Abstract
Hypoxia is well known to limit curability of tumors by ionizing radiation. Here, we show that hypoxia treatment of tumor cells causes coexpression of heat shock protein 70 (Hsp70) and phosphatidylserine (PS) on the cell surface. Colocalization of Hsp70 and PS, as determined by confocal microscopy, also occurs when exogenous FITC-labeled Hsp70 protein is added to normoxic and hypoxic tumor cells. Moreover, the interaction of Hsp70 with PS was demonstrated in artificial unilamellar phosphatidylcholine/ phosphatidylserine (PC/PS) liposomes at the physiological ratio of 8/2. Indeed, the Hsp70-liposome interaction gradually increased with elevating PS molar ratios (8/2≥7/3<5/5<4/6<3/7<2/8). In contrast, only a weak Hsp70 interaction was detected in phosphatidylcholine/phosphatidylglycerol (PC/PG) liposomes, thus demonstrating that the interaction was not a charge-related effect. The interaction of Hsp70 with surface PS significantly reduces clonogenic cell survival in normoxic (EC50 of Hsp70=85 μg/ml) and hypoxic (EC50 of Hsp70=55 μg/ml) tumor cells. The radiation-induced tumor cell killing was significantly enhanced by the addition of Hsp70 protein (50 μg/ml). Since apoptosis was not significantly enhanced in normoxic and hypoxic tumor cells by the addition of Hsp70, we hypothesize that the Hsp70 protein-induced reduction in clonogenic cell survival might be through necrosis rather than apoptosis.—Schilling, D., Gehrmann, M., Steinem, C., De Maio, A., Pockley, A. G., Abend, M., Molls, M., Multhoff, G. Binding of heat shock protein 70 to extracellular phosphatidylserine promotes killing of normoxic and hypoxic tumor cells.
Keywords: nonapoptotic tumor cell death, stress proteins, ionizing radiation, cancer therapy
Under normal conditions, heat shock protein 70 (Hsp70) predominantly resides in the cytosolic compartment, where it supports folding, refolding, and assembly of nascent polypeptides, prevents protein aggregation, and assists transport of other proteins across membranes (1). Following nutritional deprivation, chemical stress or physical interventions, such as ionizing radiation, hypoxia, and hyperthermia, the synthesis of Hsp70 is markedly increased, whereas synthesis of other proteins is down-regulated. Compared to other stress proteins, the synthesis of Hsp70 is more rapid, and Hsp70 accumulates at higher levels after stress. Thus, this protein is considered to be the major stress-inducible member of the heat shock protein family. Beside the cytosol, Hsp70 also has been found on the plasma membrane of tumor cells (2, 3) and in the extracellular milieu (4, 5). Extracellular tumor-derived HSPs are considered to be danger signals (6) that elicit potent anticancer immune responses, whereas the same proteins located in the intracellular compartment fulfill a protective function for the cell (7,8,9,10).
In the present report, we investigate the binding characteristics of extracellular-added Hsp70 to phosphatidylserine (PS)-containing vesicles and to hypoxic tumor cells and study the functional consequences. In most nonstressed eukaryotic cells, PS is exclusively located on the inner side of the plasma membrane. This asymmetry of PS is maintained by the ATP-dependent aminophospholipid translocase (11). Following exposure to environmental stress factors, PS is translocated to the outer plasma membrane leaflet via an activation of the Ca2+-dependent phospholipid scramblase and reduced ATP levels, resulting in the loss of the plasma membrane PS lipid asymmetry (12). Although the appearance of PS on the outer membrane leaflet is typically considered to be a sign of cellular stress, cell activation processes are also associated with a transient translocation of PS to the outer leaflet (13). Furthermore, exposure of PS on the surface has been found to trigger cellular recognition systems, removal by macrophages, and production of autoantibodies (14, 15).
Previous work has demonstrated that constitutive Hsc70 and inducible Hsp70 produce an aggregation of artificial liposomes containing high levels of PS in the presence of salt (0.5 mM MgCl2 and 1 mM CaCl2) (16). Here, we show that even in the absence of ions, Hsp70, but not Hsp90 or Hsp60, selectively inserts into unilamellar PC/PS vesicles (8/2) that mimic the PC/PS ratio in plasma membranes. Binding of Hsp70 to PC/PS vesicles has been found to be dependent on the PS content. In line with these in vitro results, an enhanced coexpression of Hsp70 and PS has been found on the outer leaflet of PS-positive hypoxia-treated tumor cells. Hypoxia is considered to be an important factor involved in the resistance to radiation therapy (17,18,19). Among other stressors, hypoxia initiates the synthesis and export of Hsp70 and causes PS to translocate into the outer membrane leaflet. As a consequence of these findings, we were interested in studying the effects of extracellular Hsp70 on hypoxia-treated tumor cells. We found that Hsp70 protein added from outside binds to PS in normoxic and hypoxia-treated tumor cells. Functionally, extracellular-added Hsp70 protein significantly reduces the clonogenic cell survival of normoxic, hypoxic, and irradiated tumor cells in a concentration-dependent manner. Taken together, these findings indicate that extracellular-added Hsp70 might improve killing of hypoxic tumor cells.
MATERIALS AND METHODS
Chemicals and reagents
All protein chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) or from Carl Roth (Karlsruhe, Germany) unless stated otherwise; lipids were from Avanti Polar Lipids Inc. (Alabaster, AL, USA).
HSP proteins and BSA
His-tagged Hsp70 protein, which is equivalent to HspA1A (Multimmune, Munich, Germany) was isolated from a Sf9 insect cell system (CEL-10006; Orbigen, San Diego, CA, USA). Briefly, Sf9 insect cells were transfected with baculovirus containing cDNA coding for human Hsp70 protein with a His-tag on the N terminus (BVC-10058, Orbigen). Cell lysates were administered to Ni-loaded Sepharose columns (17-5247-01; GE Healthcare, Chalfont St. Giles, UK) in binding buffer (20 mM sodium phosphate and 0.5 NaCl, pH 7.4) and His-tagged Hsp70 was eluted with increasing concentration of elution buffer (20 mM sodium phosphate, 0.5 M NaCl, 0.5 M imidazole). Fractions with high amounts of Hsp70-His were pooled, dialyzed against PBS and stored in aliquots at −20°C. Recombinant Hsp60 (NSP-540) Hsp70 (NSP-555), and Hsp90 (SPP-770), purified from HeLa cells, were obtained from Assay Designs (Ann Arbor, MI, USA).
Bovine serum albumin (BSA) (A9647; Sigma), or His-tagged Hsp70 protein were labeled with fluorescein isothiocyanate (FITC) (F6434; Invitrogen, Eugene, OR, USA), according to the manufacturer’s instructions.
