Abstract
Uterine receptivity to embryo implantation depends on appropriate progesterone (P4) and estrogen stimulation. P4 rapidly stimulates production of the morphogen Indian hedgehog (IHH) in murine uterine epithelium as well as downstream molecules in the hedgehog pathway such as Patched homolog 1 (PTCH1) and nuclear receptor subfamily 2, group F, member 2 (NR2F2) in uterine stroma. Studies using IHH-null mice indicate that IHH is obligatory for the normal P4 response in the uterus. To determine whether IHH induction in uterine epithelium is mediated through P4 receptor (PR) in epithelium (E) and/or stroma (S), we produced tissue recombinants using uteri from neonatal PR knockout (ko) mice and wild-type (wt) mice containing PR in S and/or E or lacking PR altogether using a tissue recombinant methodology and assessed their response to P4. In tissue recombinants containing wt-S (wt-S + wt-E and wt-S + ko-E), P4 induced Ihh mRNA expression at 6 h that was 6-fold greater than in oil-treated controls (P < 0.05; n = 6) in both types of tissue recombinants despite the absence of epithelial PR in wt-S + ko-E grafts. Conversely, Ihh mRNA expression was unaffected by P4 in ko-S + ko-E and ko-S + wt-E grafts despite epithelial PR expression in the latter. Nr2f2 and Ptch1 mRNA expression was similar in that it was stimulated by P4 only in recombinants containing stromal PR. These results indicate that stromal PR is both necessary and sufficient for P4 stimulation of epithelial IHH as well as downstream events such as PTCH1 and NR2F2 increases in stroma.
Uterine stromal progesterone receptor (PR) is both necessary and sufficient for progesterone-mediated induction of uterine epithelial Indian hedgehog and downstream molecules, while epithelial PR plays no role in this process.
Progesterone (P4) is required for the establishment and maintenance of pregnancy in mammals. In the uterus, P4 prepares the endometrium for embryo implantation, regulates epithelial and stromal cell proliferation, and is involved in myriad other uterine processes both before and during gestation. The functions of P4 are mediated through P4 receptor (PR), a member of a large family of nuclear receptors that are ligand-activated transcription factors. Despite extensive investigations over the years, the mechanism by which P4 induces its effects are not completely understood, although recent results have indicated that Indian hedgehog (IHH) may be a critical regulator of P4 actions in the uterus.
In 2002, two separate groups identified IHH as a P4-induced gene in the uterus (1,2). IHH expression in response to P4 was confined to uterine epithelium, but P4 also induced stromal expression of a receptor for IHH, Patched homolog 1 (PTCH1), as well as other downstream regulators of the P4 effect, such as GLI1, GLI2, and nuclear receptor subfamily 2, group F, member 2 (NR2F2; also known as COUP-TFII) (3,4). Subsequent work using a knockout of IHH in cells that expressed PR in the uterus (4) demonstrated that loss of IHH resulted in a total loss of typical uterine P4 responses, indicating that IHH is an obligatory mediator of uterine P4 actions.
Due to its centrality in the normal uterine P4 response, it is critical to understand how IHH is regulated. PR is normally expressed in both uterine stroma and epithelium, and an obvious mechanism would be for P4 to signal through epithelial PR to induce epithelial IHH expression (5). However, extensive work has demonstrated that many P4 effects on uterine epithelium are mediated indirectly, through PR in stroma. For example, stromal PR mediate the inhibitory effect of P4 on uterine epithelial proliferation induced by 17β-estradiol (6). Similarly, P4 antagonizes the effects of 17β-estradiol on the expression of the secretory protein lactoferrin and on PR expression in the epithelium, and these P4 actions in the epithelium have been shown to be entirely mediated through PR in uterine stroma (7).
Uterine epithelial cells show decreases in PR down to minimal or even undetectable levels in numerous mammalian species, including humans, before implantation (8,9,10,11). The continued action of P4 on various epithelial parameters during later gestational periods again suggests the possibility that these P4 actions on epithelium could be mediated through stroma (12). However, the possibility must be considered that PR expression in the periimplantation uterus may be sufficient to directly mediate P4-induced epithelial effects, even though it is below the limit of detection in our current assay (12).
The extensive availability of mice with targeted deletions of various genes has greatly facilitated experiments designed to elucidate the role of a particular gene in a tissue or physiological process. These knockout mice have been used extensively in conjunction with a tissue recombination technique to gain insights into the role of stromal and epithelial steroid (6,7,13,14,15,16) and protein (17) hormone receptors in various processes in reproductive and nonreproductive organs. This tissue recombination methodology involves enzymatically separating and recombining the epithelium and stroma from uteri or other reproductive organs of a wild-type (wt) mouse with those of a mouse in which a critical hormone receptor gene, such as PR, has been knocked out. These tissue recombinants are subsequently grafted into host mice, and by modulating the hormonal environment of the host animal and observing the response of the tissue recombinants composed of various combinations of wt and knockout (ko) epithelium and stroma, the role of the particular hormone receptor in each tissue compartment can be definitively determined.
