Abstract
Activin-βA and activin-βB (encoded by Inhba and Inhbb genes, respectively) are closely related TGF-β superfamily members that participate in a variety of biological processes. We previously generated mice with an insertion allele at the Inhba locus, InhbaBK. In this allele, the sequence encoding the Inhba mature domain is replaced with that of Inhbb, rendering the gene product functionally hypomorphic. Homozygous (InhbaBK/BK) and hemizygous (InhbaBK/−) mice are smaller and leaner than their wild-type littermates, and many tissues are disproportionately small relative to total body weight. To determine the mechanisms that contribute to these phenomena, we investigated the metabolic consequences of the mutation. Although the growth of InhbaBK mice is improved by providing a calorie-rich diet, diet-induced obesity, fatty liver, and insulin resistance (hallmarks of chronic caloric excess) do not develop, despite greater caloric intake than wild-type controls. Physiological, molecular, and biochemical analyses all revealed characteristics that are commonly associated with increased mitochondrial energy metabolism, with a corresponding up-regulation of several genes that reflect enhanced mitochondrial biogenesis and function. Oxygen consumption, an indirect measure of the metabolic rate, was markedly increased in InhbaBK/BK mice, and polarographic analysis of liver mitochondria revealed an increase in ADP-independent oxygen consumption, consistent with constitutive uncoupling of the inner mitochondrial membrane. These findings establish a functional relationship between activin signaling and mitochondrial energy metabolism and further support the rationale to target this signaling pathway for the medical treatment of cachexia, obesity, and diabetes.
Activin signaling affects body fat and mitochondrial function.
Members of the TGF-β superfamily are synthesized as prepropeptide precursors that are processed into mature, biologically active homodimers or heterodimers, which activate serine/threonine kinase receptors (1). Activated receptors, in turn, exert their biological effects through the actions of the Sma- and Mad-related (SMAD) family of proteins (2,3) and play important roles in the growth, differentiation, and functions of a variety of cell types (4). These include maintaining body composition, adiposity, and energy metabolism. Myostatin, for example, is well known for its role as a negative regulator of skeletal muscle mass through its affects on myocyte differentiation and growth; however, adiposity is also significantly reduced in aging myostatin knockout mice, as is their susceptibility to diet-induced diabetes (5,6,7). Overexpressing myostatin, in vivo, results in the loss of muscle and adipose tissue mass, a process presumably mediated by increased signaling through activin receptors and which can be inhibited by follistatin, an activin/myostatin antagonist (8,9,10). Bone morphogenetic protein (BMP)-7 is sufficient to drive brown adipocyte differentiation in vitro and in vivo, including the gene expression and function of fully differentiated brown adipose tissue (11). Mice that over express growth differentiation factor-3, another member of the TGF-β superfamily that also signals through activin receptors (12), have an increased sensitivity to the adipogenic effect of high-fat diets (HFDs) (13), and growth differentiation factor-3 knockout mice exhibit the reciprocal phenotype of protection from diet-induced obesity (14,15). Mice lacking inhibin-α, an activin antagonist, have cachexia that is due to increased activin receptor signaling (16,17). Thus, several TGF-β superfamily members have substantial effects on adiposity and body composition.
The activins are TGF-β superfamily members that function in a wide variety of physiological processes (4,18,20). Activin-βA and -βB are closely related, sharing 87% similarity within their mature peptides. These proteins associate to form activin A or B homodimers (βA-βA or βB-βB) or activin AB heterodimers (βA-βB). To better understand activin functions because of the birth defects and newborn lethality that occur in Inhba−/− mice, we previously engineered mice with a hypomorphic insertion allele at the Inhba locus, termed InhbaBK (21,22). In this allele, the sequence encoding the activin-βA mature domain was replaced with the corresponding sequence from activin βB, and all other sequences of the Inhba gene remained intact. Thus, the InhbaBK allele results in the misexpression of activin-βB in locations in which activin-βA is normally produced but does not adversely affect the expression level or processing of the protein (21). The basis of the hypomorphism of the allele is thought to be the lower bioactivity of the activin-βB protein relative to activin-βA when expressed in a foreign environment, resulting in altered activin signaling in target tissues. InhbaBK/BK and InhbaBK/− mice are significantly smaller and have less white adipose than wild-type littermates. In this paper, we investigate the physiological, molecular, and biochemical processes that contribute to the smaller body size and leanness of the InhbaBK mice. Our findings are consistent with essential roles for activin signaling in maintaining normal energy balance, primarily through its downstream effects on mitochondrial energy metabolism.
Materials and Methods
Mice
The construct and targeting strategies of the InhbaBK allele, and the production of InhbaBK mice have been described previously (21). For the studies described herein, a PCR-based genotyping strategy was used. A 308-bp product resulting from the wild-type allele was amplified from tail DNA, using the following primers: forward, 5′-ctgttgagtggaagga-gag-3′, reverse, 5′-gagatgggaagaagaa-aga-3′. A 193-bp product from the InhbaBK allele was amplified using the following primers: forward, 5′-ctgttgagtggaagga-gag-3′, reverse, 5′-cgatgagccgaaagtc-gatg-3′. Cycling conditions were as follows: 94 C for 3 min, followed by 35 cycles of 94 C for 30 sec, 54 C for 30 sec, 72 C for 25 sec, and then 72 C for 7 min, 25 C for 2 min, and 4 C at least 5 min. All mice were housed in a single pathogen-free room within the transgenic mouse core facility at the Baylor College of Medicine, with a 12-h dark, 12-h light cycle.
Tissue dissection, processing, and histology
Intraperitoneal injection of 0.02 ml/g body weight of 2.5% Avertin (Sigma-Aldrich, St. Louis, MO) was used for anesthesia, followed by cardiac puncture or retroorbital bleeding for collection of serum. The mice were euthanized by cervical dislocation. For body composition analyses, tissues were dissected in a standardized fashion and weighed on an analytical balance. The organs collected included liver, kidney, spleen, pancreas, gastrocnemius, and biceps. White adipose depots included reproductive (inguinal plus parametrial or testicular) and retroperitoneal. Histology specimens were fixed in 4% paraformaldehyde in PBS at 4 C (Sigma, St. Louis, MO), or 10% neutral buffered formalin (Richard Allen Scientific, Kalamazoo, MI) embedded in Paraplast X-TRA (Kendall, Mansfield, MA), and mounted. Eight-micrometer sections were stained with hematoxylin and eosin. Digital images were captured directly from the microscope using Spot Image analysis software (version 4.1.1; Diagnostic Instruments, Inc., Sterling Heights, MI).
