Abstract
Alzheimer’s disease and other tauopathies are characterized by the presence of intracellular neurofibrillary tangles composed of hyperphosphorylated, insoluble tau. General anesthesia has been shown to be associated with increased risk of Alzheimer’s disease, and we have previously demonstrated that anesthesia induces hypothermia, which leads to overt tau hyperphosphorylation in the brain of mice regardless of the anesthetic used. To investigate whether anesthesia enhances the long-term risk of developing pathological forms of tau, we exposed a mouse model with tauopathy to anesthesia and monitored the outcome at two time points—during anesthesia, or 1 wk after exposure. We found that exposure to isoflurane at clinically relevant doses led to increased levels of phospho-tau, increased insoluble, aggregated forms of tau, and detachment of tau from microtubules. Furthermore, levels of phospho-tau distributed in the neuropil, as well as in cell bodies increased. Interestingly, the level of insoluble tau was increased 1 wk following anesthesia, suggesting that anesthesia precipitates changes in the brain that provoke the later development of tauopathy. Overall, our results suggest that anesthesia-induced hypothermia could lead to an acceleration of tau pathology in vivo that could have significant clinical implications for patients with early stage, or overt neurofibrillary tangle pathology.—Planel, E., Bretteville, A., Liu, L., Virag, L., Du, A. L., Yu, W. Y., Dickson, D. W., Whittington, R. A., Duff, K. E. Acceleration and persistence of neurofibrillary pathology in a mouse model of tauopathy following anesthesia.
Keywords: Alzheimer’s disease, hypothermia, tau hyperphosphorylation, microtubules
The two histopathological hallmarks of Alzheimer’s disease (AD) are senile plaques (SPs) composed of extracellular aggregates of the β-amyloid peptide (Aβ) (1) and intraneuronal neurofibrillary tangles (NFTs), composed of abnormally hyperphosphorylated tau protein assembled into paired helical filaments (PHFs) (2). Only a small proportion of AD is due to genetic variants—the large majority of cases (∼95%) is late onset and sporadic in origin. The cause of sporadic AD is likely to be multifactorial, with external factors interacting with biological or genetic susceptibility to accelerate the manifestation of the disease.
Postoperative cognitive dysfunction, confusion, and delirium are common after general anesthesia in the elderly, with symptoms persisting for months or years in some patients (3). Some reports suggest that AD patients may be particularly at risk of deterioration after anesthesia (4, 5), and several investigators have examined whether there is a link between anesthesia and AD, with some studies suggesting that exposure to anesthetics increases the risk of AD (5,6,7,8).
Although these studies have investigated the relationship between anesthesia and AD, little is known about the impact of anesthesia on the two pathological hallmarks of AD: Aβ accumulation and abnormal tau phosphorylation. Exposure to some anesthetics has been demonstrated to induce accumulation of Aβ oligomers in vitro (9) and increase Aβ production in cell culture (10). Moreover, it was shown that repeated exposure to volatile anesthetics can enhance Aβ plaque formation in Tg2576 mice (11). We have also recently demonstrated that anesthesia-induced hypothermia leads to rapid and robust tau hyperphosphorylation in the brain of normal mice, independent of the anesthetic used (12).
In this study, we investigated the short- and long-term effect of anesthesia-induced hypothermia on tau phosphorylation, solubility, and function in a mouse model of tauopathy (line JNPL3) expressing the TauP301L mutation that causes frontal temporal lobe dementia (13, 14). We found that exposure to isoflurane led to increased tau phosphorylation and accumulation of aggregated tau species, and this was accompanied by detachment of tau from microtubules. Overall, our results suggest that anesthesia-induced hypothermia could lead to an acceleration of tau pathology in vivo. These results warrant a thorough examination of the effect of anesthesia-induced hypothermia on the risk and progression of AD in human populations.
MATERIALS AND METHODS
Animals
Four- to 8-mo-old homozygous JNPL3 (TauP301L) female or male mice were used. Female, hemizygous JNPL3 mice develop severe NFTs in the basal telencephalon, diencephalon, brain stem, and spinal cord by 10–12 mo of age (13, 14). In homozygous mice, the pathology develops as early as 4 mo in females and 7–8 mo in males. The pathology that develops in the JNPL3 line is progressive, and it leads to degeneration of neurons in the spinal cord and brain stem, resulting in ataxia and hind limb paralysis. Mice at an early age/stage of the disease do not show any deficits and are indistinguishable from nontransgenic mice when assessed on a battery of motor tests. All the mice used were not different from wild-type mice in terms of motor function (see below). Animals were used in full compliance with National Institutes of Health/Institutional Animal Care and Use Committee Guidelines.
Motor function test
To evaluate the degree of motor impairment shown by the mice 1 wk after treatment, we performed a battery of tests designed for the JNPL3 line (Dr. Jada Lewis, Mayo Clinic, Jacksonville, FL, USA; personal communication). The test includes rope hanging, tail hanging, and righting reflex. Assessment of ability to perform these tests is reflected in an overall performance score. Scores of 0 to 12 show the mice have normal motor function, scores from 13 to 20 show they have mild motor impairment, and scores 20 and above show they have moderate to severe deficits. As the JNPL3 mice develop motor deficits, they are unsuitable for cognitive performance tests such as the Morris water maze.
Anesthesia
Anesthesia was induced by exposure to isoflurane (1-chloro-2,2,2-trifluoroethyl difluoromethyl ether; AErrane; Baxter Pharmaceutical, Deerfield, IL, USA). The mice received either isoflurane 1 MAC (minimum alveolar concentration, 1.3% isoflurane) in 30% O2 in air, or 30% O2 in air for a period of 4 h in a dedicated chamber, with or without control of the body temperature. Animals were sampled either immediately or 1 wk after exposure to anesthesia, and the experiments were designed so that all of the groups (control, anesthesia, anesthesia+1 wk or 4× anesthesia+1 wk) were euthanized on the same day. Temperature was monitored with a rectal probe (Thermalert TH-5; Physitemp, Clifton, NJ, USA).
