Abstract
The preovulatory GnRH/LH surge depends on the presence of estradiol (E2) and is gated by a circadian oscillator in the suprachiasmatic nucleus (SCN) that causes the surge to occur within a specific temporal window. Although the mechanisms by which the clock times the LH surge are unclear, evidence suggests that the SCN is linked to GnRH neurons through a multisynaptic pathway that includes neurons in the anteroventral periventricular nucleus (AVPV). Recently, Kiss1 neurons in the AVPV have been implicated in the surge mechanism, suggesting that they may integrate circadian and E2 signals to generate the LH surge. We tested whether Kiss1 neurons display circadian patterns of regulation in synchrony with the temporal pattern of LH secretion. Mice housed in 14 h light, 10 h dark were ovariectomized, given E2 capsules (or nothing), and transferred into constant darkness. Two days later, the mice were killed at various times of day and their LH and Kiss1 levels assessed. In E2-treated females, LH levels were low except during late subjective day (indicative of an LH surge). Similarly, AVPV Kiss1 expression and c-fos coexpression in Kiss1 neurons showed circadian patterns that peaked coincident with LH. These temporal changes in Kiss1 neurons occurred under steady-state E2 and constant environmental conditions, suggesting that Kiss1 neurons are regulated by circadian signals. In the absence of E2, animals displayed no circadian pattern in LH secretion or Kiss1 expression. Collectively, these findings suggest that the LH surge is controlled by AVPV Kiss1 neurons whose activity is gated by SCN signals in an E2-dependent manner.
AVPV Kiss1 neurons, which have been implicated in regulating the preovulatory LH surge, display a circadian pattern of activation that depends on the presence of estradiol.
In female mammals, ovulation is induced by a surge of LH secretion from the pituitary, an event which is itself stimulated by a surge of GnRH secretion from neurons in the forebrain (1). It is well established that estradiol (E2) is critical for triggering the preovulatory GnRH/LH surge; in rodents, the surge normally occurs only on proestrus, as E2 levels are peaking, and females that are ovariectomized (OVX) do not display LH surges (2,3). Although E2 is an absolute prerequisite for generating the LH surge, in rodents, the surge event is also gated by a circadian oscillator in the suprachiasmatic nucleus (SCN). This gatekeeper ensures that the LH surge is precisely timed to occur within a 2- to 4-h window, near the onset of darkness (and coincident with the initiation of locomotor activity in nocturnal animals). Intact female rodents display an LH surge in the late afternoon of proestrus but not at other times of that day, and OVX females treated with constant steady-state E2 display a late afternoon LH surge, which repeats daily at the same time (4). In addition, lighting paradigms that alter the natural period of the circadian clock, or phase-shift the clock’s temporal output, modify the timing of the LH surge (5,6,7,8); in all cases, the surge onset remains tightly coupled to the onset of daily locomotor activity, which is also timed by the SCN, implying that the timing of the surge and activity rhythms are both gated by the SCN. Finally, lesions of the SCN or genetic disruptions of the circadian molecular clockwork disrupt estrous cyclicity and prevent the E2-induced LH surge (9,10,11,12,13,14,15). Collectively, these observations argue that proper functioning of the SCN′s circadian clockwork is essential for the timing and generation of the GnRH/LH surge in rodents, but precisely how this occurs is poorly understood.
There are two possible routes by which the SCN could regulate GnRH neurons and hence the LH surge. One pathway involves direct innervation of GnRH neurons by the SCN (16,17,18); however, anatomical and physiological evidence suggests that a second route is perhaps more critical (4). This second pathway involves an indirect circuit linking the SCN with GnRH neurons through a relay station in the anteroventral periventricular nucleus (AVPV), a hypothalamic region that is critical for the GnRH/LH surge in rodents (reviewed in Refs. 3,19, and 20). Some neurons in the AVPV express estrogen receptor-α (ERα), the primary receptor implicated in mediating positive feedback effects of E2 on the LH surge (3); moreover, some ERα-expressing cells in the AVPV receive direct neural input from the SCN (16,21). Thus, an E2-sensitive population of neurons within the AVPV could represent the cellular conduit linking the SCN to GnRH neurons, and recent evidence suggests that these particular AVPV cells may be Kiss1 neurons.
The Kiss1 gene encodes kisspeptin, a neuropeptide that plays a critical role in the neuroendocrine regulation of reproduction. In rodents, sheep, and primates (including humans), treatment with kisspeptin elicits a rapid increase in gonadotropin secretion, mediated by kisspeptin’s direct stimulation of GnRH neurons (reviewed in Refs. 19 and 22). Kiss1 neurons are located in several regions of the hypothalamus (23), but in rodents, the population of Kiss1 cells in the AVPV has been implicated as the critical component that drives the preovulatory GnRH/LH surge. First, virtually all Kiss1 neurons in the AVPV express ERα, and Kiss1 mRNA in this region is strongly up-regulated by E2 (24,25). Second, Kiss1 expression in the AVPV of cycling female rats is induced, along with Fos in Kiss1 neurons, at the time of the preovulatory LH surge (26). Third, infusions of antiserum to kisspeptin prevent the LH surge in intact and E2-treated female rats (27,28). Finally, the expression of Kiss1 in the AVPV is sexually differentiated, with females having significantly more Kiss1 neurons than males (29,30), corresponding with the ability of females (but not males) to display an LH surge.
