Abstract
Schistosomes are parasitic platyhelminths that constitute an important public health problem globally. Infection is characterized by the presence of adult worms within the vasculature of their hosts, where they can reside for many years. The worms are covered by an unusual dual lipid bilayer through which they import nutrients. How the parasites import other vital molecules, such as water, is not known. Recent proteomic analysis of the schistosome tegumental membranes revealed the presence of an aquaporin homologue at the host-interactive surface whose cDNA we have cloned and characterized. The cDNA encodes a predicted 304-aa protein (SmAQP) that is found largely in the parasite tegument by immunolocalization and is most highly expressed in the intravascular life stages. Treatment of parasites with short interfering RNAs targeting the SmAQP gene results in potent (>90%) suppression. These suppressed parasites resist swelling when placed in hypotonic medium, unlike their control counterparts, which rapidly double in volume. In addition, SmAQP-suppressed parasites, unlike controls, resist shrinkage when incubated in hyperosmotic solution. While suppressed parasites exhibit lower viability in culture relative to controls and exhibit a stunted appearance following prolonged suppression, they are nonetheless more resistant to killing by the drug potassium antimonyl tartrate (PAT). This is likely because SmAQP acts as a conduit for this drug, as is the case for aquaporins in other systems. These experiments reveal a heretofore unrecognized role of the schistosome tegument in controlling water and drug movement into the parasites and highlight the importance of the tegument in parasite osmoregulation and drug uptake.—Faghiri, Z., Skelly, P. J. The role of tegumental aquaporin from the human parasitic worm, Schistosoma mansoni, in osmoregulation and drug uptake.
Keywords: platyhelminth, RNAi, metabolite transport, antimonial, tegument
Schistosomiasis is a chronic, often debilitating, disease caused by parasitic platyhelminths that afflicts more than 200 million people in over 70 countries worldwide (1). An estimated 600 million people are at risk of infection. Mortality is put at ∼280,000 deaths annually in sub-Saharan Africa alone, with tens of millions having debilitating chronic morbidity (2, 3). Schistosomiasis mansoni is characterized by the presence of adult Schistosoma mansoni worms, or blood flukes, within the mesenteric venous plexus. Adult worms can survive for many years within the vasculature of immunocompetent hosts.
The major interface between the schistosome and its external environment is called the tegument, and this is a unique, syncytial structure that is bounded externally by a dual lipid bilayer; the apical plasma membrane is overlain by a second membrane called the membranocalyx (4,5,6,7). This double-bilayered (or heptalaminate) outer membrane is unique to blood-dwelling trematodes, such as schistosomes, and is not found in trematode parasites occupying other habitats (8). It is a prime site of intimate host-parasite interaction and performs vital functions that ensure parasite survival (9). The tegument lacks lateral membranes. This means that the tegumental cytoplasm extends as a continuous unit, or syncytium, around the entire body (5). The cytoplasm is connected by numerous thin cytoplasmic connections to cell bodies, or cytons, that lie beneath the peripheral muscle layers. These cell bodies contain nuclei, endoplasmic reticula, Golgi complexes, and mitochondria. Cell bodies actively synthesize the secretory bodies, which move along the cytoplasmic connections to the outer cytoplasm (5).
The molecular mechanisms by which intravascular schistosomes import nutrients such as glucose and some amino acids from host blood through the tegument have been characterized (10,11,12,13,14,15). How the parasites import other vital molecules (such as water) is not known. Because of the limited ability of water to diffuse freely through lipid bilayers, most cells possess specialized proteins that facilitate the conduction of water across their membranes. Transmembrane proteins that act as pores to selectively conduct water molecules in and out of a cell are known as aquaporins (AQPs) (16). Some members of the family also permit the movement of other metabolites (17, 18). Recent proteomic analysis of the schistosome tegumental membranes has revealed the presence there of a single AQP homologue (19, 20). To test the hypothesis that this tegumental protein can act as a conduit for water and other metabolites, such as schistosome-killing drugs, we set out to clone and characterize this molecule, which we designate SmAQP.
MATERIALS AND METHODS
Parasites and mice
The Puerto Rican strain of S. mansoni was maintained at the Biomedical Research Institute (Rockville, MD, USA), and obtained from Dr. Fred Lewis. Cercariae were obtained from infected Biomphalaria glabrata, and isolated parasite bodies were prepared as described previously (21). Parasites were cultured in complete RPMI culture medium, which is RPMI medium supplemented with 10 mM HEPES, 2 mM glutamate, 5% fetal calf serum, and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin), at 37°C, in an atmosphere of 5% CO2. Culture samples were withdrawn at d 14 and 21 to assess parasite viability by staining with 4 μg/ml Hoechst 33258 dye in phosphate-buffered saline (PBS). After 5 min of incubation at 37°C, parasites were examined for viability by fluorescence microscopy, as described previously (22). Adult male and female parasites were recovered by perfusion from Balb/c mice that were infected with 125 cercariae, 7 wk previously. Parasite eggs were isolated from infected mouse liver tissue, miracidia were recovered, and sporocysts were prepared and cultured for 24 h, as described previously (23).
Cloning SmAQP
To clone SmAQP, we first used S. mansoni EST sequence information derived following proteomic analysis of the tegumental membranes (19, 24) to examine the S. mansoni genome database (version 3), and this led to the identification of the SmAQP gene. Next, using oligonucleotides designed from the predicted 5′ UTR upstream of the first predicted exon (SmAqua1: 5′-GTTATCGAAAAGCCAGTCGTAG-3′) and the 3′UTR downstream of the last predicted exon (SmAqua4: 5′-CTATTTAACAATGTTAAATATTGAGG-3′), with adult parasite cDNA in a PCR, we amplified and then sequenced, at the Tufts University Core Facility, the complete predicted SmAQP coding DNA.
