Abstract
Reproductive success depends on a robust and appropriately timed preovulatory LH surge. The LH surge, in turn, requires ovarian steroid modulation of GnRH neuron activation by the neuropeptide kisspeptin and glutamate and γ-aminobutyric acid (GABA) neurotransmission in the medial preoptic area (mPOA). Middle-aged females exhibit reduced excitation of GnRH neurons and attenuated LH surges under estrogen-positive feedback conditions, in part, due to increased GABA and decreased glutamate neurotransmission in the mPOA. This study tested the hypothesis that altered kisspeptin regulation by ovarian steroids plays a role in age-related LH surge dysfunction. We demonstrate that middle-aged rats exhibiting delayed and attenuated LH surges have reduced levels of Kiss1 mRNA in the anterior hypothalamus under estrogen-positive feedback conditions. Kisspeptin application directly into the mPOA rescues total LH release and the LH surge amplitude in middle-aged rats and increases glutamate and decreases GABA release to levels seen in the mPOA of young females. Moreover, the N-methyl-d-aspartate receptor antagonist MK801 blocks kisspeptin reinstatement of the LH surge. These observations suggest that age-related LH surge dysfunction results, in part, from reduced kisspeptin drive under estrogen-positive feedback conditions and that kisspeptin regulates GnRH/LH release, in part, through modulation of mPOA glutamate and GABA release.
Middle-aged female rats with impaired LH surges exhibit deficits in estradiol induction of Kiss1 mRNA expression in the anteroventral periventricular region of the hypothalamus, and infusion of kisspeptin into the medial preoptic area rescues LH surge amplitude while decreasing GABA and increasing glutamate release.
Initiation of a robust and appropriately timed preovulatory LH surge is achieved by estradiol and progesterone regulation of excitatory and inhibitory inputs onto GnRH neurons. Kisspeptin, a potent activator of GnRH neurons (1,2,3,4), is essential for LH surges (for review Ref. 5). Afferent inputs from the anteroventral periventricular nucleus (AVPV) (6,7) modulate the amplitude and frequency of GnRH secretion and generation of the LH surge (8). The expression of kisspeptin and Kiss1 mRNA increases in the AVPV on the day of proestrus and under estradiol-positive feedback conditions (9,10). Kisspeptin neurons in the AVPV and GnRH neurons both express c-Fos at the time of the LH surge (1,9,10). Lastly, kisspeptin antibody attenuates the LH surge when infused into the preoptic area (11). Hence, AVPV kisspeptin neurons projecting to GnRH neurons in the medial preoptic area (mPOA) are thought to be critical for estradiol-positive feedback regulation of the LH surge (12,13).
Estradiol initiates positive feedback by modulating many neurotransmitter inputs to GnRH neurons (for review see Refs. 14 and 15). The LH surge in young female rats is accompanied by increased glutamate and decreased γ-aminobutyric acid (GABA) neurotransmission in mPOA, in which most GnRH neurons are located (16,17). Glutamate, glutamate agonists (18), and GABAA antagonists (19,20,21) stimulate GnRH synthesis and LH release. GABA inhibits GnRH neurons (22), and glutamate receptor antagonists (23,24) and GABAA receptor agonists (25) block GnRH/LH release. Recent studies suggested that kisspeptin increases glutamatergic (26,27) and decreases GABAergic (28) input to GnRH neurons. These data imply that kisspeptin directly and indirectly affects GnRH neurons (3,26).
An early and consistent marker of reproductive aging in female rats is a delayed and attenuated preovulatory LH surge (for review see Ref. 14), resulting in part from a reduced ability of steroids to increase glutamatergic and decrease GABAergic neurotransmission in the mPOA (29,30,31,32,33). We recently showed that LH surge amplitude in middle-aged rats is rescued by simultaneously increasing synaptic glutamate and decreasing GABA and GABAA receptor activation in the mPOA (21). Others have suggested that reduced excitatory input from the AVPV may underlie age-related LH surge changes (34). Although the role of kisspeptin in reproductive senescence has not been investigated, Kiss1 mRNA expression is altered in the medial basal hypothalamus of aged human and nonhuman primates (35,36). Therefore, we tested the hypothesis that age-related GnRH/LH surge impairment results from reduced expression of kisspeptin in the AVPV under estrogen-positive feedback conditions and that this may result in altered mPOA glutamate and GABA release.
