Abstract
Research into the metabolic phenotype of autism has been relatively unexplored despite the fact that metabolic abnormalities have been implicated in the pathophysiology of several other neurobehavioral disorders. Plasma biomarkers of oxidative stress have been reported in autistic children; however, intracellular redox status has not yet been evaluated. Lymphoblastoid cells (LCLs) derived from autistic children and unaffected controls were used to assess relative concentrations of reduced glutathione (GSH) and oxidized disulfide glutathione (GSSG) in cell extracts and isolated mitochondria as a measure of intracellular redox capacity. The results indicated that the GSH/GSSG redox ratio was decreased and percentage oxidized glutathione increased in both cytosol and mitochondria in the autism LCLs. Exposure to oxidative stress via the sulfhydryl reagent thimerosal resulted in a greater decrease in the GSH/GSSG ratio and increase in free radical generation in autism compared to control cells. Acute exposure to physiological levels of nitric oxide decreased mitochondrial membrane potential to a greater extent in the autism LCLs, although GSH/GSSG and ATP concentrations were similarly decreased in both cell lines. These results suggest that the autism LCLs exhibit a reduced glutathione reserve capacity in both cytosol and mitochondria that may compromise antioxidant defense and detoxification capacity under prooxidant conditions.—James, S. J., Rose, S., Melnyk, S., Jernigan, S., Blossom, S., Pavliv, O., Gaylor, D. W. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism.
Keywords: autistic disorder, oxidative stress, nitric oxide
Autism is a behaviorally defined neurodevelopmental disorder characterized by impairments in social interaction and communication skills and by hyperfocused interests and compulsive behaviors. Autism is usually diagnosed before 4 yr of age and is estimated to affect 1 in 150 children in the United States, with a 4:1 male to female gender bias (1). Although multiple interacting genetic and environmental factors are thought to influence individual vulnerability to autism, none have been reproducibly identified in more than a fraction of cases. In addition to complex gene-environment interactions, the heterogeneous presentation of behavioral symptoms within the spectrum of autistic disorders suggests a variable and multifactorial pathogenesis.
Several lines of evidence suggest that underlying oxidative stress and glutathione depletion contribute to pathophysiology of several neurobehavioral disorders, including schizophrenia (2, 3), bipolar disorder (4, 5), Parkinson’s disease (6, 7), Alzheimer’s disease (8, 9), and autism. Children with autism have been shown to exhibit evidence of lipid peroxidation (10, 11), reduced antioxidant activity (10, 12, 13), elevated nitric oxide levels (14, 15), and accumulation of advanced glycation end products (AGEs) and the proinflammatory AGE receptor ligand S100A9 (16). The presence of redox imbalance and chronic oxidative stress in autism is further supported by evidence of microglial inflammation (17) and decreased glutathione-mediated redox status (18, 19). Although provocative, it is not clear whether these measures of oxidative stress are present during early development and contribute to pathogenesis of autism, or whether they are a secondary manifestation of the disorder.
Oxidative stress is traditionally defined as an imbalance between oxidant generation and antioxidant defense mechanisms that leads to macromolecular damage and dysfunction. More recently, the definition has expanded to include more subtle perturbations in redox signaling mechanisms that control and regulate a wide variety of cellular functions, including enzyme activation/inhibition (20, 21), membrane signal transduction (22, 23), transcription factor binding/gene expression (24, 25), proliferation/apoptosis (26,27,28), and precursor cell ontogeny (29, 30). The ratio of reduced glutathione (GSH) to the oxidized disulfide form of glutathione (GSSG) is considered a reproducible indicator of systemic redox status that can be used to clinically assess and treat individuals who may at risk of oxidative stress-related pathology (31, 32). Glutathione is present in millimolar concentrations in eukaryotic cells and is pivotal for the maintenance of intracellular redox homeostasis and defense against oxidative damage.
Mitochondria contain ∼10–15% of total cellular GSH, which must be imported from the cytosol because mitochondria lack the enzymes for GSH synthesis. A continuous low-level generation of superoxide (∼1%) accompanies inner membrane electron transfer for energy production, and the mitochondria are dependent on glutathione status to maintain redox balance and limit macromolecular oxidative damage. When chronic or excessive, oxidative stress can overwhelm glutathione-mediated antioxidant defense mechanisms and promote a fragile redox homeostasis that has a lower threshold of toxicity for prooxidant exposures. Autism is rarely associated with classic mitochondrial disease, although several case reports of comorbid autism have been published (33,34,35,36,37,38). There is indirect evidence linking dysfunctional energy metabolism with autism, including reports of elevated serum lactate (39,40,41,42,43) carnitine deficiency (44), and oxidized mitochondrial proteins in post mortem brain (16).
To further investigate biomarkers of oxidative stress and mitochondrial dysfunction in autism, we utilized lymphoblastoid cells (LCLs) derived from autistic individuals and unaffected controls to assess intracellular and mitochondrial redox status, free radical generation, mitochondrial membrane potential, and ATP levels. Because some cases of autism may require an environmental trigger to expose a genetic susceptibility, a secondary goal was to evaluate these endpoints after the addition of exogenous oxidative or nitrosative stress.
MATERIALS AND METHODS
Materials
Culture flasks, plates, and disposable pipettes were obtained from Corning Life Sciences (Lowell, MA, USA). RPMI 1640 culture medium, penicillin/streptomycin, Dulbecco’s phosphate buffered saline, and cell dissociation buffer were purchased from Life Technologies (Carlsbad, CA, USA). Fetal bovine serum was purchased from HyClone Inc (Logan, UT, USA). DCF (6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, diacetoxymethyl ester) was obtained from Molecular Probes (Carlsbad, CA, USA). Thimerosal was obtained from Sigma-Aldrich (St. Louis, MO, USA). SNAP (S-nitroso-N-acetylpenicillamine) was obtained from Alexis Biochemicals (San Diego, CA, USA). JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide) was obtained from BD Biosciences (San Jose, CA, USA). All other chemicals were obtained from Sigma-Aldrich.
Cell culture
Lymphoblastoid cell lines derived from children with autism were purchased from Autism Genetic Resource Exchange (AGRE; Los Angeles, CA, USA). The case cell lines were derived from white males diagnosed with autistic disorder (not Asperger’s syndrome or PDD-NOS) chosen from pedigrees with at least 1 affected male sibling (AGRE ID numbers AU-1280302, -1344302, -1215301, -008404, -1267302, -038804, -0939303, -1348303, -1165302, -1393306; mean age 7.8±3.1 yr). Control cell lines were purchased from Coriell Cell Repository (Camden, NJ, USA) and were derived from apparently healthy white male donors with no documented behavioral or neurological disorder (Coriell ID numbers GM-17508, -16119, -16118, -16113, -14643, 05048, -14907, -14648, -14926, -14782; mean age 27.7±9.1). The cell lines were cyropreserved at −80°C and cultured in RPMI 1640 medium with 10% FBS and 1% penicillin/streptomycin in a humidified 5% CO2 incubator at 37°C.