Preparation and delivery of dsRNA
Two small inhibitory RNAs (siRNAs), one designated siAqua1 (spanning SmAQP coding DNA positions 175–199), with the target sequence 5′-CATGCTCATGGAACATTCATTTCAG-3′, and the second designated siAqua2, (spanning SmAQP coding DNA positions 267–291), with the sequence 5′-CTGTAATCCAGCTGTAACATTGGCA-3′, were synthesized commercially by Integrated DNA Technologies (IDT, Coralville, IA, USA) with the help of the online IDT RNAi Design Tool (https://www.idtdna.com/Scitools/Applications/RNAi/RNAi. aspx). The off-the-shelf DS Scrambled Neg negative control siRNA 5′-CTTCCTCTCTTTCTCTCCCTTGTGA-3′ was obtained from IDT, Inc. This sequence does not match any in the S. mansoni genome assembly (version 3) as assessed by using the Basic Local Alignment Search Tool (BLAST) at http://www.sanger.ac.uk/Projects/S_mansoni/ (25).
To deliver the siRNAs, schistosomula (∼1000/group, 2–7 d old), in 100 μl electroporation buffer (Ambion, Austin, TX, USA) containing 5 μg siRNA, were electroporated in a 4-mm cuvette by applying a square wave with a single 20-ms impulse, at 125 V and at room temperature, as described previously (26). Parasites were then transferred to 500 μl complete RPMI. After overnight culture, medium was replaced with 1 ml of fresh complete RPMI culture medium. Suppression was confirmed by quantitative real-time PCR (qRT-PCR) 2 d after siRNA treatment, unless otherwise indicated, and changes in protein level and phenotype were examined at least 5 d after treatment.
Gene expression analysis
To monitor the expression of the SmAQP gene during schistosome development and at different times following siRNA treatment, RNA was first extracted from the parasites using the TRIzol method (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Any residual DNA remaining in the RNA preparations was removed by DNase digestion using a TurboDNAse kit (Ambion). cDNA was synthesized using 1 μg RNA and an oligo (dT)20 primer and Superscript reverse transcriptase (Invitrogen). Quantitative real-time PCR was performed using custom TaqMan Assays using primer sets and reporter probes labeled with 6-carboxyfluorescein (FAM), obtained from Applied Biosystems (Foster City, CA, USA).
The following primers and probes were selected to detect AQP gene expression: Smaqua-aqu1F, 5′-GTGATTTAGGACCCAGACTCATGAT-3′; Smaqua-aqu1R, 5′-GTTTGCTCCACTGAATGCTTTGTT-3′; and probe Smaqua-aqu1M2, 5′-FAM-ACCCCAACCGAATATAAA-3′. To detect expression of the endogenous control tubulin gene, the following primers and probe were used: Tubulina2-F, 5′-GGTTGACAACGAGGCCATTTATG-3′; Tubulina2-R, 5′-TGTGTAGGTTGGACGCTCTATATCT-3′; and probe Tubulina2-M2, 5′-FAM-ATATTTGTCGACGGAAT-3′. Each real-time TaqMan PCR was performed using cDNA equivalent to 10 ng total parasite RNA, according to the manufacturer’s universal conditions PCR protocol, in a final volume of 25 μl. All samples were run in triplicate and underwent 45 amplification cycles on a 7500 ABI Prism (Applied Biosystems) sequence detection system instrument. For relative quantification, the ΔΔCt method was employed, using α-tubulin as the endogenous standard for each sample. Results obtained from parasites treated with irrelevant siRNA were used for calibration (27). For graphical representation, the ΔΔCt values were normalized to controls and expressed as a percentage difference (27). To compare SmAQP gene expression across schistosome life cycle stages, the housekeeping triose phosphase isomerase gene was used as a control, as previously described (11).
Anti-SmAQP antibody production
The peptide NH2-KSDFVVDVDYDDSHRDG-COOH, comprising a sequence at the carboxyl terminus of SmAQP corresponding to aa 279–295, was synthesized by Genemed Synthesis, Inc. (San Antonio, TX, USA). This sequence is indicated in boldface in Fig. 1A. A cysteine residue was added at the amino terminus to facilitate conjugation of the peptide to bovine serum albumin (BSA). Approximately 500 μg of the peptide-BSA conjugate in Freund’s complete adjuvant was used to immunize two New Zealand white rabbits subcutaneously. The rabbits were boosted with 100 μg of peptide alone in incomplete Freund’s adjuvant 20, 40, and 60 d later. Ten days following this, serum was recovered from both rabbits and pooled, and anti-SmAQP antibodies were affinity-purified and dialyzed against PBS, as described previously (15).
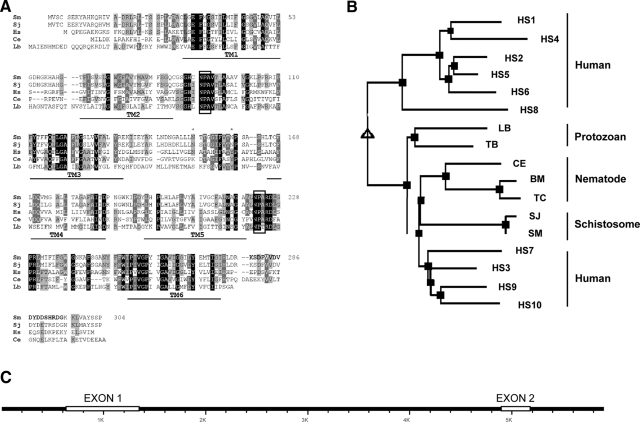
Figure 1.