Materials and Methods
Animals
Young (3–4 months) and middle-aged (9–11 months, retired breeders) female Sprague Dawley rats (Taconic Farms, Germantown, NY) were housed individually and maintained on a 14-h light, 10-h dark cycle with lights off at 2000 h. Because we wanted to identify changes in hypothalamic neurotransmitters that occur in the early stages of reproductive aging, whereas females still exhibit normal estrous cycles, only rats with at least two normal 4-d estrous cycles were used.
Drugs
Estradiol benzoate and progesterone were purchased from Steraloids, Inc. (Newport, RI), dissolved in peanut oil and injected sc. Mouse kisspeptin-10 (110–119)-NH2 (Kp-10; Phoenix Pharmaceutical, Belmont, CA), the rodent analog of human C-terminal kisspeptin decapeptide (112–121)-NH2 was chosen because of equivalent affects on LH release compared with the full-length peptide (37). Kp-10 was dissolved at 10 nm in artificial cerebrospinal fluid (ACSF) and dialyzed into the mPOA. The molecular weight of Kp-10 is 1.3 kDa, well below the 30-kDa molecular cutoff for the microdialysis membrane. MK801 (0.3 mg/kg; Sigma, St. Louis, MO), a noncompetitive N-methyl-d-aspartate acid (NMDA) receptor antagonist, was given sc at a dose previously shown to block LH surges in young females (23,38). Cetrorelix acetate (100 μg per 0.1 ml; EMD Serono, Inc., Rockland, MA), a competitive GnRH receptor antagonist, was suspended in 5% mannitol and administered sc 24 h before and just before progesterone injection (39,40,41). ACSF included 124 mm sodium chloride, 5 mm potassium chloride, 1.2 mm monopotassium phosphate, 10 mm magnesium sulfate, 1.8 mm calcium chloride, 26 mm sodium bicarbonate, and 10 mm dextrose (pH 7.4) and were from Fisher Scientific (Pittsburgh, PA).
Stereotaxic surgery and jugular vein catheterization
Rats were ovariohysterectomized (OVX) under ketamine (80 mg/kg) and xylazine (4 mg/kg) anesthesia; stereotaxic placement of the microdialysis guide cannula into the mPOA occurred during the same surgical session (21). Using Bregma as a landmark and stereotaxic coordinates from Pellegrino (42) (dorsal/ventral −8.6, anterior/posterior +2.0, and medial/lateral ±0.6), a unilateral guide cannula was implanted in the mPOA. Guide cannulae and concentric dialysis probes (2 mm dialysis surface, 340 μm outer diameter) were purchased from BASi (West Lafayette, IN). Eight days later, rats received an intraatrial jugular vein catheter for serial blood sampling (31). Catheters were kept patent with daily infusion of heparinized saline (50 IU). All animal protocols were approved by the Institutional Animal Care and Use Committee and adhered to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Steroid priming
All rats were primed with estradiol and progesterone for LH surge induction (31). At 0900 h on the day of catheterization, rats were injected with 2 μg estradiol benzoate; a second injection was given 24 h later. A single injection of 500 μg of progesterone was given at 0900, 48 h after the first estradiol benzoate injection (43). This protocol produces an LH surge in more than 80% of rats.
Microdialysis and plasma sampling
Microdialysis samples were collected at 30-min intervals from freely moving rats beginning at 0800 h (31). Microdialysis of the mPOA with Kp-10 (10 nm, 1.25 μl/min) began 1.5 h before progesterone injection and continued throughout the experiment. Controls were dialyzed with ACSF alone. Blood sampling began 1 h before or at the time of progesterone injection and continued every 1–2 h for 12 h. Blood was collected into tubes containing heparinized saline (10 IU), refrigerated overnight, and centrifuged at 10,000 × g for 20 min. Plasma was stored at −70 C until assayed for LH. An equal volume of warmed saline was infused to avoid hypovolemia. Microdialysis samples were collected, flash frozen, and stored until glutamate and GABA were determined (21). Animals were overdosed with ketamine, decapitated, and the brain rapidly frozen for later histological assessment of probe placement.
