Abstract
In the ovary, the matrix metalloproteinases (MMPs) and the tissue inhibitors of metalloproteinase (TIMPs) have been postulated to regulate extracellular matrix remodeling associated with ovulation. In the present study, we investigated the regulatory mechanisms controlling expression of Timp1 and Timp3 mRNA in periovulatory granulosa cells. Granulosa cells were isolated from immature pregnant mare serum gonadotropin-primed (10 IU) rat ovaries and treated with human chorionic gonadotropin (hCG; 1 IU/ml). At 4 h after hCG treatment, Timp1 expression was highest and then decreased gradually over the remaining 24 h of culture. In contrast, hCG induced a biphasic increase of Timp3 expression at 2 and 16 h. The hCG stimulated expression of Timp1 and Timp3 mRNA was blocked by inhibitors of the protein kinase A (H89), protein kinase C (GF109203), and MAPK (SB2035850) pathways. To further explore Timp1 and Timp3 regulation, cells were cultured with the progesterone receptor antagonist RU486, which blocked the hCG induction of Timp3 expression, whereas the epidermal growth factor receptor tyrosine kinase inhibitor AG1478 blocked the hCG stimulation of both Timp1 and Timp3 expression. The prostaglandin-endoperoxide synthase 2 inhibitor NS-398 had no effect. The potential function of TIMP3 was investigated with Timp3-specific small interfering RNA treatment. Timp3 small interfering RNA resulted in a 20% decrease in hCG-induced progesterone levels and microarray analysis revealed an increase in cytochrome P450 Cyp 17, ubiquitin conjugating enzyme E2T, and heat shock protein 70. IGF binding protein 5, stearyl-CoA desaturase, and annexin A1 were decreased. The differential regulation between Timp1 and Timp3 may correlate with their unique roles in the processes of ovulation and luteinization. For TIMP3, this may include regulating fatty acid synthesis, steroidogenesis, and protein turnover.
The hCG-stimulated pathways regulating TIMP1 and TIMP3 expression are elucidated, and reducing TIMP3 expression indicates a role in regulating fatty acid synthesis, steroidogenesis, and protein turnover.
The extracellular matrix (ECM) is a dynamic structure that not only provides a scaffold for organizing tissue architecture but also contributes signals that regulate cell function. Remodeling of the ECM is controlled, in part, by the actions of members of the matrix metalloproteinase (MMP) family (1,2,3,4). Examples of ovarian ECM remodeling are observed during follicular growth, ovulation, corpus luteum formation, and regression (5,6). It is postulated that matrix remodeling is the result of a shift in the balance between active MMPs vs. tissue inhibitors of metalloproteinase (TIMPs), which regulates local MMP action in the extracellular space (5,6). Thus, coordinate regulation of these proteinases and inhibitors is required to maintain the tissue architecture and normal ovarian function.
Currently there are four distinct TIMP family members which differ in their regulation, affinity for the MMPs and mode of action (4,7,8). TIMPs 1, 2, and 4 are secreted and act in the extracellular space, whereas TIMP3 is bound to the ECM. In addition to their classical roles of inhibiting MMP activity, TIMPs have been proposed to act as multifunctional proteins that stimulate proliferation of various cell types (4,7,8) and stimulate progesterone production by steroidogenic cells (9).
TIMP3 is unique among the TIMP family in that it is bound to the ECM rather than remaining as a soluble protein (4,7,8). It has a broader inhibition profile that extends to members of the A disintegrin and metalloproteinase with thrombospondin-like repeats families, proteases that have a role in cumulus oocyte expansion during the ovulatory process (10). Because TIMP3 is bound to the ECM, it was hypothesized that TIMP3 maintained the integrity of the ECM and supported cell growth by regulating ECM-bound growth factors (4,7,8). Support for this concept is forthcoming from numerous studies. For example, addition of TIMP3 to fibroblast cells preserved the ECM and supported cell growth (11). In contrast, the deletion of TIMP3 in the lung results in a shift of the TIMP/MMP balance favoring ECM degradation culminating in a reduced life span (12). Therefore, the ability of TIMP3 to regulate proteolysis is crucial for control of the ECM and ultimately cell growth and survival.
In the ovary, both TIMP1 and TIMP3 are hormonally regulated. The levels of Timp1 mRNA increase after an LH/human chorionic gonadotropin (hCG) stimulus in the rat and mouse and decline during the midluteal period of pregnancy or pseudopregnancy to remain low during the late luteal phase (13,14,15,16,17,18). In contrast, the expression of Timp3 mRNA in whole ovaries collected after an ovulatory stimulus exhibits little change in the rodent (19,20,21). The mRNA for both Timp1 and Timp3 are present in the theca during follicular growth but are switched on to become highly expressed in granulosa cells of preovulatory follicles in response of an LH/hCG stimulus (14,16,22). Although the patterns of Timp1 and Timp3 expression and localization have been investigated in vivo, surprisingly little is known about their regulation. In the present study, we investigated the regulatory mechanisms involved in expression of Timp1 and Timp3 mRNA in vitro using preovulatory granulosa cells and investigated the function of TIMP3 in vitro using a small interfering RNA (siRNA) approach.
Materials and Methods
Materials and reagents
Unless otherwise noted, all chemicals and reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Molecular biological enzymes, molecular size markers, oligonucleotide primers, pCRII-TOPO vector, culture media, and Trizol were purchased from Invitrogen Life Technologies, Inc. (Carlsbad, CA).
Rat granulosa cell culture
All animal procedures for these experiments were approved by the University of Kentucky Institutional Animal Care and Use Committee. Immature female rats (Harlan Sprague Dawley Inc., Indianapolis, IN) were injected with pregnant mare serum gonadotropin (PMSG; 10 IU sc) between 0900 and 1000 h on the morning of d 22–23 of age to stimulate folliculogenesis. To isolate granulosa cells, ovaries were collected from rats 48 h after PMSG administration and processed as described previously (13). Briefly, granulosa cells were isolated by follicular puncture, pooled, filtered, pelleted by centrifugation, and resuspended in Opti-MEM (Invitrogen) supplemented with 0.05 mg/ml of gentamicin and 1× insulin, transferrin, and selenium. The cells were cultured in the absence or presence of various reagents discussed in detail below for different time points at 37 C in a humidified atmosphere of 5% CO2. When reagents were dissolved in dimethylsulfoxide, the same concentration of dimethylsulfoxide was added to medium for the control cells. hCG was added after 1 h incubation with reagents. At the end of each culture period, cells were collected and snap frozen for later isolation of total RNA and protein.