Protein extraction and analysis of tau solubility
Mice were killed by cervical dislocation, and the brains were immediately removed and dissected on wet ice. Tissues were quickly weighed, frozen on dry ice, and stored at −80°C. Tau solubility was analyzed by a modification of the protocol of Greenberg and Davies (15) and Noble et al. (16). Briefly, frozen hemispheres were homogenized without thawing in 5× vol/wt of RIPA buffer (50 mM Tris-HCl, pH 7.4; 1% Nonidet P-40; 0.25% Na-deoxycholate; 150 mM NaCl; 1 mM EDTA; 1 mM PMSF; 1 mM Na3VO4; 1 mM NaF; 10 μl/ml of protease inhibitor cocktail P8340; Sigma-Aldrich, St. Louis, MO, USA), with a mechanical homogenizer (TH; Omni International, Marietta, GA, USA), and centrifuged at 20,000 g for 20 min at 4°C. An aliquot of the supernatant representing the total tau fraction was kept for analysis. The heat-stable, soluble, aggregate-free fraction was obtained by boiling another aliquot for 5 min and removing protein aggregates by centrifugation at 20,000 g for 20 min at 4°C. The rest of the supernatant was adjusted to 1% sarkosyl (N-lauroylsarcosine), incubated for 30 min at room temperature with constant shaking, and centrifuged at 100,000 g for 1 h at 20°C. The pellet containing sarkosyl-insoluble, aggregated tau was resuspended and analyzed by SDS-PAGE. Tau in the sarkosyl pellet has been shown by immuno-electron microscopy to be filamentous (17), and it is synonymous with that identified by immunohistochemistry in NFTs. All 3 fractions were diluted in O+ buffer (62.5 mM Tris-HCl, pH 6.8; 10% glycerol; 5% 2-mercaptoethanol; 2.3% SDS; 1 mM EGTA; 1 mM EDTA; 1 mM PMSF; 1 mM Na3VO4; 1 mM NaF; 10 μl/ml of protease inhibitor cocktail P8340; Sigma-Aldrich), a modified O buffer (18), boiled for 3 min, and kept at −20°C. Depending on the antibody used, 7 to 21 μg of protein were analyzed as described previously (19).
Tau/microtubule binding assays
To determine whether tau hyperphosphorylation could detach tau from microtubules, a MT binding assay was performed using a modification of a previously reported procedure (20). Following dissection, fresh cortices were immediately homogenized in 5× vol/wt of prewarmed (37°C) modified reassembly (RA) buffer (0.1 MES, pH 6.5; 0.5 mM MgSO4; 2 mM GTP; 1 mM EGTA; 2 mM DTT; 20 μM taxol; 0.1% Triton X-100; 1 mM PMSF; 1 mM Na3VO4; 1 mM NaF; 10 μl/ml Sigma Protease Inhibitor Cocktail P8340), in a warm (37°C) glass-Teflon homogenizer (20). The lysate was then immediately centrifuged at 3000 g for 2 min at 25°C to remove the debris. An aliquot (100 μl) of the supernatant was sampled, dissolved in 400 μl of O+ buffer, and boiled for 5 min. This was referred to as the total fraction, which includes both MT-free and bound fractions. Another aliquot (100 μl) of the supernatant was pelleted at 100,000 g for 20 min at 25°C. The detergent-soluble supernatant was removed, and 80 μl were diluted in 320 μl of O+ buffer and boiled for 5 min. This was referred to as the MT-free fraction. The remaining pellet was resuspended in a final volume of 100 μl of RA buffer, and diluted in 400 μl of O+ buffer and boiled for 5 min. This was referred to as the MT-bound fraction. Protein levels were quantified in all fractions.
Antibodies
The following anti-tau monoclonal antibodies (specificity given in parentheses) were a generous gift from Dr. Peter Davies (Albert Einstein College of Medicine, Bronx, NY, USA): TG-3, phospho-Ser-231 and conformation-specific (21) MC-6, phospho-Ser-235 (21), and PHF-1, phospho-Ser-396/404 (22). Total tau was detected with either Tau T57120 (monoclonal; BD Transduction Laboratories, San Jose, CA, USA), or Tau A0024 (polyclonal; Dako Cytomation, Carpinteria, CA, USA). AT8 (Pierce Biotechnology, Rockford, IL, USA) reacts with tau phosphorylated at Ser-202 and Thr-205 (23), and Tau-1 (Chemicon International, Temecula, CA, USA) recognizes tau dephosphorylated at Ser-195, Ser-198, Ser-199, and Ser-202 (24). Purified rabbit polyclonal anti-tau antibodies anti-tau pS199, pS262, and pS422 were purchased from Biosource International (Camarillo, CA, USA). Changes in tau kinases were investigated with the following antibodies: cdk-5, anti-p35C, CaMKII, phospho-CaMKII (Santa-Cruz Biotechnology, Santa Cruz, CA, USA); GSK-3β (BD Transduction Laboratories, Franklin Lakes, NJ, USA); anti-GSK-3α/β (pY219/pY216; Biosource International); and phospho-GSK-3β (Ser-9), SAPK/JNK, phospho-SAPK/JNK (T183/Y185) G9, p44/42 MAP kinase, phospho-p44/42 MAPK (T202/Y204), phospho-Akt (S473), phospho-p38 MAP kinase (T180/Y182), p38 MAP kinase (Cell Signaling Technology, Danvers, MA, USA).
Immunoblot analysis
Membrane blocking and antibody incubations were performed as described previously (16), with appropriate primary and secondary antibody dilutions. Serial dilutions of brain extracts were loaded in gels to obtain calibration curves for reliable quantification.
Immunohistochemistry
The mice were killed, and the spinal cord was immediately removed and drop-fixed in 10% neutral buffered formalin (Sigma-Aldrich) for 30 min at room temperature and then placed overnight at 4°C. Immunostaining was performed with conformation-specific (MC1; 1:50) or phospho-specific mouse monoclonal antibodies (CP13, 1:1000) (25). Deparafinized sections were exposed to steam (distilled water) for 30 min, and immunohistochemistry was performed with a DAKO Autostainer, using 3,3′-diaminobenzidine (DAB) as the chromogen, as described previously (26). Other staining methods included thioflavine S and Gallyas silver staining, as described by Lamy et al. (27). Quantification of the pathology was performed on 5 animals/condition, using 3 sections from the cervical, thoracic, and lumbar parts of the spinal cord (n=15/condition), according to a modified published protocol (28).
Phosphatase activity assay
We performed a protein phosphatase 2A (PP2A) assay with the PP2A Immunoprecipitation Phosphatase BioAssay Kit from U.S. Biological (Swampscott, MA, USA), according to the manufacturer’s instructions. Tissues were homogenized in 5× vol/wt of 20 mM imidazole-HCl, pH 7.0; 2 mM EDTA; 2 mM EGTA; 1 mM PMSF; and 10 μl/ml of protease inhibitor cocktail P8340 (Sigma-Aldrich); and centrifuged at 2000 g for 5 min at 4°C. The PP2A catalytic subunit was immunoprecipitated from the supernatant with a monoclonal antibody and protein A agarose for 2 h at 4°C. The activity of the immunoprecipitated PP2A was assessed by the release of phosphate from a chemically synthesized phosphopeptide over a period of 10 min at 30°C. The amount of phosphate released was measured by the absorbance of the molybdate-malachite green-phosphate complex at 630 nm.
