Abstract
Thiazolidinediones (TZDs) have beneficial effects on glucose homeostasis via enhancement of insulin sensitivity and preservation of β-cell function. How TZDs preserve β-cells is uncertain, but it might involve direct effects via both peroxisome proliferator-activated receptor-γ-dependent and -independent pathways. To gain insight into the independent pathway(s), we assessed the effects of short-term (≤90 min) exposure to pioglitazone (Pio) (10 to 50 μM) on glucose-induced insulin secretion (GIIS), AMP-activated protein kinase (AMPK) activation, and β-cell metabolism in INS 832/13 β-cells and rat islets. Pio caused a right shift in the dose-dependence of GIIS, such that insulin release was reduced at intermediate glucose but unaffected at either basal or maximal glucose concentrations. This was associated in INS 832/13 cells with alterations in energy metabolism, characterized by reduced glucose oxidation, mitochondrial membrane polarization, and ATP levels. Pio caused AMPK phosphorylation and its action on GIIS was reversed by the AMPK inhibitor compound C. Pio also reduced palmitate esterification into complex lipids and inhibited lipolysis. As for insulin secretion, the alterations in β-cell metabolic processes were mostly alleviated at elevated glucose. Similarly, the antidiabetic agents and AMPK activators metformin and berberine caused a right shift in the dose dependence of GIIS. In conclusion, Pio acutely reduces glucose oxidation, energy metabolism, and glycerolipid/fatty acid cycling of the β-cell at intermediate glucose concentrations. We suggest that AMPK activation and the metabolic deceleration of the β-cell caused by Pio contribute to its known effects to reduce hyperinsulinemia and preserve β-cell function and act as an antidiabetic agent.
Pioglitazone causes a right shift in β-cell metabolic activation by glucose, which results in reduced glucose-induced insulin secretion at intermediate range glucose concentrations: implications for β-cell preservation in type 2 diabetes.
Type 2 diabetes (T2D) occurs when pancreatic β-cell compensation for insulin resistance fails (1,2). Thiazolidinediones (TZDs), which are agonists of the nuclear peroxisome proliferator-activated receptor (PPAR)-γ (3), improve insulin resistance in T2D, at least in part through activation of PPARγ in adipose tissue (4). PPARγ activation stimulates the expansion of sc fat mass and diverts circulating lipids to be stored in adipocytes, away from skeletal muscles and hepatocytes (5), in which their accumulation is generally associated with insulin resistance (6).
In addition to their insulin sensitizing effect, TZDs also preserve β-cell mass and function (reviewed in Ref. 7). Many in vivo studies have described the protective effect of TZDs on β-cell function and/or the preservation of islet architecture in genetic or diet-induced rodent models of T2D (8,9,10,11,12). Ex vivo studies provide evidence that TZDs improve the insulin secretory capacity of rat (9,13) and human (14) islets and protect them from lipotoxicity and apoptosis. The A Diabetes Outcome Progression Trial (ADOPT) study showed evidence that TZDs are able to sustain glucose control in early T2D better than metformin or sulfonylureas, possibly due to β-cell preservation (15). TZDs may also lead to an increase in circulating adipokines, due to increased fat mass, and reduce glucolipotoxicity (10) and inflammation (16), and all these effects can be collectively beneficial to β-cells. Direct TZD activation of PPARγ expressed in β-cells (17,18) is also likely, although its role in β-cell function is unclear.
TZDs may mediate their cellular effects via targets other than PPARγ (19) of which the more documented is the rapid activation of AMP-activated protein kinase (AMPK) in various cell types both in vivo and in vitro (20,21,22), including β-cell (23). AMPK is a key regulator of energy metabolism with several downstream targets, and its activity is influenced by changes in the AMP to ATP ratio. Fryer et al. (21) have shown that in muscle cells AMPK activation by rosiglitazone involves a rapid increase in the AMP to ATP ratio, probably due to the TZDs’ inhibitory effect on respiratory chain complex I (24). It is possible that in the β-cell, TZDs might also modulate energy metabolism and function through AMPK. An important target of AMPK is acetyl-CoA carboxylase (ACC), which in the β-cell is involved in lipid partitioning and amplification arms of glucose-induced insulin secretion (GIIS) pathways (25).
Here we postulated that direct TZD-mediated protection of the β-cell may in part involve acute PPARγ-independent effects via AMPK and energy metabolism. We examined this hypothesis in INS 832/13 β-cells and isolated rat islets. The results show that the TZD pioglitazone (Pio) has an acute and profound impact on β-cell energy metabolism, reducing glucose oxidation, mitochondrial membrane potential, and ATP production primarily at intermediate glucose concentrations. Furthermore, Pio markedly inhibits β-cell glycerolipid (GL)/free fatty acid (FFA) cycling. These direct non-PPARγ-mediated metabolic effects of Pio are associated with a lowering of β-cell glucose sensitivity for insulin secretion and are dependent on AMPK activity.
Materials and Methods
Materials
Cell culture supplies were from Corning (Corning, NY) and Fisherbrand (Nepean, Ontario, Canada). Pio-HCl (Toronto Research Chemicals, North York, Ontario, Canada) was dissolved in dimethylsulfoxide (DMSO) (Sigma, St. Louis, MO), berberine-HCl (Wako Pure Chemical Industries, Osaka, Japan), and metformin (1,1-dimethylbiguanide-HCl; Sigma) were dissolved in water. Compound C (InSolution) was from Calbiochem (Darmstadt, Germany). d-[U-14C]glucose was from GE Healthcare (Baie d’Urfé, Québec, Canada), [1-14C]palmitate from PerkinElmer Life Sciences (Downers Grove, IL), and palmitate sodium salt from Nu-Check Prep (Elysian, MN). Bicinchoninic acid protein assay from Pierce (Rockford, IL) was used. Stock-unlabeled palmitate was prepared at 4 mm in 5% defatted BSA (Sigma) as described elsewhere (26). Defatted BSA was used in all experiments.
Cell culture
INS 832/13 cells (27) (passages 55–65) were cultured at 37 C in a humidified atmosphere containing 5% CO2 in RPMI 1640 with sodium bicarbonate (Wisent, St. Bruno, Québec, Canada), supplemented with 10% (vol/vol) fetal calf serum (Wisent), 10 mm HEPES (pH 7.4), 2 mm l-glutamine, 1 mm sodium pyruvate, and 50 μm β-mercaptoethanol (complete RPMI). Cells were grown to 80% confluence. Except when using metformin, media were changed to RPMI 1640 containing 3 mm glucose supplemented as the complete RPMI 24 h before the experiments. In experiments using metformin, media were changed 2 h before the experiments to RPMI 1640 3 mm glucose containing metformin. Experiments were conducted in Krebs-Ringer bicarbonate buffer containing 10 mm HEPES (KRBH; pH 7.4).