A) Alignment of SmAQP with other members of the AQP protein family. Sm, S. mansoni (GenBank accession no. EU780065); Sj, S. japonicum (AAW24850); Hs, Homo sapiens isoform AQP9 (NP066190); Ce, Caenorhabditis elegans (NP495972); Lb, Leishmania braziliensis (XP001562217). Predicted transmembrane domains are indicated (TM1–6); conserved NPA motifs are boxed; asterisks represent potential N-linked glycosylation sites (at positions 150 and 159). B) Phylogenetic tree of selected AQPs, generated by multiple sequence alignment with hierarchical clustering. Designations (and accession numbers) of AQP homologs compared are as in A. Additional AQPs: HS1–HS10, human AQP isoforms AQP1–AQP10 (NP932766, NP000477, CAG46822, NP001641, NP001642, NP001643, CAI13307, NP001160, NP066190, NP536354). Protozoans: LB, L. braziliensis (XP001562217); TB, Trypanosoma brucei brucei (CAG27022). Nematodes: CE, C. elegans (NP495972); BM, Brugia malayi (XP001892122); TC, Toxocara canis (AAC32826). C) SmAQP gene. White boxes indicate exons 1 and 2. K, kilobase pair.
Protein immunolocalization
Immunofluorescent detection of SmAQP in cold, acetone-fixed, whole schistosomula was carried out using the affinity-purified rabbit anti-SmAQP antiserum and Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen), essentially as described earlier (28).
Western blot analysis
Schistosomula lysates were prepared by adding 40–60 μl of ice-cold cell disruption buffer (PARIS Kit, Ambion) and incubating for 30 min on ice. The protein content was estimated using the BCA Protein Assay Kit (Pierce, Rockford, IL, USA), according to the manufacturer’s instructions. Five micrograms of each extract was resolved on NuPAGE 4–12% Bis-Tris ready-made gels (Invitrogen). Proteins were then transferred to polyvinylidene fluoride membranes, which were blocked with 5% skim milk in PBS containing 0.1% Tween 20 (PBST) for 1 h at room temperature. The membrane was then probed overnight at 4°C with affinity purified rabbit immune-serum at 1:200 (directed against SmAQP or against a control protein, SPRM1hc; ref. 11). Following 3 washes with PBST and incubation with HRP-conjugated anti-rabbit antibody for 30 min at room temperature, protein bands were visualized by ECL Plus (Amersham, Little Chalfont, UK) and autoradiography on Blue Basic Autoradfilm (ISC BioExpress, Mortsel, Belgium). Protein band quantification was achieved using the Kodak Image Station 2000RT system and Kodak ID 3.6.5 software (Eastman Kodak, Rochester, NY, USA).
Drug treatment
Potassium antimonyl tartrate (PAT; Sigma, St. Louis, MO, USA) dissolved in distilled H2O was added to parasites in RPMI at a final concentration of 10 μM for 2 h. To monitor viability, parasite aliquots were assessed before and after PAT administration, using Hoechst 33258 staining, as described above.
Size measurements of parasites
Schistosomula were transferred from complete RPMI medium to either hypoosmotic medium (distilled water) for various times, as indicated, or hyperosmotic medium (2400 mosmol/L NaCl) for 30 min. To compare parasite size before and after transfer, images were taken using an inverted microscope (TH4–100; Olympus, Tokyo, Japan) equipped with a Retiga 1300 camera (Q Imaging, Surrey, BC, Canada), and the area occupied by individual schistosomula was measured using ImageJ 1.41 software (U.S. National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Student’s t test was used to determine the statistical significance of differences between groups.
RESULTS
SmAQP gene and cDNA
To identify the entire SmAQP cDNA, EST sequence information derived from proteomic analysis of the tegumental membranes (19, 24) was first used to examine the S. mansoni genome database (version 3, http://www.sanger.ac.uk/Projects/S_mansoni/). In this manner, the putative SmAQP gene was identified and, as outlined in Materials and Methods, genomic sequence information was used to design predicted 5′ and 3′ oligonucleotides that were then used to generate the complete SmAQP cDNA by PCR. The cDNA potentially encodes the 304-aa SmAQP protein (GenBank accession no. EU780065) with a predicted molecular mass of 32,893 Da and a predicted pI of 8.67. Figure 1A shows an alignment of SmAQP with diverse members of this protein family generated using ClustalW; conserved motifs are highlighted. SmAQP is predicted to contain 6 transmembrane domains (TM1–6, Fig. 1A). The protein is 84% identical to its counterpart from Schistosoma japonicum. The schistosome proteins contain the conserved NPA motifs (boxed in Fig. 1A) that are reported to be important in water channel formation. There are two potential protein kinase II phosphorylation sites (SCSE and SHRD, at positions 3 and 291), as well as two potential protein kinase C phosphorylation sites (SEK and SHR, at positions 5 and 291) at the extreme amino and carboxyl termini of SmAQP, respectively. Both the amino and carboxyl termini are predicted to be intracellular. Two potential N-linked glycosylation sites exist between transmembrane domains 3 and 4 (at positions150 and 159; asterisks, Fig. 1A). Multiple sequence alignment with hierarchical clustering was used to generate the phylogenetic tree shown in Fig. 1B (29). SmAQP exhibits lower sequence identity with human counterparts belonging to the strict AQP family (18–23%, vs. AQPs 1, 2, 4, 6, and 8) compared with human proteins that can act as conduits for metabolites in addition to water (31–36% vs. aquaglyceroporins numbered 3, 7, 9, and 10), and this is reflected in the phylogeny shown in Fig. 1B.
