Abstract
Intrauterine growth restriction (IUGR) decreases serum insulin growth factor-1 (IGF-1) levels. IGF-1 is an epigenetically regulated gene that has two promoters, alternative exon 5 splicing, and multiple termination sites. The regulation of gene expression involves the whole gene, as evidenced by the aforementioned IGF-1 paradigm. We hypothesized that IUGR in the rat would affect hepatic IGF-1 expression and alter the epigenetic characteristics of the IGF-1 gene along its length. IUGR was induced through a bilateral uterine artery ligation of the pregnant rat, a well-characterized model of IUGR. Pups from anesthesia and sham-operated dams were used as controls. Real-time RT-PCR and ELISA was used to measure expression at day of life (DOL) 0 and 21. Bisulfite sequencing and chromatin immunoprecipitation (ChIP) quantified IGF-1 epigenetic characteristics. A nontranscribed intergenic control was used for ChIP studies. IUGR decreased hepatic and serum IGF-1. Concurrently, IUGR modified epigenetic characteristics, particularly the histone code, along the length of the hepatic IGF-1 gene. Many changes persisted postnatally, and the postnatal effect of IUGR on the histone code was gender-specific. We conclude that IUGR modifies epigenetic characteristics of the rat hepatic IGF-1 gene along the length of the whole gene.—Fu, Q., Yu, X., Callaway, C. W., Lane, R. H., McKnight, R. A. Epigenetics: intrauterine growth retardation (IUGR) modifies the histone code along the rat hepatic IGF-1 gene.
Keywords: chromatin, DNA methylation, Barker hypothesis, chromatin immunoprecipitation, insulin resistance
The early nutritional milieu of the fetus influences the adult phenotype. A relevant marker for this phenomenon is intrauterine growth restriction (IUGR) (1). IUGR is a significant cause of human morbidity and mortality that is associated with many of the most common complications of pregnancy, such as preeclampsia and other hypertensive disorders of pregnancy (2,3,4). Multiple studies demonstrate that IUGR predisposes human infants toward several postnatal morbidities, including poor postnatal growth and insulin resistance (5,6,7,8,9,10,11,12). Though moderately controversial, the positive relationship between growth retardation and adult morbidities, including insulin resistance, appears to be stronger in men than in women (5, 6, 12). Growth and insulin sensitivity are modulated by hepatic IGF-1.
In humans and rodents, absence of IGF-1 leads to poor prenatal growth (13). Moreover, multiple investigators find that human IUGR infants have lower fetal or cord IGF-1 concentrations when compared with appropriately sized infants (13,14,15). Postnatally, IUGR decreases serum IGF-1 levels in human infants during the first 9 mo of life (16). Furthermore, IUGR also decreases serum IGF-1 levels in preadolescent children who do not exhibit significant “catch-up” growth (17). Finally, adults that lack IGF-1 suffer from decreased insulin sensitivity (18).
Fetal and newborn rats with induced IUGR also suffer from decreased serum levels of IGF-1, and the organization of the rat IGF-1 gene is remarkably similar to the human IGF-1 gene (19, 20). Both human and rat IGF-1 genes are large and contain 6 exons separated by 5 introns (21, 22). Extensive nucleotide and amino acid conservation exist between the IGF-1 genes of both species, as well as comparable expression of multiple mRNA variants (22). These mRNA variants are useful markers of altered transcriptional regulation and may function to modulate translational efficiency and cell growth.
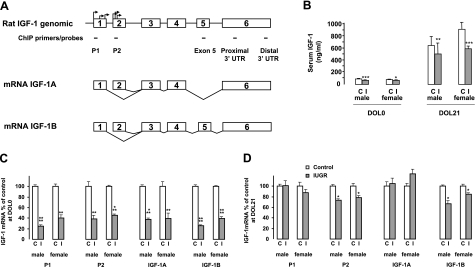
In the rat (Fig. 1A), exon 1 and exon 2 encode two different leader sequences and involve multiple transcription start sites with multiple inframe ATGs. Either exon 1 or exon 2 is spliced to the exon 3, which encodes the N terminus of the mature peptide (23,24,25). Exon 1-derived [promoter 1 (P1)] transcripts predominate in every tissue expressing the IGF-1 gene, with the exception of the liver, in which relatively high levels of Exon 2-derived [promoter 2 (P2)] transcripts are produced (26, 27).
Figure 1.
A) Rat IGF-1 gene structure and alternative splicing. Exons and introns are shown as boxes and horizontal lines, respectively. Transcription start sites are indicated by arrows. The 5 sets of ChIP primers/probes for P1, P2, exon 5, proximal, and distal 3′ UTR are shown as black bars. B) Rat serum IGF-1 levels at DOL0 and DOL21. C, D) DOL0 (C) and DOL21 rat hepatic IGF-1 mRNA variant levels (D). Graphs represent IGF-1 mRNA expressed as mean ± se percentage of control for male and female rats. P1, transcripts starting from exon 1; P2, transcripts starting from exon 2; IGF-1A, transcripts without exon 5; IGF-1B, transcripts with exon 5. White bars indicate control values; gray bars indicate IUGR. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Another variable that occurs with hepatic IGF-1 mRNA transcripts involves the inclusion or exclusion of exon 5, which changes the translational reading frame of the peptide coding region of exon 6. The IGF-1A transcript lacks exon 5, while IGF-1B contains exon 5 (23, 28, 29). Unfortunately, little evidence ties specific promoter usage to a specific IGF-1A or IGF-1B transcript, respectively. Finally, multiple polyadenylation sites in the 3′ untranslated region (UTR) of the IGF-1 gene generates different lengths of mRNAs, ranging from 0.8 to 7.5 kb. The high-molecular-weight species represents <50% of total IGF-1 mRNA in liver, in contrast to most other tissues.
The above IGF-1 variants are likely examples of epigenetic regulation, which denotes an inherited state of gene regulation that is independent of the genetic information encoded within the DNA itself (30,31,32). Epigenetic regulation involves covalent modification of DNA and histones (33,34,35). DNA methylation within a promoter and 5′ transcribed region usually represses gene transcription, whereas DNA methylation toward the 3′ end often signifies gene activation (36). A dynamic relationship exists between DNA methylation and the associated histone code (histone covalent modifications that determine how DNA and histones interact). These histone covalent modifications affect transcriptional regulation and include histone acetylation and methylation. For example, histone H3 acetylation (ac) at lysine (K) 9 and 14 and trimethylation (me3) at K4 are often associated with gene activation, while me3K36 is associated with actively transcribed regions.
The location of a modification is tightly regulated and is crucial for its effect on transcriptional regulation, and these marks affect transcription within the context of the neighboring histone codes (37,38,39,40). Furthermore, histone modifications at one site have been known to influence modifications at additional sites (41).
Because IUGR affects IGF-1 serum levels in humans and rats, we hypothesized that IUGR in rats would decrease serum levels of postnatal IGF-1, alter hepatic IGF-1 mRNA species levels, affect postnatal DNA methylation in the hepatic IGF-1 5′ flanking region, and modify multiple markers of the postnatal histone code. To understand the effect of IUGR on the hepatic IGF-1 epigenetic characteristics, we would also need to determine the histone code along the entire gene.
To test this hypothesis, we used a model of uteroplacental insufficiency and subsequent IUGR that has been well characterized by multiple groups (42,43,44). In this model, bilateral uterine artery ligation is performed on d 19 of gestation in the pregnant Sprague-Dawley rat. IUGR pups in this model are ∼25% smaller than control pups, and IUGR pups are predisposed to develop both growth failure and insulin resistance early in life, as well as overt diabetes relatively late in life (44, 45). Interestingly, gender-specific responses to IUGR become evident for multiple processes by day of life 21 (DOL21) in this model. We therefore tested our hypothesis at DOL0 and DOL21 in both genders to determine the consequences of IUGR on IGF-1 chromatin structure during the perinatal and postnatal period. We chose DOL21 purposefully to avoid the confounding effects of adolescence and senescent maturation.
MATERIALS AND METHODS
Animals
All procedures were approved by the University of Utah Animal Care committee and are in accordance with the APS Guiding Principles (46). Bilateral uterine artery ligation was performed on d 19 of gestation in pregnant Sprague-Dawley rats. Surgical procedures have been described previously (42, 43, 47,48,49). The DOL0 pups were delivered by cesarean section at term. DOL21 animals were separated from their dams for 4 h, anesthetized, and sacrificed at the two ages; livers were quickly removed, flash-frozen in liquid nitrogen, and stored at −80°C.