Islet isolation
All procedures were approved by the Institutional Committee for the Protection of Animals at the Centre de Recherche du Centre Hospitalier de l’Université du Montréal. Wistar rats from Charles River (St. Constant, Québec, Canada) were anesthetized with Somnotol (MTC Pharmaceuticals, Hamilton, Ontario, Canada) and killed by exsanguination. Pancreatic islets were isolated by collagenase (type XI; Sigma) digestion of total pancreas (28), followed by centrifugation (1040 × g) on a Histopaque 1119, 1077 (Sigma) gradient. Isolated islets were handpicked and cultured overnight in a petri dish at 37 C in a humidified atmosphere containing 5% CO2 in RPMI 1640 with sodium bicarbonate, supplemented with 10% fetal calf serum, 10 mm HEPES (pH 7.4), 2 mm l-glutamine, 1 mm sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Insulin secretion measurement
INS 832/13 cells were washed in KRBH containing 1 mm glucose and 0.5% BSA (KRBH 1G/0.5%BSA) and preincubated for 45 min in KRBH 1G/0.5%BSA in presence of pharmacological agents or vehicle. When used, compound C was added first in preincubation media and Pio added 20 min later. Insulin secretion from INS 832/13 cells was measured during 45-min static incubations in KRBH containing various glucose concentrations, 0.5% BSA, and pharmacological agents or vehicle, with or without 35 mm KCl or 0.2–0.3 mm palmitate, as specified. For islet insulin secretion, batches of 10 islets were washed in KRBH containing 3 mm glucose and 0.5% BSA (KRBH 3G/0.5%BSA), and preincubated for 45 min in KRBH 3G/0.5%BSA containing Pio or DMSO. Islets were then incubated for 45 min in KRBH containing various glucose concentrations or 3 mm glucose plus 35 mm KCl, 0.5% BSA and Pio or DMSO. At the end of the incubation, media were collected and insulin extracted from cells or islets in acid-ethanol [1.5% (vol/vol) HCl, 75% (vol/vol) ethanol]. Total insulin contents and media insulin concentrations were determined by RIA using human insulin standards (Linco Research, St. Charles, MO).
Glucose oxidation
INS 832/13 cells were washed in KRBH 1G/0.5%BSA and preincubated for 45 min in KRBH 1G/0.5%BSA plus Pio or DMSO. Preincubation media were changed to KRBH containing various glucose concentrations, 0.07% BSA, Pio or DMSO, and d-[U-14C]glucose at 0.10, 0.15, or 0.20 μCi/ml (for 1, 6, and 10 mm glucose media, respectively), and [14C]CO2 liberation was measured as previously described (29) after a 45-min incubation.
Mitochondrial membrane potential
INS 832/13 cells were washed in KRBH 1G/0.5%BSA, and preincubated for 45 min in KRBH 1G/0.5%BSA to which 10 μg/ml rhodamine 123 (Invitrogen) was added for the last 20 min. Then cells were washed and incubated for 25 min in KRBH 1G/0.5%BSA followed by a further wash and incubation for 10 min in KRBH containing 6 mm glucose and 0.5% BSA. At the end of this 10 min incubation, baseline fluorescence (excitation: 485 nm; emission: 530 nm) was measured on a FLUOstar microplate reader (BMG Labtech, Offenburg, Germany). Pio, 5 μm carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (Sigma), or DMSO was added, or medium was changed for KRBH 1G containing DMSO, and fluorescence was measured 10 min later.
Cellular ATP content
INS 832/13 cells were washed and preincubated for 45 min in KRBH 1G/0.5%BSA. ATP content of INS 832/13 cells was measured using ATPlite kit (PerkinElmer, Boston, MA) after 10 min incubation in fresh KRBH containing varying concentrations of glucose, 0.5% BSA and Pio, 2 μm oligomycin (Sigma), or DMSO.
Palmitate oxidation and esterification
INS 832/13 cells, grown in 25-cm2 flasks, were washed in KRBH 1G/0.5%BSA and preincubated for 45 min in KRBH 1G/0.5%BSA plus Pio or DMSO. Cells were then incubated for 45 min in fresh KRBH containing various glucose concentrations, 0.5% BSA, 0.2 mm unlabeled palmitate, 0.1 μCi/ml [1-14C]palmitate, 1 mm carnitine (oxidation only), and Pio or DMSO. Oxidation and esterification measurements were performed as described elsewhere (26). Briefly, for oxidation, the incubation was in sealed flasks containing a glass fiber filter soaked in 5% KOH. At the end of the incubation, perchloric acid [40% (vol/vol)] was injected into each flasks. After overnight isotopic equilibration, filters (containing trapped CO2) were removed and aliquots of the acidified media containing acid soluble β-oxidation products were collected for liquid scintillation counting. For palmitate esterification, after incubation, cells were washed and scraped in cold PBS, centrifuged, and resuspended in 3 ml Folch reagent (30). Total lipids were extracted and nonpolar lipids separated by thin-layer chromatography. Incorporation of labeled palmitate into specific lipid species was quantified after scraping by liquid scintillation counting.
Lipolysis determination
INS 832/13 cells were washed in KRBH 1G/0.5%BSA and preincubated for 45 min in KRBH 1G/0.5%BSA. Pio or DMSO was omitted from the preincubation media to avoid Pio exposure in excess of 90 min. INS 832/13 cells were then incubated for 90 min in fresh KRBH containing various glucose concentrations and 0.5% BSA in the presence of Pio or DMSO with or without 0.2 mm palmitate. Glycerol release, an index of lipolysis, was determined by a coupled enzymatic assay (31).
Immunoblot analysis
INS 832/13 cells were washed and preincubated for 45 min in KRBH 1G/0.5%BSA. Cells were then incubated for 20 min in KRBH containing various glucose concentrations, 0.5% BSA, and Pio or DMSO. Alternatively, for experiments using compound C, cells were collected at the end of the incubation. Cells were washed with cold PBS and lysed using a lysis buffer containing 20 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% (vol/vol) Triton X-100, 0.1% sodium dodecyl sulfate, protease inhibitors, 1 mm Na3VO4, and 2.5 mm Na4P2O7. Lysates were sonicated, aliquots were taken for protein assay, and samples were stored at −80 C. Proteins from total cell extracts (20 μg protein) were separated on 8% SDS-PAGE and transferred to nitrocellulose membranes (Scheicher & Schuell, Dassel, Germany) for Western blotting. Blotted proteins were probed using antibodies rabbit phospho-AMPKα (Thr172) monoclonal antibody (catalog no. 2535), AMPKα, and ACC (Cell Signaling Technology, Danvers, MA) and rabbit phospho-ACC (Ser79) (Upstate, Temecula, CA) according to suppliers’ protocols. Horseradish peroxidase-conjugated goat antirabbit IgG (Bio-Rad, Hercules, CA) was used as second antibody with SuperSignal West Pico chemiluminescence (Pierce) for detection.
Statistical analysis
Values are expressed as means ± sem. Statistical analysis was performed using one-way ANOVA with Dunnett’s posttest or two-way ANOVA with Bonferroni’s posttest for multiple comparisons using Prism version 5.01 and InStat version 3.06 (GraphPad Software, San Diego, CA).
