Abstract
Receptor for advanced glycation end-products (RAGE) is known to be involved in both micro- and macro-vascular complications in diabetes. Among numerous truncated forms of RAGE recently described, the C-terminally truncated form of RAGE has received much attention. This form of RAGE, carrying all of the extracellular domains but devoid of the transmembrane and intracytoplasmic domains, is released outside from cells, binds ligands including AGEs, and is capable of neutralizing RAGE signaling on endothelial cells in culture. This form of RAGE is generated as a splice variant and is named endogenous secretory RAGE (esRAGE). Adenoviral overexpression of esRAGE reverses diabetic impairment of vascular dysfunction, suggesting that esRAGE may be an important inhibitor of RAGE signaling in vivo and potentially be useful for prevention of diabetic vascular complications. An ELISA system to measure plasma esRAGE was recently developed, and the pathophysiological roles of esRAGE have begun to be unveiled clinically. Plasma esRAGE levels are decreased in patients with several metabolic diseases including type 1 and type 2 diabetes, metabolic syndrome and hypertension. In cross-sectional analysis, plasma esRAGE levels are inversely correlated with carotid or femoral atherosclerosis. In an observational cohort of patients with end-stage renal disease, cumulative incidence of cardiovascular death was significantly higher in subjects with lower plasma esRAGE levels. These findings suggest that plasma esRAGE may act as a protective factor against and a novel biomarker for the occurrence of metabolic syndrome and cardiovascular diseases.
Keywords: receptor for advanced glycation end-products (RAGE), soluble RAGE (sRAGE), endogenous secretory RAGE (esRAGE), AGEs, atherosclerosis, metabolic syndrome, inflammation
Receptor for Advanced Glycation End-products (RAGE) and its C-terminally Truncated Form (endogenous secretory RAGE, esRAGE)
RAGE is a multiligand cell-surface protein that was isolated from bovine lung in 1992 by the group of Schmidt and Stern.1 RAGE belongs to the immunoglobulin superfamily of cell-surface molecules, and binds to various ligands including AGEs, S100 (calgranulin), HMGB1 (amphoterin), and amyloid beta-peptides.2–4 Ligand engagement of RAGE in endothelial cells activates the transcription factor nuclear factor-κB (NF-κB), subsequently leading to increased expression of inflammatory mediators such as monocyte chemoattractant protein-1 (MCP-1) and vascular cell adhesion molecule-1 (VCAM-1).5,6 Accumulated evidence suggests that the receptor for advanced glycation end-products (RAGE) is involved in both diabetic micro-7–12 and macrovascular complications.13,14
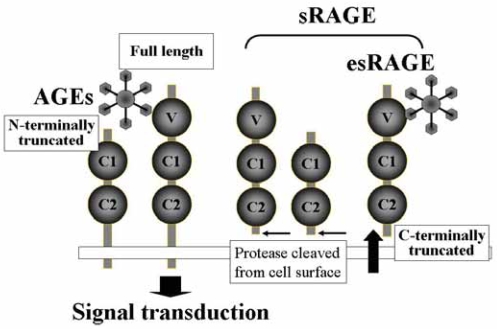
Numerous truncated forms of RAGE have recently been described15–19 (Fig. 1). Two major spliced variants of RAGE mRNA, N-terminal and C-terminal truncated forms, have been most extensively characterized.16 The N-truncated isoform of RAGE mRNA codes for a 303-amino-acid protein lacking the N-terminal signal sequence and the first V-like extracellular domain. The N-truncated form is incapable of binding with AGEs, since the V-domain is critical for binding of the ligand.1 The N-truncated form of RAGE appears to be expressed on the cell surface similar to the full-length RAGE, although its biological roles remain to be elucidated4. It has been suggested that this form of RAGE could be involved in angiogenic regulation in a fashion independent of the classical RAGE signaling pathway.4
Figure 1.
Numerous truncated forms of RAGE. There are three major spliced variants of RAGE: full length, N-terminally truncated, and C-terminally truncated. The C-terminally truncated form of RAGE is secreted from the cell and is named endogenously secreted RAGE (esRAGE). esRAGE has a V-domain, which is essential for binding with ligands, and is capable of competing with RAGE signaling as a decoy receptor. There are other forms of soluble RAGE (sRAGE) that are cleaved from cell-surface RAGE by matrix metalloproteinases. The ELISA assay for sRAGE measures all soluble forms including esRAGE in human plasma, while the ELISA for esRAGE measures only esRAGE, using polyclonal antibody raised against the unique C-terminus of the esRAGE sequence.
