Abstract
In Alzheimer’s disease (AD), oxidative stress is present early and contributes to disease pathogenesis. We previously reported that in Tg19959 transgenic AD mice, partial deficiency of the mitochondrial antioxidant enzyme manganese superoxide dismutase (MnSOD) exacerbated amyloid pathology. We therefore asked whether MnSOD overexpression would prove beneficial against AD pathogenesis, by studying the offspring of Tg19959 mice crossed with MnSOD-overexpressing mice. At 4 mo of age, there was a 2- to 3-fold increase in MnSOD protein levels in Tg19959-MnSOD mice compared to Tg19959 littermates. Tg19959-MnSOD mice also had a 50% increase in catalase protein levels, a 50% decrease in levels of oxidized protein, and a 33% reduction in cortical plaque burden compared to Tg19959 littermates. Spatial memory was impaired and synaptophysin levels were decreased in Tg19959 mice compared to wild-type littermates, but memory and synaptophysin levels were restored to wild-type levels in Tg19959-MnSOD littermates. These benefits occurred without changes in sodium dodecyl sulfate-soluble or formic acid-soluble Aβ pools or Aβ oligomers in Tg19959-MnSOD mice compared to Tg19959 littermates. These data demonstrate that facilitation of the mitochondrial antioxidant response improves resistance to Aβ, slows plaque formation or increases plaque degradation, and markedly attenuates the phenotype in a transgenic AD mouse model.—Dumont, M., Wille, E., Stack, C., Calingasan, N. Y., Beal, M. F., Lin, M. T. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer’s disease.
Keywords: Tg19959 mice, antioxidant, behavior, amyloid, synaptophysin
Oxidative stress plays a crucial role in Alzheimer’s disease (AD) (1,2,3). It is present early in pathogenesis (4, 5) and exacerbates amyloid deposition and other hallmarks of the disease. Partial deficiency of manganese superoxide dismutase (MnSOD) increased amyloid plaque deposition in Tg19959 mice (6) and tau phosphorylation in Tg2576 mice (7). It also accelerated the onset of behavioral abnormalities in hAPP mice (8). MnSOD is a mitochondrial enzyme catalyzing the dismutation of superoxide to H2O2, which is then decomposed to water via catalase or glutathione peroxidase. This mitochondrial antioxidant machinery neutralizes the production of reactive oxygen species detrimental to the mitochondrion itself and to the cell.
Considering the deleterious effects of MnSOD deficiency, we studied the effects of its overexpression in Tg19959 mice. Tg19959 mice carry the human amyloid precursor protein (APP) with 2 familial AD mutations (KM670/671NL and V717F). These mice develop early onset amyloid deposition starting in the neocortex, amydgala, and hippocampus (6, 9). At 3 mo of age, amyloid deposition is accompanied by reactive microglia and activated astrocytes (10), as well as learning and memory impairments in the Morris water maze (9). With age, amyloid plaques, levels of Aβ40 and Aβ42, and behavioral deficits increase progressively (11). At 6–7 mo of age, Tg19959 mice show working memory deficits in the 6-arm radial water maze (12) and cholinergic dysfunction associated with neuronal damage and axonal loss (13). For our study Tg19959 mice were crossed with mice overexpressing MnSOD (14), and behavior, plaque burden, oxidative markers, and Aβ levels were assessed at 4 mo of age.
MATERIALS AND METHODS
Transgenic animals
Tg19959 mice were obtained from Dr. George Carlson (McLaughlin Research Institute, Great Falls, MT, USA). Tg19959 mice were constructed by injecting FVB × 129S6 F1 embryos with a cosmid insert containing human APP695 with 2 familial AD mutations (KM670/671NL and V717F), under the control of the hamster PrP promoter (9).
MnSOD-overexpressing mice were obtained from Dr. Charles Epstein (University of California, San Francisco, CA, USA). MnSOD mice were constructed by injecting B6D2 F1 fertilized eggs with a DNA construct containing a 13-kb genomic MnSOD clone isolated from C57BL/6J mice, which included 2 kb of the native MnSOD promoter (14).
For our experiments, Tg19959 males were crossed with MnSOD females. Littermates of four different genotypes were produced and identified by PCR of tail DNA: Tg19959, Tg19959-MnSOD, MnSOD, and wild-type mice. Behavioral analyses were performed at 4 mo of age, and brain histopathology and biochemistry were then assessed on the same animals. All experiments were approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee.
Behavioral studies
Exploration and locomotor activity were evaluated in an open field (45 × 45 cm; height 20 cm). At the beginning of the test, each mouse was placed in one of the corners. The total distance traveled was recorded for 5 min/d and averaged over 3 d using a video tracking system (Ethovision 3.0, Noldus Technology, Attleborough, MA, USA).
Exploration and anxiety were measured in an elevated plus maze. The apparatus consisted of 4 arms (length 30 cm; width 5 cm; height 39 cm) in a cross-shaped form and a central region (5 cm × 5 cm). Two of these arms were enclosed on 3 sides by walls (height 15 cm), whereas the other arms were not. The 2 enclosed arms faced each other, as did the 2 open arms. At the beginning of the test, mice were placed in the central region. Time spent in the open arms was measured during a single trial of 5 min.