Results
Pio lowers the sensitivity of β-cells to glucose for insulin secretion
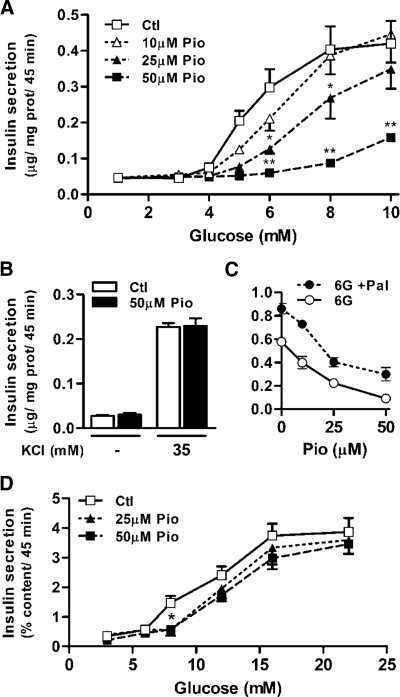
Acute exposure of β-cells to TZDs has been shown to reduce GIIS in vitro, and this was attributed to activation of AMPK (23,32,33). In the present study, we confirmed these findings and noticed that a 90-min exposure to Pio dose-dependently (10–50 μm) inhibited GIIS in INS 832/13 cells in presence of 6–10 mm glucose (Fig. 1A). Interestingly, and one of the key aspects of our study, Pio treatment caused a right shift in the glucose dose-response curve for insulin secretion, and at 10 or 25 μm Pio, there was no change in the maximal GIIS at 10 mm glucose. Thus, for the 25 μm Pio dose, insulin secretion was inhibited by 70–90% at 5–6 mm glucose, with almost total recovery of insulin release at 10 mm glucose. Recovery of almost full GIIS in INS 832/13 cells at 50 μm Pio required a higher concentration of the sugar and was apparent at 16 mm glucose (0.45 ± 0.06 and 0.36 ± 0.06 μg insulin per milligram protein per 45 min for 16 mm glucose without or with 50 μm Pio, respectively (n = 9; not significantly different).
Figure 1.
Pio reduces β-cell glucose sensitivity for insulin secretion. A–C, Insulin secretion from INS 832/13 cells incubated in the presence of the indicated glucose concentrations and 0 (open squares), 10 (open triangles), 25 (closed triangles), or 50 μm Pio (closed squares) (A) or 1 mm glucose with or without 35 mm KCl and 0 or 50 μm Pio (B), or 6 mm glucose (6G), the indicated Pio concentrations, and 0 (open circles) or 0.3 mm palmitate (Pal) (closed circles) (C). Means ± sem, n = 9 (nine different cell wells in three separate experiments). Two-way ANOVA post hoc analyses: *, P < 0.05; **, P < 0.001 vs. vehicle at same glucose concentration. D, Insulin secretion from groups of 10 rat islets treated as above. Means ± sem, n = 11 (11 different islet incubations in three separate experiments). Two-way ANOVA: Pio treatment effect, P = 0.02; one-way ANOVA post hoc analyses: *, P < 0.01 vs. vehicle for both 25 and 50 μm Pio conditions. Ctl, Control; prot, protein.
Pio had no effect on insulin release at low (nonstimulating) glucose concentrations and did not affect high potassium-induced insulin release (Fig. 1B). Pio was also without effect on palmitate amplification of insulin secretion at 6 (Fig. 1C) or 10 mm glucose (data not shown).
Similar to the results obtained with INS 832/13 cells, Pio reduced significantly insulin secretion in isolated rat islets (Fig. 1D) at intermediate (8 mm) glucose only. Pio again had no effect on KCl (35 mm)-induced insulin release at 3 mm glucose (data not shown). Total INS 832/13 cell and islet insulin contents were unaltered by Pio over the course of the experiments (data not shown). Thus, Pio specifically reduced β-cell glucose sensitivity for insulin secretion.
In as much as high concentrations of Pio used in these experiments (50 μm) did not affect cellular insulin content or basal-, palmitate-, KCl-, and high glucose-induced insulin secretion and did not affect fat oxidation and the incorporation of palmitate into diacylglycerol and triacylglycerol (see below), it is unlikely that this drug exerts nonspecific toxicity to β-cells.
Pio reduces metabolic activation by glucose in INS 832/13 cells
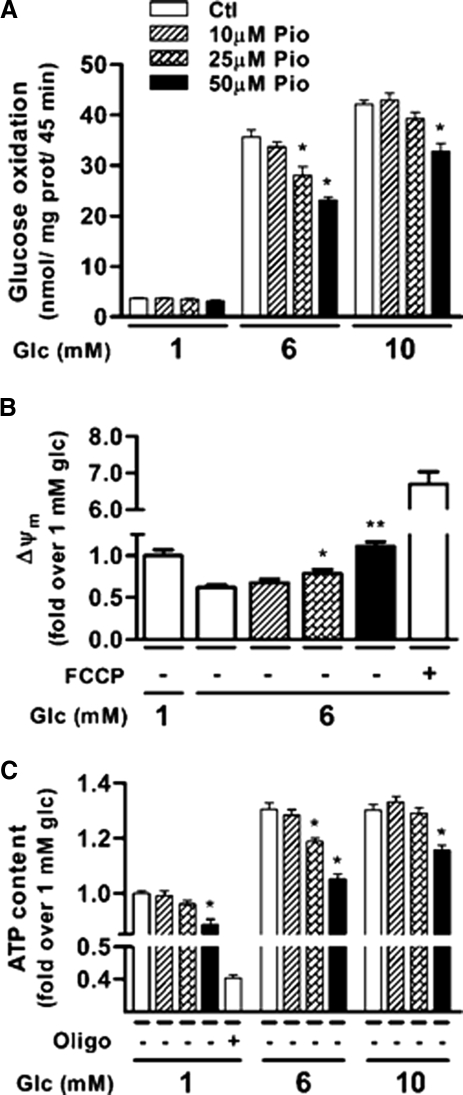
Glucose metabolism is necessary for the triggering and amplification arms of GIIS (34). Pio dose-dependently inhibited glucose oxidation in INS 832/13 cells (Fig. 2A), which was most marked at 6 mm glucose (20 and 35% in the presence of 25 and 50 μm Pio, respectively). As seen for insulin secretion, the Pio inhibitory effect on glucose oxidation was partially alleviated at 10 mm glucose.
Figure 2.
Pio reduces glucose oxidation, the mitochondrial membrane potential and ATP content of INS 832/13 cells. A, Glucose oxidation measured after 45 min using d-[U-14C]glucose. Means ± sem, n = 9 (nine different cell wells in three separate experiments), except 25 μm Pio where n = 6 (two separate experiments). Two-way ANOVA post hoc analyses: *, P < 0.001 vs. vehicle at same glucose concentration. B, Δψm measured with rhodamine 123 after 10 min incubation. Data are expressed as changes in rhodamine 123 fluorescence vs. changes measured in presence of 1 mm glucose plus DMSO. Means ± sem, n = 18 (18 different cell wells in three separate experiments). One-way ANOVA post hoc analyses: *, P < 0.05; **, P < 0.001 vs. vehicle at 6 mm glucose. FCCP, Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone, 5 μm. C, Total ATP content measured after 10 min incubation. Data are expressed as fold change over basal (1 mm glucose) control condition. Means ± sem, n = 3 (three experiments done with six replicates). Two-way ANOVA post hoc analyses: *, P < 0.001 vs. vehicle at same glucose concentration. Oligo, Oligomycin, 2 μm; Glc, glucose; Ctl, control; prot, protein.
The reduction in glucose oxidation by Pio suggested that mitochondrial oxidative metabolism may be altered. To examine this possibility, we measured mitochondrial membrane potential (Δψm) at 6 mm glucose using rhodamine 123 in INS 832/13 cells. As expected, there was a decrease in the fluorescence of the dye in cells exposed to 6 mm glucose when compared with 1 mm glucose (Fig. 2B), indicating hyperpolarization of the mitochondrial membrane. Pio dose-dependently inhibited this glucose-induced membrane polarization. In presence of 50 μm Pio, glucose-induced membrane polarization was significantly dissipated to the level observed at 1 mm glucose. Pio dose-dependently reduced the glucose-induced rise in ATP content (Fig. 2C). As for the other measured parameters, the effect of Pio was more prominent at intermediate (6 mm) than high (10 mm) glucose, with complete recovery for the 25 μm Pio conditions.
