Abstract
The cytokine, macrophage migration inhibitory factor (MIF), is encoded in a functionally polymorphic locus and subjects with high-expression MIF alleles are at an increased risk of inflammatory disease. Severe sepsis is the leading cause of death in intensive care units, and the prevailing hypothesis is that an excessive innate response contributes to its pathogenesis. To assess if MIF alleles influence the clinical course of infection, we conducted a case-control study to assess susceptibility and a parallel inception cohort study of community-acquired pneumonia (CAP) to assess risk of severe sepsis and 90-d mortality. Two distinct polymorphisms in the MIF promoter were analyzed: a G/C transition at −173 and a CATT repeat at −794. The frequency of both polymorphisms was similar in the CAP cohort (n=1739) and controls (n=639); however, the 90-d mortality was lower for the high-expression C allele (P=0.003). This association remained significant after adjusting for demographics, comorbid conditions, and disease severity score [hazard ratio=0.64 (0.44–0.91), P=0.01]. The hazard ratio was similar in different geographic subcohorts, and the association remained significant after adjusting for false discovery. These data indicate that polymorphisms associated with higher MIF expression may have a beneficial effect in community-acquired pneumonia.—Yende, S., Angus, D. C., Kong, L., Kellum, J. A., Weissfeld, L., Ferrell, R., Finegold, D., Carter, M., Leng, L., Peng, Z.-Y., Bucala, R. The influence of macrophage migration inhibitory factor gene polymorphisms on outcome from community-acquired pneumonia.
Keywords: cytokines, innate immunity, sepsis, TNF
Macrophage migration inhibitory factor (MIF) is an upstream regulator of the innate immune response with pleiotropic effects (1, 2). In autoimmune diseases, experimental and human clinical studies suggest that higher circulating MIF concentrations are associated with both increased susceptibility and severity of the disease (3). Similarly, in animal models of sepsis, immunoneutralization or genetic deletion of MIF renders mice resistant to lipopolysaccharide (LPS)- and exotoxin-induced shock, and anti-MIF antibodies are protective (4,5,6,7). In humans, high circulating MIF concentrations are associated with severe sepsis, septic shock, and reduced survival in case-control studies (8,9,10,11).
A few studies indicate that MIF has beneficial effects in certain infections. For instance, mif-knockout mice have an impaired ability to clear certain intracellular organisms, such as Salmonella and Leishmania (12,13,14). MIF may also be protective in sepsis-induced immune suppression (15). Two days after inducing peritonitis by a cecal-ligation puncture model in mice, neutralization of endogenous MIF enhanced susceptibility to bacterial superinfection, and treatment with MIF before bacterial superinfection was protective (15). A humanized anti-MIF is in preclinical development and a better understanding of the role of MIF in human infection and sepsis is desirable. Accordingly, we studied the effects of functional genetic variants in the MIF gene on outcome of infection.
The MIF gene (22q11, 1-kbp size) has two common polymorphisms, a G to C transition at position −173 and a tetranucleotide (CATT)n repeat at position −794 (16, 17). The C allele at the −173 G/C position, 7 repeat at the −794 CATT repeat, and the C/7 haplotype are associated with increased MIF expression in reporter assays and are associated with higher circulating MIF concentrations in inflammatory arthritis (17,18,19). In the present study, we examined whether these polymorphisms influenced susceptibility, inflammatory response, and outcomes of community-acquired pneumonia (CAP).
MATERIALS AND METHODS
Design
To assess susceptibility to CAP, we used a case-control design, comparing CAP cases to healthy controls. To assess risk of developing severe sepsis and death, we used a parallel cohort design, comparing the hospitalized CAP patients who did and did not develop the outcomes of interest, both overall and nested within separate geographic regions.
Subjects
CAP patients were recruited as part of the Genetic and Inflammatory Markers of Sepsis (GenIMS) study, a large, multicenter study of subjects presenting to the emergency department (EDs) of 28 academic and community hospitals in 4 regions (southwestern Pennsylvania, Connecticut, southern Michigan, and western Tennessee). Informed consent was obtained from the patient or a proxy. The Institutional Review Boards of the University of Pittsburgh and all participating sites approved the study, and all participants gave written informed consent.
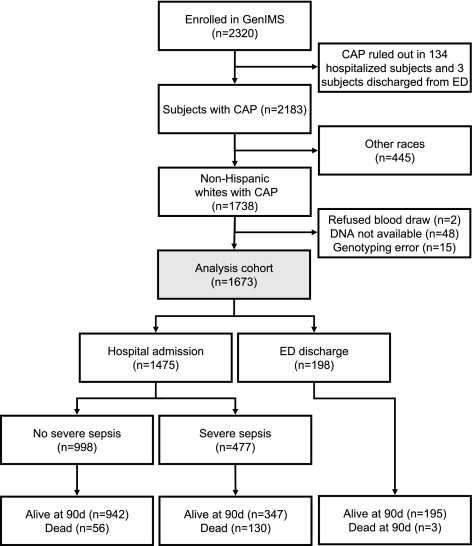
Eligible subjects were 18 yr or older and had a clinical and radiological diagnosis of pneumonia syndrome, as described by Fine et al. (20). Exclusion criteria were transfer from another hospital, discharge from a hospital within the prior 10 d, episode of pneumonia within the past 30 d, chronic mechanical ventilation dependency, cystic fibrosis, active pulmonary tuberculosis, positive HIV antibody titer, alcoholism with evidence of end-organ damage, admission for palliative care, prior enrollment in the study, incarceration, and pregnancy. Of the 2320 subjects enrolled in GenIMS, 137 were excluded because their treating physician subsequently ruled out CAP (Fig. 1). Of the 2183 remaining subjects, we restricted analysis to 1738 non-Hispanic white subjects. Race was based on self-report. We limited our analysis to non-Hispanic whites because the frequency of MIF polymorphisms differs across race (21), and we had an insufficient sample size to explore associations in other racial groups. Healthy controls comprised 639 non-Hispanic white volunteers recruited from the northeastern United States who reported no history of chronic disease or recent hospitalization.
