Abstract
The identity of store-operated calcium (Ca2+) entry (SOCE) channels in vascular smooth muscle cells (VSMCs) remains a highly contentious issue. Whereas previous studies have suggested that SOCE in VSMCs is mediated by the nonselective transient receptor potential canonical (TRPC) 1 protein, the identification of STIM1 and Orai1 as essential components of ICRAC, a highly Ca2+-selective SOCE current in leukocytes, has challenged that view. Here we show that cultured proliferative migratory VSMCs isolated from rat aorta (called “synthetic”) display SOCE with classic features, namely inhibition by 2-aminoethoxydiphenyl borate, ML-9, and low concentrations of lanthanides. On store depletion, synthetic VSMCs and A7r5 cells display currents with characteristics of ICRAC. Protein knockdown of either STIM1 or Orai1 in synthetic VSMCs greatly reduced SOCE, whereas Orai2, Orai3, TRPC1, TRPC4, and TRPC6 knockdown had no effect. Orai1 knockdown reduced ICRAC in synthetic VSMCs and A7r5 cells. Synthetic VSMCs showed up-regulated STIM1/Orai1 proteins and SOCE compared with quiescent freshly isolated VSMC. Knockdown of STIM1 and Orai1 inhibited synthetic VSMC proliferation and migration, whereas STIM2, Orai2, and Orai3 knockdown had no effect. To our knowledge, these results are the first to show ICRAC in VSMCs and resolve a long-standing controversy by identifying CRAC as the elusive VSMC SOCE channel important for proliferation and migration.—Potier, M., Gonzalez, J. C., Motiani, R. K., Abdullaev, I. F., Bisaillon, J. M., Singer, H. A., and Trebak, M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration.
Keywords: Ca2+ release-activated Ca2+ channels, store-operated Ca2+ entry channels, TRPC channels, phenotypic modulation
Store-operated calcium (Ca2+) entry (SOCE) is a ubiquitous agonist-evoked Ca2+ entry pathway originally recognized and named capacitative Ca2+ entry by Putney (1, 2). SOCE is activated by the actual fall of the Ca2+ concentration within the lumen of the endoplasmic reticulum (ER)/sarcoplasmic reticulum (SR) through the action of inositol-1,4,5-trisphosphate (IP3) on the IP3 receptor. Exposure of cells to ER/SR Ca2+-ATPase pump inhibitors such as thapsigargin, which prevent the pump from refilling the stores, activates store-operated Ca2+ (SOC) channels. The best characterized SOC current is the Ca2+ release-activated Ca2+ (CRAC) current first identified in RBL mast cells (3) and later in other cell types (4). Although the pharmacological peculiarities of SOCE such as potentiation by low concentrations of 2-aminoethoxydiphenyl borate (2-APB) (5 μM) and inhibition by higher concentrations of 2-APB (30–50 μM) (5) and low concentrations of lanthanides (e.g., 1–10 μM Gd3+) (6, 7) are well characterized, only recently has the molecular machinery of SOCE begun to be revealed with the discovery of stromal interaction molecule 1 (STIM1) as the ER Ca2+ sensor that undergoes reorganization on store depletion to convey a signal to Orai1, the pore-forming subunit of CRAC channels in mast cells, HEK293 cells, and T lymphocytes (8,9,10,11) (for review, see refs. 12,and 13). Orai1 has two ubiquitously expressed mammalian homologs, Orai2 and Orai3, that can also be activated by store depletion in a STIM1-dependent manner when ectopically expressed in HEK293 cells (14). However, the contribution of Orai2 and Orai3 to SOCE in native cells remains unknown.
Vascular smooth muscle cells (VSMCs) are one of the major cell types in blood vessels that play a central role in controlling the vascular tone and maintaining the integrity of the vessel wall (15). Although SOCE has been long described in VSMCs (16), the molecular identity and role of SOCE in VSMC function remain highly contentious issues. Previous studies suggested that transient receptor potential canonical (TRPC) 1 is involved in a thapsigargin-activated SOCE in smooth muscle cells (17,18,19) based on a modest decrease of thapsigargin-activated Ca2+ entry after treatment of cells with an antibody against TRPC1 (19, 20)or transfection of TRPC1 silencing RNA (siRNA) (17). In some cases, studies were conducted under non-voltage-clamped conditions (19, 21) and thus could be explained by a reduction in driving force. In cases in which membrane currents were recorded, there was a great heterogeneity in the biophysical and pharmacological properties of these “SOC” currents between different groups and different VSMC types (17, 18, 20, 22,23,24). Furthermore, TRPC1 involvement in VSMC SOCE is difficult to reconcile with the finding of intact SOC currents in VSMCs from TRPC1 knockout mice (25) and evidence arguing that TRPC1 mediate currents that are activated secondarily as a result of phospholipase C activation in response to a rise in cytoplasmic Ca2+ (26). The difficulty in reconciling TRPC channel properties with SOCE has been critically evaluated elsewhere (27).
With the use of molecular genetics, pharmacological reagents, Ca2+ imaging, and patch-clamp protocols that amplify whole-cell currents, we show that functional ICRAC-like currents are present in VSMCs and are inhibited by similar concentrations of inhibitors that block SOCE in other cell types; selective knockdown of two key components of the ICRAC pathway (STIM1 and Orai1) drastically reduced SOCE and ICRAC-like currents; and, notably, knockdown of other Orai and TRPC isoforms expressed in VSMCs (Orai2, Orai3, TRPC1, TRPC4, and TRPC6) had no effect on VSMC SOCE. In addition, we further show that cultured proliferative migratory rat aortic VSMCs (referred to hereafter as synthetic VSMCs) display increased SOCE and high levels of STIM1 and Orai1 compared with their quiescent contractile freshly isolated counterparts, and knockdown of STIM1 and Orai1 in synthetic VSMC inhibited proliferation and migration. Our results therefore resolve a long-standing controversy by identifying CRAC as the elusive VSMC SOCE channel and establish STIM1 and Orai1 up-regulation in synthetic VSMCs as important in VSMC proliferation and migration.
EXPERIMENTAL PROCEDURES
Reagents
The CFP-Orai1 and eYFP-STIM1 cDNA plasmids were generous gifts from Drs. Jim Putney (National Institute of Environmental Health Sciences, NIH, Research Triangle Park, NC, USA) and Tobias Meyer (Stanford University, Stanford, CA, USA), respectively. Gd3+ was purchased from Acros Organics (Fairlawn, NJ, USA). Thapsigargin and 2-APB were purchased from Calbiochem (San Diego, CA, USA). All siRNA sequences were from Dharmacon RNA Technologies (Lafayette, CO, USA), and all shRNA hairpins cloned in the pRS vector were from Origene (Rockville, MD, USA). All other chemicals products were from Fisher Scientific (Pittsburgh, PA, USA).