Pio effect on insulin secretion is antagonized by the AMPK inhibitor compound C
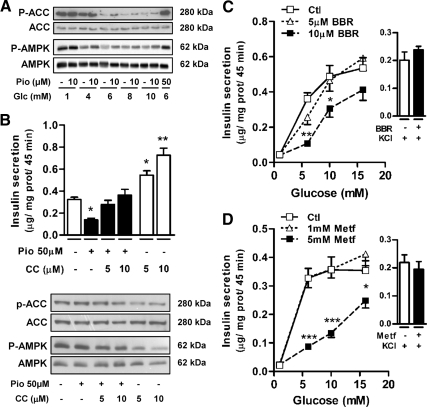
Previous studies showed that TZDs inhibit glucose metabolism in muscle (35,36) and activate AMPK (21). Thus, rosiglitazone rapidly increases the AMP to ATP ratio in H-2Kb muscle cells, leading to the activation of AMPK (21). A 20-min exposure of INS 832/13 cells to Pio increased AMPK phosphorylation (Fig. 3A) and activated this enzyme as evidenced by the phosphorylation of its downstream target ACC (Fig. 3A). Thus, Pio reversed the effect of increasing glucose concentrations (from 1 to 10 mm) to reduce the ACC phosphorylation state.
Figure 3.
Pio inhibitory effect on GIIS involves AMPK activation in INS 832/13 cells. A, Representative immunoblots for Pio-induced AMPKα (Thr172) and ACC (Ser79) phosphorylation after 20 min incubation. B, Graph, Insulin secretion from INS 832/13 cells incubated in the presence of 10 mm glucose with (black bars) or without (white bars) 50 μm Pio and the indicated compound C (CC) concentrations. Means ± sem, n = 16 (16 different cell wells in five separate experiments) except when compound C is used alone where n = 10 in three separate experiments. One-way ANOVA post hoc analyses: *, P < 0.01; **, P < 0.001 vs. vehicle. Immunoblots, AMPKα and ACC phosphorylation (P-AMPK and P-ACC) status in cells collected at the end of the incubations. C and D, Insulin secretion from INS 832/13 cells incubated in the presence of the indicated glucose concentrations and 0 (open squares), 5 (open triangles), or 10 μm berberine (BBR) (closed squares) (C) or 0 (open squares), 1 (open triangles), or 5 mm metformin (Metf) (closed squares) (D). Right to each graph, Insulin release induced by 1 mm glucose and 35 mm KCl with (black bars) or without (white bars) 10 μm BBR (C) or 5 mm Metf (D). Means ± sem, n = 9 (nine different cell wells in three separate experiments). Two-way ANOVA post hoc analyses: *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. vehicle at same glucose concentration. Glc, Glucose; Ctl, control; prot, protein.
To assess whether the effects of Pio on insulin secretion is mediated via AMPK, we used the AMPK inhibitor compound C. Low concentrations (5 and 10 μm) of compound C restored GIIS inhibited by 50 μm Pio. In the presence of 10 mm glucose, this restoration was complete (Fig. 3B, upper panel). Compound C alone amplified GIIS in INS 832/13 cells. This finding is in part anticipated because activation and inhibition of AMPK in the β-cell are thought to be associated with inhibition and stimulation of insulin secretion, respectively (37). As shown in representative immunoblots, treatment with compound C reduced the phosphorylation status of both AMPK and ACC (Fig. 3B, lower panel).
Pio action on GIIS is mimicked by the AMPK activators metformin and berberine
To determine whether other antidiabetic agents known to activate AMPK (38,39) have similar effects as Pio on GIIS, INS 832/13 cells were acutely exposed to berberine or metformin. Similarly to Pio, both compounds inhibited GIIS, particularly at submaximal glucose concentrations, without affecting basal or KCl-induced insulin release (Fig. 3, C and D).
Pio inhibits fatty acid esterification into complex lipids and lipolysis
AMPK affects lipid partitioning (40). Thus, ACC phosphorylation at Ser79 by AMPK reduces malonyl-CoA (41), an allosteric inhibitor of carnitine-palmitoyltransferase-1, which regulates the rate-limiting long-chain fatty acyl-CoAs entry into mitochondria for β-oxidation. Moreover, AMPK also inhibits glycerol-3-phosphate acyltransferase (GPAT) (42), responsible for the first committed step of GL biosynthesis. Therefore, it might be anticipated that activation of AMPK in INS 832/13 cells would favor fatty acid oxidation at the expense of their incorporation into complex lipids.
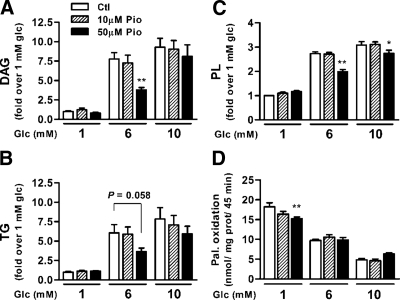
At intermediate glucose (6 mm), Pio reduced palmitate incorporation into diacylglycerol (DAG), triacylglycerol (TG), and phospholipids (PL) in INS 832/13 cells (Fig. 4). However, at 10 mm glucose, the inhibitory effect of Pio on palmitate incorporation into complex lipid species was almost totally relieved. The predicted enhancement of palmitate oxidation in the presence of Pio was not noticed (Fig. 4D). In fact, there was a small decrease in palmitate oxidation at low glucose by 50 μm Pio. As anticipated, increasing glucose concentration (from 1 to 10 mm) did lower palmitate oxidation (Fig. 4D).
Figure 4.
Pio reduces palmitate incorporation into complex lipids in INS 832/13 cells and does not increase β-oxidation. A–C, [1-14C]palmitate incorporation into DAG (A), TG (B), and PL (C) was determined after 45 min incubation. Data are expressed as fold change over basal (1 mm glucose) control condition. D, Total palmitate oxidation into CO2 plus acid soluble products calculated from the oxidation of [1-14C]palmitate after 45 min incubation. Means ± sem, n = 9 (nine different cell wells in three separate experiments). Two-way ANOVA post hoc analyses: *, P < 0.05; **, P < 0.01 vs. vehicle at same glucose concentration. Glc, Glucose; Ctl; control; prot, protein.
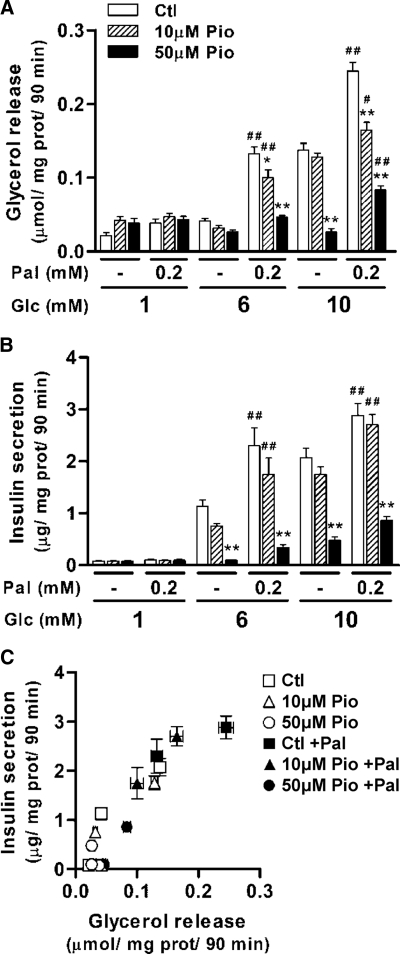
Results from our and other laboratories suggest that GL/FFA cycling, in which there is coupling of FFA esterification and lipolysis processes, plays an important role in GIIS (43,44). In addition, both hormone-sensitive lipase and adipose triglyceride lipase appear to be regulated by AMPK (45,46). Whether reduced net incorporation of palmitate into GL in the presence of Pio was accompanied by altered lipolysis was examined. As observed earlier (31,47,48,49), in the absence of Pio, there was an increase in the release of glycerol at elevated glucose in INS 832/13 cells (Fig. 5A). Pio inhibited glucose-induced glycerol release in a dose-dependent manner (Fig. 5A).