Quantification of mRNA for Timp1 and Timp3
Total RNA was isolated from cultured granulosa cells using Trizol reagent and quantified by spectrophotometry. To measure the levels of Timp1 and Timp3 mRNA in granulosa cells, Northern blot analyses were carried out as routinely performed in our laboratory. Rat cDNA for Timp1, Timp3, and the ribosomal protein L32 were labeled using random primer labeling kit (Invitrogen). Northern blot membranes were hybridized with 32P-labeled cDNA probes in NorthernMax hybridization buffer (Applied Biosystems, Austin, TX) at 68 C overnight. Excess probe was removed by washing with a stringent buffer (2× saline sodium citrate, 0.1% sodium dodecyl sulfate) twice at 68 C for 60 min. The membrane was then quantified with a phosphor imager (GE Healthcare, Piscataway, NJ). The relative levels of Timp1 and Timp3 mRNA were normalized to L32 mRNA levels.
Real-time PCR quantification of mRNA
We used real-time PCR to measure Timp3 mRNA in the siRNA experiment as well as to validate the microarray results. Briefly, total RNA was treated with 0.2 U deoxyribonuclease I to eliminate possible genomic DNA contamination. Synthesis of first-strand cDNA was performed by reverse transcription of 0.5 μg total RNA using SuperScript II with Oligo(dT)18 primer according to the manufacturer’s protocol (Invitrogen). Oligonucleotide primers corresponding to cDNA for rat L32 (accession no. BC061562; forward, 5′-GAA-GCC-CAA-GAT-CGT-CAA-AA-3′; reverse, 5′-AGG-ATC-TGG-CCC-TGG-CCC-TTG-AAT-CT-3′), rat Hspa1a (accession no. NM_031971; forward, 5′-CTA-CGC-CTT-CAA-TAT-GAA-GAG-C-3′; reverse, 5′-CAC-GAA-CTC-CTC-TTT-CTC-AGC-3′), rat Col8a1 (accession no. NM_001107100; forward, 5′-CCA-TGA-TGT-ACA-CAT-ACG-ACG-3′, reverse, 5′-TAT-TGC-CCA-GCA-TAG-AGT-CC-3′), rat Anxa1 (accession no. NM_ 012904; forward, 5′-GAC-ATC-CTT-ACC-AAG-AGA-ACC-3′; reverse, 5′-CAT-CTG-CAT-CAA-ACT-GAG-C-3′), rat Timp3 (accession no. BC097335; forward, 5′-TCT-GCA-ACT-CCG-ACA-TCG-3′, reverse, 5′-GCG-TAG-TGT-TTG-GAC-TGA-TAG-C-3′) were designed using PRIMER3 software (http://frodo.wi.mit.edu/), and the specificity for each primer set was confirmed by both electrophoresis of the PCR products and analyzing the melting (dissociation) curve after each real-time PCR. The real-time PCR contained 10% of the reverse transcription reaction product, 0.4 μm of forward and reverse primers, 0.3 μl of 1:10 diluted ROX reference dye (SYBR Green ER quantitative PCR SuperMix universal kit; Invitrogen), and SYBR Green SuperMix. PCRs were performed on a Mx3000P QPCR system (Stratagene, La Jolla, CA). The relative amount of Timp3 transcript was calculated using the 2-ΔΔCT method and normalized to the endogenous reference gene L32.
Western blot analysis
Granulosa cell lysates were prepared using radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology, Santa Cruz, CA). Twenty micrograms of protein were separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. Western blotting was performed by blocking nonspecific binding with 5% dry milk in Tris-buffered saline buffer containing 0.1% Tween 20 for 1 h. Blots were then incubated with the primary antibody, TIMP3 (1:1000; Abcam, Cambridge, MA) or β-actin (1:2000; Cell Signaling Technology, Danvers, MA), overnight at 4 C on a rocking platform. After a series of washes, blots were incubated with a secondary antibody linked to horseradish peroxidase for 1 h. After extensive washing, blots were analyzed using an enhanced chemiluminescence detection system (GE Healthcare) and exposed to x-ray film. Data for TIMP1 protein levels is not shown as we were unable to achieve a consistent band that was blocked with exogenous TIMP1.
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)- 2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) cell viability assay
Granulosa cells were collected from PMSG-primed ovaries and seeded in 96-well plates (5000 cells/well). Cells were incubated with or without human recombinant TIMP3 protein (0.1 and 1 μg/ml; Triple Point Biologics, Forest Grove, OR) for 24, 48, or 72 h. Although the ovarian concentration of TIMP3 is unknown, these doses were modeled on previous reports (24,25,26,27). Before measuring viability, treatment media were removed and replaced with 100 μl of fresh Opti-MEM (supplemented with 0.05 mg/ml of gentamicin and 1× insulin, transferrin, and selenium) and 20 μl MTS solution (Promega, Madison, WI). Cells were incubated for an additional 2 h. The absorbance was measured at 492 nm to determine the formazan concentration, which is proportional to the number of live cells (28).
Trypan blue staining
Granulosa cells were collected from PMSG-primed ovaries and seeded in 96-well plates (5000 cells/well). Cells were incubated with or without human recombinant TIMP3 protein (0.1 and 1 μg/ml) for 72 h. Treatment media were removed and granulosa cells were suspended using 3% trypsin. Trypan Blue stain was mixed with the cell suspension and incubated at room temperature for 5 min. Viable and dead cells were counted using a hemocytometer.
Knockdown of TIMP3 by siRNA in granulosa cells
Granulosa cells were collected 48 h after PMSG administration as described above. Timp3 Stealth Select RNAi or Stealth RNAi Negative Control Med GC (Invitrogen) was transfected into granulosa cells using the Lipofectamine 2000 reagent (Invitrogen). After transfection for 1 h, cells were treated with hCG (1 IU/ml) and incubated for an additional 16 h at 37 C. The cells were collected and snap frozen for isolation of total RNA for real-time PCR, DNA microarray, or processed to prepare cell lysates for Western blot analysis. The conditioned culture media were collected and analyzed for progesterone (DPC Immulite; Siemens Healthcare Diagnostics Inc., Flanders, NJ).
DNA microarray analysis of granulosa cells after siRNA knockdown of TIMP3
Granulosa cells were cultured as described above and total RNA was extracted from cells cultured with Timp3 siRNA or the negative control RNA using Trizol reagent and further purified using a RNeasy kit according to the manufacturer’s instructions (n = 3; QIAGEN Inc., Valencia, CA). Five micrograms of total RNA were used as a template for cDNA synthesis by the University of Kentucky Microarray Core facility as described previously (29). Biotinylated antisense cRNA probes were prepared and the integrity of the riboprobe was confirmed by gel electrophoresis. The Affymetrix Rat 230A and 230B oligonucleotide array sets (Affymetrix, Santa Clara, CA) were hybridized, washed, and scanned using Affymetrix equipment and protocols (Affymetrix). The DNA microarray assays were performed on total RNA pooled from granulosa cells obtained from three separate experiments. The changes observed by DNA microarray analysis were confirmed by real-time PCR for a select subset of genes.
Statistical analyses
All data are presented as means ± sem. Two-way ANOVA was used to test differences in Timp1 and Timp3 expression across time of culture and treatment. One-way ANOVA was used to test differences in Timp1 and Timp3 expression among treatments in vitro. If ANOVA revealed significant effects of time of tissue collection, time of culture, or treatment, the means were compared by Duncan’s test or Tukey’s test, with P < 0.05 considered significant.
