Abstract
High expression of Aurora-B has been observed in various cancers, and inhibition of this kinase has been shown to halt cellular proliferation. However, the mechanism of effect of Aurora-B on cellular transformation has not been fully explored. Here, we show that overexpression of Aurora-B in murine epithelial cells promotes generation of tetraploids. In search of a related mechanism, spectral karyotyping was carried out, showing premature chromatid separation (PCS). Of interest, PCS is a hallmark of Robert’s syndrome, which also involves cellular polyploidy and aneuploidy. Sorted tetraploid Aurora-B-overexpressing cells promoted significant mammary epithelial cancers when injected into nude mice, as compared to injection of nonfractionated cells, suggesting that tetraploidy is an important mediator of Aurora-B-induced tumorigenesis. Comparative chromosome hybridization performed on DNA derived from tetraploid cell-induced tumors indicates amplifications and deletions of regions throughout the genome, which include tumor-promoting or tumor-suppressing genes, respectively. Thus, sustained expression of Aurora-B induces tetraploidy, which, in turn, facilitates genomic instability and tumor development in a xenograft model.—Nguyen, H. G., Makitalo, M., Yang, D., Chinnappan, D., St. Hilaire, C., Ravid, K. Deregulated Aurora-B induced tetraploidy promotes tumorigenesis.
Keywords: polyploidy, cancer, cell cycle, premature chromatid separation
Sister chromatid separation is undoubtedly a crucial step in anaphase, where precision ensures transmission of genetic material to daughter cells. Defects in the timing of this event would lead to a gain or loss of whole chromosomes, leading to genetically unstable aneuploid cells (reviewed in ref. 1). Partial loss of mitotic checkpoint proteins MAD2, CENP-50, and Bub1 in yeast, Drosophila, and mammalian cells has been shown to cause total premature sister chromatid separation (PCS), which results in genomic instability (2,3,4,5,6,7,8,9). PCS has been described in patients (10) carrying the disorder identified as Roberts syndrome, involving marked genetic abnormalities including, trisomies, double trisomies, and monosomies, a condition similar to mosaic variegate aneuploidy (MVA) (5, 11). Infants affected by the disease generally display severe prenatal and postnatal growth retardation, microcephalus, Dandy-Walker malformation, and Wilms tumor of the kidney, and often die at 2–3 yr of age (12). Skin fibroblasts isolated from these patients were shown to have lost mitotic checkpoint. Namely, the cells underwent G1 and S phases without sister-chromatid segregation and cytokinesis, resulting in accumulation of tetraploidy (13).
Cells can become tetraploid accidentally through cytokinesis failure, mitotic slippage, chromosome missegregation, cleavage furrow regression, virus-induced cell fusion, and defects that prevent normal kinetochore–microtubule attachment (reviewed in refs. 1, 14). On the other hand, tetraploidy can arise by endomitosis, a normal programmed cellular process, as seen in the megakaryocyte developments (15). Recent evidence suggests that defects in DNA replication or repair can also trigger blockage of cytokinesis and produce tetraploidy. Loss of the adenomatous polyposis coli (APC) tumor suppressor gene has been implicated in the generation of tetraploidy and polyploidy cells through defective mitotic spindle checkpoint in early stages of tumorigenesis (16). As reported, BubR1(+/−)-APC(−/+) compound mutant mice develop 10 times more colonic tumors than Apc(−/+) mice, with a higher rate of genomic instability accompanied by premature separation of sister chromatids (4). Loss of function mutation for genes involved in DNA repair, such as BRCA1, BRCA2, and Gadd45, can lead to cleavage furrow defects, abnormal centrosome number, and tetraploidization (17, 18). In addition, tetraploidy and the subsequent genomic instability induced by overexpression c-Myc was recently observed in the megakaryocytic endomitotic pathway involving Gp1bα (19), suggesting that tetraploidy either acquired through programmed processes or accidentally come at a cost. Recently, germ line mutation of the mitotic checkpoint protein BUB1B was identified to be one of the sources of variegate aneuploidy and tetraploidy in humans (20), establishing a causal link between losses of mitotic checkpoints, leading to tetraploidy, aneuploidy, and cancer development.
A cell with a tetraploid or polyploid DNA content has been hypothesized to be a genetically unstable intermediate that often progresses to aneuploidy in the succeeding divisions (1, 21), which has been associated with the development of esophageal, cervical, colon, and hepatic cancers (1, 22). Tetraploid cells are observed in normal tissues during aging, including hepatocytes, and vascular smooth muscle cells (23). However, the issue of whether primary tetraploid cells are more prone to transformation than their diploid counterpart remains to be fully explored. A recent study (24) convincingly established the role of tetraploidy in tumorigenesis in p53-null cells. The authors were able to isolate genetically matched diploid and tetraploid cells by disrupting cytokinesis with dihydrocytochalasin B, and subjecting the cells to a tumorigenic assay.
