Abstract
In the majority of mammalian species, males are dominant over and more aggressive than females. In contrast, some reports suggest that female golden hamsters are more aggressive than males but systematic comparisons using the same methods for both sexes are rare. We observed same-sexed pairs of hamsters over repeated trials to assess whether sex differences existed in the level of agonistic behavior and in the development and maintenance of dominant-subordinate relationships with familiar partners. There were no sex differences in measures of agonistic behavior or fear responses (fleeing) during the initial series of three trials on the first day of testing. Following a four-day interval, males that had lost in session 1 showed fearful responses to a familiar dominant male and were not likely to engage in a fight with him. In contrast, females that lost the initial fights were not fearful and fought vigorously with the familiar winner in subsequent encounters. Although the amount of agonistic behavior engaged in by females did decrease over the course of the three sessions, females that lost did not demonstrate an increase in fear, as measured by the latency to flee. Males that lost fights did show increased fear during later trials and sessions. These results suggest that female hamsters are less affected by losing fights than males are and thus that females are less likely than males to develop highly polarized dominant-subordinate relationships. Further work is needed to understand the mechanisms underlying these sex differences.
Keywords: Hamster, Aggression, Sex differences, Behavior, Fear, Anxiety, Dominance
Introduction
The agonistic behavior of hamsters has been well described, starting with a study by Melton (Melton, 1950). Both male and female hamsters have been shown to reliably engage in intense agonistic behavior during same-sex encounters (Payne and Swanson, 1970; Takahashi and Lisk, 1983). Fighting in both sexes is affected by numerous factors, including (a) the hormonal status of the subject (Meisel, Sterner, and Diekman, 1988; Payne and Swanson, 1971a; Payne and Swanson, 1971b; Payne and Swanson, 1971c; Takahashi, 1990; Vandenbergh, 1971), (b) the hormonal status of the opponent (Kislak and Beach, 1955; Marques and Valenstein, 1977; Payne, 1974), (c) changes in photoperiod (Garrett and Campbell, 1980; Jasnow, Huhman, Bartness, and Demas, 2002; Landau, 1975), (d) prior housing conditions (Payne, 1973; Wise, 1974), and (e) the size and complexity of the testing environment (Johnston, 1975a; Johnston, 1975b; Payne, 1973; Payne and Swanson, 1970). A single intra-sexual agonistic bout progresses through a well defined and restricted set of stereotyped behaviors, including investigation, offensive and defensive posturing, and actual fighting, during which the two individuals are oriented at right angles to one another and attempting to bite one another, a “rolling fight” (Floody and Pfaff, 1977). Descriptions of the behaviors and sequences of behaviors used in fights have been thoroughly documented (Grant and MacKintosh, 1963; Johnston, 1976; Lerwill and Makings, 1971; Floody & Pfaff, 1977). A single fight typically results in a clearly identifiable winner and loser (Payne and Swanson, 1970) with the loser attempting to flee from the winner and the test area. Once a male subject defeats an opponent, the relationship established in the initial fight is usually maintained in future encounters between that pair of males (Floody and Pfaff, 1977; Johnston, 1975a; Johnston, 1975b; Payne and Swanson, 1970).
Nonetheless, it is still not clear whether sex differences exist in the intensity or duration of fights or in the effects of winning or losing on the subsequent behavior of participants. This is because few studies have used the same methods to directly compare the behavior of males and females. Payne & Swanson (1970) showed that in same-sex encounters males and females did not differ in levels of agonistic behavior but there was a high degree of variability in female agonistic behavior over the estrous cycle (see also Lisk and Nachtigall, 1988; Takahashi and Lisk, 1983; Takahashi and Lisk, 1984). Since the studies by Payne & Swanson (1970), other researchers have described females as being more aggressive than males. These claims were based on results showing that females were dominant over males and on observations indicating that females were more likely to cannibalize nests of other females than males (Brain, 1972; Floody and Pfaff, 1977; Goldman and Swanson, 1975a; Goldman and Swanson, 1975b; Marques and Valenstein, 1977; Payne and Swanson, 1970; Tiefer and Johnson, 1975). In addition, in some studies subjects were housed in groups, a procedure that increases aggression in females and increases indicators of social stress in both sexes (e.g., changes in adrenal weight and total body weight; (Gattermann, Fritzsche, Weinandy, and Neumann, 2002). Thus, due to the variability of the methods used, the lack of careful comparisons using the same methods, and in some cases the lack of controlling for the reproductive state of females, sex similarities and difference in agonistic behavior are not clearly understood.
