps8 disappeared at the apical ES before germ cell departure. In Sertoli cell cultures with established permeability barrier mimicking the BTB in vivo, the knockdown of Eps8 by RNAi led to F-actin disorganization and the mislocalization of the tight junction proteins occludin and ZO-1, suggesting the function of Eps8 in maintaining BTB integrity. In vivo knockdown of Eps8 in the testis caused germ cell sloughing and BTB damage, concomitant with occludin mislocalization, further validating that Eps8 is a novel regulator of cell adhesion and BTB integrity in the seminiferous epithelium.—Lie, P. P. Y., Mruk, D. D., Lee, W. M., Cheng, C. Y. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium.
Keywords: spermatogenesis, Sertoli cells, germ cells, actin, actin dynamics
During spermatogenesis, extensive restructuring takes place at the Sertoli-Sertoli and the Sertoli-germ cell interfaces in the seminiferous epithelium. For instance, the blood-testis barrier (BTB) undergoes extensive restructuring at stage VIII of the seminiferous epithelial cycle in adult rat testes to facilitate the transit of primary preleptotene spermatocytes across the BTB (1, 2). At the same time, fully developed spermatids (i.e., spermatozoa) “detach” from the epithelium during spermiation to enter the tubule lumen and are transported to the epididymis for maturation (3, 4). These events are intimately related to intercellular junctions, which are structurally linked to the underlying actin-based cytoskeleton. Thus, a better understanding on actin dynamics is critical to decipher how these events, namely, BTB restructuring and spermiation, are coordinated and regulated.
Actin is ubiquitously expressed and is the most abundant protein in all eukaryotic cells, including Sertoli cells and developing germ cells in the seminiferous epithelium. Besides its role as a cytoskeletal element to confer cell shape, it is involved in multiple cellular processes, including cell movement, cell polarity, intracellular trafficking, cell cycle events (e.g., cytokinesis), and cell-cell and cell-matrix adhesion (5,6,7,8). In response to external stimuli, actin can rapidly reorganize via numerous processes, such as nucleation, polymerization, capping, severing, bundling, and cross-linking. To achieve this, more than 60 classes of actin-binding proteins exert different effects (9). Epidermal growth factor receptor pathway substrate 8 (Eps8), a 97-kDa protein, is an important actin dynamics regulator. For instance, Eps8 controls actin-based motility by capping the barbed ends of actin filaments (10, 11). Eps8 also regulates actin bundling and Rac-mediated actin remodeling by activating Rac GTPase (12,13,14).
In the seminiferous epithelium, filamentous actin (F-actin) is mostly concentrated at the testis-specific atypical adherens junction type known as ectoplasmic specialization (ES) (15, 16). The ES is present at the inter-Sertoli cell interface at the BTB (basal ES; the BTB is constituted by coexisting basal ES, tight junction, and desmosome-like junction at the Sertoli-Sertoli cell interface), as well as at the Sertoli cell-elongating spermatid interface (apical ES), but only between Sertoli cells and more advanced spermatids (from steps 8–19 elongating spermatids) (17,18,19). On the ultrastructural level, the ES is typified by the presence of actin filament bundles sandwiched between the cisternae of the endoplasmic reticulum and the Sertoli cell plasma membrane. However, this structure is found only at the Sertoli cell side at the apical ES (19). Once the apical ES appears, it serves as the only anchoring device at the Sertoli cell-elongating spermatid interface. Developing spermatids rely on the apical ES for their proper orientation (i.e., cell polarity) and their timely migration from the basal to the adluminal edge of the seminiferous epithelium until spermiation (20). As such, it is envisioned that actin dynamics are not only crucial for Sertoli-germ cell adhesion at the apical ES, but also the maintenance of basal ES integrity and its timely restructuring. We thus examined the functional significance of Eps8 in regulating cell adhesion at the apical and the basal ES via its effects on actin dynamics. These findings led us to unveil some unexpected turns of events regarding the function of Eps8 in the testis.
MATERIALS AND METHODS
Animals and antibodies
Sprague-Dawley rats were purchased from Charles River Laboratories (Kingston, NY, USA). The use of animals for the studies reported herein was approved by the Rockefeller University Laboratory Animal Use and Care Committee (protocol number 06018). Antibodies used in this study are listed in Table 1.
TABLE 1.
Antibodies, their sources, applications, and working dilution, used for different experiments in this report
| Antibody | Vendor | Catalog no. | Application | Working dilution for IB |
|---|---|---|---|---|
| Mouse anti-Eps8 | BD Biosciences (San Jose, CA, USA) | 610143 | IB, IHC, IF | 1:5000 |
| Mouse anti-Sos1 | BD Biosciences | 610095 | IB | 1:250 |
| Rabbit anti-occludin | Invitrogen (Carlsbad, CA, USA) | 71-1500 | IB, IF | 1:125 |
| Rabbit anti-JAM-A | Invitrogen | 36-1700 | IB | 1:125 |
| Rabbit anti-ZO-1 | Invitrogen | 61-7300 | IB, IF | 1:125 |
| Rabbit anti-N-cadherin | Santa Cruz Biotechnology (Santa Cruz, CA, USA) | sc-7939 | IB | 1:200 |
| Rabbit anti-α-catenin | Santa Cruz Biotechnology | sc-7894 | IB | 1:200 |
| Rabbit anti-β-catenin | Invitrogen | 71-2700 | IB | 1:125 |
| Mouse anti-espin | BD Biosciences | E17520 | IB | 1:250 |
| Mouse anti-vinculin | Sigma-Aldrich (St. Louis, MO, USA) | V9131 | IB | 1:200 |
| Rabbit anti-FAK | Santa Cruz Biotechnology | sc-558 | IB | 1:200 |
| Rabbit anti-p-FAK-Tyr397 | Invitrogen | 44-625G | IB | 1:1000 |
| Mouse anti-c-Src | Santa Cruz Biotechnology | sc-8056 | IB | 1:200 |
| Mouse anti-p-Src-Tyr416 | Millipore (Billerica, MA, USA) | 05-677 | IB | 1:1000 |
| Goat anti-actin | Santa Cruz Biotechnology | sc-1616 | IB | 1:300 |
| Mouse anti-Abi-1a | Medical & Biological Laboratories (Nagoya, Japan) | D147-3 | N/A | N/A |
| Mouse anti-SSH3BP1a | Abcam (Cambridge, MA, USA) | ab11222 | N/A | N/A |
| Rabbit anti-IRSp53a | Upstate (Temecula, CA, USA) | 07-786 | N/A | N/A |
Antibodies used for various experiments as reported herein are listed. Note that these antibodies cross-reacted with the corresponding rat proteins as noted by the manufacturers if the source of antigens used to raise the antibodies was not from rats. IB, immunoblotting; IHC, immunohistochemistry; IF, immunofluorescence; N/A, not applicable. For IHC and IF as specified, antibodies were used at a working dilution of 1:100.
Antibodies against the functional binding partners of Eps8 were tested in our pilot experiments by immunoblot analysis. However, these antibodies cross-reacted with multiple nonspecific bands in immunoblots using rat testicular cell lysates (e.g., Sertoli and germ cells); thus, they could not be used for subsequent experiments.