Cell extraction and HPLC quantification of intracellular glutathione redox status
Approximately 2 × 106 viable cells were lysed by 3 s sonication in 100 μl ice-cold PBS followed by the addition of 100 μl ice-cold 10% meta-phosphoric acid to precipitate proteins. The solution was mixed well, incubated for 30 min on ice, and centrifuged for 15 min at 18,000 g at 4°C. The methodological details for HPLC elution and electrochemical detection have been described previously (45, 46). Briefly, free reduced glutathione (fGSH) and oxidized glutathione (GSSG) were eluted using a Shimadzu solvent delivery system (ESA model 580; ESA, Chelmsford, MA, USA) and a reverse-phase C18 column (3 μm, 4.6×150 mm; MCM, Tokyo, Japan). A 20 μl aliquot of cell extract was directly injected onto the column using a Beckman Autosampler (model 507E; Beckman Instruments, Fullerton, CA, USA). The metabolites were quantified using a model 5200A Coulochem II and CoulArray electrochemical detection system (ESA) equipped with a dual analytical cell (model 5010), a 4-channel analytical cell (model 6210), and a guard cell (model 5020). Glutathione concentrations were calculated from peak areas of standard calibration curves using HPLC software. Results are expressed as nanomoles per milligram of protein using the BCA Protein Assay Kit (Pierce, Rockford, IL, USA).
Mitochondria isolation
Mitochondria were isolated from ∼20 × 106 cells using the Qproteome Mitochondrial Isolation Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. Briefly, cells were washed in ice-cold PBS and incubated in lysis buffer with protease inhibitor (supplied by the kit) for 10 min on an orbital shaker at 4°C. Samples were centrifuged for 10 min at 1000 g at 4°C, and the pellet was resuspended in a disruption buffer with protease inhibitor (supplied by the kit). Cells were disrupted on ice by 10 rounds of aspiration and ejection through a 21-gauge needle. The lysate was centrifuged for 10 min at 1000 g at 4°C, and the resulting supernatant was centrifuged at 6000 g at 4°C to pellet mitochondria. Mitochondria were washed with ice-cold storage buffer (supplied by the kit) and centrifuged for 20 min at 6000 g at 4°. Pellets were snap-frozen on dry ice and stored at −80°C until HPLC determination of glutathione redox status.
Exposure to oxidative and nitrosative stress
Thimerosal (49% ethyl mercury) is a potent sulfhydryl reagent that is well known to induce oxidative stress by binding to and depleting intracellular-free glutathione (47). Each cell line was cultured in 24-well plates at 1 × 106 cells/ml in 2 ml Ca-Mg-free PBS and exposed to 5 replicates of thimerosal at final concentrations of 0, 0.156, 0.312, 0.625, 1.25, and 2.5 μM. After a 3-h incubation in the 37°C CO2 incubator, cells were harvested with the cell dissociation buffer, washed in 10 ml ice-cold PBS, transferred to 1.5-ml tubes, and centrifuged at 250 g at 4°C for 10 min. The pellets were snap-frozen on dry ice and stored at −80°C until HPLC analysis. Because excessive nitric oxide exposure can deplete intracellular glutathione (48, 49), an additional experiment evaluated GSH redox response to acute nitric oxide exposure. SNAP is a nitrothiol derivative that generates nitric oxide under physiological conditions and was used to induce nitrosative stress. In a 12-well plate, 1 × 106 cells/well in 4 ml PBS were incubated 30 min in 37°C incubator with or without 1 mM SNAP. Cells were harvested for HPLC analysis as described for thimerosol exposure.
Intracellular reactive oxygen species (ROS) detection
DCF is a membrane-permeable ROS-sensitive fluorescent probe that does not fluoresce until it is oxidized by intracellular free radicals. The intensity of DCF fluorescence is directly proportional to the level of free radical oxidation. Each week, 1 autistic and 1 control cell line were plated at 5 × 105 cells/ml in T25 flasks and cultured for 2 d in phenol red-free RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. The cells were harvested, washed in Ca-Mg-free PBS, and transferred to a 12-well plate at 5 × 105 cells/ml. The cells were then loaded with 1 μM DCF for 30 min in the 37°C CO2 incubator. The cells were harvested with cell dissociation buffer and washed once in Ca-Mg-free PBS. Each cell line was resuspended in Ca-Mg-free PBS at 5 × 104 cells/100 μl at thimerosal concentrations described above and plated in replicates of 5 in a 96-well plate. After 15 min in the incubator, the plate was placed in a Gemini XPS Microplate Spectrofluorometer (Molecular Devices, Sunnyvale, CA, USA), and the increase in fluorescence intensity was monitored at 37°C for 4 h at an excitation wavelength of 490 nm and an emission wavelength of 530 nm.
Mitochondrial membrane potential
Pairs of autistic and control LCLs were washed with PBS, and 1 × 106 cells/well in a total volume of 1.5 ml were plated into 24-well plates and incubated for 30 min with or without 0.5–1.0 mM SNAP in 0.5M HCl. Cells were harvested and incubated with the mitochondria-specific fluorescent dye JC-1 for 15 min at 37°C. JC-1 is a lipophilic cationic dye that accumulates in mitochondria in a membrane potential (Δψm) -dependent manner. In cells with high mitochondrial membrane potential, JC-1 selectively enters the mitochondria, where it forms aggregates with a high red/green (FL2/FL1) fluorescence intensity. Membrane depolarization can be quantified as a reversible decrease in the percentage red/green fluorescence, which can progress to green monomers as the cells become apoptotic. Before analysis by flow cytometry, cells were washed twice in assay buffer. JC-1-dependent fluorescence changes were recorded using a Partec Cy-Flow flow cytometer (Görlitz, Germany) using 488-nm excitation wavelength with 530/30-nm (FL1, green) and 585/42-nm (FL2, red) emission filters. For each analysis, the fluorescence properties of 10,000 cells were collected, and the respective gates were defined based on baseline red/green fluorescence without SNAP for each sample. The data were analyzed using the FCS Express software (De Novo Software, Los Angeles, CA, USA).