Statistical analysis
Statistical analysis was performed either with 1-way ANOVA followed by a Newman-Keuls post hoc test; or a Mann-Whitney U test. Data are expressed as means ± sd. Values of P < 0.05 were considered significant.
RESULTS
Anesthesia-induced hypothermia leads to long-lasting tau hyperphosphorylation and aggregation in female mice
We have previously demonstrated that short-term (30–60 min) anesthesia by either chloral hydrate, pentobarbital, or isoflurane induces hypothermia that leads to tau hyperphosphorylation in nontrangenic mice (12). For this study, we aimed to better replicate clinical conditions; therefore, mice (female JNPL3 line, 4.4±0.1 mo old) were exposed to 1 MAC isoflurane for 4 h. The temperature of the control animals was 37.6 ± 0.3°C (n=6), whereas the temperature of the anesthetized animals was 30.5 ± 1.9°C after 4 h exposure to anesthesia (n=5). Hypothermia was accompanied by a pronounced enhancement of immunoreactivity of all phosphorylation-dependent antibodies studied (compare lanes 1 and 2 of Fig. 1), in brain stem extracts of the mice: AT8 (Fig. 1A; phospho-Ser-202/Thr-205), CP13 (Fig. 1B; phospho-Ser-202), MC-6 (Fig. 1C; phospho-Ser-235), PS262 (Fig. 1D; phospho-Ser-262), and PHF-1 (Fig. 1E; phospho-Ser-396/404). This led to a large mobility shift in tau, as observed using a phosphorylation-independent total tau antibody (Fig. 1F). Tau hyperphosphorylation is thought to induce the formation of insoluble aggregates in vivo (29). JNPL3 mice readily develop such aggregates in the brain stem and constitute a good model to study whether hyperphosphorylation accelerates tau aggregation in vivo. After 4 h of treatment, there was no change in the level of sarkosyl-insoluble tau (Fig. 1H, lane 2; Sarkosyl Pellet fraction).
Figure 1.
Immunoblot analysis of tau phosphorylation and solubility after isoflurane treatment in 4.4-mo-old homozygous JNPL3 female mice. Protein extracts from brain stem of control mice (Ctl; lane 1, n=6) and mice sampled at the end of 4 h of anesthesia (Anes; lane 2, n=5), or 1 wk after (A+1w; lane 3, n=6) were extracted according to a modified method of Greenberg and Davies (15). Tau from total (A–F), heat-stable soluble (G), and sarkosyl-insoluble (H) fractions were evaluated by immunoblot analysis with the following antibodies: A) AT8 (pS202/pT205); B) CP13 (pS202); C) MC6 (pS235); D) pS262; E) PHF-1 (pS396/pS404); F–H) tau (phospho-independent). Scatterplots represent quantification of the immunoblot bands displayed above them. Levels of phosphoepitopes (A–E) were normalized to total tau. Scatterplots display immunoreactivity expressed as percentage of control (100%). Numbers in graphs indicate percentage of the tick line with which they are aligned. Data represented are means ± sd (1 representative value displayed; each lane represents an individual mouse). *P < 0.05, **P < 0.01 vs. Ctl; ANOVA with Neumans-Keuls post hoc test. #P < 0.05, ##P < 0.01 vs. Ctl; Mann-Whitney U test.
To examine whether phospho-tau persisted after recovery from anesthesia, the mice were examined 1 wk after exposure (n=6). The rectal temperature of the mice (38.0±0.4°C) was not significantly different from the controls. Compared to controls, the level of phosphorylated tau was significantly enhanced at AT8, CP13, MC6, and PHF-1 (quantification data not shown). The level of total tau was also increased (Fig. 1F, lane 3; ∼25% increase). When the level of phospho-tau was normalized to total tau, the AT8 epitope was the only epitope that was significantly hyperphosphorylated (Fig. 1A, lane 3). One week after exposure to anesthesia, the level of soluble (heat stable) tau was unchanged (Fig. 1G, lane 3), whereas the level of sarkosyl-insoluble tau had doubled (Fig. 1H, lane 3).
Our results demonstrate that a single exposure to anesthesia-induced hypothermia resulted in tau hyperphosphorylation that persisted for a week at some epitopes. Although insoluble tau levels did not increase significantly immediately following anesthesia exposure, they were elevated a week later.
Anesthesia-induced tau hyperphosphorylation after anesthesia was not due to kinase activation or phosphatase inhibition
We recently demonstrated that tau hyperphosphorylation induced by anesthesia with chloral hydrate exposure for 1 h is due to inhibition of Ser/Thr protein phosphatases by hypothermia rather than activation of specific kinases (12). Since the time of exposure and the anesthetic used in the present study were different from our published study, similar studies were performed to determine the activation state of a panel of candidate tau kinases. Among the kinases able to phosphorylate tau, glycogen synthase kinase-3β (GSK-3β), cyclin-dependent kinase 5 (cdk5), mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), p38, and c-Jun N-terminal kinase (JNK) are considered to be major physiological and pathological tau kinases (30,31,32). CaMKII is also thought to have a major role in regulating the phosphorylation of tau at epitopes that modulate tau binding to microtubules (33, 34). Phosphorylation of GSK-3β at Tyr216 facilitates substrate binding, while phosphorylation at Ser-9 leads to partial inhibition (32). There was no change at the Tyr216 epitope in mice analyzed either immediately or 1 wk after anesthesia (data not shown), but a significant increase of Ser-9 phosphorylation could be detected after exposure to isoflurane (Fig. 2A). JNK was slightly activated during anesthesia, but not after 1 wk of recovery (Fig. 2C). Phosphorylation and therefore activation of MAPK was reduced during anesthesia but was not significantly different 1 wk afterward (Fig. 2E). There was no change in CaMKII activation (Fig. 2I), nor a significant change for total GSK-3β (Fig. 2B), JNK (Fig. 2D), MAPK (Fig. 2F), CaMKII (Fig. 2J), or AKT (Fig. 2L). In the brain, cdk5 forms a heterodimeric complex with the neuron-specific activator p35, which can be cleaved into p25 to enhance cdk5 activity toward its substrates (30). There was no significant change in the levels of cdk5 or p35 during anesthesia (Fig. 2G, H), and there was no detectable p25. Similarly, Akt/PKB, which has been reported to phosphorylate tau (35), did not change during anesthesia (Fig. 2K, L). We also investigated the activation state of p38, which is known to phosphorylate tau at multiple sites (36). There was no change in either p38 levels or phosphorylation (data not shown). To summarize, of all the kinases tested, inhibition of GSK-3β and MAPK and activation of JNK were the only changes detected during anesthesia. These results confirm our previous published data following 1 h of anesthesia with choral hydrate (12), suggesting that the duration of anesthesia or the anesthetics used does not change the pattern of kinase activity induced by hypothermia. One week after the 4-h exposure to isoflurane, all of the kinase profiles were identical to control-treated animals (Fig. 2; compare lanes 1 and 3).
