Abstract
Taking advantage of the bioluminescence resonance energy transfer (BRET) phenomenon, we report the development of a highly photon-efficient, self-illuminating fusion protein combining a mutant red fluorescent protein (mOrange) and a mutant Renilla reniformis luciferase (RLuc8). This new BRET fusion protein (BRET3) exhibits severalfold improvement in light intensity in comparison with existing BRET fusion proteins. BRET3 also exhibits the most red-shifted light output (564-nm peak wavelength) of any reported bioluminescent protein that utilizes its natural substrate coelenterazine, a benefit of which is demonstrated at various tissue depths in small animals. The imaging utility of BRET3 at the single-cell level is demonstrated using an intramolecular sensor incorporating two mammalian target of rapamycin pathway proteins (FKBP12 and FRB) that dimerize only in the presence of rapamycin. With its increased photon intensity, red-shifted light output, and good spectral resolution (∼85 nm), BRET3 shows improved spatial and temporal resolution for measuring intracellular events in single cells and in living small animal models. The development of further BRET3-based assays will allow imaging of protein-protein interactions using a single assay directly scalable from intact living cells to small living subjects, allowing accelerated drug discovery.—De, A., Ray, P., Loening, A. M., Gambhir, S. S. BRET3: a red-shifted bioluminescence resonance energy transfer (BRET) based integrated platform for imaging protein-protein interactions from single live cells and living animals.
Keywords: Renilla luciferase, red fluorescent protein, self-illuminating fluorescent protein, cooled charge-coupled device camera
A successful strategy for imaging interacting protein partners requires an integrated sensor that scales from the single-cell level to the whole organism. However, standard in vitro and cellular assay methods for studying protein-protein interactions are challenging to adapt, and in many cases are not compatible with noninvasive imaging in small animal disease models. Reporter gene-based functional imaging assays are of particular interest, as these approaches are versatile, fast, and nontoxic, and improved instrumentation is now available to efficiently measure reporter activity both at the single-cell level and in small animal models. To date, several bioluminescence reporter gene based imaging strategies, including split reporter complementation and inducible yeast two-hybrid systems, have been devised for assessing protein-protein interactions (reviewed in refs. 1,2,3,4); however, the sensitivity of these assays for measuring events both from single live cells and from deep-tissue structures within small animals is somewhat limited (5). Bioluminescence resonance energy transfer (BRET), analogous to fluorescence resonance energy transfer (FRET), is an emerging technique based on the nonradiative energy transfer between the electromagnetic dipoles of a luminescent energy donor and a fluorescent energy acceptor (reviewed in refs. 4, 6, 7). This cellular assay has been applied in real-time imaging of cells (5, 8,9,10,11,12,13), high-throughput screening (14, 15), and small animal (5, 16) and plant (12, 17) models.
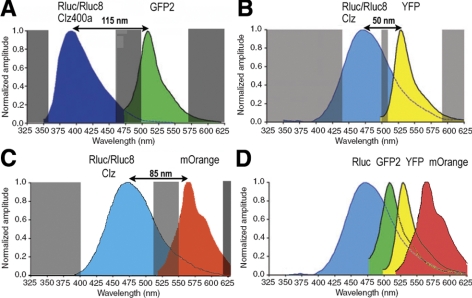
Primarily two BRET variants have been used in a wide variety of applications, both using Renilla luciferase (RLuc) as the energy donor. In BRET1, RLuc is fused to yellow fluorescent protein (YFP) as the energy acceptor, and the substrate coelenterazine (CLZ) is used, leading to a donor emission (peak ∼480 nm) and acceptor emission (peak ∼530 nm; ref. 18). Subsequently, with the use of Enduren (live-cell CLZ-h substrate) to slow down RLuc flash kinetics, the eBRET strategy was developed (19). In BRET2, YFP is replaced with GFP2 (excitation 400 nm, emission 511 nm) as the energy acceptor and the modified substrate known as DeepBlueC (CLZ400; bisdeoxycoelenterazine) that shifts the donor peak emission from 480 to 400 nm (20) is used. We recently showed that the BRET2 assay sensitivity can be significantly improved by utilizing RLuc mutants with improved quantum efficiency and/or stability, (e.g., RLuc8 and RLuc-M) as a donor (5). To extend the time available for measurement, we also developed CLZ400 analogs and showed that signal from our improved BRET2 vector can be monitored for up to 6 h (21). RLuc8 in combination with enhanced YFP (EYFP) has also been utilized for improving the BRET1 system to enhance light output (11). However, even after these improvements of the existing BRET1 and BRET2 systems (see imaging schemes shown in Fig. 1), they are still suboptimal in the context of small animal imaging because of the short wavelengths of the emission light and the strong attenuation of biological tissue to photons of wavelengths <600 nm.
Figure 1.
Schematic diagrams showing imaging schemes of various BRET systems, highlighting the spectral resolution achieved in each case. A) Spectral spans of donor and acceptor imaging windows (clear areas) for BRET2/modified BRET2 using RLuc8 as donor. Note that overlap between donor and acceptor light is minimal. Shaded areas indicate wavelength of light being blocked by spectral filters used for imaging instrument. B) Typical spectral spans of donor and acceptor imaging windows in the case of BRET1/modified BRET1. Donor signal significantly contaminates acceptor signal. C) Spectral spans of imaging windows to measure donor and acceptor light in the case of BRET3. Donor spectrum is same as in BRET1, and red-shifted mOrange acceptor signal improves spectral resolution to 85 nm, thereby reducing donor bleedthrough in acceptor window. D) Schematic showing various control BRET-based fusion proteins using RLuc8 reporter BRET3 provides most appropriate emission wavelengths for animal imaging due to its emission wavelength peak at 564 nm.