Testicular cell isolation and primary cell cultures
Primary Sertoli cells were isolated from the testes of 20-day-old rats as described previously (21). It is noted that Sertoli cells isolated from rats at 20 days of age are fully differentiated and cease to divide (22). Sertoli cells were plated on Matrigel-coated dishes or coverslips at 0.025–0.5 × 106 cells/cm2 and cultured in serum-free F12/DMEM (Sigma-Aldrich, St. Louis, MO, USA) supplemented with growth factors, bacitracin, and gentamicin at 35°C in a humidified atmosphere with 95% air/5% CO2 (v/v) (21) at time 0. About 48 h thereafter, Sertoli cells were subjected to hypotonic treatment to lyse residual germ cells, as described previously (23), so that these cultures had a Sertoli cell purity >98% and were contaminated with negligible Leydig and germ cells (24). Total germ cells were isolated from testes of adult rats [∼300 g body weight (BW)] by a mechanical procedure without using trypsin and were not subjected to the glass-wool filtration step so that elongating/elongated spermatids were retained in the germ cell population (25). As such, the relative percentage of spermatogonia (2C, diploid), preleptotene spermatocytes (S-phase), primary spermatocytes (4C, tetraploid), and round/elongating/elongated spermatids (1C) was similar to the normal intratubular range when assessed by flow cytometry as described previously (25, 26). Total germ cells were plated on 100-mm dishes at 2.5 × 106 cells/ml and cultured in F12/DMEM supplemented with 2 mM sodium pyruvate and 6 mM sodium DL-lactate as described previously (25). Germ cells were harvested for lysate preparation after ∼16 h in culture at 35°C with a viability of greater than 95% by the erythrosine red dye exclusion test, as described previously (26).
Treatment of adult rats with 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide (adjudin)
A single dose of adjudin (50 mg/kg BW) was administered to adult rats of ∼300 g BW via gavage, as described previously (27). Testes were obtained from rats terminated at specified time points after treatment for lysate preparation or sectioning. This is an established in vivo model to study anchoring junction dynamics at the Sertoli-germ cell interface in the seminiferous epithelium (28).
Eps8 silencing in primary cultured Sertoli cells in vitro
Primary Sertoli cells were isolated from the testes of 20-day-old male Sprague-Dawley rats as described previously (21, 29). For lysate preparation, cells were plated at 0.5 × 106 cells/cm2 on Matrigel (BD Biosciences, San Jose, CA, USA; diluted 1:7 with F12/DMEM, v/v)-coated 12-well culture plates. Cells were cultured for 3 d in F12/DMEM supplemented with growth factors to allow the formation of a functional tight junction (TJ)-permeability barrier with tight and anchoring junctions (e.g., basal ES) when examined by electron microscopy (30). Thereafter, cells were transfected with 100 nM nontargeting control siRNA duplexes (catalog no. D-001810-10; Dharmacon, Lafayette, CO, USA) or Eps8-specific siRNA duplexes (catalog no. J-095844-10; Dharmacon) with Ribojuice siRNA transfection reagent (Novagen, Madison, WI, USA) according to the manufacturer’s instructions. Three days after transfection, cells were terminated for lysate preparation using IP lysis buffer (see below). In RNAi experiments to be used for F-actin and immunofluorescent staining, cells were plated at 0.03–0.05 × 106 cells/cm2 on Matrigel-coated coverslips. Three days thereafter, cells were cotransfected with 60 nM siRNA duplexes (control vs. Eps8; see above) and 1 nM siGLO red transfection indicator (catalog no. D-001630-02; Dharmacon). Cells were subjected to F-actin or immunofluorescent staining 3 d after transfection.
Eps8 silencing in adult rat testes in vivo
Adult male Sprague-Dawley rats (∼300 g BW) were treated with control and Eps8 RNAi transfection mix via intratesticular injection using a 28-gauge needle, as described previously (31, 32). Each transfection mix (final volume ∼200 μl) consisted of 100 nM siRNA duplexes and 7.5 μl Ribojuice siRNA transfection reagent suspended in 190 μl Opti-MEM (Invitrogen, Carlsbad, CA, USA). The volume of each testis was assumed to be ∼1.6 ml. Different pilot experiments were performed to assess the optimal ratio of Opti-MEM to Ribojuice. Control and Eps8 RNAi transfection mix were administered to a different testis in the same rat, so that each animal consisted of a treated and a control testis. Three days after treatment, rats were either terminated for histological analysis using either frozen or paraffin sections (n=5 rats), or used for the in vivo BTB integrity assay (n=4 rats).
Immunohistochemistry, immunofluorescent staining, and F-actin staining
Frozen testis sections (∼8 μm obtained in a cryostat at −20°C) or primary cultured Sertoli cells were fixed with 4% paraformaldehyde (w/v) in PBS for 10 min at room temperature and permeabilized with 0.1% triton X-100 (v/v) in PBS for 4 min at room temperature. Immunohistochemistry was carried out with a mouse-to-mouse detection system AEC substrate (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. For immunofluorescent staining, sections or cells were blocked with 1% BSA (w/v) in PBS, to be followed by an overnight incubation of primary antibodies at 1:100 dilution in PBS, and then a 30 min incubation of Alexa Fluor conjugated (green fluorescence, Alexa Fluor 488; red fluorescence, Alexa Fluor 555) goat secondary antibodies (Invitrogen) at 1:100 to 1:300 dilution in PBS. F-actin was stained with rhodamine (Invitrogen) or FITC (Sigma-Aldrich)-conjugated phalloidin. Sections or cells were mounted in ProLong Gold antifade reagent with DAPI (Invitrogen). To eliminate interexperimental variations, all cross sections of testes within a treatment group (such as after adjudin treatment or following transfection of testes with specific siRNA duplexes vs. nontargeting siRNA duplexes) were mounted onto the same microscope slides and processed simultaneously. All staining experiments were repeated at least 3 times using different animals and/or batches of Sertoli cells.
Hematoxylin and eosin staining
Paraffin sections (6–8 μm) were obtained from Bouin’s fixed testes embedded in paraffin. After dewaxing with xylene, sections were stained with Hematoxylin 7211 for 3 min, to be followed by 1-min incubations of Clarifier 1 and Bluing Reagent, and then stained with Eosin-Y for 30 s (reagents from Richard-Allan Scientific, Richland, MI, USA). Hematoxylin and eosin staining experiments were performed in 2 independent experiments, and the results were analyzed in conjunction with 3 sets of frozen testis sections stained with DAPI in separate experiments to quantify the percentage of tubules displaying germ cell loss from the seminiferous epithelium.
BTB integrity assay
Each rat was under anesthesia with ketamine HCl (60 mg/kg BW) with xylazine (10 mg/kg BW) administered i.m. Thereafter, a small incision on the skin over the jugular vein was made, and the vein was carefully exposed. About 1.5 mg FITC-conjugated inulin (Mr 4.6 kDa)(Sigma-Aldrich) in 300 μl PBS was administered into the jugular vein using a 28-gauge needle. About 30 min thereafter, rats were euthanized by CO2 asphyxiation. The integrity of the BTB was assessed by its ability to block inulin-FITC from entering the adluminal compartment, as visualized by fluorescent microscopy in frozen testis sections. Damaged tubules were defined herein by the penetration of green fluorescence across at least 3 layers of cells behind the BTB toward the tubule lumen. A BTB integrity assay was performed in 4 independent experiments. Each experiment also consisted of both positive (rats treated with CdCl2 at 5 mg/kg BW i.p. for 3 d) and negative (normal rats) controls.
Image acquisition and analysis
Transillumination and fluorescent images were acquired by MicroSuite FIVE software (version 1.224; Olympus Soft Imaging Solutions Corp., Lakewood, CO, USA) and an Olympus BX61 fluorescent microscope equipped with an Olympus DP70 12.5MPa digital camera (Olympus America, Melville, NY, USA). Images acquired in TIFF formats were imported to Photoshop in the Adobe Creative Suite Design Premium software package (version 3.0; Adobe Systems, San Jose, CA, USA) for brightness/contrast adjustments and image overlay. For in vitro RNAi experiments in cultured Sertoli cells, ∼500 cells were examined from 10 randomly selected fields in each experimental group, including controls. For studies using cross sections of testes, ∼150 seminiferous tubules were examined from 30 randomly selected fields on each section to assess tubule damage.