Results
hCG-induced mRNA expression of Timp1 and Timp3 in granulosa cells
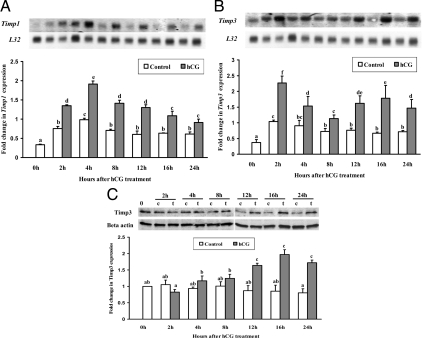
We previously demonstrated that Timp1 and Timp3 mRNA expression is induced in preovulatory follicles after hCG injection using in situ hybridization (2). To determine the signaling pathways by which hCG regulates Timp1 and Timp3 mRNA expression, granulosa cells isolated from rat ovaries 48 h after PMSG were cultured in the absence or presence of hCG (1 IU/ml). Timp1 mRNA expression was highest at 4 h after hCG treatment and then decreased gradually over the 24 h of culture (Fig. 1A). hCG induced a biphasic increase of Timp3 mRNA expression at 2 h (early response) and 16 h (late response, Fig. 1B) after treatment. A drop in Timp3 mRNA levels occurred at 8 h after hCG treatment. Western blot analysis showed that TIMP3 protein levels increased 12 h after hCG treatment and remained high through 24 h of hCG treatment (Fig. 1C).
Figure 1.
Stimulation of Timp1 (A) and Timp3 (B) mRNA expression by hCG in rat granulosa cells in vitro. Autoradiograph of a representative Northern blot analysis shows the expression of Timp1 and Timp3 mRNA and ribosomal protein L32 mRNA in granulosa cells obtained from rat preovulatory ovaries (48 h after PMSG) and cultured in medium alone (control) or with hCG (1 IU/ml) for 0, 2, 4, 8, 12, 16, or 24 h. Relative levels of mRNA for Timp1 and Timp3 were normalized to the L32 band in each sample and expressed as a fold change relative to the 0 h control (mean ± sem; n = 3 independent culture experiments). C, Western blot shows TIMP3 protein levels in cells cultured with media alone [control (c)] or after hCG treatment (t, hCG treatment) in granulosa cell culture (mean ± sem; n = 3 independent culture experiments). Bars with no common superscript letters are significantly different (P < 0.05). The figure illustrates different temporal patterns of hCG induction of Timp1 and Timp3 mRNA.
Intracellular signaling pathways controlling Timp1 and Timp3 mRNA expression in vitro
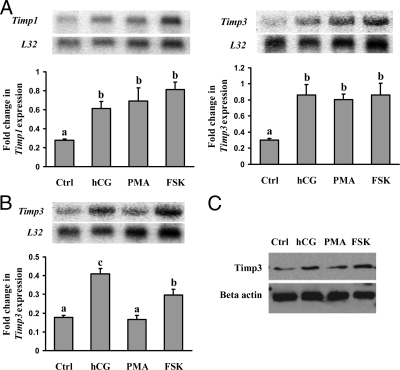
The preovulatory LH/hCG stimulus is known to activate both the protein kinase A (PKA) and protein kinase C (PKC) signaling pathways in preovulatory granulosa cells (30). To determine which signaling pathway(s) is involved in the up-regulation of Timp1 and Timp3 mRNA in response to hCG stimulation, granulosa cells were cultured with an activator of adenylate cyclase, forskolin (FSK), or an activator of PKC, phorbol 12-myristate 13-acetate (PMA). Cells were collected at 2 or 16 h after treatment. Both FSK and PMA induced Timp1 and Timp3 mRNA expression at 2 h (Fig. 2A), suggesting Timp1 and Timp3 expression at this early time point was mediated by the hCG-induced activation of both the PKA and PKC pathways. In contrast to the early response of Timp3 induction, only FSK up-regulated the late Timp3 mRNA expression at 16 h (Fig. 2B), indicating that the late Timp3 mRNA surge is mediated predominantly through the PKA pathway. TIMP3 protein levels were also stimulated by FSK at 16 h of hCG treatment (Fig. 2C).
Figure 2.
Regulation of Timp1 and Timp3 mRNA expression by activators of intracellular signaling pathways in granulosa cells in vitro. Autoradiograph of a representative Northern blot analysis shows mRNA for Timp1 and Timp3 and ribosomal protein L32 in granulosa cells from rat preovulatory ovaries (48 h after PMSG) cultured in medium alone (Ctrl) or with hCG (1 IU/ml), FSK (10 μm), or PMA (20 nm) for 2 h (A) or 16 h (B). Relative levels of mRNA for Timp1 and Timp3 were normalized to the L32 band in each sample and expressed as a fold change relative to the untreated control (mean ± sem; n = 4 independent culture experiments). C, Western blot shows TIMP3 protein levels in cells cultured in media alone (Ctrl), hCG, PMA, or FSK. Bars with no common superscript letters are significantly different (P < 0.05).). The figure demonstrates that both FSK and PMA induced Timp1 and Timp3 mRNA expression at 2 h, whereas only FSK up-regulated the Timp3 mRNA expression at 16 h.
To further investigate the mechanisms regulating Timp1 and Timp3 mRNA expression by hCG, granulosa cells were cultured with medium alone (Ctrl), inhibitors of various hCG induced signaling molecules [inhibitors of PKA (H89, 10 μm), PKC (GF109203X, 1 μm), phosphatidylinositol 3 kinase (PI3 kinase; LY294002, 25 μm), MAPK kinase (MEK; PD98059, 20 μm), and p38 kinase (SB2035850, 20 μm)], hCG, or hCG in combination with the above inhibitors. Northern blot results showed that both PKA and PKC pathway inhibitors could completely block Timp1 (Fig. 3A) and Timp3 mRNA induction either at 2 or 16 h (Fig. 3, B and C). The Western blot results showed that TIMP3 protein levels were reduced by H89 and GF109203X at 16 h of hCG treatment (Fig. 3D) corresponding to the mRNA results. The other pathway inhibitors also diminished Timp1 expression, although not to the same extent as the general PKA and PKC pathway inhibitors (Fig. 3A). Regulation of the two phases of Timp3 expression appeared to be under differential control. Of interest was the observation that treatment of the cells with the phosphatidylinositol 3-kinase inhibitor LY294002 stimulated Timp3 expression at 2 h after hCG, whereas the inhibitor decreased expression at 16 h (Fig. 3, B and C). Likewise, the MEK inhibitor PD98059 reduced Timp3 expression at the 2-h time point, whereas the inhibitor stimulated expression at 16 h after hCG (Fig. 3C).
Figure 3.