VSMC dispersion and culture
Male rats (Charles River Breeding Laboratories, Inc., Wilmington, MA, USA) were euthanized by suffocation in a CO2 chamber. Aortas were dissected out into ice-cold physiological saline solution. The fat tissues and endothelium were removed. The artery was cut into small pieces and digested with a papain solution for 20 min at 37°C followed by a second digestion with collagenase II and H mix for 15 min at 37°C. After removal of digestion solution and washes, cells were gently liberated with fire-polished glass pipette and transferred into culture plates. Isolated VSMCs underwent phenotypic modulation in culture that was complete within 30 h (28). Isolated VSMCs were either used within 6 h for experiments (freshly dispersed) or maintained in culture (45% DMEM and 45% Ham’s F12 with 10% FBS supplemented with l-glutamine) at 37°C, 5% CO2, and 100% humidity, passaged and used within 1 to 5 passages (synthetic).
RT-PCR and real-time PCR
These experiments were conducted as described previously (29). In brief, total RNA was extracted from cells using a Qiagen RNeasy Mini Kit following the manufacturer’s protocol. cDNA was made from 0.5 μg of RNA reverse transcribed using oligo(dT) primers (Invitrogen, Carlsbad, CA, USA) and SuperScript III reverse transcriptase (Invitrogen). PCR reactions were completed using Illustra PuReTaq Ready-To-Go PCR beads (GE Healthcare, Little Chalfont, Buckinghamshire, UK). The sense and antisense primers targeting rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH), TRPC, STIM, and Orai isoforms are described in Supplemental Table 1. All primers were synthesized by Integrated DNA Technologies (Coralville, IA, USA). The PCR amplification was done using a MyCycler Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, USA). Amplification started with initial denaturation at 94°C for 5 min and then 40 cycles of denaturation at 94°C for 30 s, annealing at 54.3°C for 1 min, and extension at 72°C for 2 min. Gel electrophoresis was used to identify the PCR products in a 1% agarose gel using ethidium bromide staining. Real-time PCR analysis was performed using a Bio-Rad iCycler and iCycler iQ Optical System Software (Bio-Rad Laboratories). PCR reactions were performed using Bio-Rad iQ SYBR Green Supermix. The PCR protocol started with 5 min at 94°C, followed by 45 cycles of 30 s at 94°C, 30 s at 54.3°C, and 45 s at 72°C. Quantification was measured as sample fluorescence crossed a predetermined threshold value that was just above the background. Expressions of TRPC, STIM, or Orai were compared to those of the housekeeping gene GAPDH and were measured using comparative threshold cycle values as described previously (29).
Cell transfections
Sets of four different siRNAs per target gene were initially assessed for their ability to reduce mRNA levels using quantitative PCR (qPCR) as described above; the primers are listed in Supplemental Table 1. siRNA sequences that induced significant decreases in their target mRNA were used in Western blotting to confirm protein knockdown as described below. All transfections in VSMCs were done using a Nucleofector device II (Amaxa Biosystems, Gaithersburg, MD) according to the manufacturer’s instructions. As a marker of cell transfection, 0.5 μg of green fluorescent protein (GFP) was cotransfected with siRNA or shRNA for identification of successfully transfected cells during experiments. As a control, we used either a scrambled sequence (for siRNA) or the empty pRS vector (for shRNA). Sequences of all siRNA and shRNA used in this study are listed in Supplemental Table 1. For calcium imaging experiments, cells were transfected with 10 μg of siRNA or 20 μg of shRNA of choice per 1 × 106 cells, seeded to either round glass coverslips (for Ca2+ imaging and patch-clamp studies) or plates (for Western blotting, proliferation, and migration assays), and typically assayed 72 h post-transfection. Transfections with cDNA for eYFP-STIM1 (1 μg) and CFP-Orai1 (1 μg) were also performed using the Amaxa system, and Ca2+ imaging was performed 48 h post-transfection. There were no apparent side effects of any of the siRNA treatments on cell shape or morphology.
Ca2+ measurements
Ca2+ measurements were performed as described previously (29, 30). Briefly, coverslips with attached cells were mounted in a Teflon chamber and incubated at 37°C for 45 min in culture media containing 4 μM Fura-2/acetoxymethyl ester (Molecular Probes, Eugene, OR, USA). Cells were then washed and bathed in HEPES-buffered saline solution (140 mM NaCl, 1.13 mM MgCl2, 4.7 mM KCl, 2 mM CaCl2, 10 mM d-glucose, and 10 mM HEPES, adjusted to pH 7.4 with NaOH) for at least 10 min before Ca2+ measurements were made. For Ca2+ measurements, fluorescence images of several cells were recorded and analyzed with a digital fluorescence imaging system (InCyt Im2; Intracellular Imaging Inc., Cincinnati, OH, USA). Fura-2 fluorescence at an emission wavelength of 510 nm was induced by exciting Fura-2 alternately at 340 and 380 nm. The 340/380 ratio images were obtained on a pixel-by-pixel basis. All experiments were conducted at room temperature. All figures depicting Ca2+ imaging traces are an average from several cells from one coverslip and are representative of several independent recordings.
Whole-cell patch-clamp electrophysiology
The cells used for whole-cell patch-clamp recordings have a capacitance of 47.6 ± 6.1 for synthetic VSMCs and 61.7 ± 5.6 for A7r5 cells; 2.5- to 4-MΩ patch pipettes were pulled from borosilicate glass capillaries (World Precision Instruments, Inc., Sarasota, FL, USA) with a P-97 flaming/brown micropipette puller (Sutter Instrument Company, Novato, CA, USA). Axopatch 200B and Digidata 1440A (Molecular Devices Corp., Sunnyvale, CA, USA) with pCLAMP 10 software were used for data acquisition and analysis. Synthetic VSMCs and A7r5 cells were seeded on round coverslips for 24–96 h before the experiment. Immediately before the experiments, cells were washed with bath solution containing 145 mM Na-methanesulfonate, 10 mM CsCl, 1.2 mM MgSO4, 10 mM HEPES, 10 mM CaCl2, and 10 mM glucose (pH was adjusted to 7.4 with NaOH). Pipette solution contained 145 mM Cs-methanesulfonate, 20 mM Cs-1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), 8 mM MgCl2, and 10 mM HEPES (pH adjusted to 7.2 with CsOH). Divalent-free (DVF) bath solution contained 155 mM Na-methanesulfonate, 10 mM N-hydroxyethylene-diaminetriacetic acid, 1 mM EDTA, and 10 mM HEPES (pH 7.4, adjusted with HCl). Only cells with tight seals (>3 GΩ) were selected to break in. On obtaining a GΩ seal and break-in, recordings were made from cells with <15 MΩ series resistance. Cells were maintained at a 0 mV holding potential during experiments and subjected to voltage ramps from +80 to −140 mV lasting 250 ms every 3 s. “Reverse” ramps were designed to inhibit Na+ channels, 10 μM verapamil was included in bath solution to inhibit L-type calcium channels, and 3 μM nimodipine was added to the bath solution to generally stabilize membrane patches and reach better seals. Pulses of DVF solutions were delivered to cells with the least possible pressure to minimize accidental seal damage, which could lead to leak currents contaminating recordings.
