Abstract
Volatile anesthetics (VAs), such as isoflurane, induce a general anesthetic state by binding to specific targets (i.e., ion channels) in the central nervous system (CNS). Simultaneously, VAs modulate immune functions, possibly via direct interaction with alternative targets on leukocytes. One such target, the integrin lymphocyte function-associated antigen-1 (LFA-1), has been shown previously to be inhibited by isoflurane. A better understanding of the mechanism by which isoflurane alters protein function requires the detailed information about the drug–protein interaction at an atomic level. Here, we describe the crystal structure of the LFA-1 ligand-binding domain (I domain) in complex with isoflurane at 1.6 Å. We discovered that isoflurane binds to an allosteric cavity previously implicated as critical for the transition of LFA-1 from the low- to the high-affinity state. The isoflurane binding site in the I domain involves an array of amphiphilic interactions, thereby resembling a “common anesthetic binding motif” previously predicted for authentic VA binding sites. These results suggest that the allosteric modulation of protein function by isoflurane, as demonstrated for the integrin LFA-1, might represent a unified mechanism shared by the interactions of volatile anesthetics with targets in the CNS.—Zhang, H., Astrof, N. S., Liu, J.-H., Wang, J.-H., Shimaoka, M. Crystal structure of isoflurane bound to integrin LFA-1 supports a unified mechanism of volatile anesthetic action in the immune and central nervous systems.
Keywords: allosteric modulation, conformational change, ferritin, halogen bond, stereoisomer
Understanding the mechanism by which volatile anesthetics (VAs), such as the halogenated ether isoflurane [2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane; Fig. 1, top], modulate protein activity is one of the major challenges facing molecular pharmacology. The established molecular targets of VAs in the central nervous system (CNS) are membrane-embedded ion channels (1,2,3). In lieu of high-resolution crystal structures of these CNS targets bound to VA, an extensive array of biochemical, biophysical, and computational approaches has been used to identify canonical anesthetic binding sites on both ion channels and model protein systems (1, 2). The aggregate of biochemical evidence demonstrates that VAs modulate ion channel functions in an allosteric (i.e., perturbation of the gating machinery), as opposed to a competitive (i.e., direct interaction with the ligand binding site), manner. However, the lack of high-resolution structural data on the VA-CNS target interactions precludes a more detailed mechanistic understanding of how the relatively weak binding of VAs to proteins has such profound effects on protein functions.
Figure 1.
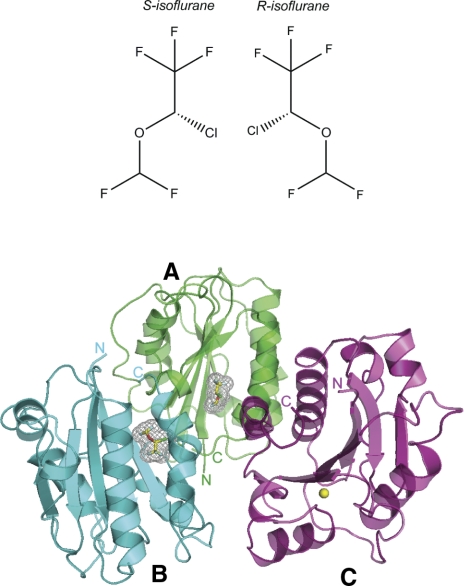
Crystal structure of the LFA-1 I domain in complex with isoflurane. Top: chemical structures of isoflurane enantiomers. Bottom: Ribbon diagram showing three I domains colored green (A), cyan (B), and magenta (C) forming an asymmetric unit. Bound isoflurane molecules in A, B are depicted as a stick model with carbon (yellow), oxygen (red), chloride (green), and fluorine (sky blue) atoms color coded. Mesh representation of Van der Waals surface for the bound isoflurane is also shown. N and C termini of each I domain are labeled. All figures were prepared with Pymol.
While inducing a general anesthetic state by binding to the CNS targets, VAs modulate immune responses possibly by interacting with alternative targets on leukocytes and/or with a subset of the CNS targets that are also expressed on leukocytes (4,5,6,7). By studying the interactions of VAs with alternative leukocyte targets, not only can the mechanisms underlying VA-induced immune-modulation be potentially elucidated, but a fuller understanding of VA action on the CNS targets can also be gained. Obtaining high-resolution structural data on the VA-alternative target interactions is of great importance to achieving these goals.