Indirect calorimetry
Oxygen consumption was assessed for individual mice using computer-controlled, open-circuit indirect calorimetry (Oxymax; Columbus Instruments, Columbus, OH) with an air flow of 0.6 liters/min at ambient temperature. After 30 min of adaptation to the metabolic chamber, O2 consumption and CO2 production were assessed at 4-min intervals for a 20- to 24-h period. The mice were fasted during the experiment and had free access to water. Total oxygen consumption represents the mean of all measurements taken during the experiment.
Weights and caloric intake experiments
Mice were given either regular or high fat diets ad libitum and were weighed weekly. Regular diet (Laboratory Autoclavable Rodent Diet 5010; Purina Mills Inc., St. Louis, MO) has a caloric content of 3.4 kcal/g, whereas high-fat diet (Mouse Diet High Carbohydrate High Fat F3282; BioServ, Frenchtown, NJ) has 5.3 kcal/g. Isolated male and female wild-type and InhbaBK/BK mice were assessed for oral intake of regular diet, measured every Tuesday afternoon for 26 wk, beginning at 3 wk of age. The caloric intake was calculated and expressed as kilocalories consumed per gram body weight per day.
Glucose and insulin tolerance testing
Glucose tolerance tests were conducted with tail vein blood and a glucometer (OneTouch Ultra; Lifescan, Milpitas, CA). After a 16-h fast, the initial time point was collected and designated 0 min, 2 g glucose per kilogram body weight was injected ip, and blood glucose levels were measured at 15, 30, 60, and 120 min. For insulin tolerance testing, 12-month-old mice that had been maintained on HFD were given 0.75 IU/kg dose of regular insulin, followed by assessment of blood glucose levels for 60 min.
Insulin ELISA
Blood samples from 12-month-old mice were collected by the retroorbital route after a 16-h fast and 30 min after an ip injection of d-glucose (2 g glucose per kilogram body weight). The sera were stored at −20 C until use. Insulin levels were measured using a rat insulin ELISA kit, following the manufacturer’s instructions (Crystalchem, Inc., Chicago, IL).
Total T4 RIA
Blood samples were obtained from 6-month-old mice by retroorbital collection. The sera were stored at −20 C until use. Total T4 was measured using a Coat-a-Count solid phase RIA kit according to the manufacturer’s instructions (Diagnostic Products Corp., Los Angeles, CA).
RNA preparation and quantitative RT-PCR analysis
Freshly collected tissues were immediately placed in RNA STAT (Leedo Medical, Houston, TX) for RNA extraction according to the manufacturer’s instructions. cDNA synthesis was carried out for individual RNA samples at 50 C using SuperScript III reverse transcriptase according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). For quantitative PCR, software, reagents (SYBR Green), and equipment (ABI PRISM 7000 sequence detection system) were used from Applied Biosystems (Foster City, CA). PCR primer design was aided by Primer Express analysis software and synthesized by the Child Health Research Center (Texas Children’s Hospital). The genes measured by real-time analysis encoded carnitine palmitoyltransferase-1a; carnitine palmitoyltransferase-1b; forkhead-box transcription factor (Fox)-O1; nuclear respiratory factor (NRF)-1; NRF-2; peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α; steroid receptor coactivator-1; steroid receptor coactivator-2; uncoupling protein (UCP)-1; UCP2; and UCP3. The primer sequences, with the final nanomolar primer concentrations per reaction, will be provided upon request.
Statistical analysis
Error bars in all graphs except covariate and quantitative PCR analyses depict the sem. All data from tissue weight, food intake, glucose, insulin, lipid, O2 consumption (see Fig. 4B), and polarography studies were assessed for normal distribution using the Shapiro-Wilk W test. A variance ratio (F) test was applied to paired data sets to determine equal or unequal variance. Two-tailed Student t tests were applied to paired groups that neither showed evidence of abnormal distribution nor unequal variance. Paired groups that failed to meet these criteria were analyzed using Wilcoxon signed ranks test. The Krustal-Wallis test was applied to lipid profile and polarography data, which contained three comparison groups each. Analyses were carried out with Excel data analysis software (Microsoft, Redmond, WA), StatsDirect statistical software (StatsDirect Ltd., Cheshire, UK), or the R statistical programing software environment (http://www.r-project.org). Analysis of quantitative PCR data was accomplished using Relative Expression Software Tool (REST) 2008 (Corbett Research, Technical University, Munich, Germany). The analysis addresses issues regarding the measurement of uncertainty in expression ratios inherent in quantitative PCR analysis by using randomization and boot-strapping techniques, including 50,000 hypothesis tests to achieve a high level of consistency.
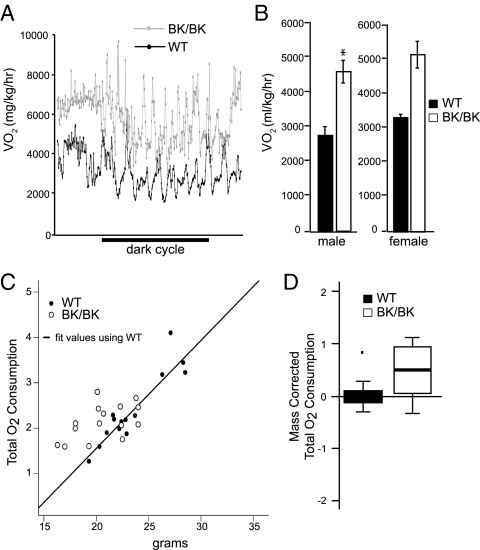
Figure 4.
Assessment of metabolic rates. Computer-controlled, open-circuit indirect calorimetry. A, Representative tracings from 3-month-old InhbaBK/BK (BK/BK) and wild-type (WT) female mice. B, Mean O2 consumption (VO2) was significantly higher in InhbaBK/BK mice than wild-type controls (P < 0.01, n = 6). The asterisk denotes the significant difference between wildtype and mutant (BK). C, Linear regression analysis for oxygen consumption fit using the wild-type mass vs. O2 consumption values (filled circles). The open circles represent the InhbaBK/BK mice, which fall above the line and have statistically higher O2 consumption for a fixed mass from what would be expected in the wild-type regression model. D, Box plots of residuals after body mass correction of total oxygen consumption in InhbaBK/BK and wild-type mice, derived from data in C. Box plots are a standard graphical method for displaying the center and spread of data values in a compact fashion. The oxygen consumption in InhbaBK/BK mice was significantly higher than wild-type mice, even after weight differences were considered (P < 0.0015, n = 6). The black line in the InhbaBK/BK plot represents the median value, relative to the wild-type value of zero, and the box represents the middle 50% of observations. The bars extend to 1.5 times the inner quartile range or to the maximum data value, whichever is smaller. The single outlier in the wild-type group is depicted as an individual point.
