Abstract
A collection of neurons in the upper lumbar spinal cord of male rats projects to the lower lumbar spinal cord, releasing gastrin-releasing peptide (GRP) onto somatic and autonomic centers known to regulate male sexual reflexes such as erection and ejaculation. Because these reflexes are androgen dependent, we asked whether manipulating levels of androgen in adult rats would affect GRP expression in this spinal center. We found that castration resulted, 28 d later, in a profound decrease in the expression of GRP in the spinal cord, as reflected in immunocytochemistry and competitive ELISA for the protein as well as real-time quantitative PCR for the transcript. These effects were prevented if the castrates were treated with testosterone propionate. Genetically male (XY) rats with the dysfunctional testicular feminization allele for the androgen receptor (AR) displayed GRP mRNA and protein levels in the spinal cord similar to those of females, indicating that androgen normally maintains the system through AR. We saw no effect of castration or the testicular feminization allele on expression of the receptor for GRP in the spinal cord, but castration did reduce expression of AR transcripts within the spinal cord as revealed by real-time quantitative PCR and Western blots. Taken together, these results suggest that androgen signaling plays a pivotal role in the regulation of GRP expression in male lumbar spinal cord. A greater understanding of how androgen modulates the spinal GRP system might lead to new therapeutic approaches to male sexual dysfunction.
Androgen signaling plays a pivotal role in the regulation of gastrin-releasing peptide expression in male lumbar spinal cord.
In mammals, some sex differences are the result of testicular steroid hormones that masculinize the central nervous system at several stages of ontogeny. Early in life, androgens such as testosterone, which induce the external and internal genitalia to develop a masculine form, also masculinize the developing nervous system, sometimes permanently altering neural populations and synaptic connections (1,2). In these instances, androgens are said to organize the nervous system in a masculine fashion (3). At maturity, gonadal steroids again act on the nervous system to regulate behavior: androgens potentiate masculine copulatory behaviors, whereas ovarian steroids such as estrogen and progesterone promote feminine behaviors. In these cases, gonadal steroids are said to activate masculine or feminine behaviors. When androgens organize and activate the body in a masculine fashion, they usually do so by acting through androgen receptors (ARs). However, in the rodent brain, testosterone is sometimes aromatized into estrogens, which then organize and activate neural circuits by acting through estrogen receptors (ERs; ERα/ERβ). Interestingly, androgenic organization and activation of spinal cord centers related to sexual behavior seem to be mediated by ARs rather than ERs. For example, one spinal center mediating male sexual reflexes is the spinal nucleus of the bulbocavernsous (SNB), which innervates striated muscles attached to the base of the penis. These muscles are known to mediate reflexive erections and flips of the penis (4), which are eliminated by castration but return in response to exogenous treatment with androgens such as testosterone or the nonaromatizable dihydrotestosterone but not to estrogens (5,6). Likewise, the SNB system responds both during development (7,8) and in adulthood to dihydrotestosterone (9). Furthermore, there is also evidence that androgen acts directly on the spinal cord to activate masculine copulatory behaviors such as erection and ejaculation (10), but little is known about spinal neurons other than the SNB that might respond to androgen to promote these behaviors.
We recently found evidence that gastrin-releasing peptide (GRP), a member of the bombesin-like peptide family first discovered in the skin of the frog Bombina bombina (11,12), mediates spinal centers promoting male copulatory reflexes. GRP is distributed widely in the central nervous system and gastrointestinal tract of mammals (13). GRP plays a role in many physiological processes, including food intake (14), circadian rhythms (15), itch (16), and anxiety (17). We demonstrated that neurons within the ejaculation generator (18) in the upper lumbar spinal cord [lumbar segments (L) 3 and 4] project axons containing GRP to the lower lumbar spinal cord (L5-6), innervating the SNB and autonomic regions that are also known to control erection and ejaculation (19). All these target regions express the specific receptor for GRP (GRP-R). Remarkably, pharmacological stimulation of GRP-Rs restores penile reflexes and ejaculation rate in castrated male rats, and antagonistic blockage of GRP-Rs via intrathecal catheters to this spinal region significantly attenuates penile reflexes and ejaculation rate in normal male rats (19). Thus, this system of neurons in the upper lumbar spinal cord uses a specific peptide, GRP, to drive lower spinal centers that coordinate male reproductive functions such as erection and ejaculation (19).
Because the spinal GRP system seems to mediate masculine sexual reflexes and because these reflexes are dependent on androgen in adulthood, fading after castration and returning with androgen replacement, we speculated that androgens may activate spinal reflex centers by modulating the GRP system (19). Thus, we asked whether androgen signaling might regulate GRP expression both at the mRNA and protein levels in the lumbar spinal cord of adult male rats. We quantified the expression level of GRP in separate regions of the lumbar spinal cord by dividing the lumbar spinal cord into upper (L3-4; somal region of GRP-secreting neurons for detection at the mRNA level) and lower (L5-6; axonal region of GRP synaptic terminals for detection at the protein level) regions. First, to probe the impact of androgen on the maintenance of the GRP system in the lumbar spinal cord during adulthood, we investigated long-term castrates and the response to testosterone propionate (TP). To clarify whether such effects were mediated by AR, we also used genetically male (XY) rats carrying the testicular feminization mutation (Tfm) of the AR gene. These mutants develop testes embryologically and secrete testosterone prenatally but, because their AR protein is dysfunctional, develop a wholly feminine exterior phenotype, including a clitoris rather than a penis (20,21,22,23,24). These two different rat models demonstrate that androgen signaling through ARs regulates GRP expression both at the mRNA and protein levels in the male lumbar spinal cord. We conclude that androgen signaling plays a pivotal role in the regulation of spinal GRP expression in males and that this represents one of the myriad ways in which androgens activate masculine copulatory behavior.