The C-terminal truncated form of RAGE lacks the exon 10 sequences encoding the transmembrane and intracytoplasmic domains.16 This spliced variant mRNA of RAGE encodes a protein consisting of 347 amino acids with a 22-amino-acid signal sequence, and is released from cells. This C-truncated form is now known to be present in human circulation and is named endogenous secretory RAGE (esRAGE).16 esRAGE was found to be capable of neutralizing the effects of AGEs on endothelial cells in culture.16 Adenoviral overexpression of esRAGE in vivo in mice reverses diabetic impairment of vascular dysfunction.20 Thus, the decoy function of esRAGE may exhibit a feedback mechanism by which esRAGE prevents the activation of RAGE signaling. It has also been suggested that some soluble RAGE (sRAGE) isoforms that could act as decoy receptors may be cleaved proteolytically from the native RAGE expressed on the cell surface,21 suggesting heterogeneity of the origin and nature of sRAGE. This proteolytic generation of sRAGE was initially described as occurring in mice.22 The molecular heterogeneity of the diverse types of sRAGE in human plasma could exert significant protective effects against RAGE-mediated toxicity.
sRAGE and esRAGE as Potential Biomarkers for Cardiovascular and Metabolic Diseases: Cross-sectional Clinical Studies (Table)
Table.
Levels of Circulating soluble RAGE in cardiovascular and metabolic diseases.
| SRAGE | references | |
|---|---|---|
| CAD (non-DM) | decreased | 23 |
| increased | 35 | |
| Diabetes (type 1) | increased | 33 |
| Diabetes (type 2) | increased | 34,35 |
| decreased | 36 | |
| Hypertension | decreased | 32 |
| Alzheimer’s disease | decreased | 24 |
|
EsRAGE | ||
| Metabolic syndrome | decreased | 26 |
| Diabetes (type 1) | decreased | 25,27 |
| Diabetes (type 2) | decreased | 26 |
| Hypertension | decreased | 26 |
| Atherosclerosis (IMT) | inverse relation | 26–28 |
Since sRAGE and esRAGE may be involved in feedback regulation of the toxic effects of RAGE-mediated signaling, recent clinical studies have focused on the potential significance of circulating sRAGE and esRAGE in a variety of pathophysiological conditions. First, Falcone et al.23 reported that total sRAGE levels are significantly lower in patients with angiographically proven coronary artery disease (CAD) than in age-matched healthy controls. The association between circulating sRAGE and angiographic observations was shown to be dose-dependent, with individuals in the lowest quartile of sRAGE exhibiting the highest risk for CAD. Importantly, this cohort consisted of a non-diabetic population, suggesting that the significance of sRAGE may not be confined to diabetes. Falcone et al also showed that the association between sRAGE and the risk of CAD was independent of other classical risk factors. The same research group also showed that patients with Alzheimer disease have lower levels of sRAGE in plasma than patients with vascular dementia and controls, suggesting a role for the RAGE axis in this clinical entity as well.24 Following development of an ELISA system to specifically measure human esRAGE,25 we measured plasma esRAGE level and cross-sectionally examined its association with atherosclerosis in 203 type 2 diabetic and 134 non-diabetic age- and gender-matched subjects.26 In this study, type 2 diabetes was diagnosed by fasting plasma glucose >126 mg/dl (7 mmol/L), causal plasma glucose >200 mg/dl (11.1 mmol/L), or 2-hour plasma glucose >200 mg/dl during 75 g oral glucose tolerance test, or previous treatment for diabetes. esRAGE levels were inversely correlated with carotid and femoral atherosclerosis, as measured as intimal-medial thickness (IMT) by arterial ultrasound (Fig. 2). Stepwise regression analyses revealed that plasma esRAGE was the third strongest and an independent factor associated with carotid IMT, following age and systolic blood pressure. Another Japanese research group also found an inverse correlation between plasma esRAGE and carotid atherosclerosis in type 127 and type 2 diabetic subjects.28 Thus, plasma esRAGE or sRAGE may protect against the occurrence of cardiovascular diseases, though this hypothesis needs to be tested in a longitudinal cohort study.
Figure 2.
Plasma esRAGE level is inversely correated with carotid and femoral atherosclerosis. Atherosclerosis was determined as intimal-medial thickness measured by arterial ultrasound. N = 337 including 203 type 2 diabetic patients. Reproduced from a reference26.
Several metabolic components well-established as risk factors for cardiovascular diseases have also been shown to be associated with altered plasma sRAGE or esRAGE levels. We have shown that plasma esRAGE levels are decreased in subjects with metabolic syndrome and are inversely correlated with several components of metabolic syndrome including body mass index, blood pressures, insulin resistance index, fasting plasma glucose, serum triglyceride, and lower HDL-cholesterol levels.26 The majorities of these correlations remained significant even when the non-diabetic or type 2 diabetic subpopulation was extracted for analyses, again suggesting that plasma esRAGE plays important roles even in the non-diabetic population. An inverse correaltion between esRAGE (or sRAGE) and body mass index was also found for control subjects,29 those with type 1 diabetes,30 and those with end-stage renal disease (ESRD).31 Moreover, patients with hypertension have been found to have lower plasma sRAGE or esRAGE levels.26,32
The findings regarding plasma levels of the soluble form of RAGE in diabetes are quite confusing. We and other groups have found that plasma esRAGE level is significantly lower in type 1 and type 2 diabetic patients than in non-diabetic controls.26,27 However, plasma sRAGE levels have been shown to be increased in type 133 and type 2 diabetic patients,34,35 although conflicting findings have been reported.36 Of note, when diabetic subjects alone were extracted for analyses, a direct association was not observed between plasma soluble RAGE (both sRAGE and esRAGE) levels and the status of glycemic control (ie, glycohemoglobin A1c).26,30,36–38 Thus, these complex findings in diabetic subjects suggest that levels of plasma soluble forms of RAGE are not determined simply by status of glycemic control, and that even plasma esRAGE and sRAGE levels may be under the control of distinct mechanisms.
