Abstract
Controlling the HIV/AIDS epidemic remains a major challenge, with approximately 5 million new HIV infections annually. Cyclopentenone prostaglandins (CyPG), such as 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2), are arachidonic acid-derived endogenous electrophiles that possess anti-HIV activity by an unknown mechanism. Given that the reactive α,β-unsaturated ketone in the cyclopentenone ring of 15d-PGJ2 covalently modifies key Cys thiols in select proteins, we hypothesized that 15d-PGJ2 inhibits HIV transcription and replication by targeting Cys thiols in HIV-1 Tat. Tat is a potent transactivator of viral gene expression required for HIV transcriptional elongation and replication. Our studies indicate that 15d-PGJ2 treatment of cells inhibits Tat-dependent transcription and replication of HIV-1, while 9,10-dihydro-15d-PGJ2, PGE2, PGF2α, or PGD2 that lack the reactive α,β-unsaturated ketone were ineffective. The inhibition of Tat activity by 15d-PGJ2 was dose-dependent, with an IC50 of 1.2 μM and independent of NF-κB pathway. Furthermore, using a biotinylated derivative of 15d-PGJ2, we demonstrate that 15d-PGJ2 modifies free Cys-thiols in Tat to form covalent Michael adducts and that the interaction was further increased on reduction of Tat. 15d-PGJ2-modified Tat was unable to transactivate the HIV long terminal repeat in U937 human macrophages. These data demonstrate that Tat acts as a molecular target of CyPG leading to the inhibition of transcription and also suggest a novel therapeutic approach to complement current antiretroviral strategies for HIV/AIDS.—Kalantari, P., Narayan, V., Henderson, A. J., Prabhu, K. S. 15-Deoxy-Δ12,14-prostaglandin J2 inhibits HIV-1 transactivating protein, Tat, through covalent modification.
Keywords: alkylation, cysteine thiols, arachidonic acid metabolite, cyclopentenone prostaglandins
HIV provirus expression is regulated at the transcriptional level, which is controlled by the upstream long terminal repeat (LTR). The HIV-1 LTR is often divided into four functional elements: the Tat activating region (TAR), the promoter, the enhancer, and the negative/modulatory regulatory element (1). The promoter, enhancer, and modulatory elements recruit host-transcription factors, such as Sp-1, NF-κB, and C/EBPβ, that are necessary to initiate transcription (2, 3), whereas the TAR element forms an RNA stem loop structure that recruits the HIV-encoded transcriptional activator Tat. Tat recruits pTEFb to the LTR, enhancing processive transcription. In the absence of Tat, transcription elongation by RNA polymerase II from the HIV promoter is very inefficient, leading to an accumulation of short initiated transcripts (4, 5). The activity of Tat substantially increases the production of elongated transcripts (4, 6, 7). Furthermore, Tat recruits chromatin-remodeling machinery to ensure efficient transcription (8,9,10). Because Tat is necessary for HIV-1 replication, compounds that specifically target its activity are attractive therapeutic compounds that would be complementary to current antiretroviral treatments targeting reverse transcriptase, integrase, and protease.
The primary structure of Tat is made up of 101 amino acids. On the basis of mutagenesis studies, six distinct domains have been mapped, which include N-terminal domain (residues 1–19), cysteine-rich domain (residues 20–39), core domain (residues 40–47), basic domain (residues 48–56), auxiliary domain (residues 57–67), and a C-terminal glutamine-rich (RGD) domain (residues 68–101) (11). The first four domains make up the essential domain, which is necessary for Tat function, whereas the auxiliary domain enhances the activity of the essential domain (11). Of particular interest is the Cys-rich domain, which contains seven Cys residues that are poorly defined with regard to intramolecular disulfide bonds. Functional analysis by deletion and site-specific mutations of the Cys-rich domain revealed that Cys residues are necessary for Tat activity. Although required for Tat transactivation function, the structural basis for this function is still not well understood. The Cys-rich domain could form a metal-linked dimer with a tetrahedral geometry in which each metal is liganded to 12 Cys residues; while the other 2 Cys act as terminal ligands (12). However, the transcriptionally active form of Tat has been shown to be a monomer, and reducing agents dramatically inhibit Tat activity (13), suggesting that Cys residues in Tat form two intramolecular disulfide bonds that are essential for transactivation function (14). Given the formation of two disulfide bonds per molecule of Tat, the role of free Cys residues in the transcription elongation is unknown.
Arachidonic acid is enzymatically metabolized by cyclooxygenases (COX) to PGH2 that is further metabolized by specific PG isomerases to PGE2, PGD2, PGF2α, thromboxane A2 (TXA2), and prostacyclin I2 (15). Members of the PGJ2 class, particularly 15d-PGJ2 (also called cyclopentenone PGs, CyPG), are derived from PGD2 by two dehydration reactions that lead to the formation of a reactive α,β-unsaturated ketone functionality (16, 17). CyPGs exhibit a unique spectrum of biological effects, including inhibition of IκB-kinase-β (18), induction of synoviocyte and endothelial cell apoptosis (13), induction of glutathione S-transferase gene expression (14), and potentiation of apoptosis in neuronal cells (19). In the case of IKKβ, 15d-PGJ2 directly interacted with Cys179 of IKKβ to inhibit NF-κB activation (18). In addition, 15d-PGJ2 serves as an endogenous ligand for the nuclear hormone receptor PPAR-γ (20). The anti-inflammatory property of 15d-PGJ2 also stems from its ability to initiate sumoylation of the liganded receptor that maintains a corepressor (NcoR) complex on NF-κB response elements (21).
Rajakariar et al. (22) have provided definitive proof in support of the in vivo production of 15d-PGJ2 and its role as a crucial checkpoint controller of cytokine/chemokine synthesis, as well as leukocyte influx and efflux. In addition, natural and synthetic CyPGs also display antiviral activity (17, 23) by targeting different steps of DNA and RNA virus replication, including synthesis, glycosylation, intracellular translocation of viral proteins, and maturation of the virus particle (24, 25). Relevant to HIV, it has been demonstrated that 15d-PGJ2 and PGA1 suppress HIV-1 replication by inhibiting transcription (26). In this study, we demonstrate that the mechanism by which 15d-PGJ2 inhibits HIV transcription is independent of NF-κB and is due to covalent modification of the Cys residues in Tat.
