Abstract
Background and Objective
The objective of this study was to improve port wine stain (PWS) therapeutic outcome in response to laser therapy. Our specific aim was to determine whether the combined use of pulsed dye laser (PDL) therapy and topical Imiquimod versus PDL alone can improve PWS therapeutic outcome.
Study Design/Materials and Methods
This pilot study involved a retrospective review of 20 subjects, all Asian, with PWS. Subject ages ranged between 3 and 56 years. Upon enrollment, three test sites were prospectively identified on each subject for treatment assignments to the following regimens: (A) PDL+Imiquimod; (B) PDL alone; and (C) Imiquimod alone. PDL test sites received a single treatment with a 585 nm wavelength; 1.5 milliseconds pulse duration; spot size 7 mm using a light dosage of 10 J/cm2 with cryogen spray cooling. For the PDL+Imiquimod and Imiquimod alone test sites, subjects were instructed to apply Imiquimod topically to the sites once daily for 1 month after PDL exposure. Subjects were followed-up at 1, 3, 6, and 12 months after PDL exposure to evaluate each of the three test sites. The primary efficacy measurement was the quantitative assessment of blanching responses as measured by a DermoSpectrometer to calculate the hemoglobin-index of each site at 1, 3, 6, and 12 months after PDL exposure. Subjects were also closely monitored for any adverse effects.
Results
Based on paired sample test analysis, there were clinically, and statistically significant, differences in blanching responses over time favoring PWS receiving PDL+Imiquimod as compared to either PDL or Imiquimod alone (P<0.05). At 12 months, it should be noted that there was some evidence of redarkening of PWS test sites treated by PDL+Imiquimod and PDL alone, presumably due to revascularization of blood vessels. However, based on comparison of the hemoglobin-indices determined at 1 and 12 months after PDL exposure, there was less revascularization of PWS test sites treated with PDL+Imiquimod as compared to PDL alone (P<0.05). Transient hyperpigmentation was noted in 10% (n = 2) and 40% (n = 8) of subjects on the PDL+Imiquimod and PDL alone test sites, respectively. On all sites, hyperpigmentation resolved spontaneously without medical intervention within 6 months. Permanent hypopigmentation or scarring was not observed on any test site.
Conclusion
Based on the results of this pilot study, PDL+Imiquimod resulted in superior blanching responses over time as compared to PDL alone for treatment of test sites on PWS lesions. Although the PDL+Imiquimod approach is intriguing, clinical validation in large PWS patient samples is required.
Keywords: pulsed dye laser, port wine stain
INTRODUCTION
Port wine stain (PWS) is a congenital, progressive vascular malformation of the dermis [1–3]. Since two-thirds of these malformations occur on the face, PWS is a clinically significant problem. PWS should not be considered a cosmetic problem but a disease with potentially devastating psychological and physical complications. Personality development is adversely influenced in virtually all patients by the negative reaction of others to a “marked” person [4–6]. In childhood, PWS are faint pink macules, but the lesions can potentially darken progressively to red-purple [7]. The subsequent hypertrophy of the underlying bone and soft tissue further disfigures the facial features of many patients. Histopathologic studies of PWS show a normal epidermis overlying an abnormal plexus of subsurface blood vessels located in the upper dermis [8].
In the past, PWS treatment has included cosmetic cover-up, skin grafting, ionizing radiation, dermabrasion, cryosurgery, tattooing and electrotherapy but none of these modalities provided cosmetically acceptable results. The development of lasers and their ability to damage selectively PWS blood vessels offered a new approach to the clinical management of these patients. Multiple laser devices have been utilized for the treatment of PWS birthmarks but the pulsed dye laser (PDL) has produced the best clinical results with the lowest incidence of adverse effects [9,10]. Yellow light produced by the PDL is preferentially absorbed by hemoglobin, allowing selective destruction of the dilated ectatic capillaries in the upper dermis.
Although the PDL has become the treatment of choice for PWS birthmarks, only 10–20% of patients obtain 100% fading of their PWS even after many treatments [11–13]. PWS can recur after laser therapy due to reformation of blood vessels [14,15]. There have also been anecdotal reports from both physicians and patients of PWS becoming darker and redder after PDL treatment.