Hypothalamic dissection, RNA purification, reverse transcription (RT), and real-time PCR
Independent groups of OVX, young and middle-aged rats were primed with peanut oil (control) or estradiol benzoate and progesterone as described above. Rats were killed 4 or 7 h after the progesterone or final oil injection. The entire hypothalamus and preoptic area were dissected and then transected just posterior to the optic chiasm. The anterior hypothalamus, which includes the AVPV, was immediately frozen on dry ice and kept at −80 C for later determination of Kiss1 mRNA expression. Although this tissue fragment includes cell groups in addition to the AVPV, the only Kiss1 mRNA-expressing cells are those in the AVPV (for review see Ref. 44).
DNA-free total RNA was purified using the RNeasy lipid minikit from QIAGEN (Valencia, CA) including a deoxyribonuclease step. RT was performed using the high-capacity cDNA reverse transcription kit with ribonuclease inhibitor (Applied Biosystems, Foster City, CA) using 500 ng of RNA per 20 μl of RT reaction. Gene expression was measured by real-time PCR using TaqMan gene expression assays and master mix (Applied Biosystems) according to the manufacturer’s instructions. The final reaction mix contained proprietary TaqMan probes and primers for the normalizer [rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) endogenous control, VIC®/MGB probe, reference sequence Rn01775763_g1, context sequence NM_017008.3, and specific target (Kiss1, Fam probe, reference sequence Rn00710914_m1; context sequence NM_181692.1)]. Real-time PCR was performed using an ABI PRISM 7900HT (Applied Biosystems) in multiplex conditions using 50 ng of cDNA per 20 μl of total reaction mix. Amplified transcripts for Kiss1 were quantified using the comparative threshold cycle method and GAPDH as normalizer. The fold change in Kiss1 expression was then calculated as 2−ΔΔCT where CT = threshold cycle, ΔCT = CT (Kiss1) − CT (GAPDH), ΔΔCT = ΔCT (experimental) − ΔCT (reference). ΔCT (reference) was calculated using the mean of the ΔCT for the anterior hypothalamus of OVX animals treated with oil.
LH assay
LH was assayed at Northwestern University (Chicago, IL) using rat LH RIA reagents provided by the National Hormone and Peptide Program (Torrance, CA). The lower limit of the assay was 0.2 ng/ml, and the intra- and interassay coefficients of variation were 7.6 and 5.8%, respectively. LH is reported as nanograms per milliliter per hour−1 [area under the curve (AUC)] or nanograms per milliliter. A LH surge was defined as an increase in plasma LH equal to or greater than 1.5 times baseline (average LH value between 0800 and 1000 h) for at least two consecutive samples. Equivalent percentages of young (13 of 16) and middle-aged (30 of 34) rats with verified probe placements exhibited a LH surge.
Analysis of glutamate and GABA
Amino acids were separated by HPLC and their content in microdialysis samples was quantified as previously described (21). Amino acid identification and quantification were achieved by comparing peak retention times and heights in samples to known standards (Sigma). Amino acid content is reported as picomoles per microliter or picomoles per microliter per hour−1 (AUC). The lower limit for detection of glutamate and GABA was 0.08 pmol. The recovery rate for glutamate and GABA is approximately 15% and is consistent across probes, based on in vitro calibrations of randomly selected probes.
Histological verification of probe placements
Every third 40-μm section throughout the extent of the dialysis probe track was stained with thionin to map probe placement in the mPOA (Fig. 1). Only rats with a confirmed LH surge and appropriate probe placement were included in the data analysis. Two to four rats from each age group were discarded due to inaccurate probe placement and/or clogging of the probe.
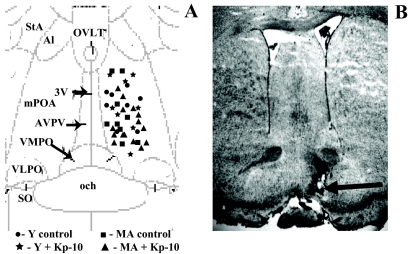
Figure 1.