Spatial learning and memory were assessed in the Morris water maze. An opaque basin (diameter 84 cm; height of wall 51 cm) was filled with opacified water (23°C). During the acquisition period, extramaze visual cues, such as light fixtures and wall posters, were arranged in the room. The hidden platform was located in the middle of the northwest (NW) quadrant, 1 cm beneath water level. Each day mice were placed next to and facing the wall of the basin in 4 different starting positions: north, east, south, and west, corresponding to 4 successive trials. Latencies and total distances before reaching the platform were recorded for 5 d with a video tracking system. After each trial, animals were placed in a plastic holding cage filled with paper towels to keep them dry and warm, with an intertrial interval of 20 min. Whenever the mouse failed to reach the platform within the maximally allowed time of 60 s, it was placed on the platform by the experimenter for 5 s. A probe trial was assessed 24 h after the acquisition period, removing the platform from the pool. The mice were released on the north side, and the percentage of time spent in each quadrant was measured for the first 15 s. To ensure that any differences were not due to visual deficits, the visible platform version of the water maze was performed 2 h after the probe trial. A pole (13 cm) was added on the platform. Animals were tested during 4 trials where the platform was located in the southeast (SE) quadrant. The duration of a single trial was 60 s with an intertrial interval of 20 min. Latencies and total distances before reaching the platform were recorded and averaged over the 4 trials.
Motor coordination was evaluated in an accelerated rotorod (Economex, Columbus Instruments, Columbus, OH, USA). The beam of the apparatus (diameter 4 cm; width 8 cm; height 38 cm) was made of ribbed plastic, and plates were flanked on either side to prevent escape. Mice were placed on top of the revolving beam for 4 successive trials per day, with 20-min intertrial intervals. The rod accelerated gradually from 4 to 40 rpm over 2 min. Latencies before falling from the rod were recorded during 2 d and averaged.
Immunohistochemistry for Aβ42 deposits, Aβ oligomers, and microgliosis
After anesthesia by intraperitoneal injection of sodium pentobarbital, mice were transcardially perfused with ice-cold 0.9% sodium chloride. Brains were removed and dissected on ice. Tissues for histology were fixed in 4% paraformaldehyde in 1 mM phosphate buffer (pH 7.4) for 24 h and stored in cryoprotectant solution (30% glycerol, 30% ethylene glycol in 20 mM phosphate buffer, pH 7.4) until further processing. Corresponding tissues for biochemistry were snap frozen in liquid nitrogen and stored at −80°C.
Regions analyzed included the retrosplenial/motor cortex and CA1/dentate region of the hippocampus. The retrosplenial/motor cortex was analyzed in 5 sections/mouse (350 μm apart) beginning at the level of bregma −1.06 to bregma −1.94. The CA1/dentate region was analyzed in 5 sections/mouse (350 μm apart) beginning at the level of bregma −1.34 to bregma −2.7.
For Aβ42 deposits, sections were pretreated with 50% formic acid for 5 min before labeling with anti-Aβ42 rabbit polyclonal antibody AB5078P (1:1000) (Chemicon, Temecula, CA, USA). Immunostaining with AB5078P is similar to that seen with the mouse monoclonal anti-Aβ antibody 6E10 (1:1000) (Covance, Emeryville, CA, USA) (data not shown). However, 6E10 also binds APP and β-C-terminal fragments in addition to Aβ and not surprisingly produces more background staining. Because Aβ42 is considered to be the pathogenic species of interest, quantitation was performed with AB5078P. To examine Aβ oligomers and microgliosis, adjacent sections were labeled with anti-oligomer rabbit polyclonal antibody A11 (1:500) (Invitrogen, Carlsbad, CA, USA) (15) and with anti-CD40 rat monoclonal antibody (1:100) (Serotec, Raleigh, NC, USA).
Immunolabeling was detected by the avidin-biotin complex peroxidase method and visualized with diaminobenzidine (DAB) incubation for 5 min (Vector, Burlingame, CA, USA). Sections were viewed with the ×10 objective on a Nikon Eclipse E600 microscope (Nikon, Melville, NY, USA), and digital images were captured using Stereo Investigator 4.35 (Microbrightfield, Burlington, VT, USA). Quantitative analysis was performed using NIH Image 1.63 (National Institutes of Health, Bethesda, MD, USA). Percentage area occupied by plaques, by A11-immunoreactivity, or by reactive microglia was calculated as well as plaque and A11-immunoreactive patch numbers (number/0.75 mm2).
ELISA of Aβ42 and Aβ40 peptides
Snap-frozen brain tissues were homogenized in 6% sodium dodecyl sulfate (SDS) with protease inhibitor cocktail (Complete Protease Inhibitor Cocktail tablet, Roche Diagnostics, Mannheim, Germany), sonicated for 1 min, and centrifuged at 200,000 g for 1 h at 20°C. The pellet was dissolved in 70% formic acid with protease inhibitor cocktail, sonicated for 1 min on ice, and centrifuged at 200,000 g for 1 h at 4°C. The supernatant was neutralized with 1 M Tris base, pH 11 (1:20 v:v). SDS-soluble and SDS-insoluble-formic acid-soluble Aβ42 and Aβ40 ELISAs were performed using commercial kits (Invitrogen) following the manufacturer’s instructions.