RNA extraction and RT-PCR
For RNA extraction, testes, Sertoli cells, and germ cells were lysed in TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA was reverse transcribed to cDNA with M-MLV reverse transcriptase (Promega, Madison, WI, USA). The cDNA of selected target gene was amplified by PCR using GoTaqDNA polymerase (Promega) with specific primers (see Table 2). The authenticity of PCR products was verified by DNA sequencing performed at Genewiz.
TABLE 2.
Primers used for amplification of Eps8 and its interacting partners by RT-PCR
| Target gene | GenBank accession no. | Primer orientation | Primer sequence (5′-3′) | Nucleotide position | Expected size (b.p.) |
|---|---|---|---|---|---|
| Eps8 | XM_001072957 | S | AGTGGATGACCGAGCTGTGA | 651–670 | 489 |
| AS | TGCACATCCCTGTCAATCCG | 1120–1139 | |||
| Sos1 | NM_001100716 | S | AGTGCTTCAGATGTGGAGG | 209–227 | 629 |
| AS | GGACTGCCTTCATCTGTC | 820–837 | |||
| Abi-1 | NM_024397 | S | GTGGCGGACTACTGTGAA | 149–166 | 490 |
| AS | AAGTTCCTCGGCCTGACA | 621–638 | |||
| IRSp53 | NM_057196 | S | GACGTCCTCTTCCAGATG | 281–298 | 444 |
| AS | CTGGGCTAGTAACTCCTTG | 706–724 | |||
| S-16 | XM_001078234 | S | TCCGCTGCAGTCCGTTCAAGTCTT | 177–200 | 385 |
| AS | GCCAAACTTCTTGGATTCGCAGCG | 538–561 |
S, sense; AS, antisense.
Lysate preparation and immunoblotting
Lysates of testes, Sertoli cells, and germ cells were prepared in IP lysis buffer: 50 mM Tris, pH 7.4 at 22°C, containing 150 mM NaCl, 2 mM EGTA, 10% glycerol (v/v), 1% Nonidet P-40 (v/v), and freshly supplemented with protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitor cocktail 1 and cocktail 2 (Sigma-Aldrich) using a lysis buffer:inhibitor ratio (v/v) at 100:1. Immunoblotting was performed as described previously (30) with corresponding antibodies (see Table 1), using ∼100 and 25 μg protein of lysates from testes and Sertoli cells, respectively, for SDS-PAGE. Equal protein loading was assessed by stripping blots and reprobed with an antiactin antibody.
Statistical analysis
Each experiment reported herein was repeated 3–5 times, excluding pilot experiments. For Sertoli cell culture experiments, each treatment per time point and the corresponding controls consisted of duplicate dishes. For in vivo studies, each time point consisted of 5 rats. Statistical analysis was performed using the GB-STAT software package (version 7.0; Dynamic Microsystems, Silver Spring, MD, USA). For multiple comparisons, 1-way ANOVA was performed followed by Dunnett’s test. This thus compared multiple treatment groups against the control (e.g., steady-state protein level of a target gene). In selected experiments, Student’s t test was used for paired comparisons.
RESULTS
Eps8 is concentrated at F-actin-rich cell junction structures, namely, basal ES and apical ES, in the seminiferous epithelium
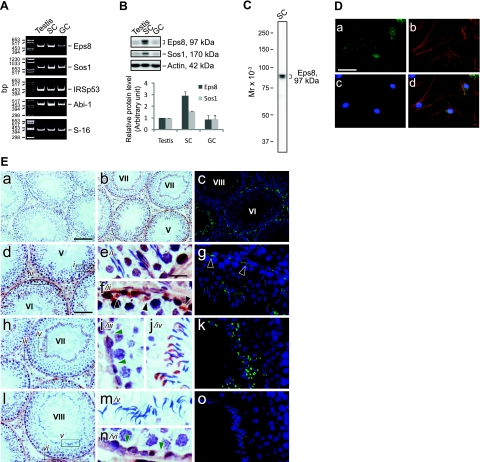
The expression of Eps8 and its functional partners in the seminiferous epithelium was examined by RT-PCR as described previously (21), using the corresponding primer pairs as shown in Table 2. The transcripts of Eps8, Sos1, IRSp53, and Abi-1 were all detected in adult rat testis, Sertoli cells, and germ cells (Fig. 1A). Despite that these results corroborated reports in the literature that the testis is equipped with Eps8 and its functional partners, exploration of the canonical functions of Eps8 was impeded by the lack of suitable antibodies for its partners in rat tissues because several antibodies obtained commercially from different vendors failed to work satisfactorily in our pilot experiments by immunoblot analysis (Table 1). Thus, we continued the study of Eps8 in the testis by other approaches. First, it was shown that the protein level of Eps8 was ∼3-fold higher in Sertoli cells than in lysates of testes and germ cells (Fig. 1B), using a specific anti-Eps8 antibody (Fig. 1C). In Sertoli cells cultured at a low cell density in vitro at ∼0.025 × 106 cells/cm2, Eps8 is the most prominent at the edges of spreading cells, colocalizing with actin stress fibers (Fig. 1D), whereas in the seminiferous epithelium in vivo, Eps8 is concentrated at F-actin-rich cell junction structures found in Sertoli cells with a stage-specific expression pattern (Fig. 1E). At stages V–VI of the seminiferous epithelial cycle, Eps8 is highly expressed at both the apical ES at the Sertoli cell-elongating spermatid interface and the basal ES at the BTB. ES is a testis-specific atypical adherens junction type rich in F-actin bundles (16). Starting from stage VII, Eps8 at the apical ES is shifted to the concave side of the elongated spermatid heads, corresponding to the site of the growing apical tubulobulbar complexes (TBCs), which are also rich in F-actin (15). Concomitantly, Eps8 was found to become diminished at the BTB. At stage VIII before the onset of spermiation, Eps8 became barely detectable both at the Sertoli cell-elongated spermatid interface and at the BTB. This expression pattern suggested that Eps8 is important for maintaining apical ES and BTB integrity, and its disappearance may be a prerequisite for cell junction disassembly during spermiation and the transient restructuring of the BTB to allow the transit of leptotene spermatocytes.
Figure 1.
Expression of Eps8 and its partners, as well as its cellular localization in adult rat testes. A) Gene expression of Eps8 and its partners in adult rat testis, Sertoli cells (SC), and germ cells (GC) was analyzed by RT-PCR. S-16 served as a control. B) Immunoblotting of Eps8 and Sos1 in lysates of testis, SC, and GC (∼50 μg proteins/lane) with actin as a loading control. Histogram in bottom panel summarizes the immunoblot results after normalizing each data point against actin. Protein level in the testis was arbitrarily set as 1. Bars represent means ± sd; n = 3. C) Specificity of the anti-Eps8 antibody as demonstrated by an immunoblot of SC lysate (∼50 μg protein). D) Cellular localization of Eps8 in SCs cultured in vitro. Primary SCs were cultured at 0.025 × 106 cells/cm2 for 5 d and stained for Eps8 (a), F-actin (b), and DAPI (c), with merged images also shown (d). E) Cellular localization of Eps8 in the seminiferous epithelium of adult rat testes with a stage-specific expression pattern. Immunohistochemistry (b, d–f, h–j, l–n) and immunofluorescent staining (c, g, k, o) were performed using frozen testis sections. Primary antibody was replaced with normal mouse IgG as negative control (a). Specific staining for Eps8 appears as brownish-red precipitates (b). Roman numerals indicate tubule stages. Fluorescent images (c, g, k, o) are merged images of Eps8 (green) and nuclei stained with DAPI (blue). Eps8 is highly expressed in the apical ES and at the BTB (black arrowheads) in stage V–VI tubules (d–g). Boxed areas marked by italic lowercase Roman numerals in left panels are enlarged in adjacent middle panels. Eps8 at the BTB site (green arrowheads) is diminished in stage VII tubules, whereas the Eps8 associated with the apical ES is shifted to the concave side of the spermatid heads, corresponding to the developing apical tubulobulbar complex (TBC) (h–k). In stage VIII tubules before spermiation, Eps8 is absent both at the BTB and at the apical ES (l–o). Scale bars = 20 μm (D; Ee–g, i–k, m–o); 100 μm (Ea–c); 80 μm (Ed, h, l).