Figure 2.
Effect of isoflurane anesthesia on tau kinases and phosphatases in 4.4-mo-old homozygous JNPL3 female mice. Extracts from brain stem of control mice (Ctl; lane 1, n=6) and mice sampled at the end of 4 h of anesthesia (Anes; lane 2, n=5), or 1 wk after (A+1w; lane 3, n=6) were separated by SDS-PAGE and identified with antibodies indicated in boxes. A) Inhibited GSK-3β (Ser9). B) Total GSK-3β. C) Activated JNK (pThr183 and pTyr185). D) Total JNK. E) Activated MAPK (pThr202 and pTyr204). F) Total MAPK. G) cdk5. H) p35. I) Activated CaMKII (pThr286). J) Total CaMKII. K) Activated Akt (pSer473). L) total Akt. M) PP1 catalytic subunit. N) PP2B catalytic subunit. O) PP2A catalytic subunit. P) PP2A activity assay. Scatterplots represent quantification of the immunoblot bands displayed above them (except P, which displays PP2A activity). Levels of phosphokinases (A, C, E, I, K) were normalized to total kinase levels. Graphs show results expressed as percentage of control (100%). Numbers in graphs indicate percentage of the tick line with which they are aligned. Data represented are means ± sd (1 representative value displayed; each lane represents an individual mouse). *P < 0.05, **P < 0.01 vs. Ctl; ANOVA with Neumans-Keuls post hoc test.
We have previously demonstrated that tau hyperphosphorylation during chloral hydrate or pentobarbital anesthesia was due to a direct effect of hypothermia on phosphatase activity (12). Indeed, hypothermia was demonstrated to lead to linear inhibition of kinases along with exponential inhibition of PP2A, which results in an imbalance leading to increased phosphorylation of tau (37). We, therefore, examined whether isoflurane directly affected protein phosphatases (PPs), either during anesthesia or 1 wk later. PPs are classified into four types (PP1, PP2A, PP2B, and PP2C) on the basis of their specificity toward certain substrates and sensitivity to specific activators and inhibitors. Tau can be dephosphorylated by PP1, PP2A, and PP2B, but PP2A shows increased propensity to dephosphorylate tau, and in vivo and slice culture studies have demonstrated that PP2A is the main regulator of tau phosphorylation under physiologically relevant conditions, whereas PP1 and PP2B are minimally involved (19, 37,38,39). There was no change in PP1, PP2A, or PP2B levels during or after isoflurane treatment (Fig. 2M, O), and there was no change in the activity of PP2A, as determined using a commercially available PP2A assay system (Fig. 2P). The inhibition of PP2A during hypothermia and the consequent tau hyperphosphorylation are a direct effect of low temperature on the kinetics of the enzymes, inhibiting exponentially PP2A while kinases are inhibited only linearly (37). It is not detectable by any in vitro phosphatase activity assay, unless the samples taken from the hypothermic animals are incubated at the temperature of the animals at the time of sacrifice (12). Here, we performed the PP2A assay at 30°C for all the samples to see whether isoflurane or the increased tau pathology could affect PP2A activity independently of temperature. Overall, the present results confirm our previously published data during anesthesia, and suggest that specific kinase activation or phosphatase inhibition is probably not the mechanism leading to tau hyperphosphorylation and aggregation 1 wk after isoflurane-induced hypothermia.
Effect of repeated anesthesia on tau hyperphosphorylation and aggregation in male mice
To verify whether results obtained in female JNPL3 mice could be reproduced in male mice, 8-mo-old male JNPL3 mice were treated according to the same protocol: a single 4-h exposure to 1 MAC isoflurane followed by analysis 1 wk later. We were not able to detect a significant increase in aggregated tau levels (data not shown), suggesting that a single exposure to anesthesia was not sufficient to accelerate tau pathology in male mice. Male mice are known to develop pathology at a slower rate than females due to reduced overall tau levels. Interestingly, multiple exposures to general and spinal anesthesia before the age of 50 have been associated with a decreased age of onset of AD (6). To evaluate whether multiple exposures to isoflurane would promote accelerated pathology development in the male mice, we exposed 8.2 ± 0.1 mo-old male mice for 4 h to 1 MAC isoflurane twice a week for 2 wk and analyzed them 1 wk after the last exposure. The temperature of the mice analyzed 1 wk after anesthesia (38.1±0.3°C; n=10) was not different from the control mice (38.2±0.2°C; n=8). In contrast to the single exposure, there was an increase in phospho-tau in the brain stem at most of the phosphoepitopes analyzed (Fig. 3A–E), and this was accompanied by an elevation of aggregated tau (Fig. 3H). There was no significant increase in total tau in either the total or soluble fraction (Fig. 3F, G). To investigate whether increased tau phosphorylation and aggregation after multiple exposure to anesthesia are due to isoflurane and/or hypothermia, we exposed another group of homozygous male JNPL3 mice to four exposures of 4 h of isoflurane, while keeping them normothermic, and collected their brain stem 1 wk later (Ctl, n=8; 4×A+1w, n=7). There was no elevation of either phospho- or insoluble tau (data not shown), demonstrating that the effect of anesthesia on tau pathology was solely due to hypothermia. These results confirm that exposure to anesthesia-induced hypothermia can enhance tau pathology in JNPL3 mice, and suggest that repeated exposure is necessary to increase the pathology in male mice, in which tau aggregates are slower to form relative to female mice.
Figure 3.