Males and females do appear to differ in the development of and long-term physiological and behavioral reactions to fighting, particularly in the responses of losers and their subsequent reactions to stimulus animals. Potegal and colleagues (Potegal, Huhman, Moore, and Meyerhoff, 1993) showed that after a single, prolonged bout of agonistic behavior, nearly 90% of defeated males displayed submissive postures when presented with a novel, non-aggressive stimulus male, an effect called conditioned defeat. Recently, it was shown that females do not show the same level of conditioned defeat (Huhman, Solomon, Janicki, Harmon, Lin, Israel, and Jasnow, 2003). This sex difference in behavioral response was present despite similar increases in adrenocorticotropic hormone (ACTH) in both males and females. In this study there were four relatively long aggressive interactions (5min) and a single test trial with a novel stimulus animal. The behavior during fights, however, was not reported and thus it is not known whether the differences observed between males and females in response to a non-aggressive intruder were due to differences in levels of aggression during the initial fights. Work by Taravosh-Lahn & Delville has recently shown that sex differences do exist in the development of adult-like aggression and the response of juvenile animals to social subjugation. Females have been shown to develop adult-like attack styles earlier in life than males and they are less affected by early social defeat than males are. These data suggest that sex differences in aggression and responses to agonistic encounters are present early in life (Taravosh-Lahn and Delville, 2004). Previous studies with adult hamsters, however, showed no sex differences in measures of aggressive behavior in like-sex encounters (Floody & Pfaff, 1977). The females used in the studies by Floody & Pfaff were tested across all four days of the estrous cycle, and were subjected to different housing conditions compared to males. Thus, no firm conclusions can be reached about sex differences in agonistic behavior or the effects of fighting on later responses to familiar or novel individuals.
In this paper we took a systematic approach to study like-sexed agonistic encounters in golden hamsters. We compared interactions in pairs of males and pairs of females over 3 encounters during a single day and during additional interactions 4 and 8-days later. We recorded behaviors that occurred during these like-sex encounters and assessed the (a) level of agonistic behavior in male-male versus female-female encounters, (b) fear responses to a known winner and (c) the level of fear responses over days. Unlike all previous studies, the end of each fight was determined by the behavior of the animals (one animal fleeing), making these agonistic encounters more similar to those observed in the wild (Johnston, unpublished observations).
Materials & Methods
Subjects
Subjects were 26 adult male and 30 adult female golden hamsters (Mesocricetus auratus) weighing between 140–190 grams that were born and raised in the lab. This colony is derived from and periodically bred with new stock from Charles River, Inc. Female subjects were intact and had normal estrous cycles. Sexual receptivity (estrus) was assessed by testing for lordosis in response to males and sometimes by assessing the consistency of the vaginal secretion (Orsini, 1961). Normal cycling was insured by repeated testing of females for at least 1 month prior to use of the animals as subjects. We used females that were one day pre-estrous because in pilot studies females during this part of the estrous cycle demonstrated the highest levels of agonistic behavior toward each other (Floody and Pfaff, 1977; Kislak and Beach, 1955; Takahashi and Lisk, 1983; Takahashi and Lisk, 1984). Thus, if females are more aggressive than males they should definitely demonstrate it in these interactions. All hamsters were born in the colony, weaned at 28–30 days, and housed individually after weaning. They were maintained on a reversed 14:10 light-dark cycle; food and water were available in their cages at all times. As part of standard laboratory procedures, all animals received minimal social experience with other males and females during their first 3 months of life; this consisted of placing male and female juveniles into a testing arena divided into 4 compartments by a wire-mesh barrier on two separate days for 5 minutes on each occasion. This allowed social investigation to occur but did not permit fighting or mating. Some of the hamsters used in this study had been previously used as scent donors or as stimulus animals in open field tests one month or more before this experiment.
Ethical note
Animal care was in accordance with Cornell University IACUC and FDA standards. Veterinarians were continually available in the case of injury. Our protocol required that, if an animal were injured during testing, that animal would be removed from the study and receive immediate medical attention. No animals were injured or removed from the study.
Testing Procedure
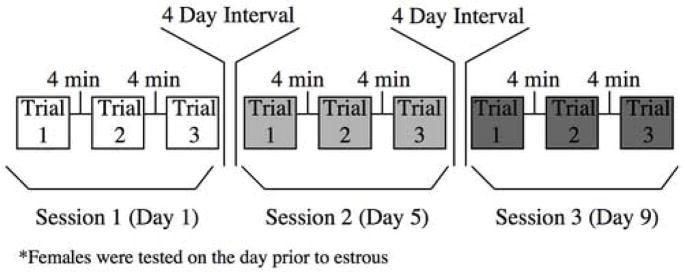
Male and female hamsters were tested during the first two hours of the dark portion of their light:dark cycle; illumination was provided by a 25 Watt bulb 10 feet distant and pointed away from the arena. There were three sessions over a period of nine days, with four-day intervals between sessions (tests occurred on Day1, Day 5, & Day 9). This schedule allowed us to test females three times on the same day of their cycle. Each session consisted of three trials separated by four-minute intervals (Fig. 1). Two male or two female hamsters were placed simultaneously into a neutral testing arena and were allowed to interact. A trial was over when one of the animals jumped from the arena, made three successive attempts to climb or jump out of the arena lasting a minimum of five seconds, or the maximum trial length of four minutes was reached. Following each trial, subjects were returned to their home cage until the next trial.