MATERIALS AND METHODS
Cells
The human U937 promonocytic cell line (American Type Culture Collection, Manassas, VA, USA) was cultured in RPMI 1640 medium supplemented with 5% defined fetal calf serum (FCS; Hyclone, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.2 M l-glutamine. The 293T human embryonic kidney cell line (American Type Culture Collection) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine.
Chemicals
Free and biotinylated 15d-PGJ2, 9,10-dihydro-15d-PGJ2, PGD2, PGE2, and PGF2α were purchased from Cayman Chemicals (Ann Arbor, MI, USA). 9,10-Dihydro-15d-PGJ2-biotinamide was synthesized in our laboratory using 9,10-dihydro-15d-PGJ2, as described previously (27). Briefly, biotinpentylamine (Thermo-Pierce, Rockford, IL, USA) was used to conjugate the carbodiimide-activated carboxylic group of 9,10-dihydro-15d-PGJ2 to form an amide bond. Such a biotin derivative of 9,10-dihydro-15d-PGJ2 with a 5-carbon spacer arm was purified using RP-HPLC and quantitated by spectrophotometry. Tris(2-carboxyethyl) phosphine hydrochloride (TCEP) was purchased from Thermo-Pierce (Thermo-Pierce). All other chemicals used were of analytical grade.
Expression, purification, and refolding of rTat
Recombinant Tat (rTat) was expressed in Escherichia coli as a His-tagged protein using the autoinduction system. We have been successful in expressing large amounts of Tat protein by combining two different methods previously described by Patki and Lederman (28) and Kirsh et al. (14). The pETTat-1His construct from the U.S. National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (Rockville, MD, USA) was used to transform BL21 (DE3) bacterial cells. Briefly, the E. coli pellet from a 24-h culture was subjected to lysis in 6 M GuHCl for 12 h. All steps were performed at 4°C unless otherwise mentioned. The clarified cell lysates were subjected to metal affinity chromatography. The eluate was dialyzed against 0.1 N HCl for 2 d at 4°C followed by an overnight lyophilization. The lyophilized powder was reconstituted in 6 M urea followed by reduction by TCEP (25 mol/mole of Tat) in the dark for 8 h at room temperature. The mixture was subjected to sequential dialysis in phosphate buffer (0.1 M, pH 6.3) containing 4 M urea, 2 M urea, 1 M urea, and finally in buffer containing 200 mM NaCl. The dialysate was filter sterilized, purged with nitrogen, and stored in small aliquots at −20°C for further use. The rTat solution tested negative for the presence of endotoxin LPS using the QCL-1000 chromogenic LAL endpoint assay from Lonza (Basel, Switzerland).
Plasmids, transient transfections, and luciferase assay
Replication competent HXB.2 virus, bacterial and mammalian HIV-1 Tat expression vectors, pTat86R His, pCI vector control, and pCI-Tat (29), respectively, were obtained from the AIDS Research Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NAIAD), NIH. pCI vector (Promega, Madison WI, USA) was used as an empty vector control. LTR reporter constructs, LTR-Luc, and ΔκBLTR-Luc, which represent the wild-type −205LTR and ΔκB mutated −205LTR promoters, respectively, were prepared as described previously (30). DNA for transfections was prepared using plasmid purification systems from Marligen Biosciences (Ijamsville, MD, USA) following protocols provided by the manufacturer. Transfection of U937 cells was performed using TransIT-Jurkat transfection reagent from Mirus Corporation (Madison, WI, USA). Transfection efficiency was assessed by cotransfecting pEGFP-N3 (Clontech, Palo Alto, CA, USA) and monitoring EGFP expression by fluorescence microscopy. Cell viability was confirmed by Trypan blue staining. In addition, cell viability was assessed in PG-treated cells using the WST-8 cell counting kit from Dojindo (Gaithersburg, MD, USA). Luciferase assay was performed using the luciferase assay kit (Promega) and a TD-20/20 luminometer (Turner BioSystems, Sunnyvale, CA, USA). The human inducible nitric oxide synthase promoter luciferase reporter, pHiNOS-Luc (iNOS-Luc), was obtained from Dr. Kalipada Pahan (Rush University Medical Center, Chicago, IL, USA).
Generation of HIV infectious titer and infections
Infectious HXB.2 virus was generated by cotransfecting HEK293T cells with 15 μg of cDNA for HXB.2, 3 μg of vesicular stomatitis virus-glycoprotein (VSV-G), and 3 μg RSV-Rev by CaPO4 transfection. Transfection efficiency was assessed by measuring p24 levels using an ELISA method (p24 ELISA; Perkin Elmer, Wellesley, MA, USA). Supernatants were collected and filtered through a 0.45-μm syringe filter (Whatman, Clifton, NJ, USA). One milliliter of undiluted virus stock was added to 1.0 × 106 U937 cells for 24 h and then replaced with fresh medium. Supernatants were collected at d 3 or 4 postinfection and assayed for viral replication by p24 ELISA. All protocols were preapproved by the Institutional Biosafety Committee at Pennsylvania State University.
Immunoblotting
Cells were washed twice with phosphate-buffered saline, and protein extracts were prepared by treating cells with M-PER reagent (Thermo-Pierce) at 4°C for 30 min. Lysates were mixed with 2× SDS loading buffer containing DTT and boiled at 100°C for 5 min before resolving by SDS-PAGE, followed by immunoblotting onto a nitrocellulose membrane. Mouse anti-Tat monoclonal antibody from the NIH AIDS Research and Reference Reagent Program was used to analyze expression, and horseradish peroxidase-conjugated goat-anti-mouse (Sigma-Aldrich, St. Louis, MO, USA) was used as the secondary antibody. In the case of analysis of biotinylated Tat, streptavidin-HRP (Thermo-Pierce) was used.