We recently reported that a stable mutant of Aurora-B induces tetraploidy and aneuploidy in normal murine epithelial cells (25). This is of high interest since various cancers are associated with elevated Aurora-B (reviewed in refs. 1, 26, 27). In the present study, we employed this system to investigate the effect of overexpressed Aurora-B on chromosome segregation, as well as the role of Aurora-B-induced tetraploidy in tumorigenesis. We found that overexpressed Aurora-B induces premature chromosome separation, and generation of tetraploid and aneuploid cells, and these tetraploid cells develop transformation properties in vitro and in vivo.
MATERIALS AND METHODS
Cell sorting based on ploidy content (staining and culturing procedures)
This procedure was applied in experiments involving cell sorting based on DNA content for subsequent protein or mRNA extraction or culturing. The emphasis here was to keep the cells alive at all times to minimize protein and mRNA degradation. Trypsinizing procedures were followed as described previously (25). Before trypsinization, cells were treated with 10 ng/ml of Hoechst stain (stock solution must be kept away from light and aliquoted to avoid free-thaw cycles) for 30 min in a tissue culture incubator. For sorting to work optimally, all cells were trypsinized completely to avoid clumps. Cells were resuspended in their cultured medium after trypsinization while placed on ice throughout the procedure, including during the transfer to the sorting facility. At the sorting facility, a specific setting was used to reject clumps (population of doublet or triplet diploid cells) in order to obtain a pure population of sorted cells. In the case of subsequent culturing of sorted cells, extra precautions (e.g., system flushes with sterile PBS) were employed to avoid unwanted contamination. After the cells were plated (usually 8 h later), the medium was replaced as soon as possible to avoid any effects of the Hoechst stain. To further confirm whether the sorted cells were pure, an aliquot of the sorted cells was placed onto a slide, mounted with appropriate mounting medium, and visualized at ×40–60, using a UV filter to document the outcome of the sorting. Alternatively, resorting the sorted cells was performed to document the purity of sorting.
Chromosome analysis using spectral karyotyping (SKY), DAPI banding, fluorescent in situ hybridization (FISH), and array comparative genomic hybridization (CGH)
Cells were grown to 70% confluency and treated with 15 ng/ml colcemid (Gibco Life Technologies, Inc., Gaithersburg, MD, USA). Cells were spun down at 500 g for 10 min and resuspended in 12 ml hypotonic solution (0.075 M KCl) and incubated at 37°C for 20 min. At the end of the incubation period, a few drops of cold Carnoy’s fixative (3:1 ratio of methanol:acetic acid) was added to the cells. This is an important priming step prior to adding the Carnoy’s fixative. Cells were then washed twice in Carnoy’s fixative. Chromosome spreading and DAPI staining were performed by conventional methods, as we described previously (25). Briefly, cells were reconcentrated in Carnoy’s fixative at ∼5 × 106 cells/ml, using a plastic Pasteur pipette to drop cells at ∼2.5 ft onto the slide that has been cooled/submerged in ice-cold methanol. The samples were then analyzed by fluorescence microscopy at ×1000. SKY, DAPI banding, and FISH were done at Roswell Park Cancer Institute (Buffalo, NY, USA). To perform array CGH of tumors derived from 4N cells, genomic DNA was isolated according to standard protocol as supplied by Roswell Park Cancer Institute.
In vivo tumorigenesis assay using nude mice
Nude mice, nu/nu BulbC inbred (homozygous), 7- to 8-wk-old females were purchased from Charles River Laboratory (Wilmington, MA, USA). To test for tumorigenic property of normal mouse epithelial (NmuMG) cells stably expressing wild-type Aurora-B-GFP or stable mutant Abox-Aurora-B-GFP or 4N cells in vivo, 5 mice were used for each group, and injection was performed as follows: cells were thoroughly trypsinized and resuspended in a total volume of 100 μl sterile PBS containing 2.0 × 106 cells. This volume was injected subcutaneously into the mammary fat pad of each nude mouse. Tumor development was examined throughout a period of 8–10 wk, at which time tumors were measured by 3 maximum diameters at x, y, and z axes to derive tumor volume by size, followed by surgical removal of the tumor masses, and weighing for determination of tumor volume by weight as described previously (28). The remaining tumors were paraffin-embedded and thin-sectioned as described previously (29), followed by eosin/hematoxylin staining for pathology analysis in pathology lab. These thin sections were visualized using an ×20 objective, Olympus IX70 microscope (Olympus, Tokyo, Japan).
RESULTS
Aurora-B induces PCS, both in 2N and 4N populations of primary mouse epithelial cells
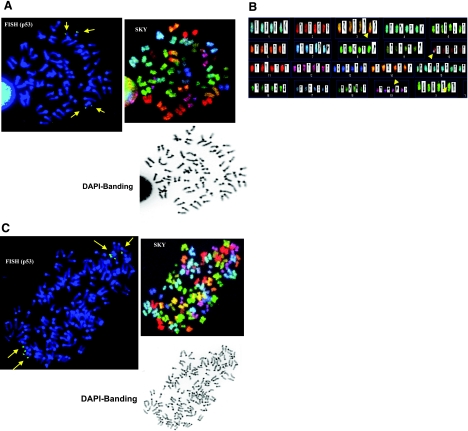
We previously reported that overexpression of a stable Aurora-B mutant promoted an aneuploidy phenotype in epithelial cells (25). There are numerous possible mechanisms that could lead to this cellular state (1). Given that Aurora-B kinase is involved in regulating chromosome separation at metaphase/anaphase transition, chromosome analysis of cells overexpressing Aurora-B kinase, as described previously (25), was performed. To this end, we employed SKY analysis and DAPI banding of 2N and 4N NmuMG cells synchronized at metaphase to assess potential chromosomal rearrangements, breakage or amplifications. NmuMG cells are nontransformed, have slow growth in semisolid media, and do not form tumors in nude mice (30). As shown in Fig. 1, of 590 cells screened, 72.7% showed total PCS, which is characterized by separated and splayed chromatids and a discernable centromere involving all chromosomes. The percentage of PCS in the 2N and 4N populations is 72.4 and 71.4, respectively. Hence, with Aurora-B overexpression, the numbers of cells showing sign of PCS is roughly the same, regardless of the ploidy status.