Figure 5.
Effect of Pio in the presence and absence of exogenous palmitate on glycerol release and glucose-induced insulin secretion in INS 832/13 cells. A, Glycerol release. B, Insulin secretion. C, Relationship of glycerol release and insulin secretion. Glycerol release and insulin secretion were measured in the same experiments. Pio concentrations of 0 (squares), 10 μm (triangles), and 50 μm (circles) in the absence of palmitate (open symbols) and presence of 0.2 mm palmitate (Pal) (closed symbols) are shown. Means ± sem, n = 9 (nine different cell wells in three separate experiments). Pio effect: *, P < 0.05; **, P < 0.001 vs. vehicle at same glucose and palmitate concentrations. Palmitate effect: #, P < 0.05; ##, P < 0.01 vs. same glucose and Pio concentrations in the absence of palmitate. Glc, Glucose; prot, protein; Ctl, control.
Exogenous FFAs enhance both lipolysis and insulin secretion in Pio-treated β-cells
Lipolysis and insulin secretion were measured from control and Pio-treated INS 832/13 β-cells in the presence and absence of 0.2 mm palmitate. In control cells, incubation in the presence of palmitate was associated with marked increases in both glycerol release and GIIS (Fig. 5, A and B). Glycerol release from Pio-treated cells was also substantially higher in the presence of palmitate at 6 and 10 mm glucose for the 10 μm Pio and at 10 mm glucose for the 50 μm Pio (Fig. 5A). These palmitate-induced increases in glycerol release also paralleled increases in insulin secretion in Pio-treated cells with complete recovery of secretion at 10 μm Pio and partial recovery at 50 μm Pio (Fig. 5B). Of note, there was a remarkably strong correlation between glycerol release and insulin secretion when combining all the data of this experiment (Fig. 5C).
Because Pio inhibits both net fatty acid esterification and glycerol release, an index of lipolysis, we concluded that Pio acutely impairs both the esterification and lipolysis arms of GL/FFA cycling in the β-cell. At low to moderate concentrations, the inhibitory effect of Pio on GL/FFA cycling is partially recovered at high glucose. Provision of exogenous FFA to Pio-treated cells also promotes some recovery of GL/FFA cycling and GIIS.
Discussion
The results indicate that Pio reduces GIIS in the β-cell at intermediate glucose concentrations by shifting the dose-dependence curve of glucose responsiveness to the right. Thus, Pio at the lower 10 and 25 μm doses had no influence on insulin secretion at the maximal stimulatory glucose concentration of 10 mm in INS 832/13 cells. In this β-cell line, 16 mm glucose could overcome the inhibitory effect of 50 μm Pio on GIIS. In isolated rat islets, Pio (both 25 and 50 μm) reduced GIIS by about 60% at 8 mm glucose but did not affect secretion at 22 mm glucose. It is interesting to note that a similar right shift in GIIS with increasing glucose infusion rates was reported in a 12-wk rosiglitazone clinical trial in insulin-resistant nondiabetic subjects (50).
What are the mechanism(s) involved in Pio action to acutely reduce GIIS only at intermediate concentrations of the sugar?
We first considered Pio actions on energy metabolism and mitochondrial function. Pio decreased the ATP content of INS 832/13 cells, likely due to the partial dissipation of Δψm. This effect of the drug on ATP content and Δψm was noticed within 10 min of incubation, thus ruling out the involvement of the transcription factor PPARγ. It is possible that reduced ATP levels could have resulted from the known inhibitory effect of TZDs on complex I of the respiratory chain (51). Another TZD, troglitazone, was also found to dissipate the mitochondrial potential and to activate AMPK within 10 min in muscle cells (24). Interestingly, berberine and metformin, which also inhibit complex I and activate AMPK (24,38,52), caused a right shift in the glucose dose-response curve of insulin secretion in INS 832/13 cells, similar to that seen with Pio.
Earlier attempts (53,54) to delineate the role of AMPK in the acute regulation of GIIS have led to contradictory observations. The present study performed under acute conditions is in accordance with the view that AMPK activation reduces GIIS, whereas its inhibition promotes insulin release (37). Thus, pharmacological inhibition of AMPK using compound C led to elevated insulin secretion, whereas activation of AMPK by three unrelated compounds, metformin, Pio, and berberine, was able to lower the sensitivity of INS 832/13 cells to glucose for insulin secretion. It is interesting to note that there is residual and significant AMPK phosphorylation at 10 mm glucose in INS 832/13 cells, which also reflects in the phosphorylation status of ACC (see Fig. 3B). This residual activity of AMPK likely keeps insulin secretion in check, even at 10 mm glucose because the addition of low amounts of the chemical inhibitor of AMPK, compound C, led to a steep increase in insulin secretion. Similarly, lowering glucose concentration to less than 6 mm, with a decrease in insulin secretion, does not fully activate AMPK (see Fig. 3A) because addition of Pio further increases AMPK phosphorylation and decreases insulin secretion. The other AMPK activators used, metformin and berberine, yielded essentially similar insulin secretion results.
Pio may also inhibit pyruvate dehydrogenase (PDH), as a similar compound, troglitazone has been reported to acutely inhibit PDH activity in L6 myotubes (36) in an AMPK-independent manner. Inhibition of PDH by Pio could explain the reduced CO2 production from glucose (at 6 mm) in INS 832/13 cells. Finally, glucokinase itself could be implicated in Pio action. AMPK has been shown to block the translocation of glucokinase from nucleus to cytosol in hepatocytes and to inhibit glucose metabolism (55). Considering a similar situation in β-cells, the reduction in glucose metabolism could be due in part to Pio-activated AMPK (either directly or indirectly via mitochondrial metabolism inhibition), reducing flux through glucokinase, glucose oxidation, ATP levels, and GIIS.
To explain the mild effect of Pio at high glucose on both β-cell metabolism and GIIS, we propose that at elevated concentration of the sugar the mitochondrial and AMPK effects of the drug listed above are dampened and overridden due to the push of elevated substrate on metabolic pathways, with gradual reestablishment of energy metabolism and GIIS. An increase in the ATP to ADP ratio acts as a coupling factor between glucose metabolism and insulin secretion, via the closure of ATP-sensitive potassium channels (34). Thus, the right shift in the glucose dose-response response curve observed with Pio can be explained at least in part by a reduction in the glucose-induced increase in cellular ATP.
Besides an action on energy metabolism, the data are compatible with a role of altered lipid metabolism and signaling in Pio action to reduce GIIS. AMPK activation promotes energy-producing catabolic pathways (e.g. β-oxidation) and inhibits energy-consuming anabolic pathways (e.g. complex lipid synthesis) (56). Acute treatment of isolated muscles with troglitazone was shown to stimulate fatty acid oxidation via activation of AMPK (22). However, in the present study, Pio did not enhance fat oxidation in INS 832/13 cells. It is perplexing that despite ACC phosphorylation (which decreases its activity) and anticipated reduced malonyl-CoA levels, acute Pio treatment did not accelerate palmitate oxidation. It is possible that in the β-cell, decreased glucose metabolism induced by Pio and AMPK activation might have resulted in reduced availability of anaplerotic sparkers for Krebs cycle activity, thereby alleviating any AMPK-mediated stimulation of β-oxidation. Thus, optimal operation of the Krebs cycle requires the continuous supply of glucose-derived cycle intermediates via anaplerosis.