Regulation of Timp1 and Timp3 mRNA expression by inhibitors of intracellular signaling pathways in granulosa cells in vitro. Granulosa cells were cultured with medium alone (Ctrl), inhibitors of various signaling molecules [an inhibitor of PKA (H89, 10 μm), MEK (PD98059 [PD], 20 μm), p38 kinase (SB2035850 [SB], 20 μm), PI3 kinase (LY294002 [LY], 25 μm), PKC (GF109203X [GF], 1 μm)], hCG, or hCG + inhibitors of various signaling molecules for 2 h (A and B) or 16 h (C). Autoradiograph of a representative Northern blot analysis shows mRNA for Timp1 (A), Timp3 early (B), and Timp3 late (C) and their associated ribosomal protein L32 mRNA. Relative levels of mRNA for Timp1 or Timp3 were normalized to the L32 band in each sample and expressed as a fold change relative to the untreated control (mean ± sem; n = 3 independent culture experiments). D, Western blot shows TIMP3 protein levels in cells cultured for 16 h in media alone (Ctrl), hCG, hCG + H89 or hCG + GF109203X. Bars with no common superscript letters are significantly different (P < 0.05). The figure depicts that common pathways exist in the induction of Timp1 and Timp3 mRNA; however, differences were noted in the stimulation of Timp3 mRNA to PD and LY treatment.
Hormonal regulation of Timp1 and Timp3 expression in granulosa cells
The LH surge or hCG stimulates a number of steps that are crucial for ovulation including activation of epidermal growth factor (EGF) signaling, induction of progesterone receptors, and generation of prostaglandin-endoperoxide synthase 2 (PTGS2) (31,32,33). We tested whether the up-regulation of Timp1 and Timp3 mRNA is mediated by the hCG-induced activation of these signaling pathways. Timp1 and Timp3 expression was not affected by the PTGS2 inhibitor NS-398 (Fig. 4). The progesterone receptor antagonist (RU486) had no effect on Timp1 expression but reduced Timp3 late mRNA expression, which suggested progesterone involvement in the regulation of Timp3 expression induced at 16 h (Fig. 4). The EGF receptor tyrosine kinase inhibitor AG1478 blocked Timp1 and Timp3 early response but had no effect on Timp3 late response (Fig. 4), indicating EGF receptor activation was involved in Timp1 and early Timp3 mRNA expression at 2 h after hCG treatment.
Figure 4.
Hormonal regulation of the hCG-induced Timp1 and Timp3 mRNA expression in cultured rat granulosa cells. The cells were cultured in medium alone (Ctrl), with the progesterone receptor antagonist RU486 (RU; 1 μm), the prostaglandin-endoperoxide synthase 2 inhibitor NS-398 (NS; 1 μm), the EGF receptor tyrosine kinase selective inhibitor AG1478 (AG; 1 μm), the new protein synthesis inhibitor cyclohexamide (CHX; 1 μg/ml), hCG (1 IU), or hCG + the inhibitors of various hormonal signaling molecules for 2 h (A and B) or 16 h (C). Autoradiograph of a representative Northern blot analysis shows mRNA for Timp1 (A), Timp3 early (B), and Timp3 late (C), and their associated ribosomal protein L32 in granulosa cells cultured with the treatments above. Relative levels of mRNA for Timp1 and Timp3 were normalized to the L32 band in each sample and expressed as a fold change relative to the untreated control (mean ± sem; n = 3 independent culture experiments). D, Western blot shows TIMP3 protein levels in cells cultured for 16 h in media alone (Ctrl) or with hCG, hCG + CHX or hCG + RU486. Bars with no common superscript letters are significantly different (P < 0.05). The figure shows that induction of Timp1 and the early peak of Timp3 mRNA were inhibited by AG, whereas the late peak of Timp3 mRNA was blocked with RU486. The induction of both peaks of Timp3 mRNA was blocked with cycloheximide, suggesting a dependence on new protein(s) synthesis.
To determine whether the hCG-induced increase in Timp1 and Timp3 mRNA levels in preovulatory granulosa cells requires de novo protein synthesis, granulosa cells were incubated for 2 or 16 h with or without hCG treatment in the absence or presence of cyclohexamide (1 μg/μl), an inhibitor of new protein synthesis. Cyclohexamide completely blocked hCG induced early (2 h) and late (16 h) Timp3 mRNA expression (Fig. 4, B and C), whereas cyclohexamide actually increased Timp1 expression (Fig. 4A). TIMP3 protein levels were also blocked by cyclohexamide and RU486 at 16 h of hCG treatment (Fig. 4D). These results suggest that expression of Timp3 mRNA observed at 2 and 16 h after hCG is dependent on the action of a newly synthesized protein(s).
Effect of TIMP3 on granulosa cell mitochondrial activity
TIMP3 has been reported in other tissues to both support cell growth (11) and induce apoptosis (34). To assess TIMP3’s ability to induce changes in granulosa cell mitochondrial activity associated with cell growth and apoptosis, cells were cultured with exogenous TIMP3 in the presence or absence of hCG and assayed by the MTS assay. The MTS assay is based on mitochondrial reductase activity and is widely used as a measure of cell growth and apoptosis (28). After 24 h of culture, there was an increase in reductase activity in cells treated with hCG or hCG+ a low dose of TIMP3 (0.1 μg/ml) (Fig. 5A). However, addition of the high dose of TIMP3 (1 μg/ml) decreased reductase activity in hCG-treated cells. By 48 h, there was an increase in reductase activity in cells treated with hCG. Addition of the high dose of TIMP3 (1 μg/ml) decreased reductase activity in both basal (no hCG) and hCG-treated cells, whereas the lower dose of TIMP3 (0.1 μg/ml) was without effect (Fig. 5A). A similar pattern was observed after 72 h of culture (Fig. 5A). Cell viability was also measured by a trypan blue exclusion assay at 72 h of culture. The results showed that hCG treatment could increase the percent of viable granulosa cells, whereas addition of the high dose of TIMP3 (1 μg/ml) decreased the number of viable granulosa cells in both basal (no hCG) and hCG-treated cells (Fig. 5B). The lower dose of TIMP3 (0.1 μg/ml) was without effect (Fig. 5B).
Figure 5.
Effect of TIMP3 on granulosa cell viability. A, The cells were cultured in medium alone (control), with exogenous TIMP3 (0.1 and 1 μg/ml) in the presence or absence of hCG for 24, 48, and 72 h. Granulosa cell viability was measured using the MTS assay (mean ± sem; n = 3 independent culture experiments). B, The cells were cultured in medium alone (control), with exogenous TIMP3 (0.1 and 1 μg/ml) in the presence or absence of hCG for 72 h. Granulosa cell viability was measured using Trypan Blue staining (mean ± sem; n = 3 independent culture experiments). Bars with no common superscript letters are significantly different within a time point (P < 0.05). The figure illustrates that physiological stimulation with hCG does not alter cell viability and only the highest dose of TIMP3 decreases cell viability.