Materials and Methods
Animals
For experiments probing the role of the AR, adult male testicular feminization mutant (Tfm) Long Evans (LE) rats and littermate wild-type (Wt) males and females bred in the colony at Michigan State University were examined. In all other studies, adult Wt Sprague Dawley (SD) rats (Shimizu Laboratory Supplies, Kyoto, Japan) were used in this study. All rats were maintained on a 12-h light, 12-h dark cycle (lights on at 0600 h and off at 1800 h), same-sex group housed, and provided with unlimited access to water and rodent chow. All experimental procedures were authorized by the Committee for Animal Research, Kyoto Prefectural University of Medicine, Japan, and/or the Institutional Animal Care and Use Committee of Michigan State University.
Surgery
Rats were anesthetized with ip injections of 50 mg/kg body weight sodium pentobarbital and bilaterally gonadectomized (male; GDX), and a TP pellet (100 mg/pellet, 60 d release; Innovative Research of America, Sarasota, FL) or empty control pellet (placebo) was sc implanted into the interscapular region (GDX + TP, GDX + placebo). Sham surgeries (sham) were also performed under sodium pentobarbital anesthesia. Twenty-eight days after surgery, rats were killed by decapitation for ELISA and real-time quantitative PCR or perfusion fixed for immunocytochemistry (ICC) analysis under deep anesthesia with sodium pentobarbital.
Immunocytochemistry and immunofluorescence
Rats were deeply anesthetized with ip injections of sodium pentobarbital, and transcardially perfused with physiological saline followed by 4% paraformaldehyde in 0.1 m phosphate buffer. Lumbar spinal cords were quickly removed and immersed in the same fixative overnight at 4 C. After immersion in 25% sucrose in 0.1 m phosphate buffer at 4 C for cryoprotection until they sank, the preparations were quickly frozen using powdered dry ice and cut in 30-μm-thick cross or horizontal sections on a cryostat (CM3050 S; Leica, Nussloch, Germany). We performed the ICC analysis according to established methods (19). In brief, endogenous peroxidase activity was eliminated from the sections by incubation in a 1% H2O2 absolute methanol solution for 30 min followed by three 5-min rinses with PBS (pH 7.4). These processes were omitted for the immunofluorescence method. After blocking nonspecific binding components with 1% normal goat serum and 1% BSA in PBS containing 0.3% Triton X-100 for 1 h at room temperature, the sections were incubated with the primary rabbit antiserum against GRP (1:5000; Phoenix Pharmaceuticals, Burlingame, CA) for 48 h at 4 C. Immunoreactive products were detected with a streptavidin-biotin kit (Nichirei, Tokyo, Japan), followed by diaminobenzidine development according to our previous method (19). Control procedures consisted of preabsorbing the working dilution (1:5000) of the primary antiserum with a saturating concentration of GRP antigen peptide (porcine GRP; 10 μg/ml) overnight at 4 C before use and substituting primary serum for the normal rabbit antiserum at the same dilution (1:5000) (data not shown). GRP-immunoreactive cells in the spinal cord were localized using an optical BH-2 microscope (Olympus, Tokyo, Japan).
To determine the effect of gonadectomy on the projection site of GRP-immunoreactive axons, double-immunofluorescence staining of GRP and neuronal nitric oxide synthase (nNOS) (A-11, mouse monoclonal antibody; Santa Cruz Biotechnology, Santa Cruz, CA) (1:8000 dilution), a marker protein for neurons in the sacral parasympathetic nucleus (SPN), was performed as described previously (19).
For quantitative analysis, GRP-immunoreactive cells with clearly visible round nuclear profiles were counted in the anterior part of the lumbar spinal cord (L3-4 level). To determine the OD of positive GRP-immunoreactive fibers in the SPN, at least six sections per animals were analyzed using ImageJ software (ImageJ 1.36b; National Institutes of Health, Bethesda, MD) with a set threshold level. GRP-immunoreactive fiber OD was quantified as the average OD in the SPN and was calculated as the ratio to the OD of nNOS immunoreactivity in the same GRP/nNOS-double immunostained sections. At least three animals were used for these analyses in each group.
Peptide extraction and ELISA
Rats were killed by decapitation under deep anesthesia with sodium pentobarbital. Lower lumbar spinal cords (L5-6) were quickly removed on ice, weighed, snap frozen immediately in liquid nitrogen, and used for peptide extraction. Peptides were extracted according to our previous methods (25). Frozen tissues were homogenized in 5% acetic acid using a disposable homogenizer (BioMasher; Nippi, Tokyo, Japan) and boiled for 10 min. The homogenate was centrifuged at 15,000 × g for 10 min at 4 C. The supernatant was collected in a tube, and the precipitate was again homogenized and centrifuged. The two supernatants were pooled and forced through a disposable C-18 cartridge (SPE, 1 ml to 100 mg; SILICYCLE, Québec, Canada). The retained material was then eluted with 60% methanol. The elute was concentrated in a vacuum centrifuge and subjected to competitive ELISA specific for GRP using a kit for GRP (Phoenix Pharmaceuticals) according to the manufacturer’s protocol. The concentration of GRP was calculated in terms of picomoles per gram wet weight (picomoles per gram tissue) of each spinal cord. We included the standard curve in each experiment.