Mitochondrial respiration assay
Mitochondrial isolation and respiration assay were performed essentially as previously described (23). Freshly dissected mouse livers (100–300 mg) from 3-wk-old wild-type and mutant mice were homogenized in 10 ml of isolation buffer [210 mm mannitol, 70 mm sucrose, 1 mm EGTA, 0.5% (wt/vol) BSA (fraction V, fatty acid free), and 5 mm HEPES (pH 7.2)] using a glass hand-held Dounce homogenizer (10 passes). Mitochondria were recovered using differential centrifugation as previously described (23). Mitochondrial pellets were resuspended in 100 μl of isolation buffer. Polarographic assay of mitochondrial oxygen consumption (expressed as nanograms atomic oxygen per minute per milligram mitochondrial protein) in the presence of 5 mm succinate was performed using a microelectrode (Instech, Plymouth Meeting, PA) and microchamber with a YSI 5300 oxygen monitor (Yellow Springs Instruments, Yellow Springs, OH) as previously described (23). After measuring state III and state IV oxygen consumption, 2,4-dinitrophenol (a proton ionophore) was added to a final concentration of 50 μm and the subsequent maximal uncoupled rate of oxygen consumption was measured. The mitochondrial protein concentration for the assay was 0.15 mg/ml.
We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during this research, following the policies of the Institutional Animal Care and Use Committee at the Baylor College of Medicine.
Results
Compromised activin signaling has selective effects on organ growth
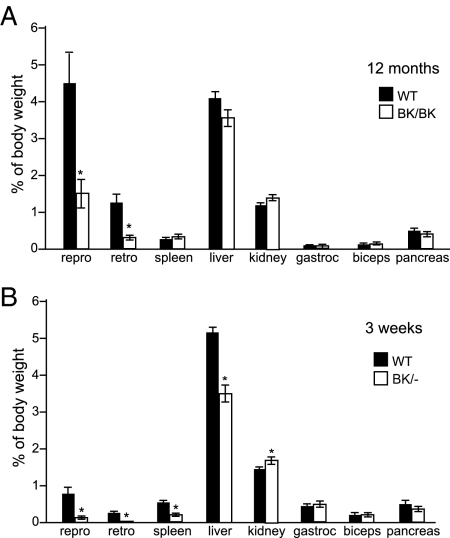
We previously described disproportionate effects of activin signaling on the growth and function of specific organs (21,22). To further assess the mechanisms by which activins affect growth, we compared the organ weights of 1-yr-old wild-type and InhbaBK/BK mice (Fig. 1A). Because about 50% of InhbaBK/BK mice die by the age of 6 months (21), we chose to examine mice that survived to 1 yr of age because this subgroup represents a relatively healthy cohort at the mild end of the growth deficiency spectrum, thus allowing a fairer comparison of organ-selective growth effects. We also examined 3-wk-old InhbaBK/− mice (mice with one InhbaBK allele and one Inhba null allele, Fig. 1B), which have severe growth deficiency, cachexia, and a 4-wk life span, thus representing the severe end of the spectrum (21). InhbaBK/+ mice (mice with one InhbaBK allele and one wild-type Inhba allele) were not fully characterized in these studies because they are overtly phenotypically normal. In 12-month-old InhbaBK/BK mice, the inguinal/perigonadal and retroperitoneal white adipose depots were obviously much smaller than those of wild-type controls. However, no other significant differences in organ weights were evident in this age group. In contrast, liver, spleen, and white adipose tissues were all significantly smaller in InhbaBK/− mice. Grossly, InhbaBK/BK and InhbaBK/− mice had less body fat than wild-type mice. Skin histology of 3-wk-old InhbaBK/− mice revealed the near absence of fat in the dermal layer (supplemental Fig. 1, J and M, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). White adipose from InhbaBK/BK mice had small, multiloculated, immature-appearing adipocytes histologically, most striking in the mesenteric fat depots (supplemental Fig. 1N), and in general, the white adipose depots were effectively absent in InhbaBK/− mice (supplemental Fig. 1D and data not shown). The thin layer of white adipose tissue that normally overlies the suprascapular brown fat depot was also dramatically thinner or absent in InhbaBK/− mice (supplemental Fig. 1, B and E). Brown adipocytes from InhbaBK/BK and InhbaBK/− mice contained smaller lipid droplets and fewer coalescing fat droplets per cell (supplemental Fig. 1, L and O, and data not shown).
Figure 1.
Organ to body weight ratios. Reproductive (repro) and retroperitoneal (retro) white adipose fat pads, spleen, liver, kidney, gastrocnemius (gastroc), biceps, and pancreas were dissected and weighed from 12- to 13-month-old wild-type (WT) and InhbaBK/BK female mice (n = 6) (A) and 3-wk-old wild-type and InhbaBK/− mice (n = 7) (B). Similar significant differences in the weights of the same tissues were observed in male and female mice for both groups (data not shown). *, Significant differences between values [WT vs. BK/BK (P < 0.02); WT vs. BK/− (P < 0.03, range 0.03–0.0005)].