Gene expression patterns after down-regulation of TIMP3 in cultured granulosa cells
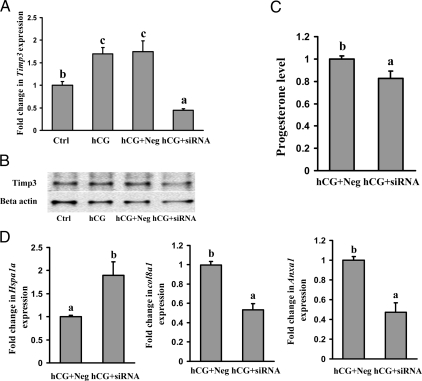
To investigate the potential function of TIMP3, granulosa cells from preovulatory ovaries were transfected with siRNA specific for Timp3 to block the hCG-induced expression of Timp3 mRNA. As determined by real-time PCR and Western blot analyses, the levels of Timp3 mRNA and protein were reduced approximately 75% in Timp3-specific siRNA-treated granulosa cells compared with that in negative control siRNA-treated cells (Fig. 6, A and B). Transfection of siRNA to primary granulosa cells was confirmed by BLOCK-iT Fluorescent Oligo (Invitrogen) (data not shown). There was no significant difference in levels of Timp3 mRNA between hCG alone compared with hCG with the negative control siRNA-treated cells (Fig. 6, A and B). Timp3 siRNA resulted in a 20% decrease in the progesterone level in the conditioned culture media, suggesting that TIMP3 may modulate steroidogenesis by luteinizing granulosa cells (Fig. 6C).
Figure 6.
Effect of reduction in Timp3 mRNA expression by siRNA in cultured granulosa cells. Granulosa cells obtained from rat preovulatory ovaries (48 h after PMSG) were cultured with no treatment (Ctrl) or with hCG (1 IU/ml), hCG + negative control siRNA, or hCG + Timp3 siRNA. A, Real-time PCR results show a decrease in Timp3 mRNA expression in granulosa cells cultured for 16 h. Relative levels of mRNA for Timp3 were normalized to the L32 band in each sample (mean ± sem; n = 3 independent culture experiments). B, A representative Western blot of three separate culture experiments shows TIMP3 protein levels in preovulatory granulosa cells cultured with hCG (1 IU/ml) + negative control siRNA or hCG + Timp3 siRNA for 16 h. C, Concentrations of progesterone in granulosa cell conditioned culture media are decreased in cells treated with hCG + Timp3 siRNA compared with cells cultured with hCG + negative control siRNA for 16 h (mean ± sem; n = 3 independent culture experiments). D, The expression of three genes, Col8a1, Hspa1a, and Anxa1, was compared using real-time PCR between the negative control siRNA and Timp3 siRNA (mean ± sem; n = 3 independent culture experiments). Bars with no common superscript letters are significantly different within a time point (P < 0.05). The figure shows that reduction of TIMP3 by siRNA reduces progesterone levels reduces Col8a1 and Anxa1 mRNA expression but increases the levels of Hspa1a mRNA.
Total RNA from the negative control siRNA-treated cells and Timp3 siRNA-treated cells were analyzed using the Affymetrix Rat 230A and 230B oligonucleotide array sets. The genes up-regulated greater than 2.5-fold or down-regulated by more than 60% after Timp3 siRNA are shown in supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. Data from the microarray exhibited an increase in cytochrome P450 Cyp 17, an enzyme involved in the hydroxylation of progestin. Another set of genes that was up-regulated controls protein folding and ubiquitination: heat shock protein 70, the ubiquitin conjugating enzyme, and ubiquitin ligase. Additionally, genes associated with signaling pathways such as the inhibitor of cAMP-dependent protein kinase activity, which inhibits both cAMP and cGMP-dependent protein kinase (35) and the adenylate cyclase activating polypeptide 1 were up-regulated.
Genes whose expression decreased by 60% or greater after Timp3 siRNA treatment of granulosa cell are shown in supplemental data. The most highly down-regulated gene was IGF binding protein 5. There were also transcription factors (e.g. ring finger and FYVE-like domain containing protein, ATP-binding cassette, and inhibitor of DNA binding mRNA) as well as regulators of cell differentiation and proliferation (e.g. neurofilament mRNA, glutamyl aminopeptidase, synaptopodin-2, and neuregulin) that were down-regulated. Because the microarray data are based on a pooled sample from three different experiments, the changes observed by microarray were confirmed by real-time PCR for genes of interest. Specifically, the genes down-regulated by microarray such as collagen 8a1 and annexin A1 were similarly down-regulated when analyzed by real-time PCR (Fig. 6D). Likewise genes that were up-regulated by microarray, such as heat shock protein 70, were also increased when analyzed by real-time PCR (Fig. 6D).
Discussion
Coordinated spatiotemporal expression patterns of the MMPs and their inhibitors are important for ECM turnover in numerous reproductive tissues including the ovary (5,6). In the present study, we demonstrate that hCG induced a rapid and transient expression of Timp1 and Timp3 mRNA in cultured rat granulosa cells; however, the expression patterns differed between the two TIMPs. Induction of Timp1 and Timp3 mRNA by hCG occurs in part through the activation of the PKA pathway and downstream mediators such as MEK and p42/44 MAPK (36). This is evident from the stimulation of Timp1 and Timp3 mRNA expression by FSK and the decrease by inhibitors that block the PKA pathway and MEK. An LH/hCG stimulus also activates the PKC pathway (30), which is able to induce expression of Timp1 and Timp3 mRNA as observed by the stimulation of Timp1 and Timp3 mRNA expression by PMA and the diminution in mRNA expression after administration of the PKC inhibitor GF109203X. Our observation that PMA increased Timp3 mRNA within 2 h but did not stimulate expression of Timp3 mRNA after 16 h of incubation may reflect the inability of PMA to mimic the long-term, sustained effect of hCG to stimulate additional pathways that maintain or facilitate Timp3 mRNA induction. Furthermore, there is a discordance between the increase in TIMP3 mRNA and a corresponding increase in TIMP3 protein at the early time point. This has been observed in other systems (37,38,39,40,41) and has been proposed to reflect differences in ubiquitination (39) or posttranscriptional changes (37,40).
An LH/hCG stimulus initiates other kinase signaling pathways such as the cAMP dependent, PKA-independent activation of PI3 kinase (42), receptor tyrosine kinase (43), and the phosphorylation of p38 kinase by a PKA- and PKC-independent pathway (36). The cAMP-dependent, PKA-independent pathway occurs through the actions of cAMP-guanine nucleotide exchange factors (cAMP-guanine nucleotide exchange factors), which activate PI3 kinase and phosphoinositide-dependent kinase (45). These alternative pathways may account for the distinct differences in the regulation of the Timp1 and Timp3 as well as the control of the early and late peaks of Timp3 mRNA. For example, the administration of the PI3 kinase inhibitor LY294002 reduced the late expression of Timp3 mRNA but, interestingly, increased both the basal and hCG-stimulated early expression of Timp3 mRNA. This stimulation would suggest that the PI3 kinase pathway exerts some tonic inhibition of Timp3 mRNA expression, which may be independent of hCG action. Although the nature of the PI3 kinase signal acting to dampen Timp3 mRNA expression is unknown, integrin-linked kinase and downstream signals such as PKB/akt may be involved because this pathway regulates the induction of MMPs in human ovarian surface epithelial cells (46).