Western blotting
Snap-frozen brain tissues were homogenized by sonication in 6% SDS containing protease inhibitor cocktail. Protein concentration was measured (DC Protein Measurement Kit, Bio-Rad, Hercules, CA, USA), and equal protein amounts of the homogenates were electrophoresed through 4–12% Bis-Tris NuPage or 10–20% Tris-Tricine polyacrylamide gels (Invitrogen). After transfer to polyvinylidene fluoride, membranes were blocked in 5% nonfat dry milk in phosphate buffered saline with 0.05% Tween 20 (PBST) and exposed overnight to primary antibody at 4°C. Horseradish peroxidase-conjugated secondary antibody binding was visualized with enhanced chemiluminescence.
Primary antibodies and concentrations used for Western blotting were the following: rabbit polyclonal anti-MnSOD (1:10000; Novus Biologicals, Littleton, CO, USA), sheep polyclonal anti-CuZnSOD (1:5000; Calbiochem, San Diego, CA, USA), rabbit polyclonal anti-catalase (1:1000) and rabbit polyclonal anti-glutathione peroxidase-1 (1:500; Abcam, Cambridge, MA, USA), rabbit polyclonal anti-APP 369 (1:1000; gift from S. Gandy, Thomas Jefferson University, Philadelphia, PA, USA), mouse monoclonal anti-Aβ 6E10 (1:1000; Covance), mouse monoclonal anti-synaptophysin (1:1000; Millipore, Billerica, MA, USA), and mouse monoclonal anti-α-tubulin (1:10,000) and mouse anti-β-actin (1:5000; Sigma, St. Louis, MO, USA).
Films were scanned at 600 dpi, and densitometry was quantified with Scion Image 4.0.2 (Scion, Frederick, MD, USA). Ratios were calculated using densitometric values of the protein of interest divided by densitometric values of α-tubulin or β-actin.
Measurement of oxidized proteins
Brain protein carbonyl levels were measured using the Oxyblot Protein Oxidation Detection Kit (Millipore) according to the manufacturer’s protocol, with the following modifications: 5% nonfat dry milk/PBST was used as blocking solution and antibody diluent, the membrane was blocked for 1 h, and the primary antibody incubation was overnight. Bands were visualized by enhanced chemiluminescence. Films were scanned at 600 dpi, and Scion Image 4.0.2 (Scion) was used for densitometry.
Statistical analysis
ANOVA was used to compare the 4 groups: Tg19959, Tg19959-MnSOD, MnSOD, and wild-type mice. Post hoc Fisher PLSD (Fisher) tests were used for further analyses between groups. When only 2 groups were involved, 2-tailed t tests were used to compare Tg19959 and Tg19959-MnSOD mice (Statview 5.0.1; SAS Institute, Cary, NC, USA).
RESULTS
MnSOD overexpression increased MnSOD and catalase levels and decreased oxidative stress in Tg19959 mice
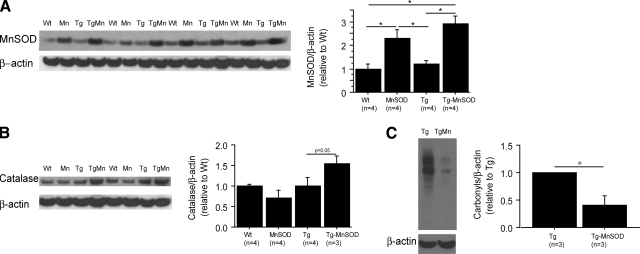
We confirmed a 2–3-fold increase of MnSOD levels at 4 mo of age in MnSOD and Tg19959-MnSOD mice compared to wild-type and Tg19959 littermates (Fig. 1A; P<0.05). Levels of CuZnSOD remained unchanged between groups (Supplemental Fig. 1A).
Figure 1.
MnSOD overexpression increased levels of MnSOD and catalase and decreased oxidative stress. A, B) Western blots of MnSOD (A) and catalase (B). Densitometric values are normalized to β-actin. MnSOD and catalase protein levels were increased in Tg19959-MnSOD mice compared to Tg19959 littermates. C) Western blots of brain protein carbonyl levels. Densitometric values are normalized to β-actin. MnSOD overexpression decreased oxidative stress in Tg19959 mice. *P < 0.05.
MnSOD catalyzes the dismutation of superoxide to H2O2. Catalase or glutathione peroxidase is then required to decompose H2O2 to water. Levels of catalase were increased in Tg19959-MnSOD compared to Tg19959 littermates (Fig. 1B; P=0.05). Levels of glutathione peroxidase-1 remained unchanged (Supplemental Fig. 1B).
Brain oxidative stress was assessed by protein carbonyls. At 4 mo of age, carbonyl levels were increased in Tg19959 mice relative to wild-type littermates (data not shown). Overexpression of MnSOD reduced markedly carbonyl levels in Tg19959 mice (Fig. 1C; P=0.028).