Interaction of 15d-PGJ2 and 9,10-dihydro-PGJ2 with rTat
Studies to determine the covalent interaction of 15d-PGJ2 with rTat were carried out as follows. First, the interaction of 15d-PGJ2 biotinamide with rTat was examined in vitro. rTat was incubated with 15d-PGJ2 at a molar ratio of 1:7 (protein:PG) for 3 h at 37°C in phosphate-buffered saline with or without DTT pretreatment. The reaction mixture was applied onto a Biogel P column (Bio-Rad, Hercules, CA, USA) to separate the unreacted 15d-PGJ2-biotinamide from the protein-PG complex. The flow-through fractions, which tested positive for the presence of rTat, were analyzed by Western immunoblotting techniques with streptavidin-HRP and anti-Tat. Second, the interaction between 15d-PGJ2 and rTat in the context of the cells was determined by using 15d-PGJ2-biotinamide to pull down Tat from cell lysates. Cells were cultured for 12 h with rTat (2 μg/106 cells). After a medium change, cells were lysed and treated with 15d-PGJ2-biotinamide (Cayman Chemicals) or 9,10-dihydro-15d-PGJ2-biotinamide at a molar ratio of 1:7 (rTat:PG) for 3 h at 4°C. The cell lysates were subjected to biotin-pulldown assays using 20 μl immobilized neutravidin (Pierce), followed by SDS-PAGE under reducing conditions and Western blot analysis. Biotinylated rTat was detected using streptavidin-HRP (Pierce). The blot was stripped and probed with anti-Tat antibodies.
Statistical analysis
When necessary, data are expressed as means ± sd. Student’s t test was used in statistical analysis for comparison, and P < 0.05 was used as the criterion for statistical significance.
RESULTS
15d-PGJ2 inhibits HIV transcription and replication
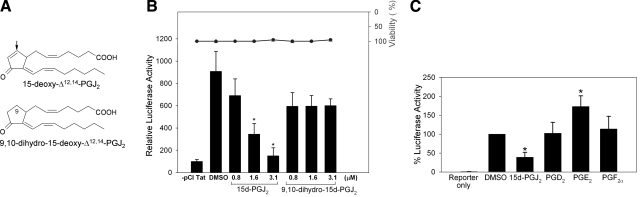
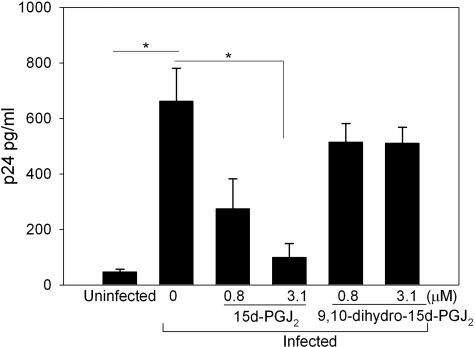
To examine the effect of 15d-PGJ2 on HIV transcription, promonocytic U937 cells were transfected with an HIV LTR-Luc reporter and Tat expression vector (pCI-Tat), followed by treatment of cells with different concentrations of 15d-PGJ2. As a control, we used 9,10-dihydro analog of 15d-PGJ2 that lacks the ability to bind to protein thiols (see structures in Fig. 1A). 15d-PGJ2 inhibited the Tat-dependent transactivation of the reporter in a dose-dependent manner, inhibiting LTR activity by >80% at the highest concentration tested, while the 9,10-dihydro-15d-PGJ2 displayed only minimal activity in repressing Tat-dependent LTR activity (Fig. 1B). Treatment of U937 cells with 15d-PGJ2 or 9,10-dihydro-PGJ2 did not cause any significant changes in cell viability. Because the major difference between 15d-PGJ2 and 9,10 dihydro is the α,β-unsaturated enone moiety in 15d-PGJ2, these data suggest that the reactive moiety is required for 15d-PGJ2-dependent inhibition of Tat-dependent transcription. In addition, we examined the role of PGD2, PGE2, and PGF2α on the transactivation function of Tat. Results shown in Fig. 1C clearly indicate the inhibitory activity is specific to 15d-PGJ2. To determine whether 15d-PGJ2 inhibited HIV replication in infected cells, we exogenously added 15d-PGJ2 at concentrations of 0.8 and 3.1 μM to HIV-infected U937 macrophages 24 h postinfection. HIV replication was monitored by measuring p24 Gag release using ELISA. Treating infected cells with 15d-PGJ2 decreased p24 levels 3 d postinfection by greater than 75% when compared with cells treated with the vehicle control (DMSO) (Fig. 2). 9,10-Dihydro-15d-PGJ2 did not cause any significant reduction in p24 levels again, implicating the importance of the enone moiety in 15d-PGJ2 as being necessary for the antiviral property.
Figure 1.
15d-PGJ2 inhibits HIV transcription. A) Structures of 15d-PGJ2 and 9,10-dihydro-15d-PGJ2. Arrow indicates electrophilic carbon at C-9 in 15d-PGJ2. B) U937 cells were transfected with HIV-LTR-Luc and pCI-Tat using the Jurkat cell transfection reagent (Mirus). Transfected cells were treated with 0.8, 1.6, and 3.1 μM 15d-PGJ2 or 9,10-dihydro-15d-PGJ2 for 24 h. Luciferase activities in lysates were measured after 24 h and normalized to total protein. Lanes 1 and 2 represent LTR-Luc + pCI empty vector and LTR-Luc + pCI-Tat in the presence of 0.1% (v/v) DMSO, respectively. Line and circles represent cells treated with PGs or vehicle control subjected to cell viability assays using CCK-8 kit (Dojindo). C) Transiently transfected U937 cells from the above experiment were treated with 3 μM of 15d-PGJ2, PGD2, PGE2, or PGF2α. Changes in luciferase activity with each treatment were compared with pCI-Tat transfected cells treated with DMSO. Data are averages of triplicate experiments. *P < 0.05 vs. DMSO control).
Figure 2.