Through selective photothermolysis, PDL exposure destroys subsurface PWS blood vessels in human skin. As a result, hypoxia, inflammation and edema are induced in the upper layers of PWS skin. Inflammatory cells migrate into the area secreting cytokines, which are potent up-regulators of proangiogenic factors. The laser-induced wound healing response to PDL treatment often results in reformation of the PWS blood vessels. As a result, the degree of PWS blanching seen following PDL treatment remains variable and unpredictable. In a recent report, the use of combined PDL irradiation and a topically applied angiogenesis inhibitor was proposed [16].
Angiogenesis inhibitors have been derived from a number of sources, including cleaved proteins, monoclonal antibodies, and natural products which contain a variety of chemopreventive compounds that can prevent the development of malignancies [17]. These compounds exert anti-angiogenic and chemopreventive properties through a variety of mechanisms.
One suchmolecule is Imiquimod (Aldara, 3M Health Care Limited, Leicestershire, UK), a topical immune response modifier agent that inhibits neovascularization [18–20]. Imiquimod disrupts pathologic neovascularization which can promote disease progression. The antiangiogenic mechanisms of Imiquimod include: (1) induction of cytokines that inhibit angiogenesis (interferons, IFN-α,β,γ, IL-10, IL-12, IL-18); (2) local up-regulation of endogenous angiogenesis inhibitors (IFNs, IP10, TIMP, TSP-1); (3) local down-regulation of pro-angiogenic factors (bFGF, MMP-9); and (4) promotion of endothelial apoptosis [21].
The purpose of this pilot study was to inhibit the reformation and recanalization of PWS blood vessels after PDL therapy using Imiquimod as an antiangiogenic agent. The primary efficacy measurement was the quantitative assessment of blanching responses as measured by a DermoSpectrometer (Cortex Tech., Hadsund, Denmark) to calculate the hemoglobin-indices of test sites treated by PDL+Imiquimod as compared, on a blinded basis, to PDL alone or Imiquimod alone. Safety was also evaluated by searching for adverse effects such as scarring or dyspigmentation on the test sites.
PATIENTS AND METHODS
The study protocol was approved by the Institutional Review Board (IRB) at Chang Gung Memorial Hospital, Taipei, Taiwan. All subjects (or parental guardians) signed the IRB approved consent form on study enrollment.
This pilot study involved a retrospective review of 20 subjects (12 females and 8 males) with PWS birthmarks treated with the PDL over a preceding 20-month period (May 2006 to December 2007). Subjects’, all of whom were Asian, ages ranged between 3 and 56 years. Information regarding the following variables was extracted from charts: age, sex, PWS severity grade prior to laser treatment, number of treatments, duration of treatment, and improvement following laser therapy.
The following inclusion criteria were used: (1) PWS suitable for comparison testing; (2) PWS greater than 20 cm2; and (3) apparent good health as documented by medical history. The following exclusion criteria were used: (1) inability to commit to a 1 year follow-up period; (2) pregnancy; (3) history of photodermatoses or skin cancer; (4) concurrent use of known photosensitizing drugs; and (5) any therapy within the previous two months to the proposed PWS test sites.
Upon enrollment, three test sites were prospectively identified on each subject for treatment assignments to the following regimens: (A) PDL+Imiquimod; (B) PDL alone; and (C) Imiquimod alone. Sites were assigned to one of the three treatment regimens by randomization. Every effort was madeto place the test sites on optically uniform areas of the PWS. This was done to ensure that clinically relevant PWS characteristics and geometry (i.e., epidermal melanin concentration, blood vessel size and depth) did not substantially vary between each of the test sites on an individual patient basis. Photographs were taken of the test sites after treatment regimen assignment and at each follow-up visit.
PDL test sites received a single treatment using the ScleroPLUS® [Candela (Wayland, MA)] laser (585 nm wavelength; 1.5 milliseconds pulse duration; spot size 7 mm) at a light dosage of 10 J/cm2. Dynamic cooling device parameters were a 30 milliseconds cryogen spurt with a delay of 30 milliseconds between spurt termination and delivery of the laser pulse.
