Abstract
Identifying the molecular mechanisms activated in compensatory hypertrophy and absent during decompensation will provide molecular targets for prevention of heart failure. We have previously shown enhanced ubiquitination (Ub) during the early growth period of pressure overload (PO) hypertrophy near intercalated discs of cardiomyocytes, where integrins are important for mechanotransduction. In this study, we tested the role of integrins upstream of Ub, whether enhanced Ub contributes to survival signaling in early PO, and if loss of this mechanism could lead to decreased ventricular function. The study used a β3 integrin (−/−) mouse and a wild-type mouse as a control for in vivo PO by transverse aortic constriction (TAC) and for cultured cardiomyocytes in vitro, stimulated with the integrin-activating peptide RGD. We demonstrate β3 integrin mediates transient Ub of targeted proteins during PO hypertrophy, which is necessary for cardiomyocyte survival and to maintain ventricular function. Prosurvival signaling proceeds by initiation of NF-κB transcription of the E3 ligase, cIAP1. In PO β3−/− mice, absence of this mechanism correlates with increased TUNEL staining and decreased ventricular mass and function by 4 wk. This is the first study to show that a β3 integrin/Ub/NF-κB pathway contributes to compensatory hypertrophic growth.—Johnston, R. K., Balasubramanian, S., Kasiganesan, H., Baicu, C. F., Zile, M. R., Kuppuswamy, D. β3 Integrin-mediated ubiquitination activates survival signaling during myocardial hypertrophy.
Keywords: NF-κB, cIAP1, pressure overload
Hypertrophy begins as a compensatory response to an increase in afterload, such as hypertension, in order to augment cell size to maintain cardiac output. However, hypertrophic growth results in heart failure by a poorly understood mechanism when the heart can no longer compensate for sustained pressure overload (PO). Increased cardiomyocyte loss via programmed cell death can be a mechanism for the development of heart failure (1, 2), and increased levels of cell death have been identified in human heart failure (3, 4). However, the molecular mechanisms for cardiomyocyte survival critical for inhibiting cell death and delaying the development of heart failure have not been defined.
During compensatory growth, protein synthesis and degradation must be coordinated to change the signaling pathways active in the cell (5). It has also been shown that proteasome function is required for pressure-induced hypertrophic growth (6). Furthermore, accumulation of ubiquitinated proteins occurs due to insufficient proteasome function (7,8,9) and precedes heart failure (9). Thus, regulated degradation of deleterious proteins via the ubiquitin-proteasome system (UPS) may be essential to avoid heart failure and maintain ventricular function.
The proteasome is the organelle responsible for protein degradation in the ubiquitin pathway. Proteins targeted for degradation are enzymatically modified with a chain of ubiquitin tags in a highly regulated process referred to as polyubiquitination, henceforth referred to as Ub throughout the text. E3 ligases are the enzymes responsible for ubiquitin substrate recognition and Ub. Several E3 ligases, including the inhibitor of apoptosis proteins (IAPs), which ubiquitinate molecules in the caspase death pathway (10, 11), and murine double minute (MDM2), the ligase for p53 (12), are upregulated in PO hypertrophy (13, 14) and potentially protect cardiomyocytes against cell death. So far, the signaling mechanisms responsible for UPS-mediated protein degradation required for hypertrophic growth and survival have not been elucidated.
One key regulator of survival that generally requires Ub for its activation is the nuclear factor of κB (ΝF-κΒ). In the canonical pathway, NF-κB is held constitutively inactive in the cytoplasm by the inhibitor of κB (IκB). Once IκB is phosphorylated, ubiquitinated, and degraded, NF-κB translocates to the nucleus (15, 16). This transcription factor induces expression of antiapoptotic genes, including cIAP1, survivin, and Bcl-2 (17, 18). Although nuclear localization of ΝF-κΒ (19, 20) and up-regulation of known target genes (13) have been shown during PO in the myocardium, the activation by an integrin or ubiquitin-mediated pathway has not been described in PO.
We have previously reported Ub is increased during the first 48 h of growth near intercalated discs of cardiomyocytes during PO (13). Integrins are present in both the sarcolemma and intercalated disc (21) and are important receptors for hypertrophic signaling. Once activated, integrins cluster on the cell surface and recruit signaling molecules onto the actin cytoskeleton to form a focal adhesion complex for downstream signaling. In this way, integrins make a physical link between the extracellular matrix and the intracellular cytoskeleton to transduce mechanical signals into intracellular biochemical signals. As an important tool, integrin activation and focal adhesion complex formation can be recapitulated in vitro by embedding cardiomyocytes in a collagen matrix with a synthetic Gly-Arg-Gly-Asp-Ser (RGD) peptide (22, 23). This cell culture model and our previous studies in 48 h PO animals demonstrate formation of focal adhesion complexes, which seems to be β3 integrin specific (22,23,24), although the more highly expressed β1 integrin is widely accepted as the integrin isoform that controls hypertrophic signaling (25,26,27). Considering that Ub and focal adhesion complex formation occur within the same early growth period during PO, are associated with the cytoskeleton, and localize to the intercalated disc region, we sought to determine the role of integrins, specifically β3 integrin, in enhanced Ub and survival signaling during hypertrophy.
MATERIALS AND METHODS
Reagents
All chemicals were obtained from Sigma (St. Louis, MO, USA), except the following: MG132 (Biomol, Plymouth Meeting, PA, USA), crude GRGDS peptide (RGD) was synthesized in Dr. Christian Schwabe’s lab (Medical University of South Carolina, Charleston, SC, USA) and purified by FPLC with 0.2% TFA over a Superdex peptide HR10/30 size exclusion column.
Adenoviruses
The reporter adenovirus Ad-NF-κB-luciferase was a generous gift from Dr. P.B. McCray, Jr. (University of Iowa, Iowa City, IA, USA) (16). The adenovirus for the expression of nonphosphorylatable IκB (Ad-IκB-S32A) was purchased from Cell Biolabs (San Diego, CA, USA). β-Galactosidase (β-Gal) adenovirus (28) was used as a control adenovirus at matching multiplicity of infection (MOI).
Murine transverse aortic constriction (TAC) model
This is an established mouse model of TAC to produce left ventricle (LV) PO. Microsurgical TAC or sham operation was described previously (29). For TAC, the transverse aorta was constricted by tying a suture around the vessel over a 27-gauge blunted needle causing occlusion of the aorta. The needle was withdrawn, resulting in a severely stenotic aortic lumen. Two days to 4 wk after surgery, animals were sacrificed by removal of the heart in deep anesthesia.
Echocardiography
These measurements were made as described previously (29). Briefly, echocardiography was performed using a 15-MHz transducer (Sonos 5500; Agilent, Santa Clara, CA, USA) prior to TAC and at 4 wk TAC. LV dimensions and wall thickness were measured at end systole and end diastole using the American Society of Echocardiography criteria (30). The transverse aortic band pressure gradient was calculated via a modified Bernoulli equation: Δpressure = 4 × (Vpeak)2, where Vpeak is the peak continuous-wave Doppler velocity at the band site (31). Pressure gradient was determined in all mice, and only mice with a pressure gradient of >100 mmHg were used for further analysis.
Cardiomyocyte cross-sectional area
Cardiomyocyte cross-sectional area was measured in Dr. Francis G. Spinale’s laboratory from paraffin-embedded LVs, as described previously (32). Briefly, hematoxylin-and-eosin–stained LV sections were examined by light microscopy on an inverted microscope (IM-35; Zeiss, Oberkochen, Germany). For each animal, 100 cardiomyocytes in a cross-sectional orientation were analyzed by SigmaScan Pro-5 to determine cross-sectional area.
Knockout mice
The β3 integrin-knockout (β3−/−) mice, originally created in the laboratory of Dr. Richard Hynes (MIT), were obtained from Jackson Laboratories (Bar Harbor, ME, USA). All mice are homozygous with a C57BL/6 background, and C57BL/6 mice were wild-type (WT) controls. Genotyping for β3−/− mice was conducted per manufacturer’s instructions.
Cell isolation
Adult feline cardiomyocytes were isolated via a hanging heart preparation using enzymatic digestion and cultured by the protocols described previously (33). We used a modified procedure (34) for adult mouse cell isolation, which is described in detail in Supplemental Material.
Cardiomyocyte/collagen culture model
The collagen culture model was performed as described previously (22, 23), with some changes. Briefly, isolated mouse or cat ventricular cardiomyocytes were cultured on laminin-coated plates. For proteasome inhibitor studies, after plating for 12 h, cells were pretreated for 30 min with MG132, and the medium was replaced with type I collagen ± RGD peptide and allowed to polymerize. For adenovirus studies, cells were plated for 4 h, and the medium was changed and adenovirally infected at the indicated MOI. Thirty-six hours postinfection, the medium was replaced with collagen ± RGD peptide and allowed to polymerize. Cardiomyocytes were extracted from collagen by incubating in collagenase solution at 37°C for 15 min and washed, and cells were lysed in Triton X-100 buffer, as explained in Preparation of Cell and Tissue Lysates. For RNA preparation, TRIzol reagent (Invitrogen, Carsbad, CA, USA) was added directly on top of the whole collagen/cell matrix for fast extraction, as explained in Quantitative Real-Time RT-PCR.