15d-PGJ2 decreases HIV replication. U937 cells were infected with HIV-1 and treated with indicated amounts of 15d-PGJ2 or 9,10-dihydro-15d-PGJ2. Three days postinfection, supernatants were collected and assayed for viral replication by p24 ELISA. DMSO was added to infected cells in the absence of the CyPGs as a negative vehicle control. Experiment was performed in triplicate, and averages were computed. *P < 0.05.
Inhibition of Tat-dependent transcription is independent of the NF-κB pathway
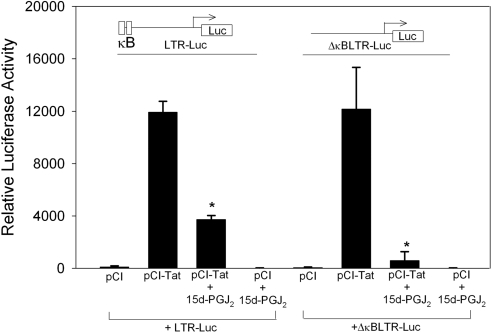
Given that 15d-PGJ2 inhibits multiple steps of the NF-κB signaling pathway and that NF-κB activation positively affects HIV transcription, we decided to examine the contribution of NF-κB inhibition in 15d-PGJ2-dependent modulation of Tat function. To address the role of NF-κB in HIV provirus transcription, we used an LTR-Luc reporter that lacked NF-κB sites (ΔκBLTR-Luc). As shown in Fig. 3, even in the absence of NF-κB binding sites, there was >90% decrease in Tat-dependent LTR activity, suggesting that 15d-PGJ2 was not inhibiting the Tat activity through an NF-κB-dependent mechanism. 15d-PGJ2 had no effect on the luciferase reporter activity in the absence of Tat expression. Taken together, these results confirm previous reports that CyPGs, such as 15d-PGJ2, inhibit HIV transcription, and further demonstrate the results of earlier studies by showing that inhibition of NF-κB is not the operative mechanism for repressing Tat-dependent HIV transcription.
Figure 3.
NF-κB binding sites are not required for 15d-PGJ2-mediated inhibition of HIV LTR. U937 cells were transfected with 1 μg of LTR-Luc or ΔκBLTR-Luc and 2 μg pCI vector or pCI-Tat for 24 h. Transfected cells were incubated with 15d-PGJ2 at a final concentration of 3 μM for 24 h. Luciferase activities in lysates were measured as described in text. Experiment was performed in triplicate, and averages were computed. *P < 0.05.
Interaction of 15d-PGJ2 and 9,10-dihydro-15d-PGJ2 with rTat in vitro
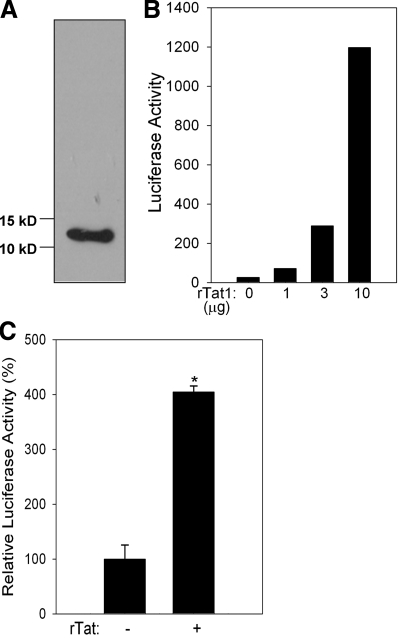
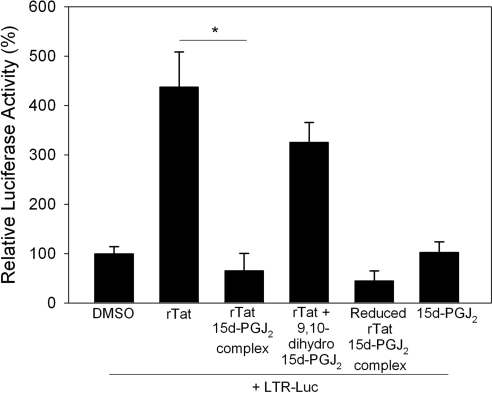
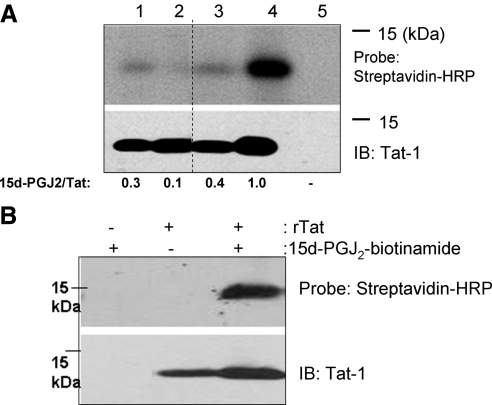
To address the possibility of a direct interaction of 15d-PGJ2 with the Cys residues, and thus affecting the biological activity of Tat, we first examined whether 15d-PGJ2 interacted with rTat. Given that purified rTat is not commercially available, we used a bacterial expression system to express rTat. The purified rTat was subjected to the protein for oxidative refolding. Western blot analysis with anti-Tat monoclonal antibodies confirmed that the bacterially expressed, affinity purified, and oxidatively refolded protein indeed was Tat (Fig. 4A). Furthermore, to demonstrate that the rTat expressed in a bacterial system was biologically active, we utilized the U937 cells transfected with the LTR-Luc reporter. The ability of ectopically added rTat to activate the HIV LTR-Luc reporter and the iNOS promoter in U937 cells suggested that the rTat was biologically active (Fig. 4B, C). Further biochemical characterization of rTat using Ellman’s reagent (dithionitrobenzoic acid; DTNB) and redox Western immunoblotting techniques demonstrated that purified and refolded rTat contained 3 free thiols/mol protein (data not shown), which is consistent with that reported earlier (13). Such a bacterially expressed active rTat was used to prepare a complex with 15d-PGJ2 or 9,10-dihydro-PGJ2 in an in vitro reaction. The reaction mixture was subjected to gel-filtration chromatography using a Biogel P6 spin column, to separate the unreacted 15d-PGJ2 or 9,10-dihydro-PGJ2. Tat activity in the eluate was measured by adding eluate to U937 cells transfected with LTR-Luc. As a control, the flow-through fraction of rTat protein without 15d-PGJ2 caused a significant increase in the luciferase activity when added exogenously to cells; while DMSO-treated buffer, processed through the column, had no effect on the luciferase activity (Fig. 5). Previous studies have shown that rTat is rapidly taken up by serum-starved cells to transactivate the HIV LTR (31). Interestingly, treatment of cells with the 15d-PGJ2-rTat complex caused a significant inhibition of LTR-Luc activity, while the rTat incubated with 9,10-dihydro-PGJ2 still retained ∼80% of the activity. rTat that was not incubated with CyPG but passed through the Biogel P6 column, was used as a positive control. These data suggest the possibility of alkylation of Tat by 15d-PGJ2 as a likely mechanism of inactivation. The physical interaction between 15d-PGJ2 (via electrophilic carbon-9) with the free thiol groups in Tat was more rigorously examined using an in vitro binding assay with purified rTat and biotinylated 15d-PGJ2 (Cayman Chemicals). rTat was treated with excess of 15d-PGJ2 (7 mol 15d-PGJ2/mol rTat, based on a total of 7 cysteine residues in completely reduced Tat) in PBS for 3 h at 37°C. Samples were separated by PAGE followed by Western blot analysis with streptavidin-HRP. As shown in Fig. 6A, 15d-PGJ2 covalently bound rTat; while 9,10-dihydro analog failed to interact with rTat. Furthermore, increasing the thiol reactivity of rTat with the reducing agent DTT (1 mM) enhanced the binding of 15d-PGJ2, suggesting that all seven cysteines were likely alkylated.