Imiquimod cream 5% w/w (Aldara, 3M Health Care Limited, Leicestershire, UK) was obtained from the pharmacy of the Chang Gung Memorial Hospital. Imiquimod is currently approved by the US Food and Drug Administration for the treatment of actinic keratoses, superficial basal cell carcinomas and genital warts [22–24]. This study represented an off-label use of Imiquimod. Subjects were provided with samples of Imiquimod for the study duration at no cost. For the PDL+Imiquimod and Imiquimod alone test sites, subjects were instructed to apply Imiquimod topically as a thin layer once daily for one month after PDL exposure.
Subjects were followed-up at 1, 3, 6, and 12 months after PDL exposure. Although PWS appearance is used as a diagnostic indicator, lesion color is strongly dependent on the observer’s viewing orientation and spectral radiance of ambient room lighting. Therefore, the primary efficacy measurement was the quantitative assessment of blanching responses as measured by a DermoSpectrometer (Cortex Tech., Hadsund, Denmark) [25,26] to calculate the hemoglobin-index at each follow-up visit for each of the three test site treatment regimens [(A) PDL+Imiquimod; (B) PDL alone; and (C) Imiquimod alone]. The device emits light from diode sources at three defined wavelengths. The amount of light backscattered from the skin is then used to determine the indices for melanin (not studied herein) and hemoglobin.
As stated above, test sites were assigned by randomization such that the individual performing the DermoSpectrometer measurements was blinded as to the treatment regimen used on an individual test site. It should be noted that it was possible to compress blood out of the skin during the DermoSpectrometer measurement resulting in an artifact in the hemoglobin-index measurement. Therefore, care was taken to make each measurement with the device in contact with the skin but without the application of pressure to the test site. Because single spectrometer measurements can be unreliable due to difficulty locating the exact position five separate measurements were made on each test site and averaged at each follow-up visit. Differences between the mean blanching responses for the three treatment regimens were then determined and analyzed by a paired sample test analysis.
Subjects were also closely monitored for any adverse effects. Safety was evaluated by examining each of the test sites for any abnormal wound healing (blistering, scabbing, erosion), scarring, dyspigmentation, or allergy to Imiquimod cream. Scarring was defined as a permanent raised hypertrophic, depressed or atrophic skin texture on the test sites. Dyspigmentation was defined as a transient (resolving within 1 year post-treatment) or permanent change in skin color on the test sites as compared to adjacent normal skin.
RESULTS
For each of the assigned treatment regimens [(A) PDL+Imiquimod; (B) PDL alone; and (C) Imiquimod alone], the hemoglobin-indices of the test site blanching responses were determined Pre-PDL, and 1, 3, 6, and 12 months following PDL (Table 1). Five separate measurements were made on each test site and averaged at each follow-up visit. The means reported are the average values for all 20 subjects enrolled in the study.
TABLE 1.
Mean Hemoglobin-Indices of Test Site Blanching Responses by Treatment Regimen (N=20 subjects)
| PDL + Imiquimod | PDL alone | Imiquimod alone | |
|---|---|---|---|
| Pre-PDLa | 29.17 ± 5.93 | 29.17 ± 5.93 | 29.17 ± 5.93 |
| 1 month | 8.42 ± 1.47* | 12.58 ± 2.31 | 28.12 ± 2.70 |
| 3 months | 8.78 ± 1.54* | 13.79 ± 2.52 | 28.38 ± 2.79 |
| 6 months | 9.46 ± 1.79* | 15.26 ± 2.74 | 28.67 ± 2.37 |
| 12 months | 9.96 ± 1.75* | 17.68 ± 2.51 | 28.85 ± 3.58 |
| Δ12 vs. 1 month | 1.54* | 5.10 | 0.73 |
All subjects received each of the three treatment regimens: (A) PDL + Imiquimod; (B) PDL alone; and (C) Imiquimod alone.
Pre-PDL values represent the mean hemoglobin-indices for all 20 subjects based on measurement on each lesion before assignment of test sites to one of the three treatment regimens. Every effort was made to place the test sites on optically uniform areas of the PWS.
P < 0.05.
For the Imiquimod alone test sites, there was no reduction in the hemoglobin-index. Inasmuch as there was no PDL-induced blood vessel disruption, as expected there were no observed blanching responses on the Imiquimod alone treated test sites. Differences between the mean blanching responses for the three treatment regimens were then determined and analyzed by a paired sample test analysis.