Figure 1.
Stable Aurora-B induces PCS in NmuMG cells, in both 2N and 4N cells. A) Mitosis carrying 2N range of chromosomes (42, XX with trisomies of no. 15 and 19), which show the phenomenon of total PCS, characterized by the separated and splayed chromatids and a discernable centromere involving all chromosomes (also known as C-anaphase cells). This is demonstrated by FISH, using BAC RP23-51O13 for p53 status (top left), SKY (top middle), and DAPI banding (top right). Because of the mitotic event leading to PCS, each chromatid of no. 11 chromosome is recognized to carry an individual FISH signal of p53. B) As clearly shown by SKY (bottom panel), the total number of chromatids is 84. C) Mitosis carrying 4N range of chromosomes (84, XXXX), as demonstrated by FISH using BAC RP23-51O13 for p53 status (top left), also showed PCS in all chromosomes, SKY (upper right) and DAPI banding (bottom left). Total number of p53 signals (i.e., 8) is demonstrated to coincide with the total number of chromatids of no. 11 chromosome, which clearly show total PCS events. Long arrows indicate p53 gene location. Chromosomal rearrangements were also noted in the SKY analysis, as indicated by short arrows in A, bottom panel.
Normal p53 gene distribution is observed in both diploid and tetraploid cells expressing Aurora-B
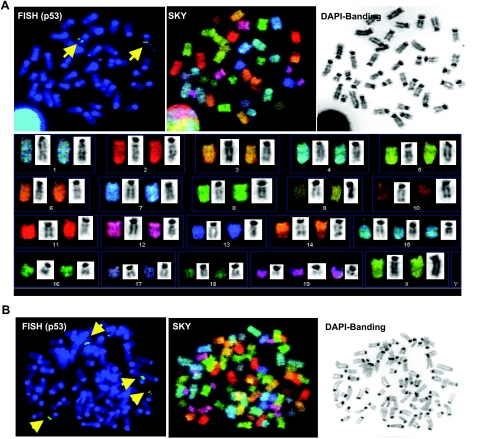
Given the role of p53 in tumorigenesis (reviewed in ref. 31,32,33), we examined whether chromosomal arrangement surrounding the p53 gene is associated with cells carrying the PCS phenotype. p53 gene status was assessed by FISH analysis using the BAC RP23-51O13 clone for hybridization, using cells overexpressing stable Aurora-B (Fig. 2). This method does not identify small alterations or mutations in coding sequence of the gene, but rather ensures chromosomal integrity. The location of p53 on chromosome 11B3 was also confirmed by SKY analysis, as shown in Fig. 2A. Interestingly, p53 gene number was proportional to ploidy status. We also examined the degree of chromosomal rearrangements in our samples. SKY analysis is the standard method to detect gross rearrangements. As shown in Fig. 2A, SKY analysis of cells overexpressing Aurora-B revealed few incidents of structural rearrangements of chromosomes, including translocation in chromosomes 3, 10, and 19. There is no evidence of breakage or amplification, as indicated by SKY analysis of these cells.
Figure 2.
Normal p53 gene distribution is observed in both diploid and tetraploid A-box mutated NmuMG cells. Normal NmuMG cells or stably overexpressing nondegradable mutant Aurora-B (A-box-mutated) were cultured in DMEM medium with 10% FBS and treated with colcemid (inhibitor of tubulin polymerization, 15 ng/ml) for 18 h, and chromosomes were prepared by acetic acid/methanol fixation and subjected to SKY, FISH, and DAPI banding analysis. A) Mitosis carrying 2N normal (42, XX with trisomies of chromosomes no. 15 and 19), as demonstrated by FISH using BAC RP23–51O13 for p53 status (top left), SKY (top middle), and DAPI banding (top right). SKY (bottom panel) demonstrates that p53 is located on chromosome 11B3, consistent with the data published on the NCBI site (http://genome.ucsc.edu/cgi-bin/hgGateway? clade=vertebrate&org=Mouse&db=0&hgsid=69124056). On the basis of the intensity of FISH signals, we conclude that each cell carrying 2N range of chromosomes contains 2 copies of p53 genes per cell, or 4 copies of p53 genes per mitosis following duplication of the gene through the prior S phase. B) Mitosis carrying 4N range of chromosomes (84, XXXX), as demonstrated by FISH using BAC RP23–51O13 for p53 status, SKY, and DAPI banding. Number of p53 signals coincides with copy number of chromosome no. 11.