Therefore, in the current study, we develop and validate a new BRET acceptor/donor pair, termed BRET3, by combining RLuc8 with the mutant red fluorescent protein (DsRed2) variant mOrange (22). This combination leads to an acceptor emission residing in the orange region of the spectrum. In conjunction with spectral imaging, the new vector is shown to be capable of imaging BRET signals from live single cells as well as from superficial and deep-tissue structures of small animal models. Furthermore, by incorporating a sensor utilizing this new BRET3 system, we test the utility of the BRET3 system for imaging small molecule dimerizer drug efficacies in single live cells.
MATERIALS AND METHODS
Materials
pcDNA3.1, pBad, and pIB vectors were from Invitrogen (Carlsbad, CA, USA). pRSET-B-mOrange plasmid was a kind gift from Dr. Roger Tsien (University of California, San Diego, CA, USA). CLZ was from Prolume (Pinetop, AZ, USA). CLZ400 was from the Molecular Imaging Products Company (Bend, OR, USA). Enduren was from Promega (Madison, WI, USA). HT1080 human fibrosarcoma cells were from American Type Culture Collection (ATCC; Manassas, VA, USA). High Five insect expression cells were from Invitrogen. Superfect transfection reagent was from Qiagen (Valencia, CA, USA). Renilla luciferase antibody (mAb 4400) was from Millipore (Billerica, MA, USA). A series of 20-nm band-pass spectral filters (from 450 to 650 nm) as well as BRET3 specific 400- to 520-nm (510ASP) donor and 550- to 620-nm (580BP60) acceptor filters were from Omega (Brattleboro, VT, USA). Three- to 4-wk-old nude mice (nu/nu) were from Charles River Laboratory (Wilmington, MA, USA).
Plasmid construction
The previously developed pCMV-GFP2-MCS-RLuc8 BRET2 vector (5) was used as a template for making the BRET3 constructs. The GFP2 sequence was replaced with PCR-amplified mOrange between the 5′ NheI and 3′ BspE1 sites. The RLuc8 donor sequence was replaced with RLuc8.6-535 by restriction digest. All clonings were done keeping the 18-aa linker sequence (ser-gly-ser-ser-leu-thr-gly-thr-arg-ser-asp-ile-gly-pro-ser-arg-ala-thr) unchanged. The two mammalian targets of mTOR pathway proteins, FKBP12 and single FRAP-binding domain (FRB), were PCR amplified using suitable restriction enzyme sites from the multiple cloning site of the control BRET3 vector. For insect cell expression of BRET3, the mOrange-RLuc8 fusion was transferred into a pIB insect cell expression vector using 5′ EcoRI and 3′ SacII restriction sites, thereby adding a 6xHis tag.
Cell culture, transfection, clonal isolation, and luciferase assay
HT1080 cells were grown in DMEM high-glucose supplemented with 10% FBS and 1% penicillin streptomycin. Transient transfection was done using Superfect. Each transfection mix consisted of experimental plasmid along with pCMV-FLuc plasmid as transfection control. Measurements of total photon counts were done from the cell lysates using a luminometer and normalized to the FLuc signal. Stable selection of HT1080 cells expressing pCMV-RLuc8, pCMV-RLuc8.6-535, and pCMV-RLuc8-YFP was done with 500 μg/ml geneticin, and of pCMV-mOrange-RLuc8 and pCMV-mOrange-FRB-FKBP12-RLuc8 plasmids with 350 μg/ml zeocin. Cell colonies with the highest expression were judged by measuring RLUC activity using the CLZ substrate.
Western blotting
The expression of fusion constructs in HT1080 cells was verified using the procedure described before (5), probing with a monoclonal Renilla antibody.
BRET assay
For live-cell assays, mammalian cells were imaged 90 min after addition of Enduren using an IVIS200 (Caliper, Alameda, CA, USA). This was done with either a set of 20-nm bandwidth filters spanning 450–650 nm (number denotes filter midpoint) or using the previously mentioned BRET3 custom filters. For BRET imaging and ratiometric calculations, typically 10,000 cells/well (unless otherwise mentioned) were seeded in black 96-well plates, 4 h before substrate addition (0.5 μg/well Enduren). BRET2-expressing cells were assayed with the substrate CLZ400 as described previously (5). All scans were performed in luminescent mode, with 1 min/filter integration times, binning of 5, and field of view at 15 cm, unless otherwise mentioned. In cases where normalization was done by cotransfection with Fluc plasmid, total light signals from parallel wells containing the same number of cells were measured by addition of 0.1 μg d-luciferin/well.
Single-cell luminescence microscopy
An Olympus LV200 luminescent microscope (Olympus America, Inc., Melville, NY, USA) with a 460- to 495-nm donor filter and 520IF acceptor filter was used for single-cell imaging. ImageJ (NIH, Bethesda, MD, USA) was used for measuring mean integrated pixel densities on regions of interest. An IVIS200 system was used as well, with the field of view at 4 cm; resulting images were analyzed using Living Image v2.5.