Real-time quantitative PCR
Rats were killed by decapitation under deep anesthesia with sodium pentobarbital. Lumbar spinal cords (L3-4 for GRP and AR, L5-6 for GRP-R) were quickly removed on ice, weighed, snap frozen immediately in liquid nitrogen, and used for RNA extraction. Total RNA was extracted with TRIzol LS reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Oligo-deoxythymidine-primed cDNA synthesis was carried out with SuperScript III reverse transcriptase (Invitrogen). To quantify copies of GRP, GRP-R, and AR transcripts in the lumbar spinal cord, cDNAs from the lumbar spinal cord were subjected to real-time PCR, which was performed in 15-μl reaction volumes consisting of 1× TaqMan universal PCR master mix (Applied Biosystems, Foster City, CA), 300 nm each of forward and reverse primer, 200 nm of TaqMan probe by using an ABI Prism 7900 sequence detection system (Applied Biosystems). We used TaqMan real-time PCR methodology for rat GRP, GRP-R, AR, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; gene expression assays no. Rn00592059, Rn00562292, Rn00560747, and Rn99999916; amplicon lengths 75, 119, 75, and 87, respectively). Amplification was carried out at 50 C for 2 min, 95 C for 10 min, 40 cycles of 95 C for 15 sec, and 60 C for 1 min. The absolute amounts of transcripts were determined by using several concentrations of standard cDNA (100, 10, 1, 0.1, 0.01, and 0.001 ng) that were reverse transcribed from whole lumbar spinal cord of Wt naive males. The expression in each reaction was normalized by the expression of GAPDH as an internal control. We performed quantitative PCR analyses by triplicates in each sample, independently at least five times and included the standards in each experiment.
Western blotting
Western blot analyses were conducted as previously described (26,27). Rats were killed by decapitation under deep anesthesia with sodium pentobarbital. Lower lumbar spinal cords (L5-6) were quickly removed on ice, weighed, immersed in four volumes of PBS, and lysed with five volumes of sample buffer containing 200 mm Tris-HCl, 4% sodium dodecyl sulfate, 20% glycerol, 10% 2-mercaptoethanol, and 0.005% bromophenol blue, and then the lysates were boiled for 5 min. The lysates (5 μl) were run on a 7.5% SDS-PAGE. Samples were electroblotted onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA) from the gel by a semidry blotting apparatus (Bio-Rad Laboratories, Hercules, CA). The blotted membrane was blocked with 5% skim milk and 0.05% Tween 20 in Tris-buffered saline (TBST) for 30 min at room temperature and then incubated with the affinity purified rabbit polyclonal antibody raised against the third cytoplasmic domain of rat/mouse GRP-R (1:2000) (GTX78155; GeneTex, San Antonio, TX) at 4 C overnight. Blots were washed three times with TBST and incubated with horseradish peroxidase-conjugated goat antirabbit IgG second antibody (1:1000) (Vector Laboratories, Burlingame, CA) for 2 h at room temperature. After being washed three times with TBST, blots were visualized by an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Uppsala, Sweden). After stripping the GRP-R antibody with the buffer containing 62.5 mm Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate, and 0.8% 2-mercaptoethanol, blots were then subjected to GAPDH (1:5000, 6C5; Abcam, Cambridge, MA) immunoreactions as the internal control to normalize the expression of the GRP-R protein. Control procedures consisted of substituting normal rabbit serum for the primary antiserum at the same dilution, indicating no specific reactions (data not shown). Results were quantified by densitometric analysis using ImageJ software (ImageJ 1.36b) and were expressed as the OD for ratio to each GAPDH expression.
Enzyme immunoassay (EIA)
For EIA of testosterone, blood was collected from the cardiac left ventricle before perfusion or the trunk blood after decapitation, and blood plasma was stored at −80 C until assay. Extraction of the steroid hormones was performed as described previously (28). To measure the testosterone concentration, aliquots of plasma extracts were assayed in a testosterone EIA by using a testosterone EIA kit (Cayman Chemical, Ann Arbor, MI) as described previously (28).
Statistics
All values are expressed as mean ± sem, and comparisons were made by a one-way ANOVA with n = number of animals per group. If significance was reached with the ANOVA test, the analysis was followed by a post hoc Tukey honestly significant difference test. Plasma concentrations of testosterone in the sham-operated, GDX + placebo and GDX + TP groups were analyzed by a Kruskal-Wallis test, followed by a Dunn’s multiple comparison test. P < 0.05 was considered statistically significant.
Results
Effects of castration and long-term TP replacement on GRP expression in the lumbar spinal cord
ICC analysis of GRP
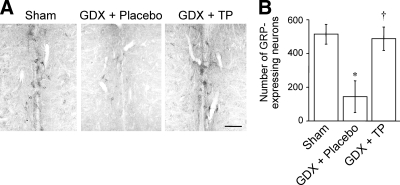
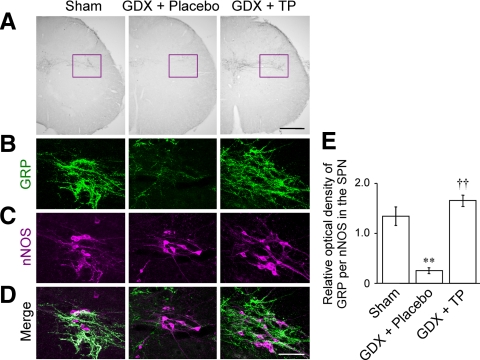
We first examined the effects of gonadectomy and long-term androgen replacement on GRP expression in the upper lumbar spinal cord (L3–4, containing GRP synthesizing neuronal somata) and lower lumbar spinal cord (L5–6; containing axon terminals of the GRP neurons) of males. In the upper lumbar spinal cord, the intensity of GRP-immunoreactive somata and neuropil labeling in the castrates was qualitatively reduced compared with sham males (n = 5 each) 28 d after surgery (Fig. 1A). TP treatment completely blocked the effects of gonadectomy on GRP labeling in the upper lumbar spinal cord (n = 5) (Fig. 1A). Similar changes in the number of GRP-expressing neurons were seen (n = 3) (Fig. 1B) (F2,6 = 7.55, sham vs. GDX + placebo, P < 0.05; GDX + placebo vs. GDX + TP, P < 0.05). Consonant with these changes in the GRP somata, the intensity of GRP-immunoreactive fibers in the lower lumbar spinal cord was greater in gonadally intact males than castrated males in the autonomic centers of the dorsal gray commissure and SPN but not in the somatic sensory layers of the dorsal horn (n = 5 each) (Fig. 2A). Again, androgen replacement prevented the decline in these immunoreactive fibers in the lower lumbar spinal cord caused by castration. Double immunofluorescence for GRP and nNOS confirmed that these changes were evident in the SPN (Fig. 2, B–D). Quantitative analysis of GRP immunoreactivity in the SPN also revealed that castrates displayed a significantly lower OD than sham male controls and the GDX + TP-treated group (n = 3) (Fig. 2E) (F2,6 = 44.97, sham vs. GDX + placebo, P < 0.01; GDX + placebo vs. GDX + TP, P < 0.01).