The entire SmAQP1 gene was identified on contig 00176 (S. mansoni genome assembly version 3.1; http://www.sanger.ac.uk/Projects/S_mansoni/). As illustrated in Fig. 1C, the gene is 4.54 kb and consists of two exons; exon 1 is 708 bp, and exon 2 is 277 bp, and these are separated by a 3554-bp intron. The AT content of the exons varies from 57% (exon 1) to 60% (exon 2), whereas, and in keeping with the generally high AT content of the schistosome genome, the AT content of the intron is substantially higher (75%). The intron possesses canonical GT:AG splice donor and acceptor sites. The SmAQP gene has some Zozak consensus (30), including an A 3 bases upstream of the initiator ATG, and this start codon is followed by another G. Despite the AT richness of the schistosome genome, no canonical TATA box is discernable up to 250 bases upstream of the initiator codon.
Developmental expression profile of SmAQP
The level of expression of the SmAQP gene, relative to expression of the housekeeping control triose phosphate isomerase gene, was examined by qRT-PCR in a number of schistosome life stages, and results are shown in Fig. 2. SmAQP gene expression is barely detectable in eggs and sporocysts but increases dramatically soon after cercarial transformation and remains high in adult parasites.
Figure 2.
Expression of the SmAQP gene in different schistosome developmental stages relative to expression of control triose phosphate isomerase gene. Spor, 24-h cultured sporocyst; Cer, cercaria; Som, 24-h cultured schistosomulum); male and female, 7 wk old.
SmAQP localization in schistosomula
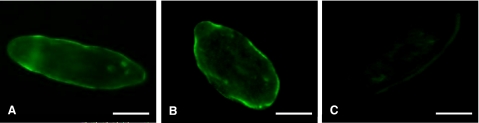
SmAQP is clearly detected most strongly in the tegument of both 2-d (Fig. 3A) and 7-d (Fig. 3B) cultured schistosomula by immunostaining. Control parasites, exposed to secondary antibody alone, remain unstained (Fig. 3C).
Figure 3.
A, B) Immunolocalization of SmAQP in 2-d (A) and 7-d (B) cultured schistosomula. Strong tegumental staining is evident. C) Control 7-d schistosomulum probed with secondary antibody alone. Scale bars = 50 μm.
Suppression of SmAQP1 gene expression
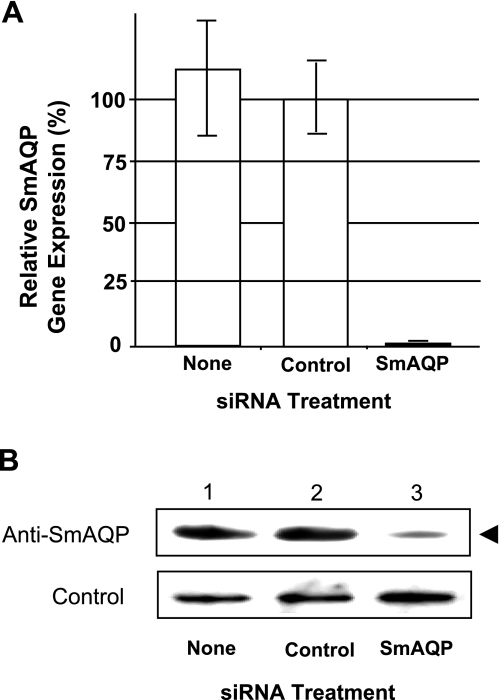
SmAQP gene expression was suppressed in schistosomula in vitro by introducing target-specific siRNAs using electroporation. Figure 4A shows the specific and robust suppression of SmAQP (98% suppression), measured 2 d after treatment with the siRNAs designated. Two SmAQP siRNAs (siAqua1 and 2), targeting different regions of the AQP mRNA, were tested, and both exerted comparable effects on SmAQP gene expression (not shown). Parasites maintained in culture and examined 8, 14, and 21 d after siRNA treatment exhibited sustained (>90%) suppression at each time point (not shown). Western blot analysis demonstrates that this treatment also results in substantial suppression of SmAQP protein production. An ∼5-fold lower level of SmAQP protein was detected using densitometry in extracts of SmAQP-suppressed parasites vs. controls (Fig. 4B). The protein was detected at its expected molecular size (∼33,000 Mr).
Figure 4.
A) Relative SmAQP expression in cultured schistosomula 2 d after no treatment (none) or treatment with control or SmAQP siRNA. Data represent means ± se. B) Detection by Western blot analysis of SmAQP protein (top panel) and a control protein (SPRM1hc; bottom panel) in extracts prepared from parasites 5 d after no treatment (none; lane 1) or treatment with control (lane 2) or SmAQP siRNA (lane 3). Arrowhead indicates relatively diminished level of SmAQP protein in lane 3 (∼33,000 Mr).
SmAQP is a water transporter
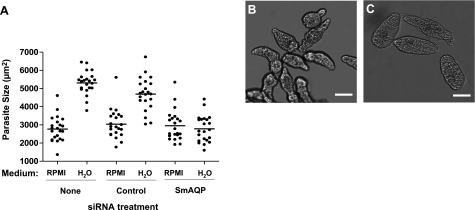
To test the hypothesis that SmAQP mediates water entry into schistosomes, we first suppressed SmAQP expression in schistosomula, as described earlier, and then transferred an aliquot containing >100 parasites from complete RPMI culture medium into hypotonic medium (distilled water) 5 d posttreatment. Parasites were photographed before and 5 min after transfer into water, and the sizes of a sample of individual parasites from control and SmAQP-suppressed groups were compared. Differences between the mean sizes of the parasites from the various groups were assessed for statistical significance using Student’s t test.