In this study, we used the tissue recombination technique in conjunction with PR ko mice to determine relative roles of stromal and epithelial PR in the increase in epithelial Ihh and downstream stromal genes that occurs after P4 treatment. Our results indicate that stromal PR is both necessary and sufficient to mediate both the production of uterine epithelial Ihh and downstream genes. Conversely, epithelial PR plays no role in epithelial Ihh induction after P4 treatment.
Materials and Methods
Tissue separation and recombination
All animal experiments described here were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Illinois Laboratory Animal Care Advisory Committee.
The procedure used for separation and recombination of stroma and epithelium from neonatal mouse uteri has been described previously (15,18). Briefly, uteri from neonatal wt and ko littermate mice (5 d old) were dissected free of adherent connective tissue and fat and placed in Hanks’ balanced salt solution. Tissues were enzymatically dissociated in a solution of 1% Trypsin 250 (Difco Laboratories, Detroit, MI) in calcium- and magnesium-free Hanks’ balanced salt solution for 90 min at 4 C. After trypsin digestion, stroma and epithelium were physically separated using fine surgical instruments. Four types of tissue recombinants were prepared: wt stroma (wt-S) + wt epithelium (wt-E), wt-S + ko epithelium (ko-E), ko stroma (ko-S) + wt-E, and ko-S + ko-E. Stroma and epithelium were recombined on nutrient agar plates and allowed to adhere during overnight culture at 37 C in a humidified 5% CO2 atmosphere. Tissue recombinants were grafted under the renal capsules of syngeneic wt female 129/Sv mice. Approximately 4 wk after grafting, all hosts were ovariectomized and 3 wk later received P4 (1 mg in 0.1 ml oil per mouse) or an equivalent amount of oil vehicle alone. The hosts were killed and tissue recombinants recovered at 6 h for IHH analysis and at 30 h for PTCH1 and NR2F2 analysis. Some recovered grafts were frozen in liquid N2 for subsequent quantitative PCR analysis, whereas others were fixed in neutral buffered formalin for immunohistochemistry or immunofluorescence. A minimum of six replicates of each of the four types of uterine tissue recombinants was obtained from both the P4-treated and vehicle-treated groups.
To determine the expression of Ihh in neonatal uterine epithelium and stroma, 5-d-old female pups were injected with P4 (40 mg/kg sc) or oil. After 6 h, uterine epithelium and stroma were separated as described and snap frozen for subsequent RNA analysis.
RNA extraction and real-time PCR
RNA was extracted from the grafts using the RNeasy mini kit and neonatal uterine epithelium and stroma using the RNeasy micro kit according to the manufacturer’s instructions (QIAGEN, Valencia, CA). Samples were treated with deoxyribonuclease to prevent DNA contamination. First-strand cDNA was synthesized from total RNA (0.5 μg) using Superscript reverse transcriptase III and random primers (Invitrogen, Carlsbad, CA). The expression value of each gene was normalized to the amount of an internal control gene (18S rRNA) cDNA to calculate a relative amount of RNA in each sample. The expression value of Ihh mRNA in each recombinant was normalized against Cadherin 1 (Cdh1; also known as E-cadherin) mRNA and Ptch1 and Nr2f2 mRNA against Vimentin (Vim) mRNA. The expression value of each gene in vehicle-treated controls was arbitrarily defined as 1. Real-time PCR assays were carried out in triplicate and the normalized expression values for all samples averaged. A relative quantitative fold change was determined using the ΔΔCt method (ABI Chemistry Guide 4330019; Applied Biosystems, Foster City, CA). ABI TaqMan gene expression assays used for specific transcripts were Mm00439613_m1 (Ihh), Mm00486906_m1 (Cdh1), Mm00772789_m1 (Nr2f2), Mm00436026_m1 (Ptch1), Mm0449201_m1 (Vim), and Hs 9999901_s1 (18S).
Immunohistochemistry
Grafts were fixed in 10% neutral-buffered formalin, dehydrated through graded alcohols, and embedded in paraffin. Grafts were sectioned at 4 μm, deparaffinized, and rehydrated. To facilitate antigen detection, slides were placed in boiling 10 mm sodium citrate buffer (pH 6.0) for 10 min and then cooled to ambient temperature. Endogenous peroxidase activity was quenched by incubation with 0.3% H2O2 for 20 min. Primary antibodies used were PR (Dako, Carpinteria, CA) and NR2F2 (Perseus Proteomics, Tokyo, Japan). For PR immunostaining, a horseradish peroxidase-conjugated antirabbit IgG was used as the secondary antibody and 3-amino, 9-ethyl-carbazole as the detection system. Immunohistochemistry for NR2F2 was performed using the Tyramide Signal Amplification System (PerkinElmer, Waltham, MA) according to the manufacturer’s instructions. Negative controls were processed without the corresponding primary antibody.