Vesicle aggregation assays
Unilamellar liposomal vesicles were freshly prepared at indicated ratios using a standard extrusion method (20). Briefly, lipid preparations comprising of phosphatidylcholine (PC) and palmitoyl-oleoyl-phosphatidylserine (PS), or PC and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphorylglycerol (PG) were dissolved in chloroform and dried under nitrogen gas. Dried lipids were hydrated in 25 mM Tris/HCl, pH 7.4, and 250 mM NaCl (1 ml/10 mg of lipid) for a minimum of 1 h at 25°C, and the suspension was passed 12 times through a 0.05-mm polycarbonate filter in a lipid extruder (Avanti Polar Lipids) in order to generate liposomes of a uniform size of 200 nm. The final lipid concentration in 1 mM Bis/Tris buffer, pH 7.4, was 1 mg/ml.
Recombinant Hsp60, Hsp70, or Hsp90 (1 μg) was incubated with 100 μl of unilamellar vesicles having the indicated composition for 30 min at room temperature. After the addition of 700 μl H2O, protein/vesicle solutions were ultracentrifuged at 200,000 g at 4°C for 2 h. The supernatants were retained, and the pellets were resuspended in 20 μl PBS for further analysis. The presence of protein in the membranous pellet fraction is indicative of protein/lipid interactions. For the quantification of protein content, supernatants were concentrated using a Speedvac (Christ Alpha; B. Braun Biotech International, Melsungen, Germany) and subjected to Western blot analysis in parallel with the pelleted fraction.
SDS-PAGE and Western blot analysis
Proteinous solutions were denatured in sample buffer [25 mM Tris hydrochloride (pH 6.8), 2% w/v SDS, 10% v/v glycerol, 10% v/v dithiothreitol (DTT), and 0.2% w/v bromphenol blue], and heated for 10 min at 95°C. Proteins were separated on a 10% SDS-PAGE under reducing conditions (21), blotted onto nitrocellulose membranes (22), and detected with monoclonal antibodies (mAbs) directed against Hsp60 (SPA-806; Assay Designs), Hsp70 (SPA-810; Assay Designs), and Hsp90 (SPA-830; Assay Designs). Bound mAbs were visualized using horseradish peroxidase-conjugated secondary antibodies (Dako, Glostrup, Denmark) and a chemiluminescence developing kit (ECL; Amersham Biosciences, Little Chalfont, UK). The amount of protein in the samples was quantified by densitometry and compared to the signals generated by corresponding recombinant heat shock proteins that were run in parallel (200 ng per lane; Assay Designs).
Cells and cell culture
The tumor subline CX−, derived from the parental human colon carcinoma cell line CX2 (Tumorzellbank, DKFZ, Heidelberg, Germany), was generated by fluorescence-activated cell sorting using the FITC-conjugated Hsp70-specific mAb cmHsp70.1 (Multimmune). The tumor subline CX+ contains consistently higher proportions of Hsp70 membrane positive cells (87±8%, n=3) than the subline CX− (23±6%, n=3) (2). Furthermore, CX+ tumor cells were PS membrane negative (0.5%), whereas viable CX− tumor cells always contained ∼10% PS membrane-positive cells.
The mycoplasma-free tumor cell line CX− was maintained under exponential growth conditions by regular cell passages in RPMI 1640 (Life Technologies, Inc., Eggenstein, Germany) supplemented with 5% v/v heat-inactivated FCS (PAA, Pasching, Austria), 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 1 mM sodium pyruvate (Life Technologies, Eggenstein, Germany). Plating efficiency and doubling time (20 h) were comparable in Hsp70 low- and high-expressing tumor sublines CX− and CX+, under physiological conditions.
Hypoxia and irradiation
To achieve hypoxic conditions, CX− tumor cells (1.2×106 cells) were plated in 10-cm-diameter culture dishes (TPP, Trasadingen, Switzerland), for 20 h. Then dishes were placed into air-tight aluminum chambers at 37°C connected to a vacuum pump and a gas cylinder containing a mixture of 95% N2 and 5% CO2. By alternating 11 times between oxygen evacuation and N2 inflow, an oxygen concentration below 0.66% (5 mm Hg) was reached in the medium after 22 min. Normoxic control cells were treated identically without the addition of N2. Cells were either kept under hypoxic and normoxic conditions for 48 h or were irradiated with the sublethal dose of 4 Gy. Cell viability was determined by Trypan blue exclusion assay.
Flow cytometry and apoptosis assays
Following a 48-h normoxia or hypoxia treatment and/or incubation with Hsp70 protein or BSA (50 μg/ml), 0.2 × 106 cells were washed with Annexin V-binding buffer and incubated with Annexin V-FITC (Roche Diagnotics, Mannheim, Germany) for 15 min at room temperature. After a washing step in PBS/10% v/v FCS and 2.5 mM CaCl2, propidium iodide (PI) was added for 1 min, and cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). For double-staining experiments, Annexin V-PE prestained cells were incubated with the membrane-Hsp70-specific mouse monoclonal IgG1 antibody cmHsp70.1-FITC (Multimmune), or with a FITC-conjugated IgG1 isotype-matched negative control immunoglobulin (BD Biosciences), for another 30 min at 4°C. Then cells were incubated with 7-amino-actinomycin D (7-AAD), and viable cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences).
Active caspase 3: Cells were washed, fixed, permeabilized, and stained with FITC-conjugated mouse monoclonal antiactive-caspase-3 mAb, according to the manufacturer’s instructions (BD Biosciences; 550480) and analyzed on a FACSCalibur flow cytometer (BD Biosciences).
DAPI staining: Cells were washed with PBS, fixed in 4% w/v paraformaldehyde, resuspended in 10 μl Vectashield containing 4′-6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA) and mounted on glass slides. The morphology of the nuclei was inspected using an Axioskop 2 plus fluorescence microscope (Carl Zeiss Jena GmbH, Jena, Germany) at an excitation wavelength of 350 nm and photographed as described below. At least 200 nuclei were inspected and counted to determine DNA fragmentation.
Immunofluorescence
Following hypoxic or normoxic treatment, tumor cells were incubated with 1 μg/ml Annexin V-Cy3.18 (Sigma) for 15 min at room temperature. After washing, the cells were incubated with the membrane-Hsp70-specific mouse monoclonal IgG1 antibody, cmHsp70.1-FITC (Multimmune), FITC-conjugated BSA, or FITC-conjugated Hsp70 protein (120 μg/ml, total volume 5 μl). After fixation in 0.1% w/v paraformaldehyde, cells were transferred onto glass slides, covered with Vectashield with or without DAPI (Vector Laboratories), and analyzed using an Axioskop 2 plus fluorescence microscope (Carl Zeiss) and the appropriate filters (Chroma Technology, Rockingham, VT, USA; BFI Optilas, Evry, France). Photographic documentation was carried out using an AxioCam MRc5 camera, an Achroplan ×100 oil objective and the AxioVision 4.4 software (Carl Zeiss). Fluorescence images of normoxic and hypoxic cells were exposed for the same time.