Preparation of cell and tissue lysates
Triton X-100 soluble and insoluble fractions were prepared from fresh tissue and cell samples as established previously (22, 35) and described in Supplemental Material. Nuclear preparations have also previously been described (36) and are described in Supplemental Material.
Confocal microscopy
Fresh frozen tissue samples of the LV free wall were used for confocal studies, as described previously (13). Sections (10 μm thick) were fixed with 2% paraformaldehyde and permeabilized with 2% SDS prepared in phosphate-buffered saline for ubiquitin staining, except for TUNEL analysis (ApopTag kit, S7110; Chemicon, Temecula, CA, USA), which was permeabilized with ethanol:acetic acid (2:1) at −20°C for 5 min. All sections were blocked with 10% donkey serum. Primary antibodies anti-ubiquitin (1:100; Dako, Glostrup, Denmark), anti-N-cadherin (1:150; Zymed, San Francisco, CA, USA), and anti-α-actinin (1:200; Sigma, St. Louis, MO, USA) were added overnight at 4°C. For p65 NF-κB staining, the protocol for the Cellomics NF-κB activation kit (Thermo Scientific, Hudson, NH, USA) was followed, except fixation was performed with methanol at −20°C for 2 min. Alexa Fluor secondary antibodies (1:250, Invitrogen) were applied 1 h at room temperature, sections were washed and TOPRO3 (1:1000, Invitrogen) was applied 1 h at room temperature, sections were washed and mounted, and laser scanning confocal microscopy (Zeiss LSM 510 META) was performed. Images were obtained at sequential Z-plane optical sections, and those sections corresponding to the central plane of the cell were processed with Adobe Photoshop (Adobe Systems, San Jose, CA, USA).
Quantitative real-time RT-PCR
Total RNA was extracted from collagen culture plates using TRIzol reagent (Invitrogen). RNA quality and quantity were determined using the Agilent System Bioanalyzer, and only RNA with an RNA integrity number (RIN) > 9 was used. Real-time RT-PCR was performed with the iCycler iQ real-time PCR detection system (Bio-Rad, Richmond, CA, USA) using the Quantitect SYBR Green RT-PCR kit (Qiagen, Germantown, MD, USA) as described previously (37). Primer sequences are available in Supplemental Material. The fold change of expression with RGD stimulation over control was calculated using the ΔΔCt method (38).
Immunoblotting
Procedures were followed as described previously (13). The following primary antibodies were used at 1:1000 dilution: ubiquitin (P4D1; Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho-IκB (S32/S36) (Santa Cruz Biotechnology), cIAP1 (R&D Systems, Minneapolis, MN, USA), IκB, p105/50 NF-κB, phospho-p65 NF-κB, and histone H4 (Cell Signaling Technology, Danvers, MA, USA), total actin (Sigma), and GAPDH (Fitzgerald Industries, Fitzgerald, MA, USA). Horseradish peroxidase-labeled antibodies (Promega) were used as secondary antibodies.
Statistics
Statistical comparisons were performed within groups using Student’s paired t test and among groups using 1-way ANOVA followed by Tukey’s post hoc test for echocardiography measurements or Student’s unpaired t test for protein or mRNA quantifications. A χ2 test was used for comparison of dichotomous variables between groups in the Kaplan-Meyer plot. Statistical significance was defined as P < 0.05.
RESULTS
Integrin activation induces increased ubiquitination in cardiomyocytes
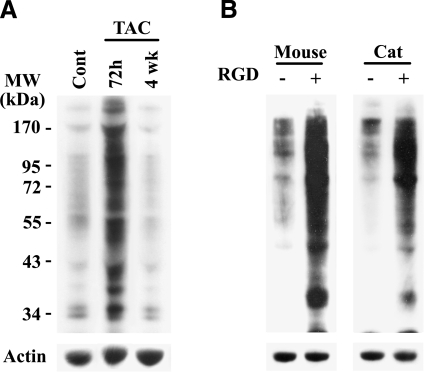
In PO feline myocardium, our previous work shows that both complexed focal adhesion proteins (22, 23, 35) and ubiquitinated proteins (13) are transiently present in the Triton-insoluble fraction on the first few days of PO and that the increased Ub occurs proximal to intercalated discs of cardiomyocytes (13). Before testing whether integrin activation is responsible for this activation and recruitment of ubiquitinated proteins to the Triton-insoluble cytoskeletal fraction, we first verified that Ub is enhanced in two additional models: a murine TAC model of in vivo PO and an in vitro cell culture model, developed in our laboratories for specific activation of integrins in isolated cardiomyocytes (22,23,24). Tissue or cell samples were homogenized in 2% Triton X-100 buffer, and the soluble proteins were separated by centrifugation from the insoluble fraction, which consists of cytoskeletal-bound proteins (35). To study the short-term PO effect on Ub, TAC was performed for 72 h, since this time point matched our previous observation of Ub in a feline PO model (13). We also performed 4 wk TAC in order to characterize the long-term effect of PO on UPS-mediated changes in hypertrophic growth and LV function. The Triton-insoluble fraction is actin rich, and total actin levels per unit mass remain constant during PO (35). Therefore, actin was used as an endogenous control, while adjusting protein loading for Western blots. Reminiscent of our previous work in the feline model (13), PO in mice for 72 h robustly increased Ub in the Triton-insoluble fraction transiently, which returns to a basal level at 4 wk (Fig. 1A). Analysis of other time points of PO shows increased Ub as early as in 48 h and returned to basal level by 1 wk PO (data not shown).
Figure 1.
Ub is increased with PO in vivo and integrin stimulation with RGD peptide in vitro. A) C57BL/6 mice were subjected to sham surgery (control) or TAC to induce PO for 72 h or 4 wk (n≥4/group). LV samples were homogenized in Triton X-100 buffer and centrifuged to obtain soluble and insoluble fractions, as described in Materials and Methods. Loading of equal total protein in the insoluble fractions was accomplished by adjusting actin levels, and a Western blot with anti-ubiquitin antibody was performed. B) Isolated adult mouse or cat ventricular cardiomyocytes were plated on laminin-coated plates. After 12 h, medium was removed and replaced with 0.2% collagen with or without solubilized RGD peptide (mouse, 6 mM; cat, 9 mM). Following 1 h incubation and collagen polymerization, cardiomyocytes were harvested by collagenase treatment and lysed in Triton X-100 buffer, as described in Materials and Methods. A Western blot with anti-ubiquitin antibody was performed on the insoluble fraction. Samples were normalized to actin for equal loading. Representative images are shown. Cell experiments were done in quadruplicate.
We next used the collagen cell culture model in vitro, which recapitulates integrin activation during mechanical stimulation (22). This model, which was slightly modified in the present study, consists of plating adult ventricular cardiomyocytes, followed by overlaying purified collagen with or without a specific integrin-activating RGD peptide. The RGD engages integrin heterodimers on the cell surface, primarily αvβ3 and α5β1, and the accompanying focal adhesion complex formation during collagen polymerization causes intracellular signaling. For signaling analysis, cardiomyocytes are recovered from collagen gels by collagenase treatment, and this digestion process does not appear to affect anabolic signaling in cardiomyocytes (22). Isolated cardiomyocytes can then be fractionated and analyzed biochemically. Our present studies with isolated adult murine cardiomyocytes show that RGD treatment can cause a large increase in Ub in the Triton-insoluble fraction (Fig. 1B), similar to that observed in the in vivo PO mouse model (Fig. 1A). Enhanced Ub was also confirmed in isolated feline cardiomyocytes (Fig. 1B), which were used in all our earlier studies (22,23,24). Furthermore, studies with both in vivo and in vitro models show no detectable changes in Ub in the Triton-soluble fractions (data not shown). Also, the increased Ub of proteins in the Triton-insoluble fraction was not observed when cardiomyocytes cultured on laminin-coated dishes were stimulated with hypertrophic agonists, including endothelin, phenylephrine, insulin, or RGD without collagen (data not shown). Previous studies show RGD in the absence of a semisolid collagen matrix engages integrins but no focal adhesion complex forms (22, 24). Therefore, the TAC mouse model and this three-dimensional collagen culture system, which results in integrin activation and focal adhesion complex formation to reflect mechanical stimulation (39), provide models useful to decipher the mechanism of Ub and identify specific target proteins.