We have proposed that GL/FFA cycling plays a role in the lipid amplification of GIIS (43,44,57). Some of the intermediates of this pathway, particularly DAG and fatty acyl-CoA, are thought to stimulate insulin granule exocytosis (58). The extent of GL/FFA cycling can be indirectly assessed by measuring the dynamic pool of GL and lipolysis. Pio decreased palmitate incorporation in DAG, TG, and PL at intermediate (6 mm) glucose. High glucose overcame this inhibition, as for glucose and energy metabolism, and GIIS. GPAT, which catalyzes the first step of GL biosynthesis, is negatively modulated by AMPK (42). Thus, Pio/AMPK-mediated inhibition of GPAT may underlie the action of this TZD on β-cell lipid esterification processes. In addition, because lipid esterification processes require two ATP molecules per fatty-acyl moiety added on the glycerol backbone, the possibility exists that reduced GL synthesis by Pio results from reduced energy production.
Whether Pio also affects the lipolysis segment of GL/FFA cycling was examined by measuring the release of glycerol, an end product of GL hydrolysis. Results showed that Pio markedly inhibited glycerol release in INS 832/13 cells, indicating that it affects GL/FFA cycling because it also inhibited fatty acid esterification. Interestingly, at the lower dose of 10 and 25 μm Pio for which GIIS was normalized at 10 mm glucose, there was evidence of partial recovery of lipolytic activity at this higher concentration of the sugar. Further recovery from Pio inhibition of both lipolysis and GIIS occurred with the addition of palmitate to the incubation medium. This was even observed with the highest Pio dose (50 μm) that, in the absence of palmitate, had completely and almost abolished lipolysis and GIIS, respectively. The very large effect of Pio to reduce lipolysis may be due to inhibition by AMPK of hormone-sensitive lipase (45) and/or adipose triglyceride lipase (46) and lower ATP to ADP and ATP to AMP ratios, which would curtail the esterification arm of the energy-dependent GL/FFA cycling. A significant correlation between glycerol release and GIIS, shown earlier by Winzell et al. (47), is strongly supported by our present results using Pio with and without palmitate. These data, although not proving cause-and-effect relationship, add substantial support to previous studies that have indicated an important role for GL/FFA cycling in GIIS (43,44,57).
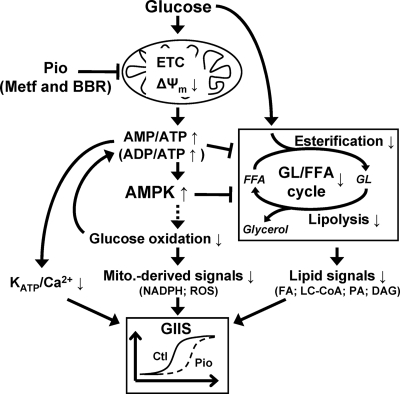
In conclusion and as illustrated in Fig. 6, Pio acutely reduces GIIS only at intermediate concentrations of glucose, without affecting basal or maximal secretion induced by the sugar. Collectively, the data are compatible with the view that this action of the TZD is due to alterations in mitochondrial energy metabolism, ATP production, and the ATP-sensitive potassium channel/Ca2+-triggering pathway of insulin secretion and AMPK activity as well as a reduced activity of the amplification arm of glucose signaling that implicates GL/FFA cycling and lipid metabolites such as DAG.
Figure 6.
Proposed mechanism for Pio’s PPARγ-independent effects in the β-cell. Pio reduces the Δψm, possibly via complex I inhibition in the electron transport chain (ETC), thereby reducing the rate of ATP synthesis. Consequent increase in AMP to ATP ratio may be the cause of the AMPK activation and increased ADP to ATP ratio restrains the closure of ATP-sensitive potassium channels (KATP) and therefore the triggering Ca2+ pathway for GIIS. Reduced glucose oxidation could be the result of ETC inhibition coupled to reduced Krebs cycle activity or a consequence of AMPK activation [via inhibition of glucokinase translocation to plasma membrane (55)] or due to the inhibition of PDH by Pio (36). Inhibition of glucose oxidation is associated with reduced pyruvate cycling and mitochondrial metabolism that generate mitochondria (Mito.)-derived signals such as oxidation of nicotinamide adenine dinucleotide phosphate (reduced form; NADPH) and reactive oxygen species (ROS), instrumental in the amplification pathways for GIIS (29). AMPK activation and possibly reduced ATP to ADP and ATP to AMP ratios inhibit FFA esterification, and AMPK activation inhibits lipolysis (45,46). FFA incorporation into GL and their subsequent lipolysis are parts of the GL/FFA cycle that generates lipid signaling molecules such as free fatty acids (FA), long chain fatty acyl-CoA (LC-CoA), phosphatidic acid (PA), and DAG involved in the amplification pathways for GIIS (43). At high glucose the substrate push counteracts the mild metabolic inhibition of Pio. It is likely that metformin (Metf) and berberine (BBR) affect the β-cell via the same processes stemming from reduced mitochondrial function. Ctl, control.
A direct and PPARγ-independent protection of β-cells by pioglitazone?
Pio concentrations used in this study are higher than the maximal blood concentrations (3–6 μm) reported in humans (59,60), but they are not unreasonable because threshold effects on the various studied processes occurred at 10–25 μm. Even after chronic administration in rats, Pio and its metabolites did not accumulate in plasma and white adipose tissue (61). However, the intracellular distribution of the compound in vivo could be higher than the reported blood values.
The acute effects of Pio on fuel metabolism and GIIS might be beneficial for β-cell function and glucose homeostasis. Pio-mediated reduction of GIIS might prevent hyperinsulinemia, a possible cause for the development of both insulin resistance and T2D (1,62,63). Although it sounds in part counterintuitive to decrease insulin secretion in hyperinsulinemic prediabetic patients or patients with T2D, one must consider that both GIIS in the presence of elevated glucose and fatty acid amplification of GIIS are not affected by Pio. Fatty acid amplification is likely an important characteristic of β-cell hypersecretion during insulin resistance (1). Thus, a right shift in the glucose response curve of insulin secretion likely ensures that the elevated blood FFAs will not lead to excess insulin secretion at low and intermediate glucose concentrations, thereby protecting the β-cell from exhaustion. Thus, the potential harmful effect (endoplasmic reticulum and oxidative stresses) of overstimulating the β-cell in the pathogenesis of T2D (63) and beneficial effect of pausing insulin secretion (64) begin to be recognized. The possibility that TZD might work in part through the prevention of β-cell burnout in the face of a fuel surfeit (57) needs consideration.
Therapeutic implications
Finally, the results suggest that Pio, metformin, and berberine share at least in part common mechanisms as antidiabetic agents because all appear to be AMPK activators and mild mitochondrial inhibitors, cause a right shift in the dose dependence of GIIS, and have been shown to restore glycemic control (65). Thus, we would like to propose that mild mitochondrial inhibition is an attractive avenue of drug development to preserve mitochondria and the functional state of various tissues in the (pre)diabetic/metabolic syndrome glucolipotoxic environment.