Figure 1.
ICC staining for GRP in the upper lumbar spinal cord (L3-4) of adult male rats. The density (A) and number (B) of GRP-immunoreactive neurons was markedly decreased 28 d after castration (GDX + placebo) but was averted by androgen replacement with TP (GDX + TP) in the upper lumbar spinal cord (L3-4). Scale bar, 100 μm. *, P < 0.05 vs. sham; †, P < 0.05 vs. GDX + placebo.
Figure 2.
Distribution of GRP immunoreactivity in the lower lumbar spinal cord (L5-6) of adult male rats. A, ICC revealed an androgen effect on GRP-immunoreactive fiber distribution in lower lumbar spinal cord autonomic nuclei because sham males have more GRP-immunoreactive fibers in the SPN (magenta inset) and dorsal gray commissure but not the dorsal horn than do castrates 28 d after surgery (GDX + placebo). The effect was averted by androgen replacement with TP (GDX + TP). Gonadectomy markedly decreased the distribution of GRP-immunoreactive fibers within the SPN (B) but did not affect expression of nNOS (C), a marker for autonomic preganglionic neurons. Double ICC reveals close appositions of GRP-containing fibers with the cell bodies and proximal dendrites of nNOS-positive neurons in the SPN (D). Androgen replacement prevented the decline in these immunoreactive fibers in the SPN caused by castration (B–D). The quantitative analysis also confirmed androgen response in the SPN (E). Scale bars, 200 μm (A); 100 μm (D). **, P < 0.01 vs. sham; ††, P < 0.01 vs. GDX + placebo.
Real-time quantitative PCR analysis of GRP mRNA
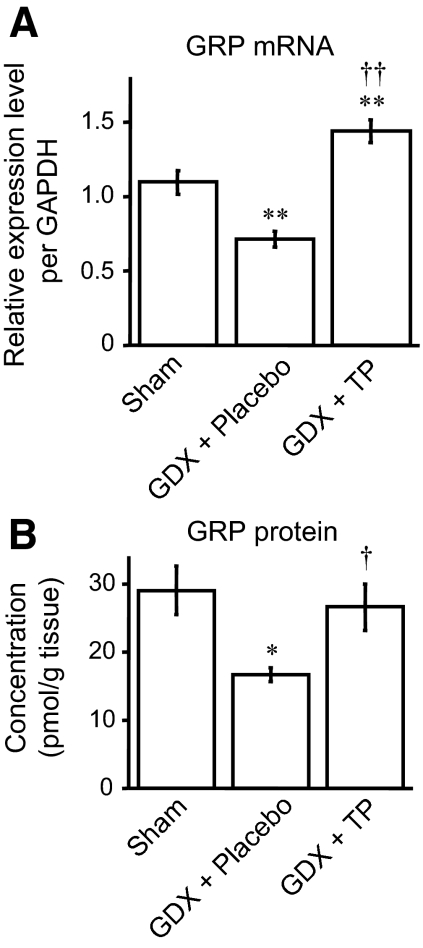
Using real-time quantitative PCR, we compared the expression level of GRP transcripts in sham (n = 8), GDX + placebo (n = 6), and GDX + TP animals (n = 7). Mirroring the results obtained from ICC analysis, the expression level of GRP mRNA 28 d after GDX was significantly reduced from gonadally intact levels (F2,18 = 29.36, P < 0.01 vs. sham), an effect that was averted by TP treatment of castrates (Fig. 3A). In fact, the expression level of GRP mRNA in the GDX + TP-treated group was significantly higher than the sham operated control males (Fig. 3A) (P < 0.01 vs. sham), perhaps responding to the supraphysiological level of androgen in TP-treated castrates in this study.
Figure 3.
Effects of castration on GRP expression in the lumbar spinal cord (L3-4) of male rats. Both real-time quantitative PCR (A) and competitive ELISA (B) revealed that the expression level of GRP mRNA and protein 28 d after gonadectomy (GDX + placebo) was significantly reduced from that seen in sham-operated males (sham), whereas TP treatment of castrates (GDX + TP) maintained GRP expression to the level seen in sham-operated males. Strikingly, although there was a significant difference between sham and GDX + TP groups at the mRNA level, no significant difference of those was observed at the protein level. *, P < 0.05 vs. sham; **, P < 0.01; †, P < 0.05 vs. GDX + placebo; ††, P < 0.01.