Figure 4.
Bacterial expression and biological activity of rTat. A) Western blot showing the immunoreactive band corresponding to purified and refolded rTat. B) rTat activates LTR-Luc in U937 cells. Cells were transfected with LTR-Luc reporter, then treated with increasing amounts of rTat. C) Ability of rTat to activate iNOS promoter. U937 cells were transfected with 2 μg iNOS-Luc for 24 h. Transfected cells were then treated with or without refolded rTat protein (180 nM). Luciferase activity was measured 48 h post-rTat treatment. Data are representative of n = 3. *P < 0.05.
Figure 5.
15d-PGJ2 inhibits rTat activity. rTat was incubated with 15d-PGJ2-biotinamide (1:7 mol/mol) or 9,10-dihydro-15d-PGJ2 (1:7 mol/mol). Some rTat samples were reduced with DTT (1 mM) for 30 min prior to incubating CyPGs. Reaction mixtures were passed through a Biogel P column; flow-through fraction was added to serum-starved U937 cells transfected with the LTR-Luc reporter vector. Cells were lysed 48 h postaddition, and luciferase activity was measured. rTat that was not incubated with CyPG, but passed through the Biogel P column, was used as a positive control. Data are averages of 3 independent experiments. *P < 0.05.
Figure 6.
15d-PGJ2 interacts with rTat. A) Purified rTat was incubated with 15d-PGJ2-biotinamide (1:7 mol/mol) or 9,10-dihydro-15d-PGJ2 (1:7 mol/mol) before and after reduction with DTT (10 μM and 1 mM) for 30 min. Interaction was analyzed on an SDS-PAGE gel followed by Western blot analysis with streptavidin-HRP. Concentration of rTat was 180 nM. Membrane was stripped and reprobed with anti-Tat-1 monoclonal antibody. Densitometric evaluation of the bands is shown at bottom. Lanes 1–5 represent rTat + 15d-PGJ2-biotinamide, rTat + 9,10-dihydro-15d-PGJ2-biotinamide, rTat reduced with 10 μM DTT + 15d-PGJ2-biotinamide, rTat reduced with 1 mM DTT + 15d-PGJ2-biotinamide, and 15d-PGJ2-biotinamide alone, respectively. B) U937 cells were incubated with rTat (180 nM) for 12 h. Cells were washed with PBS and lysed using M-PER. 15d-PGJ2-biotinamide was added to lysate at a final concentration of 1 μM and incubated on ice for 3 h. Biotinylated proteins were isolated using neutravidin beads, and interaction was analyzed on an SDS-PAGE gel followed by Western blot analysis with streptavidin-HRP. Blot was stripped and reprobed with anti-Tat monoclonal antibody. Data are representative of n = 2.
To confirm that Tat and 15d-PGJ2 physically interact in the presence of other cellular proteins, U937 cells were cultured with or without rTat, and cell lysates were prepared and incubated with 15d-PGJ2-biotinamide. Tat-15d-PGJ2 complexes were pulled down using a Neutravidin-pull down assay, separated on PAGE, and Tat was detected by immunoblotting. As shown in Fig. 6B, rTat interacted with 15d-PGJ2-biotinamide and specifically pulled down rTat from cell extracts, providing additional evidence that 15d-PGJ2 directly interacts with HIV-1 Tat.
DISCUSSION
15-Deoxy-prostaglandin J2, a downstream metabolite of hematopoietic PGD2 synthase-catalyzed conversion of PGH2 to PGD2, has been shown to be synthesized in the mammalian system, with a pivotal role in controlling the onset and resolution of acute inflammation (22). 15d-PGJ2 induces apoptosis in many cell models. One mechanism by which 15d-PGJ2 regulates cell activity is by acting as a ligand for PPARγ and influencing the expression of proinflammatory and apoptotic genes (32). In addition, 15d-PGJ2 has been shown to posttranslationally modify proteins. Targets of 15d-PGJ2 include NF-κB p50 and p65 subunits (33, 34), c-Jun of the AP-1 complex (34), and p53 (35). The interaction of 15d-PGJ2 with the above-mentioned proteins involves covalent binding with the thiol group in Cys (referenced in ref. 36). It is particularly interesting to note that in Trx, which contains two thiol/disulfide motifs (37), 15d-PGJ2 modifies only one Cys in each of these motifs (27). As in the case of Trx, the Cys-rich domain of Tat includes several thiols that are potential targets for covalent modification by 15d-PGJ2. Our data demonstrate that Tat is targeted by 15d-PGJ2, and such an interaction inhibits Tat function and HIV transcription and replication.