Based on hemoglobin-indices determined with the DermoSpectrometer at each follow-up visit, blanching responses over time were more favorable for PDL+Imiquimod as compared to PDL alone (Table 1). Based on our paired sample test analysis, the blanching responses were found to be statistically significant favoring PDL+Imiquimod as compared to PDL alone (P<0.05). At 12 months, it should be noted that there was some evidence of redarkening of PWS test sites treated by PDL+Imiquimod and PDL alone, presumably due to revascularization of blood vessels. However, based on hemoglobinindices determined at 1 and 12 months after PDL exposure, there was less revascularization (P<0.05) of PWS test sites treated with PDL+Imiquimod [mean value of 1.54 (9.96–8.42)] as compared to PDL alone [mean value of 5.10 (17.68–12.58)].
Photographs were also taken of the test sites 12 months after PDL. Representative photographs presented in Figure 1–Figure 3 demonstrate the enhanced blanching response obtained on some of the test sites treated with PDL+Imiquimod as compared to PDL alone.
Fig. 1.
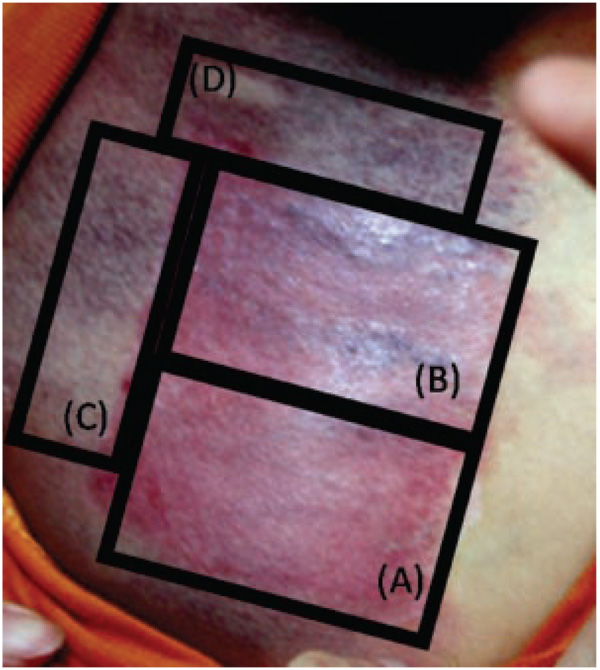
Three-year-old Asian male with PWS on the upper chest. Photograph of test sites 12 months after treatment with: (A) PDL+Imiquimod; (B) PDL alone; (C) Imiquimod alone; and (D) untreated PWS skin. There was superior blanching on the PWS test site receiving PDL+Imiquimod (A) as compared to PDL alone (B). The hemoglobin-indices of the sites in the photograph are as follows: (A) 10.5; (B) 15.9; (C) 19.1; and (D) 19.4.
Fig. 3.
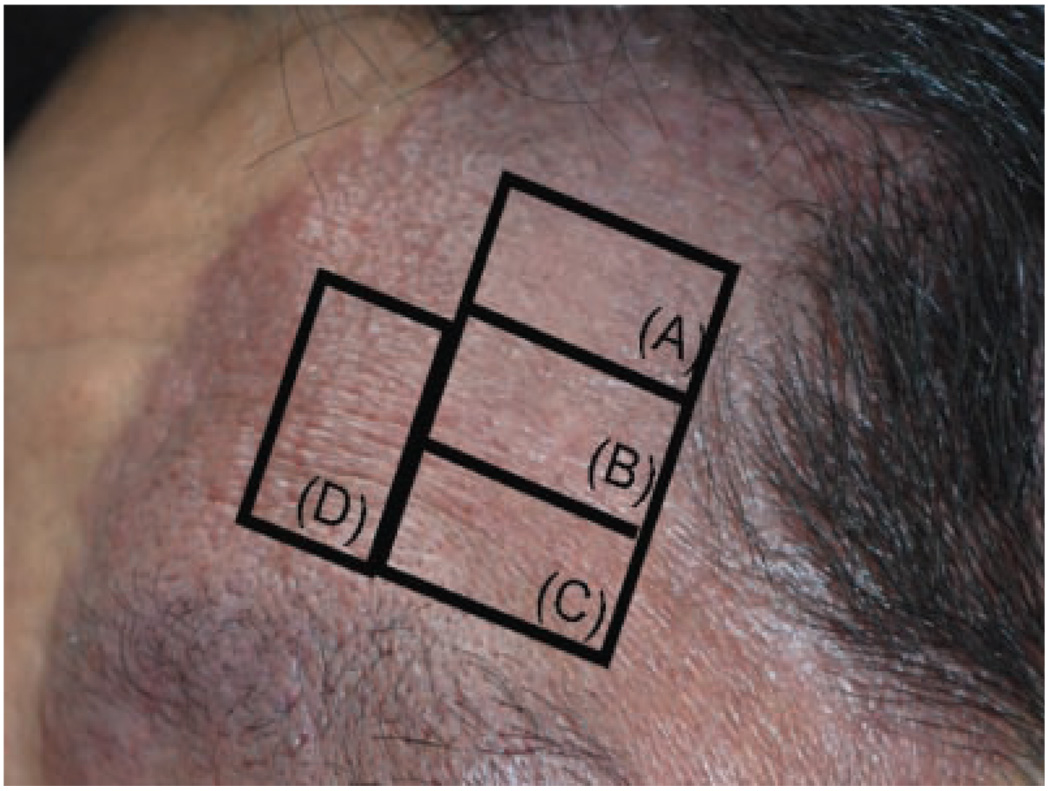
Forty-three-year-old Asian male with PWS on the face. Photograph of test sites twelve months after treatment with: (A) PDL+Imiquimod; (B) PDL alone; (C) Imiquimod alone; and (D) untreated PWS skin. There was superior blanching on the PWS test site receiving PDL+Imiquimod (A) as compared to PDL alone (B). The hemoglobin-indices of the sites in the photograph are as follows: (A) 8.1; (B) 11.1; (C) 14.9; and (D) 14.9.
Safety was evaluated for each test site by searching for any abnormal wound healing (blistering, scabbing, erosion), scarring, dyspigmentation, or allergy to Imiquimod cream. Transient hyperpigmentation was noted in 10% (n = 2) and 40% (n = 8) of subjects on the PDL+Imiquimod and PDL alone test sites, respectively. On all sites, hyper-pigmentation resolved spontaneously without medical intervention within 6 months. Abnormal wound healing or