Figure 5A shows the quantitative data acquired from this experiment, as well as sample images of control parasites in rich medium (Fig. 5B), or 5 min after incubation in distilled water (Fig. 5C). It is notable that the mean size of the group of parasites whose SmAQP gene is suppressed does not change significantly when these parasites are moved from culture medium to distilled water (Fig. 5A, right group). In contrast, when control parasites (either untreated or treated with an irrelevant siRNA) are similarly transferred from complete RPMI medium to distilled water, there is a rapid movement of water into the parasites, which swell to about twice their original size (Fig. 5A, middle and left groups). The mean size of these parasites in water is significantly different from that of their SmAQP-suppressed counterparts (P<0.05).
Figure 5.
A) Size of cultured schistosomula 5 d after no treatment (none) or treatment with control or SmAQP siRNA, when incubated either in complete RPMI medium (RPMI) or after 5 min in distilled water (H2O). Each point represents an individual parasite; line indicates the mean for that group; n = 22/treatment. Mean size of AQP-suppressed group in water differs significantly from both control counterparts; P < 0.05. B, C) Representative group of control untreated schistosomula in RPMI medium (B) and after 5 min in distilled water (C). Scale bars = 50 μm.
To determine the capacity of schistosomula to swell following prolonged incubation in distilled water, control parasites were measured at various time points after their transfer from rich medium to water. Figure 6A shows the results of this analysis. Compared to the 5-min time point, it is apparent that the parasites do not swell further with longer incubation. To determine whether the parasites can recover from the rapid influx of water, aliquots of schistosomula in water (for 5, 30, or 60 min) were then returned to complete RPMI medium, and their sizes were measured 30 min later. Figure 6B shows that at least until the 1-h time point, these parasites do recover; once returned to RPMI, their mean size does not differ significantly from that of counterparts that remained in RPMI.
Figure 6.
A) Size of cultured schistosomula at different time points after incubation in distilled H2O. Data represent means ± sd. B) Size of cultured schistosomula in RPMI or after 5 min in H2O or on return to RPMI following incubation in H2O for the time periods indicated; n = 15/treatment.
When SmAQP-suppressed parasites are incubated in hyperosmotic solution, they undergo no significant change in size, as shown in Fig. 7A (right group). In contrast, similarly treated control parasites shrink in size (Fig. 7A, left group). Figure 7 shows representative images of control parasites in rich medium (Fig. 7B), or after incubation in hyperosmotic solution (Fig. 7C).
Figure 7.
A) Size of cultured schistosomula 5 d after treatment with control or SmAQP siRNA when incubated either in complete RPMI medium (−) or after 30 min in hyperosmotic solution (+). Each point represents an individual parasite; line indicates mean for that group; n = 15/treatment. Mean size of AQP-suppressed group in water differs significantly from the control group in hyperosmotic solution; P < 0.05. B, C) Images of a representative group of control untreated schistosomula in RPMI medium (B) and after 20 min in hyperosmotic solution (C). Scale bars = 50 μm.
SmAQP1 as a drug transporter
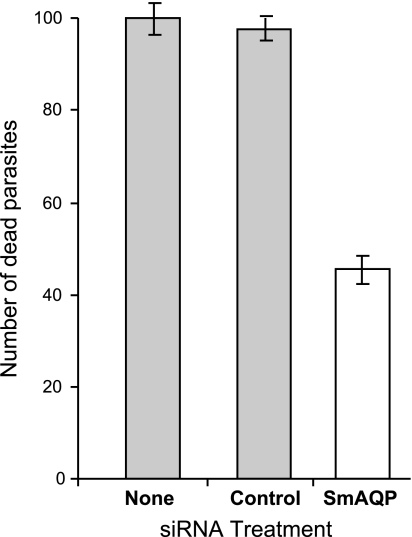
The AQP of the Leishmania parasite acts as a conduit for parasite-killing antimonial drugs (31). To test the hypothesis that SmAQP likewise permits entry of the schistosome-killing drug PAT, the viability of schistosomula that had been treated with SmAQP siRNA was compared with control, untreated parasites and control parasites treated with an irrelevant siRNA, before and after 2 h exposure to 10 μM PAT. The difference in viability before and after drug treatment distinguishes parasite debility from gene suppression vs. debility from exposure to the drug. Drug-induced deaths are recorded in Fig. 8. It is apparent that significantly fewer parasites from the SmAQP-suppressed group are killed following exposure to PAT, compared to either control group (P<0.05, Fig. 8).
Figure 8.
Number of dead schistosomula following 2 h incubation in RPMI containing 10 μM PAT, 8 d after no treatment (none) or treatment with control or SmAQP siRNA (open bar). Significantly fewer parasites were killed in the SmAQP-suppressed group vs. either control group; P < 0.05. Data represent means ± sd.
Suppressing SmAQP lowers parasite viability
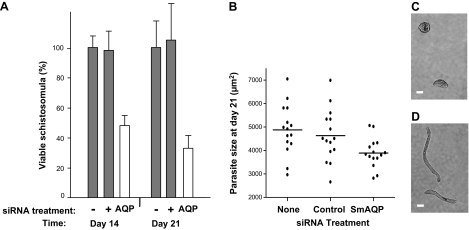
The viability of SmAQP-suppressed parasites was compared with that of control counterparts at d 14 and 21 following siRNA exposure, and results are shown in Fig. 9A. At both time points, the SmAQP-suppressed parasites have significantly lower viability compared with control parasites that have been treated with an irrelevant siRNA (+, Fig. 9A) or control parasites that were untreated (−, Fig. 9A; P<0.05 vs. both controls).