CyPGs have been shown to inhibit replication of multiple RNA and DNA viruses, including poxviruses (38) paramyxoviruses (39), togaviruses (40), rhabdoviruses (25) and HIV (26), although the mechanisms by which CyPGs target these viruses are still unclear. It is assumed that some of the antiviral activity of CyPG is associated with the ability of CyPGs to suppress NF-κB signaling. As mentioned earlier, 15d-PGJ2 has been shown to covalently modify cysteines in the p50 and p65 subunits of NF-κB, as well as a Cys in the activation loop of IKKβ (18). Binding of 15d-PGJ2 to reduced Cys residues in p50, p65, and IKKβ inhibits the activation and consequent DNA recognition and binding of NF-κB (41). NF-κB is also a critical cellular transcription factor that binds to the HIV LTR and promotes efficient HIV transcription (42, 43). Inhibitors of NF-κB signaling decrease HIV provirus transcription and replication (44). Despite the interaction of 15d-PGJ2 with IKKβ in the U937 monocytic system (data not shown), the ability of 15d-PGJ2 to inhibit HIV transcription did not appear to require NF-κB binding to the LTR since HIV-LTR reporter lacking the two NF-κB binding sites was still repressed by 15d-PGJ2. However, these data do not exclude a role for NF-κB in setting the intracellular oxidative tone that could influence early Tat-independent transcription initiation, and regulation of HIV provirus.
These studies extend original observations by Rozera et al. (26) that conclusively demonstrated the inhibitory role of CyPGs on HIV replication and suggested that the effect was possibly mediated by targeting proviral transcription. Studies by Hayes et al. (45) have indicated the role of CyPG-activated PPARγ to cause inhibition of proviral transcription. Here, we demonstrate that 15d-PGJ2 inhibits HIV transcription and replication by targeting thiols in Tat. Tat enhances provirus transcription processivity by recruiting P-TEFb and chromatin remodeling complexes to the HIV LTR. Inhibiting Tat function leads to premature transcription termination and accumulation of short HIV transcripts. In support, preliminary studies suggest that the addition of 15d-PGJ2 significantly reduces transcription processivity consistent with our conclusion that 15d-PGJ2 is specifically acting at the level of Tat (data not shown). Prostaglandins that lack the cyclopentenone enone, such as PGE2, PGF2α, and PGD2, did not affect the activity of Tat. Surprisingly, PGD2, the precursor of 15d-PGJ2, did not cause a decrease in Tat activity, which could be explained based on the relatively slow second dehydration reaction (to eliminate the C-15 hydroxyl) in PGJ2 to 15d-PGJ2. In addition, PGE2 treatment caused a statistically significant increase in Tat-dependent transcription. It is possible that increased expression of COX-2 in HIV-infected macrophages may act in autocrine or paracrine loop to positively affect transcription, which has been reported earlier (46).
The ability of 15d-PGJ2 to inhibit Tat was dependent on the presence of the α, β-unsaturated enone moiety since the 9,10-dihydro-15d-PGJ2 did not significantly inhibit HIV transcription. Furthermore, on the basis of the binding studies with biotinylated 15d-PGJ2 and 9,10-dihydro-15d-PGJ2, it is clear that the α,β-unsaturated group in 15d-PGJ2 is absolutely essential for its interaction with the Cys residues. Reduction of rTat with DTT prior to incubating with 15d-PGJ2 increased the binding of 15d-PGJ2 to Tat. This observation indicates that in a reduced state all the Cys in Tat can potentially bind 15d-PGJ2. Furthermore, DTT has been shown to inhibit Tat activity, suggesting a need to maintain the integrity of disulfide bonds in Tat (13). The results showing an increased interaction of 15d-PGJ2 with Tat is different from the selective interaction of 15d-PGJ2 with Trx, where 15d-PGJ2 modifies two Cys residues from each of the two dithiol/disulfide motifs (27, 37). In contrast to Trx, it appears that 15d-PGJ2 does not discriminate between different Cys thiols for alkylation in Tat, which may be attributed to the structural difference between the two proteins, particularly within the thiol/disulfide motifs. It should be noted that the interaction of 15d-PGJ2 with protein thiols depends on complementary interactions between the protein thiol and its surrounding environment to accommodate the fatty acid side chain of 15d-PGJ2 (36). Taken together, these findings support the premise that binding of 15d-PGJ2 to Tat, which in all likelihood brings about a structural change in Tat that ultimately leads to inhibition of proviral transcription. Further studies to characterize the extent of structural change in Tat, brought about by the interaction with 15d-PGJ2, which could affect the ability to interact with other cellular factors, are currently under way in our laboratory.
In summary, we have demonstrated the ability of 15d-PGJ2, an endogenous CyPG, to modify free thiols in Tat, leading to a transcriptional block. These findings open new avenues for the understanding of the antiviral effects of 15d-PGJ2 and may help define their therapeutic potential. More important, treatment with CyPGs might complement other antiretroviral strategies by targeting key Cys residues in Tat that are conserved in HIV strains worldwide. Further work will be necessary to explore this intriguing possibility.
Acknowledgments
These studies were supported, in part, by Public Health Service grants from the NIH to A.J.H. and K.S.P. and an intramural seed grant from the College of Agricultural Sciences, Pennsylvania State University, to K.S.P. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: monoclonal antibody to HIV-1 Tat (NT3 2D1.1; catalog no. 4138) from Dr. Jonathan Karn; pHis-Tat from Drs. Abhay Patki and M. M. Lederman, courtesy of the UK National Institute for Biological Standards and Control (NIBSC) Centralized Facility for AIDS Reagents. We are grateful to Dr. Kalipada Pahan (Rush University, Chicago, IL, USA) for providing the iNOS promoter-Luc construct. We also thank Professor C. Channa Reddy for his invaluable suggestions.