Enzyme immunoassay (EIA)
Serum IGF-1 levels at DOL0 and DOL21 were measured by using mouse/rat enzyme immunoassay (EIA) kit (DSL, Webster, TX, USA) following the manufacturer’s protocol.
RNA isolation
Total RNA isolation was performed as described earlier (42, 43). Total RNA was extracted from DOL0 and DOL21 liver using the Nucleospin RNAII kit (Macherey-Nagel, Bethlehem, PA, USA), including DNase I treatment. RNA was then quantified spectrophotometrically and checked by gel electrophoresis for integrity.
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Real-time RT-PCR was performed as described earlier (42, 43). Target primers and probes were designed using Primer Express software (Applied Biosystems, Foster City, CA, USA) (Table 1). Briefly, to test the transcripts starting from P1, primers spanned the junction of exon 1 and exon 3. For transcripts initiated from P2, primers spanned the junction of exon 2 and exon 3. For IGF-1A transcripts, the primers spanned the junction of exon 4 and exon 6, while for IGF-1B, the primers spanned the junction of exon 5 and exon 6. Real-time RT-PCR quantification was then performed using glyceraldehyde-3 phosphate dehydrogenase (GAPDH) as an internal control. Relative quantification of PCR products was based on value differences between the target and GAPDH control using the comparative Ct method (TaqMan Gold RT-PCR manual; PE Biosystems, Foster City, CA, USA).
TABLE 1.
Real-time RT-PCR primer/probe sets
| Transcript | Sequence |
|---|---|
| IGF-1A | For 5′ GTGTCCGCTGCAAGCCTAC |
| Rev 5′ CAAGTGTACTTCCTTCTGAGTCTTGG | |
| Probe 5′ 6FAM-AAGTCAGCTCGTTCCATCCGGGC | |
| IGF-1B | For 5′ CACTGACATGCCCAAGACTCA |
| Rev 5′ CCTTCTCCTTTGCAGCTTCCT | |
| Probe 5′ 6FAM-AAGTCCCAGCCCCTATCGACACACAA | |
| IGF-1 P1 | For 5′ TTTGTACTTCAGAAGCGATGGG |
| Rev 5′ CGACATGATGTGTATCTTTATCTTCAAG | |
| Probe 5′ 6FAM-TTCCAACTCAATTATTTAAGATCTGCCTCTGTGA | |
| IGF-1 P2 | For 5′ ACCCACTCTGACCTGCTGTGT |
| Rev 5′ ATGTGTATCTTTATTGGAGGTGCG | |
| Probe 5′ 6FAM-AACGACCCGGGACGTACCAAAATGA | |
| GAPDH | For 5′ CAAGATGGTGAAGGTCGGTGT |
| Rev 5′ CAAGAGAAGGCAGCCCTGGT | |
| Probe 5′ 6FAM-GCGTCCGATACGGCCAAATCCG |
Bisulfite modification
Bisulfite modification was performed as described earlier (42). IGF-1 P1 and P2 have multiple transcription start sites. We elected to analyze CpG sites that were upstream of the longest known 5′ UTR of transcripts from each promoter. Relative to the cDNA clone accession M15647, the 12 CpG sites in P1 were at −528, −523, −470, −302, −260, −231, −143, −86, −31, −29, +13, and + 22. Bisulfite-treated genomic DNA was amplified with the following primers: CpG sites between −528 and −470: set 1 forward 5′-ACCTTCTTTCATAATTCACTTTCC, reverse 5′-GGGTAAGTGGTTGGTAGT ATGG; CpG sites between −302 and −143: set 2 forward 5′-TAGTTGTGGTTATGGGGT AGTATTAA, reverse 5′-AATTACAAAAACCCAAATCAAATACT; CpG sites between −86 and + 22: set 3 forward 5′-AAAAATATCTC TCTTCCTACCTATTAC, reverse 5′-TTAGATAGGAATATTAGAAATTTGGGG. Relative to the cDNA clone accession no. NM_178866, the 6 CpG sites in P2 were at −231, −142, −133, −112, −70, and −41 on the genomic DNA. The primer sequences for CpG sites −231 to −112: set 4 forward 5′-GGAGGGTTTAATTTATAAAAGATTTTAG, reverse 5′-CCCAAACCACTTCCTTACCTAA; CpG sites between −70 and −41: set 5 forward 5′-GTTGTTGTTGTTATTGTTYGTGGTA, reverse 5′-CTAAAATCTTTTATAAATTAAACCCTCC. PCR conditions for the primers were 95°C for 10 min, followed by 94°C for 30 s, annealing at 53°C (sets 1, 4, and 5) or 54°C (sets 2 and 3) for 30 s, 72°C for 30 s, 35 cycles. For each group, 4 animals were analyzed by bisulfite sequencing. The PCR products from bisulfite-treated genomic DNA were cloned into the vector pSC-A (Stratagene, Cedar Creek, TX, USA). Six colonies from each PCR cloning were inoculated into SeqPrep™ 96 plates (Edge BioSystems, Gaithersburg, MD, USA). The plasmid DNA was prepared by using SeqPrep 96 Plasmid Prep Kit (Edge BioSystems) and sequenced according to the manufacturer’s instructions for double-stranded plasmid DNA using the BigDye® Terminator v3.1 Cycle Sequencing kit (Applied Biosystems) with M13 forward or reverse primers. Using these primers, we often encountered multiple sequence traces per reaction. Therefore, the alternative sequencing primer 5′-TGCAGCCCAATGTGGAATTCG was used successfully to sequence the majority of the failed colonies.
Chromatin immunoprecipitation (ChIP) assay and real-time PCR
ChIP with anti-acK9H3 (Cell Signaling Technologies, Beverly, MA, USA), anti-acK14H3, anti-me2K4H3, anti-me3K4H3, anti-me3K9H3 (Millipore Upstate, Charlottesville, VA, USA), or anti-me3K36H3 (Abcam, Cambridge, MA, USA) was performed as described earlier (42). The chromatin equivalent of 100 μg DNA based on the absorption at A260 was used in each immunoprecipitation (IP). For anti-acK14H3, 20 μg of antibody was used. For all of the others, the volume of antibody was equal to the volume of formaldehyde cross-linked chromatin. After purification from IP chromatin, DNA was determined against a standard curve with SYBR Safe DNA gel stain (Molecular Probes, Eugene, OR, USA). The SYBR Safe fluorescence was measured with a Tecan plate reader (Genios Pro-Basic w/o FP; Tecan Austria GmbH, Grodig, Austria) and Magellan V 6.2 software (Tecan). DNA fragments containing IGF-1 site-specific sequences, including P1, P2, exon 5, and proximal and distal 3′ UTR of the IGF-1 gene and an intergenic region, were quantified by real-time PCR. Primer and probe sequences listed in Table 2, and their location on IGF-1, shown in Fig. 1A, were designed using Primer Express software (Applied Biosystems). Intergenic sequences have been used as internal controls in ChIP assays (50). Therefore, we used a site 263.8 kb (accession no. BH351084) upstream of the IGF-1 gene, which is not transcribed, as an intergenic control. This region was found to contain low levels of all 6 histone covalent modifications, with the signal proportional to the amount of input DNA from each ChIP analysis (Table 3). Relative quantification of PCR products was based on value differences between the target and the intergenic control using the comparative Ct method (TaqMan Gold RT-PCR manual; PE Biosystems).
TABLE 2.
ChIP/real-time PCR primer/probe sets
| Transcript | Sequence |
|---|---|
| P1 | For 5′ CAGGTCTGGCTCATTTCCATC |
| Rev 5′ GCGCTTTCCATGGCTGTC | |
| Probe 5′ 6FAM-CCCCTGGGAAAGCACACCTGGA | |
| P2 | For 5′ GCCGGAGGGCTTAATTCATAA |
| Rev 5′ GGAAGCATTTGAAAGCAGCAC | |
| Probe 5′ 6FAM-AGATCCCAGTCAAAGAGTGCAGCGTTTC | |
| Exon 5 | For 5′ GCCCCTATCGACACACAAGAA |
| Rev 5′ TCCTGGGTGTGCCTTTGAC | |
| Probe 5′ 6FAM-CACCTTTCCTTCTCCTTTGCAGCTTCCT | |
| Proximal 3′ UTR | For 5′ GACCTACAGAATGTAGGAGGAGCC |
| Rev 5′ GATGTTTTGCAGGTTGCTCAAG | |
| Probe 5′ 6FAM-ATGCCACGTCACCGCAAGATCCTTT | |
| Distal 3′ UTR | For 5′ ACAATAGAGGTGGCCTTCTCCA |
| Rev 5′ CACTGGGAATCATCGAAGCC | |
| Probe 5′ 6FAM-ATCGGTGGGCTTCCTGCCATGG | |
| Intergenic region | For 5′ AAGTGGCAACTCCATGACTCAA |
| Rev 5′ GCCTGGTGTCACACCCAAA | |
| Probe 5′ 6FAM-ATGCCTTCCAGAGAGGTTTGGTACTGCC |
TABLE 3.