Figure 9.
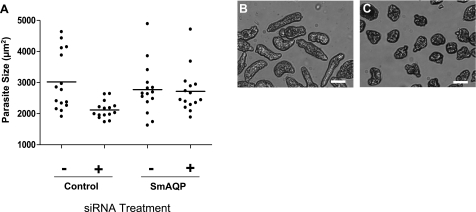
A) Viability of 14- and 21-d cultured schistosomula after no treatment (−) or treatment with control (+) or SmAQP siRNA (AQP) on d 2. Significantly fewer SmAQP-suppressed parasites (open bars) are viable at both time points vs. controls (shaded bars); P < 0.05. Data represent means ± sd. B) Size of 21-d cultured schistosomula after no treatment (none) or treatment with control or SmAQP siRNA on d 2. Mean size of SmAQP-suppressed group differs significantly from both controls; P < 0.05; n = 15/treatment. C, D) Representative images of 21-d-old SmAQP-suppressed parasites (C) vs. their generally more elongated control counterparts (D). Scale bars = 50 μm.
Morphological change in the SmAQP-suppressed parasites becomes apparent following prolonged maintenance in vitro. Parasites from all groups were measured at 21 d after treatment; as shown in Fig. 9B, the mean size of the SmAQP-suppressed group was significantly smaller than that of either control group (P<0.05). Furthermore, suppressed parasites tend to have a more squat appearance (Fig. 9C) compared with their generally more elongate control counterparts (Fig. 9D).
DISCUSSION
Here, we describe the cloning and functional characterization of a novel schistosome protein, SmAQP, which shows strong sequence conservation with members of the AQP protein family. This family is divided into two groups based on member permeability properties: strict AQPs (for instance, human proteins AQP1, -2, -4, -5, -6, and -8) only allow water to permeate the pore. Members of the second group are permeable to other small solutes, such as glycerol, and these proteins are termed aquaglyceroporins (for instance, human proteins AQP3, -7, -9, and -10) (16,17,18). The schistosome protein has higher sequence similarity with the latter group, suggesting that its permeability extends beyond water alone.
The expression of SmAQP is highest in the schistosome intravascular life stages, suggesting that the protein plays a role in regulating the movement of water to and from the definitive host. The tegument stains strongly with anti-SmAQP antibodies, suggesting that this is a site of prime water exchange between the intravascular parasites and their hosts. These immunolocalization data support earlier proteomic work, which detected the AQP in the intravascular schistosome tegument (19, 20).
SmAQP is a tegumental water transporter
Treating schistosomula once with siRNAs targeting SmAQP mRNA results in sustained suppression of gene expression. At least until the 21-d time point, gene expression remains at <90% that of controls. Data derived from such suppression of SmAQP gene expression support the hypothesis that the protein functions as a water pore in schistosomes. The function of the first cloned AQPs of other species was demonstrated following their heterologous expression in Xenopus oocytes (32). These oocytes rapidly swell and burst, relative to control oocytes, when placed in hypoosmotic medium (distilled water); the heterologous AQPs in the oocytes permit the unrestricted inward movement of water, resulting in osmotic lysis (32). If SmAQP fulfills a similar function in schistosomes, we hypothesized that diminishing the level of this tegumental protein using RNAi would lessen the parasite’s ability to swell and experience osmotic lysis when placed in distilled water. Experimental data show that SmAQP-suppressed parasites do lose the ability to rapidly swell when transferred from rich medium to hypoosmotic medium (distilled water), supporting the hypothesis. Similarly, these parasites do not shrink when transferred from rich medium into hyperosmotic medium. These data suggest that SmAQP is important for both entry and exit of water in schistosomes. The data reveal a heretofore unrecognized role of the schistosome tegument in water movement and volume regulation. This work also illustrates the power of the RNAi approach to test specific hypotheses concerning the function of individual proteins in these important human parasites.
Schistosomes in water
When schistosomula are transferred from rich medium into water, they rapidly swell. Maximal swelling is observed within 5 min incubation in water. Thereafter, the parasites do not change significantly in size, and no dramatic osmotic lysis of the parasites is ever detected, even after 72 h incubation in water. This swelling is not irreversible; once parasites are returned to complete RPMI medium after 5, 30, or 60 min in water, they regain their original size. This is not to say that these parasites survive long term after this treatment. While a majority of the parasites that have been in water for just 5 min survive following further culture in complete medium, those that have been incubated in water for 30 or 60 min, although normal in size, rapidly die over the following 24–48 h, suggesting that these worms have been terminally stressed by the procedure.
It is clear that the role of SmAQP is vital for schistosome survival since significantly fewer parasites whose SmAQP gene are suppressed survive in vitro culture compared to controls. Fewer than half of the suppressed parasites survive at the time points examined. Those that do survive appear stunted, at least after prolonged suppression (at d 21 posttreatment). Unlike controls, these suppressed worms generally do not possess an elongate appearance. An inability to regulate water movement likely impairs normal parasite biochemistry, resulting in increased mortality and the inhibition of normal parasite development.