Animal bioluminescence imaging
An aliquot of a calculated number (0.2×106) of HT1080 cells constitutively overexpressing either BRET3, RLUC8.6-535, or RLUC8 providing equal light output was injected subcutaneously in a set of three anesthetized (isoflurane) nude mice. One hour after cell injection, CLZ (20 μg/mouse) diluted in sterile Dulbecco’s-PBS (100 μl total volume) was injected via the tail vein, and the mice were imaged immediately using open, 590–610, 610–630, 630–650, and open filters in sequence, with 1 min acquisition per filter. Similarly, adjusted numbers of RLuc8, BRET2, and BRET3 cells emitting equal amounts of light (∼0.2×106) were implanted in the flank (n=4) and imaged after injection of either CLZ400 or CLZ via tail vein. To evaluate from deeper tissue depths, adjusted numbers (∼0.5 × 106) cells of each type were injected intramuscularly in another set of mice (n=3) and scanned 1 h later as above. In another study, adjusted numbers (∼1 × 106) of the three cell types were injected intravenously separately into nude mice (3 per group). These animals were imaged at various times over 2 wk by intravenous injection of CLZ and acquired images as mentioned above.
Statistical testing
Average radiance values were obtained for both cell culture assays and in vivo mouse experiments by drawing regions of interest on the images. All group comparisons were performed using the 2-sided Student’s t test with values of P ≥ 0.05 considered statistically significant.
RESULTS
BRET3 is a new class of intramolecular, self-illuminating reporter with a red-shifted spectral advantage
Mutation studies performed in our laboratory on Renilla luciferase led to the development of the variant RLuc8 that exhibits significantly enhanced light output and stability compared with the native enzyme (23, 24). Subsequent studies on Rluc8 led to the development of RLuc8.6-535, a variant that exhibits a ∼55 nm red-shift, yielding a green-peaked emission spectrum (23, 24). Here we report development of a new fusion, termed BRET3, that utilizes the mutant fluorescent protein mOrange to shift the RLuc8 emission spectrum by a total of ∼85 nm resulting in an orange peaked emission light (Fig. 1C, D), with the substrate CLZ or the Enduren analog. To compare the spectral pattern, clonal populations of cells overexpressing the BRET fusions RLuc8-YFP and mOrange-RLuc8 (BRET3) or donor proteins alone (RLuc8 and RLuc8.6-535) were established. When the substrate Enduren was added, BRET3 cells displayed donor- and acceptor-specific dual emission peaks. The narrow spectral separation of RLuc8-YFP does not allow resolution of the donor and acceptor peaks due to the limited spectral resolution of the instrument used. The emission pattern was also compared with the red-shifted mutant luciferase RLuc8.6-535 (Supplemental Fig. 1A). Open-filter images at the beginning (112 min) and the end (115 min) of the sequential acquisitions confirmed that signal variation during scan time is insignificant, thus having negligible effect on sequential measurements of light using the spectral filters (Supplemental Fig. 1B). Partnering RLuc8.6-535 with mOrange in the same orientation and using the same linker sequence as the BRET3 construct shows a drop in total light output even though the donor emission significantly overlaps with the mOrange absorbance (data not shown). Presumably, the emission light from RLuc8.6-535, with a peak at 535 nm, is suboptimal for transferring resonance energy to the acceptor moiety, at least when mOrange is fused to the N terminus of RLuc8.6-535. Further, with only a 40-nm spectral resolution, the spectral unmixing of donor and acceptor emission would have been difficult to achieve, and therefore this pairing was not pursued further.
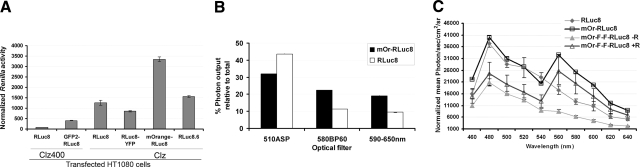
BRET3 fusion protein shows the highest light output as compared with the other BRET systems tested
The photon efficiency of various BRET combinations (e.g., GFP2-RLuc8, RLuc8-YFP, and mOrange-RLuc8 fusion vectors) was compared in transiently transfected HT1080 cells (Fig. 2A). Interestingly, the mOrange-RLuc8 combination shows the highest photon intensity compared with the improved BRET1 and BRET2 system that also utilizes RLuc8 as the donor. BRET3 shows a 7-fold improvement over GFP2-RLuc8 utilizing CLZ400 and a ∼3-fold improvement over RLuc8-YFP. The observed gain in the total light output in the case of BRET3 is consistent with the other BRET systems, i.e., GFP2-RLUC8 (5) and RLUC8-EYFP (11) reported previously. Further, the component light signals using BRET3 specific customized filters show that the donor and acceptor emission is 32.0 ± 0.1 and 22.4 ± 0.02% of the total light, respectively (Fig. 2B). In cells expressing RLUC8 donor alone, these percentages are 53.6 ± 1.7 and 14.0 ± 0.5, indicating true resonance energy characteristics featured by decreased donor output and increased acceptor output from the BRET3 protein. Based on these measurements, the BRET ratio determined is 1.4. Also, considering the emission of a longer wavelength light is advantageous for small animal imaging, we also compared the optical window (590–650 nm), where BRET3 cells show a nearly 2-fold higher light output in comparison with cells transfected with RLUC8.
Figure 2.