ChIP analysis of intergenic region 5′ of IGF-1 confirms presence of histone covalent modifications with signals proportional to the amount of input DNA
| DNA from ChIP | Amount (ng) | Ct of intergenic region |
|---|---|---|
| acK9H3 | 1 | 35.9 |
| 4 | 34.1 | |
| acK14H3 | 1 | 30.9 |
| 4 | 29.1 | |
| me2K4H3 | 1 | 34.6 |
| 4 | 31.6 | |
| me3K4H3 | 1 | 35.3 |
| 4 | 32.7 | |
| me3K9H3 | 1 | 34.7 |
| 4 | 32.8 | |
| me3K36H3 | 1 | 32.7 |
| 2 | 31.1 |
The distribution pattern of histone covalent modifications along the IGF-1 gene was determined by looking at the same six modifications at the five sites indicated above. Values were expressed as percentage of the P1 site. To absolutely quantitate the five sites in each sample and establish that all primers and probes annealed equivalently, a synthetic template was generated by cloning the five sites into a single plasmid of pBSKII (Stratagene) (synthetic template cloning primers are shown in Table 4). The synthetic template was diluted serially, and each site was quantified by real-time PCR. Ct values for all five primer/probe sets were found to be equivalent (Table 5).
TABLE 4.
Primers used to clone IGF-1 sequences to make synthetic template used in standard curve for real-time PCR quantification
| Transcript | Sequence |
|---|---|
| P1 | For 5′ gctctagaCAGGTCTGGCTCATTTCCATC |
| Rev 5′ cgggatccGCGCTTTCCATGGCTGTC | |
| P2 | For 5′ cgggatccGCCGGAGGGCTTAATTCATAA |
| Rev 5′ gtctgcagcGGAAGCATTTGAAAGCAGCAC | |
| Exon 5 | For 5′ gtctgcagcGCCCCTATCGACACACAAGAA |
| Rev 5′ ccaagcttTCCTGGGTGTGCCTTTGAC | |
| Proximal 3′ UTR | For 5′ ccaagcttGACCTACAGAATGTAGGAGGAGCC |
| Rev 5′ ccggtcgacGATGTTTTGCAGGTTGCTCAAG | |
| Distal 3′ UTR | For 5′ ccctcgagACAATAGAGGTGGCCTTCTCCA |
| Rev 5′ gtggtacctCACTGGGAATCATCGAAGCC |
Lowercase indicates restriction sites added for cloning purposes.
TABLE 5.
IGF-1 real-time/ChIP primer/probe efficiency
| Synthetic template/rxn (fmol) | Site | Mean Ct | % of P1 |
|---|---|---|---|
| 0.1 | P1 | 18.7 | 100.0 |
| 0.1 | P2 | 19.0 | 101.6 |
| 0.1 | Exon 5 | 19.1 | 100.3 |
| 0.1 | Proximal 3′ UTR | 18.9 | 99.0 |
| 0.1 | Distal 3′ UTR | 18.8 | 99.5 |
Statistics
Data were expressed as mean ± se percentage of control or of P1. ANOVA (Fisher’s protected least-significant difference) and Student’s unpaired t test were used for real-time RT-PCR. Student’s 2-tailed t test was used for statistical significance for DNA methylation. A value of P < 0.05 was considered statistically significant.
RESULTS
DOL0 and DOL21 serum IGF-1 levels reduced in IUGR
IUGR significantly decreased serum IGF-1 levels in both male and female rats vs. controls at DOL 0 (67.4±2.8 vs. 88.2 ng/ml in males; P<0.001; 69.1±3.4 vs. 78.4±1.5 ng/ml in females; P<0.05). At DOL21, serum IGF-1 levels in both male and female rats continued to be significantly decreased (501±175 vs. 638±136 ng/ml in males; P<0.01; 591±38 vs. 913±103 ng/ml in females; P<0.001) (Fig. 1B).
Hepatic IGF-1 mRNA levels
At DOL0, IUGR significantly decreased P1-initiated transcripts to 25 and 40% of controls in males and females, respectively (Fig. 1C). Similarly, P2-initiated transcripts were reduced to 39 and 46% of male and female controls. IGF-1A variant transcripts were reduced to approximately one-third of male and female control levels, and IGF-1B was 26 and 39% of control male and female levels, respectively. At DOL21, in IUGR animals, P2-initiated transcripts (75 and 79% of controls in males and females, respectively) and IGF-1B transcripts (70 and 85% of controls in males and females) were still less than controls (Fig. 1D).
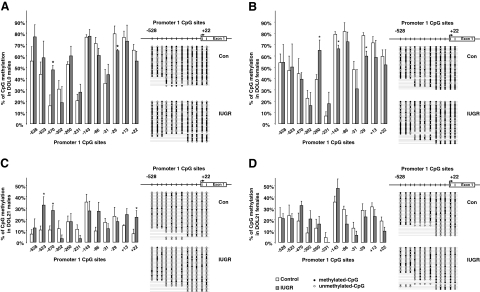
IGF-1 P1 DNA methylation
Twelve CpG sites were analyzed for methylation status within P1 of IGF-1. At DOL0 in males, uteroplacental insufficiency significantly increased CpG methylation at the −470 CpG site with a trend toward hypermethylation at −523 and −528 sites relative to controls (Fig. 2A). In DOL0 females, the −260 CpG site was hypermethylated, while sites −143 and −29 were hypomethylated (Fig. 2B). At DOL21, sites +22, −470, and −523 were significantly hypermethylated in IUGR males relative to controls (Fig. 2C), with no significant difference in IUGR females relative to controls (Fig. 2D).
Figure 2.
Rat hepatic IGF-1 P1 CpG methylation. Graphs represent mean ± se percentage of CpG methylation between −528 and +22. A) DOL0 male. B) DOL0 female. C) DOL21 male. D) DOL21 female. White bars indicate control values; gray bars indicate IUGR. Right panels show methylation pattern; each horizontal row of beads represents 12 CpG sites. Open circles represent unmethylated CpG sites; solid circles represent methylated CpG sites. *P < 0.05.
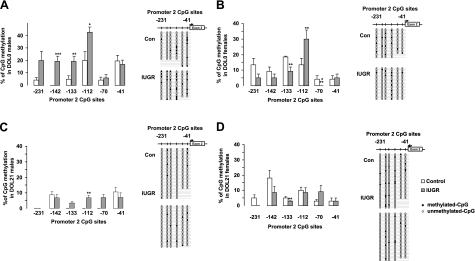
IGF-1 P2 DNA methylation
Six CpG sites were analyzed for methylation status within P2 of IGF-1. At DOL0 in males, uteroplacental insufficiency significantly increased CpG methylation at sites −112, −133, and −142 relative to controls (Fig. 3A). In females, only site −112 was hypermethylated, whereas site −133 was hypomethylated (Fig. 3B). At DOL21, site −112 remained significantly hypermethylated in IUGR males (Fig. 3C), while site −133 remained hypomethylated in females (Fig. 3D).
Figure 3.
Rat hepatic IGF-1 P2 CpG methylation. Graphs represent mean ± se percentage of CpG methylation between −231 and −41. A) DOL0 male. B) DOL0 female. C) DOL21 male. D) DOL21 female. White bars indicate control values; gray bars indicate IUGR. Right panels show methylation pattern; each horizontal row of beads represents 6 CpG sites. Open circles represent unmethylated CpG sites; solid circles represent methylated CpG sites. *P < 0.05; **P < 0.01; ***P < 0.001.