Although these observations provide compelling evidence that Kiss1 neurons in the AVPV participate in the E2-mediated induction of the LH surge, it remains ambiguous whether Kiss1 neurons also represent part of the circadian circuit linking the SCN to GnRH neurons. If Kiss1 neurons in the AVPV mediate the circadian effects of the clock in the SCN on the daily LH surge, then Kiss1 gene expression or transcriptional activity within Kiss1 cells may display a circadian pattern of regulation. To address this possibility, we evaluated the temporal patterns of Kiss1 expression and induction of the immediate-early gene c-fos (as a marker of neuronal activation) within Kiss1 neurons in the AVPV of female mice. To eliminate the possible effects of fluctuating environmental conditions, all experiments were performed under constant environmental conditions (constant darkness and temperature). In addition, because animals lacking E2 do not display LH surges, we reasoned that this might reflect the absence of E2-dependent circadian activation of Kiss1 cells in the AVPV. As an alternative, we supposed that Kiss1 neurons could well maintain their daily circadian activation, regardless of the presence or absence of E2, but could generate a preovulatory LH surge only when E2-induced kisspeptin production was sufficiently high enough to drive GnRH neurons (e.g. on proestrus). To distinguish between these possibilities, we evaluated whether Kiss1 neurons display circadian activation in both the presence and absence of E2 and propose a model of GnRH/LH surge generation via an E2-dependent circadian activation of Kiss1 neurons in the AVPV.
Materials and Methods
Animals
Adult (2 month old) C57/BL6J female mice purchased from Charles River Laboratories (Wilmington, MA) were individually housed with access to a running wheel in a 14-h light, 10-h dark cycle (lights off at 2100 h). Mice had ad libitum access to standard rodent chow and water throughout the study. Running wheel activity was continuously recorded for the duration of the study using Clocklab (Actimetrics, Wilmette, IL) and analyzed using El Temps software (http://www.el-temps.com/). All experiments were approved by the University of Washington Animal Care and Use Committee.
OVX and LH surge protocol
Ovaries were removed from isoflurane-anesthetized mice that were pretreated with Buprenex analgesic (1.5 μg, sc). Briefly, the anesthetized animal’s ventral surface was shaved and cleaned and the ovaries dissected out through midline incisions in the skin and abdominal musculature. After ovary removal, the muscle was sutured and the skin closed with sterile wound clips.
To induce a predictably timed daily LH surge, mice in experiments 1 and 2 were sc implanted at the time of OVX with a SILASTIC brand (Dow Corning, Midland, MI) capsule containing 0.625 μg E2 (in sesame oil, following the protocol of Ref. 31). This E2-treatment paradigm results in constant serum levels of E2 (∼30 pg/ml) between 2 and 5 d after implantation and reliably induces daily afternoon LH surges (defined as ≥8-fold above baseline levels) beginning on d 2 (31,32). All surgeries were performed in the morning during the first several hours after lights on.
Blood and tissue collection
At the time of killing (see specific experiments for details), mice were anesthetized with isoflurane and their blood collected via retroorbital bleeding. Blood was assayed for LH concentrations via a sensitive mouse LH RIA, performed by the University of Virginia Ligand Assay Lab as described previously (33,34). After blood collection, animals were rapidly decapitated and their brains immediately collected and frozen on dry ice. Frozen brains were stored at −80 C until sectioning on a cryostat. Five sets of 20-μm sections were cut in the coronal plane, thaw-mounted onto Superfrost-plus slides, and stored at −80 C. One set was used for each in situ hybridization (ISH) assay.
Single-label ISH
Slide-mounted brain sections were processed for Kiss1 ISH, as previously described (23,26,30). Briefly, sections were fixed in 4% paraformaldehyde, rinsed in phosphate buffer, treated with acetic anhydride, rinsed in 2× saline sodium citrate (SSC), delipidated in chloroform, dehydrated in ethanols, and air dried. Antisense mouse Kiss1 probe, spanning bases 76–486 of the mouse cDNA sequence, was generated using 33P, as previously described (23,24). The radiolabeled Kiss1 riboprobe was combined with 1/20 volume yeast tRNA (Roche Biochemicals, Indianapolis, IN) in TE (0.1 m Tris/0.01 m EDTA, pH 8.0), heat denatured, added to hybridization buffer at a ratio of 1:4, and applied to each slide (100 μl/slide; 0.03 pmol probe/ml). Slides were then coverslipped and placed in a humidity chamber at 55 C for 16 h. After hybridization, the slides were washed in 4× SSC at room temperature and placed into ribonuclease (RNase) [37 mg/ml RNase A (Roche Biochemicals) in 0.15 m sodium chloride, 10 mm Tris, 1 mm EDTA, pH 8.0] for 30 min at 37 C and then into RNase buffer without RNase at 37 C for 30 min. Slides were then washed in 0.1× SSC at 62 C, dehydrated in ethanols, and air dried. Dry slides were dipped in Kodak NTB emulsion (VWR, West Chester, PA), air dried, and stored at 4 C for 10–12 d (depending on the assay), after which they were developed and coverslipped.
Double-label ISH
For double-label ISH, slide-mounted brain sections were treated similarly to single-label ISH with the following modifications. Digoxigenin (DIG)-labeled antisense Kiss1 probe was synthesized along with radiolabeled c-fos riboprobe (labeled using 33P), and both riboprobes were dissolved in the same hybridization buffer along with tRNA and applied to slides for overnight hybridization. The radiolabeled c-fos riboprobe was generated by using 33P as described previously (35). After the 0.1× SSC washes on d 2, slides were incubated in 2× SSC with 0.05% Triton X-100 and 2% sheep serum for 1 h at room temperature and then washed in buffer 1 (100 mm Tris-HCl, pH 7.5, and 150 mm NaCl). Slides were then incubated overnight at room temperature with alkaline-conjugated anti-DIG antibody fragments (Roche Biochemical) that were diluted 1:350–400 in buffer 1 containing 1% sheep serum and 0.3% Triton X-100. The next day, slides were washed with buffer 1 and incubated with Vector Red alkaline phosphatase substrate (Vector Laboratories, Burlingame, CA) for 2 h. The slides were then air dried, dipped in NTB emulsion, stored at 4 C, and developed 10–13 d later (depending on the assay).