Figure 1.
Schematic representation of the experimental design. Subjects were tested in three different sessions 4 days apart so that females would always be tested on the same day of their estrous sycle. In each session, subjects had three aggressive encounters with a four-minute inter-trial interval
Apparatus
Interactions were carried out in a 35 × 35 × 18 cm arena made of wood on three sides and glass on the fourth side. The wooden sides of the arena were painted to allow for easier cleaning between trials. The arena was cleaned with a 50% ethanol solution and wiped dry with paper towels before each trial. Male-male and female-female pairs were of similar age and weight. In nearly all pairings, subjects were matched to within 5 grams of one another but allowable differences were up to differences of 2 weeks of age and 20 grams of weight. The members of a pair remained the same across all nine encounters. Subjects were transferred from their home cage to the testing arena with the aid of large plastic beakers; they were simultaneously released into the arena. One of the two subjects had a small (2cm × 2cm) square of white tape placed on its back to allow the experimenter to differentiate between the two subjects.
Behavioral Measures
Criteria for scoring behaviors were adapted from previous work describing agonistic behavior in the golden hamster (Floody and Pfaff, 1977; Grant and MacKintosh, 1963; Johnston, 1976; Lerwill and Makings, 1971).
Several specific behaviors were recorded during each trial to assess the level and intensity of fighting behavior. Latency to fight was defined as the time from introduction into the arena until the onset of pinning, biting, or rolling fights. Latency to flee was defined as the amount of time from the start of the trial until one of the animals either jumped out of the arena or made 3 attempts of 5 or more seconds each to climb out of the arena. If no attempt was made to flee, a flee attempt was unsuccessful (the subject did not escape from the arena), or an attempt was less than 5 seconds we recorded a flee latency as 240 seconds, the duration of the trial. The number of fights per trial was the total number of times that subjects engaged in bouts of pinning, biting, or rolling fights (see Grant & MacKintosh, 1967); bouts of fighting that were separated by 10 seconds or more, in the absence of fleeing behavior, were counted as separate events. The total duration of agonistic behavior per trial was defined as the total amount of time spent engaged in pinning, boxing, biting and rolling fights.
In addition, as a simple categorical measure of fighting in each trial, we gave a score of one for a trial during which agonistic behavior (including pinning, biting, or rolling fighting) of any intensity was observed and a score of zero for a trial in which no agonistic behavior occurred. These scores were then averaged across subjects to get a mean probability of agonistic behavior for each trial. The probability of a fleeing was calculated in the same manner. A trial in which an animal fled from the arena was scored as one and a trial with no fleeing was scored as a zero.
Finally, we used a 6-point qualitative ranking measure to assess the intensity of fighting in each trial. Trials during which no agonistic behavior occurred were scored as a zero and a trial with at least one fight lasting 5–10 seconds with repeated biting, rolling fighting, and vocalizations were scored as a five. Encounters that fell between these two endpoints received scores ranging between 1 and 4. The criteria for the ratings were as follows. A fight of any intensity was scored as 2 points; biting, vocalization, or mild injury added a single point for each event, up to the 5 point maximum. Pauses during a fight led to a 1-point subtraction for each occurrence. A single observer rated the intensity of agonistic behavior across all fights to insure reliability of scoring; this observer had observed more than twenty trials of varying levels of agonistic behavior prior to assigning intensity scores. For randomly selected trials, scores of two observers were compared and yielded a 95% inter-rater reliability score.
Statistical analysis
Quantitative data were analyzed using a repeated measures general linear model (GLM) ANOVA. For each measure, an omnibus ANOVA was computed to assess the presence of any general effects of trial (3 levels- trial 1, trial 2, and trial 3), session (3 levels- Session 1, Session 2, Session 3), sex (2 levels- male or female), and interactions between these variables. Following initial statistical tests, separate repeated measures general linear model ANOVA’s were applied to data collected on individual sessions. Significant within subject effects were then subjected to paired t-tests with Bonferroni corrections for multiple tests. After consulting a statistician, binomial data for the probability of engaging in a fight or a flee response was treated as a continuous variable and tested using repeated measures ANOVA’s.
Results
For both male pairs and female pairs, the subject that was dominant on the initial encounter continued to be the dominant animal on all subsequent encounters.