scarring was not observed on any test site. One patient did report very minor symptoms of itching and burning on the PDL+Imiquimod and Imiquimod alone test sites one week after beginning the daily topical application of the medication. However, this patient was able to complete the 1 month course of topical Imiquimod without any disruption in the daily treatment regimen for the following 3 weeks. The patient’s symptoms, believed to be most consistent with a minor allergic reaction, resolved completely within 1 week after topical Imiquimod was withdrawn.
DISCUSSION
The standard treatment for PWS is the PDL, which induces photocoagulation of the subsurface-targeted blood vessels. However, the laser-induced wound healing response of human skin to treatment often results in reformation of PWS blood vessels within 1 month after exposure. The perplexing clinical results achieved after PWS laser therapy raise the following question: can the wound healing response of human skin after laser therapy be modulated? Preliminary studies have suggested that the application of a topical angiogenesis inhibitor might suppress the reformation and reperfusion of blood vessels previously disrupted by photothermolysis [16]. The scientific rationale for this approach is that the combined use of PDL to induce PWS blood vessel injury, and an inhibitor to prevent PWS blood vessel reformation and recanalization after laser therapy, will improve PWS lesion blanching. Herein, the combined use of PDL to induce PWS blood vessel injury, and Imiquimod to prevent PWS blood vessel reformation and recanalization after laser therapy, was evaluated in this pilot study.
Based on hemoglobin-indices determined by the DermoSpectrometer (Table 1) in 20 subjects, the results of this pilot study showed more favorable PWS blanching responses over time on those test sites treated with PDL+Imiquimod as compared to either PDL alone or Imiquimod alone (P<0.05). It should also be noted that the enhanced blanching responses obtained on the test sites treated with PDL+Imiquimod were maintained up to 12 months after laser exposure. Although some PWS redarkening did occur presumably due to revascularization of the blood vessels, the test sites treated with PDL+Imiquimod showed better long-term blanching responses as compared to test sites treated by PDL alone (P<0.05). For test sites treated with Imiquimod alone, as expected no drug-induced PWS blanching was observed.