The β3 integrin isoform is necessary for increased Ub and survival signaling in early PO hypertrophy
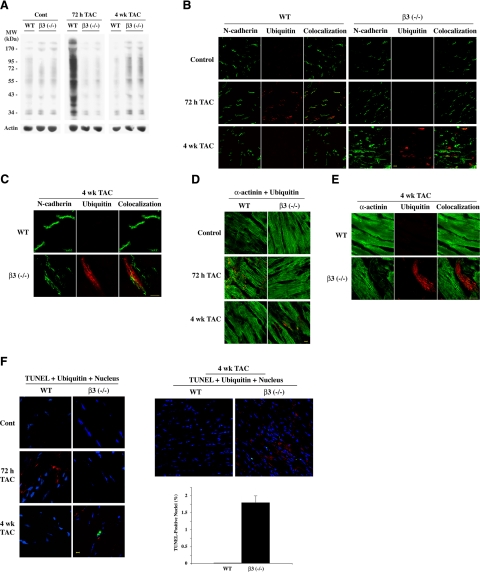
Our previous studies have suggested that β3 integrin contributes to focal adhesion signaling in cardiomyocytes (22,23,24, 35). We therefore sought to characterize the contribution of β3 integrin for increased Ub in PO myocardium by using β3−/− mice (Jackson Laboratories). In WT mice, immunostaining ventricular tissue sections with β3 integrin antibody showed the presence of β3 integrin along the sarcolemma of cardiomyocytes, and we confirmed its absence in the β3−/− mice (Supplemental Fig. 1). PO was induced in β3−/− mice and age-matched WT mice by TAC. The pressure gradient was measured by echocardiography to ensure equal pressure was induced after banding in both WT and β3−/− mice. Basal Ub was similar in both WT and β3−/− sham mice [Fig. 2A, shown in duplicates for β3 (−/−)]. However, by 72 h of PO, only WT mice showed a robust increase in Ub, which was absent in the β3−/− mice. Data were similar at 48 h PO (data not shown). This data, confirmed in three additional mice, indicates that the β3 isoform is responsible for increased Ub during early PO hypertrophy. In contrast, while Ub returned to basal levels at 4 wk PO in WT mice, Ub levels modestly increased in the β3−/− mice. These data indicate that β3 integrin mediates an early response of protein Ub to PO and that this mechanism is either absent or delayed in β3−/− mice.
Figure 2.
The β3 integrin isoform is necessary for increased Ub in the early compensated period of cardiac hypertrophy during in vivo PO. Age-matched WT (C57BL/6) mice and β3−/− [β3 (−/−)] mice were subjected to sham surgery or TAC for the indicated times (n≥4/group) and analyzed by Western blot or by immunofluorescence. A) LV samples were processed to obtain Triton-soluble and insoluble fractions. Insoluble fraction was normalized for actin, and a Western blot with anti-ubiquitin antibody was performed. B) Fresh frozen LV tissue sections were stained with anti-ubiquitin antibody (red) and anti-N-cadherin antibody (green). C) Higher-magnification image of 4 wk sections from WT and β3−/− stained with anti-ubiquitin antibody (red) and anti-N-cadherin antibody (green). D) Fresh frozen LV tissue sections were stained with anti-ubiquitin antibody (red) and anti-α-actinin antibody (green). E) Higher-magnification image of 4 wk sections from WT and β3−/− stained with anti-ubiquitin antibody (red) and anti-α-actinin antibody (green). F) Programmed cell death was analyzed; TOPRO3 (blue) stains nuclei, anti-ubiquitin antibody (red) stains ubiquitin, and TUNEL (green) indicates cell death. Low-magnification immunograph shows a larger field of WT and β3−/− at 4 wk TAC. Approximately 13,000 nuclei were counted from multiple sections from 3 mice/group. Bar graph shows average percentage of TUNEL-positive nuclei in LV of WT and β3−/− at 4 wk TAC; error bars = se. No TUNEL reactivity was present in controls or at 72 h TAC in either WT or β3−/− mice. Scale bars = 10 μm.
We have previously shown that Ub is concentrated proximal to the intercalated disc region of cardiomyocytes in early PO feline myocardium, and these changes were accompanied by increased levels of specific E3 ligases (13). These earlier studies suggest there is selective Ub of target proteins near the intercalated discs during PO. However, in heart failure, there is increased accumulation of ubiquitinated proteins throughout cardiomyocytes due to insufficient protein degradation by proteasomes (8, 9), and as a result, this accumulation contributes to cell death (8, 40). Therefore, we characterized the localization of the transient Ub in WT mice and the delayed low level Ub in β3−/− mice by performing immunofluorescence studies. Confocal microscopy confirmed the enhanced Ub at 72 h PO in WT mice occurred near the intercalated discs, as shown by ubiquitin staining near N-cadherin localization (Fig. 2B), and Ub occurred in cardiomyocytes, based on ubiquitin costaining with α-actinin (Fig. 2D). This staining pattern was absent in 72 h PO β3−/− mice (Fig. 2B, D). However, when compared to WT mice, long-term PO for 4 wk resulted in the accumulation of ubiquitin in the LV samples of β3−/− mice, which was distributed throughout the cell in contrast to localized ubiquitin staining in WT mice (Fig. 2B). The higher magnification images of N-cadherin in 4 wk WT and β3−/− mice (sections are aligned for the same orientation of cardiomyocytes) exhibit disarrayed intercalated discs, where N-cadherin was found to spread laterally (Fig. 2C). Further, the α-actinin staining was also disrupted in β3−/− cells that costain with ubiquitin at 4 wk (Fig. 2D, E). The high-magnification image (Fig. 2E) exemplifies loss of sarcomeric structure, evidenced by a decreased α-actinin signal in an ubiquitin-positive cardiomyocyte at 4 wk PO in the β3−/− mice, while α-actinin staining and sarcomeric structure are retained in the 4 wk PO WT mice. These data suggest that aggregated ubiquitin in β3−/− mice occurs in cardiomyocytes with sarcomeric disarray. Therefore, an altered pattern of global Ub occurs in the cardiomyocyte interior of long-term (4 wk) PO β3−/− mice, which is much different than the specific transient Ub observed at or near intercalated discs in short-term (72 h) PO WT mice (Fig. 2B). TUNEL assays indicated that only the Ub in the β3−/− mice at 4 wk PO, but not the Ub at 72 h in WT mice, was accompanied by increased cell death (Fig. 2F). TUNEL-positive cells were cardiomyocytes, as indicated by costaining with α-actinin (data not shown). Quantification performed on 4 wk PO WT and β3−/− mice estimated almost 2% of cardiomyocytes were undergoing cell death in the β3−/− mice, while cardiomyocyte death was undetectable in WT mice (Fig. 2F, right panel).
β3 Integrin contributes to compensatory cardiac hypertrophy
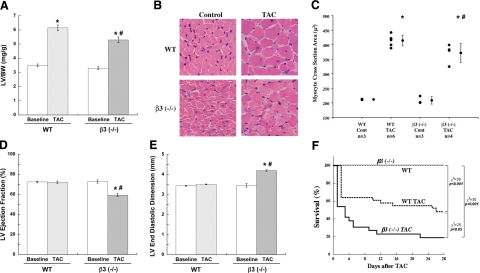
Because increased cell death and sarcomeric disarray were present in 4 wk PO ventricles in the β3−/− mice, we measured whether these changes in cardiomyocytes affected hypertrophic growth and LV function. By 4 wk TAC, morphometric parameters should be significantly affected (41), and a role for β3 can be deduced. Our data on LV to body weight ratio (LV/BW) showed a significant increase in LV mass following 4 wk PO in WT mice (Fig. 3A). Although an increase of LV mass in β3−/− mice was observed, it was significantly reduced (65% increase vs. 77% increase in WT mice), as was the LV wall thickness (Supplemental Table 1). Measurement of cardiomyocyte cross-sectional area confirmed this decreased LV mass was primarily due to the decrease in cardiomyocyte size (Fig. 3B, C). To analyze whether the loss of both the targeted Ub and cardiomyocyte growth with increased programmed cell death in β3−/− mice correlated with the loss of LV function, echocardiography was performed on WT and β3−/− mice before TAC and at the time of sacrifice at 4 wk TAC. While WT mice with 4 wk PO remained compensated, the LV ejection fraction of β3−/− mice was significantly decreased (Fig. 3D) with a corresponding increase in end diastolic dimension (Fig. 3E). The ejection fraction is calculated using end-diastolic volume (EDV) and end-systolic volume (ESV). Usually, a decreased ejection fraction results from an increased ESV (Supplemental Table 1), which can be due to decreased contractility, and the heart responds by remodeling and increasing the end diastolic dimension (Fig. 3E). With these changes in morphometry, there was a corresponding decrease in cardiac output (Supplemental Table 1). The survival curve supports β3−/− mice having a significantly reduced survival rate with PO compared to WT mice (Fig. 3F). Together, these data indicate that in PO myocardium, β3 integrin activation is necessary to mediate compensatory hypertrophic growth and to maintain cardiac output and geometry for survival during PO.