The TIMPs may be regulated by the action of autocrine and/or paracrine factors synthesized in preovulatory granulosa cells in response to the LH surge. Induction of the progesterone receptor, PTGS2, and EGF-related peptides has been shown to be rapid and transient in preovulatory granulosa cells in response to hCG in vivo and in vitro (47). Their importance in the ovulatory process is underscored by the inhibition of oocyte release in models in which these factors are lacking or attenuated (48,49,50). In the present study, the early induction of Timp1 and Timp3 mRNA occurs at a time immediately before maximal induction of the progesterone receptor and PTGS2, so it was not unexpected that neither the progesterone receptor antagonist nor PTGS2 inhibitor regulated these TIMPs at this early time point. However, RU486 reduced Timp3 late mRNA expression, which supports progesterone involvement in the regulation of Timp3 expression induced at 16 h after hCG.
Members of the EGF-like growth factor family are rapidly induced by LH/hCG and are postulated to function in an autocrine and paracrine manner to propagate LH signals throughout the preovulatory follicle (51). In the present study, the EGF receptor inhibitor AG1478 blocked Timp1 and Timp3 early response but had no effect on Timp3 late response, indicating EGF receptor activation was involved in the early expression of Timp1 and Timp3 mRNA. This increase in mRNA levels may occur through either the LH-induced rapid stimulation of EGF-related peptides, such as amphiregulin or epiregulin, or the cleavage of EGF-related peptides by a metalloproteinase from the granulosa cell surface (43). However, the simple shedding of the EGF-related peptide is unlikely to account for the regulation of both Timp1 and Timp3 mRNA expression because Timp3 mRNA induction requires new protein synthesis. For example, cells treated with cyclohexamide demonstrated that the initial stimulation of Timp1 mRNA was independent of new protein synthesis, whereas the hCG-induced Timp3 mRNA expression was completely blocked by cyclohexamide. Similar findings of a rapid, protein synthesis-independent activation of Timp1 mRNA have also been observed in the limbic system after kainate-induced seizures in rats (53). However, the ability of protein synthesis inhibitors to block MMP or TIMP production exhibits a complexity based on the cell type, the MMP or TIMP under investigation, and the type of agent used to inhibit translation. Sampieri et al. (54) reported discordant effects of cycloheximide treatment on induction of Timp1 and Timp3 mRNA expression in human fibroblasts. Thus, it is not unexpected that there are differences in the regulation of Timp1 and Timp3 mRNA expression in granulosa cells.
Differences in TIMP regulation may reflect differences in their potential role in ovarian function. TIMPs have been shown to be multifunctional in other tissues, and TIMPs in the ovary may act as autocrine/paracrine factors in cellular proliferation, differentiation, or steroidogenesis. In support of this concept are findings that mice that lack TIMP1 exhibit a disruption of the estrous cycle length, increased estradiol, decreased progesterone, and an alteration in uterine morphology (55), suggesting that TIMP1 acts to modulate ovarian steroidogenesis.
TIMP3 was first discovered as an ECM bound protein produced by oncogenic cells undergoing transformation (56). Since this first description, various functions have been attributed to TIMP3 in other tissues including inhibition of angiogenesis (57), regulation of tissue response to injury (58), and acting as a proapoptotic agent by stabilization of death receptors (59). With this backdrop, we began to investigate the actions of TIMP3 in ovarian function by decreasing the expression of Timp3 mRNA by siRNA to approximately 25% of the levels observed after an hCG stimulus. We observed a diminution of progesterone levels afterTimp3 siRNA, which can be interpreted that TIMP3 impacts progesterone production either directly or via an indirect effect on cellular interaction with the extracellular environment. Similar findings have been reported that mice lacking TIMP1 have lower serum levels of progesterone (21).
The down-regulation of TIMP3 resulted in an increase in cytochrome P450 Cyp 17. This increase in Cyp 17 when levels of progesterone are decreased can be interpreted that progesterone regulates Cyp 17 or that the stimulation of Cyp 17 is a response to overall changes in granulosa cell function after diminution of TIMP3. Another class of enzymes that was up-regulated after Timp3 siRNA treatment controls protein ubiquitination (60), suggesting a role for TIMP3 in normal cellular protein turnover. The up-regulation of heat shock protein 70 may reflect the granulosa cell response to stress or may also represent the cell’s reaction to protein modification in conjunction with the ubiquitin-proteasome system (61).
The down-regulation of TIMP3 resulted in a decrease in the expression of numerous genes. The down-regulation of type VIIIα1 procollagen may reflect changes in the structure of the ECM. In mice lacking TIMP3, fibronectin is decreased in the lung, resulting in alterations in bronchiole formation (23). Thus, TIMP3 may change the ECM, thereby impacting cell function.
Annexin A1 was another gene that was down-regulated after TIMP3 diminution. Annexin A1 has been demonstrated by some groups to be proapoptotic, whereas other evidence links annexin A1 with resistance to apoptosis (44,52). Because one of the reported actions of TIMP3 in other tissues is to induce apoptosis (59), the possibility exists that TIMP3 reduction would decrease genes associated with apoptosis such as the annexins. However, genes associated with the classical apoptotic pathways such as Bcl-2-associated X protein and the caspases were not changed after decreasing TIMP3 by siRNA (data not shown). Furthermore, our previous findings suggested that the expression of Timp3 mRNA was not associated with apoptosis but rather was positively correlated to the health status of the follicle (2). These previous observations in vivo were supported by the present findings that there was no effect of exogenous TIMP3 on cell viability after 24 h of culture. These findings highlight that hCG alone increases TIMP3 without inducing apoptosis and that even after hCG induction of TIMP3, exogenous TIMP3 is insufficient to change cell viability. Therefore, the physiological induction of TIMP3 after an hCG stimulus does not induce granulosa cell apoptosis.
In conclusion, the temporal pattern of Timp1 and Timp3 induction by hCG differs. Although these temporal patterns vary, the hCG stimulation of Timp1 and Timp3 share a common induction through the PKA pathway. There are, however, differences in their regulation because Timp1 induction is independent of new protein synthesis, whereas Timp3 is inhibited by cycloheximide. Furthermore, the late peak of Timp3 induction is sensitive to RU486 treatment, suggesting progesterone involvement. Functional studies indicate that the hCG induction of Timp3 is not associated with a decrease in cell viability. Down-regulation of TIMP3 expression resulted in changes in gene expression patterns associated with fatty acid synthesis, steroidogenesis, IGF binding proteins, and protein turnover. At the present time, it is unknown whether the effects of the TIMP3 silencing experiments are direct actions of TIMP3 or indirect effects by altering granulosa cell interaction and function with the extracellular environment.