Illustration of microdialysis probe placements in the medial preoptic area. A, The diagram corresponds to a coronal section at approximately 0.0 mm relative to Bregma (plate 33) in the atlas of Paxinos and Watson (81). 3V, Third ventricle; och, optic chiasm; VMPO, ventromedial preoptic nucleus; VLPO, ventrolateral preoptic nucleus; SO, supraoptic nucleus; Al, alar nucleus; StA, strial part preoptic nucleus; MA, middle-aged rats; Y, young rats; OVLT, organum vasculosum laminae terminalis. B, Photomicrograph of thionin-stained coronal section showing a representative probe placement between plates 32 and 33 by Paxinos and Watson (81). Magnification, ×40, shows the approximate location of a microdialysis probe. The arrow indicates the site of probe tip.
Statistical analysis
The AUC for total glutamate and GABA and serum LH release was calculated using Sigma Plot 10.0 (Systat Software, Inc., Chicago, IL). Two-way ANOVA (age × treatment) was used to determine differences in Kiss1 mRNA, total and peak glutamate, GABA and LH levels. Total LH, glutamate, and GABA in middle-aged rats treated with ACSF, Kp-10, Kp-10 + cetrorelix, and Kp-10 + MK801 were analyzed by one-way ANOVA. P ≤ 0.05 was considered statistically significant. Latency to LH surge onset was evaluated with Kruskal-Wallis. Bonferroni or Newman Keuls post hoc tests were performed as appropriate.
Results
Estradiol induces less Kiss1 mRNA in middle-aged females
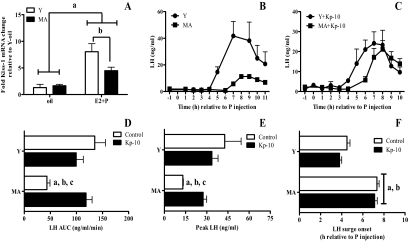
Figure 2A shows the effect of age and hormone treatment on the expression of Kiss1 mRNA in the anterior hypothalamus. Young rats were killed 4 h after the progesterone or oil injection. Middle-aged rats were killed at both 4 and 7 h after progesterone or oil injection. These time points reflect the onset time of the LH surge in young (4 h) and middle-aged (7 h) rats. There were no differences in Kiss1 mRNA levels in middle-aged rats killed at 4 or 7 h after progesterone; therefore, these data were pooled. Hormone treatment significantly increased Kiss1 mRNA levels in both age groups (Fig. 2A). However, hormone treatment induced significantly less Kiss1 mRNA in middle-aged than young rats. Age did not affect the expression of Kiss1 mRNA in control OVX rats.
Figure 2.
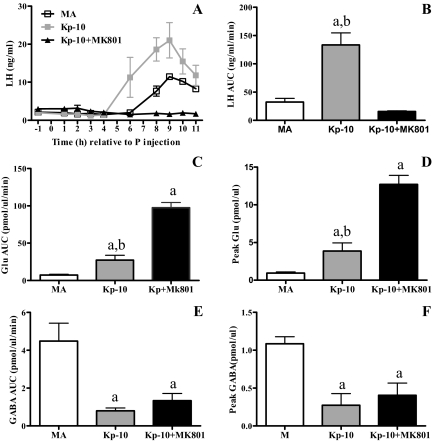
The attenuated LH surge is rescued by Kp-10 and correlates with reduced production of Kiss1 mRNA under estradiol-positive feedback conditions. A, Kiss1 mRNA in the anterior hypothalamus, which includes the AVPV. Data are expressed as mean ± sem from OVX young (Y) rats primed with estradiol and progesterone (E2 + P; n = 4) or oil (n = 4) and OVX middle-aged (MA) rats primed with E2 + P or oil and killed at 4 h (oil; n = 4, E2 + P; N 4) or 7 h (oil; n = 4, E2 + P; n = 4) after the P or last oil injection. There was no statistical difference in Kiss1 mRNA levels in E2-primed MA rats killed at 4 (n = 4) or 7 h (n = 4) after P; therefore, these data were pooled. The same was true for MA rats primed with oil (n = 4/time point). There was a significant main effect of hormone treatment (F = 44, P < 0.0001), age (F = 5, P < 0.03), and an interaction between hormone treatment and age (F = 7, P < 0.01). a, P < 0.005 vs. all E2 + P groups; b, P < 0.05 vs. Y E2 + P. B–F, Plasma LH levels are expressed as mean ± sem from OVX and E2 + P primed young control rats (Y; n = 6) and middle-aged control rats (MA; n = 7) dialyzed with ACSF and young (Y + Kp-10; n = 6) and middle-aged rats (MA + Kp-10; n = 10) dialyzed with 10 nm Kp-10. Progesterone was injected at 0900 h (time 0). B, LH surge in control rats. C, LH surge in Kp-10-treated rats. D, Total LH (AUC); there was a main effect of age (F = 11, P < 0.01) and an interaction between age and Kp-10 (F = 12, P < 0.001). E, Peak LH; there was a main effect of age (F = 11, P < 0.005) and an interaction between age and Kp-10 (F = 5.5, P < 0.05). F, LH surge onset (h relative to P injection) (Kruskal Wallis = 21.8, P < 0.005). a, P < 0.05 vs. Y; b, P < 0.05 vs. Y + Kp-10; c, P < 0.01 vs. MA + Kp-10.