We have shown previously that the integrin LFA-1 represents one alternative leukocyte target for isoflurane (8). LFA-1 binds to its major ligand ICAM-1 on endothelial cells and antigen-presenting cells, and in this way plays pivotal roles both in leukocyte trafficking and in immune responses to infection (9). Extensive crystallographic investigations have recently established the structural basis underlying the activation-dependent conformational changes that led to high-affinity ligand binding by LFA-1 (10, 11). The ligand-binding domain of LFA-1, termed the inserted (I) domain, adopts a Rossmann fold with a central β-sheet flanked by α-helices (10, 12). A Mg2+ ion is located at the top of the I domain, forming the metal ion-dependent adhesion site (MIDAS), while at the bottom are found the C and N termini of the domain. The ligand-binding affinity at the MIDAS is up-regulated 10,000-fold via activation-dependent conformational changes (10). Downward movement of the C-terminal helix is coupled allosterically to the conversion of the metal coordinating loops at the MIDAS to the high-affinity configuration, thus enabling tight binding to ICAM-1. We have provided biochemical evidence that isoflurane suppresses this activation-dependent conversion to high-affinity LFA-1, thereby inhibiting cell adhesion to ICAM-1 (8). Furthermore, using [1H,15N]-NMR spectroscopy of isotopically labeled I domain, we showed that isoflurane induced chemical shift perturbations at a cavity underneath the C-terminal helix and potentially interfered with its downward shift, as does a certain class of small-molecule allosteric LFA-1 antagonists (i.e., α I allosteric antagonists; refs.11, 13).
Here, we report the high-resolution crystal structure of the LFA-1 I domain in complex with the VA isoflurane. Our structure reveals, for the first time, a detailed atomic view of VA binding to the well-established allosteric pocket critical for conformational transition of the integrin between an active and an inactive state. The isoflurane binding site within the LFA-1 I domain involves an array of amphiphilic interactions and correlates remarkably well with a postulated “common anesthetic binding motif” observed in model proteins examined by X-ray crystallography. These results suggest that the mechanisms underlying the allosteric modulation of protein function by isoflurane demonstrated in the LFA-1 I domain structure might be generalized to VA-ion channel interactions in the CNS.
MATERIALS AND METHODS
Protein production and crystallization
The wild-type I domain of LFA-1 (residues 128–307 of the integrin αL subunit) was expressed in BL21 (DE3) and refolded as described previously (10). The protein was concentrated to 20 mg/ml. Single crystals were obtained via a hanging drop vapor diffusion method in a 24-well VDX plate (Hampton Research, Aliso Viejo, CA, USA) at 295 K using a reservoir solution of 25% PEG3350, 0.2 M ammonium acetate, and 0.1 M sodium acetate, pH 7.5. For cocrystallization with isoflurane, 10 mM isoflurane was added to both the crystallization reservoir as well as the cryoprotectant solutions (see below). Needle-like crystals were formed in 3–7 days.
Data collection and model refinement
Crystals were harvested and soaked in a cryoprotection solution (25% PEG3350, 15% glycerol, and 0.1 M sodium acetate, pH 7.5) with or without 10 mM isoflurane and flash-frozen into liquid nitrogen. X-ray diffraction data were collected at beam line ID24 at the Argonne National Laboratory (Argonne, IL, USA). The diffraction data were processed with HKL2000 (14). Molecular replacement was performed using the program Phaser from CCP4 (STFC Daresbury Laboratory, Daresbury, UK), and the wild-type I domain [Protein Data Bank (PDB) code 1LFA] was used as the searching model. The model was refined with Refmac and Arp/Warp (15, 16) and then cycled with rebuilding in Coot (17). The isoflurane molecule was built into positive-difference electron-density maps after a few restrained refinement runs of the I domain. TLS refinement was incorporated into the later stages of the refinement process. The final model was analyzed with MolProbity (18). Data collection and model refinement statistics are summarized in Supplemental Table 1. The coordinates of the I domain structure, both on its own and in complex with isoflurane, were deposited in the PDB with the codes 3F74 and 3F78, respectively.