Experiment 1: evaluation of the circadian regulation of Kiss1 mRNA in the AVPV of E2-treated animals
Kisspeptin signaling arising from the AVPV has been proposed to mediate the generation of the LH surge. Because the LH surge is regulated by the circadian clock, we hypothesized that Kiss1 neurons in the AVPV are themselves under circadian control. To test this hypothesis, we used single-label ISH to analyze levels of Kiss1 mRNA in female mice that were housed under constant lighting conditions and experiencing constant E2 exposure. After 13–20 d acclimation to a 14-h light, 10-h dark cycle, mice were OVX in the morning and implanted with E2-containing capsules that induce a daily LH surge. On the night of surgery, mice were released into constant dim light (<1 lux, equivalent to constant darkness). At least 36 h after transfer to constant darkness, animals were killed under dim, red-light illumination at one of eight circadian time points throughout the day: circadian time (cT) 0, CT 4, CT 8, CT 9, CT 11, CT 12, CT 16, and CT 20 (n = 4–6 per time point). By convention in chronobiology, the onset of locomotor activity, as determined by each animal’s running wheel activity rhythm, was denoted as CT 12. We determined in advance that the free-running period of the female mice housed in constant darkness, both in the presence or absence of E2, is significantly close to 24 h and that the phase drift after 48 h in constant darkness is insignificant. Thus, the onset of locomotor activity the day before killing (when animals were already in constant darkness) was used to determine each animal’s specific CT 12 the following day. All kills were carried out ±15 min from the various calculated time points (see Fig. 1 for example). Blood and brains were collected at the time of killing and used to measure serum levels of LH and Kiss1 mRNA expression in the AVPV for each circadian time point.
Figure 1.
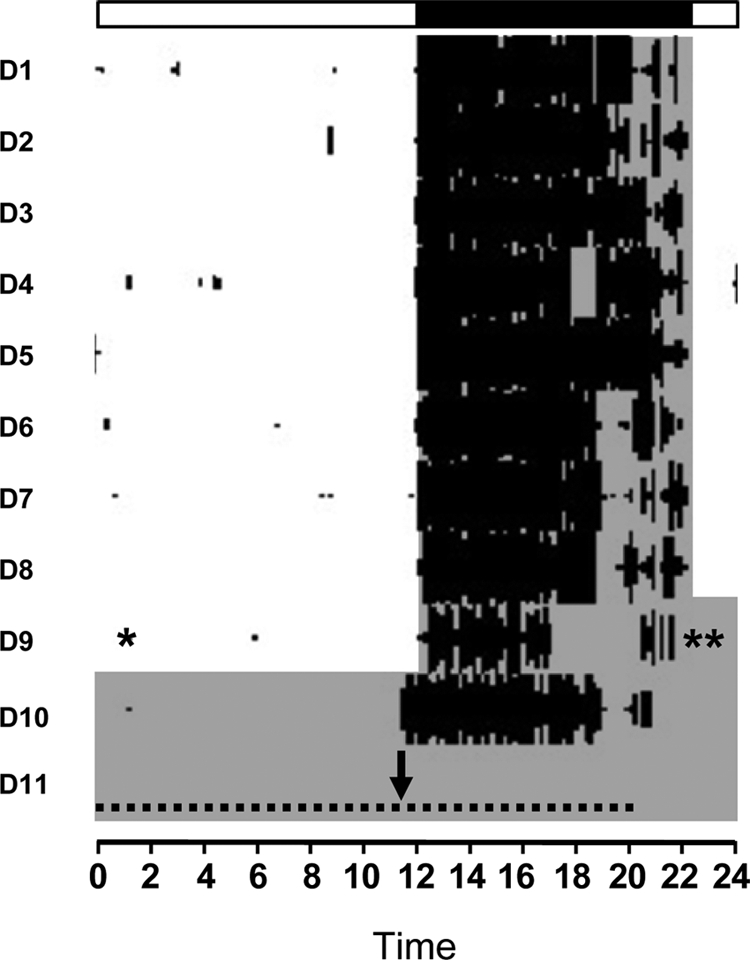
Representative locomotor actogram of a female mouse housed in 14-h light, 10-h dark for approximately 1 wk. The animal was OVX and implanted with an E2-containing capsule (indicated by an asterisk) on the morning of d 9 and then transferred into constant darkness later that night (indicated by two asterisks). The mouse was killed 2 d after receiving the E2 capsule, which was at CT 12 (indicated by the black arrow). Animals in other groups were killed at various time points throughout the second day in constant darkness, as indicated by the horizontal dashed line (see Materials and Methods for specific times). Shaded areas represent the time of darkness (<1 lux dim red light). Note that CT 12 (indicated here by the black arrow) is slightly earlier than the extrapolated time of lights off. Therefore, CT 12 was calculated individually for each animal based on its own specific activity onset.
Experiment 2: circadian activation of Kiss1 neurons in the AVPV of E2-treated animals
Experiment 1 determined that there is a circadian pattern of Kiss1 gene expression in the brains of E2-treated female mice housed in constant darkness and that this Kiss1 pattern coincided with the circadian pattern of LH secretion. We next asked whether Kiss1 neurons also undergo a circadian activation, as evidenced by the induction of the immediate-early gene c-fos in Kiss1 cells. To address this question, we used double-label ISH to determine colocalization of Kiss1 mRNA and c-fos mRNA in the brains of animals from experiment 1. One set of brain tissue from E2-treated females that were housed in constant darkness was processed for double-label ISH and the percentage of AVPV Kiss1 neurons coexpressing c-fos was compared across the eight circadian time points.