Supplementary Material
Footnotes
This work was supported by Grant P20 RR15592 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 23, 2009
Abbreviations: ECM, Extracellular matrix; EGF, epidermal growth factor; FSK, forskolin; hCG, human chorionic gonadotropin; MEK, MAPK kinase; MMP, matrix metalloproteinase; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; PI3 kinase, phosphatidylinositol 3 kinase; PKA, protein kinase A; PKC, protein kinase C; PMA, phorbol 12-myristate 13-acetate; PMSG, pregnant mare serum gonadotropin; PTGS2, prostaglandin-endoperoxide synthase 2; siRNA, small interfering RNA; TIMP, tissue inhibitor of metalloproteinase.
References
- Sternlicht MD, Werb Z 2001 How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JD, Werb Z 2004 Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 16:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G 2006 Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69:562–573 [DOI] [PubMed] [Google Scholar]
- Clark IM, Swingler TE, Sampieri CL, Edwards DR 2008 The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol 40:1362–1378 [DOI] [PubMed] [Google Scholar]
- Smith MF, McIntush EW, Ricke WA, Kojima FN, Smith GW 1999 Regulation of ovarian extracellular matrix remodelling by metalloproteinases and their tissue inhibitors: effects on follicular development, ovulation and luteal function. J Reprod Fertil Suppl 54:367–381 [PubMed] [Google Scholar]
- Curry Jr TE, Osteen KG 2003 The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 24:428–465 [DOI] [PubMed] [Google Scholar]
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP 1997 Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol 74:111–122 [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H 2000 Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477:267–283 [DOI] [PubMed] [Google Scholar]
- Boujrad N, Ogwuegbu SO, Garnier M, Lee CH, Martin BM, Papadopoulos V 1995 Identification of a stimulator of steroid hormone synthesis isolated from testis. Science 268:1609–1612 [DOI] [PubMed] [Google Scholar]
- Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS 2003 Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem 278:42330–42339 [DOI] [PubMed] [Google Scholar]
- Yang TT, Hawkes SP 1992 Role of the 21-kDa protein TIMP-3 in oncogenic transformation of cultured chicken embryo fibroblasts. Proc Natl Acad Sci USA 89:10676–10680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leco KJ, Waterhouse P, Sanchez OH, Gowing KL, Poole AR, Wakeham A, Mak TW, Khokha R 2001 Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3). J Clin Invest 108:817–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JS, Kindy MS, Edwards DR, Curry Jr TE 1991 Hormonal regulation of matrix metalloproteinase inhibitors in rat granulosa cells and ovaries. Endocrinology 128:1825–1832 [DOI] [PubMed] [Google Scholar]
- Simpson KS, Byers MJ, Curry Jr TE 2001 Spatiotemporal mRNA expression of the tissue inhibitors of metalloproteinases (TIMPs) in the ovary throughout the rat estrous cycle. Endocrinology 142:2058–2069 [DOI] [PubMed] [Google Scholar]
- Waterhouse P, Denhardt DT, Khokha R 1993 Temporal expression of tissue inhibitors of metalloproteinases in mouse reproductive tissues during gestation. Mol Reprod Dev 35:219–226 [DOI] [PubMed] [Google Scholar]
- Liu K, Olofsson JI, Wahlberg P, Ny T 1999 Distinct expression of gelatinase A [matrix metalloproteinase (MMP)-2], collagenase-3 (MMP-13), membrane type MMP 1 (MMP-14), and tissue inhibitor of MMPs type 1 mediated by physiological signals during formation and regression of the rat corpus luteum. Endocrinology 140:5330–5338 [DOI] [PubMed] [Google Scholar]
- Nothnick WB, Edwards DR, Leco KJ, Curry Jr TE 1995 Expression and activity of ovarian tissue inhibitors of metalloproteinases during pseudopregnancy in the rat. Biol Reprod 53:684–691 [DOI] [PubMed] [Google Scholar]
- Inderdeo DS, Edwards DR, Han VK, Khokha R 1996 Temporal and spatial expression of tissue inhibitors of metalloproteinases during the natural ovulatory cycle of the mouse. Biol Reprod 55:498–508 [DOI] [PubMed] [Google Scholar]
- Curry Jr TE, Nothnick WB 2000 Changes in ovarian tissue inhibitor of metalloproteinase (TIMP) expression during follicular growth, ovulation, and the luteal period in the rat. In: Hawkes SP, Edwards DR, Knokha R, eds. Tissue inhibitors of metalloproteinases in development and disease. Amsterdam: Harwood Academic Publishers; 119–126 [Google Scholar]
- Hägglund AC, Ny A, Leonardsson G, Ny T 1999 Regulation and localization of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse ovary during gonadotropin-induced ovulation. Endocrinology 140:4351–4358 [DOI] [PubMed] [Google Scholar]
- Nothnick WB, Soloway P, Curry Jr TE 1997 Assessment of the role of tissue inhibitor of metalloproteinase-1 (TIMP-1) during the periovulatory period in female mice lacking a functional TIMP-1 gene. Biol Reprod 56:1181–1188 [DOI] [PubMed] [Google Scholar]
- Curry Jr TE, Song L, Wheeler SE 2001 Cellular localization of gelatinases and tissue inhibitors of metalloproteinases during follicular growth, ovulation and early luteal formation in the rat. Biol Reprod 65:855–865 [DOI] [PubMed] [Google Scholar]
- Gill SE, Pape MC, Khokha R, Watson AJ, Leco KJ 2003 A null mutation for tissue inhibitor of metalloproteinases-3 (Timp-3) impairs murine bronchiole branching morphogenesis. Dev Biol 261:313–323 [DOI] [PubMed] [Google Scholar]
- Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B 2003 A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med 9:407–415 [DOI] [PubMed] [Google Scholar]
- Yuan LQ, Liu YS, Luo XH, Guo LJ, Xie H, Lu Y, Wu XP, Liao EY 2008 Recombinant tissue metalloproteinase inhibitor-3 protein induces apoptosis of murine osteoblast MC3T3-E1. Amino Acids 35:123–127 [DOI] [PubMed] [Google Scholar]
- Johnson JL, Sala-Newby GB, Ismail Y, Aguilera CM, Newby AC 2008 Low tissue inhibitor of metalloproteinases 3 and high matrix metalloproteinase 14 levels defines a subpopulation of highly invasive foam-cell macrophages. Arterioscler Thromb Vasc Biol 28:1647–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drynda A, Quax PH, Neumann M, van der Laan WH, Pap G, Drynda S, Meinecke I, Kekow J, Neumann W, Huizinga TW, Naumann M, König W, Pap T 2005 Gene transfer of tissue inhibitor of metalloproteinases-3 reverses the inhibitory effects of TNF-α on Fas-induced apoptosis in rheumatoid arthritis synovial fibroblasts. J Immunol 174:6524–6531 [DOI] [PubMed] [Google Scholar]
- Mosmann T 1983 Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63 [DOI] [PubMed] [Google Scholar]