ATP measurement
1 × 106 viable cells were resuspended in 500 μl PBS with or without 1 mM SNAP and incubated in the 37°C CO2 incubator for 30 min. ATP was extracted by adding 4.5 ml boiling 100 mM Tris-HCl and 4 mM EDTA, pH 7.75, and the sample was boiled in a 100°C water bath for 3 min. Samples were centrifuged at 1000 g for 60 s, and the supernatant was placed on ice until ATP measurement. The quantity of ATP was measured in a luminometer (LMAX II luminometer, Molecular Devices) using the ATP Bioluminescence Assay Kit CLS II (Roche Diagnostics, Mannheim, Germany) as directed by the manufacturer. A standard curve of ATP concentration was obtained by serial dilutions of a 2 μM ATP solution (raw data in relative light units). ATP concentration was expressed as nanomoles per 1 × 106 cells, calculated from a log-log plot of the standard curve.
RESULTS
Glutathione redox status in whole-cell extracts and mitochondria
Table 1 presents the relative concentrations of fGSH, GSSG, the percentage oxidized glutathione, and the glutathione redox ratio in whole-cell extracts and in isolated mitochondria from control and autistic LCLs. The percentage oxidized glutathione is expressed in glutathione equivalents as 2GSSG/(fGSH+2GSSG). Relative to control cells, cell extracts derived from autistic individuals showed a significant decrease in intracellular fGSH concentration associated with a significant increase in GSSG. Calculated percentage oxidized glutathione equivalents was increased (P=0.003), and the GSH/GSSG redox ratio was decreased to ∼60% that of the control cell ratio (P<0.001). In mitochondria isolated from the autism LCLs, GSH levels were also lower (P<0.001) and GSSG levels higher (P=0.059), resulting in a fGSH/GSSG redox ratio that was ∼50% that of control mitochondria (11.6 vs. 5.06; P<0.001). The percentage oxidized glutathione was almost 2-fold higher in mitochondria from autism cells compared to control (29.1 vs. 15.8; P<0.001). Comparing whole-cell extracts with mitochondria, fGSH levels in the mitochondria were ∼10% that of the whole-cell extracts in both cell lines, whereas the cellular and mitochondrial GSSG concentrations were approximately the same in both cell lines. In control cell extracts, the fGSH/GSSG redox ratio was 99.4, confirming previous observations that GSSG content was ∼1% that of the fGSH concentration (50). In contrast, the fGSH/GSSG ratio in the control mitochondria was only 11.6 and reflects the 10-fold lower GSH levels in mitochondria relative to the whole-cell extracts.
TABLE 1.
Intracellular glutathione redox status
| Group | fGSH (nmol/mg protein) | GSSG (nmol/mg protein) | Oxidized GSH (%) | fGSH/GSSG |
|---|---|---|---|---|
| Whole-cell extract | ||||
| Control | 26.48 ± 3.5 | 0.287 ± 0.07 | 2.2 ± 0.7 | 99.14 ± 33.5 |
| Autism | 21.72 ± 4.3 | 0.356 ± 0.06 | 3.2 ± 0.6 | 61.81 ± 10.6 |
| P value | 0.021 | <0.001 | 0.003 | <0.001 |
| Mitochondria | ||||
| Control | 2.64 ± 0.7 | 0.258 ± 0.12 | 15.8 ± 4.6 | 11.63 ± 3.9 |
| Autism | 1.75 ± 0.3 | 0.366 ± 0.11 | 29.1 ± 4.7 | 5.06 ± 1.3 |
| P value | 0.001 | 0.059 | <0.001 | <0.001 |
Percentage oxidized GSH equivalents was calculated as 2GSSG/(GSH+2GSSG). n = 10/group.
Intracellular free radical production with thimerosal-induced oxidative stress
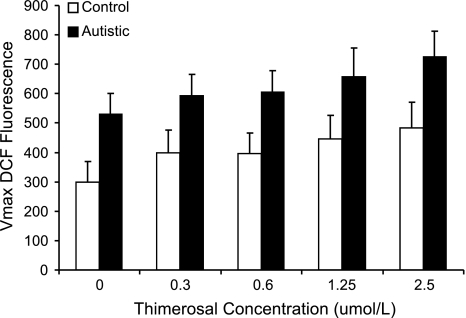
The relative rate of intracellular free radical production in autistic and control cell lines was assessed by measuring the Vmax (relative fluorescence units/s) generated by oxidized DCF 3–4 h after preloading the cells with DCF. Figure 1 presents DCF fluorescence measured in 6 different cell lines/group before (baseline) and after the addition of increasing doses of thimerosal. A significant difference in mean free radical production (DCF fluorescence) between autistic and control cell lines was observed at baseline, and this difference was maintained as thimerosal concentration was increased. However, the magnitude of the fluorescence increase with thimerosal exposure was similar between control and autistic cells and was primarily defined by the difference in fluorescence at baseline without thimerosal.
Figure 1.
Relative rate of intracellular free radical production in autistic and control cell lines is presented as mean ± sd DCF fluorescence measured in 6 different cell lines/group before (baseline) and after the addition of increasing doses of thimerosal.
Alteration in glutathione redox status with thimerosal-induced oxidative stress
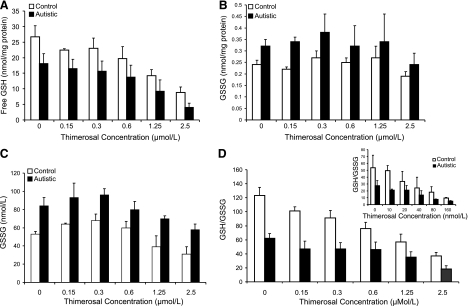
Figure 2A shows the intracellular levels of fGSH/mg protein in LCLs derived from autistic and control individuals at baseline (PBS) and after a 3-h exposure to increasing doses of thimerosal. Intracellular GSH concentrations were significantly lower in the autistic cells at baseline and with each increment of thimerosal (P<0.01). Dose-response trend analysis indicated that fGSH was significantly decreased as a function of thimerosal dose with first-order kinetics in both control (P<0.003) and autism (P<0.001) LCLs. In Fig. 2B, the GSSG concentrations from the same cell extracts were significantly higher in the autistic cells at baseline and after exposure to 0.15 and 0.3 μM thimerosal (P<0.05) but were not statistically different at the higher doses of thimerosal and did not display a dose-response trend. As shown in Fig. 2C, this may be partially explained by the greater GSSG export from the cytosol to the medium by the autism cells. Figure 2D shows the significant reduction in the GSH/GSSG redox ratio in autism compared to control cells at baseline and with increasing doses of thimerosal (P<0.02). The GSH/GSSG redox ratios exhibited a highly significant dose-response trend with increasing thimerosal dose in control and autism LCLs (P<0.001). The inset to Fig. 2D shows that a similar decrease in GSH/GSSG ratio was obtained when the thimerosal concentration was reduced from micromolar to the nanomolar range, and exposure time was increased from 4 h (μM) to 24 h (nM).
Figure 2.