Confocal laser scanning microscopy
Confocal imaging was carried out with a Leica TCS NT with appropriate lasers, filters, and an ×100 oil-immersion objective (Leica Microsystems, Wetzlar, Germany). Confocal laser scanning imaging was run in the horizontal scanning mode using two channels for simultaneous FITC (argon-krypton ion laser, excitation, 488 nm and emission 530 nm utilizing a long-pass filter) and Cy3 (excitation 549 nm, emission 565 nm) excitation. Overlays of the rendered images allowed exact spatial localization of Hsp70 and PS in yellow.
Protein binding assay
Following a 48-h treatment period, cells were counted, and 0.2 × 106 cells were incubated for 30 min on ice with either Hsp70-FITC or BSA-FITC at the indicated concentrations. Following a washing step with PBS/10% v/v FCS, PI-negative, viable cells were gated and analyzed on a FACSCalibur flow cytometer (BD Biosciences). Binding was determined as the percentage of FITC positively stained cells. The amount of cells incubated with PBS (unstained) was set to 1% and served as control for setting the marker.
Proliferation assay
Cells were seeded into 96-well plates (1000 cells/well). Twenty hours after seeding, various concentrations of Hsp70 protein or BSA (10, 25, 50 μg/ml) were added, and cells were incubated for a further 2 h. As a control, the same volume of PBS was added. Next, the cells were exposed to hypoxia or normoxia, as described above. After 48 h, the plates were removed from the hypoxic chambers and incubated under standard conditions for up to 2 d. The proliferation was measured using the MTT-like colorimetric alamarBlue assay, according to the manufacturer’s instructions (Biosource, Camarillo, CA, USA). Briefly, alamarBlue was added, and 4 h after incubation at 37°C, the absorption at 570 and 630 nm (reference wavelength) was measured using an absorbance microplate reader (ELx808; BioTek, Winooski, VT, USA). The proliferation of untreated, normoxic cells incubated with PBS was set to 100% in each experiment.
Clonogenic cell survival assay
CX− tumor cells (2×103) were seeded into 6-well plates in triplicate. Hsp70 protein or BSA was added at indicated concentrations, and tumor cells were exposed to hypoxic or normoxic conditions for 48 h, as described above. Subsequently, cells were irradiated with the indicated doses using the RT100 irradiation device (Philips, Hamburg, Germany) at a dose rate of 1 Gy/min. Irradiations were performed at room temperature under atmospheric conditions. Control cells were sham irradiated at 0 Gy. On d 6 after treatment, colonies were fixed in methanol, stained with crystal violet, and counted automatically using Immuno Spot Series I analyzer (Bio-Sys GmbH, Karben, Germany). Survival fractions were calculated relative to untreated, normoxic tumor cells (23).
Statistics
Statistical analysis was performed using SPSS 15.0.1 software (SPSS, Inc., Chicago, IL, USA). Student’s t test was used to evaluate significant differences (values of P≤0.05 were considered significant), and Pearson’s correlation coefficient r was used to determine linear correlations.
RESULTS
Hsp70 and PS are colocalized on the surface of hypoxic tumor cells
Two sublines of the human colon carcinoma cell line CX2 were generated by flow cytometric sorting based on differences in the cell surface expression level of Hsp70 as detected by the mAb cmHsp70.1 (1). Under normal conditions, 87 ± 8% of the CX+ and only 23 ± 6% of the CX− tumor cells exhibited an Hsp70 membrane-positive phenotype. A small percentage of CX− tumor cells displayed PS on the outer leaflet of the plasma membrane under physiological conditions. Following stress, the percentage of PS-positive cells is significantly elevated in CX− cells in comparison with the stress-resistant CX+ tumor cells (24). The CX− subline was chosen as a model system to study the effect of hypoxia on Hsp70 and PS surface expression.
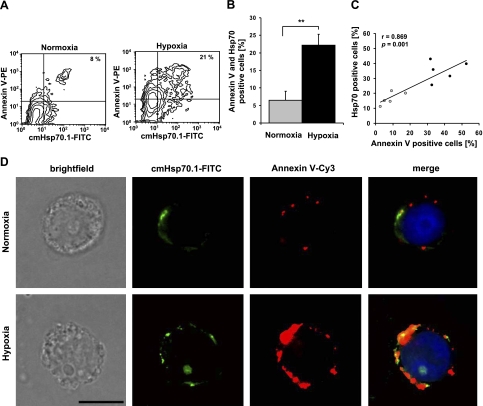
Coexpression of PS and Hsp70 on the cell surface of normoxic and hypoxic tumor cells was determined using PE-conjugated Annexin V, a Ca2+-dependent phospholipid binding protein or a PS-specific mAb (data not shown) and the FITC-conjugated cmHsp70.1 mAb. A representative example of an increase in Annexin V and Hsp70 membrane positivity in CX− tumor cells following 48 h of hypoxia is illustrated in Fig. 1A. Approximately 8% of the cells were found to be double positive for Hsp70 and Annexin V under normoxic conditions (PS; Fig. 1A, left panel), which increased to 21% after hypoxia treatment (Fig. 1A, right panel). The results of 5 independent experiments indicate a significant increase in the proportion of Annexin V and Hsp70 membrane-positive cells after hypoxia, from 6.47 ± 2.57 to 22.17 ± 3.10% (P=0.005); these results are summarized in Fig. 1B. Linear regression analysis reveals a significant correlation between the proportion of Annexin V (PS) and Hsp70 membrane-positive cells (r=0.869; P=0.001) in normoxic and hypoxic CX− tumor cells (Fig. 1C, n=5). These findings are in line with results from double-staining immunofluorescence analyses using Cy3-conjugated Annexin V and the FITC-conjugated cmHsp70.1 mAb (Fig. 1D). The leftmost panels illustrate a light microscopic view of normoxic (top panel) and hypoxic (bottom panel) CX− tumor cells with intact cell morphology. The two sets of micrographs to the right single staining of normoxic and hypoxic CX− cells with cmHsp70.1-FITC mAb (green) and Annexin V-Cy3 (red). As has already been demonstrated by flow cytometry, dual staining was markedly increased following hypoxic treatment. A costaining of the red and green fluorescence, as shown in the rightmost panels (merge), reveals an overlap of the fluorescent dyes in the hypoxic CX− tumor cells (yellow), thus indicating that Hsp70 and PS are coexpressed on the cell surface following hypoxia (Fig. 1D, right panels).