Figure 1.
Flowchart of subjects enrolled in GenIMS. Although the study enrolled 2320 subjects, 647 were excluded from our analysis because community-acquired pneumonia was not confirmed, they did not self-identify as non-Hispanic whites, or genetic material was not available.
Clinical and outcome variables
We gathered baseline and sequential clinical information on CAP subjects by structured subject or proxy interviews, bedside assessment by study nurses, and review of medical records. Prehospitalization comorbid conditions were ascertained using the Charlson comorbidity index (22). This index is based on 19 chronic illnesses, including chronic kidney disease, chronic pulmonary disease, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, connective tissue disease, ulcer disease, liver disease, AIDS, diabetes, and cancer. Severity of illness was assessed using APACHE III and the Pneumonia Severity Index (20, 23). The outcome variables for the cohort design were development of severe sepsis and 90-d mortality. We defined severe sepsis as pneumonia with acute organ dysfunction, following the 2001 International Consensus Criteria (24). Study nurses ascertained deaths in hospital. Post discharge deaths were ascertained by telephone and a National Death Index search (25). The National Death Index-coded causes of death were used to ascertain cause of death. We used blood and sputum cultures to determine microbiological etiology. Lower respiratory tract secretions by bronchoscopy and pleural fluid cultures were obtained in a very small subset and did not yield additional information in our cohort. Strict criteria to define sputum quality and to exclude skin contaminants in blood cultures based on consensus guidelines were used (26). Two reviewers reviewed all microbiology results and independently assigned etiology. Any discrepancy was resolved by consensus and a third reviewer.
Laboratory procedures
For MIF genotyping, we extracted genomic DNA using QIAamp DNA Blood Mini Kits (Qiagen, Crawley, UK). We genotyped the −173 G/C and −794 CATT repeat polymorphisms using previously described techniques with error rates of 1.4 and 0.6% (17, 19). We also genotyped putative functional polymorphisms associated with susceptibility and outcomes of severe sepsis within CD14, Toll-like receptor (TLR)-4, lymphotoxin, tumor necrosis factor (TNF), interleukin (IL)-1α, IL-1β, IL-6, IL-18, IL-10, IL-1 receptor antagonist, and plasminogen-activator inhibitor (PAI)-1 to assess whether the association between the MIF genotypes and outcomes were independent of previously reported associations between genes and sepsis incidence or outcome (Table 1) (27).
TABLE 1.
Additional polymorphisms in innate immune response genes analyzed for this study
| Marker | Chromosome | Position relative to transcription site and nucleotide substitution | Reference sequence (rs no.) |
|---|---|---|---|
| CD14 | 5q31.1 | −159 C/T | 2569190 |
| Toll-like receptor-4 | 9q32 | +896 A/G | 4986791 |
| +11996 C/T | 4986790 | ||
| Lymphotoxin | 6p21.3 | +252 G/A | 909253 |
| Tumor necrosis factor | 6p21.3 | −238 G/A | 361525 |
| −308 G/A | 1800629 | ||
| −376 G/A | 1800750 | ||
| −-857 C/A | 1799724 | ||
| Interleukin-1α | 2q12 | −889 C/T | 1800587 |
| −4845 G/T | 17561 | ||
| Interleukin-1β | 2q12 | −511 C/T | 16944 |
| −3957 C/T | 1143634 | ||
| Interleukin-1 receptor antagonist | 2q14.2 | +89495 A/G | 895495 |
| 11100 C/T | 315952 | ||
| 86 bp repeat | — | ||
| Interleukin-6 | 7p21 | −174 G/C | 13447446 |
| Interleukin-18 | 11q22.2 | −137 G/C | 187238 |
| Interleukin-10 | 1q31 | −819 C/T | 1800871 |
| −1082 G/A | 1800896 | ||
| Plasminogen activator inhibitor-1 | 7q21.3 | 4G/5G | 1799889 |
As markers of the circulating inflammatory response, we measured plasma TNF, IL-6, and IL-10 concentrations daily during the first week using an automated chemiluminescent immunoassay analyzer (IMMULITE; Siemens Inc., New York, NY, USA) (28). Details of sample processing have been described previously (28). We measured plasma MIF concentrations in a subset of 48 subjects (homozygotes for −173 G/C polymorphism matched by age, gender, and comorbidity) on the first day of hospitalization using an ELISA assay (R&D Systems, Minneapolis, MN, USA). To identify subjects who are more likely to have bacterial infections, we also measured plasma procalcitonin concentrations on the first day of hospitalization using a time-resolved amplified cryptate emission assay (BRAHMS, Hennigsdorf, Germany) (29).