RESULTS
The isoflurane-bound I domain adopts a closed conformation
The LFA-1 I domain was crystallized on its own and in complex with isoflurane under the same crystallization conditions. The crystal with isoflurane diffracted up to 1.6 Å (PDB 3F78) and was found to contain three I domain molecules in the asymmetric unit, termed molecules A–C (Fig. 1, bottom). These three molecules do not exemplify a crystallographic 3-fold symmetry in that molecule C is slightly distant from molecules A and B. In molecules A and B, one isoflurane molecule is bound to the cavity underneath the C-terminal helix (Figs. 1, bottom, and 2A). This cavity has been shown to contain the drug-binding site for the αL allosteric antagonists that block LFA-1 by stabilizing the low-affinity closed conformation (11, 13) (Supplemental Fig. 1A, B). Although molecules A and B did not contain the Mg2+ ion at the MIADS, they showed the typical closed configurations of the MIDAS residues as well as the closed position of the C-terminal β6-α7 loop, indicating that the isoflurane-bound I domain adopts a low-affinity closed conformation (Fig. 2A, C).
Figure 2.
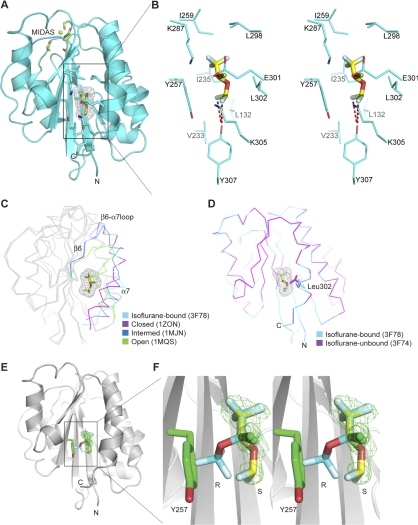
Interactions of isoflurane with the LFA-1 I domain. A) Ribbon diagram showing a side view of molecule B with isoflurane bound in the cavity underneath the α7 helix. An Mg2+ ion at the MIDAS is modeled in and shown with a green sphere. Side chains of MIADS residues and isoflurane-interacting residues are show as green and cyan sticks, respectively, with red oxygen and blue nitrogen atoms. B) Stereo view of the isoflurane-binding site. View is a blow-up of boxed region in A. Only side chains of isoflurane-interacting residues are shown. Polar interactions of isoflurane with Glu301 and Y307 are represented by red dashed lines. C) The isoflurane-bound I domain backbone (3F78) shown superimposed with the closed (1ZON) (34), intermediate (1MJN) (10), and open (1MQ9) (10) LFA-1 I domain conformations. Only C-terminal fragments encompassing the β6-α7 loop are color coded as indicated. D) Leu302 rotamer change by isoflurane. I domain backbones with (3F78, cyan) and without (3F74, magenta) isoflurane are shown superimposed. Rotamers of Leu302 are shown as cyan (3F78) and magenta (3F74) stick models. Bound S-isoflurane molecules are shown as a stick model with carbon (yellow), oxygen (red), chloride (green), and fluorine (sky blue) atoms color coded (A–D). Mesh representation of Van der Waals surface is shown for bound isoflurane (A, C, D). E, F) Stereoselective binding by S-isoflurane. Ribbon diagrams of an overview (E) and a zoomed stereoview (F) showing I domain (3F78) with bound S-isoflurane and modeled R-isoflurane. R-isoflurane was modeled into bound S-isoflurane such that trifluoromethyl and chlorine parts were superimposed. Isoflurane molecules are shown as a stick model with same color coding as in A except that R-isoflurane has cyan carbon atoms. Fo-Fc map at a level of 3.0 for S-isoflurane is shown in a green mesh representation. R- and S-enantiomers are labeled. Tyr257 is shown as a green stick, clashing with R-enantiomer.
In contrast to molecules A and B, no isoflurane was observed in molecule C (Fig. 1, bottom). The absence did not result from poor electron density but was rather due to a crystal lattice contact that distorted the flexible C-terminal helix, the latter altering the cavity to an orientation unfavorable for isoflurane binding (Supplemental Fig. 1C).
The crystal formed without isoflurane diffracted up to 1.7Å (PDB 3F74), containing three I domain molecules (molecules A–C) in the asymmetric unit, as seen in the 3F78 structure (Supplemental Table 1 and not shown). The 3F74 structure represents the closed I domain conformation with the α7 helix well packed against the body of the domain. Like molecule C in the 3F78 structure, molecule C in the 3F74 structure also exhibited a distorted C-terminal helix. The two molecules C are the same with a RMSD value of 0.06 Å, thereby confirming that the distortion of the C-terminal regions was not induced by isoflurane but likely by lattice contacts.