Western blotting
Cell lysates in RIPA buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 0.2 mM EDTA) (25 or 50 μg of proteins in denaturing conditions) were subjected to SDS-PAGE (10%) and electrotransferred onto polyvinylidene fluoride membranes. After blocking with 5% nonfat dry milk (NFDM) dissolved in Tris-buffered saline containing 0.1% Tween 20 (TTBS) for 2 h at room temperature, Western blots were probed overnight at 4°C, with specific primary antibodies in TTBS containing 2% NFDM. The membrane was incubated for 45 min at room temperature with a horseradish peroxidase-conjugated anti-mouse (1:10,000; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) or anti-rabbit IgG (1:20,000; Jackson ImmunoResearch Laboratories Inc.) in TTBS containing 2% NFDM. The primary antibodies used were GOK/STIM1 (1:250; BD Biosciences, San Jose, CA, USA), Orai1-NT (1:500; ProSci Inc., Poway, CA, USA), Orai3-NT polyclonal (1:500; ProSci Inc.), TRPC1 (1:10,000; Abcam Inc., Cambridge, MA, USA), TRPC4 (1:250; Sigma-Aldrich Corp., St. Louis, MO, USA), and TRPC6 (1:1000; Abcam Inc.). A monoclonal anti-β-actin antibody directed against the N-terminal domain (1:2000; Sigma-Aldrich Corp.) was used for Western blot loading control experiments in which the same membranes probing for the proteins above were stripped and reprobed with the anti-actin antibody. Detection was performed with the enhanced chemiluminescence reagent (ECL Western blotting detection reagents; Amersham Biosciences Corp., Piscataway, NJ, USA). Quantification of bands was achieved by densitometry using the ImageJ software.
Migration and proliferation assays
VSMCs were transfected with scrambled control or target siRNA, seeded, and allowed to form a monolayer for 48 h. A 100-μl pipette tip was used to scrape across the dish, and the resulting wound was washed with PBS. Culture medium containing 10% FBS was added to the cells followed by incubation in a 37°C incubator with 5% CO2. At different time intervals (12 and 24 h) bright-field images were captured using a Leica DM IRB microscope (Leica Microsystems, Wetzlar, Germany) at ×10. The images were analyzed using Adobe Photoshop CS3 (Adobe Systems, San Jose, CA, USA), and the total number of pixels in empty spaces inside the wound was compared with the control. Data are presented as the percentage of migration relative to the scrambled siRNA control condition. Proliferation was evaluated by cell count after trypan blue exclusion as described previously (29). In brief, VSMCs were transfected with siRNA and seeded at 3 × 103 cells per well with 10% FBS medium (12 wells per condition). At different times after transfection the cells were washed with PBS, detached, and counted in triplicate using a Coulter counter.
Statistical analysis
Data are expressed as means ± se, and statistical analysis using Student’s t test was done with Origin software (OriginLab, Northampton, MA, USA). Differences were considered significant when P < 0.05.
RESULTS
Characterization of SOCE and ICRAC-like currents in VSMCs
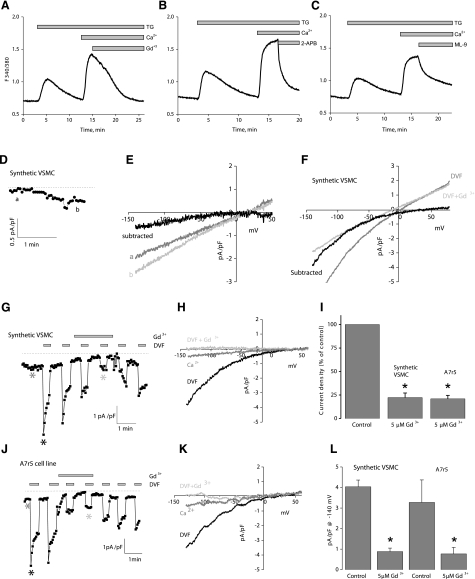
Pharmacological SOCE inhibitors revealed that rat VSMC SOCE displays classic SOCE features similar to those observed in nonexcitable cells such as HEK293 and RBL mast cells. In synthetic VSMCs, SOCE in response to thapsigargin was blocked with 5 μM Gd3+ (Fig. 1A), 30 μM 2-APB (Fig. 1B), and, as recently demonstrated in HEK293 cells (31), by 50 μM ML-9 (Fig. 1C), suggesting that STIM1 and Orai1 might be the molecular players for SOCE in VSMCs.
Figure 1.
Characterization of SOCE and ICRAC in synthetic VSMCs. A) Thapsigargin (TG) (2 μM) activates SOCE in synthetic VSMCs that is sensitive to 5 μM Gd3+ (n=72). B, C) Similar inhibition of SOCE was achieved with 50 μM 2-APB (B) (n=49) and 50 μM ML-9 (C) (n=26). D) Store depletion by dialysis of 20 mM BAPTA activates a relatively small current in 10 mM Ca2+. E) I/V relation of current shown in D before and after subtraction of the sweep right after break-in. F) Representative data of I/V relations of DVF current before and after subtraction of Gd3+-insensitive current. G) Store depletion by dialysis of 20 mM BAPTA in synthetic VSMC activates ICRAC-like currents, revealed in DVF solutions that were inhibited by 5 μM Gd3+; sweeps represented are indicated by differently shaded asterisks. H) I/V relations in Ca2+-containing solution (10 mM), before and after Gd3+ addition. I) Statistical analysis of monovalent ICRAC inhibition by 5 μM Gd3+, normalized to control. J, K) Similar results to those in G, H were obtained with the A7r5 smooth muscle cell line. L) Statistical analysis of monovalent ICRAC inhibition by 5 μM Gd3+, shown as current densities. All Ca2+ imaging traces are average data from several cells analyzed simultaneously. Data are representative of 3 to 4 independent experiments.
To characterize the current mediating SOCE in VSMCs, we used the standard method for measuring ICRAC in whole-cell mode with intracellular dialysis of high concentrations of the pH-independent, fast Ca2+ chelator BAPTA (32). On break-in with a pipette solution containing 20 mM BAPTA, a relatively small inwardly rectifying ICRAC-like current with very positive reversal potential developed in the presence of external Ca2+ (10 mM) in synthetic VSMCs (Fig. 1D, E) (0.32±0.08 pA/pF at −100 mV; n=4). Figure 1E shows the current-voltage (I/V) relation for which currents recorded immediately after break-in were subtracted from fully developed currents revealing inward rectification with a very positive reversal potential. To better study these ICRAC-like currents, we conducted subsequent current measurement using DVF bath conditions to amplify them, as described recently (29). Whole-cell current measurements were performed using reverse-voltage ramps to minimize contributions from voltage-gated Na+ channels. On break-in in synthetic VSMCs, we observed currents in DVF conditions typical of monovalent ICRAC that were inwardly rectifying with a positive reversal potential (Fig. 1G, H) (4.0±0.3 pA/pF at −140 mV; n=3). These currents displayed the peculiar phenomenon of rapid time-dependent inactivation of inward Na+ currents (called depotentiation) in DVF solutions and a very positive reversal potential of ∼+50 mV in DVF, which corresponds to a PCs/PNa permeability ratio of ∼0.1, all characteristics of ICRAC (4) (Fig. 1G). These currents were reversibly inhibited by 5 μM Gd3+. However, on Gd3+ washoff, we recovered only a partial portion of the current. Figure 1H shows a typical I/V relation taken in Ca2+-containing solution (dark gray trace), and in DVF solutions before (black trace) and after Gd3+ addition (light gray trace) taken where indicated by differently shaded asterisks in Fig. 1G. After Gd3+ addition, we observed a small Gd3+-insensitive remaining linear current (Fig. 1G) (0.9±0.2 pA/pF at −140mV; n=3), possibly reflecting a leak current; this is illustrated in Fig. 1F, depicting I/V relations before and after subtraction of the Gd3+-insensitive current. Similar experiments in the vascular smooth muscle cell line A7r5 revealed monovalent ICRAC currents that were indistinguishable from those in synthetic VSMCs (Fig. 1J, K) (3.3±1.1 pA/pF at −140 mV; n=4). A summary of the data with statistical analysis of Gd3+ inhibition is depicted in Fig. 1I (normalized to control) and Fig. 1L (absolute current densities).