Competitive ELISA analysis of GRP protein
We next used ELISA to examine the local concentration of GRP at the protein level (n = 5 in each group). Protein levels substantially mirrored the effects on GRP mRNA because castration of adult males significantly reduced local content of GRP in the lumbar spinal cord 28 d later, and these responses were prevented by long-term androgen replacement (Fig. 3B) (F2,12 = 7.08, P < 0.05 vs. sham; P < 0.05 vs. GDX + placebo). Strikingly, although there was a significant difference between sham and GDX + TP groups at the mRNA level, no significant difference of those was observed at the protein level.
AR involvement in the expression of GRP in the lumbar spinal cord revealed by Tfm rat model
Real-time quantitative PCR and competitive ELISA analyses of GRP
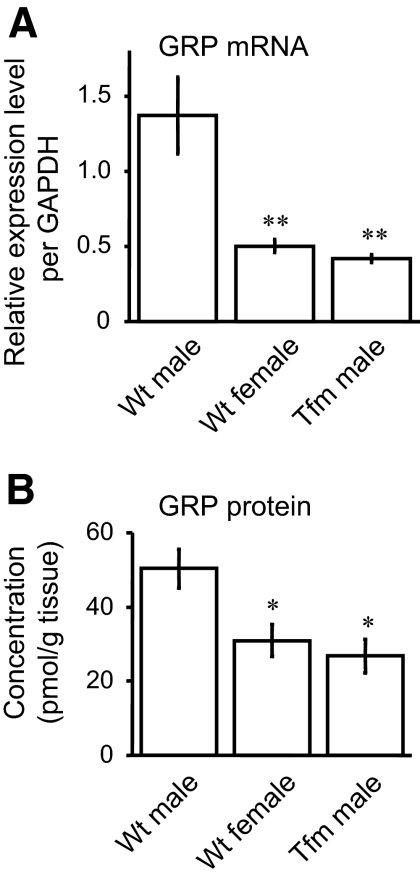
To probe whether AR mediates androgenic regulation of spinal GRP expression, similar analyses were conducted examining both mRNA and protein levels in the lumbar spinal cord of Tfm male rats. First, real-time quantitative PCR confirmed more GRP transcripts in this region in Wt males (n = 6) than Wt females (n = 6) and revealed that genetic male rats carrying the dysfunctional Tfm allele for AR (n = 7) have a female-typical level of GRP mRNA (Fig. 4A) (F2,16 = 11.88, P < 0.01 vs. Wt males). Competitive ELISA demonstrated that the local content of GRP protein was also greater in Wt males than Tfm males (n = 6 in each group) (Fig. 4B) (F2,15 = 10.58, P < 0.05 vs. Wt males).
Figure 4.
AR regulation of GRP expression in the lumbar spinal cord (L3-4) of adult rats. Both real-time quantitative PCR (A) and competitive ELISA (B) revealed more GRP transcripts in this region of Wt males than Wt females or genetic male rats carrying the Tfm allele for AR, which produces a dysfunctional protein. *, P < 0.05 vs. Wt males; **, P < 0.01.
Circulating testosterone level
Plasma concentrations of testosterone were 9.45 ± 1.92, 0.93 ± 0.20, and 15.86 ± 5.77 nmol/liter in the sham-operated (n = 8), GDX + placebo (n = 6), and GDX + TP groups (n = 7), respectively (H = 12.84). Castration significantly reduced circulating testosterone (sham vs. GDX + placebo, P < 0.01). The level of androgen after TP administration to castrated animals was slightly higher than in sham males with greater variance (sham vs. GDX + TP, P < 0.05; GDX + placebo vs. GDX + TP, P < 0.01). Plasma concentrations of testosterone were 15.74 ± 1.47, 0.43 ± 0.30, and 13.06 ± 2.00 nmol/liter in the Wt males (n = 10), Wt females (n = 10), and Tfm males (n = 10), respectively (F2,27 = 31.93, Wt males vs. Wt females, Wt females vs. Tfm males, P < 0.01), confirming several reports that T levels in Tfm male rats are similar to that of Wt males (29).
AR involvement in the expression of GRP-R in the lower lumbar spinal cord revealed by the Tfm rat model
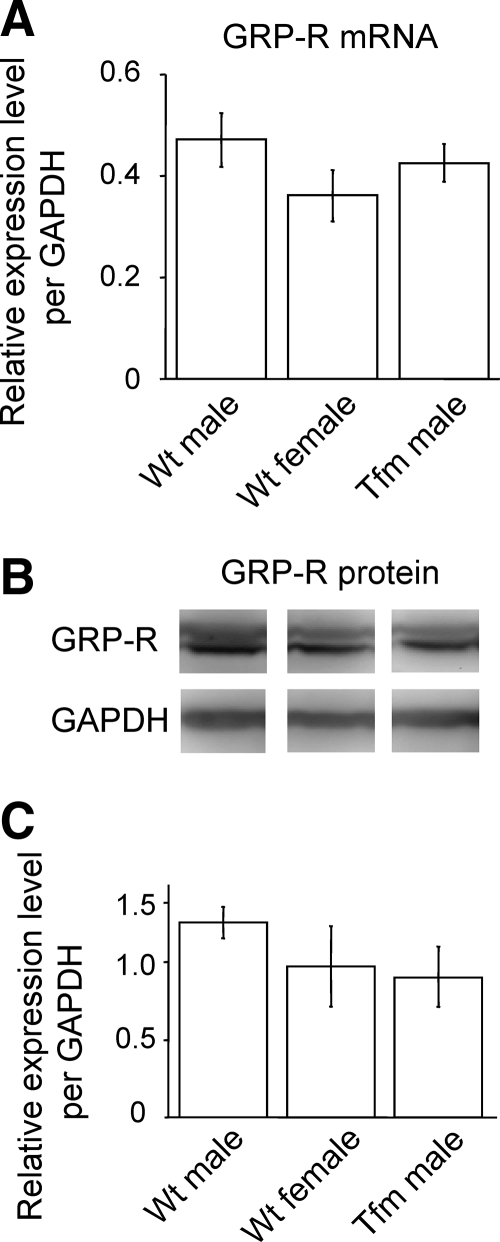
Neurons within the ejaculation generator in the upper lumbar spinal cord (L3–4) (18) project axons containing GRP to the lower lumbar spinal cord (L5–6), innervating autonomic and somatic neural regions known to control erection and ejaculation, and all these target neurons express GRP-R (19). Therefore, we compared the GRP-R mRNA expression by using real-time quantitative PCR and GRP-R protein level using Western blotting in this spinal region (L5–6) of Wt males, Wt females, and Tfm males.