Kisspeptin infusion restores LH surge amplitude in middle-aged rats
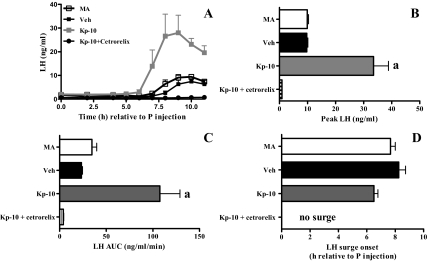
Kp-10 was dialyzed into the mPOA of OVX, steroid-primed rats beginning at 0730 h on the day of the LH surge (Fig. 2, B–F). Control middle-aged rats exhibited significantly less total and peak LH release than all other groups (Fig. 2, B–F). Kp-10 administration to middle-aged females significantly increased peak and total LH release to levels equivalent to young controls. Kp-10 had no effect on LH release in young rats. The LH surge was delayed in control middle-aged rats compared with young rats. Kp-10 did not affect LH surge onset in either age group. To verify that Kp-10 acted on GnRH neurons rather than on pituitary gonadotrophs (45), additional middle-aged rats infused with Kp-10 were also treated with the GnRH receptor antagonist cetrorelix (Fig. 3). Cetrorelix blocked the LH surge in middle-aged rats treated with Kp-10.
Figure 3.
Kisspeptin acts on the hypothalamus to enhance LH surges in middle-aged rats. Data are means ± sem from OVX, estradiol, and progesterone replaced middle-aged control (MA; n = 4) and MA rats dialyzed with 10 nm Kp-10 alone (Kp-10; n = 4), with 10 nm Kp-10 and injected with vehicle (Veh; n = 4) or 10 nm Kp-10 and injected with 100 μg of the GnRH receptor antagonist cetrorelix both 24 h before and immediately before the progesterone injection (Kp-10 + cetrorelix; n = 4). Progesterone was injected at 0900 h (time 0). A, LH surges. B, Peak LH; F = 24.5, P < 0.0001. C, Total LH (AUC); F = 15.7, P < 0.001. D, LH surge onset (h relative to P injection). a, P < 0.001 vs. all other groups.
Kisspeptin modulates extracellular glutamate and GABA
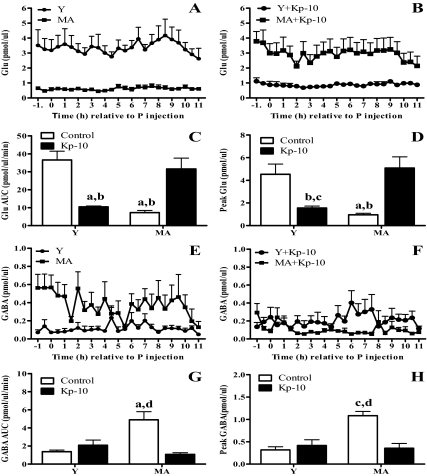
Total and peak glutamate release from the mPOA on the day of the LH surge in control middle-aged rats were less than 30% of those in young controls (Fig. 4, A–D). Kp-10-treated, middle-aged rats released significantly more glutamate than middle-aged controls and as much as young controls. In contrast, Kp-10 significantly reduced glutamate by more than 60% in young rats. Total and peak mPOA GABA on the day of the LH surge in control middle-aged rats was significantly greater than in all other groups (Fig. 4, E–H). Kp-10 reduced total and peak GABA release in middle-aged rats to levels that were equivalent to young controls. Kp-10 did not affect GABA in young rats.