Eps8 is an early target in adjudin-induced anchoring junction restructuring that leads to germ cell loss from the seminiferous epithelium
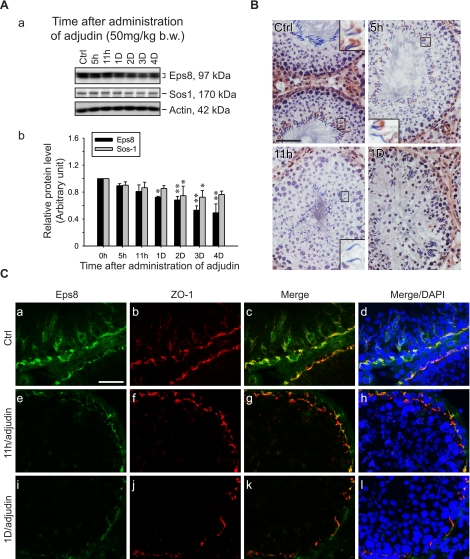
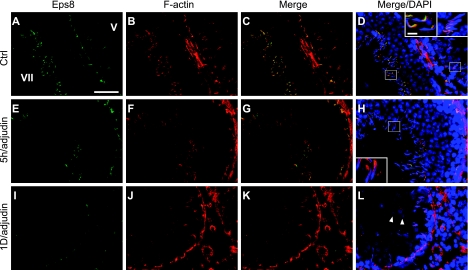
An established in vivo model for anchoring junction restructuring was used (28) to further correlate the function of Eps8 and cell adhesion in the testis. Treating adult rats with adjudin was known to cause germ cell loss by inducing anchoring junction restructuring, most notably at the apical ES, in the seminiferous epithelium (20). Following adjudin treatment, the protein levels of Eps8 and Sos1 in the testis decreased steadily (see Fig. 2A). By day 3, the Eps8 protein level dropped to only ∼50% of the controls. However, the localized decline in Eps8 at the apical ES was detected as early as 5–11 h after treatment (see Figs. 2B, C and 3). By immunohistochemistry, the Eps8 signal at the Sertoli cell-elongating/elongated spermatid interface was considerably weakened by 11 h posttreatment in some tubules with germ cells in the lumen, but were clearly not stage VIII tubules, and were thus premature for spermiation (Fig. 2B). This observation was confirmed by the dual-labeled immunofluorescent analysis of Eps8 and F-actin, as shown in Fig. 3. At 5 h, Eps8 was drastically weakened around some of the elongating spermatids that were about to dislodge from the epithelium, whereas F-actin was still clearly visible. This was in contrast to the strong colocalization signals found at nearly all apical ES in the control. The early decrease of Eps8 before germ cell detachment is reminiscent of the naturally occurring spermiation in stage VIII tubules, further supporting the role of Eps8 in elongating spermatid adhesion. At the opposite end of the seminiferous epithelium, the expression of Eps8 was also affected at the BTB (see Fig. 2C). One day after adjudin treatment, Eps8 that was associated with the BTB (as indicated by its colocalization with ZO-1, a putative TJ adaptor at the BTB) decreased in some stage V–VI tubules, which had elongating spermatids still embedded within the seminiferous epithelium (Fig. 2C).
Figure 2.
Changes in the steady-state level of Eps8 and its cellular localization in the seminiferous epithelium during adjudin-induced germ cell loss. Adult rats were treated with adjudin to induce germ cell loss resulting from anchoring junction restructuring in the seminiferous epithelium. A) a) Immunoblots of Eps8 and Sos1 in testis lysates following adjudin treatment, with actin as a loading control. b) Histogram summarizing results after normalizing each data point against actin. Protein levels at 0 h were arbitrarily set as 1. Bars are means ± sd; n = 3. *P < 0.05, **P < 0.01; 1-way ANOVA followed by Dunnett’s test. B) Immunohistochemistry of Eps8 using frozen testis sections after adjudin treatment. In control (Ctrl), Eps8 staining is clearly visible at the apical ES in most elongated spermatid heads in a stage VII tubule. The staining gradually weakens when elongated spermatids deplete from the epithelium at 5 and 11 h after adjudin treatment in non-stage VIII tubules. By 1 d, Eps8 is also diminished at the BTB site in stage V–VI tubules. Insets: magnified view of boxed area in same panel. C) Colocalization of Eps8 (green) and ZO-1 (red) in frozen testis sections after adjudin treatment. Eps8 at the BTB is much weakened at 11 h and 1 d after adjudin treatment, whereas ZO-1 remains relatively unchanged. Nuclei stained with DAPI (blue) illustrate germ cell loss from the epithelium. Scale bars = 80 μm (B); 40 μm (C).
Figure 3.
Changes in relative localization of Eps8 and F-actin in the seminiferous epithelium during adjudin-induced germ cell loss. Colocalization of Eps8 (green) and F-actin (red) in frozen testis sections after adjudin treatment. A–D) In Ctrl, epithelia of stage V and VII tubules (marked by Roman numerals) display strong colocalization signals of Eps8 and F-actin. E–H) Five hours after adjudin treatment, Eps8 staining at the apical ES is diminished in departing elongate spermatids, preceding the loss of F-actin, which will occur when spermatids fully detach. I–L) At 1 d, although Eps8 is much weakened at the BTB, F-actin remains clearly visible. Round spermatids in the tubule lumen (arrowheads) illustrate premature germ cell loss from the epithelium. Insets: magnified view of boxed area in same panel. Scale bars = 40 μm (A); 6 μm (insets).
Eps8 knockdown leads to F-actin disorganization and mislocalization of TJ proteins at the Sertoli-Sertoli cell interface in vitro
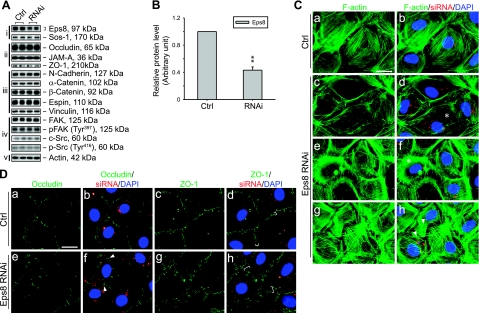
Because the decline in Eps8 at the BTB is correlated to both naturally occurring and adjudin-induced cell junction restructuring events, we sought to investigate its function using primary cultured Sertoli cells in vitro. The Sertoli cell cultures used in this study have been previously characterized to form a TJ-permeability barrier and possess the ultrastructures of both TJ and basal ES that mimic the BTB in vivo (30). After the knockdown of Eps8 by ∼60% via the transfection of Sertoli cells with specific Eps8 siRNA duplexes vs. nontargeting control siRNA duplexes (Fig. 4A, B), no change was detected in the steady-state levels of various cell junction-related proteins examined. While the knockdown of Eps8 failed to perturb the steady-state protein levels of several BTB-associated structural proteins and regulators, it caused adverse effects in F-actin organization and TJ protein localization as visualized by fluorescent microscopy (see Fig. 4C, D). Eps8-silenced cells displayed abnormalities in F-actin such as stress fiber truncation, disorganization, and overgrowth, which may be attributed to its reported function in actin capping and bundling (10, 12). Moreover, the TJ proteins occludin and ZO-1 diffused away from the cell-cell interface in Eps8-silenced cells, in which the internalized occludin appeared to form discrete cytoplasmic aggregates. Certain other BTB structural proteins, such as N-cadherin, were not affected by Eps8 silencing (data not shown). These observations illustrate that Eps8 is crucial for the maintenance of the TJ-permeability barrier at the BTB in vitro.