References
- Karn J. Tackling Tat. J Mol Biol. 1999;293:235–254. doi: 10.1006/jmbi.1999.3060. [DOI] [PubMed] [Google Scholar]
- Pierson T, McArthur J, Siliciano R F. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu Rev Immunol. 2000;18:665–708. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- Rohr O, Marban C, Aunis D, Schaeffer E. Regulation of HIV-1 gene transcription: from lymphocytes to microglial cells. J Leukoc Biol. 2003;74:736–749. doi: 10.1189/jlb.0403180. [DOI] [PubMed] [Google Scholar]
- Kao S Y, Calman A F, Luciw P A, Peterlin B M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- Marciniak R A, Sharp P A. HIV-1 Tat protein promotes formation of more-processive elongation complexes. EMBO J. 1991;10:4189–4196. doi: 10.1002/j.1460-2075.1991.tb04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg M B, Baltimore D, Frankel A D. The role of Tat in the human immunodeficiency virus life cycle indicates a primary effect on transcriptional elongation. Proc Natl Acad Sci U S A. 1991;88:4045–4049. doi: 10.1073/pnas.88.9.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laspia M F, Rice A P, Mathews M B. HIV-1 Tat protein increases transcriptional initiation and stabilizes elongation. Cell. 1989;59:283–292. doi: 10.1016/0092-8674(89)90290-0. [DOI] [PubMed] [Google Scholar]
- Agbottah E, Deng L, Dannenberg L O, Pumfery A, Kashanchi F. Effect of SWI/SNF chromatin remodeling complex on HIV-1 Tat activated transcription. Retrovirology. 2006;3:48. doi: 10.1186/1742-4690-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi T, Parra M, Vries R G, Kauder S E, Verrijzer C P, Ott M, Verdin E. The SWI/SNF chromatin-remodeling complex is a cofactor for Tat transactivation of the HIV promoter. J Biol Chem. 2006;281:19960–19968. doi: 10.1074/jbc.M603336200. [DOI] [PubMed] [Google Scholar]
- Emiliani S, Van Lint C, Fischle W, Paras P, Jr, Ott M, Brady J, Verdin E. A point mutation in the HIV-1 Tat responsive element is associated with postintegration latency. Proc Natl Acad Sci U S A. 1996;93:6377–6381. doi: 10.1073/pnas.93.13.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppuswamy M, Subramanian T, Srinivasan A, Chinnadurai G. Multiple functional domains of Tat, the trans-activator of HIV-1, defined by mutational analysis. Nucleic Acids Res. 1989;17:3551–3561. doi: 10.1093/nar/17.9.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A D, Bredt D S, Pabo C O. Tat protein from human immunodeficiency virus forms a metal-linked dimer. Science. 1988;240:70–73. doi: 10.1126/science.2832944. [DOI] [PubMed] [Google Scholar]
- Koken S E, Greijer A E, Verhoef K, van Wamel J, Bukrinskaya A G, Berkhout B. Intracellular analysis of in vitro modified HIV Tat protein. J Biol Chem. 1994;269:8366–8375. [PubMed] [Google Scholar]
- Kirsch T, Boehm M, Schuckert O, Metzger A U, Willbold D, Frank R W, Rosch P. Cloning, high-yield expression in Escherichia coli, and purification of biologically active HIV-1 Tat protein. Protein Expr Purif. 1996;8:75–84. doi: 10.1006/prep.1996.0076. [DOI] [PubMed] [Google Scholar]
- Wada M, DeLong C J, Hong Y H, Rieke C J, Song I, Sidhu R S, Yuan C, Warnock M, Schmaier A H, Yokoyama C, Smyth E M, Wilson S J, FitzGerald G A, Garavito R M, Sui de X, Regan J W, Smith W L. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282:22254–22266. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F A, Wynalda M A. Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro. J Biol Chem. 1983;258:11713–11718. [PubMed] [Google Scholar]
- Santoro M G, Benedetto A, Carruba G, Garaci E, Jaffe B M. Prostaglandin A compounds as antiviral agents. Science. 1980;209:1032–1034. doi: 10.1126/science.6157190. [DOI] [PubMed] [Google Scholar]
- Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro M G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- Kondo M, Shibata T, Kumagai T, Osawa T, Shibata N, Kobayashi M, Sasaki S, Iwata M, Noguchi N, Uchida K. 15-Deoxy-Delta(12,14)-prostaglandin J(2): the endogenous electrophile that induces neuronal apoptosis. Proc Natl Acad Sci U S A. 2002;99:7367–7372. doi: 10.1073/pnas.112212599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman B M, Tontonoz P, Chen J, Brun R P, Spiegelman B M, Evans R M. 15-Deoxy-delta 12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Pascual G, Fong A L, Ogawa S, Gamliel A, Li A C, Perissi V, Rose D W, Willson T M, Rosenfeld M G, Glass C K. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPARγ. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob M M, Gilroy D W. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc Natl Acad Sci U S A. 2007;104:20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes-Fulford M, McGrath M S, Hanks D, Erickson S, Pulliam L. Effects of dimethyl prostaglandin A1 on herpes simplex virus and human immunodeficiency virus replication. Antimicrob Agents Chemother. 1992;36:2253–2258. doi: 10.1128/aac.36.10.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica F, De Marco A, De Cesare F, Santoro M G. Inhibition of vesicular stomatitis virus replication by delta 12-prostaglandin J2 is regulated at two separate levels and is associated with induction of stress protein synthesis. Antiviral Res. 1993;20:193–208. doi: 10.1016/0166-3542(93)90020-j. [DOI] [PubMed] [Google Scholar]