Interactions between the I domain and isoflurane
Isoflurane binds to the cavity underneath the C-terminal helix, sitting in a groove formed by strands β1 and β4 at the bottom, strands β5 and β6 on one side, and helices α1 and α7 on the other (Fig. 2A, B). The average B factor for bound isoflurane (48.8 Å2) was significantly higher than that of the I domain (20.8 Å2), reflecting the weak interactions between the protein and VA (Supplemental Table 1). Despite this weakness, an array of amphiphilic interactions contributed to stabilizing the position of isoflurane within the cavity. Specifically, the trifluoromethyl head of isoflurane formed extensive hydrophobic interactions with residues Ile235, Tyr257, Ile259, Leu298, and Leu302, as well as with the aliphatic portion of Lys287. In contrast, the chloride atom was in proximity to Glu301 and to the edge of the aromatic ring of Tyr257, thus contributing to polar interactions. Another set of polar interactions was formed by the difluoromethyl fluorine atoms with the phenyl group of Tyr307. Although these polar interactions might involve halogen bonds, the distances between the chloride atom and Glu301 (i.e., 3.4 Å), as well as between the difluoromethyl fluorine and Tyr307 (i.e., 3.2 Å), were slightly longer than those previously used to define halogen bonds (19).
A comparison of the structures made in the presence (3F78) and absence (3F74) of isoflurane showed that the major structural alteration induced by isoflurane binding involved the rotamer change of Leu302, which provides a hydrophobic interaction with isoflurane (Fig. 2D). To accommodate the binding of isoflurane, the leucine side chain adopts a disfavored rotamer (2% probability) within the isoflurane-I domain complex, as opposed to the most favored rotamer (59% probability) observed in the free I domain structure.
Enantiomer selectivity vis-à-vis the I domain
Isoflurane is used clinically as a racemic mixture composed of two stereoisomers (Fig. 1, top). Racemic isoflurane was used for cocrystallization with the I domain. Although we attempted to fit R- and S-isomers to the electron density map independently, the electron-density pattern fit exclusively to the S-enantiomer (Fig. 2E, F). To substantiate this finding, we constructed a model of R-stereoisomer binding to the I domain such that the positions of the trifluoromethyl group and chloride were superimposed onto the location of the counterparts of the S-stereoisomer (Fig. 2E, F). In this model, the difluoromethyl group belonging to R-isoflurane clashed with the aromatic side chain of Tyr257. An in silico mutation of Tyr257 to alanine did not obviate the steric restriction on binding; the carbon atom of the difluoromethyl group in the R-isomer remained at a distance of 2.7 Å from the side-chain Cβ atom of Ala257 (data not shown). These results support the selective binding of the S-isomer to the I domain.
A comparison of isoflurane binding sites in the I domain and the apoferritin
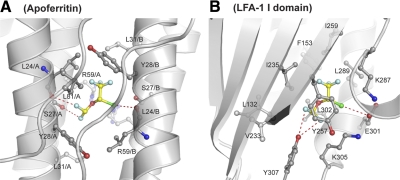
Ferritin, a soluble 4-α-helix bundle protein, has been used as a means for modeling those membrane-embedded helical bundles of ion channels wherein the anesthetic binding sites are thought to lie (20). A high-resolution structure of apoferritin bound to isoflurane showed that isoflurane is located at a 2-fold axis between protein monomers; thus, two isoflurane molecules are in the same position with 50% occupancy each. We compared the isoflurane binding sites in the I domain with the four-α-helix bundle in the apoferritin. When the positions of the bound isoflurane were aligned, the overall chemical environment of the binding pockets proved remarkably similar in both apoferritin and in the I domain (Fig. 3A, B). In apoferritin, the trifluoromethyl group is positioned in the hydrophobic core formed by the side chains of Leu24, Tyr28, Leu31, and the aliphatic portion of Arg59 (Fig. 3A). The position of Tyr28 in apoferritin is similar to the aliphatic portion of residues Lys287 and Ile259 in the I domain. Interestingly, the chloride atom of isoflurane in the four-α-helix bundle forms a polar interaction with the carboxyl oxygen of Leu24, while the chloride of isoflurane in the I domain forms a similar interaction with Glu301 and Tyr257. Finally, in the isoflurane-bound apoferritin, the two difluoromethyl fluorine atoms also contribute to the polar interactions that occur with Ser-27 and Leu24 from a symmetry-related molecule, while in the isoflurane bound I domain, these two fluorine atoms similarly form bifurcated polar interactions with Tyr307 (Fig. 3B).