Plasma esRAGE Levels in Chronic Kidney Diseases
Abnormal chronic inflammation associated with progressive, chronic kidney disease (CKD) reflects sustained activation of inflammatory cells, such as monocytes/macrophages, in which accumulation of AGEs may play an important role through binding with RAGE. It has been shown that, in peripheral monocytes from subjects with varying severities of CKD, RAGE expression is closely associated with worsening of CKD and is strongly correlated with plasma levels of pentosidine, a marker for AGEs.39 In ESRD subjects with high-grade inflammation, stimulation of mononuclear cells with AGE-modified human serum albumin causes a rapid, dose-dependent increase in NF-κB activity that could be completely blocked by an anti-RAGE antibody.40 Thus, enhanced RAGE expression in ESRD may amplify AGEs-induced perturbation and contribute to systemic inflammatory diseases like atherosclerosis. Circulating esRAGE and sRAGE levels have been shown to be increased in patients with decreased renal function, particularly those with ESRD.34,38,41 As shown in Figure 3, plasma esRAGE levels are positively correlated with urinary excretion rate, and inversely correlated with estimated glomerular fitration rate in type 2 diabetic patients. It remains to be determined whether this increase is caused by decreased renal function alone or whether esRAGE levels are upregulated to protect against toxic effects of the RAGE ligands. Successful kidney transplantation resulted in significant decrease in plasma sRAGE,42 implying that the kidneys play a role in sRAGE removal.
Figure 3.
Plasma esRAGE level is influenced by the presence of kidney disease in patients with type 2 diabetes. UAE: urinary albumin excretion, GFR: glomerular filtration rate estimated by MDRD equation.
Low circulating esRAGE Level predicts Cardiovascular Diseases: A Longitudinal Study
We recently reported an observational cohort study in patients with ESRD and for the first time longitudinally evaluated the effect of plasma esRAGE on cardiovascular mortality.31 Patients with ESRD have been reported to have a substantially increased rate of cardiovascular mortality. The cohort in that study included 206 ESRD subjects registered between June 1992 and June 1995, who had been treated by regular hemodialysis for more than 3 months. At baseline, plasma esRAGE levels in ESRD patients were higher than those in subjects without renal disease. Similar to subjects without renal disease, diabetic ESRD subjects exhibited significantly lower plasma esRAGE levels than non-diabetic ESRD subjects. Plasma esRAGE levels were inversely correlated with many of the components of metabolic syndrome, such as increase in body mass index, increase in fasting plasma glucose, increased triglyceride level and decreased HDL cholesterol, even in ESRD subjects. The subjects were followed up until December 2001, with a median follow-up period of 111 months. At the end of follow-up, 132 patients were confirmed to be alive on hemodialysis and 74 to have died. The 74 deaths during follow-up included 34 due to fatal cardiovascular events.
Even though the plasma esRAGE levels at baseline were higher in ESRD subjects than in those without kidney disease, the subjects in the lowest tertile of plasma esRAGE levels exhibited significantly higher cardiovascular mortality, but not non-cardiovascular mortality (Fig. 4). Univariate Cox proportional hazards analyses revealed that, compared with the subjects in the lowest tertile of plasma esRAGE levels, those in the middle and highest tertiles had significantly less risk of cardiovascular mortality (hazard ratios 0.26 and 0.40, respectively). Multivariate Cox proportional hazards analyses revealed that the higher risk of the subjects with lower esRAGE levels was not significant when adjusted for age, fasting plasma glucose or presence of diabetes, suggesting that esRAGE, aging, and glycemic control mutually interact in the regulation of cardiovascular mortality. Adjustment for other confounders of esRAGE (body mass index, triglyceride, and HDL cholesterol) barely affected the significant association between lower esRAGE level and cardiovascular mortality. Moreover, the association of lower esRAGE level with cardiovascular mortality was independent of other predictors of cardiovascular mortality such as serum creatinine, non-HDL cholesterol, HbA1c, and vascular complications. Our findings thus suggest that low circulating esRAGE level is a predictor for atherosclerosis and cardiovascular events in patients with ESRD. Since this study was designed to survey predictors for cardiovascular mortality in the population of ESRD patients, and the number of fatal events was relatively small and statistical power may not have been high enough to detect important cardiovascular risk factors, demonstration of the role of plasma esRAGE as a biomarker of cardiovascular mortality will require further large-scale prospective studies or nested case-control studies with required numbers of subjects calculated by power analysis.