Real-time quantitative PCR analysis of GRP-R mRNA
Using real-time quantitative PCR, we first compared the expression level of GRP-R transcripts in Wt males (n = 6), Wt females (n = 6), and Tfm males (n = 7) in the lower spinal cord (L5-6). GRP-R mRNA levels were not significantly affected by the Tfm mutation, remaining only slightly below that seen in Wt males (Fig. 5A) (F2,16 = 1.63).
Figure 5.
The expression of GRP-R in the lower lumbar spinal cord (L5-6) of adult rats. Expression of GRP-R was not significantly modulated by the Tfm mutation at either the mRNA (A) or protein levels (B and C).
Western blot analysis of GRP-R protein
To measure levels of GRP-R protein, we performed Western blot analysis in this spinal region of Wt males (n = 4), Wt females (n = 4), and Tfm males (n = 4). In the lanes that were loaded with lysates of the lower lumbar spinal cords (L5–6), specific immunoreactive bands were detected, corresponding to GRP-R (∼55 kDa) (Fig. 5B). For densitometric analysis, we calculated the expression levels of GRP-R by dividing these values by that of GAPDH as the internal control. The average expression level was less in Wt females and Tfm males compared with Wt males, but the difference was not statistically significant (Fig. 5C) (F2,9 = 2.38).
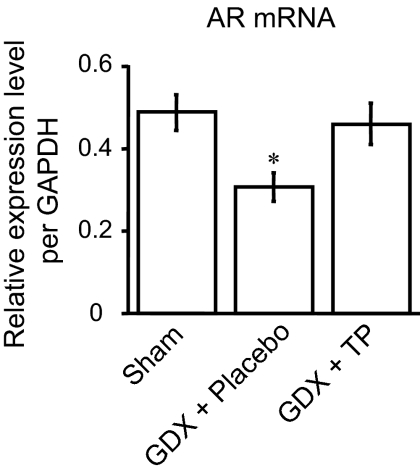
Androgen regulation of AR mRNA expression in the upper lumbar spinal cord
By means of real-time quantitative PCR analysis, we investigated whether androgen might modulate the expression of the AR gene in this spinal region, using the castration/androgen replacement model. Long-term gonadectomy of adult male rats (n = 6) significantly reduced the expression of AR mRNA compared with the sham operated males (n = 8) in the upper lumbar spinal cord (F2,18 = 4.71, P < 0.05 vs. sham), and the reduction was also averted by TP replacement (n = 7) (Fig. 6).
Figure 6.
Real-time quantitative PCR of the upper lumbar spinal cord (L3-4) revealed that gonadectomy of adult male rats (GDX + placebo) significantly reduced the expression of AR mRNA compared with sham-operated males (sham) 28 d later, and this reduction was averted by androgen replacement with TP (GDX + TP). *, P < 0.05 vs. sham.
Discussion
The aim of this study was to ask whether androgen regulates GRP expression at the mRNA and protein levels in the lumbar spinal cord of adult male rats because such regulation could contribute to androgenic modulation of male reproductive behavior (19). There is substantial evidence of androgenic effects on male reproductive behavior, although direct effects on the GRP system in the lumbar spinal cord have not been fully investigated. Thus, the present studies focused on androgen actions on the GRP system in the lumbar spinal cord by means of two different animal models: castration and testosterone replacement in adult male rats and comparison with genetically male (XY) rats carrying the Tfm allele of the AR gene. We found that androgen plays a pivotal role in the regulation of GRP expression in the male lumbar spinal cord, and the results in Tfm animals indicate that these effects are mediated by the AR. To our knowledge, this is the first demonstration of androgenic modulation of this sexually dimorphic peptidergic system in the spinal cord both at the mRNA and protein levels. It seems likely that the androgenic modulation of the spinal GRP system contributes to androgenic regulation of male sexual reflexes such as erection and ejaculation.
In general, we observed greater modulation of spinal GRP expression in terms of mRNA than of protein. This may be because GRP is also expressed in a subset of small and medium-sized dorsal root ganglion neurons that terminate in lamina I of the dorsal spinal cord to mediate itch sensation (16). Presumably these fibers, which are present in both sexes, are unrelated to sexual behavior. If so, then they would contribute to total spinal GRP protein but, with their cell bodies in the dorsal root ganglion, would not contribute to spinal GRP transcripts. Concerning GRP-R, we did not see significant effects of androgen manipulation or of the Tfm allele on the expression of mRNA or protein levels, but again these presumably reflect both the sexually dimorphic afferent fields of the L3-4 neurons and the non-sex-related afferent fields of the dorsal horn itch receptors. Although overall spinal expression of GRP-R tended to be less in Wt females and Tfm males than Wt males, future study should focus on more localized expression of the GRP-R within the lumbar spinal cord.