DNA sequencing
Because the −173 G/C polymorphism was associated with outcomes, we sequenced the MIF gene to determine whether there were additional polymorphisms with which it was in linkage disequilibrium. We sequenced the MIF gene in 96 randomly chosen subjects from Pennsylvania and Connecticut (32 subjects with the CC, GC, and GG genotypes at the −173 site). We amplified and sequenced the gene (GenBank accession number NM_002415) in two overlapping fragments using the BigDye 3.1 kit (Applied Biosystems, Foster City, CA, USA). We analyzed the sequence using an ABI 3730 DNA sequencer and aligned and curated the sequence using Sequencher V4.6 (GeneCodes, Ann Arbor, MI, USA).
Statistical analyses
We first estimated Hardy-Weinberg equilibrium using exact tests for all polymorphisms. We assessed the association between the MIF polymorphisms and susceptibility and outcomes of CAP. For the −173G/C polymorphism, we also tested for presence of C allele (codominant effect), based on prior associations with higher systemic MIF concentrations (18, 30). We performed univariate analyses using χ2, Fisher’s exact test when necessary, and Armitage’s trend test. We used 2-sample t tests, analysis of variance, and their nonparametric counterparts, when appropriate. We conducted multivariable analyses to adjust for potential confounders, using logistic regression models for susceptibility to severe sepsis and Cox regression models for survival analyses. All analyses were performed assuming significance at P < 0.05 and using SAS 9.1 and SAS genetics (SAS, Cary, NC, USA). We calculated adjusted P values to control for false discovery, as described by Hochberg and Benjamini (31). We constructed haplotypes and used exact P values to assess the association between individual haplotypes and outcomes. For haplotype analysis, we used the PROC HAPLOTYPE procedure in SAS genetics. We assessed effects of population substructure and reliability of the associations by conducting analyses stratified by geographic region. We tested for association between individual genotypes and cytokine concentrations using log-transformed data and mixed models to evaluate concentrations over time (32). Because the clinical and radiological criteria may be inaccurate, we repeated analyses after stratifying by baseline procalcitonin concentration, classifying subjects as having low (<0.1 ng/ml), intermediate (0.1–0.5 ng/ml), and high (>0.5 ng/ml) probability of bacterial pneumonia (29). For 90-d mortality, we had adequate power (β≥0.8) to ascertain relative risks of 1.75 or higher for genetic variants with a codominant effect and a minor allele frequency of 0.05 or higher.
RESULTS
Baseline characteristics
Of the 1738 non-Hispanic white participants with CAP, results for the MIF polymorphisms were available in 1673 (96.5%) participants (Fig. 1; Table 2). One hundred ninety-eight (11.8%) were discharged after treatment in the EDs. Among the remaining 1475 participants, severe sepsis occurred in 32.3%. Sputum, blood, pleural fluid, and lower respiratory tract secretions for culture to determine microbiologic etiology were obtained from 1282 (73.7%) subjects. Although positive cultures were obtained in 269 (20%) subjects, microbiologic etiology, using more stringent American Thoracic Society criteria (26), was determined in 141 (11%) subjects. The 90-d mortality rates for those discharged from EDs, hospitalized subjects, and those with severe sepsis were 1.5, 12.7, and 27.2%, respectively.
TABLE 2.
Clinical and demographic characteristics
| Characteristic | All | ED discharges | Hospital admissions | In-patients with severe sepsis | In-patients without severe sepsis |
|---|---|---|---|---|---|
| N | 1673 | 198 | 1475 | 477 | 998 |
| Age (yr) | 68.6 ± 17.1 | 52.3 ± 19 | 70.7 ± 15.6 | 73.3 ± 15 | 69.5 ± 15.7 |
| Sex (male) (n, %) | 887, 53 | 98, 49.5 | 789, 53.5 | 286, 60 | 503, 50.4 |
| Charlson comorbidity index | |||||
| Mean, median | 1.8 ± 2.2, 1 | 0.6 ± 1.1, 0 | 1.9 ± 2.2, 1 | 2.2 ± 2.3, 1 | 1.8 ± 2.1, 1 |
| Index > 0 (n, %) | 1157, 69.2 | 75, 37.9 | 1082, 73.4 | 360, 75.5 | 722, 72.3 |
| Pneumonia severity index | |||||
| Mean, median | 98.3 ± 39.6, 96 | 55 ± 26.3, 51 | 104.2 ± 37.4, 100 | 123.3 ± 39.9, 118 | 95 ± 32.5, 93 |
| Class (n, %) | |||||
| I, II | 416, 24.9 | 150, 75.8 | 266, 18 | 38, 8 | 228, 22.9 |
| III | 327, 19.6 | 25, 12.6 | 302, 20.5 | 60, 12.6 | 242, 24.3 |
| IV | 599, 35.7 | 21, 10.5 | 578, 39.2 | 190, 39.8 | 388, 38.8 |
| V | 331, 19.8 | 2, 1 | 329, 22.3 | 189, 39.6 | 140, 14 |
| APACHE III scorea | 54.2 ± 19.5 | 30.3 ± 17.7 | 57.4 ± 17.3 | 66 ± 19.3 | 53.3 ± 14.6 |
| Confirmed microbiologic etiology (n, %)b | 141, 11 | — | 141, 11 | 56, 13.4 | 85, 9.8 |
| Gram-positive organisms | 84 ± 59.5 | — | 84 ± 59.5 | 35 ± 62.5 | 49 ± 57.6 |
| Gram-negative organisms | 48 ± 34 | — | 48 ± 34 | 17 ± 30.3 | 31 ± 36.4 |
| Mixed infections | 9 ± 6.5 | — | 9 ± 6.5 | 4 ± 7.2 | 5 ± 7 |
Values are means ± sd or as indicated.
APACHE III score (23) assessed on first hospital day, regardless of whether subject was admitted to ICU.