Figure 1.
Hypoxia induces coexpression of PS and Hsp70 on the plasma membrane of CX− tumor cells. A) Representative contour blots of normoxic (left panel) or hypoxic (right panel) CX− tumor cells, costained with Annexin V-PE and cmHsp70.1-FITC mAb. Percentage of double-positive cells is indicated in upper right quadrant. Cutoff line for cmHsp70.1-FITC was set according to the corresponding isotype; cutoff line for Annexin V-PE was set according to the normoxic control. B) Histogram shows mean ± se values of Annexin V-PE and cmHsp70.1-FITC double-positive CX− tumor cells derived from 5 independent experiments. **P < 0.01. C) PS (Annexin V) and Hsp70 membrane positivity on the outer membrane leaflet under normoxic (gray dots) and hypoxic (black dots) conditions was correlated using linear regression analysis and Pearson’s coefficient of correlation. Percentages of Annexin V-positive cells vs. Hsp70-positive cells from 5 independent experiments were plotted for the regression analysis. D) Brightfield and fluorescence microscopic analysis of normoxic (top panels) and hypoxic (bottom panels) CX− cells stained with either cmHsp70.1-FITC mAb (Hsp70; green) or Annexin V-Cy3 (PS; red). Left panels: light microscopic view of normoxic (top) and hypoxic (bottom) CX− tumor cells. Middle panels: CX− cells stained with cmHsp70.1-FITC (Hsp70) and Annexin V-Cy3 (PS). Right panels: merge of both staining patterns shows colocalization on the surface of CX− cells after hypoxia (yellow spots). Scale bar = 5 μm.
Hsp70 specifically interacts with lipid vesicles consisting of PC/PS at the physiological relevant ratio of 8/2
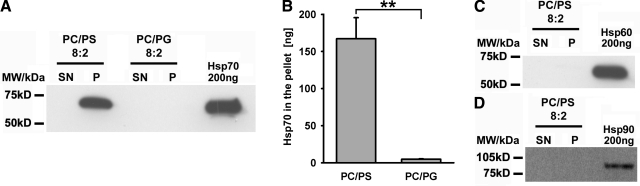
The interaction of Hsp70 and PS has been previously demonstrated using proteoliposomes or planar lipid bilayers made of PS, in the presence of 1 mM CaCl2 and 0.5 mM MgCl2 (3, 16). These results were confirmed by studying the direct binding of recombinant human Hsp70 to unilamellar lipid vesicles at a physiologically relevant PC/PS ratio of 8/2 at pH 7.4, in the absence of ions. As summarized in Table 1, the interaction of Hsp70 protein with lipid vesicles gradually increased with elevations of the PS in the molar ratio of PS within liposomes (8/2≥7/3<5/5<4/6<3/7<2/8). Furthermore, no interaction was detectable with liposomes consisting only of PC. Using identical conditions, Hsp70 does not incorporate into lipid vesicles consisting of PC/PG at a ratio of 8/2 (Fig. 2A). Only a weak interaction was detected at increasing molar ratios of PC/PG (7/3, 5/5, 4/6, 3/7, and 2/8, Table 1). The PC/PG vesicles were used as a control to exclude nonspecific binding that might be mediated through a negative charging effect of PS. Quantification of the amount of Hsp70 in PC/PS vs. PC/PG liposomes from 3 independent experiments revealed significant differences in the amount of vesicle-bound Hsp70 (pellet fraction, 167.2±22.4 vs. 4.8±0.5 ng; P=0.0046; Fig. 2B). Binding of Hsp70 was significantly weaker in PC/PG liposomes (ratios 7/3, 5/5, 4/6, 3/7, 2/8), as compared with PC/PS vesicles (Table 1). The specificity of the interaction of Hsp70 with PC/PS was demonstrated by using other recombinant HSPs, such as Hsp60 and Hsp90, as potential binding partners. As summarized in Fig. 2C, D, neither Hsp60 nor Hsp90 interacted with PC/PS (8/2) artificial liposomes under the indicated conditions. Because of dilution effects, unbound HSPs were not visible in the supernatant (SN) fraction but could be seen in a concentrated fraction (data not shown). Taken together, our findings demonstrate that Hsp70 protein preferentially interacts with unilamellar lipid vesicles containing PS.
TABLE 1.
Vesicle aggregation assay
| Vesicle ratio | Hsp70 content in vesicle (ng) |
|---|---|
| PC/PS (n=4) | |
| 8/2 | 369.3 ± 79 |
| 7/3 | 294.8 ± 39 |
| 5/5 | 415.9 ± 52 |
| 4/6 | 531.3 ± 237 |
| 3/7 | 592.9 ± 222 |
| 2/8 | 688.7 ± 275 |
| PC/PG (n=3) | |
| 8/2 | 4.8 ± 0.50 |
| 7/3 | 174.0 ± 32 |
| 5/5 | 232.4 ± 6.8 |
| 4/6 | 252.2 ± 6.7 |
| 3/7 | 234.5 ± 97 |
| 2/8 | 255.4 ± 57 |
Unilamellar PC/PS and PC/PG vesicles at different ratios ranging from 8/2 to 2/8 were incubated with 1 μg of Hsp70 protein. Values are presented as means ± se.
Figure 2.
Hsp70 protein selectively interacts with unilamellar PC/PS (8/2) liposomes. A) Representative immunoblot of the interaction of recombinant Hsp70 protein (5 μg) with unilamellar lipid vesicles consisting of either PC/PS (8/2) or PC/PG (8/2) at pH 7.4 in a liposome aggregation assay. B) Quantification of the amount of Hsp70 bound to PC/PS (8/2) and PC/PG (8/2) liposomes. Data represent means ± se of 3 independent experiments. **P = 0.0046. C, D) Interaction of recombinant Hsp60 protein (5 μg) (C) and recombinant Hsp90 protein (5 μg) (D) with PC/PS (8/2) liposomes. First lane, HSP content in 2 ml of supernatant fraction (SN); second lane, HSP content in pellet fraction (P); third lane, recombinant HSP protein (200 ng) following SDS-PAGE.