Figure 4.
Low plasma esRAGE level is a predictor of cardiovascular mortality in patients with ESRD. Cumulative mortalities in subjects with the lowest, middle, and highest tertiles of plasma esRAGE levels were estimated by Kaplan Meier analysis and the log-rank test. Reproduced from a reference.31
It is not known at present how esRAGE is involved in cardiovascular mortality. In our ESRD cohort, neither plasma pentosidine nor carboxy-methyl-lysine level predicted cardiovascular mortality. Moreover, the inverse correlation between low circulating esRAGE level and cardiovascular mortality was not dependent of plasma AGEs levels. Thus, the protective effect of esRAGE against cardiovascular mortality may not be entirely dependent on neutralization of toxic AGEs. Other endogenous ligands for RAGE, such as S100A12, may also be involved in the function of esRAGE. The plasma level of S100A12 has been shown to be increased in diabetes and inversely correlated with serum sRAGE level.36,43
sRAGE vs. esRAGE: Distinct Pathophysiological Significances?
It is unclear at present whether the pathophysiological significances of circulating esRAGE and sRAGE are distinct in different clinical settings. It appears that esRAGE represents less than half of the total sRAGE in human plasma. In our analyses, plasma esRAGE level in Japanese healthy subjects was found to be 0.25 ± 0.11 ng/ml,26 while mean plasma sRAGE level in Caucasian healthy controls has been reported to be 1.3 ng/ml.23 We and others have shown that plasma esRAGE level is decreased in diabetes.26,27 In contrast to the case of esRAGE, circulating sRAGE levels are increased, rather than decreased, in both type 1 and type 2 diabetic patients,33–35 with one conflicting report.36 Humpert et al also showed that sRAGE but not esRAGE is associated with albuminuria in patients with type 2 diabetes.38 Yamamoto et al44 recently described a head-to-head comparison of plasma esRAGE and sRAGE levels using esRAGE as a standard protein and different sets of antibodies, and showed that plasma esRAGE level was about 2-fold less than that of plasma sRAGE. In their analysis, esRAGE and sRAGE levels were positively correlated, with a stronger correlation in healthy subjects than in type 1 diabetic patients. Thus, the possibility of distinct roles for them in certain disease conditions requires further examination.
Soluble RAGE as a Therapeutic Target?
The potential usefulness of soluble RAGE for prevention and treatment of inflammatory diseases has been demonstrated in many animal models. Blockade of RAGE by administration of genetically engineereds RAGE successfully prevented the development of micro-8,9 and macrovascular complications in diabetes.45–47 We have also shown that adenoviral over-expression of esRAGE successfully restored the impaired angiogenic response in diabetic mice.20 Sakaguchi et al found that administration of sRAGE markedly suppressed neointimal formation following arterial injury in non-diabetic mice.48 Soluble RAGE has also been shown to effectively prevent the development of diabetes,49 protect against tumor growth and metastasis,50 improve the outcome of colitis,51 restore impaired wound healing,52 and suppress Alzheimer disease-like conditions.53 These effects of soluble RAGE in animal models could be explained by its decoy function, inhibiting RAGE interaction with its proinflammatory ligands, which might be applicable to human diseases as well.
Further application of soluble RAGE to the treatment of human diseases will require answers to several questions. Most importantly, limited findings are available regarding the mechanisms of regulation of circulating esRAGE or sRAGE in humans. A tissue microarray technique using a wide variety of adult normal human preparations obtained from surgical and autopsy specimens revealed that esRAGE was widely distributed in tissues, including vascular endothelium, monocyte/macrophage, pneumocytes, and several endocrine organs.54 However, it is unclear at present from which organ or tissue plasma sRAGE or esRAGE originate. Circulating AGEs may be involved in regulation of the secretion or production of soluble RAGE, since AGEs are known to upregulate RAGE expression in vitro.55 esRAGE could be simultaneously upregulated by AGEs and act as a negative feedback loop to compensate for the damaging effects of AGEs. Several studies have found positive correlations between plasma sRAGE or esRAGE and AGEs. 29–31,34 This possibility is further supported by the findings that the suppression of sRAGE expression in diabetic rat kidney is reversed by blockade of AGEs accumulation with alagebrium.56 Other inflammatory mediators, such as S100, tumor necrosis factor α, and C-reactive protein, could also be potential candidates for regulation of the plasma level of soluble RAGE in humans.36,55,57 Without doubt, further understanding of the regulation of soluble RAGE will be most helpful in delineating potential targets for therapeutic application of soluble RAGE.