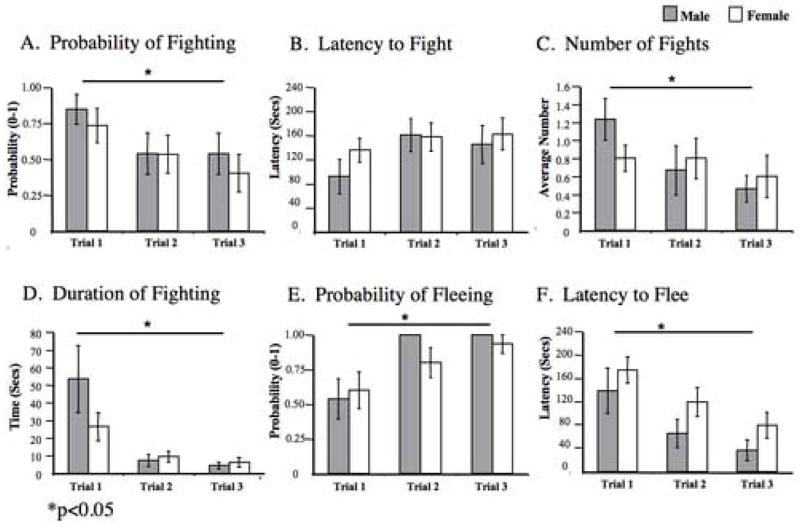
During session 1, the probability of engaging in a fight decreased significantly across trials for both sexes (F2,25= 3.602, p< 0.042, Fig. 2A). There was no trial × sex interaction or overall effect of sex for the probability of engaging in a fight. The latency to engage in a fight did not change across the three trials for either males or females (F2,25=1.679, p< 0.207, Fig. 2B), nor was there a main effect of sex or a trial × sex interaction for the latency to fight. The average number of fights engaged in by males (0.79 +/− 0.13) and females (0.79+/− 0.18) did not differ, but did decrease significantly across trials for both sexes (F2,25= 3.389, p<0.050, Fig. 2C). The total time engaged in agonistic behavior for males and females decreased significantly across trials (F2,25= 6.595, p<0.005, Fig. 2D), but there was no overall effect of sex or trial × sex interaction. The intensity of fights between males and between females decreased significantly across trials (F2,25= 5.357, p< 0.012, Table 1) and there were no sex differences in intensity.
Figure 2.
Session 1: Histograms depicting the mean (+/− SEM) scores of males (n=13 pairs) (grey) and females (n=15 pairs) (white) for (A) probability of fighting, (B) the latency to fight, (C) number of fights (D) time spent fighting, (E) the probability of fleeing, and (F) latency to flee.
Table 1.
Mean (SE) of experimenter rated fight intensity for each of three trials during three separate sessions of testing.
| Fight Intensity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | |||||||
| Trial 1 | Trial 2 | Trial 3 | Trial 1 | Trial 2 | Trial 3 | Trial 1 | Trial 2 | Trial 3 | |
| Females | 2.07 (0.45) | 1.67 (0.42) | 0.87 (0.33) | 0.73 (0.25) | 2.00 (0.49) | 0.73 (0.30) | 0.71 (0.38) | 1.21 (0.43) | 0.43 (0.20) |
|
| |||||||||
| Males | 2.46 (0.44) | 0.92 (0.34) | 1.15 (0.41) | 1.17 (0.50) | 0.23 (0.17) | 0.08 (0.08) | 1.15 (0.52) | 0.23 (0.23) | 0.23 (0.23) |
In session 1, the probability of fleeing increased on successive trials for both males and females (F2,25=6.428, p< 0.006, Figure 2E) with all subjects having fled increasingly more quickly in the three successive trials (F2,25= 19.963, P< 0.001; Fig. 2F). Fleeing behavior was equally likely in interactions between two males and two females and there were no significant sex differences for the latency to flee.
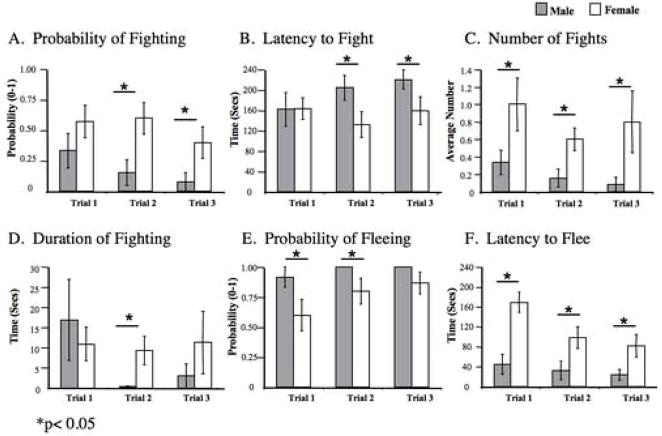
During Session 2 (4 days after Session1) males were less likely to fight (F1,26= 7.977, p< 0.010, Fig. 3A), took longer to engage in a fight (F1,26=4.771, p< 0.039, Fig. 3B), and engaged in significantly fewer fights than females (F1,26 =7.250, p<0.013, Fig 3C), No sex difference was found in the total duration of time spent engaged in agonistic behavior during Session 2 (Fig. 3D). Female fights were rated as being more intense than those of males (1.2 ± 0.3 SEM vs. 0.5 ± 0.2 SEM, respectively) during Session 2 (F1,26=5.783, p<0.024, Table 1). It should also be noted that the ratings of the intensity of fighting did not differ significantly across trials for females, but did for males, yielding a significant trial × sex interaction (F2,25=3.465, p< 0.048, Table 1).