Immunoblot analysis of tau phosphorylation and solubility after repeated isoflurane treatment in 8.2-mo-old homozygous JNPL3 male mice. Protein extracts from brain stems of control mice (Ctl; lanes 1 and 2, n=10), and mice sampled at 1 wk after the last of four isoflurane treatments (4×A+1w; lanes 3 and 4, n=8) were extracted according to a modified method of Greenberg and Davies (15). Tau from total (A–F), heat-stable soluble (G), and sarkosyl-insoluble (H) fractions were evaluated by immunoblot analysis with the following antibodies: A) AT8 (pS202/pT205); B) CP13 (pS202); C) MC6 (pS235); D) pS262; E) PHF-1 (pS396/pS404); F–H) tau (phospho-independent). Scatterplots represent quantification of the immunoblot bands displayed above them. Levels of phosphoepitopes (A–E) were normalized to total tau. Graphs display immunoreactivity expressed as percentage of control (100%). Numbers in graphs indicate percentage of the tick line with which they are aligned. Data represented are means ± sd (2 representative values displayed; each lane represents an individual mouse). *P < 0.05, **P < 0.01 vs. Ctl; Mann-Whitney U test.
Effect of repeated anesthesia on tau pathology in the spinal cord
We next examined the gross anatomical distribution and density of phospho-tau and tau in an abnormal conformation in the spinal cords of the JNPL3 male mice, using monoclonal antibodies (CP13, phospho-Ser-202; MC1, conformation dependent). Both CP13 and MC1 detected intraneuronal tau accumulation, whereas CP13 also detected hyperphosphorylated tau distributed in neuropil (Fig. 4). Compared to control animals, CP13 immunostaining was significantly elevated in animals analyzed 1 wk after treatment (Fig. 4A, B). The enhanced CP13 immunostaining in the spinal cord confirms our immunoblotting results from the brain stem (Fig. 3B). MC1 staining usually correlates with levels of insoluble tau (16), and although there was a trend to enhanced MC1 staining (Fig. 4C, D) that correlated with elevated levels of sarkosyl-insoluble tau in animals repeatedly exposed to anesthesia (Fig. 3H), the increase did not reach significance due to high variability. Likewise, Gallyas silver (Fig. 4E) and thioflavine S (Fig. 4F) staining, both markers of late stage NFTs, did not reveal significant differences between control and treated groups (data not shown). Overall, both immunoblotting and immunostaining indicate that repeated exposure to anesthesia-induced hypothermia leads to accelerated early stage tau pathology development in both the brain stem and the spinal cord of JNPL3 mice that persists for at least a week after anesthesia exposure. However, these pathological changes do not reach the stage of full-blown neurofibrillary tangles.
Figure 4.
Tau immunostaining in the spinal cord after repeated isoflurane treatment in 8.2-mo-old homozygous JNPL3 male mice. Sections from representative control mice (A, C) and mice sampled 1 wk after the last of four isoflurane treatments (B, D, E, F) isolated from lumbar portions of the spinal cord are shown. A, B) Sections were stained with CP13 (Ser202). C, D) Sections were stained with MC1, a conformational antibody that recognizes aggregated tau. E) Section was stained with Gallyas silver staining. F) Section was stained with thioflavine S. Quantification was done on 5 animals/condition, using 3 sections from cervical, thoracic, and lumbar parts of spinal cord (n=30). Level of immunoreactivity (arbitrary units): for CP13, 26.4±6.4 in controls vs. 63.7±14.6 in treated mice (P<0.05; Mann-Whitney U test); for MC1, 6.7±1.5 vs. 9.9±2.2 (not significant). View: ×200 (A–D); ×400 (E, F).
Tau pathology and microtubule binding after repeated anesthesia
Our results demonstrate that isoflurane-induced hypothermia increases tau pathology in the brain stem and spinal cord of JNPL3 mice. We next examined whether this effect would extend to the cortex. We thus exposed 6.9 ± 0.2 mo-old male JNPL3 mice to 4 h of 1 MAC isoflurane twice a week for 2 wk and analyzed cortex samples 1 wk later. The temperature of the mice analyzed 1 wk after anesthesia (38.1±0.5°C; n=12) was not significantly different from the control mice (37.6±0.5°C; n=9). There was an increase in tau phosphorylation in the cortex (data not shown), which was accompanied by an elevation of aggregated tau (Fig. 5C). No significant increase in phospho-independent tau was seen in either the total (Fig. 5A) or soluble (heat stable) tau fractions (Fig. 5B).
Figure 5.
Immunoblot analysis of tau solubility and binding to microtubules after repeated isoflurane treatment in 6.9-mo-old homozygous JNPL3 male mice. Protein extracts from cortices of control mice (Ctl; lanes 1 and 2, n=12), and mice sampled 1 wk after the last of four isoflurane treatments (4×A+1w; lanes 3 and 4, n=9) were extracted according to a modified method of Greenberg and Davies (15) (A–C). Tau from total (A), heat-stable soluble (B), and sarkosyl-insoluble (C) fractions was evaluated by immunoblot analysis with a phospho-independent tau antibody. A microtubule (MT) binding assay was performed on cortex tissue. Total (D, G), MT-free (E, H), and MT-bound (F, I) fractions were evaluated with tau (D–F, phospho-independent) and α-tubulin (G–I) antibodies. Scatterplots represent quantification of the immunoblot bands displayed above them. Levels of tau in MT-free and MT-bound fractions were normalized to total (input) tau. Graphs display immunoreactivity expressed as percentage of control (100%). Numbers in graphs indicate percentage of the tick line with which they are aligned. Data represented are means ± sd (2 representative values displayed; each lane represents an individual mouse). *P < 0.05, **P < 0.01vs. Ctl; Mann-Whitney U test.
The cortex of JNPL3 mice has much less tau pathology than the brain stem. In the brain stem, the quantity of tau that cosediments with the MT-bound fraction impairs the quantification of tau bound to microtubules. This confound is negligible in the cortex (data not shown); therefore, a MT-binding assay was performed on the cortex. Total tau did not change (Fig. 5D), but there was an increase in tau levels in the MT-free fraction (Fig. 5E) accompanied by decreased tau in the MT-bound fraction (Fig. 5F). However, there was no difference in α-tubulin (Fig. 5G–I), Ac-tubulin (acetylated tubulin), or Tyr-tubulin (tyrosinated tubulin) in any of the fractions (data not shown). These results indicate that exposure to isoflurane-induced hypothermia can lead to detachment of tau from microtubules without overall collapse of the microtubule network.