Aurora-B-induced tetraploids stimulate tumorigenesis in vivo
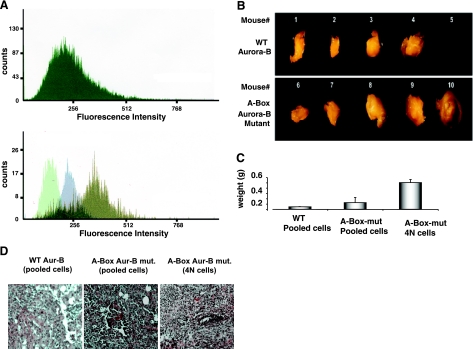
We next sought to determine whether Aurora-B-induced tetraploids play a role in the transformation properties attributed to Aurora-B. To this end, we subjected cells to fluorescence-activated cell sorting (FACS) based on ploidy content (25). A ploidy profile of sorted cells is shown in Fig. 3A, confirming our earlier report and those of others that overexpressed Aurora-B induces polyploidy. We injected equal concentrations of epithelial cells stably overexpressing wild-type or stable Aurora-B, or sorted tetraploid cells derived from Aurora-B-overexpressing cells into immunocompromised mice (nu/nu). Given that normal control NmuMG cells do not have tumorigenic capacity, either in vitro or in vivo, as described previously (25, 34), we decided to analyze NmuMG cells stably expressing wild-type and nondegradable mutant Aurora-B. Mice injected with wild-type or mutated Aurora-B-expressing cells developed tumors, confirming a previous report (35) in which a similar approach was taken using cells overexpressing wild-type Aurora-B. Interestingly, tumor size was greater in mice injected with the cells with stable expression of Aurora-B (vs. wild-type Aurora-B). The tetraploids generated the largest tumors (P<0.05) (Fig. 3B). To characterize the types of tumors, thin sections prepared from paraffin-embedded tissue were stained with hematoxylin and eosin (H&E). Fig. 3C depicts sections obtained from mammary glands of mice injected with wild-type or stable Aurora-B transfectant cells. Stable Aurora-B-expressing cells induced a more severe stage of tumor development, characterized by a high-grade adenocarcinoma with marked mitotic activities. Invasions into adjacent subcutaneous dermis and muscle layers were also observed in some sections obtained from these mice. 4N cell-derived tumors consistently comprised grade 3 or grade 4 adenocarcinoma with invasion of adjacent tissue. This result suggests that tumors from 4N cells are more prone to malignancy, consistent with a previous report (24), except that our finding is novel in showing that the tetraploidy could be the mediator of Aurora-B effect on tumor promotion. The pathology interpretation was carried out blindly at a pathology lab, as detailed in Materials and Methods.
Figure 3.
In vivo tumorigenicity of NmuMG cells stably expressing wild-type Aurora-B or Aurora-B mutant. A) Ploidy analysis of NmuMG cells, A-box-Aurora-B mutant stable transfectant before (top) and after sorting (bottom) by FACS. In the latter case, an equal number of the sorted cells was subjected to FACS analysis. Histograms are representative of 3 independent experiments. Histograms of sorted cells with 2N, 4N, and 8N DNA content are superimposed. Overlapping regions (dark green) represent cells with ploidy content other than that indicated. B) Nude mice (nu/nu) were subcutaneously injected into mammary fat pad with pooled cells overexpressing wild-type Aurora-B (top panel) or stable A-Box-Aurora-B mutant (bottom panel) or sorted tetraploid cells sorted as in panel A (tumors not depicted) at a concentration of 2 × 106 cells/mouse (∼27 g total weight). Tumors were surgically removed after 8–10 wk postinjection and visualized using a Zeiss Stemi SV6 dissecting microscope, as described in Materials and Methods. Mouse 5 did not develop a tumor. C) Average tumor weights (g). Error bars = sd. Values of P < 0.05 indicate a statistically significant difference between groups using 1-way ANOVA test. D) Tumor histology shows random and blinded H&E-stained paraffin-embedded tissue sections that were sent to pathology for independent interpretation. Tumors from 4N cells show grade 3 adenocarcinoma, poorly differentiated neoplastic epithelial cells with significant nuclear atypical and high mitotic activities. There are irregular borders and evidence of tumor invading the adjacent layers.