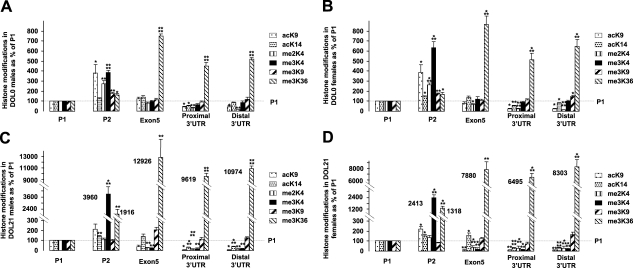
Hepatic IGF-1 histone code
Five sites along the IGF-1 gene were analyzed for six histone H3 covalent modifications in the control group to determine a “normal” histone code of the rat hepatic IGF-1 gene. The extent of each modification at the specific sites was quantified by ChIP/real-time PCR and expressed as a percentage of that observed at site P1, the predominant promoter in most tissues. For control DOL0 male and females (Fig. 4A), acetylation at K9 was significantly higher at P2 relative to P1, and acetylation of this site progressively decreased toward the 3′ UTR region of IGF-1. Acetylation at K14 was also increased in both sexes but was statistically significant only in females. Di- and trimethylation at K4 followed a similar pattern, with both forms of methylation being higher at P2 relative to P1. Though not as robust, trimethylation at K9 was increased significantly at P2 relative to P1, without the diminution of this marker in the 3′ UTR regions. In contrast to patterns of the previous described covalent modifications, trimethylation of K36 was highest at exon 5 and the 3′ UTR regions vs. the 5′ end of the hepatic IGF-1 gene. It is important to note that a similar pattern of histone code for these modifications was observed for control DOL0 male and females (Fig. 4B).
Figure 4.
Distribution pattern of histone modifications along the IGF-1 gene in control DOL0 and DOL21 rat livers. Six histone modifications at 5 sites on the IGF-1 locus were analyzed by ChIP/real-time PCR. Values for 4 sites, including P2, exon 5, and proximal and distal 3′ UTR, are presented as mean ± se percentage of P1value (set to 100%). A) DOL0 male. B) DOL0 female. C) DOL21 male. D) DOL21 female. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
At DOL21 in control males, the distribution patterns of acK9 and acK14 were essentially equivalent to DOL0, while the histone H3 methylation patterns differed (Fig. 4C). A shift away from me2K4 and toward extensive accumulation of me3K4 at P2 was noted. Trimethyl K9 was uniformly distributed across the gene. The pattern for me3K36 was similar to that seen at DOL0 but at a much higher level. Importantly, as was seen at DOL0, DOL21 females showed a pattern similar to DOL21 males (Fig. 4D).
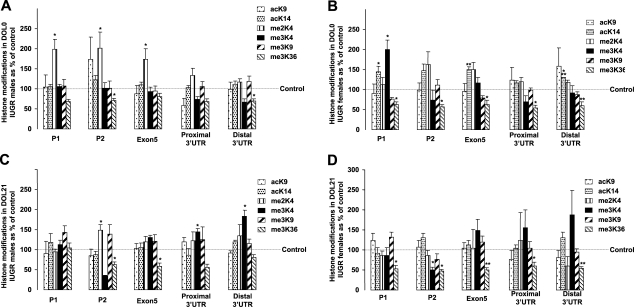
The same six histone H3 covalent modifications at the five sites were then analyzed in IUGR rat liver and quantified relative to the control animals, where the controls were considered to be 100%, after normalization with the 263.8-kb intergenic region for both groups. Data in the figures are presented as percentage of gender- and age-matched control samples. In some circumstances in which it appears that IUGR had a significant effect on a specific modification, the change was not statistically significant secondary to variability in the control animals.
In males, hepatic H3 methylation was affected by IUGR. At DOL0, P1, P2, and exon 5 had a higher concentrations of me2K4, while me3K36 decreased at all sites, with P2 and the distal 3′ UTR site being statistically significant (Fig. 5A). At DOL21, similar to the DOL0 findings, IUGR significantly increased me2K4 and decreased me3K36 at the P2 site (Fig. 5C). Furthermore, less me3K36 was also evident along the 3′ end of the hepatic IGF-1 gene, with statistical significance being reached for exon 5.
Figure 5.
Histone modifications along the IGF-1 gene in DOL0 and DOL21 rat IUGR livers relative to control. Six histone modifications at 5 sites, including P1, P2, exon 5, and proximal and distal 3′ UTR on the IGF-1 locus, were analyzed by ChIP/real-time PCR. IUGR values at each site were compared to their equivalent control values (set to 100%). A) DOL0 male. B) DOL0 female. C) DOL21 male. D) DOL21 female. *P < 0.05; **P < 0.01; ***P < 0.001.
In females, in contrast to the males, both hepatic H3 methylation and acetylation were affected by IUGR. IUGR significantly increased me3K4 of the DOL0 female IGF-1 gene at P1, as opposed to me2K4 in males (Fig. 5B). Furthermore, me3K36 was also decreased significantly at all five sites in DOL0 IUGR females. In terms of acetylation, IUGR significantly increased acK14 at P1, exon 5, and 3′UTR distal sites of the hepatic IGF-1 gene in the DOL0 females. At DOL21, in IUGR females me3K36 remained lower along the entire length of the gene, in contrast to males in which me3K36 was significantly decreased at two sites (Fig. 5D).
DISCUSSION
The primary finding of this study is that IUGR affects the epigenetic characteristics of hepatic IGF-1 along its entire length and that many of these changes persist postnatally within the context of decreased hepatic IGF-1 mRNA and serum protein levels. A secondary yet important finding of this study relates to gender. While the hepatic IGF-1 histone code does not vary between the genders under normal conditions, the postnatal effect of uteroplacental insufficiency and IUGR on the hepatic IGF-1 histone code is gender specific.
In the rat, previous studies used unilateral uterine artery ligation to investigate the effects of IUGR on IGF-1 expression. Vileisis et al. (19) used unilateral ligation to induce IUGR and found that fetal weight positively correlated with serum glucose (P<0.001), liver IGF-1 protein (P<0.001), and serum IGF-1 protein (P<0.001) levels (19). No correlation was evident for either serum insulin or lung IGF-1 protein, demonstrating that the effect of IUGR is gene- and tissue-specific. Interestingly, serum fetal glucose concentrations correlated positively with liver and serum IGF-1 protein levels, implicating fetal glucose delivery in the regulation of hepatic IGF-1 synthesis.
In our model of bilateral uterine artery ligation, we found at DOL0 that IUGR reduced serum IGF-1 levels. IUGR reduced hepatic IGF-1 mRNA transcripts initiated from alternative promoters as well as reduced levels of alternative splice variants A and B in the DOL0 animals. At DOL0, the reduction in serum IGF-1 protein levels was not as great as the reduction in mRNA. One possible explanation might involve translation efficiency. It has been shown recently in yeast under conditions of starvation that gene-specific translation efficiency appears to increase, particularly when multiple upstream open reading frames (uORFs) exist within the 5′ UTR. In the case of IGF-1 P2 transcripts, there are 8 uORFs (51). At DOL21, IUGR decreased hepatic IGF-1 transcription initiated from P2 as well as transcripts containing exon 5. The DOL21 mRNA data are more consistent with the lesser decrease in serum IGF-1. The reduction in IGF-1B transcripts in IUGR males and females would influence Eb peptide levels but not circulating mature IGF-1 protein levels.
In terms of IGF-1 transcriptional regulation from different promoters, several groups, including Adamo et al. (26, 52) and Simmons et al. (53), established the structure and start-site usage of the 5′ end of the rat hepatic IGF-1 gene. Based on combined works, multiple dispersed sites over a 350-bp 5′ region produce a P1 transcript that includes exon 1, whereas multiple highly localized sites within exon 2 produce the P2 transcript. The two different predominant IGF-1 mRNA species initiate translation at distinct AUG codons and result in IGF-1 proteins with either a 48-residue class-1 prepeptide or a 32-residue class-2 prepeptide.
Postnatally, both the P1 and P2 sites respond to diabetes, fasting, and increased caloric intake, with the P2 sites appearing to be more sensitive to the latter set of conditions (52). Furthermore, when compared to the P1 site, P2 differentially responds to GH signaling (54, 55). For example, increased serum levels of IGF-1 in C3H/HeJ mice vs. C57BL/6J mice appear to result from increased transcription from the hepatic P2 promoter (56), while other studies have shown that GH stimulates both P1 and P2 transcripts equally well (57).
In terms of the phenotypic significance related to the usage of exon 5, only limited literature is specific to the liver. Zhang et al. (58) have demonstrated that fasting decreases levels of the IGF-1B transcript in adult rat liver without affecting levels of the IGF-1A transcript. The specific transcript is important because it determines which E-peptide is produced (e.g., Ea or Eb). In the rat, the Eb peptide (or the E1 amide) has growth-promoting effects on epithelial cells. This action is not diminished significantly by competition with IGF-1, insulin, or antagonist antibodies to IGF-1 receptor. In humans, the synthetic hEb peptide can regulate cell growth and differentiation of several cancer cell lines in a manner that is also distinct from either IGF-1 or insulin (59). Furthermore, the human liver makes an Ec peptide that is 73% homologous to the rat Eb peptide from the IGF-1B mRNA species (60).