Second, it would be important to determine whether currently available pharmacological agents can regulate plasma sRAGE or esRAGE. Forbes et al58 showed that inhibition of angioten-sin-converting enzyme (ACE) in rats increased renal expression of sRAGE, and that this was associated with decreases in expression of renal full-length RAGE protein. They also showed that plasma sRAGE levels were significantly increased by inhibition of ACE in both diabetic rats and in human subjects with type 1 diabetes. Thus, one attractive scenario is that the protective effect of ACE inhibition against progression of renal dysfunction is mediated through regulation of RAGE versus soluble RAGE production. Other potential agents that may affect circulating soluble RAGE include the thiazolidinediones59 and statins60,61, both of which are known to modulate the AGEs-RAGE system in culture, although their effects on secretion of soluble RAGE are not known.
Taken altogether, the findings discussed here suggest that sRAGE or esRAGE could serve as a novel biomarker for estimation of the risk of progression of atherosclerotic disorders. Further examination of the molecular mechanisms underlying RAGE and esRAGE regulation will provide important insights into potential targets for the prevention and treatment of cardiovascular diseases.
Acknowledgments
The authors thank all colleagues in the Osaka City University Graduate School of Medicine and Kanazawa University Graduate School of Medical Science for their unflagging support of our projects. We apologize to all colleagues whose work we could not cite other than indirectly through other publications, due to limitation of space. This work was supported in part by a Grant-in-aid for Scientific Research from the Japan Society for the Promotion of Science (17590946 to H.K and H.Y.) and a grant from the Osaka Kidney Foundation (OKF06-007 to H.K.).
References
- 1.Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–97. [PubMed] [Google Scholar]
- 2.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–5004. [PubMed] [Google Scholar]
- 3.Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- 4.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83:876–86. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, De Caterina R. Advanced Glycation End Products Activate Endothelium Through Signal-Transduction Receptor RAGE: A Mechanism for Amplification of Inflammatory Responses. Circulation. 2002;105:816–822. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto Y, Kato I, Doi T, Yonekura H, Ohashi S, Takeuchi M, Watanabe T, Yamagishi S, Sakurai S, Takasawa S, Okamoto H, Yamamoto H. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest. 2001;108:261–8. doi: 10.1172/JCI11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D’Agati VD, Schmidt AM. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003;162:1123–37. doi: 10.1016/S0002-9440(10)63909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bierhaus A, Haslbeck KM, Humpert PM, Liliensiek B, Dehmer T, Morcos M, Sayed AA, Andrassy M, Schiekofer S, Schneider JG, Schulz JB, Heuss D, Neundorfer B, Dierl S, Huber J, Tritschler H, Schmidt AM, Schwaninger M, Haering HU, Schleicher E, Kasper M, Stern DM, Arnold B, Nawroth PP. Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. J Clin Invest. 2004;114:1741–51. doi: 10.1172/JCI18058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myint KM, Yamamoto Y, Doi T, Kato I, Harashima A, Yonekura H, Watanabe T, Shinohara H, Takeuchi M, Tsuneyama K, Hashimoto N, Asano M, Takasawa S, Okamoto H, Yamamoto H. RAGE control of diabetic nephropathy in a mouse model: Effects of RAGE gene disruption and administration of low-molecular weight heparin. Diabetes. 2006;55:2510–22. doi: 10.2337/db06-0221. [DOI] [PubMed] [Google Scholar]
- 11.Inagi R, Yamamoto Y, Nangaku M, Usuda N, Okamato H, Kurokawa K, van Ypersele de Strihou C, Yamamoto H, Miyata T. A severe diabetic nephropathy model with early development of nodule-like lesions induced by megsin overexpression in RAGE/iNOS transgenic mice. Diabetes. 2006;55:356–66. doi: 10.2337/diabetes.55.02.06.db05-0702. [DOI] [PubMed] [Google Scholar]
- 12.Vincent AM, Perrone L, Sullivan KA, Backus C, Sastry AM, Lastoskie C, Feldman EL. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology. 2007;148:548–58. doi: 10.1210/en.2006-0073. [DOI] [PubMed] [Google Scholar]