Hypoxic tumor cells bind extracellular Hsp70 via PS
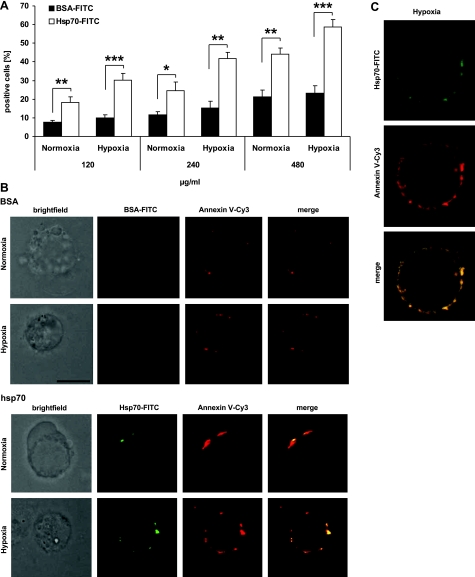
To address the question of whether or not the interaction of Hsp70 and PS is possible with the addition of extracellular Hsp70, normoxic and hypoxic CX− tumor cells were incubated with increasing amounts of FITC-conjugated Hsp70 (120, 240, 480 μg/ml) or with identical amounts of FITC-conjugated BSA control protein. To avoid endocytosis of the FITC-labeled proteins, all incubation steps were performed at 4°C. After an incubation period of 30 min, CX− tumor cells were washed extensively to remove unbound material. Binding of Hsp70-FITC (120 μg/ml, n=11) to normoxic CX− tumor cells was significantly enhanced in comparison with BSA-FITC, 18.3 ± 3.1 vs. 7.5 ± 1.0% (P=0.006), respectively. At a concentration of 240 μg/ml (n=7), binding of Hsp70-FITC to normoxic CX− cells enhanced from 11.6 ± 1.5 to 24.7 ± 4.6% (P=0.030) and at a concentration of 480 μg/ml (n=4) from 21.2 ± 3.6 to 44.0 ± 3.5% (P=0.004) as compared to BSA-FITC (Fig. 3A). As expected, binding of extracellular Hsp70-FITC to hypoxia-treated, PS-positive CX− tumor cells was even more pronounced. At 120 μg/ml (n=11), Hsp70-FITC binding was significantly enhanced from 9.9 ± 1.7 to 30.3 ± 3.4% (P<0.001), at 240 μg/ml (n=4) from 15.4 ± 3.4 to 41.7 ± 3.6% (P=0.002), and at 480 μg/ml (n=4) from 23.2 ± 4.1 to 58.8 ± 4.0% (P=0.001), as compared to BSA-FITC (Fig. 3A). A comparison of the binding of Hsp70-FITC to normoxic and hypoxic CX− tumor cells with elevated PS cell surface levels also revealed significantly increased differences at all of the tested concentrations (120 μg/ml, P=0.016; 240 μg/ml, P=0.033; 480 μg/ml, P=0.032).
Figure 3.
Colocalization of exogenously added Hsp70 protein and PS on hypoxic CX− tumor cells. A) Normoxic and hypoxic CX− tumor cells were incubated with 120, 240, and 480 μg/ml Hsp70-FITC or BSA-FITC for 30 min on ice. Following 2 washing steps, percentage of FITC-stained cells was determined by flow cytometry. Only PI negative cells were analyzed; marker was set on the basis of cells incubated with PBS. Data are means± se of 4 to 11 independent experiments. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. B) Brightfield and fluorescence microscopic analysis of normoxic and hypoxic CX− tumor cells stained with Annexin V-Cy3 (red) and 120 μg/ml Hsp70-FITC or BSA-FITC protein (green). BSA-FITC did not bind to CX− cells. Cell surface binding of Hsp70-FITC and Annexin V-Cy3 was stronger in hypoxic vs. normoxic CX− tumor cells. Bottom panels: colocalization of Hsp70-FITC and Annexin V-Cy3 (yellow), as merge of red and green fluorescence. Scale bar = 5 μm. C) Confocal microscopy of hypoxic CX− tumor cells stained as described above. BSA-FITC did not bind to CX− cells (data not shown).
Extracellular Hsp70-FITC colocalizes with PS on hypoxic CX− tumor cells. The first column in Fig. 3B shows light microscopic views of normoxic and hypoxic CX− tumor cells that were incubated with Annexin V-Cy3 and either 120 μg/ml BSA-FITC (left panels) or Hsp70-FITC (right panels). In line with the results from flow cytometry, BSA-FITC did not bind to normoxic or hypoxic CX− tumor cells (Fig. 3B, top). In contrast, Hsp70-FITC strongly bound to hypoxic CX− tumor cells (green fluorescence) that were also found to be strongly PS surface-positive (red fluorescence) (Fig. 3B, bottom). A merge of the red and green fluorescence (yellow) indicates that PS and Hsp70 colocalize on the plasma membrane of normoxic and hypoxic CX− tumor cells. A direct interaction of Hsp70 protein with Annexin V could be excluded since identical costaining could be shown when PS was stained with a PS-specific mAb (data not shown). Colocalization of extracellular Hsp70-FITC (120 μg/ml) with PS was also confirmed by confocal microscopy. Figure 3C represents a typical confocal image of CX− tumor cells that were incubated with Hsp70-FITC (top micrograph) or with Annexin V-Cy3 (middle micrograph). The bottom micrograph illustrates a merge of the two confocal images (Fig. 3C).
Exogenously added Hsp70 reduces cell proliferation in hypoxia-treated CX− tumor cells
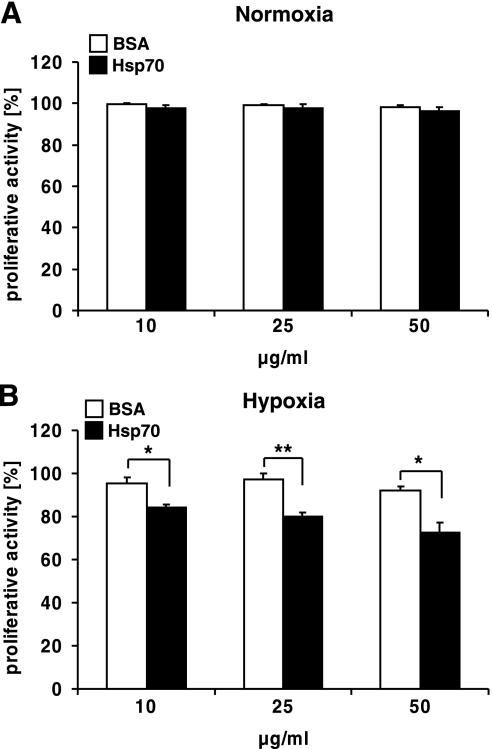
Next, we analyzed the proliferative capacity of CX− tumor cells following the addition of Hsp70. CX− cells were treated either with Hsp70 or BSA at concentrations of 10, 25, or 50 μg/ml before exposure to hypoxic or normoxic conditions for 48 h. For the proliferation assays, lower Hsp70 concentrations were used to mimic more physiological conditions since the serum levels of Hsp70 protein in tumor patients have been found to range between 1 and 20 μg/ml (35), whereas much higher Hsp70 levels are expected locally in areas where necrosis has occurred. Tumor cell proliferation was determined on d 2 after hypoxia by alamarBlue assay. This test detects the metabolic activity of the cells as a surrogate marker for cell growth. The proliferative capacity of normoxic tumor cells was neither affected by Hsp70 nor by BSA at any of the tested concentrations (Fig. 4A). In contrast, the proliferation of hypoxia-treated tumor cells was significantly inhibited by Hsp70, but not by BSA, in a concentration-dependent manner (10 μg/ml, P=0.023; 25 μg/ml, P=0.006; 50 μg/ml, P=0.015) (Fig. 4B). These findings suggest that the binding of exogenously added Hsp70 to surface PS-bearing, hypoxic tumor cells might promote an antiproliferative activity.