Motor function after repeated anesthesia
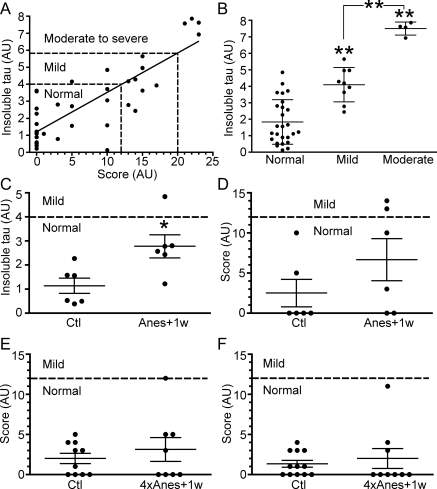
We next evaluated whether increased aggregated tau after anesthesia affects the degenerative phenotype in the mice. As the JNPL3 mice develop motor deficits, they are unsuitable for cognitive performance tests such as the Morris water maze but are amenable for evaluation of their motor function, reflected in an overall performance score. Scores of 0 to 12 show the mice have normal motor function (from 13 to 20), they have mild motor impairment, and from 20 and above, they have moderate to severe deficits. Our results show that insoluble tau levels correlate with the degree of motor impairment in JNPL3 mice (Fig. 6A, B). From these results, we could infer that levels of insoluble tau from 0 to 4 reflected normal motor function, from above 4 to 6, mild impairment, and over 6, moderate to severe motor impairment. Although we observed significant accumulation of aggregated tau 1 wk after isoflurane exposure, the levels of insoluble tau did not reach the threshold of 4 (Fig. 6C). Likewise, we did not observe worsened degenerative phenotype in all the 3 groups (Fig. 6D, F).
Figure 6.
Effect of anesthesia on motor function and tau solubility. A) Levels of sarkosyl-insoluble tau were plotted against levels of motor dysfunction (score) in 4- to 5-mo-old female JNPL3 mice (n=38, r2=0.69, P < 0.0001). B) Results from A stratified by categories. **P < 0.01 vs. Ctl; ANOVA with Neumans-Keuls post hoc test. C) Levels of insoluble tau, as detected by immunoblotting of brain stem proteins from 4.4-mo-old homozygous JNPL3 female control (Ctl; n=6), and mice examined 1 wk after a single exposure to isoflurane (Anes+1w; n=6). D) Motor dysfunction score of same group of mice as in C. E, F) motor dysfunction in homozygous male mice 8.2 mo old (E; Ctl, n=10; 4×Anes+1w, n=8), and 6.9 mo old (F; Ctl, n=12; 4×Anes+1w, n=9), respectively. Each point represents an individual mouse, with means ± se for each group. AU, arbitrary units. *P < 0.05 vs. Ctl; Mann-Whitney U test.
DISCUSSION
The purpose of this study was to examine whether anesthesia-induced hypothermia exacerbates tauopathy development in vivo, supporting the idea that exposure to anesthesia may contribute to AD pathogenesis. We found in 3 groups of mice (2 groups of males and 1 group of females) and two brain regions (brain stem and cortex), that exposure to isoflurane at a clinically relevant dose can lead to short- and long-lasting tau hyperphosphorylation, long-lasting detachment of tau from microtubules, and accumulation of insoluble tau in a mouse model with incipient neurofibrillary pathology.
NFT pathology development in AD is thought to result from the hyperphosphorylation of tau due to the deregulation of kinase and/or phosphatase activities. Hyperphosphorylated tau would dissociate from MTs and relocalize in the somatodendritic compartment. Loss of normal tau binding would then destabilize the MTs, causing neuronal dysfunction, neurodegeneration, and ultimately functional deficits (29). Our results conform to this hypothesis, demonstrating in vivo that the inhibition of phosphatase, resulting from anesthesia-induced hypothermia led to long-lasting tau hyperphosphorylation, aggregation, and dissociation from MTs. While levels of phospho-tau were at their highest during anesthesia, this did not result in alteration of levels of insoluble tau within hours of initial exposure to anesthesia. Interestingly, the levels of insoluble tau were significantly increased 1 wk later, when there was no more inhibition of phosphatase, suggesting that continuous exposure to an enhancing factor is not necessary for acceleration and persistence of neurofibrillary pathology. Indeed, it has been demonstrated that NFTs can continue to accumulate after the repression of the mutant tau, initiating the pathology in an inducible neurodegenerative mouse model (40). We propose that, in mutant mice with incipient pathology, inhibition of phosphatase activity by anesthesia-induced hypothermia leads to tau hyperphosphorylation and partial detachment from the MTs. The pool of free, hyperphosphorylated tau does not reattach to MTs when normothermia is reestablished in the animals, and it is then recruited into tau aggregates that accumulate over time.
In vitro studies have demonstrated that tau hyperphosphorylation mediated by kinase overactivation or phosphatase inhibition results in tau dissociation from MTs and destruction of MT networks (41,42,43,44). Although our data confirm the detachment of hyperphosphorylated tau from MTs in vivo, we did not observe a disassembly of MTs, as evaluated by the fractionation of three species of tubulin. One explanation for the lack of MT disassembly might be that the remaining tau bound to MTs was sufficient to stabilize the cytoskeletal structure, and indeed, tau is known to fulfill its stabilizing function in very minute amounts (45).
After detaching from MTs, tau can aggregate, and it has been suggested that aggregated tau is the toxic species that causes cognitive decline in AD (reviewed in ref. 46). This hypothesis is supported by the fact that in AD, NFT formation correlates with neuronal death and dementia (47). We thus evaluated whether increased aggregated tau after anesthesia affects the degenerative phenotype in the mice. Although we observed accumulation of aggregated tau 1 wk after isoflurane exposure, we did not observe worsened degenerative phenotype. One explanation could be that the levels of aggregated tau that accumulated in neurons was not enough to precipitate motor deficits that accompany degeneration in the hind brain and spinal cord to a degree that was measurable using our battery of tests. Indeed, our results show that insoluble tau levels correlate with the degree of motor impairment in JNPL3 mice, but that anesthesia-induced accumulation of aggregated tau did not reach the threshold necessary for motor impairment to be measurable in all 3 groups of mice.
Anesthesia appears to be associated with long-term cognitive dysfunction and the acceleration of senile dementia (reviewed in refs. 48, 49). Several reports have suggested that anesthesia could increase the risk of AD (5,6,7), (8), and hypothermia per se has also been proposed as a risk factor (50, 51). The combination of regional or general anesthetic exposure and cool operating room environment make most surgical patients mildly hypothermic. While hypothermia may be beneficial for specific, critically ill patients, perioperative hypothermia following anesthesia is clearly harmful for most patients (52). Here, anesthesia-induced hypothermia led to relatively long-term enhancement of tau pathology, suggesting that similar events could take place in the brain of patients exposed to anesthetics. This is particularly important because tau pathology correlates with the degree of cognitive impairment in AD. The observation that one exposure to isoflurane was enough to accelerate tau pathology development in female mice, which are more prone to tau accumulation (14), while repeated exposure of male mice was necessary to see a similar effect, is of particular interest. It suggests that individuals with more susceptibility, or more underlying tau pathology, such as the elderly, or those with incipient disease, are more at risk of accelerated tau accumulation after only one episode of anesthesia-induced hypothermia. Individuals with less susceptibility, or less underlying tau pathology, such as patients at an earlier age, would require multiple exposures (or possibly longer exposure) to enhance tau pathology. In support of our observations, the age of onset of AD was found to be inversely related to cumulative exposure to general and spinal anesthesia before the age of 50 (6), indicating that multiple exposures to anesthesia at an earlier age can an increase in the risk of AD.