Blood, sputum, pleural fluid, and lower respiratory tract secretions by bronchoscopy were obtained for microbiologic etiology in 1282 hospitalized subjects.
Susceptibility to community-acquired pneumonia
Genotype frequencies of the −173 G/C and −794 CATT repeat polymorphisms in the healthy controls and CAP cases are shown in Table 3. No deviation from Hardy-Weinberg equilibrium was observed within either group. There were no differences in the genotype frequency distributions between the healthy control subjects and subjects with CAP for either polymorphism. We also did not observe differences in the genotype frequency distributions after stratifying the subjects with CAP by those discharged from the ED and those requiring hospitalization (data not shown).
TABLE 3.
Frequencies of MIF-173G/C, −794CATT repeat polymorphisms, and haplotypes in CAP case and healthy control subjects
| Genotype/haplotype | Cases (n, %) | Controls (n, %) | Comparisons |
|---|---|---|---|
| −173 G/Ca,b,c | χ2 test: genotype, P = 0.86; trend, P = 0.64; allele, P = 0.64 | ||
| CC | 41, 2.5 | 16, 2.5 | |
| GC | 466, 28.4 | 189, 29.6 | |
| GG | 1132, 69.1 | 434, 67.9 | |
| −794 CATT repeatb,c,d | χ2 test: genotype, P = 0.29; trend, P = 0.33; allele, P = 0.32 | ||
| 5,5 | 102, 7.1 | 37, 6.2 | |
| 5,6 | 497, 34.4 | 185, 31.0 | |
| 5,7 | 97, 6.7 | 49, 8.2 | |
| 5,8 | 5, 0.3 | 1, 0.2 | |
| 6,6 | 536, 37.1 | 243, 40.7 | |
| 6,7 | 183, 12.7 | 69, 11.6 | |
| 6,8 | 3, 0.2 | 0, 0 | |
| 7,7 | 20, 1.4 | 13, 2.2 | |
| −173 G/C haplotype/−794 CATT repeatc,e | Overall test: P < 0.001 | ||
| C/6 | 4.9% | 5.0% | P = 0.88 |
| C/7 | 9.5% | 11.8% | P = 0.04 |
| G/5 | 25.7% | 25.1% | P = 0.74 |
| G/6 | 55.9% | 57.1% | P = 0.75 |
MIF-173 G/C distribution in Hardy-Weinberg equilibrium for both case (P=0.24) and control groups (P=0.49).
No difference in frequency of either genotype among CAP requiring hospitalization and CAP discharged from ED.
Results: 1639 subjects with MIF-173 G/C polymorphism; 1443 with −794 CATT repeat polymorphism; haplotypes were constructed for 1432 subjects with both polymorphisms.
MIF (CATT)n repeat distribution in Hardy-Weinberg equilibrium for both case (P=0.26) and control groups (P=0.07).
Haplotypes with prevalence <5% were excluded.
The −173 G/C and −794 CATT repeat polymorphisms were in linkage disequilibrium (P<0.001 for both CAP and control groups). Haplotypes are shown in Table 3. Of the eight possible haplotypes, the C/6, C/7, G/5, and G/6 haplotype frequencies were >5% among control and CAP subjects. The C/7 haplotype was less frequent among subjects with CAP (P = 0.04).
Susceptibility to severe sepsis
Genotype frequencies of MIF polymorphisms among subjects hospitalized with CAP, who did and did not develop severe sepsis, are shown in Table 4. The observed frequency of severe sepsis was lower in those with the presence of C allele at −173G/C polymorphism, but results were not significant [OR=0.8 (0.7–1.1), P=0.14]. Adjusting for potential confounders, including age, sex, comorbid conditions (Charlson index), and severity of illness at presentation, did not affect the findings. We did not detect an increased risk of severe sepsis for the −794 CATT repeat. Haplotype analysis showed that only the C/6 haplotype was associated with lower risk of severe sepsis (P = 0.02) (Table 5).
TABLE 4.
Association of MIF promoter polymorphisms with severe sepsis and 90-d mortality among subjects hospitalized with CAP
| Genotype | Severe sepsis incidence (%) | 90-d mortality (%) |
|---|---|---|
| 173 G/C | ||
| CC | 25.7a | 2.9b |
| GC | 30.2 | 9.3 |
| GG | 33.5 | 14.4 |
| −794 CATT repeat | ||
| 5,5 | 31.1c | 13.3d |
| 5,6 | 29.3 | 10.7 |
| 5,7 | 34.1 | 15.3 |
| 5,8 | 40 | 0 |
| 6,6 | 32.6 | 15.2 |
| 6,7 | 33.5 | 9.6 |
| 6,8 | 50 | 0 |
| 7,7 | 33.3 | 11.1 |
Comparisons by χ2 test.
Genotype, P = 0.34; trend, P = 0.14.
Genotype, P = 0.007; trend, P = 0.002.
Genotype, P = 0.94, P = 0.70; trend, P = 0.68.
Genotype, P = 0.40; trend, P = 0.53.
TABLE 5.
Association of MIF promoter haplotypes with severe sepsis and 90-d mortality among subjects hospitalized with CAP
| −173 G/C/−794 CATT repeat haplotype | Severe sepsis statusa
|
90-d mortalityb
|
||||
|---|---|---|---|---|---|---|
| With (%) | Without (%) | P valuec | Dead at 90 d (%) | Alive at 90 d (%) | P valuec | |
| C/6 | 3 | 5.5 | 0.01 | 2.4 | 5.0 | 0.06 |
| C/7 | 10.7 | 9.0 | 0.16 | 7.9 | 9.7 | 0.26 |
| G/5 | 25.2 | 25.6 | 0.81 | 25.7 | 25.4 | 0.91 |
| G/6 | 58.1 | 55.1 | 0.17 | 61.9 | 55.2 | 0.04 |
Haplotypes with prevalence <5% were excluded.