Increasing caloric intake improves the body weight of InhbaBK/BK mice
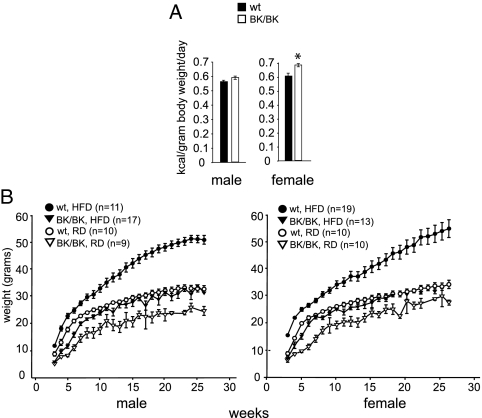
Energy balance is affected by caloric input and energy expenditure, the former primarily a function of nutritional intake and the latter influenced mostly by the basal metabolic rate. Thus, we sought to determine whether these factors contributed to the leanness of InhbaBK mice. To assess oral intake, we measured the amount of food consumed by isolated InhbaBK/BK and control mice receiving regular chow for 15 wk after weaning. The number of calories per gram body weight consumed by InhbaBK/BK mice trended greater than for the wild-type control group (Fig. 2A). We also investigated whether providing a food source with a higher caloric density would improve the weight gain of InhbaBK/BK mice and also allow us to assess the possible contribution of intestinal malabsorption. Wild-type and InhbaBK/BK mice were compared when maintained either on a regular diet (RD) or a HFD. Wild-type mice that received HFD became obese, as expected (Fig. 2B). In contrast, by 6 wk, InhbaBK/BK mice given HFD exhibited an improvement of growth so that their weight curves superimposed on those of wild-type mice receiving RD (Fig. 2B). However, the body weight and fat pad sizes of InhbaBK/BK mice remained much less than those of wild-type mice receiving the same HFD regimen. Wild-type mice receiving HFD became morbidly obese with markedly enlarged fat depots. In contrast, InhbaBK/BK mice receiving the same diet were not obese and had fat pads that were comparable with those from wild-type mice that received RD. Grossly, the fat pads from InhbaBK/BK mice on RD were considerably smaller than those from mice in the other experimental groups (supplemental Fig. 2A and data not shown). By 15 months, hepatic steatosis (accumulation of fat in the liver) was abundant in wild-type mice that received HFD and to a lesser extent in those on RD, whereas no fat accumulation was evident in the livers of InhbaBK/BK mice, irrespective of diet (supplemental Fig. 2B). Intestinal malabsorption was not a major contributing factor to the poor weight gain of InhbaBK/BK mice because their growth was improved significantly by providing an enriched calorie source, without substantial effects on stool output or appearance relative to wild-type mice on the same diet (data not shown).
Figure 2.
Caloric intake and growth characteristics of InhbaBK mice. A, Caloric intake (kilocalories per kilograms per day) of isolated, age-matched wild-type (wt; n = 6) and InhbaBK/BK (n = 6) mice receiving regular chow after weaning for 15 wk (wk 9–24). *, Significant difference when comparing InhbaBK/BK with wild-type female mice (P < 0.001; P = 0.09 for males). B, The weights (mean ± sem) of male and female wild-type (wt) and InhbaBK/BK (BK/BK) mice were measured for 26 wk on either HFD or RD.
InhbaBK/BK mice do not develop diet-induced insulin resistance but have altered lipid metabolism
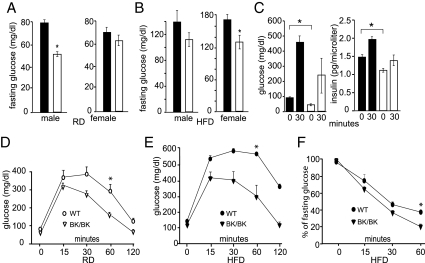
Because of activin’s ability to stimulate the release of insulin from cultured pancreatic islets (24,25) and its role in pancreatic islet cell development (26,27), we examined the effects of the InhbaBK mutation on glucose metabolism. Fasting glucose levels were measured in wild-type and InhbaBK/BK mice that were maintained on either RD or HFD, and insulin levels were measured in mice maintained on RD after an overnight fast and then 30 min after a glucose challenge. In general, fasting blood glucose and insulin levels were lower in InhbaBK/BK mice than controls, and the insulin response to glucose was blunted in InhbaBK/BK mice (Fig. 3, A–C). InhbaBK/BK mice were also tested for glucose tolerance and insulin sensitivity. All mice maintained on RD demonstrated the characteristic response: peak blood glucose levels occurring between 15 and 30 min after the glucose challenge and a return to baseline by 2 h, although InhbaBK/BK mice exhibited lower peak glucose values than controls (Fig. 3, D and E). Wild-type mice maintained on HFD for 12 months demonstrated high peak glucose levels that did not return to the baseline, indicating glucose intolerance. In contrast, InhbaBK/BK mice that were maintained on HFD for the same duration exhibited significantly better glucose tolerance than controls (Fig. 3E). To assess insulin sensitivity, we carried out insulin tolerance testing on mice that had been maintained on HFD (Fig. 3F). All experimental groups responded to insulin with a reduction in blood glucose levels; however, the InhbaBK/BK levels were 19% of the pretreatment value at 60 min compared with 39% for the wild-type control group. Collectively, these results indicate that despite activin’s positive role in insulin production, InhbaBK/BK mice do not have impaired glucose tolerance, even after receiving a diabetogenic diet for an extended time period.
Figure 3.
Glucose and insulin levels and glucose and insulin tolerance curves of InhbaBK mice. A and B, Fasting glucose levels in mice maintained on RD (n = 10–12) (A) or HFD (n = 4–8) (B) for 12 months. Note the lower glucose levels for all InhbaBK/BK mice (open bars), with significant reductions in both diet groups (RD males, P < 0.001; HFD females, P < 0.02). C, Plasma insulin response to ip glucose administration. Fasting glucose and insulin levels were significantly lower in 12-month-old InhbaBK/BK mice (open bars) than wild-type (WT) mice (shaded bars) (P < 0.05, n = 3). D, Glucose tolerance test and RD. Twelve-month-old mice were fasted for 16 h before ip injection of d-glucose (2 g/kg). Glucose levels were significantly different at 60 min after glucose administration (P < 0.005) E, Glucose tolerance tests and HFD. Nine-month-old mice that were maintained on HFD from weaning were assessed. Note lower peak glucose values and return to the normal baseline in the InhbaBK/BK group (P < 0.002). F, Insulin tolerance testing. The blood glucose values are presented as the percent change from the preinsulin administration values. *, Significant difference (P < 0.004). Results from D and E are from female mice. Similar results were observed in male mice (data not shown).
To determine the consequences of the InhbaBK mutation on lipid metabolism, we measured serum total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), and triglycerides (Table 1). No differences in triglycerides and VLDL levels were evident; however, a stepwise increase in cholesterol, HDL and LDL levels was observed in InhbaBK/BK and InhbaBK/− mice relative to wild-type mice.
Table 1.
Lipid profile
| Genotype | Cholesterol | HDL | LDL | VLDL | Triglycerides |
|---|---|---|---|---|---|
| Wild type (n = 7) | 96 ± 4 | 69 ± 4 | 17 ± 1 | 18 ± 2 | 90 ± 12 |
| InhbaBK/BK (n = 5) | 134 ± 13 | 106 ± 17 | 26 ± 5 | 17 ± 2 | 83 ± 9 |
| InhbaBK/− (n = 5) | 167 ± 13a | 130 ± 5a | 36 ± 6a | 23 ± 4 | 114 ± 18 |
Values are expressed in milligrams per deciliter ± sem.
Significant differences from wild type (P < 0.05).