SmAQP as a portal for other metabolites, such as drugs
As noted earlier, some AQP proteins can act as conduits for substrates other than water. For instance, some AQPs have been shown to play a role in controlling the exchange of metalloids (like arsenic and antimony) between an organism and its environment (33, 34). Some metalloids have been used therapeutically. For instance, medical application of arsenic can kill some parasites (such as trypanosomes), bacteria, and cancer cells (35). Organic trivalent antimonials are used in the treatment of other parasitic diseases, such as leishmaniasis (31). Although the primary treatment for schistosomiasis currently is praziquantel, until relatively recently an important treatment option for schistosome infection involved the use of trivalent antimonials, such as PAT (36,37,38,39). The cytosolic enzymes phosphofructokinase and thioredoxin glutathione reductase have both been shown to be targets of these antischistosome agents (37, 39, 40). How the drugs enter the schistosomes to effect this inhibition of intracellular enzymes is not known. In the case of Leishmania, parasite-killing trivalent antimonials enter the parasites through surface AQP proteins; mutations in AQP genes confer resistance to the drugs (31). We hypothesize that likewise in schistosomes, antimonial drugs enter the parasites through the surface SmAQP. If this is the case, then parasites with reduced levels of SmAQP should have an impaired ability to take up the drug and should therefore exhibit a greater resistance to PAT-mediated killing. Our data support this hypothesis, since significantly fewer SmAQP-suppressed parasites are killed following exposure to PAT compared to control parasites treated with an irrelevant siRNA or control, untreated parasites. Given our reliance on a single drug (praziquantel) for schistosome control, an increased knowledge of the mechanism of action of other antischistosome agents, such as PAT, is important.
In summary, data presented in this paper support the notion that the newly described tegumental protein SmAQP can function not only as a water channel but also as a conduit for antimonial drugs such as PAT. SmAQP is vital for parasite survival, likely because of the important physiological role it plays in schistosome osmotic adaptation. This vital function, coupled with the localization of SmAQP at the host-interactive tegumental surface, suggests that the protein may make an accessible and viable target for chemotherapeutic or immunological intervention.
Acknowledgments
We thank Dr. Greice Krautz-Peterson and Dr. Chuck Shoemaker for helpful comments and David Ndegwa for technical assistance. Schistosome-infected snails were provided by the Biomedical Research Institute through National Institutes of Health–National Institute of Allergy and Infectious Diseases (NIH-NIAID) contract N01-AI-30026. This work was supported by NIH-NIAID grant AI-056273.
References
- Koukounari A, Gabrielli A F, Toure S, Bosque-Oliva E, Zhang Y, Sellin B, Donnelly C A, Fenwick A, Webster J P. Schistosoma haematobium infection and morbidity before and after large-scale administration of praziquantel in Burkina Faso. J Infect Dis. 2007;196:659–669. doi: 10.1086/520515. [DOI] [PubMed] [Google Scholar]
- King C H, Dickman K, Tisch D J. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- King C H, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- Morris G P, Threadgold L T. Ultrastructure of the tegument of adult Schistosoma mansoni. J Parasitol. 1968;54:15–27. [PubMed] [Google Scholar]
- Smith J H, Reynolds E S, Von Lichtenberg F. The integument of Schistosoma mansoni. Am J Trop Med Hyg. 1969;18:28–49. [PubMed] [Google Scholar]
- Hockley D J, McLaren D J. Schistosoma mansoni: changes in the outer membrane of the tegument during development from cercaria to adult worm. Int J Parasitol. 1973;3:13–25. doi: 10.1016/0020-7519(73)90004-0. [DOI] [PubMed] [Google Scholar]
- Wilson R A, Barnes P E. The tegument of Schistosoma mansoni: observations on the formation, structure and composition of cytoplasmic inclusions in relation to tegument function. Parasitology. 1974;68:239–258. [PubMed] [Google Scholar]
- Threadgold L T. Parasitic platyhelminths. Bereiter-Hahn J, Matolsky A G, Richards K S, editors. Berlin: Springer-Verlag; 1984:132–191. [Google Scholar]
- McLaren D J, Hockley D J. Blood flukes have a double outer membrane. Nature. 1977;269:147–149. doi: 10.1038/269147a0. [DOI] [PubMed] [Google Scholar]
- Jiang J, Skelly P J, Shoemaker C B, Caulfield J P. Schistosoma mansoni: the glucose transport protein SGTP4 is present in tegumental multilamellar bodies, discoid bodies, and the surface lipid bilayers. Exp Parasitol. 1996;82:201–210. doi: 10.1006/expr.1996.0025. [DOI] [PubMed] [Google Scholar]
- Krautz-Peterson G, Camargo S, Huggel K, Verrey F, Shoemaker C B, Skelly P J. Amino acid transport in schistosomes: characterization of the permease heavy chain SPRM1hc. J Biol Chem. 2007;282:21767–21775. doi: 10.1074/jbc.M703512200. [DOI] [PubMed] [Google Scholar]
- Mastroberardino L, Spindler B, Pfeiffer R, Skelly P J, Loffing J, Shoemaker C B, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- Skelly P, Cunningham J, Kim J, Shoemaker C. Cloning, characterization and functional expression of cDNAs encoding glucose transporter proteins from the human parasite, Schistosoma mansoni. J Biol Chem. 1994;269:4247–4253. [PubMed] [Google Scholar]