Figure 3.
Session 2: Histograms depicting the mean (+/− SEM) scores of males (n=13 pairs) (grey) and females (n=15 pairs) (white) for (A) probability of fighting, (B) the latency to fight, (C) number of fights (D) time spent fighting, (E) the probability of fleeing, and (F) latency to flee.
In Session 2, males fled significantly more often than females during trials 1 and 2 (Fig. 3E).. Males also fled on nearly 100% of the trials whereas females fled on only about 75% of trials but this difference did not reach statistical significance (F1,26=3.968, p<0.058). During all encounters in Session 2, the latency to flee was much shorter for males than for females (F1,26= 9.682, p< 0.005; Fig. 3F), and decreased across trials for both sexes (F1,26= 9..992, p< 0.001).
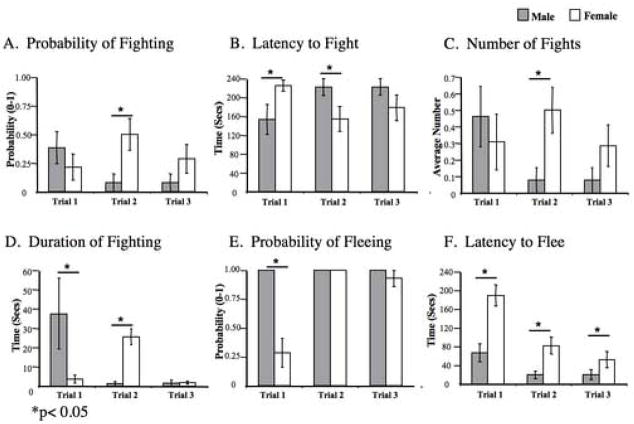
During Session 3 there was no significant sex difference in the latency to fight or probability of fighting (Fig. 4A & B). There was, however, a trial × sex interaction (F2,25=5.214, p< 0.013) for the latency to fight; the latency to fight increased over the three trials for males whereas it decreased for females. Neither the number of fights nor ratings of the intensity of fights changed significantly across the 3 trials for either males or females (Fig. 4C & Table 1). A significant effect of trial was found for the duration of fighting (F2,25= 5.733, p< 0.009; Fig. 4D); for males, the duration of fighting decreased over the three trials, but there was no significant change for females, leading to a significant trial × sex interaction (F2,25= 5.216, p< 0.013).
Figure 4.
Session 3: Histograms depicting the mean (+/− SEM) scores of males (n=13 pairs) (grey) and females (n=15 pairs) (white) for (A) probability of fighting, (B) the latency to fight, (C) number of fights (D) time spent fighting, (E) the probability of fleeing, and (F) latency to flee.
During session 3, males were significantly more likely to flee than females (F1,25=23.877, p< 0.001; Fig 4E). The difference between males and females was greatest on trial 1 of testing. During trials 2 and 3, males fled in all trials; the likelihood of fleeing by females was lowest on trial 1 and successively greater during trials 2 and 3, leading to a trial × sex interaction for this measure (F2,24=14.444, p<0.001). The latency to flee decreased on successive trials for both males and females (F2,25= 18.082, p< 0.001; Fig. 4E), with males fleeing significantly more quickly than females across all three trials (F1,24=17.223, p<0.001). In addition, a significant trial × sex interaction was found for the latency to flee (Fig. 6D; F2,25=4.406, p< 0.023), with the latency to flee for females decreasing more quickly and to a greater degree than the latency to flee for males.
Discussion
In session 1, males and females did not differ on any measure of agonistic behavior. Both sexes engaged in fights with equal probability, intensity, duration, and frequency. No reversals in the dominant-subordinate relationship occurred between any pair of subjects. These data support and extend previous findings by Floody & Pfaff (1977). In our study, the likelihood of expressing agonistic behavior over the course of three trials decreased for both sexes; this was most likely due to an increase in the probability of fleeing and a decrease in the latency to flee over the three trials. These results suggest that defeated subjects developed a fear-avoidance response to their dominant partner over the course of the three trials in Session 1. In previous studies with hamsters, the behavioral fear response in males was maintained for several days post-testing and was expressed specifically in response to the previously encountered winner (Lai and Johnston, 2002; Lai, Ramiro, Yu, and Johnston, 2005). The data from session 1 show that females also developed a fear-avoidance response similar to that shown by males. We are not aware of any studies that indicate how long this avoidance response lasts in females, if it is specific to the familiar winner, or if it is generalized to any stimulus animal.