Molecular characterization of BRET3 protein in mammalian cells. A) Comparison of total light output from individual BRET systems in HT1080 cells transfected by donor only or BRET plasmids and normalized by activity from a cotransfected pCMV-FLuc plasmid. Activity was measured 36 h post-transfection with either CLZ or CLZ400 substrates as marked. B) Comparative performance of RLUC8- and BRET3-labeled live cells in terms of light emission using different filter sets. Percentages of total light emitted through customized BRET3-specific 510ASP (donor), 580BP60 (acceptor), or spectral filter sets spanning 590–650 nm are plotted. Error bars = se. C) Spectral imaging of HT1080 cells with stable expression of RLUC8 donor alone, mORANGE-RLUC8 BRET fusion, or mORANGE-FRB-FKBP12-RLUC8 fusion sensor (mOr-F-F-RLuc8) in absence (−R) or presence (+R) of rapamycin. After addition of Enduren, cells were incubated under normal culture conditions for 110 min to allow emission signal to stabilize. Sequential scan was then performed using spectral filter sets with 20-nm bandwidth, starting at 460 nm (450–470 nm) to 640 nm (630–650 nm) with 60 s for each. Error bars = se; triplicate samples. Note that mOr-F-F-RLuc8 cells incubated with rapamycin clearly show signal gain on acceptor channel due to activation of BRET.
BRET3 can be effectively used as a drug sensor
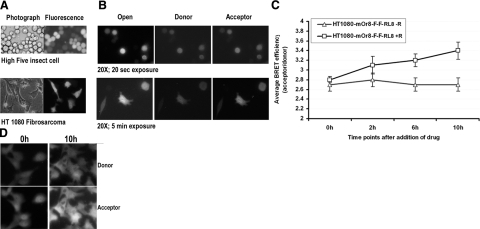
To evaluate BRET3 functionalization as a mechanism for studying drug-modulated intramolecular rearrangements, we developed a sensor construct by inserting FRB and FKBP12 into the linker region of BRET3. HT1080 cells constitutively overexpressing the mOrange-FRB-FKBP12-RLuc8 fusion protein were then tested for a response to rapamycin, as this drug causes interaction of the FRB and FKBP12 domains. Isolated clones were judged for intact fusion protein by Western blot probing against RLUC (Supplemental Fig. 2A). Imaging of cells in the presence of rapamycin shows that the ratio of acceptor to donor signal increases over time (Supplemental Fig. 2B). Spectral imaging data (Fig. 2C) show that the acceptor signal (560-nm spectral filter) value (2.58±0.6)E+04 p/s/cm2/sr from cells incubated with 20 nM rapamycin is significantly (P<0.05) greater than the value (8.6±0.8)E + 03 p/s/cm2/sr of cells not incubated with rapamycin. The spectral wavelength pattern lies within the range of 450–650 nm. BRET3 and mOrange-FRB-FKBP12-RLuc8 cells incubated with rapamycin show similar BRET-specific dual peaks, whereas the RLuc8 cells and mOrange-FRB-FKBP12-RLuc8 cells incubated without rapamycin show only a donor-specific peak.
BRET3 is capable of live intact cell spectral luminescence imaging
The live-cell imaging potential of BRET3 was checked using insect cells and mammalian cells. We cloned the BRET3 sequence into the pIB vector to enable high-yield expression of the fusion protein in insect cells. After transfection into High Five insect cells, stable populations were selected and then imaged using an Olympus luminescence microscope 2 h after the addition of Enduren (Fig. 3A). The insect cells need only a 20-s acquisition to resolve the cell, possibly due to high amounts of BRET3 protein present in the cytosol. Selected clonal isolates of HT1080 fibrosarcoma cells expressing BRET3 were also imaged, but they take a much longer exposure time (5 min) to clearly resolve the cells (Fig. 3B). Imaging of cells highly overexpressing beetle luciferase (green) or GFP2-RLUC8 required 30- to 40-min exposures (data not shown), signifying the suitability of BRET3 in live-cell applications. The donor and acceptor signals of BRET3 can be readily resolved using 460- to 495-nm and 520IF filters, respectively. Ratiometric calculations of acceptor/donor signal are determined as 3.2 from multiple datasets (n=10) of HT1080 cell images. This observed difference in BRET ratio from microscopic measurements appears different due to the slightly different filter sets available for use. We also performed cell imaging of HT1080 cells expressing mOrange-FRB-FKBP12-RLuc8 in the presence and absence of rapamycin over time. In the presence of rapamycin, the FRB-FKBP12 dimerization over 6–10 h leads to an increased acceptor signal and thus a significant increase in the BRET ratio (P≤0.05), indicating successful visualization of the FRB-FKBP12 domain interaction (Fig. 3C, D).
Figure 3.
Single-cell BRET imaging of live insect and mammalian cells expressing BRET3 fusion or sensor. A) Selected pool of High Five insect cells expressing BRET3, imaged using a light microscope, a fluorescence microscope, and an LV200 luminescence microscope with open, donor emission, and acceptor emission filters. Acquisition time was 20 s for each filter at luminescence mode. B) Similar series of photographs for HT1080 human fibrosarcoma cells expressing BRET3. For these cells, 300 s acquisition time was required for each filter. C) Mean BRET ratio determined by pixel quantitation of luminescence images taken at different time points after exposure of HT1080 stable cells (n=10) expressing mOrange-FRB-FKBP12-RLuc8 fusion to rapamycin. Acquisition time was 10 min/filter at each time point. Enduren was added once to cells 2 h before imaging. D) Luminescence donor and acceptor photographs of cells from previous experiment at 0 and 10 h time points with rapamycin incubation, showing increased acceptor signal over time.