- 13.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: A scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159–69. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 14.Nawroth P, Bierhaus A, Marrero M, Yamamoto H, Stern DM. Atherosclerosis and restenosis: is there a role for RAGE? Curr Diab Rep. 2005;5:11–6. doi: 10.1007/s11892-005-0061-9. [DOI] [PubMed] [Google Scholar]
- 15.Malherbe P, Richards JG, Gaillard H, Thompson A, Diener C, Schuler A, Huber G. cDNA cloning of a novel secreted isoform of the human receptor for advanced glycation end products and characterization of cells co-expressing cell-surface scavenger receptors and Swedish mutant amyloid precursor protein. Brain Res Mol Brain Res. 1999;71:159–70. doi: 10.1016/s0169-328x(99)00174-6. [DOI] [PubMed] [Google Scholar]
- 16.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, Okamoto H, Watanabe T, Yamamoto H. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097–109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlueter C, Hauke S, Flohr AM, Rogalla P, Bullerdiek J. Tissue-specific expression patterns of the RAGE receptor and its soluble forms-—a result of regulated alternative splicing? Biochim Biophys Acta. 2003;1630:1–6. doi: 10.1016/j.bbaexp.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Park IH, Yeon SI, Youn JH, Choi JE, Sasaki N, Choi IH, Shin JS. Expression of a novel secreted splice variant of the receptor for advanced glycation end products (RAGE) in human brain astrocytes and peripheral blood mononuclear cells. Mol Immunol. 2004;40:1203–11. doi: 10.1016/j.molimm.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Ding Q, Keller JN. Splice variants of the receptor for advanced glycosylation end products (RAGE) in human brain. Neurosci Lett. 2005;373:67–72. doi: 10.1016/j.neulet.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 20.Shoji T, Koyama H, Morioka T, Tanaka S, Kizu A, Motoyama K, Mori K, Fukumoto S, Shioi A, Shimogaito N, Takeuchi M, Yamamoto Y, Yonekura H, Yamamoto H, Nishizawa Y. Receptor for advanced glycation end products is involved in impaired angiogenic response in diabetes. Diabetes. 2006;55:2245–55. doi: 10.2337/db05-1375. [DOI] [PubMed] [Google Scholar]
- 21.Hudson BI, Harja E, Moser B, Schmidt AM. Soluble levels of receptor for advanced glycation endproducts (sRAGE) and coronary artery disease: the next C-reactive protein? Arterioscler Thromb Vasc Biol. 2005;25:879–82. doi: 10.1161/01.ATV.0000164804.05324.8b. [DOI] [PubMed] [Google Scholar]
- 22.Hanford LE, Enghild JJ, Valnickova Z, Petersen SV, Schaefer LM, Schaefer TM, Reinhart TA, Oury TD. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE) J Biol Chem. 2004;279:50019–24. doi: 10.1074/jbc.M409782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falcone C, Emanuele E, D’Angelo A, Buzzi MP, Belvito C, Cuccia M, Geroldi D. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25:1032–7. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- 24.Emanuele E, D’Angelo A, Tomaino C, Binetti G, Ghidoni R, Politi P, Bernardi L, Maletta R, Bruni AC, Geroldi D. Circulating levels of soluble receptor for advanced glycation end products in Alzheimer disease and vascular dementia. Arch Neurol. 2005;62:1734–6. doi: 10.1001/archneur.62.11.1734. [DOI] [PubMed] [Google Scholar]
- 25.Sakurai S, Yamamoto Y, Tamei H, Matsuki H, Obata K, Hui L, Miura J, Osawa M, Uchigata Y, Iwamoto Y, Watanabe T, Yonekura H, Yamamoto H. Development of an ELISA for esRAGE and its application to type 1 diabetic patients. Diabetes Res Clin Pract. 2006;73:158–65. doi: 10.1016/j.diabres.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Koyama H, Shoji T, Yokoyama H, Motoyama K, Mori K, Fukumoto S, Emoto M, Tamei H, Matsuki H, Sakurai S, Yamamoto Y, Yonekura H, Watanabe T, Yamamoto H, Nishizawa Y. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:2587–93. doi: 10.1161/01.ATV.0000190660.32863.cd. [DOI] [PubMed] [Google Scholar]
- 27.Katakami N, Matsuhisa M, Kaneto H, Matsuoka TA, Sakamoto K, Nakatani Y, Ohtoshi K, Hayaishi-Okano R, Kosugi K, Hori M, Yamasaki Y. Decreased endogenous secretory advanced glycation end product receptor in type 1 diabetic patients: Its possible association with diabetic vascular complications. Diabetes Care. 2005;28:2716–21. doi: 10.2337/diacare.28.11.2716. [DOI] [PubMed] [Google Scholar]