It is currently unknown if there are any risks related to the combined use of PDL+Imiquimod other than the known reasonable risks associated with treatment using either modality alone. The reasonable risks of PDL exposure are include: swelling, pain and discomfort, abnormal wound healing (erosion, crusting), scarring, and skin dyspigmentation. The reasonable risks of topical Imiquimod are include: skin redness, peeling, flaking, swelling, itching/ burning, scabbing, crusting, blistering, ulceration, and acute allergic reaction.
Transient hyperpigmentation was noted in 10% (n = 2) and 40% (n = 8) of subjects on the PDL+Imiquimod and PDL alone test sites, respectively. The lower incidence of hyperpigmentation seen in the PDL+Imiquimod group may be due to the chemopreventive properties of Imiquimod itself. On all sites, hyperpigmentation resolved spontaneously without any medical intervention in all subjects within 6 months. Permanent hypopigmentation or scarring was not observed on any test site. One patient may have had a minor allergic reaction to topical Imiquimod, which resolved spontaneously when the medication was withdrawn.
In summary, the results of this pilot study demonstrated that the combined use of PDL+Imiquimod resulted in superior blanching responses over time as compared to PDL alone or Imiquimod alone for treatment of test sites on PWS lesions. The combined PDL+Imiquimod approach was found to be safe.
Although the PDL+Imiquimod approach is intriguing, clinical validation in large numbers of PWS patients is required. Prospective, comparative and controlled clinical studies conducted by experienced investigators on a multi-center basis against accepted treatment regimens are required so that the role of topically applied Imiquimod in conjunction with PDL therapy of PWS may be fully defined. It has also been well documented that there can be anatomical variation in terms of PWS response to laser therapy [9]. For example, the central face does not respond as completely or to laser therapy as the lateral face. Thus, future studies evaluating the effectiveness and possible PWS recurrence following PDL+Imiquimod should take into account anatomical variation of the response to treatment. Finally, because some PWS redarkening did occur presumably due to revascularization of the blood vessels, consideration should be given to using Imiquimod for longer periods of time than 1 month following PDL. However, should longer term daily dosing of Imiquimod be considered, patients should be closely followed for the development of systemic influenza-like signs and symptoms, such as malaise, fever, nausea, myalgias and rigors, which may accompany, or even precede, local inflammatory reactions. Localized hypo- and hyper-pigmentation have been reported following extended use of topical Imiquimod and these skin color changes may be permanent in some patients [www.fda.gov/medwatch/safety/2005/aug_PI/Aldara_PI.pdf].
Fig. 2.
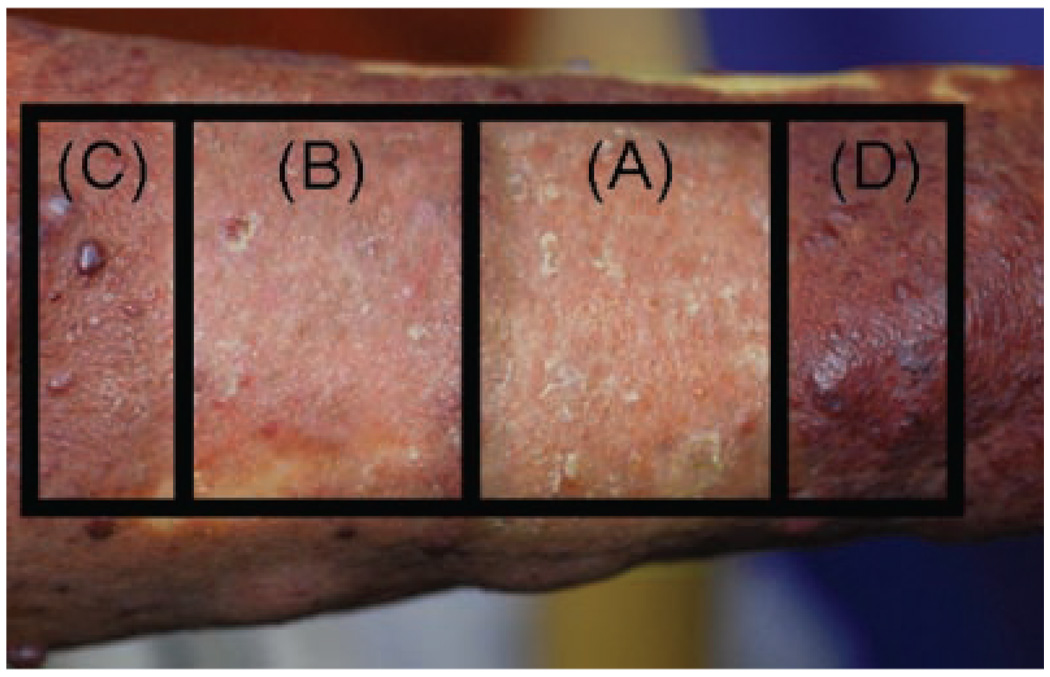