Figure 4.
Exogenously added Hsp70 protein inhibits proliferation of hypoxic CX− tumor cells. CX− tumor cells, seeded into 96-well plates, were incubated with 10, 25, or 50 μg/ml Hsp70 protein or BSA. On d 2 after a 48-h normoxic (A) or hypoxic (B) treatment, proliferation was measured, using the colorimetric alamarBlue assay. Proliferation of control cells was set to 100%. Bars represent means ± se of 3 independent experiments. *P ≤ 0.05, **P ≤ 0.01.
Exogenously added Hsp70 significantly reduces clonogenic cell survival in normoxic, hypoxic, and irradiated CX− tumor cells by a nonapoptotic pathway
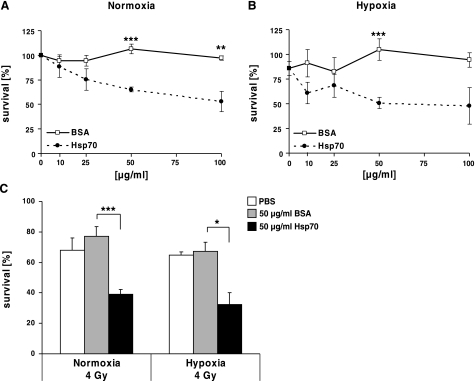
The biological impact of exogenously added Hsp70 protein was further analyzed in clonogenic cell survival assays. A significant, concentration-dependent decrease in cell viability was observed in normoxic (EC50=85 μg/ml, Fig. 5A) and hypoxic (EC50=55 μg/ml, Fig. 5B) CX− tumor cells following incubation with different concentrations of Hsp70 protein (10, 25, 50, or 100 μg/ml), whereas identical amounts of BSA did not affect clonogenic cell survival in normoxic (P<0.001, Fig. 5A) or hypoxic (48-h hypoxia treatment, P=0.001, Fig. 5B) cell groups.
Figure 5.
Exogenously added Hsp70 protein reduces clonogenic cell survival of normoxic, hypoxic, and irradiated CX− tumor cells. A, B) CX− tumor cells were seeded in 3 ml of medium in 6-well plates and incubated with Hsp70 protein (solid circles) or BSA (open squares) at 10 μg/ml (n=7), 25 μg/ml (n=4), 50 μg/ml (n=7) and 100 μg/ml (n=3). After 2 h, cells were exposed to normoxic (A) or hypoxic (B) conditions for 48 h. C) Normoxic and hypoxic CX− tumor cells were irradiated with a dose of 4 Gy Number of clones was related to completely untreated CX− tumor cells. Columns indicate radiation-sensitivity of normoxic (left panel) and hypoxic (right panel) CX− tumor cells after incubation with either Hsp70 protein or BSA (50 μg/ml). Data are means ± se of least 3 independent experiments. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
As the clinical outcome of radiation therapy is negatively influenced by tumor hypoxia, the radiation sensitivity was determined in normoxic and hypoxic CX− tumor cells that were pretreated with Hsp70 protein. In comparison to BSA, pretreatment of tumor cells with 50 μg/ml Hsp70 protein significantly enhanced the radiosensitivity of normoxic (4 Gy; P=0.006; Fig. 5C, left panel) and hypoxic (48 h hypoxia treatment, 4 Gy; P=0.024; Fig. 5C, right panel) CX− tumor cells. A radiation dose of 2.8 Gy was required to kill 50% Hsp70-treated CX−tumor cells, whereas 5.6 Gy was required to kill 50% of BSA-treated CX− tumor cells (data not shown).
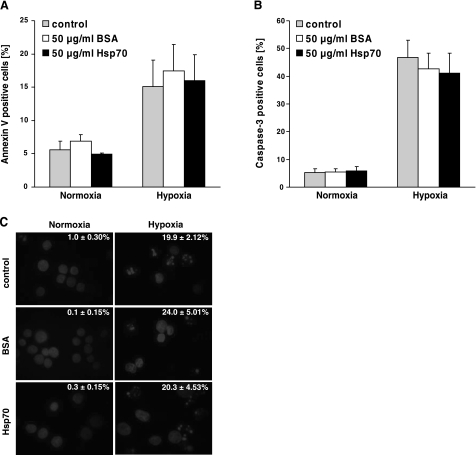
The drastic reduction in clonogenic cell survival following incubation with Hsp70 could not be explained by inhibiting the rate of cell proliferation. Therefore, we tested whether binding of extracellular Hsp70 might cause apoptotic cell death, as measured by Annexin V binding to PS, intracellular active caspase 3, and DAPI staining. Although 48-h hypoxia treatment increased the proportion of PS surface-positive (Fig. 6A) and active caspase 3-positive (Fig. 6B) cells, the addition of BSA (50 μg/ml) or Hsp70 protein (50 μg/ml) had no effect on the expression of these markers. Furthermore, hypoxia-induced DNA fragmentation was not elevated by the addition of BSA or Hsp70 protein (Fig. 6C).
Figure 6.
No increase in apoptosis was determined by addition of Hsp70 protein to normoxic and hypoxic CX− tumor cells. Normoxic and hypoxia-treated (48 h) CX− tumor cells were incubated with PBS, Hsp70 protein, or BSA (50 μg/ml), as described in text. A, B) Apoptosis was determined by flow cytometry on the basis of Annexin V (A) or active caspase-3 (B) positivity in normoxic and hypoxic tumor cells. C) Representative views of nuclear DAPI staining in normoxic and hypoxic tumor cells after incubation with PBS (control), BSA, or Hsp70 protein. Percentage of cells exhibiting DNA fragmentation is shown in each micrograph (n=3). At least 200 nuclei/treatment were analyzed and counted.