Overall trait test: P = 0.01.
Overall trait test: P = 0.009.
P value for individual haplotype trait association.
Survival
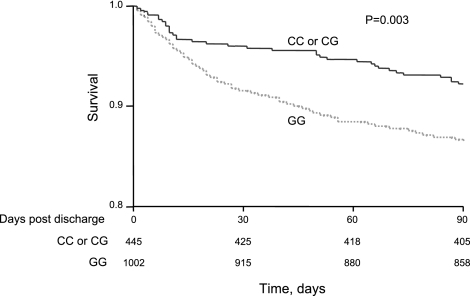
The −173G/C polymorphism was associated with 90-d mortality (P=0.007), but the −794 CATT repeat was not (Table 4). As shown in the Kaplan-Meier survival curves (Fig. 2), the presence of the C allele at the MIF −173 site was associated with higher 90-d survival (P=0.003). The association remained significant in the Cox proportional hazards model that adjusted for age, gender, comorbid conditions (Charlson index), and d 1 APACHE III score [adjusted hazard ratio (HR) with 95% CI=0.64 (0.44–0.91), P=0.01]. The association remained significant after adjusting for false discovery (P=0.04), controlling for putative functional polymorphisms in CD14, TLR-4, lymphotoxin, TNF, IL-1α, IL-1β, IL-6, IL-18, IL-10, IL-1 receptor antagonist, and PAI-1 genes. The associations also remained significant after adjusting for effects of these polymorphisms [OR=0.61 with 95% CI=0.61 (0.41–0.89), P=0.01]. Haplotype analysis showed that the G/6 haplotype was associated with higher 90-d mortality (P=0.04) (Table 5).
Figure 2.
Kaplan–Meier survival curves for MIF −173 genotypes. The CC and CG genotypes were associated with improved survival in subjects with community-acquired pneumonia. Supplemental Fig. 1 shows failure plots for MIF −173 genotypes.
Secondary and sensitivity analyses
Subjects with a MIF −173 C allele were less likely to develop septic shock [OR=0.5 (0.2–0.9), P=0.03], and there was also a trend toward a reduced likelihood of developing acute renal failure [OR=0.8 (0.6–1.005), P=0.054]. There was no difference in susceptibility to other organ failures. The associations between the −173 G/C site and mortality were similar using 30-, 60-, and 90-d time points [adjusted OR: 0.5 (0.3–0.9), P=0.02; 0.5 (0.3–0.8), P=0.002; and 0.6 (0.4–0.9), P=0.01, respectively]. Stratifying by the serum concentration of procalcitonin, which is associated with bacterial pneumonia (29), did not affect the findings with regard to susceptibility and severe sepsis occurrence. However, the lower risk of death appeared to be restricted to those with intermediate and high probability of bacterial infection [adjusted HRs for C allele within low, intermediate, and high procalcitonin groups were 1.4 (0.5–3.9), P=0.5; 0.6 (0.2–0.1.4), P=0.21; and 0.6 (0.3–0.99), P=0.04, respectively]. The association between C allele and lower risk of death was observed in all subjects with a clinical and radiological diagnosis of pneumonia syndrome, regardless whether a microbiologic etiology was determined [adjusted HRs=0.3 (0.1–1.4), P=0.15 and 0.6 (0.4–1.002), P=0.051 in subjects who did and did not have a microbiologic etiology]. The causes of death were similar between those with a C allele and those with the GG genotype (cardiovascular: 23.6 vs. 25.8%; infection: 15.7 vs. 23%; cancer: 23.6 vs. 21.6%; chronic respiratory disease: 21 vs. 12.5%; renal and diabetes: 2.6 vs. 2.8%; and other causes: 13.1 vs. 13.9% for CC and GC genotypes compared to GG genotypes; P=0.79).
Population stratification and reliability
Of the 1475 subjects who were hospitalized, 1030 (69.8%), 401 (27.2%), 33 (2.2%), and 11 (0.8%) subjects were enrolled in Pennsylvania, Connecticut, Tennessee, and Michigan, respectively. The association between the −173 G/C polymorphism and 90-d survival was independent of the enrollment site (P=0.11). The HR were similar when results were stratified by geographically distinct subcohorts, but the association was not significant in subjects recruited from Connecticut due to smaller sample size [HR with 95% CI for Pennsylvania and Connecticut sites were 0.5 (0.3–0.8), P=0.005 and 0.7 (0.4–1.3), P=0.27].
DNA sequencing
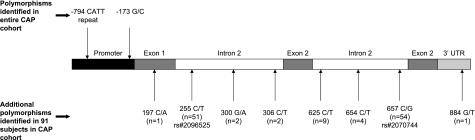
DNA was successfully sequenced in 91 (95%) subjects to identify polymorphisms within the MIF gene in linkage disequilibrium with the −173 G/C polymorphism. We identified 8 additional polymorphisms within the gene (Fig. 3). Only a single polymorphism within a single subject was identified in the coding region. These polymorphisms occurred less frequently than the studied promoter polymorphisms and had low likelihood of being functional because they were found in introns and in the 3′-untranslated region.
Figure 3.