- 28.Katakami N, Matsuhisa M, Kaneto H, Yamasaki Y. Serum endogenous secretory RAGE levels are inversely associated with carotid IMT in type 2 diabetic patients. Atherosclerosis. 2007;190:22–3. doi: 10.1016/j.atherosclerosis.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Yamagishi S, Adachi H, Nakamura K, Matsui T, Jinnouchi Y, Takenaka K, Takeuchi M, Enomoto M, Furuki K, Hino A, Shigeto Y, Imaizumi T. Positive association between serum levels of advanced glycation end products and the soluble form of receptor for advanced glycation end products in nondiabetic subjects. Metabolism. 2006;55:1227–31. doi: 10.1016/j.metabol.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Miura J, Yamamoto Y, Osawa M, Watanabe T, Yonekura H, Uchigata Y, Yamamoto H, Iwamoto Y. Endogenous secretory receptor for advanced glycation endproducts levels are correlated with serum pentosidine and CML in patients with type 1 diabetes. Arterioscler Thromb Vasc Biol. 2007;27:253–4. doi: 10.1161/01.ATV.0000251533.18013.67. [DOI] [PubMed] [Google Scholar]
- 31.Koyama H, Shoji T, Fukumoto S, Shinohara K, Emoto M, Mori K, Tahara H, Ishimura E, Kakiya R, Tabata T, Yamamoto H, Nishizawa Y. Low circulating endogenous secretory receptor for AGEs predicts cardiovascular mortality in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol. 2007;27:147–53. doi: 10.1161/01.ATV.0000251502.88818.4b. [DOI] [PubMed] [Google Scholar]
- 32.Geroldi D, Falcone C, Emanuele E, D’Angelo A, Calcagnino M, Buzzi MP, Scioli GA, Fogari R. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J Hypertens. 2005;23:1725–9. doi: 10.1097/01.hjh.0000177535.45785.64. [DOI] [PubMed] [Google Scholar]
- 33.Challier M, Jacqueminet S, Benabdesselam O, Grimaldi A, Beaudeux JL. Increased serum concentrations of soluble receptor for advanced glycation endproducts in patients with type 1 diabetes. Clin Chem. 2005;51:1749–50. doi: 10.1373/clinchem.2005.051961. [DOI] [PubMed] [Google Scholar]
- 34.Tan KC, Shiu SW, Chow WS, Leng L, Bucala R, Betteridge DJ. Association between serum levels of soluble receptor for advanced glycation end products and circulating advanced glycation end products in type 2 diabetes. Diabetologia. 2006;49:2756–62. doi: 10.1007/s00125-006-0394-1. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura K, Yamagishi SI, Adachi H, Kurita-Nakamura Y, Matsui T, Yoshida T, Sato A, Imaizumi T. Elevation of soluble form of receptor for advanced glycation end products (sRAGE) in diabetic subjects with coronary artery disease. Diabetes Metab Res Rev. 2006 doi: 10.1002/dmrr.690. [DOI] [PubMed] [Google Scholar]
- 36.Basta G, Sironi AM, Lazzerini G, Del Turco S, Buzzigoli E, Casolaro A, Natali A, Ferrannini E, Gastaldelli A. Circulating soluble receptor for advanced glycation end products is inversely associated with glycemic control and S100A12 protein. J Clin Endocrinol Metab. 2006;91:4628–34. doi: 10.1210/jc.2005-2559. [DOI] [PubMed] [Google Scholar]
- 37.Humpert PM, Kopf S, Djuric Z, Wendt T, Morcos M, Nawroth PP, Bierhaus A. Plasma sRAGE is independently associated with urinary albumin excretion in type 2 diabetes. Diabetes Care. 2006;29:1111–3. doi: 10.2337/diacare.2951111. [DOI] [PubMed] [Google Scholar]
- 38.Humpert PM, Djuric Z, Kopf S, Rudofsky G, Morcos M, Nawroth PP, Bierhaus A. Soluble RAGE but not endogenous secretory RAGE is associated with albuminuria in patients with type 2 diabetes. Cardiovasc Diabetol. 2007;6:9. doi: 10.1186/1475-2840-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou FF, Ren H, Owen WF, Jr, Guo ZJ, Chen PY, Schmidt AM, Miyata T, Zhang X. Enhanced expression of receptor for advanced glycation end products in chronic kidney disease. J Am Soc Nephrol. 2004;15:1889–1896. doi: 10.1097/01.asn.0000131526.99506.f7. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Ayala E, Anderstam B, Suliman ME, Seeberger A, Heimburger O, Lindholm B, Stenvinkel P. Enhanced RAGE-mediated NFkappaB stimulation in inflamed hemodialysis patients. Atherosclerosis. 2005;180:333–40. doi: 10.1016/j.atherosclerosis.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Kalousova M, Hodkova M, Kazderova M, Fialova J, Tesar V, Dusilova-Sulkova S, Zima T. Soluble receptor for advanced glycation end products in patients with decreased renal function. Am J Kidney Dis. 2006;47:406–11. doi: 10.1053/j.ajkd.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 42.Kalousova M, Bartosova K, Zima T, Skibova J, Teplan V, Viklicky O. Pregnancy-associated plasma protein a and soluble receptor for advanced glycation end products after kidney transplantation. Kidney Blood Press Res. 2007;30:31–7. doi: 10.1159/000098811. [DOI] [PubMed] [Google Scholar]