- Santoro M G, Benedetto A, Zaniratti S, Garaci E, Jaffe B M. The relationship between prostaglandins and virus replication: endogenous prostaglandin synthesis during infection and the effect of exogenous PGA on virus production in different cell lines and in persistently infected cells. Prostaglandins. 1983;25:353–364. doi: 10.1016/0090-6980(83)90038-2. [DOI] [PubMed] [Google Scholar]
- Rozera C, Carattoli A, De Marco A, Amici C, Giorgi C, Santoro M G. Inhibition of HIV-1 replication by cyclopentenone prostaglandins in acutely infected human cells. Evidence for a transcriptional block. J Clin Invest. 1996;97:1795–1803. doi: 10.1172/JCI118609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Yamada T, Ishii T, Kumazawa S, Nakamura H, Masutani H, Yodoi J, Uchida K. Thioredoxin as a molecular target of cyclopentenone prostaglandins. J Biol Chem. 2003;278:26046–26054. doi: 10.1074/jbc.M303690200. [DOI] [PubMed] [Google Scholar]
- Patki A H, Lederman M M. HIV-1 Tat protein and its inhibitor Ro 24–7429 inhibit lymphocyte proliferation and induce apoptosis in peripheral blood mononuclear cells from healthy donors. Cell Immunol. 1996;169:40–46. doi: 10.1006/cimm.1996.0088. [DOI] [PubMed] [Google Scholar]
- Frankel A D, Chen L, Cotter R J, Pabo C O. Dimerization of the tat protein from human immunodeficiency virus: a cysteine-rich peptide mimics the normal metal-linked dimer interface. Proc Natl Acad Sci U S A. 1988;85:6297–6300. doi: 10.1073/pnas.85.17.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A J, Zou X, Calame K L. C/EBP proteins activate transcription from the human immunodeficiency virus type 1 long terminal repeat in macrophages/monocytes. J Virol. 1995;69:5337–5344. doi: 10.1128/jvi.69.9.5337-5344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A D, Pabo C O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Ishihara S, Rumi M A, Okuyama T, Kinoshita Y. Effect of prostaglandins on the regulation of tumor growth. Curr Med Chem Anticancer Agents. 2004;4:379–387. doi: 10.2174/1568011043352902. [DOI] [PubMed] [Google Scholar]
- Straus D S, Pascual G, Li M, Welch J S, Ricote M, Hsiang C H, Sengchanthalangsy L L, Ghosh G, Glass C K. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Sala D, Cernuda-Morollon E, Canada F J. Molecular basis for the direct inhibition of AP-1 DNA binding by 15-deoxy-delta 12,14-prostaglandin J2. J Biol Chem. 2003;278:51251–51260. doi: 10.1074/jbc.M309409200. [DOI] [PubMed] [Google Scholar]
- Buzek J, Latonen L, Kurki S, Peltonen K, Laiho M. Redox state of tumor suppressor p53 regulates its sequence-specific DNA binding in DNA-damaged cells by cysteine 277. Nucleic Acids Res. 2002;30:2340–2348. doi: 10.1093/nar/30.11.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande V, Ramos M J. Molecular recognition of 15-deoxy-delta(12,14)-prostaglandin J2 by nuclear factor-κB and other cellular proteins. Bioorg Med Chem Lett. 2005;15:4057–4063. doi: 10.1016/j.bmcl.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Watson W H, Pohl J, Montfort W R, Stuchlik O, Reed M S, Powis G, Jones D P. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J Biol Chem. 2003;278:33408–33415. doi: 10.1074/jbc.M211107200. [DOI] [PubMed] [Google Scholar]
- Santoro M G, Jaffe B M, Garaci E, Esteban M. Antiviral effect of prostaglandins of the A series: inhibition of vaccinia virus replication in cultured cells. J Gen Virol. 1982;63:435–440. doi: 10.1099/0022-1317-63-2-435. [DOI] [PubMed] [Google Scholar]
- Amici C, Santoro M G. Suppression of virus replication by prostaglandin A is associated with heat shock protein synthesis. J Gen Virol. 1991;72:1877–1885. doi: 10.1099/0022-1317-72-8-1877. [DOI] [PubMed] [Google Scholar]
- Mastromarino P, Conti C, Petruzziello R, De Marco A, Pica F, Santoro M G. Inhibition of Sindbis virus replication by cyclopentenone prostaglandins: a cell-mediated event associated with heat-shock protein synthesis. Antiviral Res. 1993;20:209–222. doi: 10.1016/0166-3542(93)90021-a. [DOI] [PubMed] [Google Scholar]
- Stamatakis K, Sanchez-Gomez F J, Perez-Sala D. Identification of novel protein targets for modification by 15-deoxy-delta 12,14-prostaglandin J2 in mesangial cells reveals multiple interactions with the cytoskeleton. J Am Soc Nephrol. 2006;17:89–98. doi: 10.1681/ASN.2005030329. [DOI] [PubMed] [Google Scholar]
- Nabel G J, Rice S A, Knipe D M, Baltimore D. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science. 1988;239:1299–1302. doi: 10.1126/science.2830675. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Meisterernst M, Scheidereit C, Li G, Roeder R G. Transcriptional regulation of the HIV-1 promoter by NF-κB in vitro. Genes Dev. 1992;6:761–774. doi: 10.1101/gad.6.5.761. [DOI] [PubMed] [Google Scholar]
- Williams S A, Chen L F, Kwon H, Ruiz-Jarabo C M, Verdin E, Greene W C. NF-κB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M M, Lane B R, King S R, Markovitz D M, Coffey M J. Peroxisome proliferator-activated receptor gamma agonists inhibit HIV-1 replication in macrophages by transcriptional and post-transcriptional effects. J Biol Chem. 2002;277:16913–16919. doi: 10.1074/jbc.M200875200. [DOI] [PubMed] [Google Scholar]
- Lima R G, Moreira L, Paes-Leme J, Barreto-de-Souza V, Castro-Faria-Neto H C, Bozza P T, Bou-Habib D C. Interaction of macrophages with apoptotic cells enhances HIV Type 1 replication through PGE2, PAF, and vitronectin receptor. AIDS Res Hum Retroviruses. 2006;22:763–769. doi: 10.1089/aid.2006.22.763. [DOI] [PubMed] [Google Scholar]