We have demonstrated here that single or multiple exposures of JNPL3 mice to isoflurane-induced hypothermia led to tau hyperphosphorylation, aggregation, and detachment from MTs. Although more drastic events such as MT collapse and grossly enhanced dysfunction were not induced in our experimental paradigm, these results have clear clinical implications for anesthesia and AD, suggesting that perioperative hypothermia should be avoided when not medically necessary. Most of the studies on anesthesia and AD have not documented thermal management methods and the body temperature of patients during and after surgery. Reevaluation of the data with hypothermia as a parameter could lead to a more definitive conclusion on the link between anesthesia and the risk and progression of AD, and possible preventive measures.
Acknowledgments
The authors are grateful to Dr. Jada Lewis (Mayo Clinic, Jacksonville, FL, USA) for help with the motor function analysis. We thank Dr. Peter Davies (Albert Einstein College of Medicine, Bronx, NY, USA) for the generous gift of antibodies. We are also grateful to Helen Y. Figueroa and Mathieu Herman for technical help. This work was supported by an Institute for the Study of Aging/Alzheimer’s Drug Discovery Foundation grant (to E.P.), a National Institute of Neurological Disorders and Stroke grant (NS048447), and a National Institute on Aging grant (AG017216; to K.E.D.).
References
- Selkoe D J. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof P R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Ancelin M L, de Roquefeuil G, Ledesert B, Bonnel F, Cheminal J C, Ritchie K. Exposure to anaesthetic agents, cognitive functioning and depressive symptomatology in the elderly. Br J Psychiatry. 2001;178:360–366. doi: 10.1192/bjp.178.4.360. [DOI] [PubMed] [Google Scholar]
- Rees D I, Gaines G Y., 3rd Anesthetic considerations for patients with Alzheimer’s disease. Tex Med. 1985;81:45–48. [PubMed] [Google Scholar]
- Bone I, Rosen M. Alzheimer’s disease and anaesthesia. Anaesthesia. 2000;55:592–593. doi: 10.1046/j.1365-2044.2000.01479-5.x. [DOI] [PubMed] [Google Scholar]
- Bohnen N, Warner M A, Kokmen E, Kurland L T. Early and midlife exposure to anesthesia and age of onset of Alzheimer’s disease. Int J Neurosci. 1994;77:181–185. doi: 10.3109/00207459408986029. [DOI] [PubMed] [Google Scholar]
- Johansson C, Skoog I. A population-based study on the association between dementia and hip fractures in 85-year olds. Aging (Milano) 1996;8:189–196. doi: 10.1007/BF03339676. [DOI] [PubMed] [Google Scholar]
- Eckenhoff R G, Eckenhoff M F. Anesthesia, Amyloid and Alzheimer’s. Cellscience Rev. 2007;4:78–96. [Google Scholar]
- Eckenhoff R G, Johansson J S, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller J M, Eckenhoff M F. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Maeda U, Alfille P, Culley D J, Crosby G, Tanzi R E. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104:988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- Bianchi S L, Tran T, Liu C, Lin S, Li Y, Keller J M, Eckenhoff R G, Eckenhoff M F. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2007;29:1002–1010. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E, Richter K E G, Nolan C E, Finley J E, Liu L, Wen Y, Krishnamurthy P, Herman M, Wang L, Schachter J B, Nelson R B, Lau L-F, Duff K E. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci. 2007;27:3090–3097. doi: 10.1523/JNEUROSCI.4854-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin W L, Yen S H, Dickson D W, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Sahara N, Lewis J, DeTure M, McGowan E, Dickson D W, Hutton M, Yen S H. Assembly of tau in transgenic animals expressing P301L tau: alteration of phosphorylation and solubility. J Neurochem. 2002;83:1498–1508. doi: 10.1046/j.1471-4159.2002.01241.x. [DOI] [PubMed] [Google Scholar]
- Greenberg S G, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, Gaynor K, LaFrancois J, Wang L, Kondo T, Davies P, Burns M, Veeranna , Nixon R, Dickson D, Matsuoka Y, Ahlijanian M, Lau L F, Duff K. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38:555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P H. High-resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Planel E, Yasutake K, Fujita S C, Ishiguro K. Inhibition of protein phosphatase 2A overrides Tau protein kinase I/glycogen synthase kinase 3β and cyclin-dependent kinase 5 inhibition and results in tau hyperphosphorylation in the hippocampus of starved mouse. J Biol Chem. 2001;276:34298–34306. doi: 10.1074/jbc.M102780200. [DOI] [PubMed] [Google Scholar]
- Planel E, Krishnamurthy P, Miyasaka T, Liu L, Herman M, Kumar A, Bretteville A, Figueroa H Y, Yu W H, Whittington R A, Davies P, Takashima A, Nixon R A, Duff K E. Anesthesia-induced hyperphosphorylation detaches 3-repeat tau from microtubules without affecting their stability in vivo. J Neurosci. 2008;28:12798–12807. doi: 10.1523/JNEUROSCI.4101-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jicha G A, Lane E, Vincent I, Otvos L, Jr, Hoffmann R, Davies P. A conformation- and phosphorylation-dependent antibody recognizing the paired helical filaments of Alzheimer’s disease. J Neurochem. 1997;69:2087–2095. doi: 10.1046/j.1471-4159.1997.69052087.x. [DOI] [PubMed] [Google Scholar]
- Otvos L, Jr, Feiner L, Lang E, Szendrei G I, Goedert M, Lee V M. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39:669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett. 1995;189:167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- Szendrei G I, Lee V M, Otvos L., Jr Recognition of the minimal epitope of monoclonal antibody Tau-1 depends upon the presence of a phosphate group but not its location. J Neurosci Res. 1993;34:243–249. doi: 10.1002/jnr.490340212. [DOI] [PubMed] [Google Scholar]