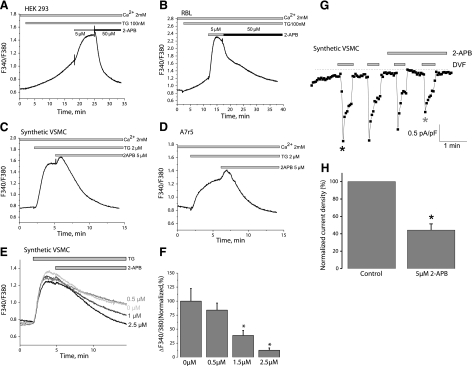
Synthetic rat aortic VSMCs express STIM1 and Orai1 mRNA but also express mRNA encoding STIM2, Orai2, and Orai3 (Supplemental Fig. 1). We thus considered the possibility that Orai2, Orai3, or both may contribute subunits to homo- or heteromultimeric SOCE channels in VSMCs. It was previously shown that Orai1 possesses a peculiar characteristic: it is potentiated by low concentrations of 2-APB (5 μM) and inhibited by high concentrations (30–50 μM), whereas Orai2 is inhibited only by high concentrations of 2-APB and Orai3 is potentiated only by high concentrations (33). We conducted experiments probing for the potentiating effect of 5 μM 2-APB to narrow the possible molecular players for SOCE in VSMCs. Control experiments in RBL and HEK293 cells, in which SOCE was activated by submaximal concentrations of thapsigargin (100 nM) in the presence of extracellular Ca2+ (2 mM), confirmed that SOCE in these cells is potentiated by 5 μM 2-APB and inhibited by 50 μM (Fig. 2A, B). Surprisingly, thapsigargin-activated SOCE in synthetic VSMCs as well as in A7r5 cells was completely blocked by 5 μM 2-APB (Fig. 2C, D). Although, on addition of 5 μM 2-APB, we consistently observed a typical early phase of enhancement of Ca2+ entry followed by subsequent inhibition (Fig. 2C, D), which is a typical characteristic of ICRAC. Dose-response analysis revealed that thapsigargin-activated SOCE in synthetic VSMCs is fully inhibited with concentrations of 2-APB as low as 2.5 μM after 7–9 min (Fig. 2E, F). Monovalent ICRAC in synthetic VSMCs was inhibited within 2 min of perfusion with 5 μM 2-APB (56%±7% inhibition) (Fig. 2G, H). This result suggested to us that SOCE in VSMCs cannot be accounted for by any of the three Orai isoforms alone, and we considered the possibility that SOCE in VSMCs might be encoded by heteromultimers of different Orai isoforms. Given previous work from other laboratories arguing for a role for TRPC1 in VSMC SOCE (17,18,19, 34), we also considered the possibility that a TRPC isoform might contribute subunits to VSMC SOC channels. We thus decided to undertake RNA silencing to knock down STIM1, all Orai, and TRPC proteins detected in VSMC (RT-PCR detected only TRPC1, TRPC4, and TRPC6 transcripts in synthetic VSMCs).
Figure 2.
SOCE and ICRAC in synthetic VSMCs are inhibited by 5 μM 2-APB. A, B) Average data in HEK293 cells (A) (n=51) and RBL cells (B) (n=53). 2-APB at 5 μM potentiates SOCE activated by submaximal concentrations of thapsigargin (TG) (100 nM), whereas 50 μM 2-APB inhibits SOCE in these cells. C, D) Thapsigargin (2 μM) activates SOCE in synthetic VSMCs (C) (n=12) and the A7r5 cell line (D) (n=14), which is inhibited by 5 μM 2-APB. E, F) Dose response of 2-APB inhibition in synthetic VSMCs (representing average from 12–54 cells). G, H) Inhibition of ICRAC recorded in DVF solutions by 5 μM 2-APB. Ca2+ imaging data are representative of 4 independent coverslips, with each coverslip analyzing 21–30 cells. Patch-clamp data are representative of 4 independent experiments.
STIM1 and Orai1 alone mediate SOCE in VSMCs
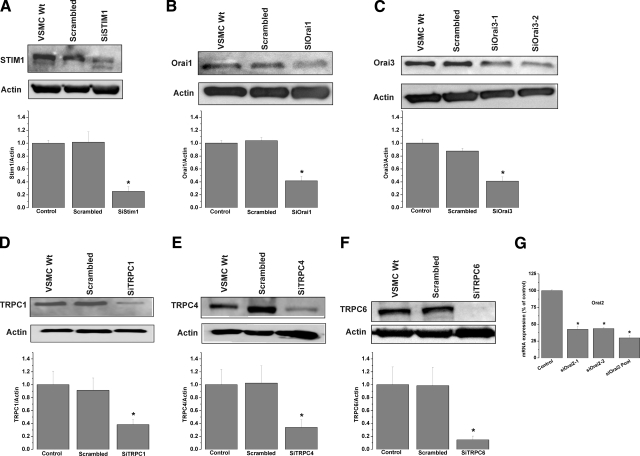
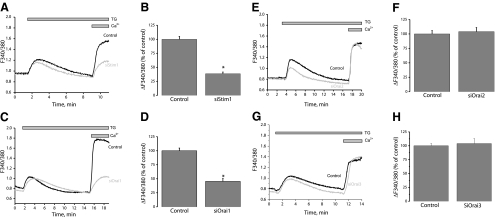
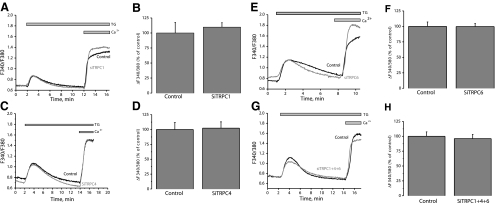
We used siRNA to assess the involvement in SOCE of STIM1, Orai, and TRPC isoforms. Knockdown of STIM1, Orai, or TRPC isoforms in VSMCs was achieved with two different siRNA sequences used individually (see Supplemental Table 1 for sequences). siRNA sequences induced a marked decrease in their target protein levels as assessed by Western blotting (Fig. 3) (decrease by 74.7±7.5% for siSTIM1-1, 58.3±7.0% for siOrai1-1, 58.8±6.4% for siOrai3-1, 61.8±7.7% for siTRPC1-1, 66.2±12.1% for siTRPC4-1, and 85.4±5.8% for siTRPC6-1, n=3) (Fig. 3A–F). An extended version of the Western blots with molecular weight markers is shown in Supplemental Fig. 2. Unfortunately, commercial antibodies against Orai2 failed to detect a specific band for rat Orai2 in synthetic VSMCs. Nevertheless, qPCR showed that both siRNAs against Orai2 either individually or pooled were efficient in decreasing Orai2 mRNA levels 72 h post-transfection (Fig. 3G) (decrease by 58.9±4.5% for siOrai2-1, 57.9±1.4% for siOrai2-2, and 71.4±3.9% for pooled siOrai2-1 and -2). Interestingly, down-regulation of either STIM1 (Fig. 4A, B) or Orai1 (Fig. 4C, D) using siRNA significantly suppressed thapsigargin-activated SOCE in synthetic VSMCs (61.5±3.06% inhibition of Ca2+ entry for siSTIM1-1 and 54.63±4.99% for siOrai1-1). Neither Orai2 (Fig. 4E, F), Orai3 (Fig. 4G, H), TRPC1 (Fig. 5A, B), TRPC4 (Fig. 5C, D), TRPC6 (Fig. 5E, F), or simultaneous TRPC1/4/6 (Fig. 5G, H) knockdown affected the amplitude of thapsigargin-induced SOCE in synthetic VSMCs. Statistical analysis of the Ca2+ release phase showed that none of the siRNAs used had any significant effect on Ca2+ release (data not shown). All results are representative of two independent siRNA sequences per target gene and are compared with cells transfected with a scrambled control siRNA (see Supplemental Table 1 for sequence). Results are representative of four to nine independent experiments. For the case of STIM1 and Orai1, results similar to those with siRNA were obtained using two shRNA sequences cloned in the pRS vector (Origene) used independently; parallel transfections with the empty vector were performed as a control. Supplemental Fig. 3 shows results for shSTIM1-1 and shOrai1-1 and are representative of three independent experiments (shRNA sequences are provided in Supplemental Table 1).