MnSOD overexpression reduced amyloid plaque burden and microgliosis in Tg19959 brains
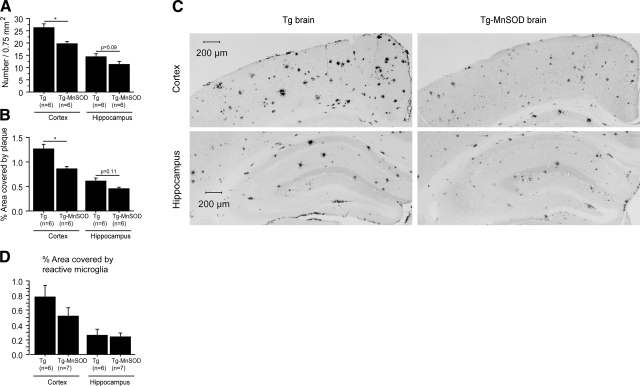
Amyloid plaques in Tg19959 and Tg19959-MnSOD mouse brains were detected by Aβ42 antibody staining (Fig. 2A–C). At 4 mo of age, overexpression of MnSOD in Tg19959 mice reduced amyloid deposition in the cortex (plaques/0.75 mm2, P=0.004; percentage area covered by plaque, P=0.002). There was a trend toward decreased amyloid deposition in the hippocampus (plaques/0.75 mm2, P=0.11; percentage area covered by plaque, P=0.09).
Figure 2.
MnSOD overexpression reduced amyloid plaque burden and microgliosis in Tg19959 mice. A, B) Amyloid plaques per 0.75 mm2 (A) and percentage area covered by plaque (B) in Tg19959 mice and Tg19959-MnSOD littermates. At 4 mo of age, MnSOD overexpression reduced amyloid plaques in Tg19959 mouse brains. C) Representative photographs of amyloid plaques detected by Aβ42 antibody AB5078P. D) Percentage area covered by reactive microglia (mean±se). There was a trend toward a decrease of cortical microgliosis in Tg19959-MnSOD mice compared to Tg19959 littermates. *P < 0.05.
We also performed immunohistochemical staining with CD40 antibody to visualize reactive microglia. There was a trend toward a decrease in cortical microgliosis in Tg19959-MnSOD mice compared to Tg19959 littermates (Fig. 2D; t test, cortex P=0.2066; hippocampus P=0.8198).
MnSOD overexpression rescued spatial memory retention and restored synaptophysin levels in Tg19959 mice
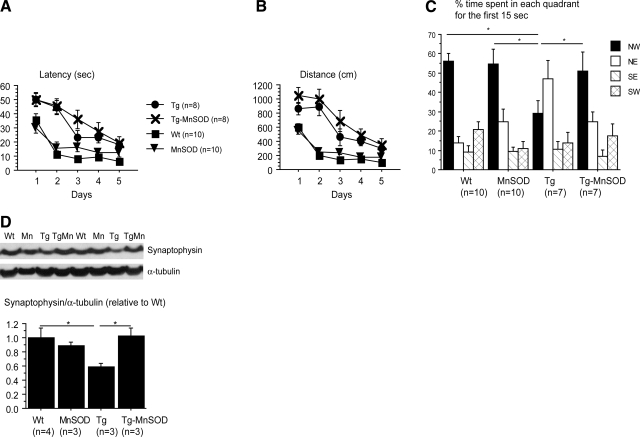
Spatial learning and memory were assessed by the Morris water maze. During the acquisition period, mice were trained for 5 d to locate the platform hidden in the NW quadrant of the pool. Both Tg19959 and Tg19959-MnSOD mice were slower to learn the location of the hidden platform compared to wild-type and MnSOD littermates (Fig. 3A, B; P<0.05). MnSOD overexpression did not improve spatial learning during the acquisition phase.
Figure 3.
MnSOD overexpression rescued spatial memory retention and restored synaptophysin levels in Tg19959 mice. A, B) Latency (A) and distance traveled (B) during the acquisition period over 5 d. Tg19959 and Tg19959-MnSOD mice were slower to learn than MnSOD and wild-type littermates. C) Percentage time spent in each quadrant during first 15 s of probe trial. Northwest quadrant was the target quadrant. Tg19959-MnSOD mice spent more time in the target quadrant compared to Tg19959 littermates. D) Western blot of synaptophysin, expressed as ratio of protein to α-tubulin. MnSOD overexpression restored synaptophysin levels in Tg19959 mice. Values are means ± se. *P < 0.05.
During the probe trial, percentage time spent in each quadrant was calculated for each group, and we focused on the percentage time spent in the target (NW) quadrant. At 4 mo, Tg19959 mice spent less time in the target quadrant than Tg19959-MnSOD, MnSOD, and wild-type littermates (Fig. 3C; P<0.05). Overexpression of MnSOD rescued spatial memory retention in Tg19959 mice.
A visible platform version of the task was also conducted to ensure that differences were not due to visual or motor abnormalities. Latency, distance, and swim speed were recorded during 4 consecutive trials. No significant differences were found between groups (data not shown). We also assessed exploration, locomotor activity (open field task), anxiety (elevated plus maze), and motor coordination (accelerated rotorod). We did not observe any significant changes in any of the tests between Tg19959 and Tg19959-MnSOD littermates (data not shown).