- Skelly P J, Shoemaker C B. Rapid appearance and asymmetric distribution of glucose transporter SGTP4 at the apical surface of intramammalian-stage Schistosoma mansoni. Proc Natl Acad Sci U S A. 1996;93:3642–3646. doi: 10.1073/pnas.93.8.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Skelly P J, Leaffer D, Cohn R G, Caulfield J P, Shoemaker C B. Immunolocalization of a Schistosoma mansoni facilitated diffusion glucose transporter to the basal, but not the apical, membranes of the surface syncytium. Parasitology. 1995;110:383–394. doi: 10.1017/s0031182000064726. [DOI] [PubMed] [Google Scholar]
- Gonen T, Walz T. The structure of aquaporins. Q Rev Biophys. 2006;39:361–396. doi: 10.1017/S0033583506004458. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Shayakul C, Berger U V, Mackenzie B, Devidas S, Guggino W B, van Hoek A N, Hediger M A. Molecular characterization of a broad selectivity neutral solute channel. J Biol Chem. 1998;273:24737–24743. doi: 10.1074/jbc.273.38.24737. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Weremowicz S, Morton C C, Hediger M A. Functional and molecular characterization of the human neutral solute channel aquaporin-9. Am J Physiol Renal Physiol. 1999;277:F685–F696. doi: 10.1152/ajprenal.1999.277.5.F685. [DOI] [PubMed] [Google Scholar]
- Braschi S, Curwen R S, Ashton P D, Verjovski-Almeida S, Wilson A. The tegument surface membranes of the human blood parasite Schistosoma mansoni: a proteomic analysis after differential extraction. Proteomics. 2006;6:1471–1482. doi: 10.1002/pmic.200500368. [DOI] [PubMed] [Google Scholar]
- Skelly P, Wilson R. Making sense of the schistosome surface. Adv Parasitol. 2006;63:185–284. doi: 10.1016/S0065-308X(06)63003-0. [DOI] [PubMed] [Google Scholar]
- Skelly P J, Da'dara A, Harn D A. Suppression of cathepsin B expression in Schistosoma mansoni by RNA interference. Int J Parasitol. 2003;33:363–369. doi: 10.1016/s0020-7519(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Jones J T, Helm C N, Kusel J R. Variation in susceptibility of Schistosoma mansoni to damage by polycations. Mol Biochem Parasitol. 1988;30:35–44. doi: 10.1016/0166-6851(88)90130-2. [DOI] [PubMed] [Google Scholar]
- Hackett F. The culture of Schistosoma mansoni and production of life cycle stages. Hyde J E, editor. Totowa, NJ, USA: Humana Press; 1993:89–99. doi: 10.1385/0-89603-239-6:89. [DOI] [PubMed] [Google Scholar]
- Braschi S, Wilson R A. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Proteomics. 2006;5:347–356. doi: 10.1074/mcp.M500287-MCP200. [DOI] [PubMed] [Google Scholar]
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Correnti J M, Brindley P J, Pearce E J. Long-term suppression of cathepsin B levels by RNA interference retards schistosome growth. Mol Biochem Parasitol. 2005;143:209–215. doi: 10.1016/j.molbiopara.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Skelly P J, Dougan P M, Maule A, Day T A, Shoemaker C B. Cloning and characterization of a muscle isoform of a Na,K-ATPase alpha subunit (SNaK1) from Schistosoma mansoni. Parasitology. 2001;123:277–284. doi: 10.1017/s0031182001008484. [DOI] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourbal B, Sonuc N, Bhattacharjee H, Legare D, Sundar S, Ouellette M, Rosen B P, Mukhopadhyay R. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J Biol Chem. 2004;279:31010–31017. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- Preston G M, Carroll T P, Guggino W B, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Sanders O I, Rensing C, Kuroda M, Mitra B, Rosen B P. Antimonite is accumulated by the glycerol facilitator GlpF in Escherichia coli. J Bacteriol. 1997;179:3365–3367. doi: 10.1128/jb.179.10.3365-3367.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Shen J, Carbrey J M, Mukhopadhyay R, Agre P, Rosen B P. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci U S A. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienert G P, Schussler M D, Jahn T P. Metalloids: essential, beneficial or toxic? Major intrinsic proteins sort it out. Trends Biochem Sci. 2008;33:20–26. doi: 10.1016/j.tibs.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Molina R, Acevedo C E, Torres J M, Lopez-Sanabria U, Ramirez-Rodriguez E. Treatment of schistosomiasis mansoni with antimony lithium thiomalate (Anthiomaline); final report. Am J Trop Med Hyg. 1950;30:881–886. doi: 10.4269/ajtmh.1950.s1-30.881. [DOI] [PubMed] [Google Scholar]
- Bueding E, Mansour J M. The relationship between inhibition of phosphofructokinase activity and the mode of action of trivalent organic antimonials on Schistosoma mansoni. Br J Pharmacol Chemother. 1957;12:159–165. doi: 10.1111/j.1476-5381.1957.tb00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaleb H A, Shoeb H, el-Kawhary N, el-Borolossy A W, el-Halawany S A, Madkour M K. Screening of three synthetic organic trivalent antimonials for potential antischistosomal activity in mice. J Egypt Med Assoc. 1979;62:1–29. [PubMed] [Google Scholar]
- Su J G, Mansour J M, Mansour T E. Purification, kinetics and inhibition by antimonials of recombinant phosphofructokinase from Schistosoma mansoni. Mol Biochem Parasitol. 1996;81:171–178. doi: 10.1016/0166-6851(96)02702-8. [DOI] [PubMed] [Google Scholar]
- Kuntz A N, Davioud-Charvet E, Sayed A A, Califf L L, Dessolin J, Arner E S, Williams D L. Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target. PLoS Med. 2007;4:e206. doi: 10.1371/journal.pmed.0040206. [DOI] [PMC free article] [PubMed] [Google Scholar]