Tumors from tetraploid cells show various degrees of chromosome amplification and deletions, as determined by array CGH
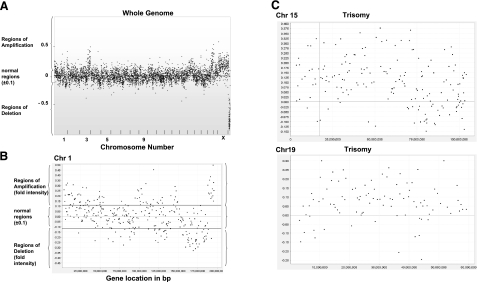
By which mechanism does Aurora-B-induced tetraploidy promote cancer development? Given that the doubling of the whole genome in cells has been proposed to be an unstable intermediate, and has been shown to give rise to whole chromosome gain/loss and gross rearrangement in vivo (24), we similarly used array-CGH analysis to obtain insight into the genetic alterations in these tetraploid-induced tumors. We attempted to look for patterns of gene amplification. As shown in Fig. 4, there are widespread regions of genomic amplification and deletion. There are also trisomies 15 and 19. Within the deleted regions in the genome, there are genes, such as peroxiredoxin 6 (redox regulation), MARK-1 (MAP/microtubule affinity-regulating kinase 1, which regulates cytoskeletal stability; refs 36,37,38), tumor suppressor candidate 4 (NPR2-like), and cell cycle-controlled gene Cdh-1 [Fizzy-related protein homologue (Fzr)], and more (see Supplemental Table 1). Amplified regions (at least 4-fold) include genes known to participate in tumor promotion, including casein kinase I isoform-α (CK1-α) and β platelet-derived growth factor receptor precursor (39,40,41,42,43). Supplemental Table 1 displays extended examples of putative known genes in these regions.
Figure 4.
Tumors from tetraploid cells showed various degrees of amplification and deletions, detected by array CGH. Tumors were harvested from sites injected with 4N cells and subjected to standard DNA extraction method. Purified DNA was sent to Roswell Park Cancer Institute for array CGH using the mouse 6.5K RPCI-23/-24 BAC array. Array CGH was performed as a sex-mismatch to provide an internal hybridization control for chromosome X and Y copy number differences. A) Data set for the whole genome. B) Typical data set from chromosome 1 with signal intensity of ±0.1 represents normal range. Signals >+0.1 represent amplification regions of the chromosome and <−0.1 represent regions of gene deletion. C) Typical pattern of signal that indicates whole chromosome amplification, including trisomies or tetrasomies. Trisomies of chromosomes 15 and 19 are shown.
DISCUSSION
Aurora-B is overexpressed in various tumors (1, 26, 27). This kinase plays an integral part in the mitotic spindle checkpoint and monitors the biorientation in spindle-kinetochord attachment needed for precise separation of chromosomes during metaphase. Disruption of Aurora-B function at the protein or gene level has been shown to impair mitotic spindle checkpoint (1). In our study, we report the novel finding that overexpression of a more stable form of Aurora-B, which allows sustained expression (as typically found in tumor cells) causes PCS, a phenomenon that was first described in Robert’s syndrome. PCS is also found in a rare autosomal recessive disorder, the cancer-prone syndrome of PCS (probably similar to Robert’s syndrome), where a monoallelic or bialletic BUB1B mutation is thought to be involved (5, 10, 20). Biallelic BUB1B mutations cause a more severe phenotypic penetration, characterized by PCS of all chromosomes, and mosaicism for various trisomies and monosomies. However, mutation of Aurora-B has not been reported in these patients.
The mechanism leading to PCS is largely unclear. However, there are speculations that it may involve deregulated cohesin removal and defective spindle checkpoint. In mitosis, cohesin removal occurs in two steps (44), in which the release of cohesin from chromosome arms take place during prophase and prometaphase and requires the actions of Polo-like kinase 1 (Plk-1) and Aurora-B kinase (45,46,47). When cells are treated with hesperadin, a small molecule that inhibits the activity of Aurora B, cohesin is present in higher amounts on chromosome arms, indicating that Aurora B is required for cohesin dissociation (46). In our study, we reported that cells with overexpression of Aurora-B have a lower level of cohesin and exhibit premature separation of chromatids, a plausible scenario in light of the above reports (46, 47). One possibility is that high expression of Aurora-B could result in a faster dissociation of the cohesin complex at prometaphase, which overrides the checkpoint arrest and allows rapid exit from mitosis with premature chromosome segregation, resulting in tetraploid cells. In the second step of cohesin removal at the onset of anaphase, biorientation of both sister kinetochores is achieved; the cells are released from spindle checkpoint and elicit the concomitant activation of the APC/c complex-separase cascade, resulting in the dissolution of the centromeric cohesin complex between sister chromatids. It is conceivable that premature degradation of cohesin complex at the chromosome arms and at the centromeric regions prior to biorientation attachment of sister kinetochores inevitably leads to defective chromosome segregation, a primary cause of aneuploidy and genomic instability as observed in this current study, and in agreement with previous reports involving Mad2 or BubR1 (1, 8, 9, 20, 48,49,50).
Polyploidization and aneuploidy have been recognized as a significant phenomenon, early in the carcinogenesis process (1, 14). Hence, mechanisms leading to aneuploidy and polyploidy are central to cancer research. It is of equal importance to understand how after a prolonged existence in precancerous tetraploid/polyploid state, these cells become cancerous. Studies (24, 51) have addressed the significance of tetraploidy in tumor development. Shi and King reported that a cell line with high rate of spontaneous nondisjunction and errors in chromosome segregation becomes tetraploid (51). Fujiwara et al. (24) examined the physiological effect downstream of the tetraploid state and questioned whether it can promote carcinogenesis. Convincingly, tetraploid cells were shown to induce a high level of tumorigenesis in the setting of p53-null mutation. In our study, tetraploid and octaploid mouse mammary epithelial cells were generated by deregulated Aurora-B expression using a stable Aurora-B mutant. In our xenograph model, tetraploid cells induce aggressive tumors. Our data are consistent with previous findings (24, 51) in that errors during segregation of chromosomes could induce tetraploidy and subsequent deleterious effects on the fate of these cells. In addition, we show that mammary mouse tumors from tetraploid cells can arise in the setting of normal p53 gene status. Future studies will explore whether p53 induction in response to DNA damage is normal in the Aurora-B-overexpressing cells.