Figure 4.
Effects of Eps8 knockdown in primary Sertoli cell cultures in vitro. Eps8 was silenced by RNAi in primary cultured Sertoli cells with a functional TJ-permeability barrier mimicking the BTB in vivo. A) Effects of Eps8 silencing on steady-state protein levels related to junction dynamics: i) Eps8 and its partner Sos1; ii) TJ proteins; iii) basal ES proteins; iv) protein kinases known to regulate BTB dynamics; v) actin served as protein loading control. B) Histogram summarizing results shown in A after normalizing each data point against actin. Protein level in Ctrl was arbitrarily set as 1. Eps8 was knocked down by ∼60%, whereas no change in the steady-state levels in all other proteins was detected. Bars are means ± sd; n = 3. **P < 0.01; Student’s t test. C–D) Cells transfected with either Ctrl or Eps8 siRNA duplexes were tracked by cotransfection with siGLO red transfection indicator (red) and stained for F-actin, occludin, and ZO-1 (green). Nuclei were visualized by DAPI (blue). C) Changes in F-actin organization in SCs following Eps8 silencing. As compared to the uniformly distributed and orderly array of F-actin in control SCs (a, b), F-actin fibers in Eps8-silenced cells appear fragmented (c, d) and disorganized (e, f), as indicated by asterisks. In some Eps8-silenced cells (g, h), F-actin fibers appear overgrown and stretch over to neighboring cells (arrowheads). D) Redistribution and internalization of occludin and ZO-1 from the cell-cell interface resulting from Eps8 silencing. Both occludin and ZO-1 are localized primarily at the cell-cell interface in control cells (a–d). In Eps8-silenced cells (e–h), considerably more occludin appears to be redistributed and internalized, forming discrete aggregates in the cytosol (arrowheads), whereas ZO-1 staining appears diffused from the cell-cell interface (white bracket). Scale bars = 20 μm.
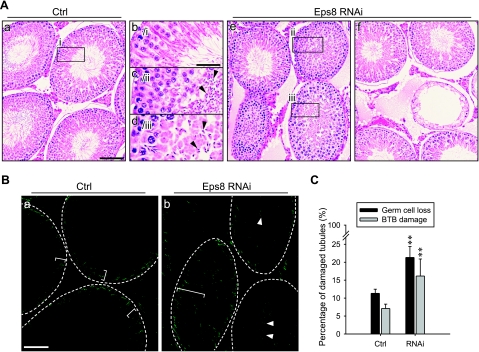
Eps8 is important to confer germ cell adhesion and BTB integrity in vivo
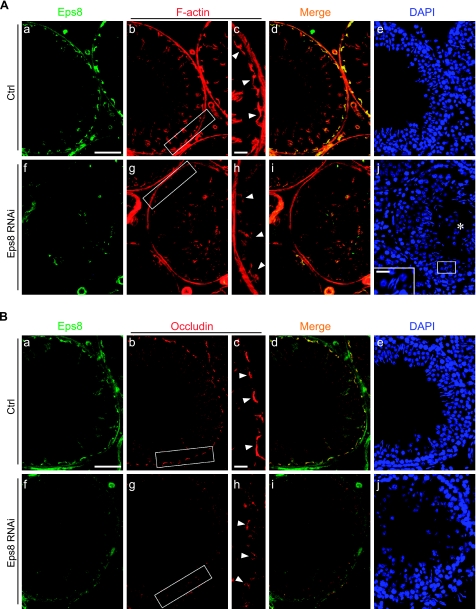
To further validate the physiological function of Eps8 in maintaining the BTB integrity, and to confirm its role in Sertoli-germ cell adhesion, Eps8 was transiently silenced in the testis in vivo by the localized administration of RNAi reagents. Following Eps8 knockdown, the morphology of seminiferous tubules was examined to assess germ cell loss in treatment groups vs. controls that received the nontargeting siRNA duplexes (see Fig. 5A, C). While a portion of tubules near the site of injection in control testes was damaged, resulting in germ cell sloughing, the percentage of damaged tubules in Eps8-silenced testes was about 1-fold higher, and the damaged tubules were randomly distributed across the sections of testes that were examined. The severity of germ cell loss ranged from the detachment of several spermatids into the lumen to tubules that were devoid of germ cells. In addition, a BTB integrity assay was performed (see Fig. 5B, C), and damage to the BTB was visualized by the influx of FITC-inulin through the BTB into the apical compartment of the seminiferous epithelium. To score the damage consistently, a damaged tubule was defined by the penetration of inulin-FITC (green fluorescence) across at least 3 layers of cells from the basal compartment in any portion of the tubule. Again, the percentage of tubules with damaged BTB was about 1-fold higher in Eps8-silenced testes than the control. These two sets of results shown in Fig. 5 verified the role of Eps8 in germ cell adhesion and BTB integrity under physiological conditions. However, the affected tubules accounted for only 15–20% of the total, which may be because of the stage-specific requirement for Eps8 (see Fig. 1 for the stage-specific expression pattern of Eps8). In light of the findings in the in vitro Eps8-silencing experiments, the changes in F-actin and occludin at the BTB in vivo were also visualized by fluorescent microscopy, focusing on stage V–VI tubules in which Eps8 should be expressed at the BTB (see Fig. 6). Following Eps8 knockdown, a portion of stage V–VI tubules manifested germ cell loss and weakened Eps8 staining at the BTB. In these tubules, the BTB-associated F-actin (Fig. 6A) and occludin (Fig. 6B) became disorganized and noticeably diminished, in sharp contrast to the compact and intense staining in the corresponding control. Thus, the mislocalization of occludin-based protein complex is one of the contributing factors that led to BTB damage following the knockdown of Eps8 in vivo.
Figure 5.
Effects of Eps8 knockdown in adult rat testes in vivo. Silencing of Eps8 in the adult rat testis by the local administration of RNAi reagents. A) Hematoxylin and eosin staining of paraffin-embedded testis sections. a, b)Testes treated with Ctrl siRNA duplexes. c–f) Testes treated with Eps8 siRNA duplexes where tubules manifest germ cell loss. Boxed areas marked by lowercase Roman numerals in a and e are enlarged in b–d. B) Localization of inulin-FITC in frozen testis sections following BTB integrity assay. Integrity of the BTB was assessed by its ability to block influx of inulin-FITC into the seminiferous epithelium. Broken ring marks approximate location of BTB in each seminiferous tubule; white brackets mark penetration of inulin-FITC from BTB. Representative area of damaged tubules after Eps8 RNAi is shown in b. FITC-inulin diffuses into the seminiferous epithelium (white bracket) and the tubule lumen (white arrowheads). C) Histogram summarizing quantitative analysis of damaged tubules in A (germ cell loss) and B (BTB damage). Bars = means ± sd; n = 4–5. **P < 0.01; Student’s t test. Scale bars = 80 μm (Aa, e–f); 30 μm (Ab–d); 70 μm (B).