Statistical analysis
Data were analyzed by one-way ANOVA followed by Tukey-Kramer’s multiple comparison tests. Statistical analyses were performed using GraphPad Prism 4.0 (GraphPad Software, Inc., San Diego, CA). Data are expressed as mean ± sem, and differences between groups were considered significant at P < 0.05.
Results
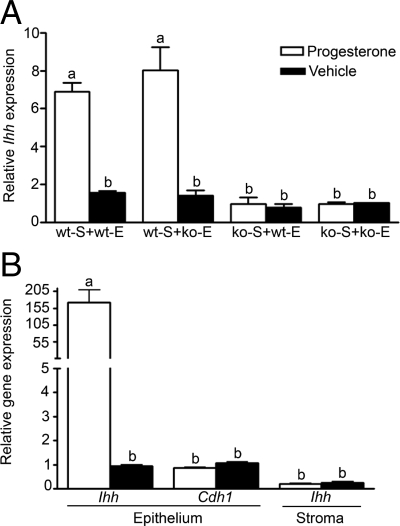
In wt-S + wt-E tissue recombinations, P4 treatment of ovariectomized hosts induced approximately a 6-fold increase in Ihh mRNA compared with similar tissue recombinations in hosts given only oil vehicle (Fig. 1). In tissue recombinations prepared with wt-S but ko-E, P4 treatment of ovariectomized hosts induced large increases in Ihh mRNA that were similar to those in wt-S + wt-E tissue recombinations in response to P4 (Fig. 1); thus, PR status of the epithelium did not affect the increase in Ihh mRNA in response to P4. This contrasted with results observed in tissue recombinations prepared with ko-S (ko-S + wt-E and ko-S + ko-E), where P4 treatment of ovariectomized hosts did not increase Ihh mRNA over low levels seen in oil-treated hosts. As with the tissue recombinations prepared with wt-S, epithelial PR status did not affect Ihh mRNA response to P4, because Ihh mRNA was similar in ko-S + wt-E and ko-S + ko-E tissue recombinations.
Figure 1.
Relative roles of stromal and epithelial PR in the induction of epithelial Ihh mRNA in response to P4. A, In uterine tissue recombinations containing wt-S (wt-S + wt-E and wt-S + ko-E), P4 induced a robust increase in Ihh mRNA compared with these tissue recombinations in oil-treated hosts. In contrast, uterine tissue recombinations lacking wt-S (ko-S + wt-E and ko-S + ko-E) did not have an increase in Ihh mRNA over levels seen in oil-treated controls, despite the presence of epithelial PR in ko-S + wt-E tissue recombinations. Thus, Ihh mRNA is regulated by P4 signaling through stromal PR. The Ihh mRNA values were normalized against Cdh1 mRNA to control for varying amounts of epithelium present in different tissue recombinants and allow valid comparisons between groups. B, In neonatal pups, P4 treatment induced a 170-fold increase in Ihh mRNA in uterine epithelium compared with oil-treated controls. Ihh mRNA in uterine stroma was low and not induced by P4. Ihh mRNA in uterine stroma is expressed relative to Ihh mRNA of oil-treated uterine epithelium. Cdh1 expression in uterine epithelium was unchanged by P4. Bars with different superscripts are significantly different at P < 0.01. Each data point is based on n ≥ 6, and Ihh mRNA expression in various groups was compared by one-way ANOVA followed by Tukey-Kramer’s multiple-comparison tests.
In neonatal uterus, P4 treatment induced an approximately 175-fold increase in Ihh mRNA in uterine epithelium compared with oil-treated controls. Ihh mRNA in uterine stroma was low and not increased by P4. The mRNA expression of Cdh1, used as a normalization standard, was not changed by P4 (Fig. 1).