In our system, the triggering step leading to tetraploidy in Aurora-B-overexpressing cells seems to be mediated through PCS or massive defects in chromosome separation and not by blockage of cytokinesis, as reported for other tetraploid cells (24). We speculate that in our experimental setting, the tetraploid cells acquired through premature chromosome separation may become genetically unstable at a faster rate than the one induced by cytokinesis failure, making p53 gene less crucial in the steps leading to cancerous transformation. Alternatively, tetraploid cells with defective chromosome separation may have massive aneuploidy, leading to misregulation of thousands of genes, including important tumor suppressor genes and oncogenes that can cause neoplastic transformation (26, 52). In the cases of cervical and other cancers in which tetraploidy has been highly prevalent, p53 gene mutation does not seem to be a prerequisite, while the large-scale whole chromosome abnormality is more essential in this transformation (1, 14, 53). The question of whether different mechanisms leading to polyploidy/tetraploidy may have differential downstream effects in the transformation processes remains to be explored.
One striking finding (24) was that tetraploid-derived tumors display massive chromosomal instability, including both numerical and structural chromosome aberrations. With a similar approach, using array CGH, we found that tetraploid-derived tumors display widespread regions of amplification and deletion throughout their genome. However, we did not observe large-scale numerical and structural chromosome rearrangement as reported previously (24).
In summary, our study reveals novel findings on the effect of sustained levels of Aurora-B on chromatid separation and generation of tetraploid cells that display tumorigenic properties, both in vitro and in vivo in a setting of a normal p53 gene. Cancer tissue derived from the tetraploid cells displays widespread regions of gene amplification and deletions.
Acknowledgments
This work was supported by National Cancer Institute seed funds to Boston University School of Medicine Cancer Center.
References
- Nguyen H G, Ravid K. Tetraploidy/aneuploidy and stem cells in cancer promotion: The role of chromosome passenger proteins. J Cell Physiol. 2006;208:12–22. doi: 10.1002/jcp.20565. [DOI] [PubMed] [Google Scholar]
- Kaitna S, Pasierbek P, Jantsch M, Loidl J, Glotzer M. The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous chromosomes during meiosis. Curr Biol. 2002;12:798–812. doi: 10.1016/s0960-9822(02)00820-5. [DOI] [PubMed] [Google Scholar]
- Resnick T D, Satinover D L, MacIsaac F, Stukenberg P T, Earnshaw W C, Orr-Weaver T L, Carmena M. INCENP and Aurora B promote meiotic sister chromatid cohesion through localization of the Shugoshin MEI-S332 in Drosophila. Dev Cell. 2006;11:57–68. doi: 10.1016/j.devcel.2006.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C V, Yang Y M, Swamy M V, Liu T, Fang Y, Mahmood R, Jhanwar-Uniyal M, Dai W. Colonic tumorigenesis in BubR1+/-ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability. Proc Natl Acad Sci U S A. 2005;102:4365–4370. doi: 10.1073/pnas.0407822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura S, Matsumoto Y, Morishima K, Izumi H, Matsumoto H, Ito E, Tsutsui K, Kobayashi J, Tauchi H, Kajiwara Y, Hama S, Kurisu K, Tahara H, Oshimura M, Komatsu K, Ikeuchi T, Kajii T. Monoallelic BUB1B mutations and defective mitotic-spindle checkpoint in seven families with premature chromatid separation (PCS) syndrome. Am J Med Genet A. 2006;140:358–367. doi: 10.1002/ajmg.a.31069. [DOI] [PubMed] [Google Scholar]
- Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe S W, Benezra R. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima Y, Hori T, Okada M, Kimura H, Haraguchi T, Hiraoka Y, Bao Y C, Kawashima T, Kitamura T, Fukagawa T. The constitutive centromere component CENP-50 is required for recovery from spindle damage. Mol Cell Biol. 2005;25:10315–10328. doi: 10.1128/MCB.25.23.10315-10328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel L S, Liberal V, Chatterjee A, Kirchwegger R, Pasche B, Gerald W, Dobles M, Sorger P K, Murty V V, Benezra R. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–359. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- Orr B, Bousbaa H, Sunkel C E. Mad2-independent spindle assembly checkpoint activation and controlled metaphase-anaphase transition in Drosophila S2 cells. Mol Biol Cell. 2007;18:850–863. doi: 10.1091/mbc.E06-07-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German J. Roberts’ syndrome. I. Cytological evidence for a disturbance in chromatid pairing. Clin Genet. 1979;16:441–447. doi: 10.1111/j.1399-0004.1979.tb01354.x. [DOI] [PubMed] [Google Scholar]