Figure 6.
Changes in F-actin and occludin organization after Eps8 knockdown in adult rat testes in vivo. Ctrl (Aa–e, Ba–e) and Eps8-silenced (Af–j, Bf–j) testis sections were stained for Eps8 (green), F-actin (red; A) or occludin (red; B), and nuclei (DAPI; blue). In Eps8-silenced tubules showing germ cell loss (round spermatids in lumen, marked by asterisk; Aj), the Eps8 staining in the seminiferous epithelium is considerably weakened (Af vs. a). Both F-actin and occludin at the BTB (arrowheads) appear diffused (Ag, h; Bg, h) as compared to intense and compact staining in rats treated with Ctrl siRNA duplexes (Ab, c; Bb, c). Boxed areas in Ab, g and Bb, g are enlarged in Ac, h and Bc, h, respectively. Scale bars = 80 μm (Aa, Ba); 60 μm (Ac, Bc); 20 μm (Aj, inset).
DISCUSSION
The 3 best-known functions of Eps8 are actin barbed-end capping, actin bundling, and modulation of Rac GTPase-mediated actin remodeling (10,11,12,13,14, 33). Herein we report that Eps8 is present in the testis along with some of its functional partners, such as Abi-1, IRSp53, and Sos1, residing in both Sertoli and germ cells in the seminiferous epithelium. More important, it was shown that Eps8 is involved in regulating cell adhesion at the apical ES and the basal ES in the seminiferous epithelium of rat testes. These two testis-specific anchoring junctions are present at the Sertoli cell-elongating spermatid interface and the BTB at the inter-Sertoli cell interface, respectively. Because F-actin is highly concentrated at both the apical ES and the basal ES, disruption of a key actin regulator was anticipated to cause a loss of cell adhesion function at these sites.
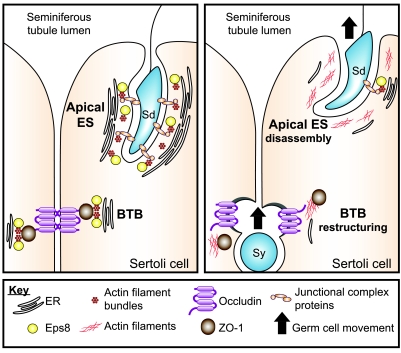
The role of F-actin in Sertoli cells at the ES has long intrigued reproductive biologists because of its unique arrangement as opposed to other epithelial cell junctions. For instance, actin filaments associated with the ES, at both the apical and basal ES (see Fig. 7), are packed into hexagonal arrays of unipolar bundles (15, 17). Early studies have shown that damage to actin filaments by cytochalasin D led to the disappearance of apical ES at the Sertoli cell-elongating spermatid interface (34), illustrating the significance of the actin filament bundles in supporting the apical ES. In this study adjudin induced junction disassembly at the apical ES was correlated with the decrease in Eps8 protein level. The opposite also holds true such that the knockdown of Eps8 by the intratesticular administration of RNAi reagents perturbed the apical ES. Because the effects were not directly exerted on existing actin filaments as in cytochalasin D treatment, these results also illustrated the dynamic nature of ES-associated actin. The mechanism by which Eps8 maintains the integrity of ES has yet to be identified, but an obvious possibility is the participation in the formation and maintenance of the characteristic actin bundles, which are sandwiched between the cisternae of endoplasmic reticulum and the plasma membrane of Sertoli cells. Together with IRSp53, Eps8 is known to form an actin-bundling complex regulated by Cdc42 (12). Apart from this, the Rac guanosine nucleotide exchange factor (GEF) activity of the Eps8-Abi-1-Sos1 complex may also be important at the junctional site, because Rac activity is required for stabilizing the cadherin-based protein complex (35). Although the limited available data we have at the moment cannot clarify the underlying signaling pathways on the precise mechanisms by which Eps8 regulates actin dynamics at the ES, a unique feature of the apical ES may offer some other clues. For instance, it is known that junctional proteins usually restricted to focal adhesions at the cell-matrix interface, such as the integrin-laminin complex (36), are integrated components of the apical ES. This, hence, led us to speculate that the ES might display focal adhesion-like capability to aid the propulsion of spermatids along the Sertoli cell surface, because developing spermatids (step 8 and beyond) are nonmotile cells. As such, they are dependent almost exclusively on Sertoli cells for their movement along the seminiferous epithelium during spermiogenesis (20). In this case, the actin barbed-end capping activity of Eps8 would also be important to regulating force transmission from the actin filaments to the cell-cell contact site. However, the possibility of the participation of apical ES in spermatid movement remains to be investigated because of the noncontractile nature of the actin bundles (37, 38). Recent studies have shown that apical ES is likely working in concert with microtubules, together with several motor proteins, such as dynein (39, 40), kinesin (41), and myosin VIIa (42), to facilitate spermatid movement in the seminiferous epithelium during spermiogenesis. This resembles a cargo train (i.e., elongating spermatid) “sliding” on a railroad track (i.e., microtubule) using the engine driven by motor oils (i.e., motor proteins: dynein, myosin VIIa, kinesin), as recently reviewed (18, 39). It also remains to be determined whether Eps8 and its functional partners mediate crosstalk between the actin-based filaments and the microtubules at the ES to coordinate spermatid movement. Nonetheless, studies reported herein have illustrated the involvement of Eps8 in conferring cell adhesion at the apical ES and its potential involvement in spermatid movement (see Fig. 7).
Figure 7.
Schematic drawing illustrating possible role of Eps8 in coordinating apical ES disassembly and BTB restructuring. Left panel: seminiferous epithelium of adult rat testes at a relatively static state, with intact cell junctions at the apical ES and the BTB, such as stages I–VII and X–XIV of the seminiferous epithelial cycle. Actin filaments at the apical ES and the basal ES/TJ at the BTB are tightly packed to provide robust support for the junction complexes. The presence of Eps8 plausibly contributes to the maintenance of F-actin organization, such as via its actin bundling capability. Right panel: a restructuring seminiferous epithelium where apical ES disassembly and BTB “restructuring/opening” take place simultaneously, such as at stages VIII–IX of the epithelial cycle. At the BTB, down-regulation of Eps8 promotes the internalization of occludin-based TJ complexes, thereby destabilizing the BTB to facilitate the transit of primary preleptotene spermatocytes. Concomitantly, the disruption of the apical ES before elongated spermatid disengagement at spermiation is also promoted by a reduced Eps8 steady-state level. In addition, the status of the junction integrity at the apical ES and/or BTB is known to be regulated, at least in part, by the relative contribution of androgens (e.g., testosterone) and estrogens (e.g., estradiol-17β) (50,51,52,53). Much work is needed in future studies to examine the role of these sex hormones on Eps8 and other actin regulatory proteins. Junctional complex proteins at the Sertoli cell-elongating/elongated spermatid interface (i.e., apical ES) are α6β1-integrin-laminin-333, nectins-afadins, and cadherins/catenins. Sy, primary preleptotene spermatocyte; Sd, elongating/elongated spermatid; ER, cisternae of endoplasmic reticulum.
In addition to the maintenance of the apical ES, Eps8 appears to be associated with the initial phase of TBC formation at late stage VII at the concave side of elongated spermatid heads by shifting its localization to the developing TBC site. Indeed, it was previously reported that Arp2/3 activity is needed for the de novo actin polymerization around the tubular invaginations at the TBC (43). Eps8 may play a role in the upstream regulation by transmitting signals to Rac, thereby activating the Arp2/3 complex (44).