MIF gene-sequencing results identified 8 additional polymorphisms in the MIF gene. Sequencing results were available in 91 of 96 randomly chosen subjects from different geographic regions (32 subjects with the CC, GC, and GG genotypes at the −173 MIF G/C site).
Circulating cytokine concentrations
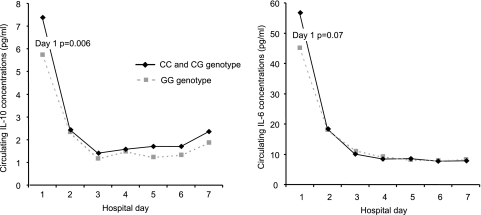
Because the −173 G/C genotype was associated with 90-d mortality, we explored the association of this genotype with circulating cytokine levels in the hospitalized CAP cohort. IL-6 and IL-10 concentrations were higher in subjects with a C allele (P=0.07 and 0.006) on d 1 of hospitalization, but concentrations declined rapidly on d 2, and no differences were seen subsequently (Fig. 4). There were no differences in TNF concentrations on d 1 of hospitalization or over the first week (5.7 vs. 5.6 pg/ml for the CC and GC genotypes vs. GG genotypes, P=0.8, on d 1 of hospitalization). We also ascertained d 1 circulating MIF concentrations in a subset of 48 subjects with CC and GG genotypes at the −173 G/C site but did not detect a difference (17.1 vs. 22.1 ng/ml for CC and GG genotypes, P=0.15).
Figure 4.
Association between the MIF-173 genotypes and IL-6 and IL-10 concentrations during the hospital stay. Subjects with the CC and GC genotypes had higher circulating IL-6 and IL-10 concentrations on hospital day 1, but no differences were detectable subsequently.
DISCUSSION
We found that the third of subjects with a commonly occurring, high-expression variant of the MIF gene had a marked reduction in death following CAP. This association was independent of demographic characteristics, comorbidities, severity of illness, and other genotypes previously associated with outcomes after CAP. These findings remained significant after adjusting for false discovery, and they were unchanged using more stringent definitions of CAP and using 30-, 60-, and 90-d time points for mortality. Our results also were consistent across different geographic regions. The haplotypes previously associated with higher MIF expression also appeared to be associated with decreased susceptibility to CAP and decreased risk of severe sepsis, whereas haplotypes associated with lower MIF expression were associated with higher 90-d mortality. Additional sequencing of the MIF gene failed to identify important variants that might have been missed by our analysis. We conclude that genetic variation in the MIF gene may influence outcome of pneumonia. By the nature of this genetic study, we cannot ascribe causality to any particular MIF allele. However, given the prior association between high-expression MIF alleles and increased MIF production (16, 17, 19, 30, 33), it appears likely that a robust MIF response is protective in CAP.
Our findings must be viewed in light of the strengths and weaknesses of the study design. For the main outcomes of severe sepsis and mortality, we used an inception cohort design, which is generally considered a stronger approach than a case-control design (34). We recruited a large number of subjects from multiple centers. Spurious associations can occur in gene-association studies. In this instance, however, the associations with both outcome measures, mortality, and severe sepsis, were in the same direction, the statistical significance of the association with mortality was high, and it persisted even after adjusting for potential confounders. Furthermore, the findings were unchanged or strengthened when analyses were repeated using more stringent definitions for CAP and infection, based on procalcitonin concentrations. Although cultures were obtained in most subjects, we identified an etiological agent in a small subgroup, and the hazard ratios were similar among subjects with and without microbiologic diagnosis. The low yield of cultures in our study is consistent with previous large studies of CAP patients (35, 36) and likely due to poor yield of current culture techniques and strict criteria to discriminate between infection and contamination. As part of ongoing work, we have been exploring genetic variation in several other genes involved in the inflammatory response in the same cohort. The association between the MIF gene and survival is preserved even after adjustment for false discovery (37).
We limited the study to subjects with CAP because we were concerned that a broader population of subjects at risk for severe sepsis due to different infections could lead to spurious associations and our findings cannot be generalized to other infections. While we also included subjects with CAP who were discharged after evaluation in the EDs, a group that is often excluded in previous studies (38, 39), we could not assess differences in the frequency of MIF polymorphisms among those who develop milder forms of CAP and do not seek treatment in the ED. It is possible that our findings may differ within specific subsets of CAP depending on the infecting organism, and we are not powered to explore this possibility.
While our study is one of the largest genetic association studies in CAP or sepsis that has been performed to date, we recognize that these results must be validated by additional investigation. The associations reported herein, nevertheless, are robust in geographically distinct subcohorts, accounting for population substructure and for heterogeneity of effect size under different study settings.
Previous studies have shown a strong association between higher circulating MIF levels and outcome from sepsis (8,9,10,11). We did not observe a consistent relation between MIF genotypes and circulating cytokine concentrations over the time period that plasma samples were available for analysis. MIF genotype appeared to be associated with higher circulating IL-10 levels at the time of clinical presentation, but no associations were seen with other cytokines, such as TNF. We did not observe an association between MIF genotypes and circulating MIF in a small subset of studied subjects; this contrasts with what has been reported in chronic inflammatory conditions (18, 19, 33), and it may reflect the clinical heterogeneity of CAP at presentation. Plasma cytokine levels also are an imperfect measure of cytokine production in inflamed tissues, and for most cytokines, including MIF, circulating concentrations vary markedly over time (8, 11).