Figure 3.
Comparison of isoflurane-binding sites in apoferritin and I domain. Ribbon diagram showing isoflurane-binding sites in apoferritin (A) and LFA-1 I domain (B). Isoflurane-interacting residue side chains are shown as a ball-and-stick model with red oxygen and blue nitrogen atoms. L24/A and L24/B main chains are also shown as a ball-and-stick model (A). Isoflurane is shown as a ball-and-stick model with yellow carbon, red oxygen, green chloride, and sky blue fluorine atoms. Polar interactions of isoflurane with proteins are represented by red dashed lines.
DISCUSSION
Isoflurane binds to the cavity underneath the C-terminal helix of the LFA-1 I domain, causing a number of hydrophobic and polar interactions. The pivot of Leu302 to the disfavored rotamer conformation represents the major structural rearrangement of the I domain cavity on the binding of isoflurane. Although no comparable structures of isoflurane were found with authentic CNS targets, the general features we observed were also present in a number of model protein systems that bind one or more VAs (20, 21). Specifically, a comparison of the I domain and apoferritin demonstrated that despite the lack of any real structural homology between the two proteins, the overall structural and chemical environments of the isoflurane binding cavities are markedly similar. In both the I domain and apoferritin, predominantly hydrophobic interactions surround isoflurane, while a select number of polar interactions also contribute to binding activity. The ability to interact with both aliphatic and polar amino acid residues is a unique property of halogen atoms, as is exemplified by those decorating the isoflurane skeleton (19, 22). The similarity between the binding sites of the two otherwise distinct structures might signify a canonical amphipathic binding pocket for halogenated VA on proteins, as was theorized prior to the advent of high-resolution protein X-ray crystallography and which was more recently observed in a number of crystallographic and computational studies of VA binding pockets in proteins (20, 23,24,25).
Our structure reveals that, although crystallized with a racemic drug, only the S-isomer of isoflurane can bind to the I domain, in contrast to the apoferritin–isoflurane structure, in which the specific stereoisomer could not be identified (20). The S-isomer of bromoform is also preferentially bound to luciferase, which has been linked to the interactions that take place between Br and glutamic acid (24). Like many drugs, the stereoisomers of isoflurane have unequal potency both in vitro and in vivo (26,27,28,29,30). However, in accordance with Pfeiffer’s rule (31), in which the potency of a drug correlates to the stereoselectivity of its pharmacological effects, the difference in anesthetic potency is modest and the S-isomer is typically ∼20–50% more effective both in in vitro and in vivo assays (32, 33). By comparison, our structure suggests that the effects of the individual stereoisomers on inhibiting LFA-1 may be more significant since it is clear that the I domain cannot accommodate the R-isomer at the observed binding site. We previously estimated a KD of binding to the I domain (based on our NMR measurements) of ∼800 μM using racemic isoflurane. It would be especially interesting to determine whether the stereoselectivity observed in this crystal structure is reflected in the inhibition of LFA-1 by isoflurane using pure isoflurane stereoisomers in a future investigation.
Earlier studies have described small-molecule allosteric LFA-1 inhibitors (i.e., α I allosteric antagonist; ref. 13) that bind to the cavity beneath the C-terminal helix, causing interference with the downward shift of the α7 helix (Supplemental Fig. 1A, B). This class of allosteric LFA-1 inhibitors stabilizes the closed LFA-1 conformation, thereby blocking any ligand binding by LFA-1. As suggested by our earlier NMR results, isoflurane shares with the α I allosteric antagonists many of the same interacting residues (e.g., Tyr257, Ile259, Lys287, Leu298, Leu302, and Ile235) (Supplemental Fig. 1A, B). Therefore, despite having no structural resemblance to the α I allosteric antagonists, isoflurane may suppress LFA-1 activation via an analogous mechanism. By interfering with the movement of the C-terminal α7 helix, isoflurane may block the transition to the open conformation. The α I allosteric antagonists (e.g., lovastatin, Supplemental Fig. 1) have ∼100 times higher affinity to the I domain (11) than isoflurane. This finding is explained, at least partly, by the fact that the antagonists have larger sizes (e.g., MW; lovastatin, 404; isoflurane 184) and presumably larger hydrophobic surface areas, thereby inducing more substantial changes in the I domain pocket and the C-terminal helix than isoflurane (Supplemental Fig. 1).