Baseline concentrations without the addition of thimerosal and after a 3-h exposure to increasing concetrations of 0.15–2.5 μM thimerosal. A) Intracellular free glutathione. B) Intracellular GSSG. C) Extracellular (medium) GSSG. D) Intracellular GSH/GSSG redox ratio. Inset: GSH/GSSG ratio after 24 h exposure to 10–160 nM concentrations of thimerosal.
Glutathione redox status with nitrosative stress
To determine whether exposure to nitrosative stress in vitro would alter intracellular glutathione redox status, LCLs from autistic and control individuals were exposed to PBS (baseline) or 1 mM SNAP for 30 min before extraction and HPLC analysis. As shown in Table 2, nitrosative stress significantly decreased fGSH and the GSH/GSSG ratio in the control cells while significantly increasing GSSG and the percentage oxidized glutathione equivalents. In the autistic cell lines, GSSG levels and the percentage oxidized glutathione were increased with SNAP exposure, and the GSH/GSSG redox ratio was decreased (P=0.04). There were significant differences between control and autism LCLs in each of the endpoints at baseline (PBS), but no difference in the magnitude of cell line response to SNAP exposure (P>0.05).
TABLE 2.
Glutathione redox status with nitrosative stress
| Group | fGSH (nmol/mg protein) | GSSG (nmol/mg protein) | Oxidized GSH (%) | fGSH/GSSG |
|---|---|---|---|---|
| Control | ||||
| PBS | 23.5 ± 4.5 | 0.19 ± 0.04 | 1.6 ± 0.5 | 115.0 ± 38 |
| SNAP | 18.3 ± 4.1 | 0.48 ± 0.30 | 4.9 ± 2.0 | 45.5 ± 23 |
| P value | 0.01 | <0.001 | <0.001 | <0.001 |
| Autism | ||||
| PBS | 19.8 ± 4.1* | 0.26 ± 0.08* | 2.7 ± 0.5* | 79.9 ± 16.8* |
| SNAP | 17.4 ± 3.9 | 0.51 ± 0.35 | 5.7 ± 4.0 | 43.7 ± 20.1 |
| P value | 0.20 | 0.04 | 0.03 | 0.04 |
P < 0.04 vs. control.
Mitochondrial membrane potential
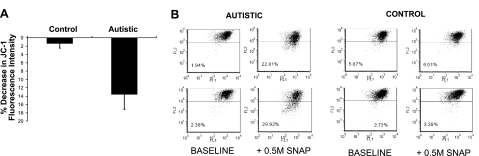
The percentage decrease in JC-1 red/green fluorescence is proportional to the degree of mitochondrial membrane depolarization. As shown in Fig. 3A, the mean shift in percentage JC-1 red/green fluorescence after 30 min SNAP exposure was significantly greater in autism LCLs relative to control. In Fig. 3B, two representative fluorograms from autism and control cell pairs suggest that membrane depolarization with nitrosative stress was partial and did not induce a complete membrane collapse as would be expected with a severe apoptotic insult. In a separate experiment, membrane depolarization with SNAP exposure was found to revert back to baseline levels after 24 h (data not shown), which suggests that the nitrosative stress was transient.
Figure 3.
Percentage decrease in JC-1 red/green fluorescence is proportional to the degree of mitochondrial membrane depolarization. A) Mean ± sd shift in percentage JC-1 red/green fluorescence after 30 min exposure to nitric oxide generated by 0.5 mM SNAP (6/group). B) Percentage decrease in JC-1 red/green fluorescence in two autism and control cell pairs at baseline and after SNAP exposure as measured by flow cytometry.
Cellular ATP concentration
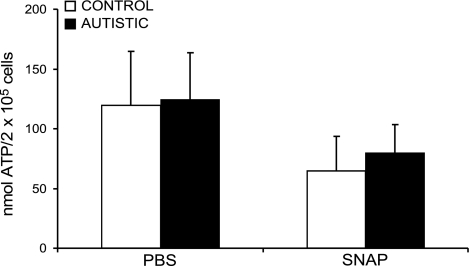
Baseline ATP levels in whole-cell extracts were not significantly different between autistic and control cell lines, as shown in Fig. 4. Exposure to 1 mM SNAP for 30 min resulted in a significant decrease in ATP levels in both control and autistic cell lines (P=0.03 and 0.002, respectively), although there was no difference in the magnitude of ATP decrease between the two cell lines.
Figure 4.
Intracellular ATP concentration (nmol/106 cells) measured after 30-min exposure to PBS or nitric oxide generated by 1.0 mM SNAP.
DISCUSSION
The glutathione redox ratio (GSH/GSSG) and the percentage oxidized glutathione equivalents are dynamic indicators of cytosolic and mitochondrial redox status as well as the severity of oxidative stress. The present findings provide the first evidence that intracellular redox status is compromised in LCLs derived from autistic children and that the redox status of isolated mitochondria is similarly compromised. In isolated mitochondria from the autism LCLs, a significant decrease in GSH and the GSH/GSSG redox ratio was associated with a significant increase in the oxidized GSSG disulfide form of glutathione. Further, the percentage oxidized glutathione equivalents, which take into account alterations in the absolute values of GSH and GSSG, was significantly increased (P<0.001). These data support and extend previous findings in extracellular fluids indicating that biomarkers of oxidative stress may be elevated in a subset of autistic children. Because mitochondria are both the major source and primary target of ROSs, the decrease in mitochondrial glutathione redox potential in LCLs from autistic individuals implies that mitochondrial antioxidant defense mechanisms are insufficient to maintain redox homeostasis.
The dynamics of GSH and GSSG transport across the mitochondrial membrane are the key determinants of mitochondrial redox status. Mitochondria lack the enzymes to synthesize GSH and depend on import from the cytosol, effectively linking GSH concentrations between the two functionally distinct compartments. However, GSSG export as a mechanism to maintain redox control is very different between the two compartments. Cytosolic GSSG is rapidly and efficiently exported out of the cell under conditions of oxidative stress (51). In contrast, mitochondria are unable to export GSSG and must depend on NADPH reducing equivalents and glutathione reductase enzyme activity to regenerate GSH in situ (52). Thus, although mitochondria generate the majority of ROSs within the cell, redox control mechanisms are inherently more limited in mitochondria than in the cytosol. A diminished capacity to counteract endogenous and exogenous ROSs renders mitochondria more vulnerable to oxidative damage/dysfunction, which can lead to a feed-forward cycle of increased ROS generation and an oxidative stress phenotype.