Acknowledgments
We thank Dr. Marie-Soleil Gauthier for helpful discussions.
Footnotes
This work was supported by grants from the Canadian Institute of Health Research (to M.P.) and the Canadian Diabetes Association (to M.P.); Grant DK019514-26 from the National Institutes of Health (to N.B.R. and M.P.); and special Program Grant 427695 from the Juvenile Diabetes Research Foundation and National Health and Medical Research Council (to C.J.N.). J.L. is supported by a training award from the Fonds de la Recherche en Santé du Québec and received support from Diabète Québec. M.P. and V.P. hold the Canada Research Chairs in Diabetes and Metabolism and Diabetes and Pancreatic β-Cell Function, respectively.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 30, 2009
Abbreviations: ACC, Acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; DAG, diacylglycerol; DMSO, dimethylsulfoxide; FFA, free fatty acid; GIIS, glucose-induced insulin secretion; GL, glycerolipid; GPAT, glycerol-3-phosphate acyltransferase; KRBH, Krebs-Ringer bicarbonate buffer containing HEPES; Δψm, change in mitochondrial membrane potential; PDH, pyruvate dehydrogenase; Pio, pioglitazone; PL, phospholipid; PPAR, peroxisome proliferator-activated receptor; T2D, type 2 diabetes; TG, triacylglycerol; TZD, thiazolidinedione.
References
- Leahy JL 2005 Pathogenesis of type 2 diabetes mellitus. Arch Med Res 36:197–209 [DOI] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ 2006 Islet β cell failure in type 2 diabetes. J Clin Invest 116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA 1995 An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ). J Biol Chem 270:12953–12956 [DOI] [PubMed] [Google Scholar]
- Semple RK, Chatterjee VK, O'Rahilly S 2006 PPAR γ and human metabolic disease. J Clin Invest 116:581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JM, Dzamko N, Cleasby ME, Hegarty BD, Furler SM, Cooney GJ, Kraegen EW 2004 Direct demonstration of lipid sequestration as a mechanism by which rosiglitazone prevents fatty-acid-induced insulin resistance in the rat: comparison with metformin. Diabetologia 47:1306–1313 [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Shulman GI 2006 Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55(Suppl 2):S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IW, Mariz S 2007 β-Cell preservation with thiazolidinediones. Diabetes Res Clin Pract 76:163–176 [DOI] [PubMed] [Google Scholar]
- Higa M, Zhou YT, Ravazzola M, Baetens D, Orci L, Unger RH 1999 Troglitazone prevents mitochondrial alterations, β cell destruction, and diabetes in obese prediabetic rats. Proc Natl Acad Sci USA 96:11513–11518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H, Takizawa M, Ozawa S, Nakamichi Y, Yamaguchi S, Katsuta H, Tanaka T, Maruyama M, Katahira H, Yoshimoto K, Itagaki E, Nagamatsu S 2004 Pioglitazone improves insulin secretory capacity and prevents the loss of β-cell mass in obese diabetic db/db mice: possible protection of β cells from oxidative stress. Metabolism 53:488–494 [DOI] [PubMed] [Google Scholar]
- Kawasaki F, Matsuda M, Kanda Y, Inoue H, Kaku K 2005 Structural and functional analysis of pancreatic islets preserved by pioglitazone in db/db mice. Am J Physiol Endocrinol Metab 288:E510–E518 [DOI] [PubMed] [Google Scholar]
- Chicco A, Basabe JC, Karabatas L, Ferraris N, Fortino A, Lombardo YB 2000 Troglitazone (CS-045) normalizes hypertriglyceridemia and restores the altered patterns of glucose-stimulated insulin secretion in dyslipidemic rats. Metabolism 49:1346–1351 [DOI] [PubMed] [Google Scholar]
- Diani AR, Sawada G, Wyse B, Murray FT, Khan M 2004 Pioglitazone preserves pancreatic islet structure and insulin secretory function in three murine models of type 2 diabetes. Am J Physiol Endocrinol Metab 286:E116–E122 [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Zhou YT, Lee Y, Unger RH 1998 Troglitazone lowers islet fat and restores β cell function of Zucker diabetic fatty rats. J Biol Chem 273:3547–3550 [DOI] [PubMed] [Google Scholar]
- Vandewalle B, Moerman E, Lefebvre B, Defrance F, Gmyr V, Lukowiak B, Kerr Conte J, Pattou F 2008 PPARγ-dependent and -independent effects of Rosiglitazone on lipotoxic human pancreatic islets. Biochem Biophys Res Commun 366:1096–1101 [DOI] [PubMed] [Google Scholar]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G 2006 Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443 [DOI] [PubMed] [Google Scholar]
- Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, Al-Haddad W, Dhindsa S, Dandona P 2004 Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab 89:2728–2735 [DOI] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W 1996 Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology 137:354–366 [DOI] [PubMed] [Google Scholar]
- Dubois M, Pattou F, Kerr-Conte J, Gmyr V, Vandewalle B, Desreumaux P, Auwerx J, Schoonjans K, Lefebvre J 2000 Expression of peroxisome proliferator-activated receptor γ (PPARγ) in normal human pancreatic islet cells. Diabetologia 43:1165–1169 [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello Russo C 2005 Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol 70:177–188 [DOI] [PubMed] [Google Scholar]
- Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB 2004 Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun 314:580–585 [DOI] [PubMed] [Google Scholar]
- Fryer LG, Parbu-Patel A, Carling D 2002 The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 277:25226–25232 [DOI] [PubMed] [Google Scholar]
- LeBrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, Tomas E 2006 Thiazolidinediones can rapidly activate AMP-activated protein kinase (AMPK) in mammalian tissues. Am J Physiol Endocrinol Metab 291:E175–E181 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhou L, Shao L, Qian L, Fu X, Li G, Luo T, Gu Y, Li F, Li J, Zheng S, Luo M 2007 Troglitazone acutely activates AMP-activated protein kinase and inhibits insulin secretion from β cells. Life Sci 81:160–165 [DOI] [PubMed] [Google Scholar]
- Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhäusl W, Fürnsinn C 2004 Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes 53:1052–1059 [DOI] [PubMed] [Google Scholar]
- Ruderman N, Prentki M 2004 AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 3:340–351 [DOI] [PubMed] [Google Scholar]
- Roduit R, Nolan C, Alarcon C, Moore P, Barbeau A, Delghingaro-Augusto V, Przybykowski E, Morin J, Massé F, Massie B, Ruderman N, Rhodes C, Poitout V, Prentki M 2004 A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes 53:1007–1019 [DOI] [PubMed] [Google Scholar]
- Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB 2000 Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49:424–430 [DOI] [PubMed] [Google Scholar]
- Gotoh M, Maki T, Satomi S, Porter J, Bonner-Weir S, O'Hara CJ, Monaco AP 1987 Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation 43:725–730 [DOI] [PubMed] [Google Scholar]
- Guay C, Madiraju SR, Aumais A, Joly E, Prentki M 2007 A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J Biol Chem 282:35657–35665 [DOI] [PubMed] [Google Scholar]
- Folch J, Ascoli I, Lees M, Meath JA, LeBaron N 1951 Preparation of lipide extracts from brain tissue. J Biol Chem 191:833–841 [PubMed] [Google Scholar]