In view of previous reports of an association between high-expression MIF alleles and increased MIF expression (17,18,19, 33), experimental data pointing to a beneficial effect of MIF in clearance of certain intracellular pathogens (12, 14), and the present human genetic data, we suggest that MIF is protective in CAP. MIF is an upstream regulator of the innate response that functions to sustain the activation of monocytes/macrophages and other cell types by inhibiting apoptosis (40), up-regulating TLR expression (41), and overriding the immunosuppressive effects of glucocorticoids (41, 42) The human genetic findings reported herein prompt caution in the clinical application of anti-MIF strategies in infectious and noninfectious inflammatory conditions in order to avoid placing patients at increased risk of adverse outcomes. MIF genotype nevertheless may provide useful information for the design of clinical trials targeting MIF or other downstream inflammatory mediators. Finally, our results lend further support to the notion that MIF alleles exist in a balanced polymorphism under different selection pressures (21), where these alleles are protective in some diseases, but confer higher risk in others.
Supplementary Material
Acknowledgments
We are indebted to the nurses, respiratory therapists, phlebotomists, physicians, and other health care professionals who participated in GenIMS, as well as the patients and their families who supported this trial. A complete list of GenIMS investigators is available online (http://www.ccm.upmc.edu/genims_investigators). GenIMS was funded by NIH grants NIGMS R01 GM61992, NIAID R01 AI042310, and NO1 AI50031, with additional support from GlaxoSmithKline for enrollment and clinical data collection, Diagnostic Products Corporation for the cytokine assays, and an internal seed grant from the Department of Critical Care Medicine at the University of Pittsburgh. Yale University (R.B.) has applied for a patent describing the diagnostic value of MIF genotype. No other potential conflicts of interest were identified on behalf of the GenIMS investigators.
References
- Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Guyatt G, Bernard G R, Calandra T, Cook D, Elbourne D, Marshall J, Nunn A, Opal S. New strategies for clinical trials in patients with sepsis and septic shock. Crit Care Med. 2001;29:880–886. doi: 10.1097/00003246-200104000-00039. [DOI] [PubMed] [Google Scholar]
- Morand E F, Leech M, Weedon H, Metz C, Bucala R, Smith M D. Macrophage migration inhibitory factor in rheumatoid arthritis: clinical correlations. Rheumatology (Oxford) 2002;41:558–562. doi: 10.1093/rheumatology/41.5.558. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Calandra T, Mitchell R A, Martin S B, Tracey K J, Voelter W, Manogue K R, Cerami A, Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- Calandra T, Spiegel L A, Metz C N, Bucala R. Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of gram-positive bacteria. Proc Natl Acad Sci U S A. 1998;95:11383–11388. doi: 10.1073/pnas.95.19.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra T, Echtenacher B, Roy D L, Pugin J, Metz C N, Hultner L, Heumann D, Mannel D, Bucala R, Glauser M P. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- Bozza M, Satoskar A R, Lin G, Lu B, Humbles A A, Gerard C, David J R. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beishuizen A, Thijs L G, Haanen C, Vermes I. Macrophage migration inhibitory factor and hypothalamo-pituitary-adrenal function during critical illness. J Clin Endocrinol Metab. 2001;86:2811–2816. doi: 10.1210/jcem.86.6.7570. [DOI] [PubMed] [Google Scholar]
- Lehmann L E, Novender U, Schroeder S, Pietsch T, von Spiegel T, Putensen C, Hoeft A, Stuber F. Plasma levels of macrophage migration inhibitory factor are elevated in patients with severe sepsis. Intens Care Med. 2001;27:1412–1415. doi: 10.1007/s001340101022. [DOI] [PubMed] [Google Scholar]
- Bozza F A, Gomes R N, Japiassu A M, Soares M, Castro-Faria-Neto H C, Bozza P T, Bozza M T. Macrophage migration inhibitory factor levels correlate with fatal outcome in sepsis. Shock. 2004;22:309–313. doi: 10.1097/01.shk.0000140305.01641.c8. [DOI] [PubMed] [Google Scholar]
- Gando S, Nishihira J, Kobayashi S, Morimoto Y, Nanzaki S, Kemmotsu O. Macrophage migration inhibitory factor is a critical mediator of systemic inflammatory response syndrome. Intens Care Med. 2001;27:1187–1193. doi: 10.1007/s001340000818. [DOI] [PubMed] [Google Scholar]
- Koebernick H, Grode L, David J R, Rohde W, Rolph M S, Mittrucker H W, Kaufmann S H. Macrophage migration inhibitory factor (MIF) plays a pivotal role in immunity against Salmonella typhimurium. Proc Natl Acad Sci U S A. 2002;99:13681–13686. doi: 10.1073/pnas.212488699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma N, Koseki H, Akasaka T, Nakayama T, Taniguchi M, Serizawa I, Akahori H, Osawa M, Mikayama T. Deficiency of the macrophage migration inhibitory factor gene has no significant effect on endotoxaemia. Immunology. 2000;100:84–90. doi: 10.1046/j.1365-2567.2000.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoskar A R, Bozza M, Rodriguez S M, Lin G, David J R. Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous Leishmania major infection. Infect Immun. 2001;69:906–911. doi: 10.1128/IAI.69.2.906-911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak N, Sterns T, Echtenacher B, Mannel D N. Improved resistance to bacterial superinfection in mice by treatment with macrophage migration inhibitory factor. Infect Immun. 2005;73:6488–6492. doi: 10.1128/IAI.73.10.6488-6492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donn R P, Shelley E, Ollier W E, Thomson W. A novel 5′-flanking region polymorphism of macrophage migration inhibitory factor is associated with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2001;44:1782–1785. doi: 10.1002/1529-0131(200108)44:8<1782::AID-ART314>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Baugh J A, Chitnis S, Donnelly S C, Monteiro J, Lin X, Plant B J, Wolfe F, Gregersen P K, Bucala R. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 2002;3:170–176. doi: 10.1038/sj.gene.6363867. [DOI] [PubMed] [Google Scholar]