It has been previously suggested that VAs allosterically alter protein function by modulating those helical motions needed for ion channel gating (1, 20). Based on the present crystallographic results, as well as on our previous biochemical and NMR analyses (8), we have demonstrated the allosteric mechanism by which isoflurane inhibits LFA-1 activation. This study not only helps to explain the mechanism underlying VA-induced immuno-modulation but also suggests the existence of a “unified” mechanism integral to the action of VAs in the central nervous and immune systems. The binding of VA within an amphipathic pocket is thought to stabilize the conformation of those α helix/helices critical for the transition to a functionally altered state. This unanticipated parallel, in conjunction with the relative ease by which the I domain and a panel of its mutants can be structurally and biochemically characterized, makes the LFA-1 I domain well tailored for functional and structural investigations into numerous critical issues facing anesthetic pharmacology.
Supplementary Material
Acknowledgments
We thank Koichi Yuki and Sulpicio G. Soriano (Children’s Hospital Boston and Harvard Medical School, Boston, MA, USA) for providing reagents and advice. This work was supported by grants from the National Institutes of Health, AI063421 (M.S.) and HL048675 (M.S., J.H.W.).
References
- Eckenhoff R G, Johansson J S. Molecular interactions between inhaled anesthetics and proteins. Pharmacol Rev. 1997;49:343–367. [PubMed] [Google Scholar]
- Franks N P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Hemmings H C, Jr, Akabas M H, Goldstein P A, Trudell J R, Orser B A, Harrison N L. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- McBride W T, Armstrong M A, McBride S J. Immunomodulation: an important concept in modern anaesthesia. Anaesthesia. 1996;51:465–473. doi: 10.1111/j.1365-2044.1996.tb07793.x. [DOI] [PubMed] [Google Scholar]
- Hayes J K, Havaleshko D M, Plachinta R V, Rich G F. Isoflurane pretreatment supports hemodynamics and leukocyte rolling velocities in rat mesentery during lipopolysaccharide-induced inflammation. Anesth Analg. 2004;98:999–1006. doi: 10.1213/01.ANE.0000104584.91385.1D. [DOI] [PubMed] [Google Scholar]
- Mobert J, Zahler S, Becker B F, Conzen P F. Inhibition of neutrophil activation by volatile anesthetics decreases adhesion to cultured human endothelial cells. Anesthesiology. 1999;90:1372–1381. doi: 10.1097/00000542-199905000-00022. [DOI] [PubMed] [Google Scholar]
- Chiang N, Schwab J M, Fredman G, Kasuga K, Gelman S, Serhan C N. Anesthetics impact the resolution of inflammation. PLoS ONE. 2008;3:e1879. doi: 10.1371/journal.pone.0001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K, Astrof N S, Bracken C, Yoo R, Silkworth W, Soriano S G, Shimaoka M. The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J. 2008;22:4109–4116. doi: 10.1096/fj.08-113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M L, Springer T A. Lymphocyte function associated-1 (LFA-1, CD11a/CD18) Kreis T, Vale R, editors. New York: Sambrook and Tooze; Guidebook to the Extracellular Matrix and Adhesion Proteins. 1999:228–232. [Google Scholar]
- Shimaoka M, Xiao T, Liu J-H, Yang Y, Dong Y, Jun C-D, McCormack A, Zhang R, Joachimiak A, Takagi J, Wang J-h, Springer T A. Structures of the aL I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell. 2003;112:99–111. doi: 10.1016/s0092-8674(02)01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen J, Welzenbach K, Ramage P, Geyl D, Kriwacki R, Legge G, Cottens S, Weitz-Schmidt G, Hommel U. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J Mol Biol. 1999;292:1–9. doi: 10.1006/jmbi.1999.3047. [DOI] [PubMed] [Google Scholar]