- Peyot ML, Nolan CJ, Soni K, Joly E, Lussier R, Corkey BE, Wang SP, Mitchell GA, Prentki M 2004 Hormone-sensitive lipase has a role in lipid signaling for insulin secretion but is nonessential for the incretin action of glucagon-like peptide 1. Diabetes 53:1733–1742 [DOI] [PubMed] [Google Scholar]
- Blumentrath J, Neye H, Verspohl EJ 2001 Effects of retinoids and thiazolidinediones on proliferation, insulin release, insulin mRNA, GLUT 2 transporter protein and mRNA of INS-1 cells. Cell Biochem Funct 19:159–169 [DOI] [PubMed] [Google Scholar]
- Bollheimer LC, Troll S, Landauer H, Wrede CE, Schölmerich J, Buettner R 2003 Insulin-sparing effects of troglitazone in rat pancreatic islets. J Mol Endocrinol 31:61–69 [DOI] [PubMed] [Google Scholar]
- Prentki M 1996 New insights into pancreatic β-cell metabolic signaling in insulin secretion. Eur J Endocrinol 134:272–286 [DOI] [PubMed] [Google Scholar]
- Fürnsinn C, Brunmair B, Neschen S, Roden M, Waldhäusl W 2000 Troglitazone directly inhibits CO(2) production from glucose and palmitate in isolated rat skeletal muscle. J Pharmacol Exp Ther 293:487–493 [PubMed] [Google Scholar]
- Fediuc S, Pimenta AS, Gaidhu MP, Ceddia RB 2008 Activation of AMP-activated protein kinase, inhibition of pyruvate dehydrogenase activity, and redistribution of substrate partitioning mediate the acute insulin-sensitizing effects of troglitazone in skeletal muscle cells. J Cell Physiol 215:392–400 [DOI] [PubMed] [Google Scholar]
- Rutter GA, Leclerc I 2009 The AMP-regulated kinase family: enigmatic targets for diabetes therapy. Mol Cell Endocrinol 297:41–49 [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE 2001 Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB 2006 Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55:2256–2264 [DOI] [PubMed] [Google Scholar]
- Carling D, Zammit VA, Hardie DG 1987 A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett 223:217–222 [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG 1996 Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol 270:E299–E304 [DOI] [PubMed] [Google Scholar]
- Muoio DM, Seefeld K, Witters LA, Coleman RA 1999 AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J 338(Pt 3):783–791 [PMC free article] [PubMed] [Google Scholar]
- Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M 2006 Fatty acid signaling in the β-cell and insulin secretion. Diabetes 55(Suppl 2):S16–S23 [DOI] [PubMed] [Google Scholar]
- Prentki M, Madiraju SR 2008 Glycerolipid metabolism and signaling in health and disease. Endocr Rev 29:647–676 [DOI] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE, Febbraio MA 2006 Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 290:E500–E508 [DOI] [PubMed] [Google Scholar]
- Narbonne P, Roy R 2009 Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature 457:210–214 [DOI] [PubMed] [Google Scholar]
- Winzell MS, Strom K, Holm C, Ahren B 2006 Glucose-stimulated insulin secretion correlates with β-cell lipolysis. Nutr Metab Cardiovasc Dis 16(Suppl 1):S11–S16 [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Leahy JL, Delghingaro-Augusto V, Moibi J, Soni K, Peyot ML, Fortier M, Guay C, Lamontagne J, Barbeau A, Przybytkowski E, Joly E, Masiello P, Wang S, Mitchell GA, Prentki M 2006 β Cell compensation for insulin resistance in Zucker fatty rats: increased lipolysis and fatty acid signalling. Diabetologia 49:2120–2130 [DOI] [PubMed] [Google Scholar]
- Mulder H, Yang S, Winzell MS, Holm C, Ahrén B 2004 Inhibition of lipase activity and lipolysis in rat islets reduces insulin secretion. Diabetes 53:122–128 [DOI] [PubMed] [Google Scholar]
- Kim SH, Abbasi F, Chu JW, McLaughlin TL, Lamendola C, Polonsky KS, Reaven GM 2005 Rosiglitazone reduces glucose-stimulated insulin secretion rate and increases insulin clearance in nondiabetic, insulin-resistant individuals. Diabetes 54:2447–2452 [DOI] [PubMed] [Google Scholar]
- Konrad D, Rudich A, Bilan PJ, Patel N, Richardson C, Witters LA, Klip A 2005 Troglitazone causes acute mitochondrial membrane depolarisation and an AMPK-mediated increase in glucose phosphorylation in muscle cells. Diabetologia 48:954–966 [DOI] [PubMed] [Google Scholar]
- Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, Hu LH, Li J, Ye JM 2008 Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 57:1414–1418 [DOI] [PubMed] [Google Scholar]
- Rutter GA 2001 Nutrient-secretion coupling in the pancreatic islet β-cell: recent advances. Mol Aspects Med 22:247–284 [DOI] [PubMed] [Google Scholar]
- Wang CZ, Wang Y, Di A, Magnuson MA, Ye H, Roe MW, Nelson DJ, Bell GI, Philipson LH 2005 5-amino-imidazole carboxamide riboside acutely potentiates glucose-stimulated insulin secretion from mouse pancreatic islets by KATP channel-dependent and -independent pathways. Biochem Biophys Res Commun 330:1073–1079 [DOI] [PubMed] [Google Scholar]
- Mukhtar MH, Payne VA, Arden C, Harbottle A, Khan S, Lange AJ, Agius L 2008 Inhibition of glucokinase translocation by AMP-activated protein kinase is associated with phosphorylation of both GKRP and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Am J Physiol Regul Integr Comp Physiol 294:R766–R774 [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D 1997 The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur J Biochem 246:259–273 [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Prentki M 2008 The islet β-cell: fuel responsive and vulnerable. Trends Endocrinol Metab 19:285–291 [DOI] [PubMed] [Google Scholar]
- Kwan EP, Xie L, Sheu L, Nolan CJ, Prentki M, Betz A, Brose N, Gaisano HY 2006 Munc13–1 deficiency reduces insulin secretion and causes abnormal glucose tolerance. Diabetes 55:1421–1429 [DOI] [PubMed] [Google Scholar]
- Budde K, Neumayer HH, Fritsche L, Sulowicz W, Stompôr T, Eckland D 2003 The pharmacokinetics of pioglitazone in patients with impaired renal function. Br J Clin Pharmacol 55:368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripalakit P, Neamhom P, Saraphanchotiwitthaya A 2006 High-performance liquid chromatographic method for the determination of pioglitazone in human plasma using ultraviolet detection and its application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 843:164–169 [DOI] [PubMed] [Google Scholar]
- Fujita Y, Yamada Y, Kusama M, Yamauchi T, Kamon J, Kadowaki T, Iga T 2003 Sex differences in the pharmacokinetics of pioglitazone in rats. Comp Biochem Physiol C Toxicol Pharmacol 136:85–94 [DOI] [PubMed] [Google Scholar]
- Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J 2008 Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 31(Suppl 2):S262–S268 [DOI] [PubMed] [Google Scholar]
- Aston-Mourney K, Proietto J, Morahan G, Andrikopoulos S 2008 Too much of a good thing: why it is bad to stimulate the beta cell to secrete insulin. Diabetologia 51:540–545 [DOI] [PubMed] [Google Scholar]
- Sargsyan E, Ortsäter H, Thorn K, Bergsten P 2008 Diazoxide-induced β-cell rest reduces endoplasmic reticulum stress in lipotoxic β-cells. J Endocrinol 199:41–50 [DOI] [PubMed] [Google Scholar]
- Yin J, Xing H, Ye J 2008 Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 57:712–717 [DOI] [PMC free article] [PubMed] [Google Scholar]