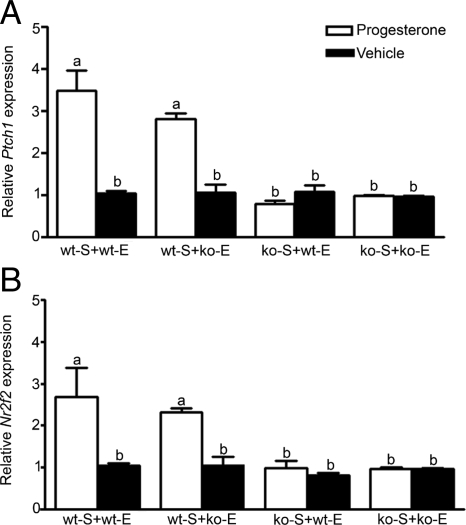
In both types of uterine tissue recombinations containing wt-S (wt-S + wt-E and wt-S + ko-E), P4 treatment induced a 3-fold increase in Ptch1 mRNA compared with these tissue recombinations in oil-treated hosts (Fig. 2). In contrast, in uterine tissue recombinations lacking wt-S (ko-S + wt-E and ko-S + ko-E), Ptch1 mRNA expression was low and not increased relative to oil-treated controls. Comparable low levels of Ptch1 mRNA expression in response to P4 were seen in ko-S + wt-E tissue recombinations as well as ko-S + ko-E tissue recombinations, despite epithelial PR expression in the former. Expression of Nr2f2 mRNA in uterine tissue recombinants in response to P4 was similar to that for Ptch1 mRNA (Fig. 2); P4 induced Nr2f2 mRNA only when stromal PR was present, and epithelial PR status did not affect Nr2f2 mRNA expression.
Figure 2.
Expression of Ptch1 (A) and Nr2f2 (B) mRNA in uterine tissue recombinants in response to P4. In uterine tissue recombinations containing wt-S (wt-S + wt-E and wt-S + ko-E), P4 induced a robust increase in Ptch1 mRNA compared with those tissue recombinations in oil-treated hosts. In contrast, uterine tissue recombinations lacking wt-S (ko-S + wt-E and ko-S + ko-E) showed Ptch1 mRNA comparable to oil-treated controls, despite epithelial PR in ko-S + wt-E tissue recombinations. Expression of Nr2f2 mRNA in uterine tissue recombinants in response to P4 was similar to that seen with Ptch1 mRNA, in that P4 induced Nr2f2 mRNA only when stromal PR was present, and epithelial PR status did not affect Nr2f2 mRNA expression. Bars with different superscripts are significantly different at P < 0.01. All Ptch1 and Nr2f2 mRNA values were normalized against Vim mRNA to control for varying amounts of stroma present in different tissue recombinants and allow valid comparisons between groups.
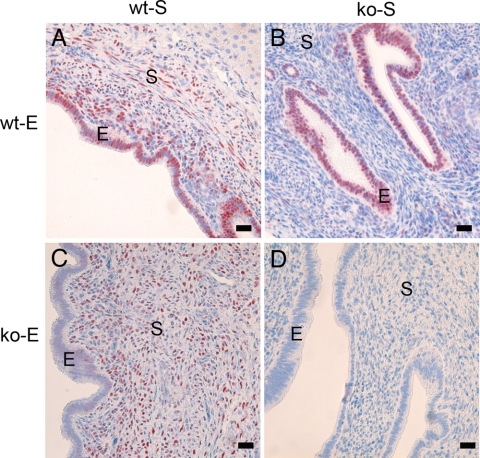
In homotypic tissue recombinations of wt tissue (wt-S + wt-E), PR immunostaining was detected in both stromal and epithelial compartments (Fig. 3A). Conversely, in homotypic tissue recombinations of ko tissue (ko-S + ko-E), PR immunostaining was not detected (Fig. 3D). In heterotypic tissue recombinations (wt-S + ko-E and ko-S + wt-E), PR immunostaining was detected only in tissues that were of wt origin (Fig. 3, B and C).
Figure 3.
Localization of PR expression in tissue recombinants. Uterine tissue recombinations of wt-S + wt-E showed PR immunostaining in both stromal (S) and epithelial (E) compartments (A). Conversely, in wt-S + ko-E tissue recombinations (C), PR immunostaining was confined to the stroma, whereas epithelium lacked PR. In ko-S + wt-E (B) tissue recombinations, PR immunostaining was detected only in epithelium. In ko-S + ko-E (D) tissue recombinations, PR immunostaining was undetectable. Scale bar, 10 μm.