One of the interesting and unexpected findings of this study was the redistribution of TJ proteins occludin and ZO-1 at the BTB resulting from Eps8 knockdown both in vitro and/or in vivo. It is noted that the technique of direct intratesticular administration is widely used by investigators in the field to assess toxicity of a chemical entity (31, 32, 45,46,47). However, the administration of specific Eps8 siRNA duplexes to testes in vivo did not induce extensive BTB damage, unlike other studies using a toxicant such as glycerol, which damaged occludin-based TJ fibrils in almost the entire testis (45). Instead, the damaging effects were limited to only ∼15% of the tubules examined in 4 different rats. This was probably due to the differential susceptibility to Eps8 knockdown in tubules at different stages of the seminiferous epithelial cycle. Likewise, it is known that Sertoli cells are cyclic cells whose functional characteristics in the seminiferous epithelium are not uniform at different stages of the epithelial cycle (20, 48). Because the silencing of Eps8 in Sertoli cells cultured in vitro did not affect some other BTB-associated proteins, such as N-cadherin (data not shown), it seems that the detrimental effect is specific for certain BTB proteins, such as occludin. Based on these findings, it is tempting to speculate that Eps8 plays a novel role of maintaining the TJ-permeability barrier integrity. It is conceivable that the down-regulation of Eps8 detected in stage VIII tubules allows the increased endocytosis of occludin, thereby “destabilizing” the BTB so as to facilitate the transit of primary preleptotene spermatocytes at the BTB (see Fig. 7). The effects of Eps8 on TJ proteins is possibly exerted via Rac GTPase, which has been reported to regulate the localization occludin and ZO-1 as well as the morphology of TJ-fibrils (49). In this context, it is of interest to note that the status of the junction integrity at the apical ES and/or BTB is known to be regulated, at least in part, by the relative contribution of androgens (e.g., testosterone), estrogens (e.g., estradiol-17β) (50,51,52,53), and cytokines (e.g., TGF-β3, TNF-α) (53,54,55,56). Thus, much work is needed in future studies to examine the role of sex hormones and/or cytokines to maintain the steady-state level of Eps8 and other actin regulatory proteins in the seminiferous epithelium, which, in turn, regulates actin dynamics as depicted in Fig. 7.
In short, we have demonstrated unequivocally that Eps8 is a crucial regulator of cell adhesion, most notably at the apical ES and the basal ES at the BTB. It is conceivable that the events of spermiation and BTB restructuring, which take place at these two sites simultaneously during stage VIII of the epithelial cycle, may be coordinated via the down-regulation of Eps8. Furthermore, in light of previous reports that the Eps8 polypyrimidine tract binding protein domain interacts with the integrin cytoplasmic domain (57) and that FAK activity is enhanced by Eps8 in colon cancer cells (58), it is very likely that Eps8 plays a role in integrin signaling. Therefore, it will be interesting to investigate whether Eps8 is involved in the integrin/laminin-mediated regulatory mechanism to coordinate events at the apical ES, the BTB, and hemidesmosome in the seminiferous epithelium (59).
Acknowledgments
This work was supported by NIH grants (NICHD, R01HD056034, R03HD051512 to C.Y.C.) and a Hong Kong Research Grants Council grant (HKU7599/06M to W.M.L.).
References
- Pelletier R M, Byers S W. The blood-testis barrier and Sertoli cell junctions: structural considerations. Microsc Res Tech. 1992;20:3–33. doi: 10.1002/jemt.1070200104. [DOI] [PubMed] [Google Scholar]
- Wong C H, Mruk D D, Siu M K Y, Cheng C Y. Blood-testis barrier dynamics are regulated by α2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–235. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- Carlier M F, Pantaloni D. Control of actin assembly dynamics in cell motility. J Biol Chem. 2007;282:23005–23009. doi: 10.1074/jbc.R700020200. [DOI] [PubMed] [Google Scholar]
- DeMali K A, Burridge K. Coupling membrane protrusion and cell adhesion. J Cell Sci. 2003;116:2389–2397. doi: 10.1242/jcs.00605. [DOI] [PubMed] [Google Scholar]
- Lanzetti L. Actin in membrane trafficking. Curr Opin Cell Biol. 2007;19:453–458. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Li R, Gundersen G G. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol. 2008;9:860–873. doi: 10.1038/nrm2522. [DOI] [PubMed] [Google Scholar]
- Pollard T D. Introduction to actin and actin-binding proteins. Kreis T, Vale R, editors. New York: Oxford University Press; Guidebook to the Cytoskeletal and Motor Proteins. 1999:3–11. [Google Scholar]
- Disanza A, Carlier M F, Stradal T E B, Didry D, Frittoli E, Confalonieri S, Croce A, Wehland J, Di Fiore P P, Scita G. Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat Cell Biol. 2004;6:1180–1188. doi: 10.1038/ncb1199. [DOI] [PubMed] [Google Scholar]
- Higgs H N. There goes the neighbourhood: Eps8 joins the barbed-end crowd. Nat Cell Biol. 2004;6:1147–1149. doi: 10.1038/ncb1204-1147. [DOI] [PubMed] [Google Scholar]
- Disanza A, Mantoani S, Hertzog M, Gerboth S, Frittoli E, Steffen A, Berhoerster K, Kreienkamp H, Milanesi F, Di Fiore P P, Ciliberto A, Stradal T E B, Scita G. Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8-IRSp53 complex. Nat Cell Biol. 2006;8:1337–1347. doi: 10.1038/ncb1502. [DOI] [PubMed] [Google Scholar]
- Scita G, Tenca P, Areces L B, Tocchetti A, Frittoli E, Giardina G, Ponzanelli I, Sini P, Innocenti M, Di Fiore P P. An effector region in Eps8 is responsible for the activation of the Rac-specific GEF activity of Sos-1 and for the proper localization of the Rac-based actin-polymerizing machine. J Cell Biol. 2001;154:1031–1044. doi: 10.1083/jcb.200103146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenhauser N, Borgonovo A, Disanza A, Romano P, Ponzanelli I, Iannolo G, Di Fiore P P, Scita G. The eps8 family of proteins links growth factor stimulation to actin reorganization generating functional redundancy in the Ras/Rac pathway. Mol Biol Cell. 2004;15:91–98. doi: 10.1091/mbc.E03-06-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl A W, Vaid K S, Guttman J A. The Sertoli cell cytoskeleton. Cheng C Y, editor. Austin, TX, USA: Landes Bioscience/Springer Science; Molecular Mechanisms in Spermatogenesis. 2008:186–211. [Google Scholar]
- Wong E W P, Mruk D D, Cheng C Y. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama Y, Maekawa M, Yuasa S. Ectoplasmic specializations in the Sertoli cells: new vistas based on genetic defects and testicular toxicology. Anat Sci Int. 2003;78:1–16. doi: 10.1046/j.0022-7722.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Vogl A W, Pfeiffer D C, Mulholland D, Kimel G, Guttman J A. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- Yan H H Y, Mruk D D, Lee W M, Cheng C Y. Ectoplasmic specialization: a friend or a foe of spermatogenesis? Bioessays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk D D, Cheng C Y. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mruk D D, Siu M K Y, Conway A M, Lee N P Y, Lau A S N, Cheng C Y. Role of tissue inhibitor of metalloproteases-1 in junction dynamics in the testis. J Androl. 2003;24:510–523. doi: 10.1002/j.1939-4640.2003.tb02703.x. [DOI] [PubMed] [Google Scholar]