InhbaBK/BK mice have increased energy expenditure
Because of their leanness and resistance to the deleterious effects of the HFD regimen, we wanted to determine whether energy homeostasis was altered in InhbaBK/BK mice. Oxygen consumption, an indirect indicator of the metabolic rate, was measured in fasting 12-wk-old mice and was found to be 50% higher in InhbaBK/BK mice relative to wild-type mice, a difference that persisted throughout the light and dark phases of the light cycle (Fig. 4, A and B). Significantly higher values were also observed in 6- and 9-month-old InhbaBK/BK mice (data not shown).
Because differences in body mass can affect the metabolic rate, it was possible that the smaller size of InhbaBK/BK mice was the main cause of their higher oxygen consumption. Therefore, to correct for the effects of body size, a regression was first performed to total oxygen consumption as a linear function of mass in the two genotypes, using an analysis of covariance. This analysis determined that there was no significant difference for the slopes between the two genotypes when considering the relationship between body mass and oxygen consumption. However, the pattern of residuals in the InhbaBK/BK mice prompted us to perform an additional analysis using the body mass to oxygen consumption relationship in the wild-type mice to determine predicted oxygen consumption in InhbaBK/BK mice. The differences between observed and predicted values showed that InhbaBK/BK mice have greater oxygen consumption than would be predicted for comparably sized wild-type animals (Fig. 4, C and D). Collectively, these results show that differences in body size have modest effects on oxygen consumption, but genotype effects other than body size also contribute to the higher metabolic rates of InhbaBK/BK mice.
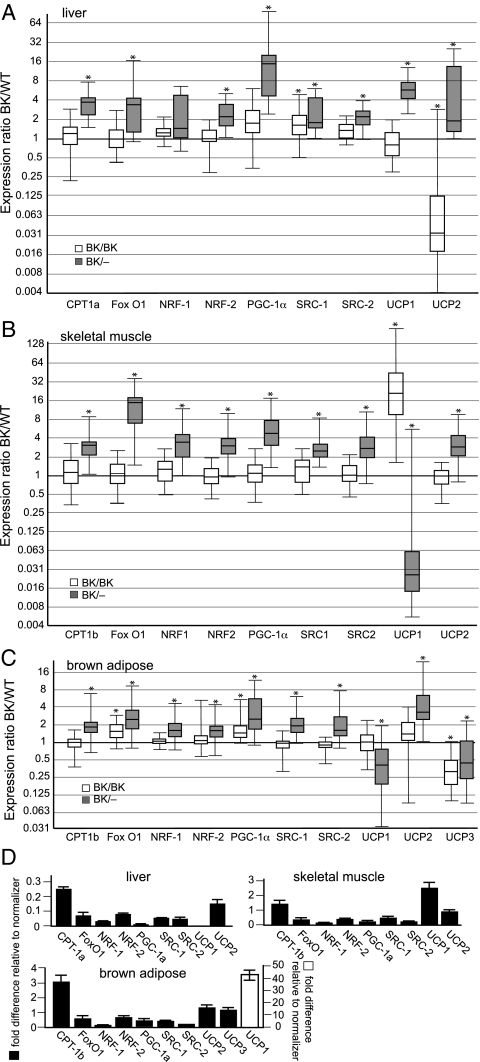
Considering the poor weight gain, reduced adiposity, and increased energy expenditure of InhbaBK/BK mice, we next sought to identify underlying processes that might be associated with these physiologic disturbances. Thyroid hormone plays a major endocrine role in regulating the metabolic rate. Furthermore, activin A has been implicated as a negative regulator of thyroid cell growth as well as the TSH-stimulated cAMP response, and activin-A and -B are thought to have important regulatory functions in human thyroid disorders (28,29,30). Therefore, we measured thyroid hormone (total T4) in 12-wk-old mice. No significant differences in total T4 levels were present among the experimental groups (total T4: wild-type male, 4.1 ± 0.08; InhbaBK/BK male, 4.2 ± 0.01; wild-type female, 3.8 ± 0.1; InhbaBK/BK female, 4.0 ± 0.17 μg/dl; n = 6). We also examined the expression of genes that play key roles in regulating energy expenditure. Genes that were selected for this analysis encode products that influence the metabolic rate by affecting mitochondrial biogenesis and the expression of UCPs and by controlling the transport and metabolism of fatty acids in the mitochondria. Because of their importance in intermediary metabolism and the prominent contributing roles of mitochondria to this process, mitochondrial gene expression profiles for liver (Fig. 5A), skeletal muscle (Fig. 5B), and brown adipose (Fig. 5C) were compared for 3-wk-old InhbaBK/BK and InhbaBK/− mice. Several increases in mRNA levels were detected in all three tissues from InhbaBK/− mice, including Ppargc1 (PGC-1α), Nrf1, and Gabpa (NRF-2), a finding consistent with a generalized increase in mitochondrial metabolism. Fewer changes in mRNA levels were evident in tissues from InhbaBK/BK mice. In contrast to the other genes, however, Ucp1, Ucp2, and Ucp3 mRNA levels were less consistent in their expression trends, including significant reductions in all three tissues.
Figure 5.
Quantitative assessment of mitochondrial gene expression. Quantitative RT-PCR of mitochondrial gene expression in liver (n = 5–6) (A), skeletal muscle (n = 5) (B), and brown adipose (n = 7) (C) are shown. The data are presented as ratios of InhbaBK/BK or InhbaBK/− mRNA levels relative to wild-type values, which were arbitrarily set at 1. Ratios are shown as whisker box plots, with boxes representing the middle 50% of observations, solid lines within the boxes representing median gene expression, and whisker bars indicating the minimum and maximum value. CPT, Carnitine palmitoyltransferase; SRC, steroid receptor coactivator. *, Significant differences from the wild-type (P < 0.05). D, Fold differences of wild-type mRNA relative to the normalizer, cyclophilin B, to show relative levels of expression when comparing individual genes.