Assessment of BRET3 in overcoming tissue photon attenuation from deep-tissue structures of small animal models
Cell number vs. photon output relations for HT1080 cells expressing RLuc8, RLuc8.6-535, or BRET3 show that cell numbers at a ratio of 9:8:10 yield equal light output. Based on these data, adjusted numbers of each cell type providing an equal photon output were injected at various tissue depths in nude mice (Supplemental Fig. 3A, B). Cells placed at subcutaneous locations (n=3) show limited differences in signal output, with RLuc8 emitting 11 ± 2%, RLuc8.6-535 emitting 27 ± 5%, and BRET3 emitting 21 ± 3% of the total light in the preferred (590–650 nm) light window. With cells implanted at an increased tissue depth of ∼4–6 mm by intramuscular injection (n=3), the percentage of the total light measured at 590- to 650-nm wavelength is increased (compared with cells implanted subcutaneously) to 26 ± 3.7, 32 ± 6.2, and 40 ± 3.9%, respectively (Supplemental Fig. 3C). The percentage increases in light signal in the 590- to 650-nm optical window clearly demonstrate that blue emission wavelengths are attenuated even with an increase of only a few millimeters of tissue depth.
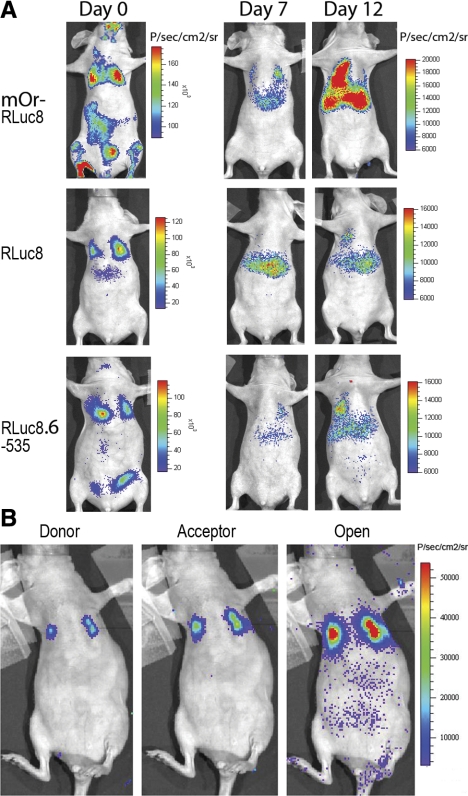
To draw an additional comparison of the performance of BRET3, RLuc8, and RLuc8.6-535 in transmitting light through mice, stable cells (adjusted for total light yield) were injected via the tail vein, and these animals were imaged for up to 12 d. The light emission from lungs of mice after injection of CLZ 30 min after cell injection demonstrates that the BRET3 and RLuc8.6-535 cells led to 3.7- and 1.6-fold higher transmitted signal relative to mice with RLuc8 cells (n=3; P<0.05). As we continued to follow the metastatic spread of HT1080 fibrosarcoma cells in nude mice, the results (Fig. 4A) indicated that BRET3-expressing cells, forming tumors predominantly at lungs and liver sites, could be detected at much earlier time points (7 d) compared with those with RLuc8 (12 d). Comparing the spectral scan results, we estimated that the percentage of total light that escapes in the 590- to 650-nm range is even higher (P<0.05) with ∼45% for of BRET3, and only 30% for RLuc8. Further, we also show that independent of depth, BRET3 specific donor and acceptor signal can be measured from tumor locations in live mouse (Fig. 4B). With the injection of 40 μg of Enduren, the signal outputs from lungs were measured as (7.34±0.4)E+02 p/s/cm2/sr as donor signals and (1.97±0.9)E+03 p/s/cm2/sr as acceptor signals. Thus, within the limits of different instruments and filter sets used, the ratiometric value in animals is 2.7, a value close to the ratiometric value of 3.2 measured from single cells.
Figure 4.
Demonstration of wavelength advantage of BRET3 over luciferase alone in early detection of tumor metastasis in deep-tissue organs. A) Representative mouse images showing total light signal after adjusted number of stable cells for BRET3, RLuc8, and RLuc8.6-535 was delivered in individual mouse via tail-vein injection. All images were acquired for 1 min after injection of 20 μg CLZ. Day 7 and day 12 mouse images were scaled to a single color scale to show differences in signal outcome from each reporter. B) Representative mouse imaged sequentially using BRET3-specific donor, acceptor, and open filter sets of mOrange-RLuc8-expressing tumors at lungs to show that BRET3 can overcome limits of signal detection from deeper tissues. Pseudocolor bar is adjusted to represent photon values for all three images.
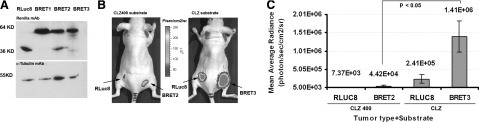
Further, direct comparisons are also made for the performance of RLuc8-based BRET systems, i.e., BRET2 and BRET3 in small animals. HT1080 stable cells expressing RLuc8, BRET1, BRET2, and BRET3 fusions show near equivalent levels of the RLUC protein (36 kDa) in each cell population. In the Western gel, the BRET fusion proteins appear as a 64-kDa band (Fig. 5A). After the protein quantities were judged, direct comparisons of BRET2 and BRET3 cells were drawn by implanting an adjusted number of cells emitting equivalent light at subcutaneous tissue depth in nude mice. Using the BRET2-specific CLZ400 substrate in mice with RLuc8 and BRET2 cells, the signal strengths from both RLuc8 and BRET2 cells appeared significantly lower (P<0.05) in comparison with mice implanted with RLuc8 and BRET3 cells where CLZ substrate was injected (Fig. 5B, C). In both cases, the total light signal from the BRET fusion was much higher (∼6-fold) than from donor-only cells.