Fifty-six-year-old Asian female with PWS on the right upper extremity. Photograph of test sites twelve months after treatment with: (A) PDL+Imiquimod; (B) PDL alone; (C) Imiquimod alone; and (D) untreated PWS skin. There was superior blanching on the PWS test site receiving PDL+Imiquimod (A) as compared to PDL alone (B). The hemoglobin-indices of the sites in the photograph are as follows: (A) 11.1; (B) 16.4; (C) 28.1; and (D) 28.7.
ACKNOWLEDGMENTS
This project was supported by research grants awarded from Chang Gung Memorial Hospital (CMRP606, CMRP812) and the National Institute of Health (AR47551 and EB002495).
Contract grant sponsor: Chang Gung Memorial Hospital; Contract grant numbers: CMRP606, CMRP812; Contract grant sponsor: National Institutes of Health; Contract grant numbers: AR47551, EB002495.
REFERENCES
- 1.Mulliken JB, Young AR. Vascular Birthmarks-Hemangiomas and malformations. Saunders: Philadelphia: WB; 1988. [Google Scholar]
- 2.Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218–222. [PubMed] [Google Scholar]
- 3.Pratt AG. Birthmarks in infants. Arch Dermatol Syphilol. 1953;67:302–305. doi: 10.1001/archderm.1953.01540030065006. [DOI] [PubMed] [Google Scholar]
- 4.Kalick SM. Toward an interdisciplinary psychology of appearances. Psychiatry. 1978;41:249–254. [PubMed] [Google Scholar]
- 5.Heller A, Rafman S, Svagulis I, Pless IB. Birth defects and psychosocial adjustment. Am J Dis Child. 1985;139:257–263. doi: 10.1001/archpedi.1985.02140050051021. [DOI] [PubMed] [Google Scholar]
- 6.Malm M, Calber NN. Port-wine stain—A surgical and psychological problem. Ann Plast Surg. 1988;20:512–516. doi: 10.1097/00000637-198806000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Geronemus RG, Ashinoff R. The medical necessity of evaluation and treatment of port-wine stains. J Dermatol Surg Oncol. 1991;17:76–79. doi: 10.1111/j.1524-4725.1991.tb01597.x. [DOI] [PubMed] [Google Scholar]
- 8.Barsky SH, Rosen S, Geer DE, Noe JM. The nature and evolution of port wine stains: A computer assisted study. J Invest Dermatol. 1980;74:154–157. doi: 10.1111/1523-1747.ep12535052. [DOI] [PubMed] [Google Scholar]
- 9.Renfro L, Geronemus RG. Anatomical differences in the treatment of port wine stains with the pulsed dye laser. Arch Dermatol. 1993;29:182–188. [PubMed] [Google Scholar]
- 10.Geronemus R, Lou W, Quintana A, Kauvar A. High fluence modified pulsed dye laser photocoagulation with dynamic cooling for port wine stains in infancy. Arch Dermatol. 2000;136:942–943. doi: 10.1001/archderm.136.7.942. [DOI] [PubMed] [Google Scholar]
- 11.van der Horst CMAM, Koster PHL, deBorgie CAJM, Bossuyt PMM, van Gemert MJC. Effect of timing of treatment of port-wine stains with the flash-lamp-pumped pulsed dye laser. N Engl J Med. 1998;338:1028–1033. doi: 10.1056/NEJM199804093381504. [DOI] [PubMed] [Google Scholar]
- 12.Morelli JG, Weston WL, Huff JC, Yohn JJ. Initial lesion size as a predictive factor in determining the response of port-wine stains in children treated with the pulsed dye laser. Arch Pediatr Adolesc Med. 1995;149:1142–1144. doi: 10.1001/archpedi.1995.02170230096014. [DOI] [PubMed] [Google Scholar]