- Donn R, Alourfi Z, Zeggini E, Lamb R, Jury F, Lunt M, Meazza C, De Benedetti F, Thomson W, Ray D. A functional promoter haplotype of macrophage migration inhibitory factor is linked and associated with juvenile idiopathic arthritis. Arthritis Rheum. 2004;50:1604–1610. doi: 10.1002/art.20178. [DOI] [PubMed] [Google Scholar]
- Radstake T R, Sweep F C, Welsing P, Franke B, Vermeulen S H, Geurts-Moespot A, Calandra T, Donn R, van Riel P L. Correlation of rheumatoid arthritis severity with the genetic functional variants and circulating levels of macrophage migration inhibitory factor. Arthritis Rheum. 2005;52:3020–3029. doi: 10.1002/art.21285. [DOI] [PubMed] [Google Scholar]
- Fine M J, Auble T E, Yealy D M, Hanusa B H, Weissfeld L A, Singer D E, Coley C M, Marrie T J, Kapoor W N. A prediction rule to identify low-risk patients with community acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- Zhong X B, Leng L, Beitin A, Chen R, McDonald C, Hsiao B, Jenison R D, Kang I, Park S H, Lee A, Gregersen P, Thuma P, Bray-Ward P, Ward D C, Bucala R. Simultaneous detection of microsatellite repeats and SNPs in the macrophage migration inhibitory factor (MIF) gene by thin-film biosensor chips and application to rural field studies. Nucleic Acids Res. 2005;33:e121. doi: 10.1093/nar/gni123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson M E, Pompei P, Ales K L, MacKenzie C R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Knaus W A, Wagner D P, Draper E A, Zimmerman J E, Bergner M, Bastos P G, Sirio C A, Murphy D J, Lotring T, Damiano A. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- Levy M M, Fink M, Marshall J C, Abraham E, Angus D, Cook D, Cohen J, Opal S M, Vincent J L, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- National Death Index. http://www.cdc.gov/nchs/ndi.htm (Accessed November 2008) [Google Scholar]
- Mandell L A, Wunderink R G, Anzueto A, Bartlett J G, Campbell G D, Dean N C, Dowell S F, File T M, Jr, Musher D M, Niederman M S, Torres A, Whitney C G. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C L, Russell J A, Walley K R. Genetic polymorphisms in sepsis and septic shock: role in prognosis and potential for therapy. Chest. 2003;124:1103–1115. doi: 10.1378/chest.124.3.1103. [DOI] [PubMed] [Google Scholar]
- Kellum J A, Kong L, Fink M P, Weissfeld L A, Yealy D M, Pinsky M R, Fine J, Krichevsky A, Delude R L, Angus D C, for the GenIMS Investigators Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the genetic and inflammatory markers of sepsis (GenIMS) study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay M M, Huber P R, Tamm M, Muller B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600–607. doi: 10.1016/S0140-6736(04)15591-8. [DOI] [PubMed] [Google Scholar]
- Donn R, Alourfi Z, De Benedetti F, Meazza C, Zeggini E, Lunt M, Stevens A, Shelley E, Lamb R, Ollier W E, Thomson W, Ray D. Mutation screening of the macrophage migration inhibitory factor gene: positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2402–2409. doi: 10.1002/art.10492. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Laird N M, Ware J H. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Meyer-Siegler K L, Vera P L, Iczkowski K A, Bifulco C, Lee A, Gregersen P K, Leng L, Bucala R. Macrophage migration inhibitory factor (MIF) gene polymorphisms are associated with increased prostate cancer incidence. Genes Immun. 2007;8:646–652. doi: 10.1038/sj.gene.6364427. [DOI] [PubMed] [Google Scholar]
- Guyatt G H, Sackett D L, Sinclair J C, Hayward R, Cook D J, Cook R J. Users’ guides to the medical literature. IX A method for grading health care recommendations Evidence-Based Medicine Working Group JAMA. 1995;274:1800–1804. doi: 10.1001/jama.274.22.1800. [DOI] [PubMed] [Google Scholar]
- Metersky M L, Ma A, Bratzler D W, Houck P M. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169:342–347. doi: 10.1164/rccm.200309-1248OC. [DOI] [PubMed] [Google Scholar]
- Joshi N, Caputo G M, Weitekamp M R, Karchmer A W. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19:368–375. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- Gallagher P M, Lowe G, Fitzgerald T, Bella A, Greene C M, McElvaney N G, O'Neill S J. Association of IL-10 polymorphism with severity of illness in community acquired pneumonia. Thorax. 2003;58:154–156. doi: 10.1136/thorax.58.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterer G W, Quasney M W, Cantor R M, Wunderink R G. Septic shock and respiratory failure in community-acquired pneumonia have different TNF polymorphism associations. Am J Respir Crit Care Med. 2001;163:1599–1604. doi: 10.1164/ajrccm.163.7.2011088. [DOI] [PubMed] [Google Scholar]
- Mitchell R A, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci U S A. 2002;99:345–350. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger T, David J, Glauser M P, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- Calandra T, Bernhagen J, Metz C N, Spiegel L A, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.