During session 2, many of the similarities between males and females found in Session 1 disappeared. Males were less likely than females to engage in an agonistic encounter, they had a shorter latency to flee, and they were more likely to flee than their female counterparts. Male agonistic encounters were less intense than female-female encounters, and males were less likely to engage in agonistic behavior. We interpret these data to mean that males that lost maintained a stronger fear-avoidance response than females. These data support recent findings by Huhman et al (2004) showing that adult females fail to exhibit conditioned defeat and results from Taravosh-Lahn & Delville (2004), indicating that females were less affected by repeated defeat than males were. Our results were obtained in very different conditions than the studies of conditioned defeat; the fear/avoidance response in females was lost despite the conditions in which they were tested, namely that subjects were tested with the same stimulus animal and in the same environment where previous agonistic encounters took place.
By Session 3, the differences in agonistic behavior between males and females that were observed on session 2 were attenuated, such that the probability of fighting and the number of fights by females decreased. Despite this apparent change in female agonistic behavior, subordinate females still demonstrated a significantly diminished fear response relative to males, in that they were significantly less likely to flee on the initial trial of session 3 than males were and they took much longer to flee a dominant opponent than males did. These data lead us to the conclusion that females did alter their behavior in response to a dominant opponent, but not nearly to the extent that males did.
From a broad, evolutionary perspective, why should male and female hamsters differ in their reactions to previous opponents after an interval of several days? We suggest that this is due to sex differences in the strategies necessary for reproductive success. Of the small number of aggressive interactions observed in the wild in which we knew the sex and identity of the individuals, all were between males in the context of competition for estrous females (Johnston, Larimer, Song & Johnston, unpublished observations). It makes sense for a subordinate male to yield to a dominant male because they may still get to mate with the female. In captivity, females mate with multiple males (Lisk & Baron, 1982; Huck, et al., 1986) and in the few cases that we observed in the wild, the subordinate male did mate with the female after the dominant male. In contrast, if females fight with another female the likely context would be to defend her burrow, food hoard and/or pups. Loss of a food hoard could be disastrous at some times of year, and loss of pups would obviously influence reproductive success. Thus, the costs of avoidance or fear of the dominant individual could be much more costly for females than males.
What mechanisms might be involved in these sex differences in behavior? One possibility is sex differences in the hypothalamic-pituitary axis (HPA). In rats, males and females show differences in stress reactivity depending on the form of stressor used. Males show a much greater stress response than females following social defeat, while females are much more affected by changes in social partners (Haller, Fuchs, Halasz, and Makara, 1999). In hamsters, males and females generate similar increases in ACTH following defeat in the conditioned defeat paradigm (Huhman et al 2003). Other studies, however, have shown that sex differences do exist in the sensitivity of the HPA. Female hamsters have higher basal glucocorticoid levels than males (Gaskin and Kitay, 1970). Following a mild stress such as introduction into a novel cage, males show a greater relative increase in glucocorticoid levels than females (Weinberg and Wong, 1986). Results by Tarvosh-Lahn and Delville (2004), who used more sensitive radio-immuno assay techniques to measure cortisol, have shown that social defeat early in life results in no sex differences in the reactivity of the HPA axis following exposure to an aggressive resident animal later in life. Based on these findings it is unlikely that the observed differences in fear of a familiar opponent in males and females are due to differences in hormonal stress responses.
Another possible mechanism underlying sex differences in response to defeat could be differences in circulating gonadal hormones. Testosterone, estrogen, and progesterone have all been shown to have potent effects on aggressive behavior in male and/or female hamsters. Alterations in estrogen and progesterone associated with the delivery of pups leads to elevations in maternal aggression (Siegel and Rosenblatt, 1980) and aggression in females varies with the estrous cycle (Takahashi, 1990; Takahashi and Lisk, 1984). Recently, it was shown that alterations in gonadal hormones have a significant effect on the expression of conditioned defeat in the female hamster (Faruzzi, Solomon, Demas, and Huhman, 2005). Specifically, the changes in estrogen and progesterone associated with normal cycling appear to diminish the expression of conditioned defeat. The effect we observed, that females were not afraid of a familiar dominant female 4 days after a series of fights, could also be due to the regular changes in these reproductive hormones during the estrous cycle. Finally, there could also be sex differences in the reactivity of the areas of the brain involved in social behavior, aggression, and the learned fear of a familiar winner (Lai, et al., 2002, 2005; Choi, et al., 2005; Bielsky, et al., 2004, 2005). More work is needed to better understand the mechanisms underlying the observed differences in male and female behavior.