Functional characteristics of mitochondria are consistent with increased energy metabolism
During oxidative phosphorylation, electrons are generated from reducing equivalents produced by the breakdown of fats, carbohydrates, and proteins. In the final step of electron transport, oxygen is reduced to water by cytochrome c oxidase. Using polarography, oxidative phosphorylation capacity can be determined in isolated mitochondria by measuring oxygen consumption in the presence of an oxidative substrate (e.g. succinate) and ADP, constituting the state III rate of oxygen consumption. The state IV rate represents the oxygen consumed by mitochondria independent of ADP phosphorylation, reflecting the basal level of mitochondrial uncoupling. To determine whether the observed increase in mitochondrial gene expression had consequential effects on mitochondrial function, we performed polarography on intact liver mitochondria from 3-wk-old wild-type, InhbaBK/BK, and InhbaBK/− mice. No significant differences in ADP-dependent (state III) oxygen consumption were evident when comparing the three genotypes (supplemental Fig. 3). Similarly, there were no differences during complete uncoupling, which occurs when mitochondria are treated with 2,4-dinitrophenol, a proton ionophore. However, ADP-independent (state IV) oxygen consumption was significantly greater in InhbaBK/− mice. The transition from state III to state IV marks the time that ADP is depleted from the oxygen sensor chamber, and is normally reflected by a flattening of the slope of the oxygen consumption curve. However, this transition was less evident in InhbaBK/− mice (supplemental Fig. 3A). A similar, although less pronounced difference was observed in mitochondria from InhbaBK/BK mice (supplemental Fig. 3B). Taken together, these data are consistent with a partial constitutive uncoupling of the inner mitochondrial membrane because oxygen consumption continues in mitochondria from InhbaBK/BK and InhbaBK/− mice after ADP depletion.
Discussion
We have previously shown that despite normal levels of the activin B protein in locations that activin-A is expressed, activin-B is functionally hypomorphic in many contexts. The severity of the disturbance depends on the requirement for activin signaling in the particular cell and tissue type as well as the number of InhbaBK alleles (21,22). The specific mechanism for the functional hypomorphism may reflect differences between activin-A and -B in their abilities to interact with receptors, coligands/coreceptors, and extracellular binding proteins. In fact, differences in receptor binding affinities and biological functions have been demonstrated for activin-A and -B, including selective responsiveness of pancreatic islets to activin-B, mediated by the activin receptor-like kinase (ALK)-7 receptor (31,32). In contrast to InhbaBK mice that have low insulin levels, hyperinsulinemia occurs in mice lacking activin-B and mice lacking ALK7 (25). Therefore, it is plausible that additional metabolic consequences can result from activin-B misexpression in InhbaBK mice, possibly also mediated by the inappropriate activation of ALK7.
A selective reduction in white adipose tissue mass was observed in 1-yr-old InhbaBK/BK mice. Additional organs were disproportionately smaller in the more severely affected InhbaBK/− mice, suggesting that the reduction of white adipose tissue mass in InhbaBK mice might be a secondary consequence of their increased metabolic rates. Alternatively, the differentiation, growth, or function of white adipose may be more sensitive than other organs to the direct effects of activin receptor signaling. Yet neither the InhbaBK/BK mice nor the InhbaBK/− mice exhibited lipodystrophy because the adipocytes of InhbaBK/BK mice retained the capacity to hypertrophy in a calorie-rich environment and there was no hyperglycemia or ectopic fat deposition in any of the InhbaBK mice (supplemental Fig. 2D and data not shown).
Body size affects energy expenditure (33,34,35,36,37,38,39), but the differences in body size between wild-type and InhbaBK/BK mice could not account entirely for the differences in the metabolic rate, confirmed by covariate analysis, which revealed genotype effects, even after adjusting for differences in body size. These observations are consistent with the contention that altered mitochondrial biogenesis and function is an important contributor to the leanness, smaller body size, and elevated metabolic rates of InhbaBK mice. First, several genes that play important roles in these processes were increased in liver, skeletal muscle, and brown adipose: three metabolically active sites with an abundance of mitochondria. PGC-1α, NRF1, and NRF2 are essential early transcriptional regulators of several genes that participate in mitochondrial biogenesis and function (40,41,42,43,44,45). As such, their up-regulation in tissues from InhbaBK mice suggests a relationship between the activins and transcriptional events that govern mitochondrial energy metabolism. Attractive candidate coregulators of this process include other members of the TGF-β superfamily, including myostatin, which binds to activin receptors and causes cachexia when overexpressed in vivo (8). Also, BMP7 is capable of binding and activating activin receptors (46), can be inhibited by myostatin during adipogenesis in vitro (47), and is sufficient to initiate and maintain the cascade of brown adipocyte differentiation in a variety of contexts, including the expression of mature brown adipocyte markers in vitro, and the formation of ectopic brown fat in vivo with consequential effects on whole-body energy metabolism (11).
These effects are mediated by p38 MAPK, part of a signal transduction cascade that activates mitochondrial biogenesis and function (48) and that is directly triggered by activin signaling in other contexts (49,50,51). Because activin and BMP cascades often occur in opposition (52,53,54), it is plausible that compromising activin signaling (as in InhbaBK mice) results in a disruption of the balance between activins, BMPs, and other TGF-β superfamily ligands that affect the basal metabolic rate. Candidate downstream transcription factors include PGC-1α, which was increased in InhbaBK mice and is activated and stabilized by p38 MAPK (48). Other candidates include members of the Fox family because they play important roles in adipogenesis and form activating transcriptional complexes with SMAD proteins (55,56,57). Interestingly, FoxC2, which forms an activating transcriptional complex with SMAD3 (a downstream mediator of activin signaling), causes a white to brown adipocyte conversion when selectively expressed in adipose tissues of transgenic mice (58). Also, we have observed ultrastructural characteristics in brown adipocytes from InhbaBK/− mice that are consistent with increased mitochondrial activity, specifically densely packed cristae and the smaller size of lipid droplets (data not shown); the latter is likely due to the increased lipolysis that is often associated with active thermogenesis in these cells. Finally, polarographic analyses of isolated liver mitochondria revealed no differences in state III or completely uncoupled oxygen consumption but instead showed a significant increase in state IV (ADP independent) oxygen consumption in InhbaBK mice. This suggests that mitochondrial oxidative phosphorylation capacity is not significantly altered but that under physiologic conditions, the process is less efficient than normal, reflecting an increase of partial mitochondrial uncoupling.
This process, which is regulated in part by UCPs (19), represents the fraction of potential energy generated by electron transport that is dissipated as heat rather than used for ADP phosphorylation. Because changes in Ucp mRNA levels are unreliable predictors of uncoupling protein activity, however, additional experiments will be required to test the hypotheses that UCPs either play a direct role in mitochondrial uncoupling in InhbaBK mice or that the InhbaBK mutation results in the dysregulation of Ucp mRNA levels through a feedback mechanism that attempts to offset the physiological consequences of a constitutive catabolic state.