Figure 3.
β3 Integrin contributes to hypertrophic growth and ventricular function in PO myocardium. A) Hypertrophic growth of WT and β3−/− [β3 (−/−)] mice was measured at 4 wk PO by the ratio of LV mass to body weight (BW) on sacrifice. B) Control and 4 wk PO LVs of WT and β3−/− mice were paraffin-embedded and cross-sections were made. Sections stained with hematoxylin and eosin were imaged by light microscopy to visualize cardiomyocyte size. C) Cross-sectional area was calculated from 100 cardiomyocytes/animal using Sigma Scan Pro-5. The graph shows average cardiomyocyte size in each animal followed by average for each group; data are means ± se. *P < 0.05 vs. control; #P < 0.05 vs. WT TAC. D, E) LV function (ejection fraction) (D) and geometry (end diastolic dimension) (E) were analyzed by echocardiography prior to TAC and immediately prior to sacrifice after 4 wk of TAC. Data are means ± se. *P < 0.05 vs. baseline; #P < 0.05 vs. WT TAC. F) Kaplan-Meyer survival curve shows survival rate of WT and β3−/− control and 4 wk TAC mice.
β3 Integrin is required for IκB degradation, NF-κB nuclear localization, and cIAP1 induction during PO
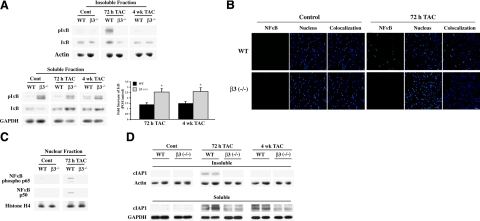
We next determined whether β3 integrin-mediated Ub contributes to the activation of cardiomyocyte survival pathways in early PO to avoid cell death. Activated integrins recruit signaling molecules onto the cytoskeleton to regulate cellular processes, such as growth and gene expression. We have previously shown both β3 integrin activation (23) and phosphorylation and recruitment of IκB into the Triton-insoluble cytoskeletal fraction of PO cardiac tissue, where increased Ub was also observed (13). Ub and degradation of IκB, subsequent to its phosphorylation at Ser-32 and Ser-36 sites, is required for ΝF-κB translocation to the nucleus and activation of survival genes (15, 42, 43). Therefore, in the present study, we explored the role of β3 integrin in IκB degradation for ΝF-κB activation by performing studies in the β3−/− mice subjected to PO and compared with WT mice. Analysis of the Triton-insoluble LV samples from control and TAC mice showed the presence of phosphorylated IκB only in WT mice at 72 h PO but not in β3−/− mice at 72 h or 4 wk of PO (Fig. 4A). When the total IκB levels in the Triton-insoluble fractions were compared, no appreciable difference was observed between WT and β3−/− mice with the exception of a low level increase only in 72 h WT TAC mice, which accounts for the recruitment of IκB from the cytoplasm.
Figure 4.
β3 Integrin is required for IκB degradation, NF-κB nuclear localization, and cIAP1 induction during PO hypertrophy. Age-matched WT and β3−/− mice were subjected to TAC for the indicated times (n≥4/group). A) Triton-insoluble and soluble fractions of LV were prepared and analyzed by Western blot with anti-IκB and pIκB (S32/S36) antibodies. Soluble fractions were normalized to GAPDH; insoluble fractions were normalized to actin. Quantification was conducted by densitometry of IκB level in soluble fraction by NIH Image J. Summary data show average ± se fold increase of IκB in soluble fraction of PO tissue over control. B) Fresh frozen LV tissue sections from sham and 72 h TAC mice were stained with anti-p65 NFκB antibody (green) and nuclear stain TOPRO3 (blue). Scale bar = 20 μM. C) Nuclear fractions were prepared from sham and 72 h TAC mice and were analyzed for movement of the NFκB heterodimer complex, p50:p65. Western blot analyses were performed with anti-p50 and anti-phospho-p65 (active form) NF-κB subunit antibodies. Histone H4 was used to normalize nuclear fractions from individual animals. D) Insoluble and soluble fractions were analyzed by Western blot with anti-cIAP1 antibody. Soluble fractions were normalized to GAPDH; insoluble fractions were normalized to actin.
Next, we analyzed whether IκB accumulated in the Triton-soluble fraction of β3−/− TAC mice in the absence of Ub. In our Triton subfractionation, the soluble fraction represents a larger volume than the insoluble fractions (35), and thus it represents the major proportion of the cellular level of IκB. The graph in Fig. 4A shows the summary data for the fold increase of IκB in the soluble fraction of WT and β3−/− mice at both 72 h and 4 wk of TAC compared to control mice. PO in both WT and β3−/− mice caused an increase in IκB at both time points of PO when compared to their basal levels. Importantly, this PO-induced increase was significantly higher in β3−/− mice at both time points of PO. That is, compared to WT mice, both 72 h and 4 wk PO in β3−/− mice showed a further increase of IκB levels from 1.3 to 2.6 fold and 1.5 to 2.7 fold, respectively. The Western blot further shows that compared to WT mice, phosphorylated IκB was higher in the soluble fraction of β3−/− mice in controls and retained throughout 4 wk of PO (Fig. 4A). Therefore, in the absence of β3 integrin-mediated Ub, IκB, even after higher levels of basal phosphorylation in the soluble fraction, was neither recruited to the insoluble complex nor eliminated from the cell.
To test whether the lack of recruitment of IκB to the insoluble fraction and accumulation of undegraded phospho-IκB in the soluble fraction of β3−/− mice inhibits PO-induced activation of NFκB, we studied the nuclear localization of NF-κB by performing immunofluorescence and biochemical analyses at 72 h PO (Fig. 4B,C). The immunographs show NF-κB translocates to the nucleus only in 72 h WT sections (Fig. 4B). The signal for cytoplasmic NF-κB in WT and β3−/− mice was very low when compared to the concentrated nuclear distribution in 72 h PO WT mice. To support these findings, nuclear fractions were also prepared from sham and 72-h PO mice and Western blotted for NF-κB subunits. Whereas in 72 h PO WT mice, NF-κB is activated, as measured by movement of its p50 and phosphorylated p65 subunits of NF-κB to the nucleus, the 72 h PO β3−/− mice lack NF-κB nuclear localization (Fig. 4C).
Previous studies in smooth muscle and epithelial cells demonstrate that IκB degradation and subsequent NFκB transcriptional activation results in the expression of cell survival molecules, including cIAP1 (17, 18). Furthermore, our earlier work showed an increased level of cIAP1 in 48 h PO myocardium (13). To promote cell survival, cIAP1, which is an E3 ligase, ubiquitinates deleterious molecules in the proapoptotic caspase pathway for their elimination (10, 11). To investigate whether β3 integrin activation contributes to cIAP1 expression during PO, the cIAP1 level was measured in both WT and β3−/− mice following PO. Analysis of cIAP1 in the Triton-insoluble fraction showed an up-regulation in 72 h PO WT mice, which was absent in 72 h PO β3−/− mice (Fig. 4D, top lanes; data shown in duplicate). The increased level of cIAP1 in this fraction returned to the basal level by 4 wk PO. When the Triton-soluble LV fraction was analyzed for changes in cIAP1 protein level, both WT and β3−/− mice showed increases from baseline levels with PO at each time point, although the change in cIAP1 from its basal level was only moderate in β3−/− mice (Fig. 4D, bottom lanes). These data indicate that β3 integrin activation contributes to cIAP1 expression and recruitment to the Triton-insoluble cytoskeletal complex in PO.
Integrin activation in adult cardiomyocytes results in UPS-mediated degradation of IκB for ΝF-κΒ transcriptional activation of cIAP1
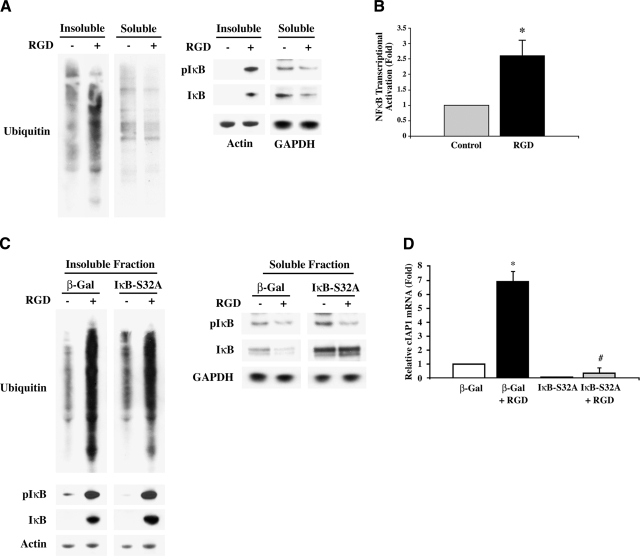
To link β3 integrin-mediated Ub to NF-κB-regulated cardiomyocyte survival, we first established Ub of cytoskeleton-bound proteins and the accompanying IκB phosphorylation and NF-κB activation in collagen-layered adult cardiomyocytes with RGD stimulation. Similar to the data shown in Fig. 1B, stimulation of adult feline cardiomyocytes with RGD resulted in a robust Ub of proteins in the Triton-insoluble fraction, and this increase was not observed in the soluble fraction (Fig. 5A). Furthermore, IκB was phosphorylated and recruited to the insoluble fraction of RGD-treated cells (Fig. 5A). The RGD treatment also caused a decrease in total IκB in the soluble fraction of cardiomyocytes. This drop in IκB level could be due to either subsequent ubiquitin-mediated degradation and/or its recruitment to the insoluble protein complex.