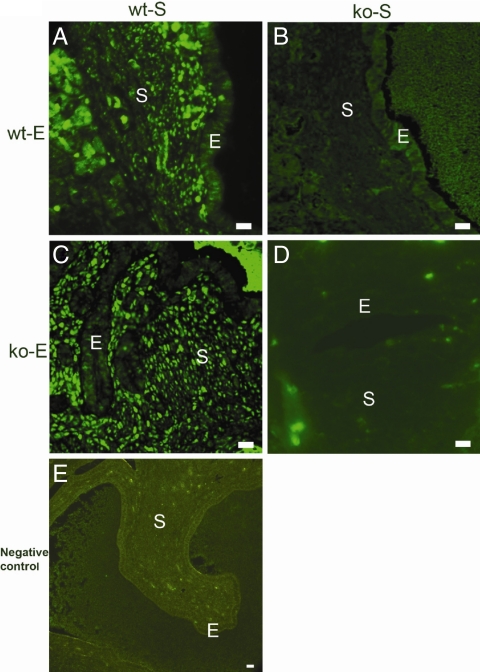
NR2F2 expression in uterine tissue recombinations was also evaluated using immunohistochemistry. P4 induced striking increases in stromal NR2F2 expression in wt-S + wt-E tissue recombinations (Fig. 4A). Epithelial cells did not express NR2F2, consistent with previous results indicating that NR2F2 is expressed only in uterine stroma (3). In tissue recombinations of wt-S + ko-E (Fig. 4c), P4 induced similar increases in stromal NR2F2 expression despite their lack of epithelial PR. Conversely, in ko-S + wt-E (Fig. 4B) or ko-S + ko-E (Fig. 4D) tissue recombinations, NR2F2 expression was not stimulated by P4, despite the presence of PR in epithelial cells of the former.
Figure 4.
Immunohistochemical localization of NR2F2 expression in uterus in response to P4. In wt-S + wt-E uterine tissue recombinations (panel A), P4 induced increased stromal (S) NR2F2 expression. NR2F2 was not expressed in the epithelium (E). In wt-S + ko-E tissue recombinations (panel C), P4 induced similar increases in stromal NR2F2 expression despite absence of epithelial PR. Conversely, in ko-S + wt-E (panel B) or ko-S + ko-E (panel D) tissue recombinations, NR2F2 expression was not stimulated by P4, despite PR expression in epithelial cells of ko-S + wt-E grafts. A negative control of a wt-S + ko-E tissue recombination processed without primary antibody did not show staining (panel E). Scale bar, 10 μm.
Discussion
Previous work has indicated that Ihh is a P4-induced gene in uterine epithelium (1,2). Subsequent work using a knockout of IHH in cells that expressed PR in the uterus (4) demonstrated that loss of IHH resulted in a total loss of typical uterine P4 responses, indicating that IHH is an obligatory mediator of P4 actions in uterus. The stimulation of Ihh mRNA in murine uterine epithelium by P4 results in secondary increases in expression of downstream molecules in the stroma that are involved in the hedgehog pathway such as the IHH receptor PTCH1 and NR2F2, which is an essential mediator of P4 effects on implantation (2). The increases in stromal PTCH1 and NR2F2 are obligatorily dependent on epithelial IHH and do not occur in mice where epithelial IHH has been ablated. NR2F2 was found to regulate bone morphogenetic protein-2 (BMP2) that is essential for decidualization of stromal cells during implantation in the murine uterus (3,4). NR2F2 is also essential for angiogenesis, and defects in angiogenesis in the uterus in IHH conditional ko mice are probably due to impaired induction of NR2F2 (4).
The elucidation of the critical role of IHH in P4 signaling in the uterus was an important step toward a mechanistic understanding of how P4 induces uterine effects. A critical question that remained to be answered was whether P4 stimulated Ihh mRNA through stromal and/or epithelial PR. To address this question, we used wt and PR ko uteri to produce tissue recombinations in which PR was present in stroma and/or epithelium or was not present in either compartment. In tissue recombinants of wt-S + wt-E, P4 induced large increases in epithelial Ihh mRNA that were comparable to those in intact uteri (1,2). These results emphasize the validity of the tissue separation/recombination and grafting technique and illustrate the similar responses of normal uteri and grafted tissue recombinations to trophic stimuli such as P4 treatment.
Our results indicate that induction of Ihh mRNA in uterine epithelium by P4 is mediated by stromal PR. This is clearly illustrated by the similar Ihh mRNA expression induced by P4 in wt-S + wt-E compared with wt-S + ko-E tissue recombinants, which both showed high levels of Ihh mRNA despite lack of epithelial PR in the latter. Thus, stromal PR is both necessary and sufficient for epithelial Ihh mRNA expression, in that Ihh mRNA increases are seen in tissue recombinants that have only stromal PR but lack epithelial PR. Conversely, lack of stromal PR in ko-S + wt-E and ko-S + ko-E tissue recombinants precludes the normal Ihh mRNA increase in response to P4, even though ko-S + wt-E tissue recombinants still express epithelial PR. These results indicate that epithelial PR is neither necessary nor sufficient for epithelial Ihh mRNA increases in response to P4 and that P4 increases epithelial Ihh mRNA entirely through P4 interactions with stromal PR and subsequent stromal-epithelial interactions.