As suggested by these multiple mRNA species, the hepatic IGF-1 gene is regulated epigenetically. Though studies have noted specific epigenetic changes in response to IUGR in other genes, the vast majority of these analyses have been limited to promoter regions. Recent studies involving epigenetic programming of the hepatic DUSP5 gene led us to the insight that many of the epigenetic consequences of perinatal environmental events are not limited to the 5′ regulatory region (42).
One of these epigenetic consequences is DNA methylation. DNA CpG methylation plays a central role in gene expression. Traditionally, DNA methylation within CpG sites within promoters is thought to silence genes in an “on–off” manner (36). Evidence of this phenomenon is seen in developmental processes regulating tissue-specific gene expression. In contrast, we have observed in two disparate genes (i.e., DUSP5, IGF-1) that environmental stresses in the perinatal period affect CpG DNA methylation in regions marginally populated by CpGs, leading to moderate decreases in gene expression, at least at a tissue level.
More specifically, the modest extent of IGF-1 P1 and P2 DNA hypermethylation appears to dampen IGF-1 expression in the IUGR liver. Similar results were seen for CpG methylation within the promoter of the glutamate decarboxylase (GAD) gene (61). GAD DNA isolated from repressive GAD nonexpressing brain tissue showed a 2- to 4-fold increase in methylation compared to GAD+ tissue but still only represented ∼5% increase in sites methylated. In addition, DNA methylation has also been used to demonstrate changes in nucleosome positioning to explain changes in gene expression (62). The changes in DNA methylation seen here between IUGR and control may be an indication of altered histone placement.
Histones and associated modifications have also been implicated in gene activation and silencing and play a role in all stages of transcription, including initiation, elongation, and termination. Site-specific histone modifications, including histone acetylation and methylation, have been identified around transcription start sites. The main sites of lysine methylation associated with gene activation include K4, K36, and K79 on histone H3, while acetylation occurs at H3K9 and K14. Though earlier works focused on the consequences of single site histone modifications, it is now evident that modifications at different sites influence their effect on transcription, which involves simultaneously reading multiple histone markers (63).
To establish a more complete histone code for IGF-1, we looked at six histone H3 markers. Trimethyl K4 (me3K4), acetyl K9 (acK9), and acK14 are traditionally associated with gene activation. Dimethyl K4 (me2K4) and me3K36 are traditionally associated with transcription elongation, while me3K9 is considered to be involved in gene silencing. Recently, however, me3K9 also has shown to be associated with active genes (66,67,68).
In the context of mapping the histone code for the “normal” rat IGF-1 gene, acetyl K9 and K14 were highest at the 5′ end, while me3K36 accumulated more at the 3′end. The two IGF-1 promoters displayed different modification patterns with P2 having a greater accumulation of all histone covalent modifications analyzed. The increased accumulation seen here was not associated with increased hepatic expression from P2 (26, 27). The differences between P1 and P2 could be due to multiple factors. First, the transcriptional activity at P1 may influence modifications at P2. Second, P2 initiates transcription from a single cluster of sites and contains a putative CAAT box and TATA box, both of which attract particular chromatin modifying complexes (52). These core promoter elements are missing from P1. Promoters lacking TATA boxes attract a different set of factors and therefore a different chromatin modifying complexes are likely attracted (64). The lack of core promoter elements and associated protein complexes in P1 may be responsible for dispersed transcription initiation from two major and two minor sites spread over several hundred bases (52, 64, 65). Finally, the greater accumulation of modifications at P2 may simply be due to an overall increase in histone residency across P2.
Interestingly, histone modifications spanning the normal IGF-1 gene were similar between male and female controls, though a great deal variability did exist among the controls. However, when challenged with IUGR, males and females responded differently. For example, IUGR increased me2K4 at P1, P2, and exon 5 in DOL0 male livers, whereas IUGR increased me3K4 at P1 and acetyl K14 across the entire locus DOL0 female livers. At DOL21, me2K4 at P2 was still increased in IUGR male livers, whereas IUGR decreased me3K4 at P2 in IUGR female livers. Interestingly, less variability was often found among the IUGR animals relative to the controls, which suggests that the response to IUGR forces a relative synchronization of the histone code. This epigenetic synchronization may limit the ability of the liver to respond to the continuum of demands placed on it throughout the life of the animal. Although the technology is not yet available to test directly the consequences of these histone modifications in a gene-specific manner in vivo, it is clear that the IGF-1 hepatic histone code differs for males and females under the stress of IUGR.
A telling example of this difference is me3K4 at P1, which is increased in IUGR female livers at DOL0, but not in male IUGR livers. me3K4 occurs secondary to the attraction of the methyltransferase Set1 by phosphorylated RNA polymerase II (66,67,68). me3K4 of the 5′ end of a gene is commonly associated with gene activation and initiation of elongation (69,70,71,72,73,74). Subsequently, the increase in me3K4 at P1 in DOL0 IUGR females relative to the controls was unexpected given the decrease in IGF-1 mRNA levels. This finding suggests that the initial stage of RNAPII polymerase migration on the IGF-1 gene may not have been affected.
In contrast, transcriptional elongation may be affected. This theory is suggested by IUGR decreasing me3K36 in the body and 3′ end of the gene in IUGR males and females at both ages, in association with the decreased levels of IGF-1 mRNA. As RNAPII polymerase migrates toward the 3′end of the gene, the methyltransferase Set2 is attracted to the transcription complex under normal conditions (66,67,68, 75, 76). Set2 methylates K36 of histone H3, and this methylation appears to be critical to transcriptional elongation. Our findings suggest that uteroplacental insufficiency and IUGR disrupt components of this system, such as Set2 or RNAPII polymerase phosphorylation, the latter of which is necessary for Set2 binding. At a minimum, our findings represent a downstream marker of aberrant transcriptional regulation of the hepatic IGF-1 gene in the IUGR rat and demonstrate the value of assessing chromatin structure at more than the 5′ promoter region. It is possible that our findings will further represent an initial disruption of the histone code that leads to subsequent failure of IGF-1 transcriptional elongation.
In our study, an IUGR insult altered components of the IGF-1 histone code that persist from the perinatal period into juvenile life. Alterations to the histone code that continue well beyond the point of insult offer insight into possible mechanisms through which uteroplacental insufficiency may predispose toward the phenotypic changes seen later in life. Two persistent changes are particularly intriguing. The first change is the persistence decrease in me3K36 at DOL0 and DOL21 in both genders at multiple sites. As discussed above, this may lead a dampening of IGF-1 transcriptional elongation and a subsequent decrease in expression. The second change is the increase in me2K4 at P2 in the male animals. Though often thought of as an “activating marker” of transcription, evidence exists that the increase in me2K4 may come at the expense of me3K4, likely an even more potent transcriptional activator (77, 78). The significant decrease of me3K4 in the DOL21 female IUGR liver in association with the decreased expression of IGF-1 mRNA is consistent with the concept that the ratio of me2K4: me3K4 may be an important regulator of transcriptional regulator. The latter highlights the concept that similar responses in gene expression to a stimulus may occur through gender-specific modifications of the histone code. However, caution needs to be exerted in speculating on the long-term effects of persistent modifications of histone marks, particularly considering that the effects of these changes are likely dependent on multiple factors, including the histone code of multiple other nucleosomes. At this juncture, no primary “epigenetic transgenic” animal model exists to demonstrate how an initial change in an epigenetic characteristic changes expression of a specific gene.
The translational relevance of our findings lies in the potential importance of IGF-1 to several components of the IUGR postnatal phenotype. The first and most obvious is growth. IGF-1 mediates many of the anabolic and mitogenic actions of growth hormone in postnatal life. Fattal-Valevski et al. (17) determined IGF-1 levels in preadolescent IUGR children (mean age 6.5±2.1 yr; n=57) vs. control children (7.6±2.8 yr; n=30), IGF-1 serum levels were significantly decreased in the non catch-up IUGR group. Similarly, Verkauskiene et al. (79) investigated a group of adults (mean age 22.6±4.3 yr) that were born small for gestational age (SGA) verses adults (mean age 22.6±4.2 yr) who were appropriately sized at birth. IUGR adults who were characterized by low weight and height also demonstrated significantly lower mean serum IGF-1 levels.