- Lee J-O, Rieu P, Arnaout M A, Liddington R. Crystal structure of the A domain from the a subunit of integrin CR3 (CD11b/CD18) Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Springer T A. Therapeutic antagonists and the conformational regulation of integrin structure and function. Nat Rev Drug Disc. 2003;2:703–716. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Murshudov G N, Vagin A A, Dodson E J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Perrakis A, Morris R, Lamzin V S. Automated protein model building combined with iterative structure refinement. Nat Struct Biol. 1999;6:458–463. doi: 10.1038/8263. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Davis I W, Leaver-Fay A, Chen V B, Block J N, Kapral G J, Wang X, Murray L W, Arendall W B, 3rd, Snoeyink J, Richardson J S, Richardson D C. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–W383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffinger P, Hays F A, Westhof E, Ho P S. Halogen bonds in biological molecules. Proc Natl Acad Sci U S A. 2004;101:16789–16794. doi: 10.1073/pnas.0407607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Loll P J, Eckenhoff R G. Structural basis for high-affinity volatile anesthetic binding in a natural 4-helix bundle protein. FASEB J. 2005;19:567–576. doi: 10.1096/fj.04-3171com. [DOI] [PubMed] [Google Scholar]
- Bertaccini E J, Trudell J R, Franks N P. The common chemical motifs within anesthetic binding sites. Anesth Analg. 2007;104:318–324. doi: 10.1213/01.ane.0000253029.67331.8d. [DOI] [PubMed] [Google Scholar]
- Kortagere S, Ekins S, Welsh W J. Halogenated ligands and their interactions with amino acids: implications for structure-activity and structure-toxicity relationships. J Mol Graph Model. 2008;27:170–177. doi: 10.1016/j.jmgm.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Streiff J H, Jones K A. Volatile anesthetic binding to proteins is influenced by solvent and aliphatic residues. J Chem Inf Model. 2008;48:2066–2073. doi: 10.1021/ci800206a. [DOI] [PubMed] [Google Scholar]
- Franks N P, Jenkins A, Conti E, Lieb W R, Brick P. Structural basis for the inhibition of firefly luciferase by a general anesthetic. Biophys J. 1998;75:2205–2211. doi: 10.1016/S0006-3495(98)77664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A A, Curry S, Franks N P. Binding of the general anesthetics propofol and halothane to human serum albumin. High resolution crystal structures. J Biol Chem. 2000;275:38731–38738. doi: 10.1074/jbc.M005460200. [DOI] [PubMed] [Google Scholar]
- Dickinson R, White I, Lieb W R, Franks N P. Stereoselective loss of righting reflex in rats by isoflurane. Anesthesiology. 2000;93:837–843. doi: 10.1097/00000542-200009000-00035. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tang P, Firestone L, Zhang T T. 19F nuclear magnetic resonance investigation of stereoselective binding of isoflurane to bovine serum albumin. Biophys J. 1996;70:532–538. doi: 10.1016/S0006-3495(96)79599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan J J, Firestone S, Firestone L L. Isoflurane’s enhancement of chloride flux through rat brain gamma-aminobutyric acid type A receptors is stereoselective. Anesthesiology. 1995;83:611–615. doi: 10.1097/00000542-199509000-00021. [DOI] [PubMed] [Google Scholar]
- Moody E J, Harris B D, Skolnick P. Stereospecific actions of the inhalation anesthetic isoflurane at the GABAA receptor complex. Brain Res. 1993;615:101–106. doi: 10.1016/0006-8993(93)91119-d. [DOI] [PubMed] [Google Scholar]
- Franks N P, Lieb W R. Stereospecific effects of inhalational general anesthetic optical isomers on nerve ion channels. Science. 1991;254:427–430. doi: 10.1126/science.1925602. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C C. Optical isomerism and pharmacological action, a generalization. Science. 1956;124:29–31. doi: 10.1126/science.124.3210.29. [DOI] [PubMed] [Google Scholar]
- Hall A C, Lieb W R, Franks N P. Stereoselective and non-stereoselective actions of isoflurane on the GABAA receptor. Br J Pharmacol. 1994;112:906–910. doi: 10.1111/j.1476-5381.1994.tb13166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien E J, Miranda J F, Ariens E J. Quantitative structure-activity correlation of optical isomers: a molecular basis for Pfeiffer’s rule. Mol Pharmacol. 1976;12:598–604. [PubMed] [Google Scholar]
- Qu A, Leahy D J. The role of the divalent cation in the structure of the I domain from the CD11a/CD18 integrin. Structure. 1996;4:931–942. doi: 10.1016/s0969-2126(96)00100-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.