Experiment 3: evaluation of the circadian regulation of Kiss1 neurons in the AVPV of OVX animals
Experiments 1 and 2 suggested that Kiss1 neurons of E2-treated mice are under circadian control and that this temporal regulation of Kiss1 cells mirrors that of LH secretion. Because OVX animals do not display an LH surge, we next asked whether the circadian stimulatory regulation of Kiss1 neurons is dependent on the presence of E2. We postulated that in the absence of E2, Kiss1 cells would not show a circadian-induced increase in either Kiss1 gene expression or c-fos induction, in contrast to what happens in the presence of E2. To test this hypothesis, we replicated experiments 1 and 2 but this time using OVX mice that were not E2 treated. Mice were housed in 14 h light, 10 h dark for 6 d and then OVX. Six to 7 d after OVX, animals were released into constant dim light (<1 lux; constant darkness). At least 36 h later, the mice were killed under dim, red-light illumination at one of eight circadian time points, as in experiment 1 (n = 3–5 per time point). Blood and brains were collected at the time of killing and processed to determine serum levels of LH (via RIA) as well as Kiss1 and c-fos mRNA (via single- and double-label ISH).
Quantification of ISH assays and statistical analysis
Slides were analyzed with an automated image processing system by a person unaware of the treatment group of each slide. For single-label experiments, custom grain-counting software was used to unilaterally count the number of radiolabeled cell clusters and the number of silver grains in each cell (a semiquantitative index of mRNA content per cell) (23,26) for all AVPV sections. Cells were considered Kiss1 positive when the number of silver grains in a cluster exceeded that of background by 3-fold. For double-label assays, DIG-containing cells (Kiss1 cells) were identified under fluorescence microscopy, and the grain-counting software was used to quantify silver grains (representing c-fos mRNA) over each cell. A cell was considered double labeled if it had a signal-to-background ratio of at least 3 (26,30). For each animal, the amount of double labeling was calculated as a percentage of the total number of Kiss1 mRNA-expressing cells and then averaged across animals to produce a group mean.
All LH and ISH data are expressed as the mean ± sem for each group. One-way ANOVAs were used to assess variation among experimental groups (time points) in each experiment, and differences in means were assessed by post hoc Fisher’s least significant difference tests (with Staview 5.0.1; SAS Institute, Cary, NC). Significance level was set at P < 0.05.
Results
Experiment 1: Kiss1 gene expression in the AVPV of E2-treated female mice is under circadian control
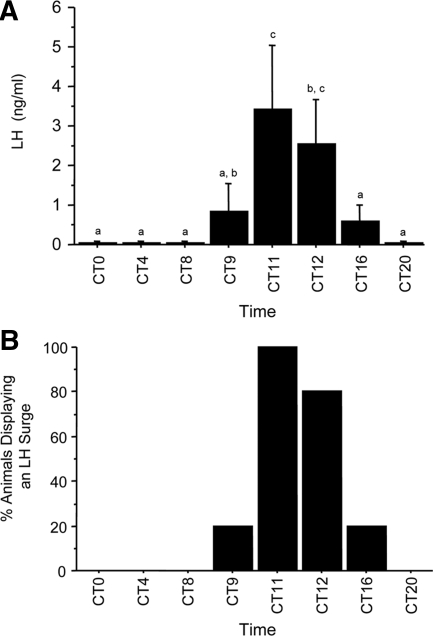
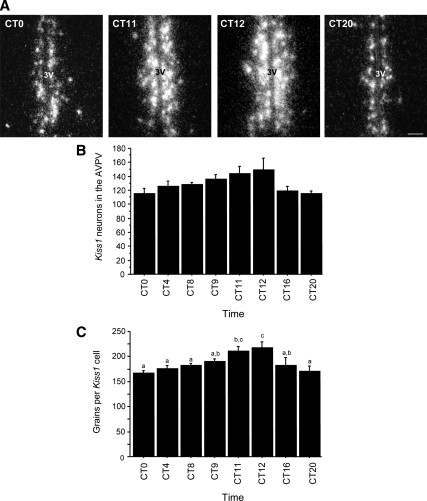
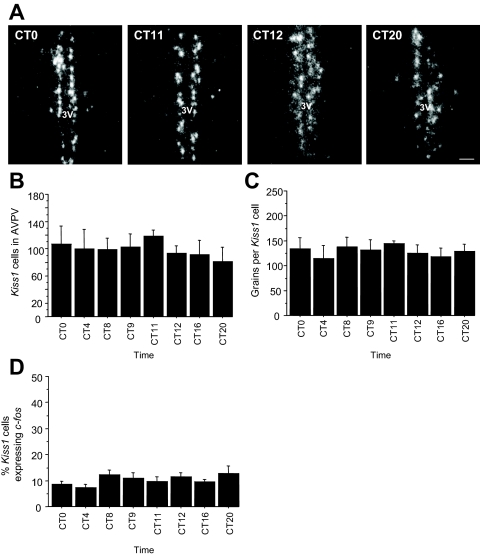
This experiment tested whether Kiss1 expression in the AVPV exhibits a circadian pattern under constant conditions and whether this pattern mirrors that of LH secretion. Female mice housed in constant darkness and treated with constant E2 displayed a significant circadian pattern of LH secretion, with serum LH levels being low to undetectable in the subjective morning (CT 0, CT 4, CT 8), high in the subjective late afternoon/early evening (CT 11, CT 12), and low again in the late subjective night (CT 20) (P < 0.05, Fig. 2A). LH levels were at intermediate levels at CT 9 and CT 16, reflecting the presence of an LH surge in a small subset (∼20%) of animals in these two groups (Fig. 2B). Kiss1 gene expression in the AVPV of E2-treated females also exhibited a similar circadian pattern, with higher expression visible in the late afternoon (CT 11, CT 12) vs. earlier or later time points (Fig. 3A). Quantitative analysis determined that there was a trend for increased number of Kiss1 cells at CT 11 and CT 12 (P < 0.09; Fig. 3B) and significantly more (24–28%) grains per Kiss1 cell (indicative of Kiss1 mRNA level per cell) in the late afternoon/ early evening compared with earlier and later time-points (P < 0.01; Fig. 3C). Similarly, total Kiss1 mRNA in the AVPV, calculated as the product of Kiss1 cell number and number of grains per Kiss1 cell, showed a significant circadian pattern with markedly higher levels (∼45–55%) at CT 11 and CT 12 vs. earlier and later time-points (P < 0.01, data not shown).