Figure 3.
Successful mRNA and protein knockdown of STIM1, Orai, and TRPC using siRNA. A–F) Western blot analysis probing for decrease protein levels of STIM1 (A), Orai (B, C), and TRPC isoforms (D–F) after RNA silencing. Actin was used as a control, and densitometry was performed using ImageJ software (for Orai3 blots, densitometry is shown for siOrai3-1). Extended versions of Western blots are shown in Supplemental Fig. 2. G) qPCR assessing effect of specific siRNA on Orai2 mRNA levels. Western blotting data are representative of 3 independent experiments; qPCR data are representative of 6 independent experiments. Wt, wild type.
Figure 4.
STIM1 and Orai1 mediate SOCE in synthetic VSMCs. Average SOCE in response to 2 μM thapsigargin (TG) after siRNA knockdown of STIM1 from one coverslip (A) and statistical analysis of several cells from different coverslips are shown (B) (control, n=25; siSTIM1, n=19 cells). Similar data are shown for Orai1 (C, D) (control, n=22; siOrai1, n=16 cells), Orai2 (E, F) (control, n=24; siOrai2, n=23 cells), and Orai3 (G, H) (control, n=34; siOrai3, n=20 cells). No siRNA had any significant effect on Ca2+ release. Data are representative of at least 4 independent experiments.
Figure 5.
TRPC1, TRPC4, and TRPC6 are not involved in synthetic VSMC SOCE. Average SOCE in response to 2 μM thapsigargin (TG) after siRNA knockdown of TRPC1 from one coverslip (A), and statistical analysis of several cells from different coverslips are shown (B) (control, n=70; siTRPC1, n=145 cells). Similar data are shown for TRPC4 (C, D) (control, n=51; siTRPC4, n=42 cells), TRPC6 (E, F) (control, n=33; siTRPC6, n=27 cells), and simultaneous knockdown of TRPC1/4/6 (G, H) (control, n=21; siTRPC1/4/6, n=24). No siRNA had any significant effect on Ca2+ release. Data are representative of at least 4 independent experiments (9 independent experiments for siTRPC1).
Orai1 mediates monovalent ICRAC currents in synthetic VSMCs and A7r5 cells
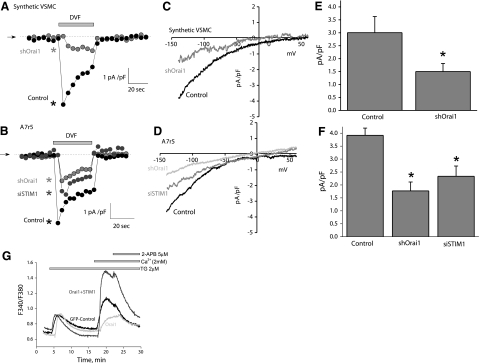
We determined in synthetic VSMCs the effect of Orai1 silencing on monovalent ICRAC using whole-cell patch-clamp recording in DVF conditions. As expected, in control cells store depletion activated monovalent ICRAC (Fig. 6A, C). Orai1 silencing inhibited ICRAC densities by 50% (control, 3.0±0.6 pA/pF; silenced, 1.5±0.3 pA/pF; n=3) (Fig. 6A, C). Figure 6C shows a typical I/V relation of ICRAC recorded in DVF bath solutions from control and Orai1-silenced synthetic VSMCs. Similarly, the smooth muscle cell line A7r5 also displayed ICRAC similar to that observed in synthetic VSMCs that were also reduced after silencing of Orai1 (control, 3.9±0.3 pA/pF; silenced, 1.8±0.3 pA/pF; n=4) (Fig. 6B, D). In addition, STIM1 silencing in A7r5 cells also inhibited ICRAC current amplitude (siSTIM1, 2.33±0.39 pA/pF; n=6). Statistical analyses are shown in Fig. 6E for synthetic VSMCs and in Fig. 6F for A7r5 cells.
Figure 6.
Orai1 knockdown inhibits ICRAC in synthetic VSMCs and A7r5 cells. A–D) Silencing of Orai1 inhibits ICRAC recorded in DVF solutions in synthetic VSMCs (A, C). Similar results were obtained in A7r5 cells (B, D). STIM1 knockdown in A7r5 cells also inhibits ICRAC recorded in DVF solutions (B, D). E, F) Statistical analysis in synthetic VSMCs (E) and A7r5 cells (F), based on 3 to 4 independent experiments. G) Effect of 5 μM 2-APB on SOCE in wild-type synthetic VSMCs transfected with GFP (n=30) and in same cells transfected with CFP-Orai1 alone (n=16) or CFP-Orai1 + eYFP-STIM1 (n=28). Data are representative of 4 independent experiments. TG, thapsigargin.
From the above results an obvious question emerges: if STIM1/Orai1 mediates SOCE and ICRAC in VSMCs, why is it uniquely inhibited by low concentrations of 2-APB? This property cannot be attributed to the rat species because RBL cells display the same pattern of 2-APB activation/inhibition observed in human cells. 2-APB was shown to reverse STIM1 puncta back into a tubular distribution and therefore disrupt the STIM1/Orai1 functional interaction (31). We reasoned that a VSMC-specific modification of either STIM1 or Orai1 might be responsible for this peculiar pharmacological property. To address this hypothesis, we expressed human fluorescently tagged Orai1 either alone or coexpressed with STIM1 in VSMCs. Expression of CFP-Orai1 in synthetic VSMCs caused somewhat of an inhibition of SOCE consistent with the dominant-negative effect on SOCE previously described in other cell types (35) (Fig. 6G). However, coexpression of eYFP-STIM1 and CFP-Orai1 in synthetic VSMCs caused a substantial amplification of the magnitude and the rate of SOCE as reported previously in other cell types (35, 36) (Fig. 6G). Notably, the amplified SOCE is also sensitive to 5 μM 2-APB, possibly suggesting a VSMC-specific post-translational modification of STIM1 and/or Orai1.