- Kajii T, Kawai T, Takumi T, Misu H, Mabuchi O, Takahashi Y, Tachino M, Nihei F, Ikeuchi T. Mosaic variegated aneuploidy with multiple congenital abnormalities: homozygosity for total premature chromatid separation trait. Am J Med Genet. 1998;78:245–249. doi: 10.1002/(sici)1096-8628(19980707)78:3<245::aid-ajmg7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kajii T, Ikeuchi T, Yang Z Q, Nakamura Y, Tsuji Y, Yokomori K, Kawamura M, Fukuda S, Horita S, Asamoto A. Cancer-prone syndrome of mosaic variegated aneuploidy and total premature chromatid separation: report of five infants. Am J Med Genet. 2001;104:57–64. doi: 10.1002/ajmg.1580. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Ito E, Tauchi H, Komatsu K, Ikeuchi T, Kajii T. Chromosomal instability syndrome of total premature chromatid separation with mosaic variegated aneuploidy is defective in mitotic-spindle checkpoint. Am J Hum Genet. 2000;67:483–486. doi: 10.1086/303022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem N J, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007;17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Zimmet J, Ravid K. Polyploidy: occurrence in nature, mechanisms, and significance for the megakaryocyte-platelet system. Exp Hematol. 2000;28:3–16. doi: 10.1016/s0301-472x(99)00124-1. [DOI] [PubMed] [Google Scholar]
- Dikovskaya D, Schiffmann D, Newton I P, Oakley A, Kroboth K, Jamieson T J, Meniel V, Clarke A, Sansom O, Nathke I S. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J Cell Biol. 2007;176:183–195. doi: 10.1083/jcb.200610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel B P, Starita L M, Parvin J D. Overexpression of a protein fragment of RNA helicase A causes inhibition of endogenous BRCA1 function and defects in ploidy and cytokinesis in mammary epithelial cells. Oncogene. 2003;22:983–991. doi: 10.1038/sj.onc.1206195. [DOI] [PubMed] [Google Scholar]
- Hollander M C, Sheikh M S, Bulavin D V, Lundgren K, Augeri-Henmueller L, Shehee R, Molinaro T A, Kim K E, Tolosa E, Ashwell J D, Rosenberg M P, Zhan Q, Fernandez-Salguero P M, Morgan W F, Deng C X, Fornace A J., Jr Genomic instability in Gadd45a-deficient mice. Nat Genet. 1999;23:176–184. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu J, Prochownik E V. c-Myc-mediated genomic instability proceeds via a megakaryocytic endomitosis pathway involving Gp1bα. Proc Natl Acad Sci U S A. 2007;104:3490–3495. doi: 10.1073/pnas.0610163104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S, Coleman K, Reid S, Plaja A, Firth H, Fitzpatrick D, Kidd A, Mehes K, Nash R, Robin N, Shannon N, Tolmie J, Swansbury J, Irrthum A, Douglas J, Rahman N. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat Genet. 2004;36:1159–1161. doi: 10.1038/ng1449. [DOI] [PubMed] [Google Scholar]
- Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- Olaharski A J, Sotelo R, Solorza-Luna G, Gonsebatt M E, Guzman P, Mohar A, Eastmond D A. Tetraploidy and chromosomal instability are early events during cervical carcinogenesis. Carcinogenesis. 2006;27:337–343. doi: 10.1093/carcin/bgi218. [DOI] [PubMed] [Google Scholar]
- Ravid K, Lu J, Zimmet J M, Jones M R. Roads to polyploidy: the megakaryocyte example. J Cell Physiol. 2002;190:7–20. doi: 10.1002/jcp.10035. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Bandi M, Nitta M, Ivanova E V, Bronson R T, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- Nguyen H G, Chinnappan D, Urano T, Ravid K. Mechanism of Aurora-B degradation and its dependency on intact KEN and A-boxes: identification of an aneuploidy-promoting property. Mol Cell Biol. 2005;25:4977–4992. doi: 10.1128/MCB.25.12.4977-4992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–250. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Katayama H, Brinkley W R, Sen S. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 2003;22:451–464. doi: 10.1023/a:1023789416385. [DOI] [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez P Y, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Nagata Y, Yu G, Nguyen H G, Jones M R, Toselli P, Jackson C W, Tatsuka M, Todokoro K, Ravid K. Aberrant quantity and localization of Aurora-B/AIM-1 and survivin during megakaryocyte polyploidization and the consequences of Aurora-B/AIM-1-deregulated expression. Blood. 2004;103:3717–3726. doi: 10.1182/blood-2003-09-3365. [DOI] [PubMed] [Google Scholar]
- Salomon D S, Perroteau I, Kidwell W R, Tam J, Derynck R. Loss of growth responsiveness to epidermal growth factor and enhanced production of α-transforming growth factors in ras-transformed mouse mammary epithelial cells. J Cell Physiol. 1987;130:397–409. doi: 10.1002/jcp.1041300313. [DOI] [PubMed] [Google Scholar]