In the present study, we used two different rat strains: SD strain (albino) for castration experiments and LE strain (pigmented) for Tfm experiments. Unexpectedly, we found a strain difference that baseline of spinal GPR levels is higher in the LE strain than the SD strain, at both the mRNA and protein levels. Furthermore, the expression level of AR mRNA in the lumbar spinal cord was also higher in the LE strain than the SD strain (our unpublished observations). It has been reported that a higher proportion of LE rats display noncontact erections that may reflect sexual arousal, whereas SD albino rats rarely do, suggesting that SD rats have reduced erectile function compared with LE rats (30). These strain differences in sexual function may reflect strain differences in the spinal GRP system and are worthy of further study.
Galanin (31)-, cholecystokinin (32)- and enkephalin (33)-expressing neurons, which are localized dorsolateral to the central canal at the L3-4 level and project to the thalamus, the so-called lumbar spinothalamic neurons in lamina X, have been suggested to convey the sexual information from the lumbar spinal level to the thalamus, generating ejaculation in male rats (18,34,35). Because lumbar spinal cord expression of these peptides is greater in males than females (31,32,33), these peptidergic systems may, like GRP, be regulated by circulating androgen in adulthood or by other factors altogether. On the other hand, the other peptides may be regulated by androgen during embryonic and/or neonatal development. Although the developmental changes in these male-specific peptidergic systems have not yet been determined, a similar reduction in the expression was reported in galanin and cholecystokinin neurons in the lumbar spinal cord of Tfm rats (36).
We found that long-term gonadectomy of adult males reduces the expression of AR mRNA in the spinal cord compared with the sham-operated males, and this reduction is averted by TP replacement, suggesting the down-regulation of AR expression is caused by low levels of circulating androgen. Therefore, decreased AR expression might play a role in the attenuation of the spinal GRP system in castrates. Furthermore, we do not yet know whether androgens directly act on the GRP gene to promote GRP synthesis or indirectly by, for example, up-regulating AR expression in the GRP neurons. For that matter, it is possible that androgen acts on AR in some other cell population, which then induces the GRP-ergic neurons to increase GRP expression.
Testosterone can be aromatized by cytochrome P450 aromatase to an active estrogen, 17β-estradiol, and the enzyme might be expressed and functional in the spinal cord, perhaps modulating nociception at the spinal level (37,38). Thus, we could not be sure beforehand whether the effects of castration and testosterone replacement were actually mediated by estrogenic hormone metabolites. However, we recently demonstrated that nearly every GRP-positive spinal neuron also contains AR, but none of these neurons contain ERα (19). Furthermore, the present study of XY rats with a dysfunctional AR gene (Tfm) indicates that the GRP expressions in this region of Wt males is mediated by AR rather than ERα and/or ERβ because Tfm males displayed GRP mRNA and protein levels in the spinal cord similar to those of females. The present and previous studies, taken together, support the hypothesis that the androgen response observed in the spinal GRP system is mediated by AR rather than ERs.
In conclusion, we found evidence that circulating androgens in adult rats can profoundly alter the expression of GRP in a spinal system that mediates male sexual reflexes. This androgenic modulation of spinal GRP expression appears to be mediated by the AR rather than ERs and may represent an important pathway by which androgens activate masculine behaviors mediated by the spinal cord.
Acknowledgments
We are grateful to Akie Takara for her technical assistance.
Footnotes
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture, and Technology, Japan (to H.S., K.-M., and M.K.) and National Institutes of Health Grant NS28421 (to S.M.B.).
Present affiliation for H.S.: Ushimado Marine Laboratory, Graduate School of Natural Science and Technology, Okayama University, Setouchi, Okayama 701-4303, Japan.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 9, 2009
Abbreviations: AR, Androgen receptor; DAB, diaminobenzidine; EIA, enzyme immunoassay; ER, estrogen receptor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GDX, gonadectomized; GRP, gastrin-releasing peptide; GRP-R, GRP receptor; ICC, immunocytochemistry; L, lumbar segment; LE, Long Evans; nNOS, neuronal nitric oxide synthase; SD, Sprague Dawley; SNB, spinal nucleus of bulbocavernosus; SPN, sacral parasympathetic nucleus; Tfm, testicular feminization mutation; TP, testosterone propionate; TBST, Tween 20 in Tris-buffered saline; Wt, wild type.