Our results indicate that Ptch1 and Nr2f2 mRNA expression is entirely mediated by P4 acting through stromal PR. As for Ihh mRNA expression, stromal PR is both necessary and sufficient, and epithelial PR appears to play no role in the increase in Ptch1 and Nr2f2 mRNA expression after P4 treatment. Thus, although epithelial production of IHH is an essential mediator of downstream P4 signaling events, such as secondary increases in Ptch1 and Nr2f2 mRNA expression, epithelial PR is not necessary for or apparently involved in this process. Thus, P4 binding to stromal PR induces epithelial Ihh mRNA expression, and this induces Ptch1 and Nr2f2 mRNA expression through stromal-epithelial cross talk, and all of these processes are mediated through stromal PR and independent of epithelial PR.
The quantitative PCR analysis of Nr2f2 mRNA expression was directly corroborated by immunohistochemical staining for NR2F2. These results clearly identified stroma as the sole source of NR2F2, consistent with earlier work (3), vividly illustrated the normal increase in stromal NR2F2 in response to P4 in both wt-S + wt-E and wt-S + ko-E tissue recombinants and also showed the lack of this response in the absence of stromal PR in ko-S + wt-E and ko-S + ko-E grafts.
Immunohistochemistry for PR was used as an essential means of validating the identity and PR expression in different tissue compartments of the various recombinants. These results indicated that PR immunostaining in tissue recombinants prepared with entirely wt tissues (wt-S + wt-E) or ko tissues (ko-S + ko-E) show PR immunostaining identical to the wt and PR ko uteri, respectively (6). Likewise, in heterotypic tissue recombinations (wt-S + ko-E and ko-S + wt-E), PR immunostaining was detected only in tissues that were of wt origin, validating the efficacy of the tissue separation/recombination procedure. Of equal importance, the PR immunostaining illustrates that PR expression in one tissue compartment is not dependent on PR expression in the other. For example, in ko-S + wt-E and wt-S + ko-E tissue recombinants, PR expression is still seen in the epithelium and stroma, respectively, despite the fact that these tissues have been recombined and grafted with tissue from ko mice lacking PR. This normal expression of either epithelial or stromal PR even in the absence of PR expression in the other tissue compartment is critical for interpretation of the overall results. For example, these results eliminate the possibility that a loss of epithelial PR expression in ko-S + wt-E tissue recombinants could preclude a PR response in the epithelium and erroneously lead to a conclusion that epithelial PR was not involved in the increase in Ihh, Ptch1, or Nr2f2 mRNA induced by P4 even if epithelial PR normally played a critical role in the intact uterus.
Tissue recombinations at the time of grafting contain varying amounts of epithelium despite efforts to add similar amounts of epithelium during tissue recombinant preparation. In addition, use of tissue from animals in which a gene such as PR that regulates epithelial proliferation has been knocked out in epithelial and/or stromal tissue can lead to marked differences in epithelial proliferation in various types of tissue recombinations. Thus, simply examining expression of an epithelial gene such as Ihh relative to a housekeeping gene ubiquitously present in all cells does not ensure valid comparisons among different groups of tissue recombinations. Hence all comparisons of Ihh mRNA here are made relative to Cdh1 mRNA. CDH1 is an epithelial protein that does not show extensive variation in response to hormones, proliferative status, etc. in the uterus and thus functions as an effective indicator of epithelial content in tissue recombinations (14). Comparison of Ihh mRNA relative to Cdh1 mRNA allows results to be expressed as essentially Ihh mRNA per a certain quantity of epithelium, a more accurate and valid measure of Ihh mRNA expression than simply comparing it to a housekeeping gene expressed in both epithelial and connective tissue cells for normalization. Similarly, in the analyses of Ptch1 and Nr2f2 mRNA in uterine tissue recombinants, all values for these stromal proteins were normalized against Vim mRNA, a marker of connective tissue cells, to take into account variations in connective tissue amounts in the tissue recombinants and provide the most accurate basis of comparison.
Our present results, in conjunction with earlier studies of Ihh, Ptch1, and Nr2f2 mRNA and protein expression and regulation (1,3,4,5), lead to the proposal of the following model for how P4 signals in the uterus (Fig. 5). Our results indicate that P4 binding to stromal PR induces epithelial IHH, and this IHH secondarily induces PTCH1 and NR2F2 in the stroma. This complex signaling cascade results in typical P4 actions on critical events such as implantation and decidualization. Early demonstrations of stromal-epithelial interactions led to speculation about and some evidence for epithelial communication with stroma (19), although the vast majority of literature in this field concerns stromal effects on epithelium, rather than vice versa. The recent demonstration of IHH involvement in P4 signaling in the uterus (1,2,4,5,20) and the elucidation of sonic hedgehog (SHH) as an epithelial signal critical for normal androgen response of the stroma in the developing prostate (21) have begun to provide mechanistic information about these long-postulated epithelial signals.