Although mitochondria are classically associated with oxidative phosphorylation and ATP synthesis, it is important to note that these organelles are also essential for normal calcium homeostasis (53), redox signaling cascades (54, 55), and regulation of neurotransmission (56). It is well established that mitochondria are highly concentrated in presynaptic terminals and that loss of redox control can negatively affect the efficiency of neurotransmission and synaptic plasticity (56, 57). Similarly, mitochondria localization and redox signaling at the immunological synapse between lymphocytes and antigen-presenting cells is required for immune activation, and localized redox imbalance can disrupt these signaling pathways (58,59,60). Thus, it is possible that our observations of mitochondrial glutathione redox deficit in the autism lymphoblastoid cell model may have broader implications for neuronal and immune cell synaptic efficiency in vivo. Although the present findings in LCLs clearly need to be confirmed in primary cells from autistic individuals, they provide preliminary evidence of abnormal mitochondrial glutathione redox imbalance in an in vitro cell model.
There is scant evidence that autism is associated with classic mitochondrial disease; however, it is plausible to propose that subtle deficits in mitochondrial function may contribute to the predisposition and pathophysiological expression of autism. If present during critical developmental periods, mitochondrial dysfunction could promote synaptic calcium deregulation and disruption of normal neural network expansion and connectivity that have been postulated to underlie cognitive/attention deficits in autism (61, 62). This possibility is supported by reports of mitochondrial dysfunction and oxidative stress in the early stages of many other neurobehavioral disorders, including schizophrenia, bipolar disorder, major depression, and Alzheimer’s disease (63,64,65).
To evaluate the effect of acute prooxidant stress on redox homeostasis, LCLs were exposed to increasing doses of thimerosal (ethyl mercury thiosalicylate), an established sulfhydryl reagent that rapidly binds to and depletes intracellular glutathione (47). At baseline, in the absence of added thimerosal, the constitutive rate of free radical production (DCF fluorescence) was significantly higher in the autism cells and associated with an increase in GSSG, a decrease in GSH, and the GSH/GSSG ratio compared to control. With the addition of thimerosal, GSH and the redox ratio were significantly decreased as a function of dose with first-order kinetics in both control and autism cell lines. The effect of prooxidant thimerosal exposure on each of these endpoints was consistently greater in the autism LCLs compared to control with both acute (4 h/mM thimerosal) and chronic (24 h/nM thimerosal) exposures. Superimposed on a background of increased free radical generation, it would be expected that the autism cells would reach a toxic threshold sooner and at a lower dose compared to the control cells. Taken together, these data indicate that intracellular glutathione redox buffering capacity is intrinsically lower in the autism cell lines and is more severely compromised with thimerosal exposure.
Thimerosal was a commonly used antimicrobial preservative in vaccines and pharmaceuticals for many decades. Questions about the cumulative dose toxicity with multiple infant vaccines in the 1990s resulted in an FDA mandate for its removal in 2001 from all infant vaccines except for the influenza vaccine. Although experimental evidence for the neurotoxicity and immunotoxicity of thimerosal is unequivocal (47, 66, 67), the potential contribution of thimerosal to the increased prevalence of autism in the 1990s is a complex issue, and quantitative ascertainment of incidence from retrospective studies remains controversial. Although cell models provide insights into mechanism, the extrapolation of thimerosal dose/response characteristics from artificial, albeit controlled, cell culture conditions to the complexities of the in vivo cell milieu is tenuous at best. Nonetheless, based on previous experimental evidence and the results reported here, it is plausible to hypothesize that exposures to prooxidant environmental toxins, including thimerosal, would have the greatest effect on individuals with a preexisting fragile redox homeostasis or depleted glutathione reserves due to concurrent infection, or who are simultaneously exposed to other prooxidant contaminants that in combination can reach a toxic threshold (68). These potentially vulnerable subpopulations need to be identified and evaluated independently because large population epidemiologic studies do not have the sensitivity to detect minor high-risk subpopulations.
In a second series of experiments, nitrosative stress was induced by in vitro exposure to the nitric oxide generator SNAP. Resting levels of NO in most tissues are thought to be in the low nanomolar range, with peak tissue values reaching low micromolar range (1–4 μM) with ischemic stimulation of nitric oxide synthase (55, 69). Other studies have shown that exposure to 1 M SNAP results in peak NO concentration of 0.54 μM in 20 min (70). Thus, in our experiments the 30-min exposure to 0.5–1.0 mM SNAP was within physiological limits of acute NO release in vivo. Given our finding of an elevated spontaneous free radical generation in the autism LCLs, it is likely that SNAP-generated NO reacted with mitochondrial superoxide to form peroxynitrate, a highly reactive and damaging free radical that is detoxified by glutathione (71). Acute NO exposure induced a decrease in free glutathione concentrations and an increase in oxidized glutathione in both autism and control LCLs. Similarly, NO exposure decreased intracellular ATP to comparable levels in both autism and control LCLs, which suggests that the adaptive response to acute nitrosative stress is intact in the autism LCLs.
In the mitochondria, NO is generated from mitochondrial nitric oxide synthase (mNOS) and is an important regulator of mitochondrial energy metabolism. Previous studies have shown that NO induces a reversible and concentration dependent inhibition of complex I (NADH dehydrogenase) and complex IV (cytochrome c oxidase) in response to acute oxidative stress (72, 73). This adaptive response arrests electron transfer to prevent additional ROS generation and oxidative damage. Although reduced, sufficient levels of ATP are maintained in most cell types because NO-mediated down-regulation of aerobic metabolism triggers a concomitant up-regulation of anaerobic glycolysis (74). Notably, astrocytes possess this adaptive protective mechanism, but neurons do not (75). Robust compensatory glycolytic capacity in both autism and control LCLs may explain why viability was not affected by acute NO exposure and why ATP levels in both cell lines were similarly affected by SNAP exposure. Although not measured in the present study, chronic or severe nitrosative stress can promote irreversible respiratory arrest, glutathione depletion, oxidative damage, and cell death (76, 77).
Nitric oxide is well known to disrupt mitochondrial respiration and membrane potential in both neurons and peripheral lymphocytes and may be a common response to inflammation in both cell types (70, 78). In neurons, acute subtoxic doses of NO have been shown to induce a rapid reversible depolarization of mitochondrial membrane potential associated with calcium dysregulation and partial ATP depletion (70, 79). These changes in mitochondrial membrane potential and energy metabolism with acute NO exposure have been attributed to reversible inhibition of cytochrome c by nitrosylation. In cardiomyocytes, reversible changes in mitochondrial membrane potential are initiated by decreases in GSH/GSSG redox status (80). These observations in other cell types may offer insights into the increased sensitivity of the autism LCLs to acute mitochondrial membrane depolarization compared to control LCLs.