Figure 5.
Demonstration of higher light output of BRET3 over improved BRET2 system. A) Western blot showing size and near equivalent quantity of reporter proteins in established cell population expressing RLUC8, BRET1, BRET2, or BRET3. Blot is probed with Renilla monoclonal antibody and α-tubulin monoclonal antibody as loading control. B) Representative images showing total light signal after adjusted number of stable RLuc8 and BRET2 or RLuc8 and BRET3 cells was implanted at subcutaneous tissue depths as marked. Images were captured for 2 min after injection of 25 μg CLZ400 for BRET2 imaging and for 30 s after injection of CLZ for BRET3 imaging. Mouse images were scaled to a single color scale to show differences in signal outcome from each system. C) Bar graph showing quantitative signal strength as obtained from animal experiment as in B. In comparison with BRET2 signal using CLZ400, BRET3 signal using CLZ substrate shows significantly enhanced light output.
DISCUSSION
To date, a large number of FRET probes exist (25,26,27), whereas the number of BRET probes reported for biological applications is relatively few. While FRET probes perform well for reporting intracellular events from single cells, extension of these applications to whole-animal imaging is greatly limited due to obscuring autofluorescence generated by external excitation. On the other hand, bioluminescence reporters are well known for their suitability for small animal imaging applications (28), and more recently, development of advanced microscopes suitable for capturing low-energy luminescent light from cellular environments has allowed the use of luminescence proteins for cellular-level imaging (8, 10,11,12,13, 29). The strategy of combining a fluorescent and a bioluminescent reporter to make self-illuminated reporter protein seems advantageous to overcome the common problems associated with in vivo fluorescent imaging. When such fluorescent-bioluminescent reporter combinations are able to couple via RET, one can easily excite the fluorescent protein by the bioluminescent donor and obtain much higher sensitivity. The combination of mOrange-RLuc8 fusion (BRET3) reported here is such a special class of optical reporters, and imaging studies can benefit from it for multiple reasons. First, BRET3 displays the most red-shifted luminescence signal reported so far obtainable from a luciferase-substrate reaction using CLZ or Enduren. Second, BRET3 maintains a wide range (85 nm) of spectral resolution, giving an opportunity to have wide optical windows necessary for small animal imaging applications. Third, BRET3 also provides at least a 2-fold higher photon yield than RLUC8, a gain that can distinctively help to improve spatial and temporal resolution when used with luminescence microscopes where submicron resolution is a requirement. Fourth, this new combination can also perform as a BRET sensor to report events from intracellular protein-protein interactions (or protein folding or signal transduction), either as an intramolecular sensor as demonstrated here or as separate constructs where interacting protein partners bring the donor and acceptor within RET proximity. The development of BRET3 would thus benefit several future applications as a powerful single-format assay that can perform efficiently over a span of experiments ranging from in vitro assays to single-cell imaging as well as in vivo animal/plant models.
Considerable efforts have been made by our group to improve the characteristics of Renilla reniformis luciferase for studies in small animal models, including a recently reported 55-nm red-shifted variant of RLuc (RLuc8.6-535) that exhibits a 535-nm emission peak when used with CLZ (24). The red-shifted emission spectrum is considerably advantageous, as the preferential absorption of tissue to short-wavelength photons entails that even small red-shifts in the emission spectrum can lead to relatively large increases in light transmission from animal tissues. Our current BRET-based intramolecular probes combining RLUC8 with the mOrange are additionally advantageous for imaging applications using animals due to shifting the emission wavelength by ∼85 nm. Previously, development of mOrange, a monomeric mutant DsRed2 protein, was described (22), which displays high quantum yield, and brightness. We considered these characteristics favorable for partnering as a BRET acceptor and constructed the fusion proteins by fusing it to the N terminus of RLuc8 and RLuc8.6-535. Even though emission with RLuc8.6-535-CLZ (535-nm peak) has better spectral overlap with the absorbance spectrum of mOrange (548-nm peak) than that of RLuc8 (484 nm), we found the mOrange-Rluc8 fusion performed better in terms of spectral separation (85-nm spectral resolution) and increased light output. It needs to be mentioned again that these two constructs are identical with the exception of the 6 additional mutations contained in RLuc8.6-535. In addition, the increased spectral separation of RLuc8 and mOrange compared with RLuc8.6-535 and mOrange is advantageous, as this separation minimizes signal bleedthrough from the donor into the acceptor channel and thus minimizes the requirement of a correction factor in determining the BRET ratio. Of the various BRET pairs tested here (Table 1) utilizing the RLUC8 as the donor, BRET3 convincingly shows the highest photon output. Although it is not very clear what exactly causes signal gain from an artificial BRET pairing, it seems reflective in many cases where either a natural or an artificial BRET pair reportedly displays similar features (5, 11, 30). Presumably, the binding of the two proteins can optimize the spatial relation between the chromophores, thus maximizing the transfer of energy via resonance and thereby featuring an apparent enhancement of quantum yield of the RET signal (31).
TABLE 1.