- Weaver C L, Espinoza M, Kress Y, Davies P. Conformational change as one of the earliest alterations of tau in Alzheimer’s disease. Neurobiol Aging. 2000;21:719–727. doi: 10.1016/s0197-4580(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Fujishiro H, Tsuboi Y, Lin W L, Uchikado H, Dickson D W. Co-localization of tau and alpha-synuclein in the olfactory bulb in Alzheimer’s disease with amygdala Lewy bodies. Acta Neuropathol. 2008;116:17–24. doi: 10.1007/s00401-008-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy C, Duyckaerts C, Delaere P, Payan C, Fermanian J, Poulain V, Hauw J J. Comparison of seven staining methods for senile plaques and neurofibrillary tangles in a prospective series of 15 elderly patients. Neuropathol Appl Neurobiol. 1989;15:563–578. doi: 10.1111/j.1365-2990.1989.tb01255.x. [DOI] [PubMed] [Google Scholar]
- Tolivia J, Navarro A, del Valle E, Perez C, Ordonez C, Martinez E. Application of Photoshop and Scion Image analysis to quantification of signals in histochemistry, immunocytochemistry and hybridocytochemistry. Anal Quant Cytol Histol. 2006;28:43–53. [PubMed] [Google Scholar]
- Trojanowski J Q, Lee V M. Paired helical filament tau in Alzheimer’s disease. The kinase connection. Am J Pathol. 1994;144:449–453. [PMC free article] [PubMed] [Google Scholar]
- Maccioni R B, Otth C, Concha I I, Munoz J P. The protein kinase Cdk5. Structural aspects, roles in neurogenesis and involvement in Alzheimer’s pathology. Eur J Biochem. 2001;268:1518–1527. doi: 10.1046/j.1432-1033.2001.02024.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee H G, Raina A K, Perry G, Smith M A. The role of mitogen-activated protein kinase pathways in Alzheimer’s disease. Neurosignals. 2002;11:270–281. doi: 10.1159/000067426. [DOI] [PubMed] [Google Scholar]
- Planel E, Sun X, Takashima A. Role of GSK-3 beta in Alzheimer’s disease pathology. Drug Dev Res. 2002;56:491–510. [Google Scholar]
- Litersky J M, Johnson G V, Jakes R, Goedert M, Lee M, Seubert P. Tau protein is phosphorylated by cyclic AMP-dependent protein kinase and calcium/calmodulin-dependent protein kinase II within its microtubule-binding domains at Ser-262 and Ser-356. Biochem J. 1996;316:655–660. doi: 10.1042/bj3160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi J J, Yen S H, Gondal J A, Wu Q, Grundke-Iqbal I, Iqbal K. Ser-262 in human recombinant tau protein is a markedly more favorable site for phosphorylation by CaMKII than PKA or PhK. FEBS Lett. 1998;436:471–475. doi: 10.1016/s0014-5793(98)01185-5. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H, Pyo H K, Feinstein B, Pasinetti G M. Akt/PKB kinase phosphorylates separately Thr212 and Ser214 of tau protein in vitro. Biochim Biophys Acta. 2003;1639:159–168. doi: 10.1016/j.bbadis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Reynolds C H, Betts J C, Blackstock W P, Nebreda A R, Anderton B H. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3β. J Neurochem. 2000;74:1587–1595. doi: 10.1046/j.1471-4159.2000.0741587.x. [DOI] [PubMed] [Google Scholar]
- Planel E, Miyasaka T, Launey T, Chui D H, Tanemura K, Sato S, Murayama O, Ishiguro K, Tatebayashi Y, Takashima A. Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: implications for Alzheimer’s disease. J Neurosci. 2004;24:2401–2411. doi: 10.1523/JNEUROSCI.5561-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennecib M, Gong C, Grundke-Iqbal I, Iqbal K. Role of protein phosphatase-2A and -1 in the regulation of GSK-3, cdk5 and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett. 2000;485:87–93. doi: 10.1016/s0014-5793(00)02203-1. [DOI] [PubMed] [Google Scholar]
- Gong C X, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J Biol Chem. 2000;275:5535–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe K H. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurland G, Gundersen G G. Protein phosphatase inhibitors induce the selective breakdown of stable microtubules in fibroblasts and epithelial cells. Proc Natl Acad Sci U S A. 1993;90:8827–8831. doi: 10.1073/pnas.90.19.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick S E, Trojanowski J Q, Lee V M. Selective destruction of stable microtubules and axons by inhibitors of protein serine/threonine phosphatases in cultured human neurons. J Neurosci. 1997;17:5726–5737. doi: 10.1523/JNEUROSCI.17-15-05726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Lee G, Bloom G S, Mumby M C. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- Kim D, Su J, Cotman C W. Sequence of neurodegeneration and accumulation of phosphorylated tau in cultured neurons after okadaic acid treatment. Brain Res. 1999;839:253–262. doi: 10.1016/s0006-8993(99)01724-2. [DOI] [PubMed] [Google Scholar]
- Levy S F, Leboeuf A C, Massie M R, Jordan M A, Wilson L, Feinstein S C. Three- and four-repeat tau regulate the dynamic instability of two distinct microtubule subpopulations in qualitatively different manners. Implications for neurodegeneration. J Biol Chem. 2005;280:13520–13528. doi: 10.1074/jbc.M413490200. [DOI] [PubMed] [Google Scholar]
- Bretteville A, Planel E. Tau aggregates: toxic, inert, or protective species? J Alzheimers Dis. 2008;14:431–436. doi: 10.3233/jad-2008-14411. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann F R, Bussiere T, Bouras C, Kovari E, Perl D P, Morrison J H, Gold G, Hof P R. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Polge C, de Roquefeuil G, Djakovic M, Ledesert B. Impact of anesthesia on the cognitive functioning of the elderly. Int Psychogeriatr. 1997;9:309–326. doi: 10.1017/s1041610297004468. [DOI] [PubMed] [Google Scholar]
- Xie Z, Tanzi R E. Alzheimer’s disease and post-operative cognitive dysfunction. Exp Gerontol. 2006;41:346–359. doi: 10.1016/j.exger.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Holtzman A, Simon E W. Body temperature as a risk factor for Alzheimer’s disease. Med Hypotheses. 2000;55:440–444. doi: 10.1054/mehy.2000.1085. [DOI] [PubMed] [Google Scholar]
- Avila J, Diaz-Nido J. Tangling with hypothermia. Nat Med. 2004;10:460–461. doi: 10.1038/nm0504-460. [DOI] [PubMed] [Google Scholar]
- Sessler D I. Perioperative heat balance. Anesthesiology. 2000;92:578–596. doi: 10.1097/00000542-200002000-00042. [DOI] [PubMed] [Google Scholar]