A potential role of subclinical mitochondrial dysfunction and altered redox homeostasis in a subset of children with autism has been previously proposed (40, 81, 82). Within the limitations of an in vitro cell model, the baseline differences in intracellular and mitochondrial glutathione redox status in autism and control LCLs cultured under identical conditions would support this possibility. Although the control cell donors were young adults, it is highly unlikely that the observed decrease in glutathione redox status and mitochondrial function could have been due to a relative age effect because these parameters are well established to decrease with age (84,85,86,87). Because reduced levels of glutathione and biomarkers of oxidative stress have been implicated in pathophysiology of several other neurobehavioral disorders (3, 4, 83), further investigation of redox status and metabolism in primary cells and other experimental models of autism may lead to better understanding of mitochondrial gene-environment vulnerabilities associated with this complex disorder.
Acknowledgments
We thank the many autism families that participated in the Autism Genetic Research Exchange program. This research was supported, in part, with funding from the National Institute of Child Health and Human Development (RO1 HD051873) to S.J.J., and by grants from Safeminds, Inc., and the Arkansas Biosciences Institute (S.J.J.).
References
- Centers for Disease Control and Prevention Prevalence of autism spectrum disorders: autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveill Summ. 2007;56:12–28. [PubMed] [Google Scholar]
- Yao J K, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, Deppen P, Preisig M, Ruiz V, Steullet P, Tosic M, Werge T, Cuenod M, Do K Q. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104:16621–16626. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreazza A C, Kauer-Sant'anna M, Frey B N, Bond D J, Kapczinski F, Young L T, Yatham L N. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008;111:135–144. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov D L, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Bush A I. N-acetyl cysteine for depressive symptoms in bipolar disorder: a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Serra J A, Dominguez R O, de Lustig E S, Guareschi E M, Famulari A L, Bartolome E L, Marschoff E R. Parkinson’s disease is associated with oxidative stress: comparison of peripheral antioxidant profiles in living Parkinson’s, Alzheimer’s and vascular dementia patients. J Neural Transm. 2001;108:1135–1148. doi: 10.1007/s007020170003. [DOI] [PubMed] [Google Scholar]
- Bains J S, Shaw C A. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Rev. 1997;25:335–358. doi: 10.1016/s0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Cecchi C, Latorraca S, Sorbi S, Iantomasi T, Favilli F, Vincenzini M T, Liguri G. Gluthatione level is altered in lymphoblasts from patients with familial Alzheimer’s disease. Neurosci Lett. 1999;275:152–154. doi: 10.1016/s0304-3940(99)00751-x. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Chauhan V, Brown W T, Cohen I. Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin: the antioxidant proteins. Life Sci. 2004;75:2539–2549. doi: 10.1016/j.lfs.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Ming X, Stein T P, Brimacombe M, Johnson W G, Lambert G H, Wagner G C. Increased excretion of a lipid peroxidation biomarker in autism. Prostaglandins Leukot Essent Fatty Acids. 2005;73:379–384. doi: 10.1016/j.plefa.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Zoroglu S S, Armutcu F, Ozen S, Gurel A, Sivasli E, Yetkin O, Meram I. Increased oxidative stress and altered activities of erythrocyte free radical scavenging enzymes in autism. Eur Arch Psychiatry Clin Neurosci. 2004;254:143–147. doi: 10.1007/s00406-004-0456-7. [DOI] [PubMed] [Google Scholar]
- Yorbik O, Sayal A, Akay C, Akbiyik D I, Sohmen T. Investigation of antioxidant enzymes in children with autistic disorder. Prostaglandins Leukot Essent Fatty Acids. 2002;67:341–343. doi: 10.1054/plef.2002.0439. [DOI] [PubMed] [Google Scholar]
- Sogut S, Zoroglu S S, Ozyurt H, Ramazan Y H, Ozugurlu F, Sivasli E, Yetkin O, Yanik M, Tutkun H, Savas H A, Tarakcioglu M, Akyol O. Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clin Chim Acta. 2003;331:111–117. doi: 10.1016/s0009-8981(03)00119-0. [DOI] [PubMed] [Google Scholar]
- Zoroglu S S, Yurekli M, Meram I, Sogut S, Tutkun H, Yetkin O, Sivasli E, Savas H A, Yanik M, Herken H, Akyol O. Pathophysiological role of nitric oxide and adrenomedullin in autism. Cell Biochem Funct. 2003;21:55–60. doi: 10.1002/cbf.989. [DOI] [PubMed] [Google Scholar]
- Boso M, Emanuele E, Minoretti P, Arra M, Politi P, Ucelli D N, Barale F. Alterations of circulating endogenous secretory RAGE and S100A9 levels indicating dysfunction of the AGE-RAGE axis in autism. Neurosci Lett. 2006;410:169–173. doi: 10.1016/j.neulet.2006.08.092. [DOI] [PubMed] [Google Scholar]
- Pardo C A, Vargas D L, Zimmerman A W. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17:485–495. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- James S J, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor D W, Neubrander J A. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- James S J, Melnyk S, Jernigan S, Cleves M A, Halsted C H, Wong D H, Cutler P, Bock K, Boris M, Bradstreet J J, Baker S M, Gaylor D W. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141:947–956. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Chida A S, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- Paolicchi A, Dominici S, Pieri L, Maellaro E, Pompella A. Glutathione catabolism as a signaling mechanism. Biochem Pharmacol. 2002;64:1027–1035. doi: 10.1016/s0006-2952(02)01173-5. [DOI] [PubMed] [Google Scholar]
- Dickinson D A, Forman H J. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- Fratelli M, Goodwin L O, Orom U A, Lombardi S, Tonelli R, Mengozzi M, Ghezzi P. Gene expression profiling reveals a signaling role of glutathione in redox regulation. Proc Natl Acad Sci U S A. 2005;102:13998–14003. doi: 10.1073/pnas.0504398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol. 2004;68:1255–1267. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Schafer F Q, Buettner G R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Aw T Y. Cellular redox: a modulator of intestinal epithelial cell proliferation. News Physiol Sci. 2003;18:201–204. doi: 10.1152/nips.01448.2003. [DOI] [PubMed] [Google Scholar]