- Jo M, Gieske MC, Payne CE, Wheeler-Price SE, Gieske JB, Ignatius IV, Curry Jr TE, Ko C 2004 Development and application of a rat ovarian gene expression database (rOGED). Endocrinology 145:5384–5396 [DOI] [PubMed] [Google Scholar]
- Morris JK, Richards JS 1995 Luteinizing hormone induces prostaglandin endoperoxide synthase-2 and luteinization in vitro by A-kinase and C-kinase pathways. Endocrinology 136:1549–1558 [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC 2002 Novel signaling pathways that control ovarian follicular development, ovulation and luteinization. Recent Prog Horm Res 57:195–220 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M 2007 Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C, Park-Sarge OK 2000 Progesterone receptor activation mediates LH-induced type-I pituitary adenylate cyclase activating polypeptide receptor [PAC(1)] gene expression in rat granulosa cells. Biochem Biophys Res Commun 277:270–279 [DOI] [PubMed] [Google Scholar]
- Ahonen M, Baker AH, Kähäri VM 1998 Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res 58:2310–2315 [PubMed] [Google Scholar]
- Kumar P, Van Patten SM, Walsh DA 1997 Multiplicity of the β form of the cAMP-dependent protein kinase inhibitor protein generated by post-translational modification and alternate translational initiation. J Biol Chem 272:20011–20020 [DOI] [PubMed] [Google Scholar]
- Salvador LM, Maizels E, Hales DB, Miyamoto E, Yamamoto H, Hunzicker-Dunn M 2002 Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology 143:2986–2994 [DOI] [PubMed] [Google Scholar]
- Castagnino P, Soriano JV, Montesano R, Bottaro DP 1998 Induction of tissue inhibitor of metalloproteinases-3 is a delayed early cellular response to hepatocyte growth factor. Oncogene 17:481–492 [DOI] [PubMed] [Google Scholar]
- Su S, DiBattista JA, Sun Y, Li WQ, Zafarullah M 1998 Up-regulation of tissue inhibitor of metalloproteinases-3 gene expression by TGF-β in articular chondrocytes is mediated by serine/threonine and tyrosine kinases. J Cell Biochem 70:517–527 [PubMed] [Google Scholar]
- Wallace JA, Alexander S, Estrada EY, Hines C, Cunningham LA, Rosenberg GA 2002 Tissue inhibitor of metalloproteinase-3 is associated with neuronal death in reperfusion injury. J Cereb Blood Flow Metab 22:1303–1310 [DOI] [PubMed] [Google Scholar]
- Nareyeck G, Zeschnigk M, von der Haar D, Schilling H, Bornfeld N, Anastassiou G 2005 Differential expression of tissue inhibitor of matrix metalloproteinases 3 in uveal melanoma. Ophthalmic Res 37:23–28 [DOI] [PubMed] [Google Scholar]
- Chong NH, Kvanta A, Seregard S, Bird AC, Luthert PJ, Steen B 2003 TIMP-3 mRNA is not overexpressed in Sorsby fundus dystrophy. Am J Ophthalmol 136:954–955 [DOI] [PubMed] [Google Scholar]
- Carvalho CR, Carvalheira JB, Lima MH, Zimmerman SF, Caperuto LC, Amanso A, Gasparetti AL, Meneghetti V, Zimmerman LF, Velloso LA, Saad MJ 2003 Novel signal transduction pathway for luteinizing hormone and its interaction with insulin: activation of Janus kinase/signal transducer and activator of transcription and phosphoinositol 3-kinase/Akt pathways. Endocrinology 144:638–647 [DOI] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Park JY, Su YQ 2006 Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol 20:715–723 [DOI] [PubMed] [Google Scholar]
- Roviezzo F, Getting SJ, Paul-Clark MJ, Yona S, Gavins FN, Perretti M, Hannon R, Croxtall JD, Buckingham JC, Flower RJ 2002 The annexin-1 knockout mouse: what it tells us about the inflammatory response. J Physiol Pharmacol 53:541–553 [PubMed] [Google Scholar]
- Richards JS 2001 New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol 15:209–218 [DOI] [PubMed] [Google Scholar]
- Ahmed N, Maines-Bandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N 2006 Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am J Physiol Cell Physiol 290:C1532–C1542 [DOI] [PubMed] [Google Scholar]
- Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A 2005 Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology 146:77–84 [DOI] [PubMed] [Google Scholar]
- Davis BJ, Lennard DE, Lee CA, Tiano HF, Morham SG, Wetsel WC, Langenbach R 1999 Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1β. Endocrinology 140:2685–2695 [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M 2004 EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 [DOI] [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M 2008 Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 22:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LH, Pervaiz S 2007 Annexin 1: the new face of an old molecule. FASEB J 21:968–975 [DOI] [PubMed] [Google Scholar]
- Rivera S, Tremblay E, Timsit S, Canals O, Ben-Ari Y, Khrestchatisky M 1997 Tissue inhibitor of metalloproteinases-1 (TIMP-1) is differentially induced in neurons and astrocytes after seizures: evidence for developmental, immediate early gene, and lesion response. J Neurosci 17:4223–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampieri CL, Nuttall RK, Young DA, Goldspink D, Clark IM, Edwards DR 2008 Activation of p38 and JNK MAPK pathways abrogates requirement for new protein synthesis for phorbol ester mediated induction of select MMP and TIMP genes. Matrix Biol 27:128–138 [DOI] [PubMed] [Google Scholar]
- Nothnick WB 2000 Disruption of the tissue inhibitor of metalloproteinase-1 gene results in altered reproductive cyclicity and uterine morphology in reproductive-age female mice. Biol Reprod 63:905–912 [DOI] [PubMed] [Google Scholar]
- Blenis J, Hawkes SP 1984 Characterization of a transformation-sensitive protein in the extracellular matrix of chicken embryo fibroblasts. J Biol Chem 259:11563–11570 [PubMed] [Google Scholar]
- Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B 2003 A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med 9:407–415 [DOI] [PubMed] [Google Scholar]
- Mohammed FF, Smookler DS, Taylor SE, Fingleton B, Kassiri Z, Sanchez OH, English JL, Matrisian LM, Au B, Yeh WC, Khokha R 2004 Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet 36:969–977 [DOI] [PubMed] [Google Scholar]
- Ahonen M, Poukkula M, Baker AH, Kashiwagi M, Nagase H, Eriksson JE, Kähäri VM 2003 Tissue inhibitor of metalloproteinases-3 induces apoptosis in melanoma cells by stabilization of death receptors. Oncogene 22:2121–2134 [DOI] [PubMed] [Google Scholar]
- Kikkert M, Hassink G, Wiertz E 2005 The role of the ubiquitination machinery in dislocation and degradation of endoplasmic reticulum proteins. Curr Top Microbiol Immunol 300:57–93 [DOI] [PubMed] [Google Scholar]
- Esser C, Alberti S, Höhfeld J 2004 Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochim Biophys Acta 1695:171–188 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.