Figure 2.
A, Mean (±sem) serum levels of LH in OVX, E2-treated mice housed in constant conditions and killed at one of eight time points throughout the circadian day. Values with different letters differ significantly from each other (P < 0.05). B, Percentage of animals at each time point displaying an LH surge (defined as an 8-fold or greater increase in LH values compared with mean CT 0 and CT 4 values) (31). n = 4–6 animals per group.
Figure 3.
A, Representative dark-field photomicrographs showing Kiss1 mRNA-expressing cells (as reflected by the presence of white clusters of silver grains) in the AVPV of OVX, E2-treated female mice that were housed in constant conditions and killed at different times throughout the circadian day. 3V, Third ventricle. B, Mean (±sem) number of Kiss1-expressing cells in the AVPV across the circadian day displayed a trend (P < 0.09) for more Kiss1 neurons during the late subjective afternoon/early evening. C, The amount of Kiss1 mRNA per cell, as indicated by the number of silver grains per cell, was significantly different across circadian time points, with highest values at CT 11 and CT 12 (P < 0.01); values with different letters differ significantly from each other. n = 4–6 animals per group.
Experiment 2: Kiss1 cells in the AVPV of E2-treated mice show a circadian pattern of neuronal activation
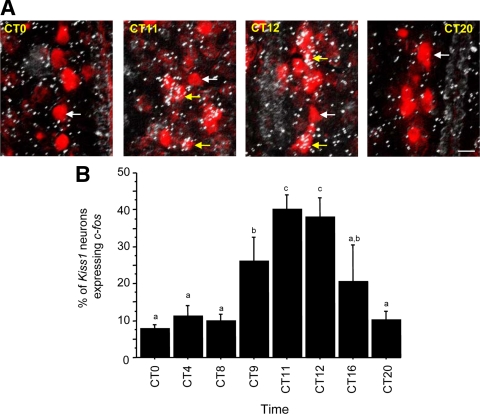
Experiment 1 identified a circadian pattern of Kiss1 expression in the brains of E2-treated female mice housed in constant darkness, coinciding with a circadian pattern of LH secretion. This experiment tested whether Kiss1 cells in E2-treated mice also undergo a circadian pattern of neuronal activation, as determined by c-fos induction in Kiss1 cells. We found that the expression of c-fos in Kiss1 neurons in the AVPV was low or nondetectable in the subjective morning and late subjective night, despite the presence of c-fos-expressing cells in other brain regions at these times (e.g. thalamus and paraventricular nucleus). In contrast, c-fos expression in Kiss1 neurons was prevalent in subjective late afternoon/early evening, coincident with the occurrence of LH surges at these times (see experiment 1) and indicative of a circadian regulation (Fig. 4). Quantitatively, the percentage of Kiss1 neurons expressing c-fos was no more than 10% at CT 0, CT 4, and CT 8, elevated to 40% at CT 11 and CT 12, and roughly 10% again at CT 20 (P < 0.01; Fig. 4). Similar to the LH data in these E2-treated females (experiment 1), the percentage of c-fos/Kiss1 coexpression was intermediate at CT 9 and CT 16 (Fig. 4), reflecting high coexpression in one animal in each of these groups (the same animals with high LH).
Figure 4.
A, Representative photomicrographs of Kiss1 mRNA and c-fos mRNA coexpression in the AVPV of OVX, E2-treated female mice housed in constant conditions and killed at different times throughout the circadian day. Kiss1-containing neurons were visualized with Vector Red substrate, and c-fos mRNA was marked by the presence of silver grains. White arrows denote example Kiss1 cells lacking c-fos; yellow arrows denote example Kiss1 cells coexpressing c-fos. B, Mean (±sem) percentage of Kiss1 mRNA-containing neurons in the AVPV that coexpress c-fos in OVX, E2-treated female mice killed at one of eight times throughout the circadian day. There was a significant effect of time (P < 0.01) with increased coexpression of Kiss1 and c-fos in the late afternoon/early evening. Values with different letters differ significantly from each other. n = 4–6 animals per group.
Experiment 3: Kiss1 neurons in the AVPV of OVX mice do not exhibit circadian patterns of regulation
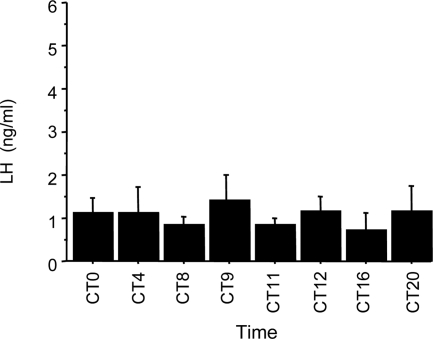
Because OVX mice are incapable of displaying an LH surge, this experiment tested whether the circadian patterns of Kiss1 gene expression and neuronal activation observed in experiments 1 and 2 are dependent on the presence of E2. Unlike our findings in E2-treated animals, serum levels of LH in OVX mice lacking E2 and housed in constant darkness showed no evidence of a circadian pattern. Although the baseline concentrations of LH were higher in all OVX animals (reflecting absence of negative feedback after OVX), there was no obvious circadian pattern or significant change in LH levels across the 24-h cycle (P > 0.95; Fig. 5). Likewise, neither Kiss1 cell number in the AVPV nor Kiss1 mRNA/cell were different among any of the circadian time points (P > 0.90 for each; Fig. 6), nor was there any significant difference in the percentage of c-fos coexpression in Kiss1 neurons across the circadian day (P > 0.90; Fig. 6D). Collectively, these observations demonstrate that there is little or no circadian pattern of activation of Kiss1 neurons in the AVPV of mice lacking E2 and housed in constant conditions.