Low IGF-1 levels also contribute to adult insulin resistance, another major morbidity associated with IUGR. Hepatic IGF-1 is of vital importance for normal carbohydrate metabolism in both mice and humans. In mice, elimination of hepatic IGF-1 production using a Cre/loxP recombination system increases serum levels of insulin without significantly affecting glucose elimination (80). In humans, recombinant IGF-1 is ∼6% as potent as insulin in the production of hypoglycemia (81). Furthermore, severe IGF-1 deficiency in humans leads to insulin resistance, which is reversible with recombinant IGF-1 (18).
Caution is always necessary when attempting to apply data from a rat model to human pathophysiology. The fetal and juvenile rat are physiologically immature relative to the human, and the insult imposed on the fetal rat in this model of uteroplacental insufficiency is severe, while the effect of uteroplacental insufficiency experienced by humans ranges across a continuum. Furthermore, we acknowledge that early postnatal nutrition is important. We have previously characterized breast milk from our control and IUGR dams after 21 d of supporting their respective pups, and no significant differences in KCAL, protein, fat, or zinc were noted between the two groups (48).
The work we present here establishes part of the histone code for the rat hepatic IGF-1 gene under normal and intrauterine growth restricted conditions. The results show unique patterns of multiple histone modifications at defined locations within the gene. Our findings strongly support the concept that alterations in the histone code along the IGF-1 gene are responsible for the reduced levels of IGF-1 observed in IUGR rats. Changes in both histone acetylation and methylation were observed, which indicates that multiple histone modifying complexes are affected by UPI.
Acknowledgments
The project was funded by the U.S. National Institutes of Health, grant HD41075. We thank the Division of Neonatology and the Developmental Origins of Health Laboratories at the University of Utah for their support and guidance.
References
- Syddall H E, Sayer A A, Simmonds S J, Osmond C, Cox V, Dennison E M, Barker D J, Cooper C. Birth weight, infant weight gain, and cause-specific mortality: the Hertfordshire cohort study. Am J Epidemiol. 2005;161:1074–1080. doi: 10.1093/aje/kwi137. [DOI] [PubMed] [Google Scholar]
- Witlin A G, Sibai B M. Hypertension in pregnancy: current concepts of preeclampsia. Annu Rev Med. 1997;48:115–127. doi: 10.1146/annurev.med.48.1.115. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Eirio V, Koskinen J, Kujansuu E, Ranta T. Doppler assessment of the uterine and uteroplacental circulation in the second trimester in pregnancies at high risk for pre-eclampsia and/or intrauterine growth retardation: comparison and correlation between different Doppler parameters. Ultrasound Obstet Gynecol. 1997;9:330–338. doi: 10.1046/j.1469-0705.1997.09050330.x. [DOI] [PubMed] [Google Scholar]
- Frusca T, Soregaroli M, Valcamonico A, Guandalini F, Danti L. Doppler velocimetry of the uterine arteries in nulliparous women. Early Hum Dev. 1997;48:177–185. doi: 10.1016/s0378-3782(96)01854-3. [DOI] [PubMed] [Google Scholar]
- Barker D J, Hales C N, Fall C H, Osmond C, Phipps K, Clark P M. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- Mi J, Law C, Zhang K L, Osmond C, Stein C, Barker D. Effects of infant birthweight and maternal body mass index in pregnancy on components of the insulin resistance syndrome in China. Ann Intern Med. 2000;132:253–260. doi: 10.7326/0003-4819-132-4-200002150-00002. [DOI] [PubMed] [Google Scholar]
- Radunovic N, Kuczynski E, Rosen T, Dukanac J, Petkovic S, Lockwood C J. Plasma apolipoprotein A-I and B concentrations in growth-retarded fetuses: a link between low birth weight and adult atherosclerosis. J Clin Endocrinol Metab. 2000;85:85–88. doi: 10.1210/jcem.85.1.6243. [DOI] [PubMed] [Google Scholar]
- Valdez R, Athens M A, Thompson G H, Bradshaw B S, Stern M P. Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia. 1994;37:624–631. doi: 10.1007/BF00403383. [DOI] [PubMed] [Google Scholar]
- Yarbrough D E, Barrett-Connor E, Kritz-Silverstein D, Wingard D L. Birth weight, adult weight, and girth as predictors of the metabolic syndrome in postmenopausal women: the Rancho Bernardo study. Diabetes Care. 1998;21:1652–1658. doi: 10.2337/diacare.21.10.1652. [DOI] [PubMed] [Google Scholar]
- Phillips D I, Barker D J, Hales C N, Hirst S, Osmond C. Thinness at birth and insulin resistance in adult life. Diabetologia. 1994;37:150–154. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- Tenhola S, Halonen P, Jaaskelainen J, Voutilainen R. Serum markers of GH and insulin action in 12-year-old children born small for gestational age. Eur J Endocrinol. 2005;152:335–340. doi: 10.1530/eje.1.01869. [DOI] [PubMed] [Google Scholar]
- Flanagan D E, Moore V M, Godsland I F, Cockington R A, Robinson J S, Phillips D I. Fetal growth and the physiological control of glucose tolerance in adults: a minimal model analysis. Am J Physiol Endocrinol Metab. 2000;278:E700–706. doi: 10.1152/ajpendo.2000.278.4.E700. [DOI] [PubMed] [Google Scholar]
- Woods K A, Camacho-Hubner C, Savage M O, Clark A J. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- Lassarre C, Hardouin S, Daffos F, Forestier F, Frankenne F, Binoux M. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr Res. 1991;29:219–225. doi: 10.1203/00006450-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Ashton I K, Zapf J, Einschenk I, MacKenzie I Z. Insulin-like growth factors (IGF) 1 and 2 in human foetal plasma and relationship to gestational age and foetal size during midpregnancy. Acta Endocrinol (Copenh) 1985;110:558–563. doi: 10.1530/acta.0.1100558. [DOI] [PubMed] [Google Scholar]
- Ozkan H, Aydin A, Demir N, Erci T, Buyukgebiz A. Associations of IGF-I, IGFBP-1 and IGFBP-3 on intrauterine growth and early catch-up growth. Biol Neonate. 1999;76:274–282. doi: 10.1159/000014169. [DOI] [PubMed] [Google Scholar]
- Fattal-Valevski A, Toledano-Alhadef H, Golander A, Leitner Y, Harel S. Endocrine profile of children with intrauterine growth retardation. J Pediatr Endocrinol Metab. 2005;18:671–676. doi: 10.1515/jpem.2005.18.7.671. [DOI] [PubMed] [Google Scholar]
- Woods K A, Camacho-Hubner C, Bergman R N, Barter D, Clark A J, Savage M O. Effects of insulin-like growth factor I (IGF-I) therapy on body composition and insulin resistance in IGF-I gene deletion. J Clin Endocrinol Metab. 2000;85:1407–1411. doi: 10.1210/jcem.85.4.6495. [DOI] [PubMed] [Google Scholar]
- Vileisis R A, D'Ercole A J. Tissue and serum concentrations of somatomedin-C/insulin-like growth factor I in fetal rats made growth retarded by uterine artery ligation. Pediatr Res. 1986;20:126–130. doi: 10.1203/00006450-198602000-00006. [DOI] [PubMed] [Google Scholar]
- El-Khattabi I, Gregoire F, Remacle C, Reusens B. Isocaloric maternal low-protein diet alters IGF-I, IGFBPs, and hepatocyte proliferation in the fetal rat. Am J Physiol Endocrinol Metab. 2003;285:E991–E1000. doi: 10.1152/ajpendo.00037.2003. [DOI] [PubMed] [Google Scholar]
- Shimatsu A, Rotwein P. Sequence of two rat insulin-like growth factor I mRNAs differing within the 5′ untranslated region. Nucleic Acids Res. 1987;15:7196. doi: 10.1093/nar/15.17.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimatsu A, Rotwein P. Mosaic evolution of the insulin-like growth factors. Organization, sequence, and expression of the rat insulin-like growth factor I gene. J Biol Chem. 1987;262:7894–7900. [PubMed] [Google Scholar]