Physical parameters of various BRET systems developed to date using mutant Renilla luciferases
| BRET system | Donor
|
Acceptor
|
||||||
|---|---|---|---|---|---|---|---|---|
| Reporter | Substrate | Emmax | Filter | Reporter | Exmax | Emmax | Filter | |
| GFP2-Rluc8 | Rluc8 | Clz400 | 400 nm | 360–450 nm | GFP2 | 400 nm | 511 nm | 500–570 nm |
| Rluc8-YFP | Rluc8 | Clz/Enduren | 480 nm | 20 nm spectral | YFP | 514 nm | 527 nm | 20 nm spectral |
| mOrange-Rluc8 | Rluc8 | Clz/Enduren | 480 nm | 400–520 nm | mOrange | 548 nm | 564 nm | 550–620 nm |
| mOrange-Rluc8.6 | Rluc8.6 | Clz/Enduren | 535 nm | 20 nm spectral | mOrange | 548 nm | 564 nm | 20 nm spectral |
Emmax, maximum emission; Exmax, maxiumum excitation.
Until recently, FRET was the only RET approach that allowed dynamic study of the subcellular distribution of interacting protein complexes. Applications to microscopic imaging for BRET were limited due to the inherent low level of light emission from bioluminescent reactions. However, the design criteria using a cooled charge-coupled device camera-based microscope for successful cellular imaging of bioluminescent light has been shown (13). Since then, many studies (8, 11, 12) have shown that spectral BRET imaging from subcellular compartments is possible. The ability to identify the subcellular location of interacting proteins and quantitative assessment of the changes undergoing in specific compartments provide a clear advantage over spectrophotometric BRET analysis from cell populations using a microplate reader (8). During this study, using insect cells and mammalian cells stably expressing the BRET3 protein, we verified the microscopic detection ability of the effecter signal using an Olympus bioluminescent microscope. In applications where migratory cancer cells are being used, high temporal resolution of cellular structures is critical to avoid blurring. Compared with our previously validated cells expressing GFP2-RLuc8 vector or click beetle luciferase (green), the cells in this study were resolved much faster using BRET3. GFP2-RLuc8 utilizing CLZ400 is known to exhibit low quantum yield, and firefly luciferase is kinetically less active than Renilla luciferase; therefore, both require long exposure times to generate high-resolution single-cell imaging. The requirement of long exposures limits the usage of these proteins to report events from metastatic cancer cell types with characteristic high cell motility (data not shown). However, to overcome the limitations of the current filter sets used for microscopic studies, future applications using BRET3 protein should use the 510ASP as the donor filter and 580BP60 as the acceptor filter for microscopic applications with minimal cross-contamination of donor and acceptor signal.
With its red-shifted emission wavelength and relative increase in light output, BRET3 is expected to exhibit improved performance in animal models compared to previous BRET systems or Renilla luciferase variants. With the use of stable cells at subcutaneous and intramuscular depths, the percentages of total light emitted in the preferred optical window (measured at 590–650 nm) were compared. With increased tissue depths, low energy photons (blue-green wavelength) are preferentially attenuated, and therefore the total light output is decreased. When BRET3 cells are trapped in mouse lungs, ∼50% of the total light emits in the 590- to 650-nm wavelength. These results clearly demonstrate that BRET3 emission is advantageous for animal imaging, providing the capability to image much lower numbers of cells at much early time points from any part of the animal body. Further development of BRET pairs with red-shifting of both the donor and the acceptor emissions will undoubtedly further empower the imaging applications utilizing the BRET phenomenon to study and understand multiprotein interactions.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grants 5RO1 CA82214 (to S.S.G.), NCI ICMIC P50 CA114747 (to S.S.G.), CCNE U54 CA119367 (to S.S.G.), and NCI SAIRP. We thank Yiwei (Kevin) Jia (Olympus), Kenya Okazaki (Olympus), and Tim Doyle (Stanford Small Animal Imaging Facility) for technical help with cell imaging.
References
- Hebert T E, Gales C, Rebois R V. Detecting and imaging protein-protein interactions during G protein-mediated signal transduction in vivo and in situ by using fluorescence-based techniques. Cell Biochem Biophys. 2006;45:85–109. doi: 10.1385/CBB:45:1:85. [DOI] [PubMed] [Google Scholar]
- Massoud T F, Paulmurugan R, De A, Ray P, Gambhir S S. Reporter gene imaging of protein-protein interactions in living subjects. Curr Opin Biotechnol. 2007;18:31–37. doi: 10.1016/j.copbio.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmurugan R, Ray P, De A, Chan C T, Gambhir S S. Imaging protein-protein interactions in living subjects. Trends Anal Chem. 2005;24:446–458. [Google Scholar]
- Piehler J. New methodologies for measuring protein interactions in vivo and in vitro. Curr Opin Struct Biol. 2005;15:4–14. doi: 10.1016/j.sbi.2005.01.008. [DOI] [PubMed] [Google Scholar]
- De A, Loening A M, Gambhir S S. An improved bioluminescence resonance energy transfer strategy for imaging intracellular events in single cells and living subjects. Cancer Res. 2007;67:7175–7183. doi: 10.1158/0008-5472.CAN-06-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Applications of bioluminescence- and fluorescence resonance energy transfer to drug discovery at G protein-coupled receptors. Eur J Pharm Sci. 2004;21:397–405. doi: 10.1016/j.ejps.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Pfleger K D, Eidne K A. Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET) Nat Methods. 2006;3:165–174. doi: 10.1038/nmeth841. [DOI] [PubMed] [Google Scholar]