- Levine A J, Momand J, Finlay C A. The p53 tumour suppressor gene. Nature. 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Sharpless N E, DePinho R A. p53: good cop/bad cop. Cell. 2002;110:9–12. doi: 10.1016/s0092-8674(02)00818-8. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler K W. p53 function and dysfunction. Cell. 1992;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Mazzoni E, Adam A, Bal de Kier Joffe E, Aguirre-Ghiso J A. Immortalized mammary epithelial cells overexpressing protein kinase C gamma acquire a malignant phenotype and become tumorigenic in vivo. Mol Cancer Res. 2003;1:776–787. [PubMed] [Google Scholar]
- Kimmins S, Crosio C, Kotaja N, Hirayama J, Monaco L, Hoog C, van Duin M, Gossen J A, Sassone-Corsi P. Differential functions of the aurora-B and aurora-C kinases in mammalian spermatogenesis. Mol Endocrinol. 2007;21:726–739. doi: 10.1210/me.2006-0332. [DOI] [PubMed] [Google Scholar]
- Egler R A, Fernandes E, Rothermund K, Sereika S, de Souza-Pinto N, Jaruga P, Dizdaroglu M, Prochownik E V. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24:8038–8050. doi: 10.1038/sj.onc.1208821. [DOI] [PubMed] [Google Scholar]
- Furuta J, Nobeyama Y, Umebayashi Y, Otsuka F, Kikuchi K, Ushijima T. Silencing of peroxiredoxin 2 and aberrant methylation of 33 CpG islands in putative promoter regions in human malignant melanomas. Cancer Res. 2006;66:6080–6086. doi: 10.1158/0008-5472.CAN-06-0157. [DOI] [PubMed] [Google Scholar]
- Jeon S, Kim Y S, Park J, Bae C D. Microtubule affinity-regulating kinase 1 (MARK1) is activated by electroconvulsive shock in the rat hippocampus. J Neurochem. 2005;95:1608–1618. doi: 10.1111/j.1471-4159.2005.03505.x. [DOI] [PubMed] [Google Scholar]
- Knippschild U, Gocht A, Wolff S, Huber N, Lohler J, Stoter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- Knippschild U, Wolff S, Giamas G, Brockschmidt C, Wittau M, Wurl P U, Eismann T, Stoter M. The role of the casein kinase 1 (CK1) family in different signaling pathways linked to cancer development. Onkologie. 2005;28:508–514. doi: 10.1159/000087137. [DOI] [PubMed] [Google Scholar]
- Klominek J, Baskin B, Hauzenberger D. Platelet-derived growth factor (PDGF) BB acts as a chemoattractant for human malignant mesothelioma cells via PDGF receptor beta-integrin alpha3beta1 interaction. Clin Exp Metastasis. 1998;16:529–539. doi: 10.1023/a:1006542301794. [DOI] [PubMed] [Google Scholar]
- Coltrera M D, Wang J, Porter P L, Gown A M. Expression of platelet-derived growth factor B-chain and the platelet-derived growth factor receptor beta subunit in human breast tissue and breast carcinoma. Cancer Res. 1995;55:2703–2708. [PubMed] [Google Scholar]
- Waizenegger I C, Hauf S, Meinke A, Peters J M. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell. 2000;103:399–410. doi: 10.1016/s0092-8674(00)00132-x. [DOI] [PubMed] [Google Scholar]
- Sumara I, Vorlaufer E, Stukenberg P T, Kelm O, Redemann N, Nigg E A, Peters J M. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol Cell. 2002;9:515–525. doi: 10.1016/s1097-2765(02)00473-2. [DOI] [PubMed] [Google Scholar]
- Gimenez-Abian J F, Sumara I, Hirota T, Hauf S, Gerlich D, de la Torre C, Ellenberg J, Peters J M. Regulation of sister chromatid cohesion between chromosome arms. Curr Biol. 2004;14:1187–1193. doi: 10.1016/j.cub.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Dai J, Sullivan B A, Higgins J M. Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Dev Cell. 2006;11:741–750. doi: 10.1016/j.devcel.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Wang X, Dai W. Shugoshin, a guardian for sister chromatid segregation. Exp Cell Res. 2005;310:1–9. doi: 10.1016/j.yexcr.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Fang G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol Biol Cell. 2002;13:755–766. doi: 10.1091/mbc.01-09-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienitz A, Vogel C, Morales I, Muller R, Bastians H. Partial downregulation of MAD1 causes spindle checkpoint inactivation and aneuploidy, but does not confer resistance towards taxol. Oncogene. 2005;24:4301–4310. doi: 10.1038/sj.onc.1208589. [DOI] [PubMed] [Google Scholar]
- Shi Q, King R W. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature. 2005;437:1038–1042. doi: 10.1038/nature03958. [DOI] [PubMed] [Google Scholar]
- Duesberg P, Li R, Fabarius A, Hehlmann R. Aneuploidy and cancer: from correlation to causation. Contrib Microbiol. 2006;13:16–44. doi: 10.1159/000092963. [DOI] [PubMed] [Google Scholar]
- Margolis R L. Tetraploidy and tumor development. Cancer Cell. 2005;8:353–354. doi: 10.1016/j.ccr.2005.10.017. [DOI] [PubMed] [Google Scholar]