- Roberts C T, Jr, Lasky S R, Lowe W L, Jr, LeRoith D. Rat IGF-I cDNA’s contain multiple 5′-untranslated regions. Biochem Biophys Res Commun. 1987;146:1154–1159. doi: 10.1016/0006-291x(87)90768-6. [DOI] [PubMed] [Google Scholar]
- Roberts C T, Jr, Lasky S R, Lowe W L, Jr, Seaman W T, LeRoith D. Molecular cloning of rat insulin-like growth factor I complementary deoxyribonucleic acids: differential messenger ribonucleic acid processing and regulation by growth hormone in extrahepatic tissues. Mol Endocrinol. 1987;1:243–248. doi: 10.1210/mend-1-3-243. [DOI] [PubMed] [Google Scholar]
- Tobin G, Yee D, Brunner N, Rotwein P. A novel human insulin-like growth factor I messenger RNA is expressed in normal and tumor cells. Mol Endocrinol. 1990;4:1914–1920. doi: 10.1210/mend-4-12-1914. [DOI] [PubMed] [Google Scholar]
- Adamo M, Lowe W L, Jr, LeRoith D, Roberts C T., Jr Insulin-like growth factor I messenger ribonucleic acids with alternative 5′-untranslated regions are differentially expressed during development of the rat. Endocrinology. 1989;124:2737–2744. doi: 10.1210/endo-124-6-2737. [DOI] [PubMed] [Google Scholar]
- Shemer J, Adamo M, Raizada M K, Heffez D, Zick Y, LeRoith D. Insulin and IGF-I stimulate phosphorylation of their respective receptors in intact neuronal and glial cells in primary culture. J Mol Neurosci. 1989;1:3–8. doi: 10.1007/BF02896850. [DOI] [PubMed] [Google Scholar]
- Bell G I, Stempien M M, Fong N M, Rall L B. Sequences of liver cDNAs encoding two different mouse insulin-like growth factor I precursors. Nucleic Acids Res. 1986;14:7873–7882. doi: 10.1093/nar/14.20.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe W L, Jr, Lasky S R, LeRoith D, Roberts C T., Jr Distribution and regulation of rat insulin-like growth factor I messenger ribonucleic acids encoding alternative carboxyterminal E-peptides: evidence for differential processing and regulation in liver. Mol Endocrinol. 1988;2:528–535. doi: 10.1210/mend-2-6-528. [DOI] [PubMed] [Google Scholar]
- Richards E J. Chromatin methylation: who’s on first? Curr Biol. 2002;12:R694–695. doi: 10.1016/s0960-9822(02)01208-3. [DOI] [PubMed] [Google Scholar]
- Ahmad K, Henikoff S. Epigenetic consequences of nucleosome dynamics. Cell. 2002;111:281–284. doi: 10.1016/s0092-8674(02)01081-4. [DOI] [PubMed] [Google Scholar]
- Grewal S I, Moazed D. Heterochromatin and epigenetic control of gene expression. Science. 2003;301:798–802. doi: 10.1126/science.1086887. [DOI] [PubMed] [Google Scholar]
- Ng H H, Bird A. DNA methylation and chromatin modification. Curr Opin Genet Dev. 1999;9:158–163. doi: 10.1016/s0959-437x(99)80024-0. [DOI] [PubMed] [Google Scholar]
- McNairn A J, Gilbert D M. Epigenomic replication: linking epigenetics to DNA replication. Bioessays. 2003;25:647–656. doi: 10.1002/bies.10305. [DOI] [PubMed] [Google Scholar]
- Cheutin T, McNairn A J, Jenuwein T, Gilbert D M, Singh P B, Misteli T. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science. 2003;299:721–725. doi: 10.1126/science.1078572. [DOI] [PubMed] [Google Scholar]
- Jones P A. The DNA methylation paradox. Trends Genet. 1999;15:34–37. doi: 10.1016/s0168-9525(98)01636-9. [DOI] [PubMed] [Google Scholar]
- Pokholok D K, Harbison C T, Levine S, Cole M, Hannett N M, Lee T I, Bell G W, Walker K, Rolfe P A, Herbolsheimer E, Zeitlinger J, Lewitter F, Gifford D K, Young R A. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517–527. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Kurdistani S K, Tavazoie S, Grunstein M. Mapping global histone acetylation patterns to gene expression. Cell. 2004;117:721–733. doi: 10.1016/j.cell.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Schneider R, Bannister A J, Sherriff J, Bernstein B E, Emre N C, Schreiber S L, Mellor J, Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- Bernstein B E, Humphrey E L, Erlich R L, Schneider R, Bouman P, Liu J S, Kouzarides T, Schreiber S L. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc Natl Acad Sci U S A. 2002;99:8695–8700. doi: 10.1073/pnas.082249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S, Eisenhaber F, O'Carroll D, Strahl B D, Sun Z W, Schmid M, Opravil S, Mechtler K, Ponting C P, Allis C D, Jenuwein T. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- Fu Q, McKnight R A, Yu X, Callaway C W, Lane R H. Growth retardation alters the epigenetic characteristics of hepatic dual specificity phosphatase 5. FASEB J. 2006;20:2127–2129. doi: 10.1096/fj.06-6179fje. [DOI] [PubMed] [Google Scholar]
- Fu Q, McKnight R A, Yu X, Wang L, Callaway C W, Lane R H. Uteroplacental insufficiency induces site specific changes in histone H3 covalent modifications and affects DNA-histone H3 positioning in day 0 IUGR rat liver. Physiol Genomics. 2004;20:108–116. doi: 10.1152/physiolgenomics.00175.2004. [DOI] [PubMed] [Google Scholar]
- Simmons R A, Templeton L J, Gertz S J. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- Tsirka A E, Gruetzmacher E M, Kelley D E, Ritov V H, Devaskar S U, Lane R H. Myocardial gene expression of glucose transporter 1 and glucose transporter 4 in response to uteroplacental insufficiency in the rat. J Endocrinol. 2001;169:373–380. doi: 10.1677/joe.0.1690373. [DOI] [PubMed] [Google Scholar]
- Guiding principles for research involving animals and human beings. Am J Physiol. 2002;283:R281–283. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- Baserga M, Hale M A, McKnight R A, Yu X, Callaway C W, Lane R H. Uteroplacental insufficiency alters hepatic expression, phosphorylation, and activity of the glucocorticoid receptor in fetal IUGR rats. Am J Physiol. 2005;289:R1348–1353. doi: 10.1152/ajpregu.00211.2005. [DOI] [PubMed] [Google Scholar]
- Ke X, Lei Q, James S J, Kelleher S L, Melnyk S, Jernigan S, Yu X, Wang L, Callaway C W, Gill G, Chan G M, Albertine K H, McKnight R A, Lane R H. Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiol Genomics. 2006;25:16–28. doi: 10.1152/physiolgenomics.00093.2005. [DOI] [PubMed] [Google Scholar]
- Ke X, McKnight R A, Wang Z M, Yu X, Wang L, Callaway C W, Albertine K H, Lane R H. Nonresponsiveness of cerebral p53-MDM2 functional circuit in newborn rat pups rendered IUGR via uteroplacental insufficiency. Am J Physiol. 2005;288:R1038–1045. doi: 10.1152/ajpregu.00701.2004. [DOI] [PubMed] [Google Scholar]
- Lan F, Collins R E, De Cegli R, Alpatov R, Horton J R, Shi X, Gozani O, Cheng X, Shi Y. Recognition of unmethylated histone H3 lysine 4 links BHC80 to LSD1-mediated gene repression. Nature. 2007;448:718–722. doi: 10.1038/nature06034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia N T, Ghaemmaghami S, Newman J R, Weissman J S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. [E-pub ahead of print] Science. 2009 doi: 10.1126/science.1168978. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo M L, Ben-Hur H, Roberts C T, Jr, LeRoith D. Regulation of start site usage in the leader exons of the rat insulin-like growth factor-I gene by development, fasting, and diabetes. Mol Endocrinol. 1991;5:1677–1686. doi: 10.1210/mend-5-11-1677. [DOI] [PubMed] [Google Scholar]
- Simmons J G, Van Wyk J J, Hoyt E C, Lund P K. Multiple transcription start sites in the rat insulin-like growth factor-I gene give rise to IGF-I mRNAs that encode different IGF-I precursors and are processed differently in vitro. Growth Factors. 1993;9:205–221. doi: 10.3109/08977199309010833. [DOI] [PubMed] [Google Scholar]
- Foyt H L, Lanau F, Woloschak M, LeRoith D, Roberts C T., Jr Effect of growth hormone on levels of differentially processed insulin-like growth factor I mRNAs in total and polysomal mRNA populations. Mol Endocrinol. 1992;6:1881–1888. doi: 10.1210/mend.6.11.1282673. [DOI] [PubMed] [Google Scholar]