Figure 5.
Integrins activate NF-κB transcription for cIAP1 expression in cardiomyocytes in vitro. Collagen/RGD model was conducted as in Fig. 1B. A) Feline cardiomyocytes were stimulated with 9 mM RGD, and Triton-insoluble and soluble samples were used for Western blots with anti-ubiquitin, anti-IκB, and anti-pIκB (S32/S36) antibodies and normalized to actin and GAPDH, respectively. Cell experiments were done in quadruplicate. B) Feline cardiomyocytes were infected with Ad-NFκB-luc (MOI 10) for 36 h prior to collagen layering ± 9 mM RGD for 2 h. Total RNA was subjected to real-time RT-PCR for luciferase expression. 18 S mRNA was used as an internal control. Summary graph represents average of 3 experiments; error bars = se. *P < 0.05 vs. infected control. C) Feline cardiomyocytes were infected with either Ad-β-Gal (MOI 150) or Ad-IκB-S32A (MOI 150) for 36 h before stimulating with collagen containing 9 mM RGD for 1 h. Western blots were performed on insoluble and soluble fractions with anti-ubiquitin, anti-IκB, and anti-pIκB (S32/S36) antibodies; loading was normalized to actin and GAPDH, respectively. D) Murine cardiomyocytes were infected with either Ad-β-Gal (MOI 150) or Ad-IκB-S32A (MOI 150) for 36 h before stimulating with 6 mM RGD in collagen for 1 h. Total RNA was extracted and subjected to real-time RT-PCR for cIAP1. Endogenous GAPDH mRNA was used as an internal control. Summary graph represents average of 3 three experiments; error bars = se. *P = 0.01 vs. β-Gal control; #P = 0.005 vs. β-Gal + RGD.
Next, we used a ΝF-κΒ-luciferase reporter adenovirus (16) to confirm the RGD-stimulated IκB phosphorylation results in ΝF-κΒ transcriptional activation. This adenoviral vector contains a TATA-like promoter with four tandem repeats of the NF-κB DNA binding sequence fused to the luciferase gene, so luciferase will only be expressed in infected cells if ΝF-κΒ translocates to the nucleus and is transcriptionally active (16). Unfortunately, we were unable to directly measure luciferase activity in these studies, since the collagenase treatment involved in the protocol interferes with luciferase activity. Therefore, we performed real-time RT-PCR to measure the luciferase transcript in cardiomyocytes stimulated with RGD. As shown by the graph in Fig. 5B, RGD stimulation for 2 h significantly increased the luciferase mRNA level, suggesting ΝF-κΒ transcriptional activation during integrin stimulation by RGD.
Finally, to directly link the β3 integrin-mediated Ub to NF-κB activation for survival signaling in cardiomyocytes, we used adenoviral gene delivery in the collagen cell culture model to study the effect of a nonphosphorylatable IκB mutant on IκB levels, cIAP1 expression, and cell viability. The nonphosphorylatable IκB construct has a S32A mutation (IκB-S32A) and renders IκB unrecognizable for Ub, thus allowing IκB to stably sequester NFκB (15, 16). Stimulation of cardiomyocytes infected with β-galactosidase (β-Gal)-expressing control virus with RGD in a collagen environment resulted in enhanced Ub of Triton-insoluble proteins (Fig. 5C), similar to noninfected cells (Fig. 5A). Expression of IκB-S32A by adenoviral infection did not appreciably affect this RGD-induced increase in Ub. Similarly, in both β-Gal and IκB-S32A-expressing cardiomyocytes, IκB was recruited to the insoluble fraction and phosphorylated (Fig. 5C, lower panels). Because the phosphospecific antibody recognizes IκB phosphorylation at both Ser-32 and Ser-36 sites and because IκB-S32A-expressing cardiomyocytes still have intact Ser-36, we did not observe a major difference between β-Gal control and IκB-S32A-expressing cardiomyocytes in terms of RGD-induced IκB phosphorylation in the Triton-insoluble fractions. However, because phosphorylation of both serine residues is required for Ub and degradation of IκB (15), we explored whether the expression of IκB-S32A blocks IκB degradation. For this, we analyzed the IκB level and phosphorylation state in the Triton-soluble fraction, which represents a larger pool of IκB. Similar to previous experiments (Fig. 5A), RGD stimulation of β-Gal-expressing cardiomyocytes showed a reduction in the IκB level, including its phosphorylated form (Fig. 5C, right panels). However, in IκB-S32A-expressing cardiomyocytes, the increased level of IκB was retained during RGD stimulation with an accompanying decreased level of phosphorylation. These data indicate that the RGD-induced decrease of IκB in the soluble fraction of β-Gal-expressing control cardiomyocytes is dependent on ubiquitin-mediated degradation of phosphorylated IκB. For further confirmation, these data were replicated with the proteasome inhibitor MG132 (Supplemental Fig. 2), in which inhibiting proteasomal degradation during RGD stimulation caused retention of IκB and its phosphorylated form in the soluble fraction, further supporting that RGD causes UPS-mediated degradation of IκB.
We next used the IκB-S32A mutant to test whether NF-κB transcriptionally regulates cIAP1 downstream of integrins in adult cardiomyocytes. In β-Gal-expressing cells, RGD treatment caused a 6.5-fold increase in cIAP1 expression when compared to unstimulated cells (Fig. 5D). However, expression of the mutant form of IκB decreased basal levels of the cIAP1 transcript and totally blocked its induction with RGD. Importantly, IκB-S32A expression during RGD stimulation resulted in a substantial reduction of cell viability (Supplemental Fig. 3). These results demonstrate that the ΝF-κΒ-dependent transcription in response to integrin activation mediates cIAP1 expression and that this pathway is important for cardiomyocyte survival.
β3 Integrin contributes to Ub and IκB degradation in isolated cardiomyocytes
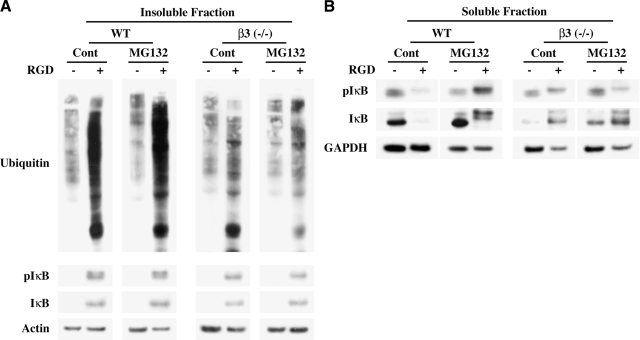
To test whether β3 integrin is required for Ub of proteins in the Triton-insoluble complex and IκB degradation, cardiomyocytes were isolated from both WT and β3−/− mice and used for integrin stimulation in the collagen system described above. Ub and IκB phosphorylation were measured in WT and β3−/− cells for side-by-side comparisons. We also included the proteasome inhibitor MG132 for these studies to evaluate whether the change in Ub level was due to altered UPS function. Analysis of the insoluble fraction of cardiomyocytes showed that the RGD-induced robust Ub in WT cells was substantially lower in β3−/− cells (Fig. 6A), and this observation is similar to in vivo observations in 72 h TAC mice (Fig. 2A). To demonstrate that the decreased Ub in β3−/− cells was not due to increased degradation of ubiquitinated proteins by the UPS, we used the proteasome inhibitor MG132 prior to RGD stimulation. MG132 pretreatment showed no appreciable change in the level of RGD-induced Ub in β3−/− cells, suggesting that the loss of Ub was not due to increased UPS-mediated degradation. We next analyzed whether there were differences in WT and β3−/− cells in terms of IκB recruitment to the insoluble fraction and phosphorylation as observed in vivo in mice with 72 h TAC (Fig. 4A). Figure 6A (bottom panels) shows RGD caused IκB recruitment and phosphorylation to a similar extent in WT and β3−/− cells, which was unaffected by MG132 treatment, suggesting that these changes occur in vitro due to the activation of other integrins by RGD.