The best pathological correlate of cognitive impairment in AD is loss of synapses, as seen by decrease in synaptophysin (16). Given the improvement in spatial memory retention in the Morris water maze task, we examined synaptophysin levels. Tg19959 mice had reduced synaptophysin levels compared to wild-type mice. This level was restored by MnSOD overexpression (Fig. 3D; P=0.02 Tg19959 vs. wild-type littermates; P=0.02 Tg19959 vs. Tg19959-MnSOD littermates).
Effects of MnSOD overexpression on APP expression and processing and Aβ levels
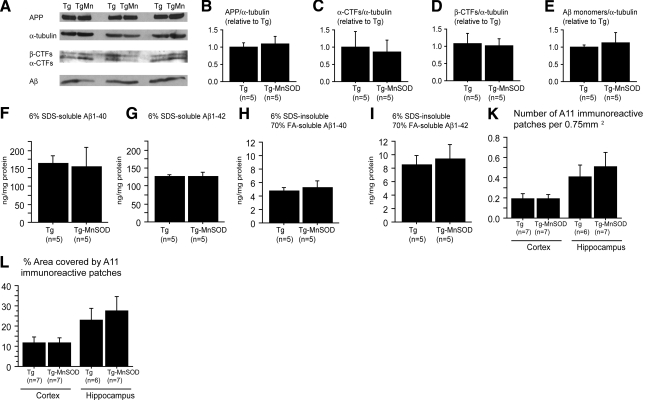
To determine potential mechanisms for the beneficial effects of MnSOD overexpression in Tg19959 mice, we first examined whether MnSOD altered APP expression. Tg19959-MnSOD mice had the same levels of full-length APP protein as Tg19959 littermates (Fig. 4A, B). Next, we examined whether MnSOD altered the amyloidogenic processing of APP. Levels of APP α-C-terminal fragments (CTFs) and β-CTFs were unchanged by MnSOD overexpression (Fig. 4A, C, D). Thus, the beneficial effects of MnSOD overexpression are not likely to be due to changes in APP expression or α- or β-secretase activities.
Figure 4.
MnSOD overexpression did not change APP processing or Aβ levels. A–E) Western blots (A) and analysis of full-length APP (B), α-CTFs (C), β-CTFs (D), and 6% SDS-soluble Aβ monomers (E). Densitometric values are normalized to α-tubulin, in Tg19959 mice and Tg19959-MnSOD littermates. In Tg19959 mice, overexpression of MnSOD did not change APP processing or 6% SDS-soluble Aβ monomer levels. F–I) Quantification of Aβ1-40 and Aβ1-42 levels by ELISA in 6% SDS-soluble and 6% SDS-insoluble 70% FA-soluble pools. In Tg19959 mice, overexpression of MnSOD did not affect 6% SDS-soluble or 6% SDS-insoluble 70% FA-soluble Aβ levels. J, K) A11-immunoreactive patches per 0.75 mm2 (J) and percentage area occupied by A11-immunoreactivity (K). No significant differences were found between Tg19959 mice and Tg19959-MnSOD littermates. APP, amyloid precursor protein; CTF, carboxyterminal fragment of APP; SDS, sodium dodecyl sulfate; FA, formic acid. Values are means ± se.
We then measured levels of 6% SDS-soluble Aβ monomers by Western blot using 6E10 antibody, and there was no change with MnSOD overexpression (Fig. 4A, E). We also used ELISA to measure brain levels of 6% SDS-soluble and 6% SDS-insoluble 70% formic acid-soluble Aβ1-40 and Aβ1-42. MnSOD overexpression did not alter levels of 6% SDS-soluble or 6% SDS-insoluble 70%-formic acid-soluble Aβ1-40 or Aβ1-42 (Fig. 4F–I).
In addition to Aβ monomers, we also assessed the effect of MnSOD overexpression on A11-positive Aβ oligomers using immunohistochemistry. No significant difference in A11-positive Aβ oligomers was found between Tg19959-MnSOD mice and Tg19959 littermates (Fig. 4J, K).
DISCUSSION
There is a large body of evidence demonstrating the involvement of mitochondrial dysfunction and oxidative stress in AD pathogenesis. Markers of oxidative stress colocalize with amyloid-β deposition (17, 18). In transgenic AD mice, partial deficiency of the mitochondrial antioxidant enzyme MnSOD exacerbated the AD phenotype, increasing amyloid deposition (6) and tau phosphorylation (7), and accelerating the onset of behavioral impairments (8). We now report that overexpression of MnSOD in a transgenic AD mouse model attenuated the AD phenotype.
Important clinical and pathological features of AD are memory loss and amyloid deposition. In 4-mo-old Tg19959 mice, MnSOD overexpression significantly improved spatial memory retention in the Morris water maze probe trial, although it did not change performance during the acquisition phase. Dissociation between learning and memory has been previously observed in transgenic AD mice (19,20,21). Given that the best pathological correlate of cognitive impairment in AD is loss of synapses (16), the improvement in spatial memory retention with MnSOD overexpression is consistent with recovery of synaptophysin levels in Tg19959 mice. MnSOD overexpression also reduced plaque burden and microgliosis in 4-mo-old Tg19959 mouse brains.