- 13.Lanigan SW. Port-wine stains unresponsive to pulsed dye laser: Explanations and solutions. Brit J Dermatol. 1998;139:173–177. doi: 10.1046/j.1365-2133.1998.02351.x. [DOI] [PubMed] [Google Scholar]
- 14.Mork NJ, Austad J, Helsing P. Do port wine stains recur after successful treatment with pulsed dye laser? J Eur Acad Dermatol Venereol. 1998;11:S142–S143. [Google Scholar]
- 15.Huikeshoven M, Koster PHL, de Borgie CAJM, Beek J, van Gemert MJC, van der Horst CMAM. Redarkening of port-wine stains 10 years after pulsed-dye-laser treatment. N Engl J Med. 2007;356:1235–1240. doi: 10.1056/NEJMoa064329. [DOI] [PubMed] [Google Scholar]
- 16.Phung TL, Oble DA, Jia W, Benjamin LE, Mihm MC, Nelson JS. Can the wound healing response of human skin be modulated after laser treatment and the effects of exposure extended? Implications on the combined use of the pulsed dye laser and a topical angiogenesis inhibitor for treatment of port wine stain birthmarks. Lasers Surg Med. 2008;40:1–5. doi: 10.1002/lsm.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 18.Kouba DJ, Yip D, Fincher EF, Moy RL. Topical imiquimod in the treatment of a long-standing capillary malformation. Br J Dermatol. 2007;157(5):1071–1072. doi: 10.1111/j.1365-2133.2007.08181.x. [DOI] [PubMed] [Google Scholar]
- 19.Sun ZJ, Zhao YF, Zhang WF. Immune response: A possible role in the pathophysiology of hemangioma. Med Hypotheses. 2007;68(2):353–355. doi: 10.1016/j.mehy.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Ho NT, Lansang P, Pope E. Topical iniquimod in the treatment of infantile hemangiomas: A retrospective study. J Am Acad Dermatol. 2007;56(1):63–68. doi: 10.1016/j.jaad.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Sauder DN. Immunomodulatory and pharmacologic properties of imiquimod. J Am Acad Dermatol. 2000;43:S6–S11. doi: 10.1067/mjd.2000.107808. [DOI] [PubMed] [Google Scholar]
- 22.Salasche SJ, Levine N, Morrison L. Cycle therapy of actinic keratoses of the face and scalp with 5% topical imiquimod cream: An open-label trial. J Am Acad Dermatol. 2002;47(4):571–577. doi: 10.1067/mjd.2002.126257. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan TP, Dearaujo T, Vincek V, Berman B. Evaluation of superficial basal cell carcinomas after treatment with imiquimod 5% cream or vehicle for apoptosis and lymphocyte phenotyping. Dermatol Surg. 2003;29(12):1181–1186. doi: 10.1111/j.1524-4725.2003.29399.x. [DOI] [PubMed] [Google Scholar]
- 24.Serrao VV, Paris FR, Feio AB. Genital vitiligo-like depig-mentation following use of imiquimod 5% cream. Eur J Dermatol. 2008;8(3):342–343. doi: 10.1684/ejd.2008.0402. [DOI] [PubMed] [Google Scholar]
- 25.Tejasvi T, Sharma VK, Kaur J. Determination of minimal erythemal dose for narrowband-ultraviolet B radiation in north Indian patients: Comparison of visual and Dermaspectrometer readings. Indian J Dermatol Venereol Leprol. 2007;73(2):97–99. doi: 10.4103/0378-6323.31893. [DOI] [PubMed] [Google Scholar]
- 26.Ramsing DW, Agner T. Effect of glove occlusion on human skin. (I). Short-term experimental exposure. Contact Dermatitis. 1996;34(1):1–5. doi: 10.1111/j.1600-0536.1996.tb02102.x. [DOI] [PubMed] [Google Scholar]