These results led us to hypothesize that a major contributing factor to the leanness and higher metabolic rates of InhbaBK mice is that their energy requirements are intrinsically greater due to increased mitochondrial uncoupling. This would correspond to inner mitochondrial membrane leakiness that requires increased energy expenditure through electron transport to maintain the proton gradient and drive oxidative phosphorylation, analogous to a leaking pipeline that requires an increase in fluid volume to maintain outflow. Protection from diet-induced obesity, small to absent white adipose depots, absent hepatic steatosis, and near-normal glucose tolerance under HFD conditions are all consistent with this model because the excess energy substrates that would normally be stored in adipose and other tissues are instead used to offset the high substrate demand.
In conclusion, we have established major effects on energy metabolism as a result of aberrant activin signaling and have also shown that these effects are associated with abnormal mitochondrial function. As such, our findings support the contention that the activin signaling pathway may be an important target for modulating energy expenditure and provides an attractive focus for the treatment of medical conditions that affect body composition and metabolism, including cachexia, obesity, and diabetes.
Supplementary Material
Acknowledgments
We thank Caifen Tang, Deb Townley, Alexis Stephens, Keith Weiser, and Keltoum Anflous for technical assistance; Jennifer Northrop, Stephanie Pangas, Melanie Heney, and Martin Matzuk for critical review of the manuscript; and Hope Martin for administrative assistance.
Footnotes
This work was supported by National Institutes of Health Grant HD01156 and the Robert Wood Johnson Foundation. This work was also supported in part by Research Grant 5-FY01-482 from the March of Dimes Birth Defects Foundation. C.W.B. was a recipient of a Burroughs Wellcome Fund Career Award in the Biomedical Sciences.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 23, 2009
Abbreviations: ALK, Activin receptor-like kinase; BMP, bone morphogenetic protein; Fox, forkhead-box transcription factor; HDL, high-density lipoprotein; HFD, high-fat diet; LDL, low-density lipoprotein; NRF, nuclear respiratory factor; PGC, peroxisome proliferator-activated receptor-γ coactivator; RD, regular diet; SMAD, Sma- and Mad-related; UCP, uncoupling protein; VLDL, very low-density lipoprotein.
References
- Shi Y, Massagué J 2003 Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113:685–700 [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Hill CS 2004 New insights into TGF-β-Smad signalling. Trends Biochem Sci 29:265–273 [DOI] [PubMed] [Google Scholar]
- Massagué J 1998 TGF-β signal transduction. Annu Rev Biochem 67:753–791 [DOI] [PubMed] [Google Scholar]
- Chang H, Brown CW, Matzuk MM 2002 Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocr Rev 23:787–823 [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ 1997 Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387:83–90 [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ 2002 Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 109:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA 2002 Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun 291:701–706 [DOI] [PubMed] [Google Scholar]
- Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ 2002 Induction of cachexia in mice by systemically administered myostatin. Science 296:1486–1488 [DOI] [PubMed] [Google Scholar]
- Krummen LA, Woodruff TK, DeGuzman G, Cox ET, Baly DL, Mann E, Garg S, Wong WL, Cossum P, Mather JP 1993 Identification and characterization of binding proteins for inhibin and activin in human serum and follicular fluids. Endocrinology 132:431–443 [DOI] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC 2001 Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98:9306–9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR 2008 New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454: 1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ware SM, Sato A, Houston-Hawkins DE, Habas R, Matzuk MM, Shen MM, Brown CW 2006 The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development 133:319–329 [DOI] [PubMed] [Google Scholar]
- Wang W, Yang Y, Meng Y, Shi Y 2004 GDF-3 is an adipogenic cytokine under high fat dietary condition. Biochem Biophys Res Commun 321:1024–1031 [DOI] [PubMed] [Google Scholar]
- Andersson O, Korach-Andre M, Reissmann E, Ibáñez CF, Bertolino P 2008 Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc Natl Acad Sci USA 105:7252–7256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen JJ, Huang L, Li L, Jorgez C, Matzuk MM, Brown CW 2009 Deficiency of growth differentiation factor 3 protects against diet-induced obesity by selectively acting on white adipose. Mol Endocrinol 23:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Mather JP, Krummen L, Lu H, Bradley A 1994 Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci USA 91:8817–8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coerver KA, Woodruff TK, Finegold MJ, Mather J, Bradley A, Matzuk MM 1996 Activin signaling through activin receptor type II causes the cachexia-like symptoms in inhibin-deficient mice. Mol Endocrinol 10:534–543 [DOI] [PubMed] [Google Scholar]
- de Kretser DM, Robertson DM 1989 The isolation and physiology of inhibin and related proteins. Biol Reprod 40:33–47 [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB 2005 The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol 6:248–261 [DOI] [PubMed] [Google Scholar]
- de Kretser DM, Buzzard JJ, Okuma Y, O'Connor AE, Hayashi T, Lin SY, Morrison JR, Loveland KL, Hedger MP 2004 The role of activin, follistatin and inhibin in testicular physiology. Mol Cell Endocrinol 225:57–64 [DOI] [PubMed] [Google Scholar]
- Brown CW, Houston-Hawkins DE, Woodruff TK, Matzuk MM 2000 Insertion of Inhbb into the Inhba locus rescues the Inhba-null phenotype and reveals new activin functions. Nat Genet 25:453–457 [DOI] [PubMed] [Google Scholar]
- Brown CW, Li L, Houston-Hawkins DE, Matzuk MM 2003 Activins are critical modulators of growth and survival. Mol Endocrinol 17:2404–2417 [DOI] [PubMed] [Google Scholar]
- Trounce IA, Kim YL, Jun AS, Wallace DC 1996 Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264:484–509 [DOI] [PubMed] [Google Scholar]
- Yasuda H, Inoue K, Shibata H, Takeuchi T, Eto Y, Hasegawa Y, Sekine N, Totsuka Y, Mine T, Ogata E, et al. 1993 Existence of activin-A in A- and D-cells of rat pancreatic islet. Endocrinology 133:624–630 [DOI] [PubMed] [Google Scholar]