Acknowledgments
The authors would like to greatly thank Keith Quencer for his help in testing of all of the hamsters used in this experiment and Joan Johnston for her work caring for the hamsters used in this study. This research was supported in part by NIMH grants #5R01MH58001-01H1, 2R01MH058001-06A2 and NIMH training grant 2T32MH15793.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu S-B, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Brain PF. Effects of isolation/grouping on endocrine function and fighting behavior in male and female golden hamsters. (Mesocricetus auratus Waterhouse) Behav Biol. 1972;7(3):349–57. doi: 10.1016/s0091-6773(72)80106-8. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong H-W, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delinieates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Faruzzi AN, Solomon MB, Demas GE, Huhman KL. Gonadal hormones modulate the display of submissive behavior in socially defeated female Syrian hamsters. Horm Behav. 2005;47(5):569–75. doi: 10.1016/j.yhbeh.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Floody OR, Pfaff DW. Aggressive behavior in female hamsters: the hormonal basis for fluctuations in female aggressiveness correlated with estrous state. J Comp Physiol Psychol. 1977;91(3):443–64. doi: 10.1037/h0077341. [DOI] [PubMed] [Google Scholar]
- Garrett JW, Campbell CS. Changes in social behavior of the male golden hamster accompanying photoperiodic changes in reproduction. Horm Behav. 1980;14 (4):303–18. doi: 10.1016/0018-506x(80)90020-3. [DOI] [PubMed] [Google Scholar]
- Gaskin JH, Kitay JI. Adrenocortical function in the hamster. Sex differences and effects of gonadal hormones. Endocrinology. 1970;87(4):779–86. doi: 10.1210/endo-87-4-779. [DOI] [PubMed] [Google Scholar]
- Gattermann R, Fritzsche P, Weinandy R, Neumann K. Comparative studies of body mass, body measurements and organ weights of wild-derived and laboratory golden hamsters (Mesocricetus auratus) Lab Anim. 2002;36(4):445–54. doi: 10.1258/002367702320389125. [DOI] [PubMed] [Google Scholar]
- Goldman L, Swanson H. Population control in confined colonies of golden hamsters (Mesocricetus auratus Waterhouse) Z Tierpsychol. 1975a;37(3):225–36. doi: 10.1111/j.1439-0310.1975.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Goldman L, Swanson HH. Developmental changes in pre-adult behavior in confined colonies of golden hamsters. Dev Psychobiol. 1975b;8(2):137–50. doi: 10.1002/dev.420080206. [DOI] [PubMed] [Google Scholar]
- Grant EC, MacKintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–59. [Google Scholar]
- Haller J, Fuchs E, Halasz J, Makara GB. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res Bull. 1999;50(1):33–9. doi: 10.1016/s0361-9230(99)00087-8. [DOI] [PubMed] [Google Scholar]
- Huck UW, Gore AUW, Lisk RD, Parente EJ, Principato DE. Determinants of mating success in the golden hamster (Mesocricetus auratus): III. Female acceptance of multiple mating partners. J Comp Psychol. 1986;100:128–136. [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44(3):293–9. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short days and exogenous melatonin increase aggression of male Syrian hamsters (Mesocricetus auratus) Horm Behav. 2002;42(1):13–20. doi: 10.1006/hbeh.2002.1797. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Scent marking by male golden hamsters (Mesocricetus auratus) I. Effects of odors and social encounters. Z Tierpsychol. 1975a;37(1):75–98. doi: 10.1111/j.1439-0310.1975.tb01128.x. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Scent marking by male Golden Hamsters (Mesocricetus auratus) III. Behavior in a seminatural environment. Z Tierpsychol. 1975b;37(2):213–21. [PubMed] [Google Scholar]
- Johnston RE. The role of dark chest patches and upright postures in the agonistic behavior of male hamsters, mesocricetus auratus. Behavioral Biology. 1976;17:161–76. [Google Scholar]
- Kislak JW, Beach FA. Inhibition of aggressiveness by ovarian hormones. Endocrinology. 1955;56(6):684–92. doi: 10.1210/endo-56-6-684. [DOI] [PubMed] [Google Scholar]
- Lai WS, Johnston RE. Individual recognition after fighting by golden hamsters: a new method. Physiol Behav. 2002;76(2):225–39. doi: 10.1016/s0031-9384(02)00721-7. [DOI] [PubMed] [Google Scholar]
- Lai WS, Ramiro LL, Yu HA, Johnston RE. Recognition of familiar individuals in golden hamsters: a new method and functional neuroanatomy. J Neurosci. 2005;25(49):11239–47. doi: 10.1523/JNEUROSCI.2124-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau IT. Light-dark rhythms in aggressive behavior of the male golden hamster. Physiol Behav. 1975;14(6):767–74. doi: 10.1016/0031-9384(75)90068-2. [DOI] [PubMed] [Google Scholar]