- Hall A G. The role of glutathione in the regulation of apoptosis. Eur J Clin Investig. 1999;29:238–245. doi: 10.1046/j.1365-2362.1999.00447.x. [DOI] [PubMed] [Google Scholar]
- Noble M, Smith J, Power J, Mayer-Proschel M. Redox state as a central modulator of precursor cell function. Ann N Y Acad Sci. 2003;991:251–271. doi: 10.1111/j.1749-6632.2003.tb07481.x. [DOI] [PubMed] [Google Scholar]
- Kwon Y W, Masutani H, Nakamura H, Ishii Y, Yodoi J. Redox regulation of cell growth and cell death. Biol Chem. 2003;384:991–996. doi: 10.1515/BC.2003.111. [DOI] [PubMed] [Google Scholar]
- Jones D P. Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation Res. 2006;9:169–181. doi: 10.1089/rej.2006.9.169. [DOI] [PubMed] [Google Scholar]
- Jones D P. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Graf W D, Marin-Garcia J, Gao H G, Pizzo S, Naviaux R K, Markusic D, Barshop B A, Courchesne E, Haas R H. Autism associated with the mitochondrial DNA G8363A transfer RNA(Lys) mutation. J Child Neurol. 2000;15:357–361. doi: 10.1177/088307380001500601. [DOI] [PubMed] [Google Scholar]
- Fillano J J, Goldenthal M J, Rhodes C H, Marin-Garcia J. Mitochondrial dysfunction in patients with hypotonia, epilepsy, autism, and developmental delay: HEADD syndrome. J Child Neurol. 2002;17:435–439. doi: 10.1177/088307380201700607. [DOI] [PubMed] [Google Scholar]
- Filipek P A, Juranek J, Smith M, Mays L Z, Ramos E R, Bocian M, Masser-Frye D, Laulhere T M, Modahl C, Spence M A, Gargus J J. Mitochondrial dysfunction in autistic patients with 15q inverted duplication. Ann Neurol. 2003;53:801–804. doi: 10.1002/ana.10596. [DOI] [PubMed] [Google Scholar]
- Pons R, Andreu A L, Checcarelli N, Vilá M R, Engelstad K, Sue C M, Shungu D, Haggerty R, De Vivo D C, DiMauro S. Mitochondrial DNA abnormalities and autistic spectrum disorders. J Pediatr. 2004;144:81–85. doi: 10.1016/j.jpeds.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Poling J S, Frye R E, Shoffner J, Zimmerman A W. Developmental regression and mitochondrial dysfunction in a child with autism. J Child Neurol. 2006;21:170–172. doi: 10.2310/7010.2006.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao C Y, Mendell J R. Autistic disorder in 2 children with mitochondrial disorders. J Child Neurol. 2007;22:1121–1123. doi: 10.1177/0883073807306266. [DOI] [PubMed] [Google Scholar]
- Correia C, Coutinho A M, Diogo L, Grazina M, Marques C, Miguel T, Ataide A, Almeida J, Borges L, Oliveira C, Oliveira G, Vicente A M. Brief report: high frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J Autism Dev Disord. 2006;36:1137–1140. doi: 10.1007/s10803-006-0138-6. [DOI] [PubMed] [Google Scholar]
- Oliveira G, Diogo L, Grazina M, Garcia P, Ataide A, Marques C, Miguel T, Borges L, Vicente A M, Oliveira C R. Mitochondrial dysfunction in autism spectrum disorders: a population-based study. Dev Med Child Neurol. 2005;47:185–189. doi: 10.1017/s0012162205000332. [DOI] [PubMed] [Google Scholar]
- Chugani D C, Sundram B S, Behen M, Lee M L, Moore G J. Evidence of altered energy metabolism in autistic children. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:635–641. doi: 10.1016/s0278-5846(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Coleman M, Blass J P. Autism and lactic acidosis. J Autism Dev Disord. 1985;15:1–8. doi: 10.1007/BF01837894. [DOI] [PubMed] [Google Scholar]
- Laszlo A, Horvath E, Eck E, Fekete M. Serum serotonin, lactate and pyruvate levels in infantile autistic children. Clin Chim Acta. 1994;222:205–207. doi: 10.1016/0009-8981(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Filipek P A, Juranek J, Nguyen M T, Cummings C, Gargus J J. Relative carnitine deficiency in autism. J Autism Dev Disord. 2004;34:615–623. doi: 10.1007/s10803-004-5283-1. [DOI] [PubMed] [Google Scholar]
- Melnyk S, Pogribna M, Pogribny I P, Yi P, James S J. Measurement of plasma and intracellular S-adenosylmethionine and S-adenosylhomocysteine utilizing coulometric electrochemical detection: alterations with plasma homocysteine and pyridoxal 5′-phosphate concentrations. Clin Chem. 2000;46:265–272. [PubMed] [Google Scholar]
- Melnyk S, Pogribna M, Pogribny I, Hine R J, James S J. A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J Nutr Biochem. 1999;10:490–497. doi: 10.1016/s0955-2863(99)00033-9. [DOI] [PubMed] [Google Scholar]
- James S J, Slikker W, III, Melnyk S, New E, Pogribna M, Jernigan S. Thimerosal neurotoxicity is associated with glutathione depletion: protection with glutathione precursors. Neurotoxicology. 2005;26:1–8. doi: 10.1016/j.neuro.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Baldelli S, Aquilano K, Rotilio G, Ciriolo M R. Glutathione and copper, zinc superoxide dismutase are modulated by overexpression of neuronal nitric oxide synthase. Int J Biochem Cell Biol. 2008;40:2660–2670. doi: 10.1016/j.biocel.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Berendji D, Kolb-Bachofen V, Meyer K L, Kröncke K D. Influence of nitric oxide on the intracellular reduced glutathione pool: different cellular capacities and strategies to encounter nitric oxide-mediated stress. Free Radic Biol Med. 1999;27:773–780. doi: 10.1016/s0891-5849(99)00123-9. [DOI] [PubMed] [Google Scholar]
- Lenton K J, Therriault H, Wagner J R. Analysis of glutathione and glutathione disulfide in whole cells and mitochondria by postcolumn derivatization high-performance liquid chromatography with ortho-phthalaldehyde. Anal Biochem. 1999;274:125–130. doi: 10.1006/abio.1999.4258. [DOI] [PubMed] [Google Scholar]
- Eklow L, Thor H, Orrenius S. Formation and efflux of glutathione disulfide studied in isolated rat hepatocytes. FEBS Lett. 1981;127:125–128. doi: 10.1016/0014-5793(81)80357-2. [DOI] [PubMed] [Google Scholar]
- Olafsdottir K, Reed D J. Retention of oxidized glutathione by isolated rat liver mitochondria during hydroperoxide treatment. Biochim Biophys Acta. 1988;964:377–382. doi: 10.1016/0304-4165(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Richter C, Kass G E N. Oxidative stress in mitochondria: its relationship to cellular Ca2+ homeostasis, cell death, proliferation, and differentiation. Chem-Biol Interact. 1991;77:1–23. doi: 10.1016/0009-2797(91)90002-o. [DOI] [PubMed] [Google Scholar]
- Hansen J M, Go Y M, Jones D P. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- Wolin M S, Ahmad M, Gupte S A. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol. 2005;289:L159–L173. doi: 10.1152/ajplung.00060.2005. [DOI] [PubMed] [Google Scholar]