- Orth J M. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec. 1982;203:485–492. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- Galdieri M, Ziparo E, Palombi F, Russo M, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl. 1981;5:249–259. [Google Scholar]
- Lee N P Y, Mruk D D, Conway A M, Cheng C Y. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25:200–215. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- Aravindan G R, Pineau C, Bardin C W, Cheng C Y. Ability of trypsin in mimicking germ cell factors that affecf Sertoli cell secretory function. J Cell Physiol. 1996;168:123–133. doi: 10.1002/(SICI)1097-4652(199607)168:1<123::AID-JCP15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Pineau C, Syed V, Bardin C W, Jegou B, Cheng C Y. Germ cell-conditioned medium contains multiple factors that modulate the secretion of testins, clusterin, and transferrin by Sertoli cells. J Androl. 1993;14:87–98. [PubMed] [Google Scholar]
- Cheng C Y, Silvestrini B, Grima J, Mo M Y, Zhu L J, Johansson E, Saso L, Leone M G, Palmery M, Mruk D D. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–461. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- Mruk D D, Silvestrini B, Cheng C Y. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C Y, Mather J P, Byer A L, Bardin C W. Identification of hormonally responsive proteins in primary Sertoli cell culture medium by anion-exchange high performance liquid chromatography. Endocrinology. 1986;118:480–488. doi: 10.1210/endo-118-2-480. [DOI] [PubMed] [Google Scholar]
- Siu M K Y, Wong C H, Lee W M, Cheng C Y. Sertoli-germ cell anchoring junction dynamics in the tesis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- Chung N P, Mruk D D, Mo M Y, Lee W M, Cheng C Y. A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermatogenesis reversibly in vivo. Biol Reprod. 2001;65:1340–1351. doi: 10.1095/biolreprod65.5.1340. [DOI] [PubMed] [Google Scholar]
- Russell L D, Saxena N K, Weber J E. Intratesticular injection as a method to assess the potential toxicity of various agents to study mechanisms of normal spermatogenesis. Gamete Res. 1987;17:43–56. doi: 10.1002/mrd.1120170106. [DOI] [PubMed] [Google Scholar]
- Di Fiore P P, Scita G. Eps8 in the midst of GTPases. Int J Biochem Cell Biol. 2002;34:1178–1183. doi: 10.1016/s1357-2725(02)00064-x. [DOI] [PubMed] [Google Scholar]
- Russell L D, Goh J C, Rashed R M, Vogl A W. The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol Reprod. 1988;39:105–118. doi: 10.1095/biolreprod39.1.105. [DOI] [PubMed] [Google Scholar]
- Lui W, Lee W M, Cheng C Y. Rho GTPases and spermatogenesis. Biochim Biophys Acta. 2003;1593:121–129. doi: 10.1016/s0167-4889(02)00348-8. [DOI] [PubMed] [Google Scholar]
- Yan H H Y, Cheng C Y. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- Russell L D, Saxena N K, Turner T T. Cytoskeletal involvment in spermiation and sperm transport. Tissue Cell. 1989;21:361–379. doi: 10.1016/0040-8166(89)90051-7. [DOI] [PubMed] [Google Scholar]
- Vogl A W, Pfeiffer D C, Redenbach D M, Grove B D. Sertoli cell cytoskeleton. Russell L D, Griswold M D, editors. Clearwater, FL, USA: Cache River Press; The Sertoli Cell. 1993:39–86. [Google Scholar]
- Guttman J A, Kimel G H, Vogl A W. Dynein and plus-end microtubule-dependent motors are associated with specialized Sertoli cell junction plaques (ectoplasmic specializations) J Cell Sci. 2000;113:2167–2176. doi: 10.1242/jcs.113.12.2167. [DOI] [PubMed] [Google Scholar]
- Miller M G, Mulholland D J, Vogl A W. Rat testis motor proteins associated with spermatid translocation (dynein) and spermatid flagella (kinesin-II) Biol Reprod. 1999;60:1047–1056. doi: 10.1095/biolreprod60.4.1047. [DOI] [PubMed] [Google Scholar]
- Vaid K S, Guttman J A, Singaraja R R, Vogl A W. A kinesin is present at unique Sertoli/spermatid adherens junctions in rat and mouse testes. Biol Reprod. 2007;77:1037–1048. doi: 10.1095/biolreprod.107.063735. [DOI] [PubMed] [Google Scholar]
- Velichkova M, Guttman J A, Warren C, Eng L, Kline K, Vogl A W, Hasson T. A human homologue of Drosophila kelch associates with myosin-VIIa in specialized adhesion junctions. Cell Motil Cytoskelet. 2002;51:147–164. doi: 10.1002/cm.10025. [DOI] [PubMed] [Google Scholar]
- D'Souza R, Pathak S, Upadhyay R, Gaonkar R, D'Souza S, Sonawane S, Gill-Sharma M, Balasinor N H. Disruption of tubulobulbar complex by high intratesticular estrogens leading to failed spermiation. [E-pub ahead of print] Endocrinology. 2009 doi: 10.1210/en.2008-1232. doi: 10.1210/en.2008-1232. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Wiebe J P, Kowalik A, Gallardi R L, Egeler O, Clubb B H. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J Androl. 2000;21:625–635. [PubMed] [Google Scholar]
- Wiebe J P, Barr K J, Buckingham K D. Sustained azoospermia in squirrel monkey, Saimiri sciureus, resulting from a single intratesticular glycerol injection. Contraception. 1989;39:447–457. doi: 10.1016/0010-7824(89)90122-4. [DOI] [PubMed] [Google Scholar]
- Wiebe J P, Barr K J. Suppression of spermatogenesis without inhibition of steroidogenesis by 1,2,3-trihydroxypropane solution. Life Sci. 1984;34:1747–1754. doi: 10.1016/0024-3205(84)90574-5. [DOI] [PubMed] [Google Scholar]
- De Kretser D M, Kerr J B. The cytology of the testis. Knobil E, Neill J B, Ewing L L, Greenwald G S, Markert C L, Pfaff D W, editors. New York: Raven Press; The Physiology of Reproduction. 1988:837–932. [Google Scholar]
- Jou T, Schneeberger E E, Nelson W J. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCalman C D, Getsios S, Farookhi R, Blaschuk O W. Estrogens potentiate the stimulatory effects of follicle-stimulating hormone on N-cadherin messenger ribonucleic acid levels in cultured mouse Sertoli cells. Endocrinology. 1997;138:41–48. doi: 10.1210/endo.138.1.4831. [DOI] [PubMed] [Google Scholar]
- Meng J, Holdcraft R W, Shima J E, Griswold M D, Braun R E. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Yeh S, Chen L, Lin H, Zhang C, Ni J, Wu C, di Sant'Agnese P A, DeMesy-Bentley K L, Tzeng C, Chang C. Androgen receptor in Sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology. 2006;147:5624–5633. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- Yan H H Y, Mruk D D, Lee W M, Cheng C Y. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Wong E W P, Mruk D D, Cheng C Y. TGF-β3 and TNFα perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu M K Y, Lee W M, Cheng C Y. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- Lui W Y, Wong C H, Mruk D D, Cheng C Y. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- Calderwood D A, Fujioka Y, de Pereda J M, Garcia-Alvarez B, Nakamoto T, Margolis B, McGlade C J, Liddington R C, Ginsberg M H. Integrin β cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci U S A. 2003;100:2272–2277. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa M, Lee J, Chen Y, Chen Y, Lee Y, Wang S, Huang C, Chow N, Leu T. Eps8 facilitates cellular growth and motility of colon cancer cells by increasing the expression and activity of focal adhesion kinase. J Biol Chem. 2007;282:19399–19409. doi: 10.1074/jbc.M610280200. [DOI] [PubMed] [Google Scholar]
- Yan H H Y, Mruk D D, Wong E W P, Lee W M, Cheng C Y. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci U S A. 2008;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]