- Lerwill CJ, Makings P. The agonistic behavior of the golden hamster. Animal Behaviour. 1971;19:714–21. [Google Scholar]
- Lisk RD, Baron G. Female regulation of mating location and acceptance of new mating partners following mating to sexual satiety: The Coolidge effect demonstrated in the female golden hamster. Behav Neural Biol. 1982;36:416–421. doi: 10.1016/s0163-1047(82)90822-6. [DOI] [PubMed] [Google Scholar]
- Lisk RD, Nachtigall MJ. Estrogen regulation of agonistic and proceptive responses in the golden hamster. Horm Behav. 1988;22(1):35–48. doi: 10.1016/0018-506x(88)90029-3. [DOI] [PubMed] [Google Scholar]
- Marques DM, Valenstein ES. Individual differences in aggressiveness of female hamsters: response to intact and castrated males and to females. Anim Behav. 1977;25(1):131–9. doi: 10.1016/0003-3472(77)90075-6. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sterner MR, Diekman MA. Differential hormonal control of aggression and sexual behavior in female Syrian hamsters. Horm Behav. 1988;22(4):453–66. doi: 10.1016/0018-506x(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Melton ER. A preliminary study of dominance subordination relationships in the golden hamster. Journal of the Colorado and Wyoming Academy of Sciences. 1950;4:77. [Google Scholar]
- Orsini MW. The external vaginal phenomena characterizing the stages of the estrous cycle, pregnancy, pseudopregnancy, lactation, and the anestrous hamster, Mesocricetus auratus Waterhouse. Proc. Anim. Care Panel. 1961;11:193–206. [Google Scholar]
- Payne AP. A comparison of the aggressive behaviour of isolated intact and castrated male golden hamsters towards intruders introduced into the home cage. Physiol Behav. 1973;10(3):629–31. doi: 10.1016/0031-9384(73)90235-7. [DOI] [PubMed] [Google Scholar]
- Payne AP. The aggressive response of the male golden hamster towards males and females of differing hormonal status. Anim Behav. 1974;22(4):829–35. doi: 10.1016/0003-3472(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Payne AP, Swanson HH. Agonistic behaviour between pairs of hamsters of the same and opposite sex in a neutral observation area. Behaviour. 1970;36(4):260–9. [PubMed] [Google Scholar]
- Payne AP, Swanson HH. The effect of castration and ovarian implantation on aggressive behaviour of male hamsters. J Endocrinol. 1971a;51(1):217–8. doi: 10.1677/joe.0.0510217. [DOI] [PubMed] [Google Scholar]
- Payne AP, Swanson HH. Hormonal control of aggressive dominance in the female hamster. Physiol Behav. 1971b;6(4):355–7. doi: 10.1016/0031-9384(71)90167-3. [DOI] [PubMed] [Google Scholar]
- Payne AP, Swanson HH. Hormonal modication of aggressive behavior between female goldne hamsters. J Endocrinol. 1971c;51(2):17–8. [PubMed] [Google Scholar]
- Potegal M, Huhman K, Moore T, Meyerhoff J. Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus) Behav Neural Biol. 1993;60(2):93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- Siegel HI, Rosenblatt JS. Hormonal and behavioral aspects of maternal care in the hamster: a review. Neurosci Biobehav Rev. 1980;4(1):17–26. doi: 10.1016/0149-7634(80)90023-8. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Hormonal regulation of sociosexual behavior in female mammals. Neurosci Biobehav Rev. 1990;14(4):403–13. doi: 10.1016/s0149-7634(05)80062-4. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Lisk RD. Organization and expression of agonistic and socio-sexual behavior in golden hamsters over the estrous cycle and after ovariectomy. Physiol Behav. 1983;31(4):477–82. doi: 10.1016/0031-9384(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Lisk RD. Intrasexual interactions among female golden hamsters (Mesocricetus auratus) over the estrous cycle. J Comp Psychol. 1984;98(3):267–75. [PubMed] [Google Scholar]
- Taravosh-Lahn K, Delville Y. Aggressive behavior in female golden hamsters: development and the effect of repeated social stress. Horm Behav. 2004;46(4):428–35. doi: 10.1016/j.yhbeh.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Tiefer L, Johnson W. Neonatal androstenedione and adult sexual behavior in golden hamsters. J Comp Physiol Psychol. 1975;88(1):239–47. doi: 10.1037/h0076210. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. The effects of gonadal hormones on the aggressive behaviour of adult golden hamsters (Mesocricetus auratus) Anim Behav. 1971;19(3):589–94. doi: 10.1016/s0003-3472(71)80116-1. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Wong R. Adrenocortical responsiveness to novelty in the hamster. Physiol Behav. 1986;37(5):669–72. doi: 10.1016/0031-9384(86)90170-8. [DOI] [PubMed] [Google Scholar]
- Wise DA. Aggression in the female golden hamster: effects of reproductive state and social isolation. Horm Behav. 1974;5(3):235–50. doi: 10.1016/0018-506x(74)90032-4. [DOI] [PubMed] [Google Scholar]