SOCE and STIM1/Orai1 are up-regulated in synthetic proliferative migratory VSMCs
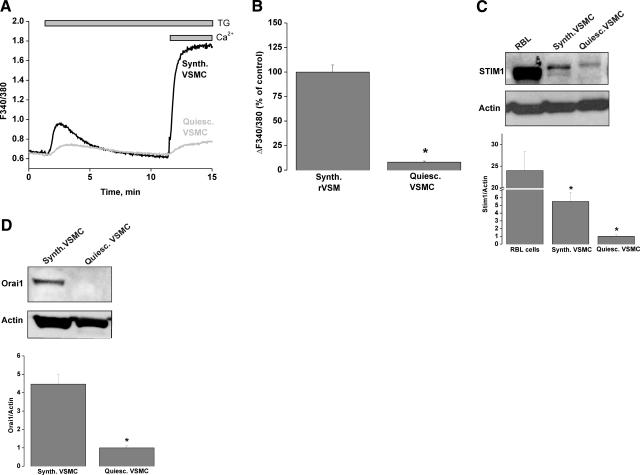
VSMCs in vivo are contractile and quiescent unlike the synthetic serum-cultured VSMCs used so far in this study, which are proliferative and migratory and are known to recapitulate most of the features observed during disease states such as atherosclerosis and restenosis after percutaneous intervention (28, 37,38,39). In vitro VSMC dedifferentiation from a quiescent to a proliferative and migratory phenotype is fully achieved after ∼30 h in culture after enzymatic dispersion (28). We sought to compare the amplitude of SOCE stimulated by thapsigargin (2 μM) in synthetic rat aortic VSMCs with that of quiescent freshly dispersed VSMCs from rat aorta using Ca2+ imaging. As seen in Fig. 7A, B, SOCE was substantially higher in synthetic VSMCs compared with quiescent freshly dispersed VSMCs. These results were obtained in the presence of the L-type Ca2+ channel inhibitor verapamil (10 μM) and the Na+ channel inhibitor tetrodotoxin (0.5 μM), ruling out the contribution of membrane depolarization to the small Ca2+ entry observed in freshly isolated VSMCs. In fact, as previously reported by Berra-Romani et al. (37) and shown in Fig. 7C, D, the difference in SOCE magnitude between freshly isolated and VSMCs correlated with differences in expression of STIM1 and Orai1 proteins between these two cell types. Western blot analysis using protein extracts from quiescent freshly isolated and synthetic aortic VSMCs showed that VSMCs have much higher protein levels of STIM1 and Orai1 compared with their quiescent freshly isolated counterparts (5.5±1.2- and 4.46±0.5-fold increase, respectively, n=4) (Fig. 7C, D). Extended versions of Western blots with molecular weight markers are shown in Supplemental Fig. 4. The up-regulation of STIM1 and Orai1 in proliferative migratory VSMCs prompted us to analyze the effects of STIM1 and Orai1 knockdown on migration and proliferation of those cells.
Figure 7.
SOCE and STIM1/Orai1 protein levels are increased in proliferative migratory VSMCs. A, B) Thapsigargin (TG) (2 μM) activates a much bigger SOCE in synthetic VSMCs (Synth.VSMC; n=154) than in quiescent freshly dispersed VSMCs (Quiesc.VSMC; n=98). Traces are averages from 25 to 30 cells and are representative of 6 independent experiments, each analyzing 21–35 cells. C) Synthetic VSMCs express higher levels of STIM1 protein compared with quiescent freshly isolated VSMCs. D) Similar results were seen in regard to Orai1 expression. Western blotting data are representative of 4 independent experiments. RBL mast cells were used as controls for STIM1 protein expression.
STIM1 and Orai1 are important for VSMC migration and proliferation
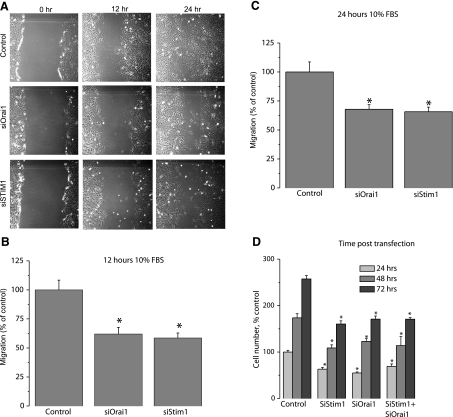
Synthetic VSMCs transfected with either scrambled control siRNA or siRNA against different STIM and Orai isoforms were used in a scratch wound assay to assess VSMC migration in response to 10% serum. To avoid contributions from VSMC proliferation, wound closures were analyzed 12 h postscratch. siRNA against either STIM1 or Orai1 led to a significant decrease in the wound surface covered by VSMCs (compared with scrambled control siRNA) in response to serum 12 and 24 h postwound (% inhibition 12 h postwound: 41.4±4.3% for STIM1; 38.0±5.7% for Orai1) (Fig. 8A–C). However, STIM2, Orai2, and Orai3 silencing had no effect on synthetic VSMC migration (Supplemental Fig. 5A, B).
Figure 8.
STIM1 and Orai1 knockdown inhibits synthetic VSMC proliferation and migration. A) Bright-field views of scratch wound migration assay at different times postwound in synthetic VSMCs transfected with either crambled control siRNA or siRNA against either STIM1 or Orai1. B, C) Data from 3 independent experiments (4 wells/condition), quantified and analyzed as described in Materials and Methods, presented as percentage of control. D) Proliferation in VSMCs transfected with scrambled control siRNA, siSTIM1, siOrai1, and siSTIM1 + siOrai1, assayed as described in Materials and Methods. Data are presented as percentage of control and are representative of 5 independent experiments in 96-well plates (12 independent wells/transfection).
VSMC proliferation was assessed in the presence of complete culture media containing 10% serum and was evaluated as described in Materials and Methods. Figure 8D shows that STIM1 and Orai1 siRNA significantly inhibited cell proliferation (% inhibition 72 h post-transfection: 37.7±2.6% for STIM1; 33.8±2.5% for Orai1; compared with scrambled control siRNA); simultaneous STIM1 and Orai1 knockdown was similar to either alone (% inhibition 72 h post-transfection: 33.6±1.3%). Knockdown of Orai2, Orai3, and STIM2 using specific siRNA had no effect on proliferation of synthetic VSMCs (Supplemental Fig. 5C).