Figure 5.
Mean (±sem) plasma LH in OVX mice housed in constant conditions and killed at one of eight times throughout the circadian day. There was no significant difference in LH levels between any of the time points. n = 4–5 animals per group.
Figure 6.
Lack of a circadian pattern in Kiss1 gene expression in OVX females. A, Representative dark-field photomicrographs showing Kiss1 mRNA-expressing cells in the AVPV of OVX mice housed in constant conditions and killed at different time points throughout the circadian day. B, Mean (±sem) number of Kiss1 mRNA-expressing neurons in the AVPV of OVX female mice housed in constant conditions and killed at one of eight time points throughout the circadian day. C, Mean (±sem) number of silver grains per Kiss1 cell in the AVPV of OVX mice killed across the circadian day. D, Mean (±sem) percentage of Kiss1 mRNA-containing neurons in the AVPV that coexpress c-fos in OVX female mice. In A–C, there was no significant difference in the measures between any of the circadian time points (P > 0.90 for all measures). n = 3–5 animals per group.
Discussion
Since the pioneering work of Everett and Sawyer in 1950 (36,37,38,39), we have known that the LH surge (and subsequently, ovulation) is dependent not only on E2 but also on a neural signal that reflects the time of day, so that the LH surge occurs only within a narrow temporal window (typically late afternoon in nocturnal rodents). Despite the fact that this LH surge phenomenon has been extensively studied over the intervening years (4), the neuronal circuitry and molecular mechanisms that represent the point of convergence between the E2 and circadian signals have remained a mystery. In the present study, we show that a population of Kiss1 neurons in the AVPV, previously implicated in the LH surge mechanism, is under circadian regulation. Moreover, we show that the ability of Kiss1 neurons to exhibit circadian regulation of both gene expression and neuronal activation is contingent on the presence of E2, perhaps explaining why an LH surge is absent in females with low or absent E2. Collectively, our findings suggest that Kiss1 neurons in the AVPV could receive and integrate both hormonal and temporal cues to generate and time the LH surge.
Considerable evidence suggests that the precisely timed pattern of LH release in female rodents reflects the interaction of E2 with an obligatory signal that emanates from the circadian clock in the SCN. First, intact female rodents normally display an LH surge only in the late afternoon of proestrus; however, barbiturate treatment during a critical window (2–3 h in the late afternoon) not only blocks the LH surge (and ovulation) but also delays its next appearance by exactly 24 h (36,37,38,39). Second, in OVX rodents, treatment with constant high levels of E2 elicits a daily LH surge, which occurs at the exact same time every day (40,41,42). However, females lacking a functional SCN (achieved by creating discrete lesions of SCN or genetic disruptions of the molecular clockwork in the SCN) are incapable of displaying an LH surge (at any time) in response to an E2 challenge (9,11,14,15,43). This establishes the absolute requirement of circadian signaling from the SCN to produce an LH surge. Finally, experimental paradigms that modify the circadian clock’s period or phase shift the clock’s temporal output correspondingly alter the timing of the LH surge in rodents. However, in all such cases, the onset of the LH surge remains tightly coupled to the onset of daily locomotor rhythm (5,6,7,8), indicating that the temporal gating of the surge and behavioral rhythms share a common circadian pacemaker, presumably the SCN. Although these findings argue persuasively that the SCN regulates the LH surge, the neural mechanisms by which this occurs are poorly understood. Because the AVPV plays a key role in generating the LH surge (reviewed in Refs. 3,19, and 44), it seems plausible that the SCN signals to GnRH neurons through intermediaries in the AVPV. In support of this, tract tracing experiments demonstrate that SCN neurons innervate a subset of AVPV neurons (16) and these specific AVPV neurons also express ERα, which mediates the E2-induced LH surge.
The phenotypic identity of the E2-responsive neurons in the AVPV that are targets for SCN-derived projections is unknown. However, our results suggest that these neurons are Kiss1 neurons. In E2-treated female mice, we found that Kiss1 gene expression in the AVPV varied across the circadian day. The expression of Kiss1 increased (by ∼25%) during the subjective late afternoon and early evening and then diminished in late subjective night. Because the animals were housed in constant darkness, these changes in Kiss1 gene expression were unlikely to have been produced by an hourglass mechanism that might be initiated each day through a daily resetting of a light-dark cycle. Furthermore, because all animals in all time points had the same constant E2 treatment, it is unlikely that these temporal changes in Kiss1 expression were caused by group differences or temporal changes in circulating levels of E2. These data indicate that Kiss1 gene expression is regulated by both sex steroid signals (up-regulated Kiss1 expression compared with OVX levels, as previously determined) (24,30) and circadian signals (up-regulated Kiss1 expression at specific times) (present study). Moreover, transcriptional activation of Kiss1 neurons, as measured by neuronal c-fos induction, also exhibited a strong circadian pattern in constant conditions (in the presence of E2). Thus, in addition to a timed increase in Kiss1 gene transcription, Kiss1 neurons are also temporally induced by afferent circadian signals to increase the expression of other genes (or perhaps alter neuronal firing). Presumably, this temporal activation of Kiss1 neurons is related to a timed stimulation of GnRH neurons, resulting in the circadian-gated LH surge. Whether the observed circadian pattern of Kiss1 gene expression and Kiss1 neuronal activation are causally related is unknown, but it is conceivable that the pattern of increased Kiss1 gene expression reflects a replenishing of Kiss1/kisspeptin stores after the neuron has fired to release kisspeptin (denoted by high neuronal c-fos induction).