- 43.Kosaki A, Hasegawa T, Kimura T, Iida K, Hitomi J, Matsubara H, Mori Y, Okigaki M, Toyoda N, Masaki H, Inoue-Shibata M, Nishikawa M, Iwasaka T. Increased plasma S100A12 (EN-RAGE) levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5423–8. doi: 10.1210/jc.2003-032223. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto Y, Miura J, Sakurai S, Watanabe T, Yonekura H, Tamei H, Matsuki H, Obata KI, Uchigata Y, Iwamoto Y, Koyama H, Yamamoto H. Assaying soluble forms of receptor for advanced glycation end products. Arterioscler Thromb Vasc Biol. 2007;27:e33–e34. doi: 10.1161/01.ATV.0000251533.18013.67. [DOI] [PubMed] [Google Scholar]
- 45.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation end-products. Nat Med. 1998;4:1025–31. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 46.Kislinger T, Tanji N, Wendt T, Qu W, Lu Y, Ferran LJ, Jr, Taguchi A, Olson K, Bucciarelli L, Goova M, Hofmann MA, Cataldegirmen G, D’Agati V, Pischetsrieder M, Stern DM, Schmidt AM. Receptor for advanced glycation end products mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2001;21:905–10. doi: 10.1161/01.atv.21.6.905. [DOI] [PubMed] [Google Scholar]
- 47.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–35. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 48.Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, Marso SP, Duda S, Arnold B, Liliensiek B, Nawroth PP, Stern DM, Schmidt AM, Naka Y. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111:959–72. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Yan SD, Colgan J, Zhang H-P, Luban J, Schmidt AM, Stern D, Herold KC. Blockade of late stages of autoimmune diabetes by inhibition of the receptor for advanced glycation end products. J Immunol. 2004;173:1399–1405. doi: 10.4049/jimmunol.173.2.1399. [DOI] [PubMed] [Google Scholar]
- 50.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 51.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 52.Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, Nowygrod S, Wolf BM, Caliste X, Yan SF, Stern DM, Schmidt AM. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159:513–25. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stern A, Luddy JS, Lue LF, Walker DG, Roher A, Buttini M, Mucke L, Li W, Schmidt AM, Kindy M, Hyslop PA, Stern DM, Du Yan SS. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. Embo J. 2004;23:4096–105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng C, Tsuneyama K, Kominami R, Shinohara H, Sakurai S, Yonekura H, Watanabe T, Takano Y, Yamamoto H, Yamamoto Y. Expression profiling of endogenous secretory receptor for advanced glycation end products in human organs. Mod Pathol. 2005;18:1385–96. doi: 10.1038/modpathol.3800450. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem. 2000;275:25781–90. doi: 10.1074/jbc.M001235200. [DOI] [PubMed] [Google Scholar]
- 56.Coughlan MT, Thallas-Bonke V, Pete J, Long DM, Gasser A, Tong DC, Arnstein M, Thorpe SR, Cooper ME, Forbes JM. Combination therapy with the advanced glycation end product cross-link breaker, alagebrium, and angiotensin converting enzyme inhibitors in diabetes: synergy or redundancy? Endocrinology. 2007;148:886–95. doi: 10.1210/en.2006-1300. [DOI] [PubMed] [Google Scholar]
- 57.Zhong Y, Li SH, Liu SM, Szmitko PE, He XQ, Fedak PW, Verma S. C-Reactive protein upregulates receptor for advanced glycation end products expression in human endothelial cells. Hypertension. 2006;48:504–11. doi: 10.1161/01.HYP.0000234904.43861.f7. [DOI] [PubMed] [Google Scholar]
- 58.Forbes JM, Thorpe SR, Thallas-Bonke V, Pete J, Thomas MC, Deemer ER, Bassal S, El-Osta A, Long DM, Panagiotopoulos S, Jerums G, Osicka TM, Cooper ME. Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J Am Soc Nephrol. 2005;16:2363–72. doi: 10.1681/ASN.2005010062. [DOI] [PubMed] [Google Scholar]
- 59.Marx N, Walcher D, Ivanova N, Rautzenberg K, Jung A, Friedl R, Hombach V, de Caterina R, Basta G, Wautier MP, Wautiers JL. Thiazolidinediones reduce endothelial expression of receptors for advanced glycation end products. Diabetes. 2004;53:2662–8. doi: 10.2337/diabetes.53.10.2662. [DOI] [PubMed] [Google Scholar]
- 60.Okamoto T, Yamagishi S, Inagaki Y, Amano S, Koga K, Abe R, Takeuchi M, Ohno S, Yoshimura A, Makita Z. Angiogenesis induced by advanced glycation end products and its prevention by cerivastatin. Faseb J. 2002;16:1928–30. doi: 10.1096/fj.02-0030fje. [DOI] [PubMed] [Google Scholar]
- 61.Cuccurullo C, Iezzi A, Fazia ML, De Cesare D, Di Francesco A, Muraro R, Bei R, Ucchino S, Spigonardo F, Chiarelli F, Schmidt AM, Cuccurullo F, Mezzetti A, Cipollone F. Suppression of RAGE as a basis of simvastatin-dependent plaque stabilization in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2006;26:2716–23. doi: 10.1161/01.ATV.0000249630.02085.12. [DOI] [PubMed] [Google Scholar]