- Butler A A, Ambler G R, Breier B H, LeRoith D, Roberts C T, Jr, Gluckman P D. Growth hormone (GH) and insulin-like growth factor-I (IGF-I) treatment of the GH-deficient dwarf rat: differential effects on IGF-I transcription start site expression in hepatic and extrahepatic tissues and lack of effect on type I IGF receptor mRNA expression. Mol Cell Endocrinol. 1994;101:321–330. doi: 10.1016/0303-7207(94)90249-6. [DOI] [PubMed] [Google Scholar]
- Adamo M L, Ma X, Ackert-Bicknell C L, Donahue L R, Beamer W G, Rosen C J. Genetic increase in serum insulin-like growth factor-I (IGF-I) in C3H/HeJ compared with C57BL/6J mice is associated with increased transcription from the IGF-I exon 2 promoter. Endocrinology. 2006;147:2944–2955. doi: 10.1210/en.2005-0742. [DOI] [PubMed] [Google Scholar]
- Woelfle J, Billiard J, Rotwein P. Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J Biol Chem. 2003;278:22696–22702. doi: 10.1074/jbc.M301362200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Whitehead R E, Jr, Underwood L E. Effect of fasting on insulin-like growth factor (IGF)-IA and IGF-IB messenger ribonucleic acids and prehormones in rat liver. Endocrinology. 1997;138:3112–3118. doi: 10.1210/endo.138.8.5348. [DOI] [PubMed] [Google Scholar]
- Siegfried J M, Kasprzyk P G, Treston A M, Mulshine J L, Quinn K A, Cuttitta F. A mitogenic peptide amide encoded within the E peptide domain of the insulin-like growth factor IB prohormone. Proc Natl Acad Sci U S A. 1992;89:8107–8111. doi: 10.1073/pnas.89.17.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew S L, Lavender P, Clark A J, Ross R J. An alternatively spliced human insulin-like growth factor-I transcript with hepatic tissue expression that diverts away from the mitogenic IBE1 peptide. Endocrinology. 1995;136:1939–1944. doi: 10.1210/endo.136.5.7720641. [DOI] [PubMed] [Google Scholar]
- Huang H S, Akbarian S. GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PLoS ONE. 2007;2:e809. doi: 10.1371/journal.pone.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi M, Pao M M, Jeong S, Gal-Yam E N, Egger G, Weisenberger D J, Jones P A. Footprinting of mammalian promoters: use of a CpG DNA methyltransferase revealing nucleosome positions at a single molecule level. Nucleic Acids Res. 2005;33:e176. doi: 10.1093/nar/gni180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Lin J C, Wei V, Yoo C, Cheng J C, Nguyen C T, Weisenberger D J, Egger G, Takai D, Gonzales F A, Jones P A. Distinct localization of histone H3 acetylation and H3–K4 methylation to the transcription start sites in the human genome. Proc Natl Acad Sci U S A. 2004;101:7357–7362. doi: 10.1073/pnas.0401866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeny M A, Soutoglou E, Nagy Z, Scheer E, Janoshazi A, Richardot M, Argentini M, Kessler P, Tora L. Identification of a small TAF complex and its role in the assembly of TAF-containing complexes. PLoS ONE. 2007;2:e316. doi: 10.1371/journal.pone.0000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S, Brand M, Mittler G, Yanagisawa J, Kato S, Meisterernst M, Tora L. TATA-binding protein-free TAF-containing complex (TFTC) and p300 are both required for efficient transcriptional activation. J Biol Chem. 2002;277:32875–32882. doi: 10.1074/jbc.M205860200. [DOI] [PubMed] [Google Scholar]
- Gerber M, Shilatifard A. Transcriptional elongation by RNA polymerase II and histone methylation. J Biol Chem. 2003;278:26303–26306. doi: 10.1074/jbc.R300014200. [DOI] [PubMed] [Google Scholar]
- Hampsey M, Reinberg D. Tails of intrigue: phosphorylation of RNA polymerase II mediates histone methylation. Cell. 2003;113:429–432. doi: 10.1016/s0092-8674(03)00360-x. [DOI] [PubMed] [Google Scholar]
- Xiao T, Hall H, Kizer K O, Shibata Y, Hall M C, Borchers C H, Strahl B D. Phosphorylation of RNA polymerase II CTD regulates H3 methylation in yeast. Genes Dev. 2003;17:654–663. doi: 10.1101/gad.1055503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R, Bannister A J, Myers F A, Thorne A W, Crane-Robinson C, Kouzarides T. Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat Cell Biol. 2004;6:73–77. doi: 10.1038/ncb1076. [DOI] [PubMed] [Google Scholar]
- Alisi A, Leoni S, Piacentani A, Conti Devirgiliis L. Retinoic acid modulates the cell-cycle in fetal rat hepatocytes and HepG2 cells by regulating cyclin-cdk activities. Liver. 2003;23:179–186. doi: 10.1034/j.1600-0676.2003.00829.x. [DOI] [PubMed] [Google Scholar]
- Pray-Grant M G, Daniel J A, Schieltz D, Yates J R, 3rd, Grant P A. Chd1 chromodomain links histone H3 methylation with SAGA- and SLIK-dependent acetylation. Nature. 2005;433:434–438. doi: 10.1038/nature03242. [DOI] [PubMed] [Google Scholar]
- Sims R J, 3rd, Reinberg D. Histone H3 Lys 4 methylation: caught in a bind? Genes Dev. 2006;20:2779–2786. doi: 10.1101/gad.1468206. [DOI] [PubMed] [Google Scholar]
- Martin D G, Baetz K, Shi X, Walter K L, MacDonald V E, Wlodarski M J, Gozani O, Hieter P, Howe L. The Yng1p plant homeodomain finger is a methyl-histone binding module that recognizes lysine 4-methylated histone H3. Mol Cell Biol. 2006;26:7871–7879. doi: 10.1128/MCB.00573-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe L, Auston D, Grant P, John S, Cook R G, Workman J L, Pillus L. Histone H3 specific acetyltransferases are essential for cell cycle progression. Genes Dev. 2001;15:3144–3154. doi: 10.1101/gad.931401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan N J, Dover J, Wood A, Schneider J, Heidt J, Boateng M A, Dean K, Ryan O W, Golshani A, Johnston M, Greenblatt J F, Shilatifard A. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol Cell. 2003;11:721–729. doi: 10.1016/s1097-2765(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Li B, Howe L, Anderson S, Yates J R, 3rd, Workman J L. The Set2 histone methyltransferase functions through the phosphorylated carboxyl-terminal domain of RNA polymerase II. J Biol Chem. 2003;278:8897–8903. doi: 10.1074/jbc.M212134200. [DOI] [PubMed] [Google Scholar]
- Dehe P M, Dichtl B, Schaft D, Roguev A, Pamblanco M, Lebrun R, Rodriguez-Gil A, Mkandawire M, Landsberg K, Shevchenko A, Rosaleny L E, Tordera V, Chavez S, Stewart A F, Geli V. Protein interactions within the Set1 complex and their roles in the regulation of histone 3 lysine 4 methylation. J Biol Chem. 2006;281:35404–35412. doi: 10.1074/jbc.M603099200. [DOI] [PubMed] [Google Scholar]
- Tresaugues L, Dehe P M, Guerois R, Rodriguez-Gil A, Varlet I, Salah P, Pamblanco M, Luciano P, Quevillon-Cheruel S, Sollier J, Leulliot N, Couprie J, Tordera V, Zinn-Justin S, Chavez S, van Tilbeurgh H, Geli V. Structural characterization of Set1 RNA recognition motifs and their role in histone H3 lysine 4 methylation. J Mol Biol. 2006;359:1170–1181. doi: 10.1016/j.jmb.2006.04.050. [DOI] [PubMed] [Google Scholar]
- Verkauskiene R, Jaquet D, Deghmoun S, Chevenne D, Czernichow P, Levy-Marchal C. Smallness for gestational age is associated with persistent change in insulin-like growth factor I (IGF-I) and the ratio of IGF-I/IGF-binding protein-3 in adulthood. J Clin Endocrinol Metab. 2005;90:5672–5676. doi: 10.1210/jc.2005-0423. [DOI] [PubMed] [Google Scholar]
- Isaksson O G, Jansson J O, Sjogren K, Ohlsson C. Metabolic functions of liver-derived (endocrine) insulin-like growth factor I. Horm Res. 2001;55:18–21. doi: 10.1159/000063468. [DOI] [PubMed] [Google Scholar]
- Guler H P, Zapf J, Froesch E R. Short-term metabolic effects of recombinant human insulin-like growth factor I in healthy adults. N Engl J Med. 1987;317:137–140. doi: 10.1056/NEJM198707163170303. [DOI] [PubMed] [Google Scholar]