Figure 6.
β3 Integrin is required for UPS-mediated IκB degradation. A, B) Isolated cardiomyocytes from age-matched WT and β3−/− mice were plated on laminin-coated plates. After 12 h of plating, cells were pretreated for 30 min ± MG132, and medium was replaced with collagen ± 6 mM RGD peptide for 1 h. Cardiomyocytes were harvested by collagenase treatment and extracted in Triton X-100 buffer. Insoluble (A) and soluble proteins (B) were separated by centrifugation and used for Western blot analyses with anti-ubiquitin, anti-IκB, and anti-pIκB (S32/S36) antibodies. Actin and GAPDH were used to normalize insoluble and soluble fractions, respectively.
Next, we explored whether the decreased level of RGD-induced Ub observed in β3−/− cardiomyocytes affected the overall level of IκB. For this, we measured the total IκB level in the soluble fraction that represents its larger pool. Figure 6B shows that in WT cardiomyocytes, consistent with earlier data (Fig. 5C), RGD caused a substantial reduction in IκB level, including phosphorylated IκB, which could be prevented by inhibiting the proteasomal degradation with MG132. However, in β3−/− cardiomyocytes, where the basal level of IκB was found to be lower, RGD treatment caused an increased level of IκB, and this increase was unaffected during MG132 treatment. Together, these data indicate that, in WT cardiomyocytes, β3 integrin stimulation by RGD causes a robust Ub of cytoskeleton-bound proteins with considerable degradation of IκB and that such a protective mechanism to promote cell survival is substantially reduced in β3−/− cardiomyocytes.
DISCUSSION
We have previously shown that Ub is transiently increased during the active growth period of pressure-induced cardiac hypertrophy (13), so it was our contention that this event is a key modulator for the change in the proteomic profile in cardiomyocytes to promote growth and survival. Our two major goals in the present study were to establish the involvement of β3 integrin in increased Ub during the active growth phase in PO myocardium and to characterize the importance of such Ub for compensatory cardiac hypertrophy. For this, we used WT and β3−/− mice to induce PO by TAC and isolated adult cardiomyocytes to study integrin activation by RGD. Our major findings from in vivo studies with TAC mice include the following: 1) PO for 72 h causes targeted Ub of proteins near the intercalated discs of cardiomyocytes, which can be observed as Triton-insoluble proteins; 2) this early response to PO of increased Ub is absent in β3−/− mice; 3) IκB phosphorylation and degradation, ΝF-κB nuclear localization, and cIAP1 expression in 72 h PO WT mice are diminished in β3−/− mice; and 4) in the β3−/− mice that survive long term (4 wk) PO, but not in WT mice, ubiquitinated proteins accumulate, which is accompanied by increased cardiomyocyte death and decreased LV function. Our in vitro studies performed with RGD stimulation of adult cardiomyocytes support these findings in the following manner: 1) RGD treatment of WT but not β3−/− cardiomyocytes causes a large increase of Ub of Triton-insoluble proteins (similar to the comparison between 72 h PO WT vs. 4 wk PO β3−/− mice); 2) RGD induces ΝF-κΒ-mediated expression of cIAP1; and 3) β3 integrin is required for the ubiquitin-mediated degradation of IκB induced by RGD.
The importance of αvβ3 integrins for survival is demonstrated in epithelial (17, 44) and smooth muscle cells (18, 45) but has not been shown in cardiomyocytes. Although it is well established that β1 integrin is essential for hypertrophic growth (25,26,27), our study establishes the importance of β3 integrin for hypertrophic growth via the activation of survival mechanisms. In the absence of β3 integrin, our studies show that 4 wk PO leads to decreased cardiac function, as evidenced by both the decrease in LV ejection fraction and cardiac output and the increase in LV end-diastolic dimension. The distribution of β3 integrin in WT mice was predominantly present along the sarcolemma compared to the intercalated disc region (Supplemental Fig. 1). Although ubiquitination in WT mice is predominantly observed at or near the intercalated discs, the absence of β3 integrin staining in this region could be due to epitope masking by the plethora of proteins present in this area. It is also possible β3 integrin is absent at the intercalated disc. In either case, our study demonstrates that the β3 integrin is required for the targeted ubiquitination of proteins at the intercalated disc, where it might mediate the recruitment of appropriate signaling proteins. In the absence of β3 integrin, as in the case of β3−/− mice, PO-induced localized ubiquitination is lost, and under these conditions, sustained PO leads to disorganization of intercalated discs with the accumulation of globally ubiquitinated proteins throughout the cardiomyocytes. Interestingly, in support of our data, one study in β3−/− mice showed ventricle function was impaired at a basal level, which was intensified following 1 wk PO (46). However, this study also demonstrated 1 wk PO caused increased LV mass in β3−/− mice compared to WT mice, while we observe a modest but significant reduction in ventricular mass, cardiomyocyte cross-sectional area, and wall thickness by 4 wk PO in β3−/− mice. At 1 wk PO, infiltration of blood-borne cells was proven to at least partially contribute to LV hypertrophy in β3−/− mice (46), suggesting that the loss of cardiomyocytes and blunted size increase are greater contributors to LV mass during sustained PO.
Previous studies have shown enhanced myofibrillar protein breakdown during cachexia (47) and UPS involvement in atrophy (48), but there is little research on the role of UPS-mediated protein degradation during an active hypertrophic growth phase in adult cardiac muscle (myocardium). In this context, previous studies show that proteasome function is necessary for PO-induced hypertrophic growth (6) and that dysfunction of the proteasome may contribute to heart failure due to accumulation of proapoptotic proteins, such as Bax and p53, in cardiomyocytes (9, 40). Thus, activation of the UPS may be necessary to alter the proteome and signaling potential in terminally differentiated cardiomyocytes during hypertrophic growth for two reasons: to eliminate proapoptotic and misfolded proteins induced by stress and to up-regulate signaling events that activate antiapoptotic and progrowth genes.
Within this context, our study shows that PO causes β3 integrin-dependent Ub, leading to decreased cellular concentrations of IκB, which, in turn, activates ΝF-κΒ and the subsequent induction of the survival E3 ligase cIAP1. In the absence of this mechanism, as observed in β3−/− mice, long-term PO causes accumulation of ubiquitinated proteins, correlating with features observed in heart failure, including a disorganized cytoskeleton (49), increased programmed cell death (1, 2, 4), and decreased ventricular function. The cell death in the β3−/− mice (∼2%) associated with accumulated ubiquitin at 4 wk PO is sufficient to cause ventricular dysfunction, as a previous study showed cell death of only 0.023% caused lethal dilated cardiomyopathy (1). Therefore, the lack of targeted Ub to activate survival pathways, such as NF-κB, and failure to eliminate various proapoptotic substrates by specific E3 ligases, such as cIAP1, in early PO in the β3−/− might be the reason for eventual accumulation of ubiquitinated proteins in dying cells and deteriorating ventricular function during long-term PO. It is, however, possible that infiltrating immune cells (46) and nonmyocytes in β3−/− mouse heart contribute to this loss of function as well. Nevertheless, inhibiting only the NF-κB pathway during integrin stimulation of isolated cardiomyocytes causes increased cell death (Supplemental Fig. 3). This is supported in vivo by the observation that there is an IκB buildup in the β3−/− at 72 h compared to the WT that is sustained up to 4 wk of PO (Fig. 3A), accompanied by blunted cIAP1 expression, even though an increase in Ub is observed in this long-term PO period. Therefore, the delayed increase in Ub in β3−/− mice at 4 wk is qualitatively different from the transient Ub seen in 72 h PO WT mice. In WT mice, whereas IκB was initially degraded at 72 h PO, there was a slight increase in IκB at 4 wk PO. This could be due to ΝF-κΒ inducing transcription of its inhibitor IκB as a regulatory negative feedback mechanism to generate a steady decline in ΝF-κΒ activation once the heart compensates for the PO (20). Hence, increasing amounts of IκB by 4 wk of PO in WT mice should decrease ΝF-κΒ activity that is no longer required, corresponding to transient cIAP1 expression (Fig. 4D). However, IκB levels were constantly elevated in the case of β3−/− mice, and NF-κB, as analyzed in 72 h PO, was not activated. It is also worthy to note the basal level of pIκB was elevated in β3−/− tissue (Fig. 4A), but in isolated cardiomyocytes from β3−/− mice, the basal level of pIκB is actually less compared to WT cells. Therefore, elevated pIκB in β3−/− LV is probably due to factors released by inflammatory cells that infiltrate the myocardium at higher levels in the β3−/− mice (46).