DISCUSSION
In this report we show that SOCE in synthetic VSMCs displays classic pharmacological features of SOCE in other cells, namely inhibition by low concentrations of lanthanides (5 μM Gd3+), 2-APB, and ML-9. We show that ICRAC in the presence of external Ca2+ and under DVF conditions is functionally present in rat synthetic VSMCs and the A7r5 smooth muscle cell line, can be amplified in DVF bath solutions, and has similar kinetics, reminiscent of ICRAC in RBL cells. As previously demonstrated in other cell types (4, 29, 32, 40), we show that VSMC monovalent ICRAC measured under DVF conditions displays rapid time-dependent inactivation of inward Na+ currents (termed depotentiation) on removal of extracellular divalent currents, strong inward rectification, positive reversal potential, and inhibition by low concentrations of lanthanides and 2-APB reminiscent of ICRAC (4). VSMC monovalent ICRAC and SOCE were substantially inhibited by silencing of Orai1 and STIM1, whereas silencing of Orai2, Orai3, TRPC1, TRPC4, and TRPC6 had no effect on SOCE. Unlike other cell types, VSMC SOCE shows a unique pharmacological feature because it is inhibited with low concentrations of 2-APB (5 μM). Surprisingly, ectopic coexpression of human eYFP-STIM1 and CFP-Orai1 in VSMCs generated a large SOCE that was also inhibited by 5 μM 2-APB, suggesting that perhaps in VSMCs STIM1 and/or Orai1 undergoes a post-translational modification or there is a factor that mediates 2-APB action on STIM1/Orai1 that is different in VSMCs. In agreement with previous studies (37), we also show that SOCE is up-regulated in synthetic proliferative and migratory VSMCs compared with the quiescent freshly isolated rat VSMCs and found that this result correlated with significantly higher protein levels of STIM1 and Orai1 in synthetic VSMCs. Notably, we showed that migration and proliferation of synthetic proliferative migratory VSMCs was inhibited on STIM1 and Orai1 knockdown but was unaffected by Orai2, Orai3, and STIM2 knockdown. We propose that ICRAC is mediating SOCE in VSMC and is encoded by STIM1 and Orai1 independently of TRPC and other Orai isoforms and plays a functional role in VSMC proliferation and migration.
In this study, we failed to detect an involvement of Orai2, Orai3, or any of the TRPC channels expressed in synthetic VSMCs (TRPC1, TRPC4, and TRPC6) in SOCE despite successful knockdown of their protein levels. Furthermore, simultaneous knockdown of all three of these TRPC isoforms had no effect on VSMC SOCE. Prior studies on VSMC SOCE suggested that TRPC1 channels can participate in VSMC SOCE (17,18,19, 34). Large nonselective outwardly rectifying currents mediated by TRPC1 were proposed to play a role in SOCE activated by thapsigargin or cyclopiazonic acid (18, 20, 41). These SOC currents identified in various VSMC types from different species and vascular beds vary significantly in their electrophysiological and pharmacological characteristics. Whether these differences are due to distinct currents from different VSMC types or to different experimental conditions used in different studies is unclear. In virtually all of these studies the use of several pharmacological agents that distinguish store-operated from non-store-operated channels at similar concentrations in Fura-2 imaging and patch-clamp recordings was not undertaken. In some instances, the pharmacological features of SOCE in Fura-2 imaging (42) did not correlate with those of membrane currents from the same cells (41). In other cases, SOCE and membrane currents were inhibited by agents known to inhibit non-SOCE pathways (43, 44). One notable exception is the studies by Byron and colleagues in the A7r5 smooth muscle cell line, in which the pharmacological characteristics of the SOC current measured correlated with those of SOCE measured under identical external ionic conditions with Fura-2 (17). One interesting finding from the Byron group is that unlike other studies, these authors reported small SOC currents (∼0.5 pA/pF at −115 mV in 20 mM external Ca2+) in A7r5 cells that are inwardly rectifying with positive reversal potential, features shared with ICRAC, and are in agreement with our findings (∼0.32 pA/pF at −100 mV in 10 mM external Ca2+) (17, 45).
Our data are in agreement with previous studies from two independent groups: the first showed the involvement of STIM1 (46) and Orai1 (47) in VSMC SOCE, and the other implicated STIM1 and Orai1 in human airway smooth muscle SOCE (48, 49). One report on TRPC1-knockout mice described normal SOC currents in VSMCs and thus questioned the role of TRPC1 as a component of SOCE in smooth muscle (25). Based on experiments carried out using a blocking polyclonal anti-TRPC1 antibody, Li et al. (20) conceded that TRPC1 contributed only partially to ionic currents activated by thapsigargin and only in a small fraction of human VSMCs (∼25%); surprisingly, the effect of TRPC1 knockdown on membrane currents as a means to corroborate the antibody results was not reported. Although we found no contribution of TRPC isoforms to SOCE, we cannot exclude the partial contribution of TRPC1 (or another TRPC isoform) in a small fraction of cells to a nonselective thapsigargin-activated current under certain experimental conditions. In the study by Li et al. (20), thapsigargin-evoked membrane currents were blocked using anti-STIM1 antibody, suggesting a role of membrane STIM1 in SOCE. Although our results are not incompatible with a role of plasma membrane STIM1 in VSMC SOCE, we showed that SOCE could be amplified by coexpression of CFP-Orai1 and eYFP-STIM1; the latter was shown to be restricted to the internal stores (36).
We found that STIM1 and Orai1 contribute to VSMC proliferation and migration in vitro. Two recent reports described an essential role for STIM1 in neointimal formation after arterial balloon injury in the rat (50, 51), providing in vivo relevance for STIM1, and one recent study reported increased expression of STIM1/Orai1 in aorta from hypertensive rats (52). Clearly, additional studies are required to understand the contribution of Orai and STIM to VSMC function and Ca2+ signaling so we might in the future use these proteins as targets for therapy of vascular occlusive diseases such as restenosis, atherosclerosis, and hypertension.
Supplementary Material
Acknowledgments
This work was supported by an NIH early career grant (K22ES014729) to M.T. We gratefully acknowledge Dr. Wayne DeHaven (National Institute of Environmental Health Sciences, NIH) for helpful advice on the patch-clamp recordings and Wendy Hobb for administrative assistance.