Figure 5.
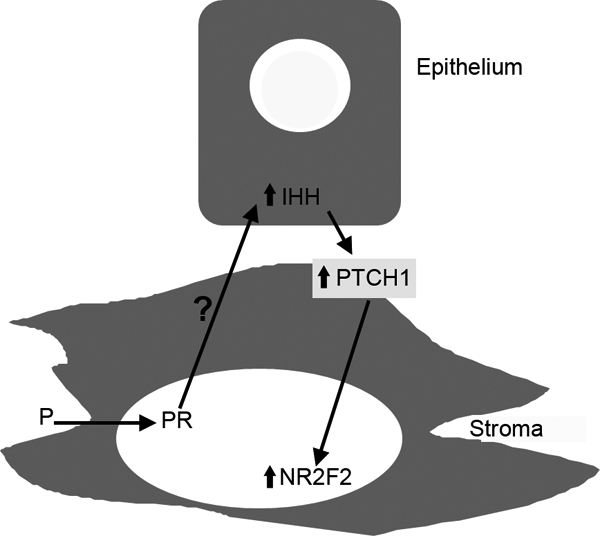
Model for P4 induction of epithelial IHH and downstream stromal molecules such as PTCH1 and NR2F2 in uterus. P4 binds to PR in stromal cells, inducing an increase in epithelial IHH through an as yet undefined stromal-epithelial interaction. IHH production in epithelium results in PTCH1 and NR2F2 expression in stroma. This mechanism of P4 response represents the first known reciprocal communication loop known in uterus, in which communication from the stroma in response to P4 leads to the production of an epithelial molecule, and that epithelial molecule then acts back on stroma to produce changes in expression of other molecules downstream of the IHH response, such as PTCH1 and NR2F2.
The present work is the first demonstration of reciprocal stromal-epithelial interactions to regulate key signaling molecules in both compartments and represents a step forward in our understanding of the intricate mechanisms regulating steroid response in target organs such as the uterus. A critical question that remains is the identity of the stromal signal produced in response to P4 that induces epithelial IHH production and starts the IHH/PTCH1/NR2F2 signaling cascade. Some progress has been made in identifying molecules such as fibroblast growth factors 7 and 10 (FGF7 and -10, respectively) and hepatocyte growth factor as stromal molecules that could function as progestamedins that are induced by P4 in stroma and then act on the epithelium to induce the eventual epithelial response (12,22,23,24,25,26). Cheng et al. (27) reported that 17β-hydroxysteroid dehydrogenase type 2 expression in human endometrial epithelial cells is regulated by the transcription factors SP1 and SP3 that are targets of P4-dependent paracrine signaling from the endometrial stromal cells. Likewise, some stromal molecules that regulate epithelial response to androgens and estrogens have also been suggested (12,26,28,29). However, the precise mechanisms by which stromal cells signal epithelium in response to hormones remain largely unknown, despite the critical nature of this process for normal response to steroid hormones (6,7,13,15,16,30,31) and protein hormones such as relaxin (17). Elucidation of these critical factors will be essential for continued progress in understanding stromal-epithelial cross talk and its role in hormone signaling.
Footnotes
This work was supported by National Institutes of Health Grant U54 HD055787 (M.K.B., I.C.B., F.J.D., P.S.C.) and the Billie A. Field Endowment, University of Illinois (P.S.C.). Work at the University of Illinois was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR16515 from the National Center for Research Resources, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 16, 2009
Abbreviations: IHH, Indian hedgehog; ko, knockout; ko-E, ko epithelium; ko-S, ko stroma; NR2F2, nuclear receptor subfamily 2, group F, member 2; P4, progesterone; PR, P4 receptor; PTCH1, Patched homolog 1; wt, wild type; wt-E, wt epithelium; wt-S, wt stroma.