We performed in vitro studies using murine adult cardiomyocytes for three reasons: 1) the β3−/− mouse codes for a germline β3 elimination, so it was necessary to demonstrate that the β3 integrin-mediated Ub occurs at the level of cardiomyocytes to further confirm the confocal data; 2) in vitro models allow characterization of the pathways activated downstream of β3 integrin-mediated Ub because direct immunopreciptitations are not possible in the Triton-insoluble fraction; and 3) we could distinguish the prosurvival role of β3 integrin-mediated Ub in cardiomyocytes in an environment devoid of the inflammatory response activated in the β3−/− mouse (46). Using this model, we connect enhanced Ub to survival signaling in cardiomyocytes by showing how β3 integrin induces NF-κB activation via UPS-mediated IκB degradation. Furthermore, with RGD stimulation in vitro, we show NF-κB is necessary for cardiomyocyte viability and induces transcription of cIAP1 because expression of a nonphosphorylatable/nondegradable IκB mutant that prevents NF-κB activation imposes cell death and blocks cIAP1 expression. cIAP1 is an E3 ligase for protein molecules in the caspase 8 pathway, and therefore, we predict expression of this E3 ligase contributes to the enhanced integrin-mediated Ub for cell survival. Although we extended our studies to β3−/− cardiomyocytes and showed that RGD stimulation neither caused an increase in Ub nor the accompanying decrease in the level of IκB, we were unable to directly link β3 integrin with cIAP1 expression in vitro. Unlike the WT cells, it was technically difficult to culture viable β3−/− cardiomyocytes for 3 days for use in the adenoviral studies, so we were unable to perform additional studies to show the absence of NF-κB activation in the β3−/− cells induced with RGD.
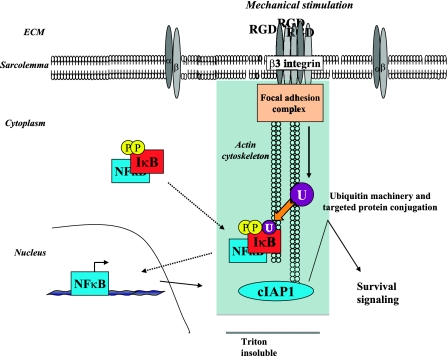
While this is the first study in adult cardiomyocytes showing β3 integrin-mediated NF-κB activation, there is precedent in other cell types. Overexpression of β3 alone caused increased ΝF-κΒ activation in smooth muscle cells (45). β3 integrin also activates NF-κB induced by cleaved collagen fragments for transcription of survival factors, which includes cIAP1, in epithelial cells (17). However, no studies have shown integrin-mediated increased Ub or ΝFκB-mediated cIAP1 expression in cardiomyocytes. As summarized in Fig. 7, we show β3 integrin engagement contributes to the enhanced Ub in PO myocardium, which plays a role in cardiomyocyte survival via ubiquitin-mediated NF-κB activation and subsequent cIAP1 expression. Dysfunction of this pathway could be one mechanism of decompensation into heart failure. Further studies in this emerging field will surely reveal other regulatory roles of β3 integrin-mediated Ub that are critical for compensatory hypertrophic growth and the prevention of heart failure.
Figure 7.
Schematic showing signaling mechanism of β3 integrin-mediated Ub for NFκB activation and cIAP1 expression during compensatory cardiac hypertrophy. The β3 integrin recognizes the RGD motif of extracellular matrix (ECM) proteins and responds by clustering, followed by recruitment of molecules onto the actin cytoskeleton to create a focal adhesion complex in order to mediate downstream intracellular signaling responses, including enhanced Ub. This can be recreated in vitro by overlaying cardiomyocytes with collagen containing an RGD peptide. During PO in vivo or RGD treatment in vitro, IκB undergoes phosphorylation, which is not β3 integrin-specific. However, when β3 integrin is activated, phosphorylated IκB is recruited to the actin-cytoskeleton complex (a protein complex resistant to Triton X-100 solubilization), where it is recognized for Ub and subsequent degradation. NFκB is then freed in the cytoplasm and translocates to the nucleus for transcription of cIAP1, an E3 ligase. cIAP1 is also recruited to the cytoskeleton, and it promotes Ub and negatively regulates the proapoptotic caspase pathway for cell survival.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health (NIH) grant PPG HL-48788, by a Merit Award from the Research Service of the Department of Veterans Affairs, by NIH grant RO1 HL-092124 (to D.K.), by American Heart Association predoctoral fellowship 0615468U (to R.K.J.), and by NIH predoctoral fellowship NIH T32HL07260 (to R.K.J.). We thank An Van Laer for proficiency with the TAC model. We also thank Dr. Robin Muise-Helmericks, Dr. Amy Bradshaw, and Phillip Moschella for thoughtful comments and useful discussions, and Dr. Francis G. Spinale for help with the measurement of myocyte cross-sectional area.
References
- Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor S M, Shirani J, Armstrong R C, Kitsis R N. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Chawla-Sarkar M, Young D, Nishiyama K, Rayborn M E, Hollyfield J G, Sen S. Myocardial cell death and regeneration during progression of cardiac hypertrophy to heart failure. J Biol Chem. 2004;279:52630–52642. doi: 10.1074/jbc.M402037200. [DOI] [PubMed] [Google Scholar]
- Narula J, Haider N, Virmani R, DiSalvo T G, Kolodgie F D, Hajjar R J, Schmidt U, Semigran M J, Dec G W, Khaw B A. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara J A, Quaini E, Di Loreto C, Beltrami C A, Krajewski S, Reed J C, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- Hedhli N, Pelat M, Depre C. Protein turnover in cardiac cell growth and survival. Cardiovasc Res. 2005;68:186–196. doi: 10.1016/j.cardiores.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Depre C, Wang Q, Yan L, Hedhli N, Peter P, Chen L, Hong C, Hittinger L, Ghaleh B, Sadoshima J, Vatner D E, Vatner S F, Madura K. Activation of the cardiac proteasome during pressure overload promotes ventricular hypertrophy. Circulation. 2006;114:1821–1828. doi: 10.1161/CIRCULATIONAHA.106.637827. [DOI] [PubMed] [Google Scholar]
- Weekes J, Morrison K, Mullen A, Wait R, Barton P, Dunn M J. Hyperubiquitination of proteins in dilated cardiomyopathy. Proteomics. 2003;3:208–216. doi: 10.1002/pmic.200390029. [DOI] [PubMed] [Google Scholar]
- Kostin S, Pool L, Elsasser A, Hein S, Drexler H C, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klovekorn W P, Schaper J. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- Tsukamoto O, Minamino T, Okada K, Shintani Y, Takashima S, Kato H, Liao Y, Okazaki H, Asai M, Hirata A, Fujita M, Asano Y, Yamazaki S, Asanuma H, Hori M, Kitakaze M. Depression of proteasome activities during the progression of cardiac dysfunction in pressure-overloaded heart of mice. Biochem Biophys Res Commun. 2006;340:1125–1133. doi: 10.1016/j.bbrc.2005.12.120. [DOI] [PubMed] [Google Scholar]
- Hu S, Yang X. Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J Biol Chem. 2003;278:10055–10060. doi: 10.1074/jbc.M207197200. [DOI] [PubMed] [Google Scholar]
- Bertrand M J, Milutinovic S, Dickson K M, Ho W C, Boudreault A, Durkin J, Gillard J W, Jaquith J B, Morris S J, Barker P A. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Fang S, Jensen J P, Ludwig R L, Vousden K H, Weissman A M. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000;275:8945–8951. doi: 10.1074/jbc.275.12.8945. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Mani S, Shiraishi H, Johnston R K, Yamane K, Willey C D, Cooper G, 4th, Tuxworth W J, Kuppuswamy D. Enhanced ubiquitination of cytoskeletal proteins in pressure overloaded myocardium is accompanied by changes in specific E3 ligases. J Mol Cell Cardiol. 2006;41:669–679. doi: 10.1016/j.yjmcc.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Spruill L S, Baicu C F, Zile M R, McDermott P J. Selective translation of mRNAs in the left ventricular myocardium of the mouse in response to acute pressure overload. J Mol Cell Cardiol. 2008;44:69–75. doi: 10.1016/j.yjmcc.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I κB-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- Sanlioglu S, Williams C M, Samavati L, Butler N S, Wang G, McCray P B, Jr, Ritchie T C, Hunninghake G W, Zandi E, Engelhardt J F. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-κB. J Biol Chem. 2001;276:30188–30198. doi: 10.1074/jbc.M102061200. [DOI] [PubMed] [Google Scholar]