To determine potential mechanisms for the beneficial effects of MnSOD overexpression, we first examined whether it affected expression of the APP transgene. MnSOD overexpression did not change levels of full-length APP protein. We next examined whether MnSOD overexpression affected APP processing. Oxidative stress has been reported to increase β-site APP cleaving enzyme expression and activity in NT2 cells through stress-activated protein kinase signaling (22, 23). However, we did not observe any change in β-CTF or α-CTF levels with MnSOD overexpression. Since Aβ is produced by the cleavage of APP, we next examined levels of different Aβ pools. MnSOD overexpression did not modulate levels of 6% SDS-soluble or 70% formic acid-soluble Aβ1-40 or Aβ1-42. There was also no difference in A11-positive oligomers with MnSOD overexpression. It should be noted that the detection of Aβ oligomers is complex and highly dependent on technical factors, including the antibody used. The A11 antibody is commonly used to assay Aβ oligomers, although it also recognizes oligomers of other proteins (15, 24). We observed that MnSOD overexpression did not affect the monomeric and oligomeric pools of Aβ. Taken together, these data indicate that the protective effects of MnSOD were exerted downstream of Aβ monomers or oligomers, likely by increasing resistance to Aβ toxicity, retarding higher molecular weight aggregation or fibrillarization of Aβ, or increasing clearance of more aggregated species.
Aβ toxicity is mediated at least in part by oxidative stress. Aβ directly generates reactive oxygen species in the presence of iron or copper ions (25), and methionine-35 is critical for these reactions (26). Free radicals are generated early in the Aβ aggregation process, when oligomers and protofibrils are formed (27). Aβ oligomers can also induce neuronal oxidative stress through N-methyl-d-aspartate receptors and calcium influx (28). Antioxidants such as vitamin E attenuate Aβ-induced toxicity in cultured cells (29, 30) and in vivo (31). Overexpression of MnSOD markedly reduces lipid peroxidation, protein nitration, and apoptosis induced by Aβ in PC6 cells (32). Thus, overexpression of MnSOD increases resistance of cultured cells to Aβ-induced toxicity by decreasing oxidative stress. We have now shown that MnSOD overexpression in a transgenic AD mouse model improves cognitive performance and reverses loss of synaptophysin without affecting APP or Aβ monomer or oligomer levels. Therefore MnSOD overexpression also increases resistance to APP or Aβ-induced toxicity in vivo. Tg19959 mice overexpressing MnSOD showed increased levels of both MnSOD and catalase, which sequentially convert superoxide to hydrogen peroxide and hydrogen peroxide to water and oxygen, thereby reducing oxidative stress. In fact, we observed decreased brain protein oxidation. It is likely that the increase in catalase also played an important role, because increasing MnSOD, without a compensatory mechanism to remove the hydrogen peroxide generated, could potentially increase oxidative stress. In PC6 cells made resistant to Aβ toxicity by overexpression of MnSOD, there was also an increase in the hydrogen peroxide scavenging enzyme glutathione peroxidase (32).
Oxidative stress may promote Aβ aggregation or fibrillarization. Aβ accelerates the copper-mediated generation of the lipid oxidation product 4-hydroxy-2-nonenal (4-HNE) from polyunsaturated lipids (33). In turn, 4-HNE modifies 3 histidine residues of Aβ by Michael addition, a reaction that causes protein misfolding and accelerates fibril formation at low protein concentrations (34). Oxidized cholesterol products can also modify Aβ by Schiff base formation and accelerate Aβ aggregation (35). Thus, by reducing oxidative stress, overexpression of MnSOD could retard high-molecular-weight aggregation or fibrillarization of Aβ. In fact, in Tg19959 mice overexpressing MnSOD, plaque deposits were reduced, whereas Aβ monomers and oligomers were not. We cannot rule out the possibility that overexpression of MnSOD might also enhance plaque clearance. Microglia are known to phagocytose plaques (36, 37). We found an overall decrease in microgliosis with MnSOD overexpression in Tg19959 mice, but this may not reflect the microglial phagocytic activity.
Because MnSOD detoxifies superoxide specifically within mitochondria, this work highlights the importance of mitochondrial antioxidant function in protection against AD pathogenesis. AD has been characterized as a synaptic failure (16, 38). In our study we demonstrated that augmenting mitochondrial MnSOD was able to restore synaptophysin levels. Synapses are important sites not only for cognitive function but also for extracellular (39, 40) and intracellular (41) Aβ accumulation. Synaptic activity is associated with high metabolic demand that could lead to the production of oxidative stress. In fact, synaptic activity induces up-regulation of antioxidant defenses (42). It should be noted that different antioxidants may act differently. For example, α-lipoic acid reduced oxidative stress but did not affect cognition or end point amyloid pathology (43), whereas blueberry supplementation improved cognition without affecting amyloid burden (44). To our knowledge, this work is the first to demonstrate in vivo that genetically augmenting the mitochondrial antioxidant defense system protects synapses and attenuates the AD-like phenotype in a transgenic mouse model.