The mechanism by which the SCN regulates Kiss1 neurons has not been elucidated. Based on previous studies, it is clear that the circadian regulation of the LH surge by the SCN involves neuronal, not humoral, signaling from the clock to GnRH neurons (45,46). Thus, if Kiss1 neurons in the AVPV serve as a cellular conduit to relay input from the SCN to GnRH neurons, these Kiss1 neurons would likely be regulated by the clock through neuronal innervation. In rodents, there are direct neuronal connections between the SCN and the AVPV and also between the AVPV and GnRH neurons (16,21,47,48), suggesting that a multisynaptic neuronal circuit links the SCN with GnRH neurons through the AVPV. Although neuroanatomical evidence documenting a direct SCN-Kiss1 circuit has yet to be reported, preliminary observations in mice suggest a direct, monosynaptic innervation of Kiss1 neurons in the AVPV by vasopressin signaling arising from the SCN (49). These investigations also implicate vasopressin as the key neurotransmitter up-regulating Kiss1 neurons, a conjecture supported by findings that vasopressin can stimulate GnRH/LH secretion in animals lacking a functional SCN (50,51). It is also worth noting that Kiss1 cells themselves may be circadian oscillators and that the observed temporal changes in Kiss1 activation could be generated intrinsically by intracellular clocks. This possibility derives support from the fact that many non-SCN cells throughout the body (e.g. GnRH or liver cells) express clock genes and in some cases exhibit endogenous circadian rhythmicity in vitro, independent of SCN input (52,53). However, given the strong evidence that SCN signaling is critical for both the generation and timing of the LH surge, and the fact that the SCN projects to the AVPV (and likely Kiss1 cells), the most parsimonious explanation is that the circadian pattern of Kiss1 neurons is controlled by the SCN.
Animals lacking E2 cannot display an LH surge. The inability to surge in the absence of E2 could reflect the absence of circadian input to the surge-generating circuitry and/or some other missing E2-dependent aspect of the surge-generating mechanism. Here, we show that the circadian pattern of Kiss1 regulation observed in E2-treated mice was completely absent in OVX animals. Thus, despite a lower, yet significant, number of detectable Kiss1 neurons in the OVX condition, these Kiss1 cells were not temporally activated in the absence of E2. This finding argues against a model in which the SCN activates Kiss1 neurons every day but generates an LH surge only when there is sufficient Kiss1 mRNA and kisspeptin release to drive a GnRH surge. Instead, our results imply that either 1) the SCN does not automatically signal to Kiss1 neurons every day but, instead, does so only in the presence of E2 or 2) the SCN sends temporal cues to Kiss1 neurons every day, but the ability of Kiss1 neurons to receive/decode the circadian signal is E2 dependent. Both the SCN and Kiss1 neurons express ERs (24,54,55), rendering either of these models plausible. It should also be noted that E2 may also act upstream of the SCN at other E2-responsive neurons that project to the clock (56). Regardless of mechanism, any of these E2-dependent models would result in Kiss1 neurons being up-regulated by circadian input only when there is E2 present, thus explaining why the LH surge occurs only during proestrus or conditions of elevated E2. Notably, these models indicate that E2 has a dual role for inducing the LH surge: 1) to stimulate Kiss1 gene expression, presumably to generate abundant kisspeptin, regardless of time of day (i.e. fewer AVPV Kiss1 neurons in OVX than E2-treated animals) and 2) to permit the SCN to activate Kiss1 neurons, thereby triggering them to signal to GnRH neurons. Although complementary and synergistic in their effects on the hypothalamo-pituitary-gonadal axis, these two actions of E2 on the Kiss1 system may involve independent processes.
In conclusion, we report a significant circadian regulation of Kiss1 gene expression and transcriptional activation of Kiss1 neurons in the AVPV of female mice housed in constant conditions and exposed to steady-state E2. These results implicate Kiss1 neurons in the AVPV as critical integrators of both sex steroids and circadian signals and suggest that the timing of the LH surge involves temporal activation of Kiss1 neurons. We also determined that this circadian pattern of Kiss1 neuron regulation is E2 dependent, implying that the SCN is incapable of activating Kiss1 neurons in the absence of the molecular/cellular effects of E2.
Footnotes
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (NIH) through cooperative agreements U54 HD12629 (to the University of Washington Center for Research in Reproduction and Contraception) and U54 HD28934 (University of Virginia Ligand Assay Core) as well as Grants K99/R00 HD056157 and R01 HD27142. Additional funding support was provided by NIH Grant R01 MH075016 and an National Science Foundation Graduate Research Fellowship (to J.L.R.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 14, 2009
Abbreviations: AVPV, Anteroventral periventricular nucleus; CT, circadian time; DIG, digoxigenin; E2, estradiol; ERα, estrogen receptor-α; OVX, ovariectomized; RNase, ribonuclease; SCN, suprachiasmatic nucleus; SSC, saline sodium citrate.