- Coulon V, Audet M, Homburger V, Bockaert J, Fagni L, Bouvier M, Perroy J. Subcellular imaging of dynamic protein interactions by bioluminescence resonance energy transfer. Biophys J. 2008;94:1001–1009. doi: 10.1529/biophysj.107.117275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroy J, Pontier S, Charest P G, Aubry M, Bouvier M. Real-time monitoring of ubiquitination in living cells by BRET. Nat Methods. 2004;1:203–208. doi: 10.1038/nmeth722. [DOI] [PubMed] [Google Scholar]
- Welsh D K, Kay S A. Bioluminescence imaging in living organisms. Curr Opin Biotechnol. 2005;16:73–78. doi: 10.1016/j.copbio.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Hoshino H, Nakajima Y, Ohmiya Y. Luciferase-YFP fusion tag with enhanced emission for single-cell luminescence imaging. Nat Methods. 2007;4:637–639. doi: 10.1038/nmeth1069. [DOI] [PubMed] [Google Scholar]
- Xu X, Soutto M, Xie Q, Servick S, Subramanian C, von Arnim A G, Johnson C H. Imaging protein interactions with bioluminescence resonance energy transfer (BRET) in plant and mammalian cells and tissues. Proc Natl Acad Sci U S A. 2007;104:10264–10269. doi: 10.1073/pnas.0701987104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson M A. detection systems optimized for low-light chemiluminescence imaging. Van Dyke K, van Dyke C, Woodfork K, editors. Boca Raton, FL, USA: CRC Press; Luminescence Bio/TechnologyInstruments and Applications. 2002:469–481. [Google Scholar]
- Hamdan F F, Audet M, Garneau P, Pelletier J, Bouvier M. High-throughput screening of G protein-coupled receptor antagonists using a bioluminescence resonance energy transfer 1-based beta-arrestin2 recruitment assay. J Biomol Screen. 2005;10:463–475. doi: 10.1177/1087057105275344. [DOI] [PubMed] [Google Scholar]
- Issad T, Blanquart C, Gonzalez-Yanes C. The use of bioluminescence resonance energy transfer for the study of therapeutic targets: application to tyrosine kinase receptors. Expert Opin Ther Targets. 2007;11:541–556. doi: 10.1517/14728222.11.4.541. [DOI] [PubMed] [Google Scholar]
- De A, Gambhir S S. Noninvasive imaging of protein-protein interactions from live cells and living subjects using bioluminescence resonance energy transfer. FASEB J. 2005;19:2017–2019. doi: 10.1096/fj.05-4628fje. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt G, Viana R M, Mootz H D, von Arnim A G, Batschauer A. Chemically induced and light-independent cryptochrome photoreceptor activation. Mol Plant. 2008;1:4–14. doi: 10.1093/mp/ssm002. [DOI] [PubMed] [Google Scholar]
- Xu Y, Piston D W, Johnson C H. A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins. Proc Natl Acad Sci U S A. 1999;96:151–156. doi: 10.1073/pnas.96.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger K D, Dromey J R, Dalrymple M B, Lim E M, Thomas W G, Eidne K A. Extended bioluminescence resonance energy transfer (eBRET) for monitoring prolonged protein-protein interactions in live cells. Cell Signal. 2006;18:1664–1670. doi: 10.1016/j.cellsig.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Dionne P, Mireille C, Labonte A, Carter-Allen K, Houle B, Joly E, Taylor SC, Menard L. BRET2: efficient energy transfer from Renilla luciferase to GFP2 to measure protein-protein interactions and intracellular signaling events in live cells. van Dyke K, van Dyke C, Woodfork K, editors. Boca Raton, FL, USA: CRC Press; Luminescence Bio/TechnologyInstruments and Applications. 2002:539–555. [Google Scholar]
- Levi J, De A, Cheng Z, Gambhir S S. Bisdeoxycoelenterazine derivatives for improvement of bioluminescence resonance energy transfer assays. J Am Chem Soc. 2007;129:11900–11901. doi: 10.1021/ja073936h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N C, Campbell R E, Steinbach P A, Giepmans B N G, Palmer A E, Tsien R Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Loening A M, Fenn T D, Wu A M, Gambhir S S. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng Des Sel. 2006;19:391–400. doi: 10.1093/protein/gzl023. [DOI] [PubMed] [Google Scholar]
- Loening A M, Wu A M, Gambhir S S. Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat Methods. 2007;4:641–643. doi: 10.1038/nmeth1070. [DOI] [PubMed] [Google Scholar]
- Selvin P R. The renaissance of fluorescence resonance energy transfer. Nat Struct Biol. 2000;7:730–734. doi: 10.1038/78948. [DOI] [PubMed] [Google Scholar]
- Siegel R M, Chan F K, Zacharias D A, Swofford R, Holmes K L, Tsien R Y, Lenardo M J. Measurement of molecular interactions in living cells by fluorescence resonance energy transfer between variants of the green fluorescent protein. Sci STKE. 2000;200:PL1. doi: 10.1126/stke.2000.38.pl1. [DOI] [PubMed] [Google Scholar]
- Zal T, Gascoigne N R. Using live FRET imaging to reveal early protein-protein interactions during T cell activation. Curr Opin Immunol. 2004;16:418–427. doi: 10.1016/j.coi.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Massoud T F, Gambhir S S. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- Welsh D K, Yoo S H, Liu A C, Takahashi J S, Kay S A. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmiya Y. Basic and applied aspects of color tuning of bioluminescence systems. Jpn J Appl Phys. 2005;44:6368–6369. [Google Scholar]
- Molinari P, Casella I, Costa T. Functional complementation of high-efficiency resonance energy transfer: a new tool for the study of protein binding interactions in living cells. Biochem J. 2008;409:251–261. doi: 10.1042/BJ20070803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.