Supplementary Material
Acknowledgments
We thank Charles Epstein (University of California, San Francisco, CA, USA) for providing MnSOD-overexpressing mice, and Anatoly Starkov, Davide Tampelini, Gunnar Gouras, and Yoon Kim for providing important scientific inputs to the manuscript. This research project was supported by U.S. National Institutes of Health grant AG20729.
References
- Lin M T, Beal M F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Moreira P I, Nunomura A, Nakamura M, Takeda A, Shenk J C, Aliev G, Smith M A, Perry G. Nucleic acid oxidation in Alzheimer disease. Free Radic Biol Med. 2008;44:1493–1505. doi: 10.1016/j.freeradbiomed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Moreira P I, Takeda A, Smith M A, Perry G. Oxidative RNA damage and neurodegeneration. Curr Med Chem. 2007;14:2968–2975. doi: 10.2174/092986707782794078. [DOI] [PubMed] [Google Scholar]
- Keller J N, Schmitt F A, Scheff S W, Ding Q, Chen Q, Butterfield D A, Markesbery W R. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Ringman J M, Younkin S G, Pratico D, Seltzer W, Cole G M, Geschwind D H, Rodriguez-Agudelo Y, Schaffer B, Fein J, Sokolow S, Rosario E R, Gylys K H, Varpetian A, Medina L D, Cummings J L. Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology. 2008;71:85–92. doi: 10.1212/01.wnl.0000303973.71803.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Calingasan N Y, Yu F, Mauck W M, Toidze M, Almeida C G, Takahashi R H, Carlson G A, Flint Beal M, Lin M T, Gouras G K. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J Neurochem. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- Melov S, Adlard P A, Morten K, Johnson F, Golden T R, Hinerfeld D, Schilling B, Mavros C, Masters C L, Volitakis I, Li Q X, Laughton K, Hubbard A, Cherny R A, Gibson B, Bush A I. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito L, Raber J, Kekonius L, Yan F, Yu G Q, Bien-Ly N, Puolivali J, Scearce-Levie K, Masliah E, Mucke L. Reduction in mitochondrial superoxide dismutase modulates Alzheimer’s disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishti M A, Yang D S, Janus C, Phinney A L, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser P E, Carlson G A, George-Hyslop P S, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- Dudal S, Krzywkowski P, Paquette J, Morissette C, Lacombe D, Tremblay P, Gervais F. Inflammation occurs early during the Abeta deposition process in TgCRND8 mice. Neurobiol Aging. 2004;25:861–871. doi: 10.1016/j.neurobiolaging.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Hyde L A, Kazdoba T M, Grilli M, Lozza G, Brusa R, Zhang Q, Wong G T, McCool M F, Zhang L, Parker E M, Higgins G A. Age-progressing cognitive impairments and neuropathology in transgenic CRND8 mice. Behav Brain Res. 2005;160:344–355. doi: 10.1016/j.bbr.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Lovasic L, Bauschke H, Janus C. Working memory impairment in a transgenic amyloid precursor protein TgCRND8 mouse model of Alzheimer’s disease. Genes Brain Behav. 2005;4:197–208. doi: 10.1111/j.1601-183X.2004.00104.x. [DOI] [PubMed] [Google Scholar]
- Bellucci A, Luccarini I, Scali C, Prosperi C, Giovannini M G, Pepeu G, Casamenti F. Cholinergic dysfunction, neuronal damage and axonal loss in TgCRND8 mice. Neurobiol Dis. 2006;23:260–272. doi: 10.1016/j.nbd.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Raineri I, Carlson E J, Gacayan R, Carra S, Oberley T D, Huang T T, Epstein C J. Strain-dependent high-level expression of a transgene for manganese superoxide dismutase is associated with growth retardation and decreased fertility. Free Radic Biol Med. 2001;31:1018–1030. doi: 10.1016/s0891-5849(01)00686-4. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson J L, McIntire T M, Milton S C, Cotman C W, Glabe C G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Terry R D, Masliah E, Salmon D P, Butters N, DeTeresa R, Hill R, Hansen L A, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Pappolla M A, Chyan Y J, Omar R A, Hsiao K, Perry G, Smith M A, Bozner P. Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer’s disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. Am J Pathol. 1998;152:871–877. [PMC free article] [PubMed] [Google Scholar]
- Smith M A, Hirai K, Hsiao K, Pappolla M A, Harris P L, Siedlak S L, Tabaton M, Perry G. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem. 1998;70:2212–2215. doi: 10.1046/j.1471-4159.1998.70052212.x. [DOI] [PubMed] [Google Scholar]