- Keating D J. Mitochondrial dysfunction, oxidative stress, regulation of exocytosis and their relevance to neurodegenerative diseases. J Neurochem. 2008;104:298–305. doi: 10.1111/j.1471-4159.2007.04997.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Quintana A, Schwindling C, Wenning A S, Becherer U, Rettig J, Schwarz E C, Hoth M. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci U S A. 2007;104:14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani G, Colavitti R, Borrello S, Galeotti T. Redox regulation of lymphocyte signaling. IUBMB Life. 2000;49:381–389. doi: 10.1080/152165400410227. [DOI] [PubMed] [Google Scholar]
- Buttke T M, Sandstrom P A. Redox regulation of programmed cell death in lymphocytes. Free Radic Res. 1995;22:389–397. doi: 10.3109/10715769509147548. [DOI] [PubMed] [Google Scholar]
- Beversdorf D Q, Narayanan A, Hillier A, Hughes J D. Network model of decreased context utilization in autism spectrum disorder. J Autism Dev Disord. 2007;37:1040–1048. doi: 10.1007/s10803-006-0242-7. [DOI] [PubMed] [Google Scholar]
- Krey J F, Dolmetsch R E. Molecular mechanisms of autism: a possible role for Ca2+ signaling. Curr Opin Neurobiol. 2007;17:112–119. doi: 10.1016/j.conb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Quiroz J A, Gray N A, Kato T, Manji H K. Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:2551–2565. doi: 10.1038/sj.npp.1301671. [DOI] [PubMed] [Google Scholar]
- Maes M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett. 2008;29:287–291. [PubMed] [Google Scholar]
- Leuner K, Pantel J, Frey C, Schindowski K, Schulz K, Wegat T, Maurer K, Eckert A, Muller W E. Enhanced apoptosis, oxidative stress and mitochondrial dysfunction in lymphocytes as potential biomarkers for Alzheimer’s disease. J Neural Transm Suppl. 2007;72:207–215. doi: 10.1007/978-3-211-73574-9_27. [DOI] [PubMed] [Google Scholar]
- Baskin D S, Ngo H, Didenko V V. Thimerosal induces DNA breaks, caspase-3 activation, membrane damage, and cell death in cultured human neurons and fibroblasts. Toxicol Sci. 2003;74:361–368. doi: 10.1093/toxsci/kfg126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani S, Gollapudi S, Yel L, Chiplunkar S, Gupta S. Biochemical and molecular basis of thimerosal-induced apoptosis in T cells: a major role of mitochondrial pathway. Genes Immun. 2002;3:270–278. doi: 10.1038/sj.gene.6363854. [DOI] [PubMed] [Google Scholar]
- Li Z, Dong T, Proschel C, Noble M. Chemically diverse toxicants converge on Fyn and c-Cbl to disrupt precursor cell function. PLoS Biol. 2007;5:e35. doi: 10.1371/journal.pbio.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S S, Wolin M S. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- Brorson J R, Schumacker P T, Zhang H. Nitric oxide acutely inhibits neuronal energy production. The Committees on Neurobiology and Cell Physiology. J Neurosci. 1999;19:147–158. doi: 10.1523/JNEUROSCI.19-01-00147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z X, Hallur S, Qiu H Z, Peng X X, Li Y B. Induction of endogenous glutathione by the chemoprotective agent, 3H-1,2-dithiole-3-thione, in human neuroblastoma SH-SY5Y cells affords protection against peroxynitrite-induced cytotoxicity. Biochem Biophys Res Commun. 2004;316:1043–1049. doi: 10.1016/j.bbrc.2004.02.156. [DOI] [PubMed] [Google Scholar]
- Husain M, Bourret T J, McCollister B D, Jones-Carson J, Laughlin J, Vazquez-Torres A. Nitric oxide evokes an adaptive response to oxidative stress by arresting respiration. J Biol Chem. 2008;283:7682–7689. doi: 10.1074/jbc.M708845200. [DOI] [PubMed] [Google Scholar]
- Antunes F, Boveris A, Cadenas E. On the biologic role of the reaction of NO with oxidized cytochrome c oxidase. Antioxid Redox Signal. 2007;9:1569–1579. doi: 10.1089/ars.2007.1677. [DOI] [PubMed] [Google Scholar]
- Bolanos J P, Delgado-Esteban M, Herrero-Mendez A, Fernandez-Fernandez S, Almeida A. Regulation of glycolysis and pentose-phosphate pathway by nitric oxide: impact on neuronal survival. Biochim Biophys Acta. 2008;1777:789–793. doi: 10.1016/j.bbabio.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Almeida A, Delgado-Esteban M, Bolanos J P, Medina J M. Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. J Neurochem. 2002;81:207–217. doi: 10.1046/j.1471-4159.2002.00827.x. [DOI] [PubMed] [Google Scholar]
- Moncada S, Bolanos J P. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- Erusalimsky J D, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- Takabayashi A, Kawai Y, Iwata S, Kanai M, Denno R, Kawada K, Obama K, Taki Y. Nitric oxide induces a decrease in the mitochondrial membrane potential of peripheral blood lymphocytes, especially in natural killer cells. Antioxid Redox Signal. 2000;2:673–680. doi: 10.1089/ars.2000.2.4-673. [DOI] [PubMed] [Google Scholar]
- Brorson J R, Sulit R A, Zhang H. Nitric oxide disrupts Ca2+ homeostasis in hippocampal neurons. J Neurochem. 1997;68:95–105. doi: 10.1046/j.1471-4159.1997.68010095.x. [DOI] [PubMed] [Google Scholar]
- Aon M A, Cortassa S, Maack C, O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282:21889–21900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman J R, Kelley R I, Bauman M L, Cohen B H, Murray K F, Mitchell R L, Kern R L, Natowicz M R. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS ONE. 2008;3:e3815. doi: 10.1371/journal.pone.0003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard J. Autism: a mitochondrial disorder? Med Hypotheses. 1998;50:497–500. doi: 10.1016/s0306-9877(98)90270-5. [DOI] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush A I. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Ames B N, Shigenaga M K, Hagen T M. Mitochondrial decay in aging. Biochim Biophys Acta Mol Basis Dis. 1995;1271:165–170. doi: 10.1016/0925-4439(95)00024-x. [DOI] [PubMed] [Google Scholar]
- Lenaz G, Bovina C, Castelluccio C, Fato R, Formiggini G, Genova M L, Marchetti M, Pich M M, Pallotti F, Castelli G P, Biagini G. Mitochondrial complex I defects in aging. Mol Cell Biochem. 1997;174:329–333. [PubMed] [Google Scholar]
- De la Asuncion J G, Millan A, Pla R, Bruseghini L, Esteras A, Pallardo F V, Sastre J, Vina J. Mitochondrial glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. FASEB J. 1996;10:333–338. doi: 10.1096/fasebj.10.2.8641567. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Sohal R S. Pro-oxidant shift in glutathione redox state during aging. Adv Drug Delivery Rev. 2008;60:1545–1552. doi: 10.1016/j.addr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]