DISCUSSION
Here, we studied the biological impact of exogenously added Hsp70 on tumor cell survival. It is well established that, among other stress factors, hypoxia initiates the synthesis of Hsp70 in eukaryotic cells (25,26,27,28) and causes the translocation of PS from the inner to the outer plasma membrane leaflet (29, 30). Depending on the severity of stress, the translocation of PS to the outer membrane leaflet can be either reversible or result in apoptotic cell death. Hypoxic tumor cells with the capacity to recover from stress are generally considered to be radiotherapy resistant.
Hsp70 has been found to fulfill dual roles depending on its intracellular or extracellular localization (1, 31). On the one hand, increased levels of Hsp70 in the cytosol play a key role in protecting cells from lethal damage induced by environmental stresses. On the other hand, extracellular Hsp70 has been found to act as a danger signal for the innate and adaptive immune system (7). Although the mechanism via which Hsp70 is exported into the extracellular milieu is not fully understood, many laboratories have reported the release of Hsp70 proteins from tumor cells with intact cell membranes (32,33,34,35). An alternative vesicular pathway has been proposed which enables export of Hsp70 but that does not involve the classical ER Golgi compartment (33, 36). These findings concur with those from Asea’s group (37) demonstrating that inhibitors that perturb ER Golgi transport, including monensin and brefeldin A, do not affect extracellular Hsp70 release. We have recently shown that a mild heat shock can cause a translocation of Hsp70 from the cytosol into the extracellular milieu, in which it stimulates macrophages in a membrane-associated form (3). In line with these previous findings, herein we show that hypoxia initiates colocalization of Hsp70 and PS in the outer membrane leaflet of hypoxic tumor cells. In nonstressed eukaryotic cells, the location of PS is restricted to the inner plasma membrane leaflet (38). This asymmetry is maintained by an ATP-dependent flippase and floppase, as well as a Ca2+-dependent scramblase (11). Following hypoxia, PS translocates to the outer membrane leaflet, causing the loss of PS asymmetry. We have observed the simultaneous translocation of both, Hsp70 and PS to the cell surface. These two molecules colocalized in the plasma membrane of hypoxic tumor cells. Consequently, we speculate that in stressed tumor cells Hsp70 might be translocated to the outer membrane leaflet by a mechanism associated with PS flipping.
The specificity of the interaction of Hsp70 with PS could be confirmed by in vitro vesicle aggregation assays, showing that Hsp70, but not other HSP family members, insert into artificial lipid vesicles. Previous studies have shown that Hsp70 interacts with artificial lipid bilayers containing high amounts of PS in the presence of CaCl2 (1 mM) and MgCl2 (0.5 mM) (39). In accordance with these data, we demonstrate that the inducible Hsp70 interacts with PS even in the absence of ions at a PC/PS ratio of 8/2, which resembles the PS/PC content of naturally occurring plasma membranes. The finding that Hsp70 does not bind to PC/PG (8/2) indicates that the interaction of Hsp70 with lipids is not related to a charge-related effect.
The concentration-dependent interaction of extracellular Hsp70 with PS was confirmed in normoxic and hypoxic tumor cells that expose higher levels of PS on their outer membrane leaflet. We have been able to show that Hsp70, but not a control protein, preferentially bind to hypoxic tumor cells in a concentration-dependent manner and colocalizes with PS in the plasma membrane.
Currently, it cannot completely be ruled out that apart from PS, Hsp70 also binds to Hsp70 present on the cell surface via a protein-protein interaction. Thus, we observed binding of Hsp70 (120 μg/ml) to PS-negative, surface-Hsp70 membrane positive CX+ tumor cells (data not shown). The extracellular residing part of Hsp70, which is specifically recognized by the cmHsp70.1 mAb (40), is part of the oligomerization domain of Hsp70 (41). Therefore, it is reasonable to assume that a homomeric interaction of Hsp70 with the C-terminal-located oligomerization domain might also occur. This hypothesis is further supported by the finding that binding of exogenously added Hsp70 protein to tumor cells could only be inhibited by antibody blocking studies in which both Hsp70- and PS-specific mAbs were simultaneously added (data not shown).
The functional consequence of Hsp70 binding to tumor cells was studied in proliferation assays and in clonogenic cell survival assays. Although the proliferative capacity of normoxic tumor cells was not affected by the addition of BSA control protein or Hsp70 protein, the clonogenic cell survival was found to be significantly reduced. These data are in line with prior studies indicating that the addition of extracellular Hsp70 decreases the viability of PC12 tumor cells (16). Furthermore, the addition of Hsp70 protein prior to radiation significantly reduces tumor cell survival. With respect to these results, we assumed that extracellular Hsp70 might cause tumor cell apoptosis. However, incubation of normoxic tumor cells with Hsp70 did not induce programmed cell death. Similar results have been found with hypoxic tumor cells. In all cases, incubation with Hsp70 significantly decreased cell viability, although the rate of apoptosis was not increased above the level that was induced by hypoxia alone. Therefore, we speculate that the Hsp70-induced loss in tumor cell survival is mediated via a nonapoptotic pathway.
Taken together, our findings might have future clinical relevance in that hypoxic tumors could be rendered more sensitive to radiotherapy by a prior treatment with Hsp70 protein. One approach could be to include Hsp90 inhibitors as part of the radiotherapy protocol (42). Apart from the inhibition of the interaction of the molecular chaperone Hsp90 with client proteins that are critical for cell cycle control, signaling and growth factors, these inhibitors are known to strongly up-regulate the synthesis of Hsp70 in tumor cells. Preliminary data from our laboratory (unpublished results) and others (43) indicate that tumors which overexpress Hsp70 release Hsp70 into the serum. We hypothesize that elevated extracellular Hsp70 levels might enhance radiation-induced tumor cell killing.
Acknowledgments
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (DFG MU1238 7/2 to G.M.), the Bundesministerium für Forschung und Technologie (BMBF BioChance plus, 0313686A to G.M.), the European Union (Transnet 512253; Stemdiagnostics LSHB CT 2007 037703; Cardiorisk 211404 to G.M.), the U.S. National Institutes of Health (NIH GM 050878 to A.D.), and Multimmune GmbH.