References
- Putney J W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Parekh A B, Putney J W., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis R S. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad L M, Cannon T R, Taylor C W. A non-capacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin. J Physiol. 1999;517:121–134. doi: 10.1111/j.1469-7793.1999.0121z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, Bird G S, McKay R R, Putney J W., Jr Comparison of human TRPC3 channels in receptor-activated and store-operated modes: differential sensitivity to channel blockers suggests fundamental differences in channel composition. J Biol Chem. 2002;277:21617–21623. doi: 10.1074/jbc.M202549200. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S H, Tanasa B, Hogan P G, Lewis R S, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim M L, Heo W D, Jones J T, Myers J W, Ferrell J E, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio P J, Yeromin A V, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak J A, Wagner S L, Cahalan M D, Velicelebi G, Stauderman K A. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa D L, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet J P. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwack Y, Feske S, Srikanth S, Hogan P G, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Potier M, Trebak M. New developments in the signaling mechanisms of the store-operated calcium entry pathway. Pflügers Arch. 2008;457:405–415. doi: 10.1007/s00424-008-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven W I, Smyth J T, Boyles R R, Putney J W., Jr Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- Wamhoff B R, Bowles D K, Owens G K. Excitation-transcription coupling in arterial smooth muscle. Circ Res. 2006;98:868–878. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- Casteels R, Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann L I, Markun D R, Henderson K K, Cribbs L L, Byron K L. Pharmacological and electrophysiological characterization of store-operated currents and capacitative Ca2+ entry in vascular smooth muscle cells. J Pharmacol Exp Ther. 2006;317:488–499. doi: 10.1124/jpet.105.095067. [DOI] [PubMed] [Google Scholar]
- Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel S S, Yuan J X. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- Xu S Z, Beech D J. TrpC1 is a membrane-spanning subunit of store-operated Ca(2+) channels in native vascular smooth muscle cells. Circ Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- Li J, Sukumar P, Milligan C J, Kumar B, Ma Z Y, Munsch C M, Jiang L H, Porter K E, Beech D J. Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res. 2008;103:e97–104. doi: 10.1161/CIRCRESAHA.108.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunichika N, Yu Y, Remillard C V, Platoshyn O, Zhang S, Yuan J X. Overexpression of TRPC1 enhances pulmonary vasoconstriction induced by capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2004;287:L962–L969. doi: 10.1152/ajplung.00452.2003. [DOI] [PubMed] [Google Scholar]
- Albert A P, Large W A. A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes. J Physiol. 2002;538:717–728. doi: 10.1113/jphysiol.2001.013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina V A, Platoshyn O, Bailey C L, Wang J, Limsuwan A, Sweeney M, Rubin L J, Yuan J X. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- Trepakova E S, Gericke M, Hirakawa Y, Weisbrod R M, Cohen R A, Bolotina V M. Properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J Biol Chem. 2001;276:7782–7790. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Storch U, Mederos y Schnitzler M, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflügers Arch. 2007;455:465–477. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- Zarayskiy V, Monje F, Peter K, Csutora P, Khodorov B, Bolotina V. Store-operated Orai1 and IP3 receptor-operated TRPC1: separation of Siamese twins. Channels. 2007;1:e1–e7. doi: 10.4161/chan.4835. [DOI] [PubMed] [Google Scholar]
- Nilius B. Store-operated Ca2+ entry channels: still elusive! Sci STKE. 2004;2004:pe36. doi: 10.1126/stke.2432004pe36. [DOI] [PubMed] [Google Scholar]
- House S J, Ginnan R G, Armstrong S E, Singer H A. Calcium/calmodulin-dependent protein kinase II-δ isoform regulation of vascular smooth muscle cell proliferation. Am J Physiol. 2007;292:C2276–C2287. doi: 10.1152/ajpcell.00606.2006. [DOI] [PubMed] [Google Scholar]
- Abdullaev I F, Bisaillon J M, Potier M, Gonzalez J C, Motiani R K, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–1299. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, St. Bird J G, McKay R R, Birnbaumer L, Putney J W., Jr Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels. J Biol Chem. 2003;278:16244–16252. doi: 10.1074/jbc.M300544200. [DOI] [PubMed] [Google Scholar]
- Smyth J T, Dehaven W I, Bird G S, Putney J W., Jr Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci. 2008;121:762–772. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis R S. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J Gen Physiol. 1995;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinelt C, Lis A, Beck A, Fleig A, Penner R. 2-Aminoethoxydiphenyl borate directly facilitates and indirectly inhibits STIM1-dependent gating of CRAC channels. J Physiol. 2008;586:3061–3073. doi: 10.1113/jphysiol.2008.151365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert A P, Saleh S N, Peppiatt-Wildman C M, Large W A. Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells. J Physiol. 2007;583:25–36. doi: 10.1113/jphysiol.2007.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa D L, Beck A, Nadler M J, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet J P. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J C, Dehaven W I, Smyth J T, Wedel B, Boyles R R, Bird G S, Putney J W., Jr Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra-Romani R, Mazzocco-Spezzia A, Pulina M V, Golovina V A. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol. 2008;295:C779–C790. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House S J, Potier M, Bisaillon J, Singer H A, Trebak M. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflügers Arch. 2008;456:769–785. doi: 10.1007/s00424-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G K. Molecular control of vascular smooth muscle cell differentiation and phenotypic plasticity. Novartis Found Symp. 2007;283:174–191. doi: 10.1002/9780470319413.ch14. discussion 191–173, 238–141. [DOI] [PubMed] [Google Scholar]
- Bakowski D, Parekh A B. Permeation through store-operated CRAC channels in divalent-free solution: potential problems and implications for putative CRAC channel genes. Cell Calcium. 2002;32:379–391. doi: 10.1016/s0143416002001914. [DOI] [PubMed] [Google Scholar]
- Bergdahl A, Gomez M F, Wihlborg A K, Erlinge D, Eyjolfson A, Xu S Z, Beech D J, Dreja K, Hellstrand P. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol. 2005;288:C872–880. doi: 10.1152/ajpcell.00334.2004. [DOI] [PubMed] [Google Scholar]
- Flemming R, Xu S Z, Beech D J. Pharmacological profile of store-operated channels in cerebral arteriolar smooth muscle cells. Br J Pharmacol. 2003;139:955–965. doi: 10.1038/sj.bjp.0705327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel S S, Platoshyn O, Wang J, Yu Y, Sweeney M, Krick S, Rubin L J, Yuan J X. Capacitative Ca2+ entry in agonist-induced pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol. 2001;280:L870–L880. doi: 10.1152/ajplung.2001.280.5.L870. [DOI] [PubMed] [Google Scholar]
- Ng L C, Gurney A M. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res. 2001;89:923–929. doi: 10.1161/hh2201.100315. [DOI] [PubMed] [Google Scholar]
- Brueggemann L I, Markun D R, Barakat J A, Chen H, Byron K L. Evidence against reciprocal regulation of Ca2+ entry by vasopressin in A7r5 rat aortic smooth-muscle cells. Biochem J. 2005;388:237–244. doi: 10.1042/BJ20041360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csutora P, Peter K, Kilic H, Park K M, Zarayskiy V, Gwozdz T, Bolotina V M. Novel role for STIM1 as a trigger for calcium influx factor production. J Biol Chem. 2008;283:14524–14531. doi: 10.1074/jbc.M709575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarayskiy V V, Yang B, Peter K, Herbert A G, Bolotina V M. ADAR-mediated RNA editing of Orai1 is a key to different selectivity of store-operated channels. Biophys J. 2008;94:2938-Pos. (abstr.) [Google Scholar]
- Peel S E, Liu B, Hall I P. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res. 2006;7:119. doi: 10.1186/1465-9921-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel S E, Liu B, Hall I P. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:744–749. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R W, Wang H, Gao P, Li M Q, Zeng C Y, Yu Y, Chen J F, Song M B, Shi Y K, Huang L. An essential role for STIM1 in neointima formation following arterial injury. Cardiovasc Res. 2008;81:660–668. doi: 10.1093/cvr/cvn338. [DOI] [PubMed] [Google Scholar]
- Aubart F C, Sassi Y, Coulombe A, Mougenot N, Vrignaud C, Leprince P, Lechat P, Lompre A M, Hulot J S. RNA interference targeting stim1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol Ther. 2008;17:455–462. doi: 10.1038/mt.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachini F R, Chiao C W, Carneiro F S, Lima V V, Carneiro Z N, Dorrance A M, Tostes R C, Webb R C. Increased activation of stromal interaction molecule-1/Orai-1 in aorta from hypertensive rats: a novel insight into vascular dysfunction. Hypertension. 2009;53:409–416. doi: 10.1161/HYPERTENSIONAHA.108.124404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.