References
- Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR 2008 Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 149:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2009 Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J Neuroendocrinol 21:305–311 [DOI] [PubMed] [Google Scholar]
- Herbison AE 2008 Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Schwartz WJ 2006 Timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 147:1148–1153 [DOI] [PubMed] [Google Scholar]
- Alleva JJ, Waleski MV, Alleva FR 1971 A biological clock controlling the estrous cycle of the hamster. Endocrinology 88:1368–1379 [DOI] [PubMed] [Google Scholar]
- Fitzgerald K, Zucker I 1976 Circadian organization of the estrous cycle of the golden hamster. Proc Natl Acad Sci USA 73:2923–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moline ML, Albers HE 1988 Response of circadian locomotor activity and the proestrous luteinizing hormone surge to phase shifts of the light-dark cycle in the hamster. Physiol Behav 43:435–440 [DOI] [PubMed] [Google Scholar]
- Moline ML, Albers HE, Todd RB, Moore-Ede MC 1981 Light-dark entrainment of proestrous LH surges and circadian locomotor activity in female hamsters. Horm Behav 15:451–458 [DOI] [PubMed] [Google Scholar]
- Brown-Grant K, Raisman G 1977 Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci 198:279–296 [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS 2006 Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod 75:778–784 [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS 2004 Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol 14:1367–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G, Brown-Grant K 1977 The ‘suprachiasmatic syndrome’: endocrine and behavioural abnormalities following lesions of the suprachiasmatic nuclei in the female rat. Proc R Soc Lond B Biol Sci 198:297–314 [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E 1982 Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 34:395–404 [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW 1980 Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 31:147–157 [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Stirland JA, Darrow JM, Menaker M, Loudon AS 1999 Free running circadian rhythms of melatonin, luteinizing hormone, and cortisol in Syrian hamsters bearing the circadian tau mutation. Endocrinology 140:758–764 [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Blaustein JD, Bittman EL 1995 The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport 6:1715–1722 [DOI] [PubMed] [Google Scholar]
- Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM 1997 Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol 384:569–579 [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, van der Donk HA, van den Hurk R, Buijs RM 1993 Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J Neuroendocrinol 5:137–144 [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA 2007 Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci 30:504–511 [DOI] [PubMed] [Google Scholar]
- Simerly RB 1998 Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res 92:195–203 [DOI] [PubMed] [Google Scholar]
- Watson Jr RE, Langub Jr MC, Engle MG, Maley BE 1995 Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Res 689:254–264 [DOI] [PubMed] [Google Scholar]
- Popa SM, Clifton DK, Steiner RA 2008 The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol 70:213–238 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA 2004 A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA 2005 Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA 2006 Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K 2007 Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378 [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K 2005 Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146:4431–4436 [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M 2007 Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM 2005 Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102:15682–15687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM 2008 Classical estrogen receptor α signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology 149:5328–5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Buenzle J, Fraley GS, Rissman EF 2005 Effects of galanin-like peptide (GALP) on locomotion, reproduction, and body weight in female and male mice. Horm Behav 48:141–151 [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Rissman EF 2004 A critical role for the evolutionarily conserved gonadotropin-releasing hormone. II. Mediation of energy status and female sexual behavior. Endocrinology 145:3639–3646 [DOI] [PubMed] [Google Scholar]
- Finn PD, Steiner RA, Clifton DK 1998 Temporal patterns of gonadotropin-releasing hormone (GnRH), c-fos, and galanin gene expression in GnRH neurons relative to the luteinizing hormone surge in the rat. J Neurosci 18:713–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett JW, Sawyer CH 1950 A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology 47:198–218 [DOI] [PubMed] [Google Scholar]
- Siegel HI, Bast JD, Greenwald GS 1976 The effects of phenobarbital and gonadal steroids on periovulatory serum levels of luteinizing hormone and follicle-stimulating hormone in the hamster. Endocrinology 98:48–55 [DOI] [PubMed] [Google Scholar]
- Stetson MH, Gibson JT 1977 The estrous cycle in golden hamsters: a circadian pacemaker times preovulatory gonadotropin release. J Exp Zool 201:289–294 [DOI] [PubMed] [Google Scholar]
- Stetson MH, Watson-Whitmyre M 1977 The neural clock regulating estrous cyclicity in hamsters: gonadotropin release following barbiturate blockade. Biol Reprod 16:536–542 [PubMed] [Google Scholar]
- Legan SJ, Coon GA, Karsch FJ 1975 Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology 96:50–56 [DOI] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ 1975 A daily signal for the LH surge in the rat. Endocrinology 96:57–62 [DOI] [PubMed] [Google Scholar]
- Norman RL, Blake CA, Sawyer CH 1973 Estrogen-dependent 24-hour periodicity in pituitary LH release in the female hamster. Endocrinology 93:965–970 [DOI] [PubMed] [Google Scholar]
- Stetson MH, Watson-Whitmyre M 1976 Nucleus suprachiasmaticus: the biological clock in the hamster? Science 191:197–199 [DOI] [PubMed] [Google Scholar]
- Kauffman AS 2009 Sexual differentiation and the Kiss1 system: Hormonal and developmental considerations. Peptides 30:83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Schwartz WJ 2003 Lateralization of circadian pacemaker output: activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J Neurosci 23:7412–7414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL 1999 Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology 140:207–218 [DOI] [PubMed] [Google Scholar]
- Gu GB, Simerly RB 1997 Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol 384:142–164 [PubMed] [Google Scholar]
- Simerly RB, Swanson LW 1987 The distribution of neurotransmitter-specific cells and fibers in the anteroventral periventricular nucleus: implications for the control of gonadotropin secretion in the rat. Brain Res 400:11–34 [DOI] [PubMed] [Google Scholar]
- Kallo I, Vida B, Deli L, Kalamatianos T, Hrabovszky E, Caraty A, Coen CW, Liposits Z, Evidence that kisspeptin neurons in the AVPV form part of a bisynaptic pathway connecting suprachiasmatic vasopressin neurons with preoptic GnRH cells. Program of the 38th Annual Meeting of the Society for Neuroscience, Washington, DC, 2008 (Abstract 618.4) [Google Scholar]
- Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A 2001 The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res 901:109–116 [DOI] [PubMed] [Google Scholar]
- Palm IF, Van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A 1999 Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience 93:659–666 [DOI] [PubMed] [Google Scholar]
- Chappell PE, Goodall CP, Tonsfeldt KJ, White RS, Bredeweg E, Latham KL 2009 Modulation of gonadotropin-releasing hormone (GnRH) secretion by an endogenous circadian clock. J Neuroendocrinol 21:339–345 [DOI] [PubMed] [Google Scholar]
- Chappell PE, White RS, Mellon PL 2003 Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci 23:11202–11213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I 1997 The distribution of estrogen receptor-β mRNA in forebrain regions of the estrogen receptor-α knockout mouse. Endocrinology 138:5649–5652 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- De La Iglesia HO, Blaustein JD, Bittman EL 1999 Oestrogen receptor-α-immunoreactive neurones project to the suprachiasmatic nucleus of the female Syrian hamster. J Neuroendocrinol 11:481–490 [DOI] [PubMed] [Google Scholar]