- Bertolino P, Holmberg R, Reissmann E, Andersson O, Berggren PO, Ibáñez CF 2008 Activin B receptor ALK7 is a negative regulator of pancreatic β-cell function. Proc Natl Acad Sci USA 105:7246–7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka T, Idehara C, Yano M, Matsushita T, Yamada T, Ii S, Moritani M, Hata J, Sugino H, Noji S, Itakura M 1998 Hypoplasia of pancreatic islets in transgenic mice expressing activin receptor mutants. J Clin Invest 102:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Cleary MM, Si Y, Liu G, Eto Y, Kritzik M, Dabernat S, Kayali AG, Sarvetnick N 2004 Inhibition of activin signaling induces pancreatic epithelial cell expansion and diminishes terminal differentiation of pancreatic β-cells. Diabetes 53:2024–2033 [DOI] [PubMed] [Google Scholar]
- Franzén A, Piek E, Westermark B, ten Dijke P, Heldin NE 1999 Expression of transforming growth factor-β1, activin A, and their receptors in thyroid follicle cells: negative regulation of thyrocyte growth and function. Endocrinology 140:4300–4310 [DOI] [PubMed] [Google Scholar]
- Schulte KM, Jonas C, Krebs R, Röher HD 2001 Activin A and activin receptors in thyroid cancer. Thyroid 11:3–14 [DOI] [PubMed] [Google Scholar]
- Matsuo SE, Ebina KN, Kulcsar MA, Friguglietti CU, Kimura ET 2003 Activin βB Expression in rat experimental goiter and human thyroid tumors. Thyroid 13:239–247 [DOI] [PubMed] [Google Scholar]
- Mathews LS, Vale WW 1991 Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell 65:973–982 [DOI] [PubMed] [Google Scholar]
- del Re E, Sidis Y, Fabrizio DA, Lin HY, Schneyer A 2004 Reconstitution and analysis of soluble inhibin and activin receptor complexes in a cell-free system. J Biol Chem 279:53126–53135 [DOI] [PubMed] [Google Scholar]
- James WP, Trayhurn P 1981 Thermogenesis and obesity. Br Med Bull 37:43–48 [DOI] [PubMed] [Google Scholar]
- Shibata H, Bukowiecki LJ 1987 Regulatory alterations of daily energy expenditure induced by fasting or overfeeding in unrestrained rats. J Appl Physiol 63:465–470 [DOI] [PubMed] [Google Scholar]
- Blaxter KL 1989 Energy metabolism in animals and man. Cambridge (England) and New York: Cambridge University Press [Google Scholar]
- Freeman KB, Heffernan M, Dhalla Z, Patel HV 1989 Effects of exposure temperature on brown adipose tissue uncoupling protein mRNA levels. Biochem Cell Biol 67:147–151 [DOI] [PubMed] [Google Scholar]
- Porter RK, Brand MD 1993 Body mass dependence of H+ leak in mitochondria and its relevance to metabolic rate. Nature 362:628–630 [DOI] [PubMed] [Google Scholar]
- al-Adsani H, Hoffer LJ, Silva JE 1997 Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metab 82:1118–1125 [DOI] [PubMed] [Google Scholar]
- Rippe C, Berger K, Böiers C, Ricquier D, Erlanson-Albertsson C 2000 Effect of high-fat diet, surrounding temperature, and enterostatin on uncoupling protein gene expression. Am J Physiol Endocrinol Metab 279:E293–E300 [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM 2005 Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370 [DOI] [PubMed] [Google Scholar]
- Puigserver P 2005 Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-α. Int J Obes (Lond) 29(Suppl 1):S5–S9 [DOI] [PubMed] [Google Scholar]
- Scarpulla RC 2002 Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta 1576:1–14 [DOI] [PubMed] [Google Scholar]
- Kelly DP, Scarpulla RC 2004 Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 18:357–368 [DOI] [PubMed] [Google Scholar]
- Virbasius JV, Virbasius CA, Scarpulla RC 1993 Identity of GABP with NRF-2, a multisubunit activator of cytochrome oxidase expression, reveals a cellular role for an ETS domain activator of viral promoters. Genes Dev 7:380–392 [DOI] [PubMed] [Google Scholar]
- Scarpulla RC 2006 Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem 97:673–683 [DOI] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, Heldin CH, Miyazono K 1995 Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol 130:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L 2003 Myostatin signals through a transforming growth factor β-like signaling pathway to block adipogenesis. Mol Cell Biol 23:7230–7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM 2001 Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARγ coactivator-1. Mol Cell 8:971–982 [DOI] [PubMed] [Google Scholar]
- Cocolakis E, Lemay S, Ali S, Lebrun JJ 2001 The p38 MAPK pathway is required for cell growth inhibition of human breast cancer cells in response to activin. J Biol Chem 276:18430–18436 [DOI] [PubMed] [Google Scholar]
- Zhang L, Deng M, Parthasarathy R, Wang L, Mongan M, Molkentin JD, Zheng Y, Xia Y 2005 MEKK1 transduces activin signals in keratinocytes to induce actin stress fiber formation and migration. Mol Cell Biol 25:60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guise C, Lacerte A, Rafiei S, Reynaud R, Roy M, Brue T, Lebrun JJ 2006 Activin inhibits the human Pit-1 gene promoter through the p38 kinase pathway in a Smad-independent manner. Endocrinology 147:4351–4362 [DOI] [PubMed] [Google Scholar]
- Candia AF, Watabe T, Hawley SH, Onichtchouk D, Zhang Y, Derynck R, Niehrs C, Cho KW 1997 Cellular interpretation of multiple TGF-β signals: intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development 124:4467–4480 [DOI] [PubMed] [Google Scholar]
- Piek E, Afrakhte M, Sampath K, van Zoelen EJ, Heldin CH, ten Dijke P 1999 Functional antagonism between activin and osteogenic protein-1 in human embryonal carcinoma cells. J Cell Physiol 180:141–149 [DOI] [PubMed] [Google Scholar]
- Faure S, Lee MA, Keller T, ten Dijke P, Whitman M 2000 Endogenous patterns of TGFβ superfamily signaling during early Xenopus development. Development 127:2917–2931 [DOI] [PubMed] [Google Scholar]
- Seoane J, Le HV, Shen L, Anderson SA, Massagué J 2004 Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117:211–223 [DOI] [PubMed] [Google Scholar]
- Labbé E, Silvestri C, Hoodless PA, Wrana JL, Attisano L 1998 Smad2 and Smad3 positively and negatively regulate TGF β-dependent transcription through the forkhead DNA-binding protein FAST2. Mol Cell 2:109–120 [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Tsukazaki T, Nishimatsu S, Attisano L, Wrana JL, Thomsen GH 1999 Dominant-negative Smad2 mutants inhibit activin/Vg1 signaling and disrupt axis formation in Xenopus. Dev Biol 207:364–379 [DOI] [PubMed] [Google Scholar]
- Fujita H, Kang M, Eren M, Gleaves LA, Vaughan DE, Kume T 2006 Foxc2 is a common mediator of insulin and transforming growth factor β signaling to regulate plasminogen activator inhibitor type I gene expression. Circ Res 98:626–634 [DOI] [PubMed] [Google Scholar]
- Chang H, Brown CW, Matzuk MM 2002 Genetic analysis of the transforming growth factor β superfamily. Endocr Rev 23:787–823 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.