- Hartman R E, Shah A, Fagan A M, Schwetye K E, Parsadanian M, Schulman R N, Finn M B, Holtzman D M. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2006;24:506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Daumas S, Sandin J, Chen K S, Kobayashi D, Tulloch J, Martin S J, Games D, Morris R G. Faster forgetting contributes to impaired spatial memory in the PDAPP mouse: deficit in memory retrieval associated with increased sensitivity to interference? Learn Mem. 2008;15:625–632. doi: 10.1101/lm.990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M, Wille E, Calingasan N Y, Tampellini D, Williams C, Gouras G K, Liby K, Sporn M, Flint Beal M, Lin M T. Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer’s disease. J Neurochem. 2009;109:502–512. doi: 10.1111/j.1471-4159.2009.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A, Danni O, Smith M A, Perry G, Tabaton M. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem. 2005;92:628–636. doi: 10.1111/j.1471-4159.2004.02895.x. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato M A, Danni O, Smith M A, Perry G, Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- Yoshiike Y, Minai R, Matsuo Y, Chen Y R, Kimura T, Takashima A. Amyloid oligomer conformation in a group of natively folded proteins. PLoS ONE. 2008;3:e3235. doi: 10.1371/journal.pone.0003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Atwood C S, Hartshorn M A, Multhaup G, Goldstein L E, Scarpa R C, Cuajungco M P, Gray D N, Lim J, Moir R D, Tanzi R E, Bush A I. The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- Varadarajan S, Yatin S, Kanski J, Jahanshahi F, Butterfield D A. Methionine residue 35 is important in amyloid beta-peptide-associated free radical oxidative stress. Brain Res Bull. 1999;50:133–141. doi: 10.1016/s0361-9230(99)00093-3. [DOI] [PubMed] [Google Scholar]
- Tabner B J, El-Agnaf O M, Turnbull S, German M J, Paleologou K E, Hayashi Y, Cooper L J, Fullwood N J, Allsop D. Hydrogen peroxide is generated during the very early stages of aggregation of the amyloid peptides implicated in Alzheimer disease and familial British dementia. J Biol Chem. 2005;280:35789–35792. doi: 10.1074/jbc.C500238200. [DOI] [PubMed] [Google Scholar]
- De Felice F G, Velasco P T, Lambert M P, Viola K, Fernandez S J, Ferreira S T, Klein W L. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis J, Cole G M, Schubert D. Vitamin E protects nerve cells from amyloid beta protein toxicity. Biochem Biophys Res Commun. 1992;186:944–950. doi: 10.1016/0006-291x(92)90837-b. [DOI] [PubMed] [Google Scholar]
- Yatin S M, Aksenov M, Butterfield D A. The antioxidant vitamin E modulates amyloid beta-peptide-induced creatine kinase activity inhibition and increased protein oxidation: implications for the free radical hypothesis of Alzheimer’s disease. Neurochem Res. 1999;24:427–435. doi: 10.1023/a:1020997903147. [DOI] [PubMed] [Google Scholar]
- Sung S, Yao Y, Uryu K, Yang H, Lee V M, Trojanowski J Q, Pratico D. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004;18:323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- Keller J N, Kindy M S, Holtsberg F W, St Clair D K, Yen H C, Germeyer A, Steiner S M, Bruce-Keller A J, Hutchins J B, Mattson M P. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray I V, Liu L, Komatsu H, Uryu K, Xiao G, Lawson J A, Axelsen P H. Membrane-mediated amyloidogenesis and the promotion of oxidative lipid damage by amyloid beta proteins. J Biol Chem. 2007;282:9335–9345. doi: 10.1074/jbc.M608589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Komatsu H, Murray I V, Axelsen P H. Promotion of amyloid beta protein misfolding and fibrillogenesis by a lipid oxidation product. J Mol Biol. 2008;377:1236–1250. doi: 10.1016/j.jmb.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Bieschke J, Zhang Q, Powers E T, Lerner R A, Kelly J W. Oxidative metabolites accelerate Alzheimer’s amyloidogenesis by a two-step mechanism, eliminating the requirement for nucleation. Biochemistry. 2005;44:4977–4983. doi: 10.1021/bi0501030. [DOI] [PubMed] [Google Scholar]
- Jucker M, Heppner F L. Cerebral and peripheral amyloid phagocytes: an old liaison with a new twist. Neuron. 2008;59:8–10. doi: 10.1016/j.neuron.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Laouar Y, Pittenger C, Mori T, Szekely C A, Tan J, Duman R S, Flavell R A. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D J. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Cirrito J R, Yamada K A, Finn M B, Sloviter R S, Bales K R, May P C, Schoepp D D, Paul S M, Mennerick S, Holtzman D M. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Takahashi R H, Almeida C G, Kearney P F, Yu F, Lin M T, Milner T A, Gouras G K. Oligomerization of Alzheimer’s beta-amyloid within processes and synapses of cultured neurons and brain. J Neurosci. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Soriano F X, Leveille F, Martel M A, Dakin K A, Hansen H H, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner B A, Wyllie D J, Ikonomidou C, Hardingham G E. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedlak S L, Casadesus G, Webber K M, Pappolla M A, Atwood C S, Smith M A, Perry G. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer’s disease. Free Radic Res. 2009;43:156–164. doi: 10.1080/10715760802644694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J A, Denisova N A, Arendash G, Gordon M, Diamond D, Shukitt-Hale B, Morgan D. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr Neurosci. 2003;6:153–162. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.