- Toruner M, Fernandez-Zapico M, Sha J J, Pham L, Urrutia R, Egan L J. Antianoikis effect of nuclear factor-κB through up-regulated expression of osteoprotegerin, BCL-2, and IAP-1. J Biol Chem. 2006;281:8686–8696. doi: 10.1074/jbc.M512178200. [DOI] [PubMed] [Google Scholar]
- Von Wnuck Lipinski K, Keul P, Ferri N, Lucke S, Heusch G, Fischer J W, Levkau B. Integrin-mediated transcriptional activation of inhibitor of apoptosis proteins protects smooth muscle cells against apoptosis induced by degraded collagen. Circ Res. 2006;98:1490–1497. doi: 10.1161/01.RES.0000229267.77982.0d. [DOI] [PubMed] [Google Scholar]
- Li Y, Ha T, Gao X, Kelley J, Williams D L, Browder I W, Kao R L, Li C. NF-κB activation is required for the development of cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol. 2004;287:H1712–H1720. doi: 10.1152/ajpheart.00124.2004. [DOI] [PubMed] [Google Scholar]
- Ha T, Li Y, Gao X, McMullen J R, Shioi T, Izumo S, Kelley J L, Zhao A, Haddad G E, Williams D L, Browder I W, Kao R L, Li C. Attenuation of cardiac hypertrophy by inhibiting both mTOR and NFκB activation in vivo. Free Radic Biol Med. 2005;39:1570–1580. doi: 10.1016/j.freeradbiomed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Zhang J Q, Elzey B, Williams G, Lu S, Law D J, Horowits R. Ultrastructural and biochemical localization of N-RAP at the interface between myofibrils and intercalated disks in the mouse heart. Biochemistry. 2001;40:14898–14906. doi: 10.1021/bi0107445. [DOI] [PubMed] [Google Scholar]
- Laser M, Willey C D, Jiang W, Cooper G, 4th, Menick D R, Zile M R, Kuppuswamy D. Integrin activation and focal complex formation in cardiac hypertrophy. J Biol Chem. 2000;275:35624–35630. doi: 10.1074/jbc.M006124200. [DOI] [PubMed] [Google Scholar]
- Willey C D, Balasubramanian S, Rodriguez Rosas M C, Ross R S, Kuppuswamy D. Focal complex formation in adult cardiomyocytes is accompanied by the activation of beta3 integrin and c-Src. J Mol Cell Cardiol. 2003;35:671–683. doi: 10.1016/s0022-2828(03)00112-3. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Kuppuswamy D. RGD-containing peptides activate S6K1 through beta3 integrin in adult cardiac muscle cells. J Biol Chem. 2003;278:42214–42224. doi: 10.1074/jbc.M303428200. [DOI] [PubMed] [Google Scholar]
- Ross R S, Pham C, Shai S Y, Goldhaber J I, Fenczik C, Glembotski C C, Ginsberg M H, Loftus J C. Beta1 integrins participate in the hypertrophic response of rat ventricular myocytes. Circ Res. 1998;82:1160–1172. doi: 10.1161/01.res.82.11.1160. [DOI] [PubMed] [Google Scholar]
- Yutao X, Geru W, Xiaojun B, Tao G, Aiqun M. Mechanical stretch-induced hypertrophy of neonatal rat ventricular myocytes is mediated by beta(1)-integrin-microtubule signaling pathways. Eur J Heart Fail. 2006;8:16–22. doi: 10.1016/j.ejheart.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Ogawa E, Saito Y, Harada M, Kamitani S, Kuwahara K, Miyamoto Y, Ishikawa M, Hamanaka I, Kajiyama N, Takahashi N, Nakagawa O, Masuda I, Kishimoto I, Nakao K. Outside-in signalling of fibronectin stimulates cardiomyocyte hypertrophy in cultured neonatal rat ventricular myocytes. J Mol Cell Cardiol. 2000;32:765–776. doi: 10.1006/jmcc.2000.1119. [DOI] [PubMed] [Google Scholar]
- Iijima Y, Laser M, Shiraishi H, Willey C D, Sundaravadivel B, Xu L, McDermott P J, Kuppuswamy D. c-Raf/MEK/ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells. J Biol Chem. 2002;277:23065–23075. doi: 10.1074/jbc.M200328200. [DOI] [PubMed] [Google Scholar]
- Cheng G, Zile M R, Takahashi M, Baicu C F, Bonnema D D, Cabral F, Menick D R, Cooper G., 4th A direct test of the hypothesis that increased microtubule network density contributes to contractile dysfunction of the hypertrophied heart. Am J Physiol Heart Circ Physiol. 2008;294:H2231–H2241. doi: 10.1152/ajpheart.91515.2007. [DOI] [PubMed] [Google Scholar]
- Sahn D J, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- DeGroff C G, Shandas R, Kwon J, Valdes-Cruz L. Accuracy of the Bernoulli equation for estimation of pressure gradient across stenotic Blalock-Taussig shunts: an in vitro and numerical study. Pediatr Cardiol. 2000;21:439–447. doi: 10.1007/s002460010104. [DOI] [PubMed] [Google Scholar]
- Spinale F G, Coker M L, Thomas C V, Walker J D, Mukherjee R, Hebbar L. Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res. 1998;82:482–495. doi: 10.1161/01.res.82.4.482. [DOI] [PubMed] [Google Scholar]
- Kent R L, Mann D L, Urabe Y, Hisano R, Hewett K W, Loughnane M, Cooper G., 4th Contractile function of isolated feline cardiocytes in response to viscous loading. Am J Physiol Heart Circ Physiol. 1989;257:H1717–H1727. doi: 10.1152/ajpheart.1989.257.5.H1717. [DOI] [PubMed] [Google Scholar]
- Sambrano G R, Fraser I, Han H, Ni Y, O'Connell T, Yan Z, Stull J T. Navigating the signalling network in mouse cardiac myocytes. Nature. 2002;420:712–714. doi: 10.1038/nature01306. [DOI] [PubMed] [Google Scholar]
- Kuppuswamy D, Kerr C, Narishige T, Kasi V S, Menick D R, Cooper G. Association of tyrosine-phosphorylated c-Src with the cytoskeleton of hypertrophying myocardium. J Biol Chem. 1997;272:4500–4508. doi: 10.1074/jbc.272.7.4500. [DOI] [PubMed] [Google Scholar]
- Willey C D, Palanisamy A P, Johnston R K, Mani S K, Shiraishi H, Tuxworth W J, Zile M R, Balasubramanian S, Kuppuswamy D. STAT3 activation in pressure-overloaded feline myocardium: role for integrins and the tyrosine kinase BMX. Int J Biol Sci. 2008;4:184–199. doi: 10.7150/ijbs.4.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxworth W J, Jr, Saghir A N, Spruill L S, Menick D R, McDermott P J. Regulation of protein synthesis by eIF4E phosphorylation in adult cardiocytes: the consequence of secondary structure in the 5′-untranslated region of mRNA. Biochem J. 2004;378:73–82. doi: 10.1042/BJ20031027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- Birks E J, Latif N, Enesa K, Folkvang T, Luong le A, Sarathchandra P, Khan M, Ovaa H, Terracciano C M, Barton P J, Yacoub M H, Evans P C. Elevated p53 expression is associated with dysregulation of the ubiquitin-proteasome system in dilated cardiomyopathy. Cardiovasc Res. 2008;79:472–480. doi: 10.1093/cvr/cvn083. [DOI] [PubMed] [Google Scholar]
- Lindsey M L, Goshorn D K, Comte-Walters S, Hendrick J W, Hapke E, Zile M R, Schey K. A multidimensional proteomic approach to identify hypertrophy-associated proteins. Proteomics. 2006;6:2225–2235. doi: 10.1002/pmic.200500013. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I κB alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- Traenckner E B, Wilk S, Baeuerle P A. A proteasome inhibitor prevents activation of NF-κB and stabilizes a newly phosphorylated form of I κB-α that is still bound to NF-κB. EMBO J. 1994;13:5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatena M, Almeida M, Chaisson M L, Fausto N, Nicosia R F, Giachelli C M. NF-κB mediates αvβ3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courter D L, Lomas L, Scatena M, Giachelli C M. Src kinase activity is required for integrin αVβ3-mediated activation of nuclear factor-κB. J Biol Chem. 2005;280:12145–12151. doi: 10.1074/jbc.M412555200. [DOI] [PubMed] [Google Scholar]
- Ren J, Avery J, Zhao H, Schneider J G, Ross F P, Muslin A J. β3 integrin deficiency promotes cardiac hypertrophy and inflammation. J Mol Cell Cardiol. 2007;42:367–377. doi: 10.1016/j.yjmcc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Acharyya S, Ladner K J, Nelsen L L, Damrauer J, Reiser P J, Swoap S, Guttridge D C. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–378. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H H, Willis M S, Lockyer P, Miller N, McDonough H, Glass D J, Patterson C. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J Clin Invest. 2007;117:3211–3223. doi: 10.1172/JCI31757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemler M S, Bies R D, Frid M G, Sastravaha A, Zisman L S, Bohlmeyer T, Gerdes A M, Reeves J T, Stenmark K R. Myocyte cytoskeletal disorganization and right heart failure in hypoxia-induced neonatal pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2000;279:H1365–H1376. doi: 10.1152/ajpheart.2000.279.3.H1365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.