References
- Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- Vega V L, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz J C, Steinem C, Multhoff G, Arispe N, De Maio A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180:4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem T H, Fleshner M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 2003;8:272–286. doi: 10.1379/1466-1268(2003)008<0272:sehiaf>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maio A. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999;11:1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- Moseley P. Stress proteins and the immune response. Immunopharmacology. 2000;48:299–302. doi: 10.1016/s0162-3109(00)00227-7. [DOI] [PubMed] [Google Scholar]
- Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81:15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- Srivastava P K. Heat shock proteins in immune response to cancer: the fourth paradigm. Experientia. 1994;50:1054–1060. doi: 10.1007/BF01923461. [DOI] [PubMed] [Google Scholar]
- Calderwood S K, Khaleque M A, Sawyer D B, Ciocca D R. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15:3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- Pomorski T, Holthuis J C, Herrmann A, van Meer G. Tracking down lipid flippases and their biological functions. J Cell Sci. 2004;117:805–813. doi: 10.1242/jcs.01055. [DOI] [PubMed] [Google Scholar]
- Schlegel R A, Williamson P. Phosphatidylserine, a death knell. Cell Death Differ. 2001;8:551–563. doi: 10.1038/sj.cdd.4400817. [DOI] [PubMed] [Google Scholar]
- Fischer K, Voelkl S, Berger J, Andreesen R, Pomorski T, Mackensen A. Antigen recognition induces phosphatidylserine exposure on the cell surface of human CD8+ T cells. Blood. 2006;108:4094–4101. doi: 10.1182/blood-2006-03-011742. [DOI] [PubMed] [Google Scholar]
- Asano K, Miwa M, Miwa K, Hanayama R, Nagase H, Nagata S, Tanaka M. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med. 1994;200:459–467. doi: 10.1084/jem.20040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoven B, Schlegel R A, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N, Doh M, Simakova O, Kurganov B, De Maio A. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J. 2004;18:1636–1645. doi: 10.1096/fj.04-2088com. [DOI] [PubMed] [Google Scholar]
- Sovik A, Malinen E, Skogmo H K, Bentzen S M, Bruland O S, Olsen D R. Radiotherapy adapted to spatial and temporal variability in tumor hypoxia. Int J Radiat Oncol Biol Phys. 2007;68:1496–1504. doi: 10.1016/j.ijrobp.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Gray L H, Conger A D, Ebert M, Hornsey S, Scott O C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Kelleher D K, Hockel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol. 2001;28:29–35. doi: 10.1016/s0093-7754(01)90210-6. [DOI] [PubMed] [Google Scholar]
- Herrig A, Janke M, Austermann J, Gerke V, Janshoff A, Steinem C. Cooperative adsorption of ezrin on PIP2-containing membranes. Biochemistry. 2006;45:13025–13034. doi: 10.1021/bi061064a. [DOI] [PubMed] [Google Scholar]
- Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenman R, Burk D, Virolainen E, Buick R N, Church J, Schwartz D R, Carey T E. Clonogenic cell assay for anchorage-dependent squamous carcinoma cell lines using limiting dilution. Int J Cancer. 1989;44:131–136. doi: 10.1002/ijc.2910440123. [DOI] [PubMed] [Google Scholar]
- Gehrmann M, Marienhagen J, Eichholtz-Wirth H, Fritz E, Ellwart J, Jaattela M, Zilch T, Multhoff G. Dual function of membrane-bound heat shock protein 70 (Hsp70), Bag-4, and Hsp40: protection against radiation-induced effects and target structure for natural killer cells. Cell Death Differ. 2005;12:38–51. doi: 10.1038/sj.cdd.4401510. [DOI] [PubMed] [Google Scholar]
- Price B D, Calderwood S K. Gadd45 and Gadd153 messenger RNA levels are increased during hypoxia and after exposure of cells to agents which elevate the levels of the glucose-regulated proteins. Cancer Res. 1992;52:3814–3817. [PubMed] [Google Scholar]
- Terui K, Haga S, Enosawa S, Ohnuma N, Ozaki M. Hypoxia/re-oxygenation-induced, redox-dependent activation of STAT1 (signal transducer and activator of transcription) confers resistance to apoptotic cell death via hsp70 induction. Biochem J. 2004;380:203–209. doi: 10.1042/BJ20031891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccia A J, Auger E A, Koong A, Terris D J, Minchinton A I, Hahn G M, Brown J M. Activation of the heat shock transcription factor by hypoxia in normal and tumor cell lines in vivo and in vitro. Int J Radiat Oncol Biol Phys. 1992;23:891–897. doi: 10.1016/0360-3016(92)90667-7. [DOI] [PubMed] [Google Scholar]
- Benjamin I J, Kroger B, Williams R S. Activation of the heat shock transcription factor by hypoxia in mammalian cells. Proc Natl Acad Sci U S A. 1990;87:6263–6267. doi: 10.1073/pnas.87.16.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran S, Thorpe P E. Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int J Radiat Oncol Biol Phys. 2002;54:1479–1484. doi: 10.1016/s0360-3016(02)03928-7. [DOI] [PubMed] [Google Scholar]
- Walton M, Sirimanne E, Reutelingsperger C, Williams C, Gluckman P, Dragunow M. Annexin V labels apoptotic neurons following hypoxia-ischemia. Neuroreport. 1997;8:3871–3875. doi: 10.1097/00001756-199712220-00007. [DOI] [PubMed] [Google Scholar]
- Hartl F U, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Barreto A, Gonzalez J M, Kabingu E, Asea A, Fiorentino S. Stress-induced release of HSC70 from human tumors. Cell Immunol. 2003;222:97–104. doi: 10.1016/s0008-8749(03)00115-1. [DOI] [PubMed] [Google Scholar]
- Broquet A H, Thomas G, Masliah J, Trugnan G, Bachelet M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem. 2003;278:21601–21606. doi: 10.1074/jbc.M302326200. [DOI] [PubMed] [Google Scholar]
- Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- Pockley A G. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Hightower L E, Guidon P T. Selective release from cultured mammalian cells of heat-shocked (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- Asea A, Ara G, Teicher B A, Stevenson M A, Calderwood S K. Effects of the flavonoid drug quercetin on the response of human prostate tumours to hyperthermia in vitro and in vivo. Int J Hyperthermia. 2001;17:347–356. doi: 10.1080/02656730110053146. [DOI] [PubMed] [Google Scholar]
- Devaux P F. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- Arispe N, Doh M, De Maio A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones. 2002;7:330–338. doi: 10.1379/1466-1268(2002)007<0330:lidtca>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels R D. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Fouchaq B, Benaroudj N, Ebel C, Ladjimi M M. Oligomerization of the 17-kDa peptide-binding domain of the molecular chaperone HSC70. Eur J Biochem. 1999;259:379–384. doi: 10.1046/j.1432-1327.1999.00053.x. [DOI] [PubMed] [Google Scholar]
- Camphausen K, Tofilon P J. Inhibition of Hsp90: a multitarget approach to radiosensitization. Clin Can Res. 2007;13:4326–4330. doi: 10.1158/1078-0432.CCR-07-0632. [DOI] [PubMed] [Google Scholar]
- Wang M H, Grossmann M E, Young C Y. Forced expression of Hsp70 increases the secretion of Hsp70 and provides protection against tumor growth. Brit J Cancer. 2004;90:926–931. doi: 10.1038/sj.bjc.6601583. [DOI] [PMC free article] [PubMed] [Google Scholar]