References
- Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK 2002 Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol 245:280–290 [DOI] [PubMed] [Google Scholar]
- Takamoto N, Zhao B, Tsai SY, DeMayo FJ 2002 Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol 16:2338–2348 [DOI] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY 2007 COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 3:e102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ 2006 Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 38:1204–1209 [DOI] [PubMed] [Google Scholar]
- Lee K, Jeong J, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ 2006 Molecular mechanisms involved in progesterone receptor regulation of uterine function. J Steroid Biochem Mol Biol 102:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Young P, Brody JR, Lydon JP, O'Malley BW, Cunha GR 1998 Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology 139:4708–4713 [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Lydon JP, Cunha GR 2000 Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod 62:831–838 [DOI] [PubMed] [Google Scholar]
- Okulicz WC, Scarrell R 1998 Estrogen receptor α and progesterone receptor in the rhesus endometrium during the late secretory phase and menses. Proc Soc Exp Biol Med 218:316–321 [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW 1995 Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod 53:1527–1543 [DOI] [PubMed] [Google Scholar]
- Spencer TE, Becker WC, George P, Mirando MA, Ogle TF, Bazer FW 1995 Ovine interferon-τ inhibits estrogen receptor up-regulation and estrogen-induced luteolysis in cyclic ewes. Endocrinology 136:4932–4944 [DOI] [PubMed] [Google Scholar]
- Tan J, Paria BC, Dey SK, Das SK 1999 Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology 140:5310–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazer FW, Slayden OD 2008 Progesterone-induced gene expression in uterine epithelia: a myth perpetuated by conventional wisdom. Biol Reprod 79:1008–1009 [DOI] [PubMed] [Google Scholar]
- Buchanan DL, Kurita T, Taylor JA, Lubahn DB, Cunha GR, Cooke PS 1998 Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification, and cornification. Endocrinology 139:4345–4352 [DOI] [PubMed] [Google Scholar]
- Buchanan DL, Setiawan T, Lubahn DB, Taylor JA, Kurita T, Cunha GR, Cooke PS 1999 Tissue compartment-specific estrogen receptor-α participation in the mouse uterine epithelial secretory response. Endocrinology 140:484–491 [DOI] [PubMed] [Google Scholar]
- Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR 1997 Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA 94:6535–6540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR 2000 Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod 62:821–830 [DOI] [PubMed] [Google Scholar]
- Yao L, Agoulnik AI, Cooke PS, Meling DD, Sherwood OD 2008 Relaxin acts on stromal cells to promote epithelial and stromal proliferation and inhibit apoptosis in the mouse cervix and vagina. Endocrinology 149:2072–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigsby RM, Cooke PS, Cunha GR 1986 A simple efficient method for separating murine uterine epithelial and mesenchymal cells. Am J Physiol 251:E630–E636 [DOI] [PubMed] [Google Scholar]
- Bigsby RM 2002 Control of growth and differentiation of the endometrium: the role of tissue interactions. Ann NY Acad Sci 955:110–117; discussion 118, 396–406 [DOI] [PubMed] [Google Scholar]
- Wakitani S, Hondo E, Phichitraslip T, Stewart CL, Kiso Y 2008 Upregulation of Indian hedgehog gene in the uterine epithelium by leukemia inhibitory factor during mouse implantation. J Reprod Dev 54:113–116 [DOI] [PubMed] [Google Scholar]
- Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W 1999 Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol 209:28–39 [DOI] [PubMed] [Google Scholar]
- Chen C, Spencer TE, Bazer FW 2000 Expression of hepatocyte growth factor and its receptor c-met in the ovine uterus. Biol Reprod 62:1844–1850 [DOI] [PubMed] [Google Scholar]
- Chen C, Spencer TE, Bazer FW 2000 Fibroblast growth factor-10: a stromal mediator of epithelial function in the ovine uterus. Biol Reprod 63:959–966 [DOI] [PubMed] [Google Scholar]
- Ka H, Al-Ramadan S, Erikson DW, Johnson GA, Burghardt RC, Spencer TE, Jaeger LA, Bazer FW 2007 Regulation of expression of fibroblast growth factor 7 in the pig uterus by progesterone and estradiol. Biol Reprod 77:172–180 [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Hayashi K, Song G, Black SG, Bazer FW, Spencer TE 2008 Progesterone regulates FGF10, MET, IGFBP1, and IGFBP3 in the endometrium of the ovine uterus. Biol Reprod 79:1226–1236 [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW 2002 Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci 7:d1879–d1898 [DOI] [PubMed] [Google Scholar]
- Cheng YH, Imir A, Suzuki T, Fenkci V, Yilmaz B, Sasano H, Bulun SE 2006 SP1 and SP3 mediate progesterone-dependent induction of the 17β hydroxysteroid dehydrogenase type 2 gene in human endometrium. Biol Reprod 75:605–614 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Ricke W, Thomson A, Marker PC, Risbridger G, Hayward SW, Wang YZ, Donjacour AA, Kurita T 2004 Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol 92:221–236 [DOI] [PubMed] [Google Scholar]
- Koji T, Chedid M, Rubin JS, Slayden OD, Csaky KG, Aaronson SA, Brenner RM 1994 Progesterone-dependent expression of keratinocyte growth factor mRNA in stromal cells of the primate endometrium: keratinocyte growth factor as a progestomedin. J Cell Biol 125:393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Cooke PS, Kurita T 2004 Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol 67:417–434 [DOI] [PubMed] [Google Scholar]
- Kurita T, Cooke PS, Cunha GR 2001 Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Dev Biol 240:194–211 [DOI] [PubMed] [Google Scholar]