References
- Morris JA, Jordan CL, Breedlove SM 2004 Sexual differentiation of the vertebrate nervous system. Nat Neurosci 7:1034–1039 [DOI] [PubMed] [Google Scholar]
- Matsuda K, Sakamoto H, Kawata M 2008 Androgen action in the brain and spinal cord for the regulation of male sexual behaviors. Curr Opin Pharmacol 8:747–751 [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC 1959 Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382 [DOI] [PubMed] [Google Scholar]
- Sachs BD 1982 Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil 66:433–443 [DOI] [PubMed] [Google Scholar]
- Hart BL 1973 Effects of testosterone propionate and dihydrotestosterone on penile morphology and sexual reflexes of spinal male rats. Horm Behav 4:239–246 [DOI] [PubMed] [Google Scholar]
- Hart BL 1979 Activation of sexual reflexes of male rats by dihydrotestosterone but not estrogen. Physiol Behav 23:107–109 [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP 1983 Hormonal control of a developing neuromuscular system. I. Complete memasculinization of the male rat spinal nucleus of the bulbocavernosus using the anti-androgen flutamide. J Neurosci 3:417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP 1983 Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J Neurosci 3:424–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Fishman RB, Breedlove SM 1992 Differential effects of testosterone metabolites upon the size of sexually dimorphic motoneurons in adulthood. Horm Behav 26:204–213 [DOI] [PubMed] [Google Scholar]
- Hart BL, Haugen CM 1968 Activation of sexual reflexes in male rats by spinal implantation of testosterone. Physiol Behav 3:735–738 [Google Scholar]
- Anastasi A, Erspamer V, Bucci M 1971 Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia 27:166–167 [DOI] [PubMed] [Google Scholar]
- McDonald TJ, Jörnvall H, Nilsson G, Vagne M, Ghatei M, Bloom SR, Mutt V 1979 Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun 90:227–233 [DOI] [PubMed] [Google Scholar]
- Panula P, Nieminen O, Falkenberg M, Auvinen S 1988 Localization and development of bombesin/GRP-like immunoreactivity in the rat central nervous system. Ann NY Acad Sci 547:54–69 [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Taylor JE, Coy DH, Moore KA, Moran TH 1996 Hindbrain GRP receptor blockade antagonizes feeding suppression by peripherally administered GRP. Am J Physiol 271:R180–R184 [DOI] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Isobe Y, Inouye ST 1993 Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide Y. J Neurosci 13:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Chen ZF 2007 A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448:700–703 [DOI] [PubMed] [Google Scholar]
- Merali Z, Bédard T, Andrews N, Davis B, McKnight AT, Gonzalez MI, Pritchard M, Kent P, Anisman H 2006 Bombesin receptors as a novel anti-anxiety therapeutic target: BB1 receptor actions on anxiety through alterations of serotonin activity. J Neurosci 26:10387–10396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt WA, Coolen LM 2002 Identification of a potential ejaculation generator in the spinal cord. Science 297:1566–1569 [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Matsuda K, Zuloaga DG, Hongu H, Wada E, Wada K, Jordan CL, Breedlove SM, Kawata M 2008 Sexually dimorphic gastrin releasing peptide system in the spinal cord controls male reproductive functions. Nat Neurosci 11:634–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin CW, Bullock L, Blackburn WR, Sherins RJ, Vanha-Perttula T 1971 Testosterone metabolism in the androgen-insensitive rat: a model for testicular feminization. Birth Defects Orig Artic Ser 7:185–192 [PubMed] [Google Scholar]
- Beach FA, Buehler MG 1977 Male rats with inherited insensitivity to androgen show reduced sexual behavior. Endocrinology 100:197–200 [DOI] [PubMed] [Google Scholar]
- Krey LC, Lieberburg I, MacLusky NJ, Davis PG, Robbins R 1982 Testosterone increases cell nuclear estrogen receptor levels in the brain of the Stanley-Gumbreck pseudohermaphrodite male rat: implications for testosterone modulation of neuroendocrine activity. Endocrinology 110:2168–2176 [DOI] [PubMed] [Google Scholar]
- Griffin JE, Leshin M, Wilson JD 1982 Androgen resistance syndromes. Am J Physiol 243:E81–E87 [DOI] [PubMed] [Google Scholar]
- Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM 1990 A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem 265:8893–8900 [PubMed] [Google Scholar]
- Cui H, Sakamoto H, Higashi S, Kawata M 2008 Effects of single-prolonged stress on neurons and their afferent inputs in the amygdala. Neuroscience 152:703–712 [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Matsuda K, Hosokawa K, Nishi M, Morris JF, Prossnitz ER, Kawata M 2007 Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology 148:5842–5850 [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Matsuda K, Zuloaga DG, Nishiura N, Takanami K, Jordan CL, Breedlove SM, Kawata M 2009 Stress affects a gastrin-releasing peptide system in the spinal cord that mediates sexual function: Implications for psychogenic erectile dysfunction. PLoS ONE 4:e4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Mezaki Y, Shikimi H, Ukena K, Tsutsui K 2003 Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology 144:4466–4477 [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, Breedlove SM 2008 The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Horm Behav 53:613–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD 1996 Penile erection in response to remote cues from females: albino rats severely impaired relative to pigmented strains. Physiol Behav 60:803–808 [DOI] [PubMed] [Google Scholar]
- Newton BW 1992 A sexually dimorphic population of galanin-like neurons in the rat lumbar spinal cord: functional implications. Neurosci Lett 137:119–122 [DOI] [PubMed] [Google Scholar]
- Phan DC, Newton BW 1999 Cholecystokinin-8-like immunoreactivity is sexually dimorphic in a midline population of rat lumbar neurons. Neurosci Lett 276:165–168 [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Zhang X, Hökfelt T 1999 An immunohistochemical investigation of the opioid cell column in lamina X of the male rat lumbosacral spinal cord. Neurosci Lett 270:9–12 [DOI] [PubMed] [Google Scholar]
- Ju G, Melander T, Ceccatelli S, Hökfelt T, Frey P 1987 Immunohistochemical evidence for a spinothalamic pathway co-containing cholecystokinin- and galanin-like immunoreactivities in the rat. Neuroscience 20:439–456 [DOI] [PubMed] [Google Scholar]
- Truitt WA, Shipley MT, Veening JG, Coolen LM 2003 Activation of a subset of lumbar spinothalamic neurons after copulatory behavior in male but not female rats. J Neurosci 23:325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton BW, Phan DC 2006 Androgens regulate the sexually dimorphic production of co-contained galanin and cholecystokinin in lumbar laminae VII and X neurons. Brain Res 1099:88–96 [DOI] [PubMed] [Google Scholar]
- Evrard HC 2006 Estrogen synthesis in the spinal dorsal horn: a new central mechanism for the hormonal regulation of pain. Am J Physiol Regul Integr Comp Physiol 291:R291–R299 [DOI] [PubMed] [Google Scholar]
- Evrard HC, Balthazart J 2003 Aromatase (estrogen synthase) activity in the dorsal horn of the spinal cord: functional implications. Ann NY Acad Sci 1007:263–271 [DOI] [PubMed] [Google Scholar]