Figure 4.
Kisspeptin differentially affects glutamate and GABA release in the mPOA of young and middle-aged rats on the day of the LH surge. Data are means ± sem and are from the same animals shown in Fig. 2. Time course of extracellular glutamate (Glu) (A and B) and GABA (E and F) levels in the mPOA of control and Kp-10-treated young (Y) and middle-aged rats (MA). C, Total Glu (AUC); there was an interaction between age and Kp-10 [F = 25.12, P < 0.0001]. (D, Peak Glu release; there was an interaction between age and Kp-10 [F = 14.4, P < 0.001]. (G, Total GABA; there were main effects of age [F = 7.7, P < 0.01] and treatment [F = 11.8, P < 0.005] and an interaction between age and Kp-10 [F = 24.9, P < 0.001]. (H, Peak GABA release; there were main effects of age [F = 8.5, P < 0.01] and treatment [F = 7, P < 0.02] and an interaction between age and Kp-10 [F = 11.8, P < 0.003]. a, P < 0.05 vs. Y control; b, P < 0.01 vs. MA + Kp-10; c, P < 0.001 vs. Y control; d, P < 0.01 vs. Y+ Kp-10 vs. MA + Kp-10. P, Progesterone.
MK801 receptor blockade
There is good evidence that the NMDA subtype of glutamate receptor is essential for the generation of LH surges in young females in that the NMDA receptor antagonist MK801 blocks the LH surge (23,46). Because Kp-10 restoration of the LH surge in middle-aged rats was associated with increased glutamate release in the mPOA, we asked whether activation of NMDA receptors was necessary for Kp-10 rescue of the LH surge (Fig. 5). Systemic administration of the NMDA receptor antagonist MK801 blocked Kp-10-induced restoration of LH surges in middle-aged rats. MK801 also increased mPOA glutamate release but did not significantly affect GABA release.
Figure 5.
Kisspeptin facilitation of LH release in middle-aged rats requires activation of NMDA receptors. Data are means ± sem. A, Time course of LH release from control (n = 4), Kp-10 (n = 7), and Kp-10 + MK801-treated (n = 6) middle-aged rats (MA). P, Progesterone. B, Total LH release (F = 13.9, P < 0.005). C, Total glutamate (Glu) release (F = 74.8, P < 0.001). D, Peak Glu (F = 43.2, P < 0.005). E, Total GABA release (F = 13.9, P < 0.01). F, Peak GABA (F = 9.2, P < 0.005). a, P < 0.01 vs. MA; b, P < 0.001 vs. MA + Kp-10 + MK801.
Discussion
These data suggest that age-related attenuation of LH surge amplitude results from impaired hypothalamic sensitivity to estrogen-positive feedback, resulting in reduced excitatory input to GnRH neurons. Specifically, age-related changes in LH surge amplitude may be causally linked to reduced Kiss1 mRNA expression in the AVPV, reduced mPOA glutamate, and increased mPOA GABA release in response to estradiol and progesterone priming. Kisspeptin infusion into the mPOA of hormone-primed rats rescues GnRH/LH release and elevates local glutamate and decreases local GABA release, thereby restoring the balance of local excitatory and inhibitory amino acid release in the mPOA of middle-aged rats to levels typical of young females. Thus, age-related LH surge changes most likely result from reduced kisspeptin availability and/or release rather than reduced Kiss1r expression or compromised Kiss1r function in GnRH neurons.
Middle-aged females have compromised endocrine (LH) and neural (Kiss1 mRNA) responses to estradiol
A hallmark of impending reproductive failure in middle-aged rats is a delayed and attenuated LH surge, which is not due to reductions in GnRH neuron number or pituitary dysfunction (for review see Ref. 14). Instead, age-related LH surge dysfunction reflects compromised excitatory input to GnRH neurons under estradiol-positive feedback conditions (21,29,33,47). On the day of the surge, fewer GnRH neurons in middle-aged females express cFos, a marker of neuronal activation, than in young females (34,48). AVPV neurons, which provide excitatory afferent input to GnRH neurons (6,49,50), also exhibit reductions in the percentage of cFos-positive neurons in middle-aged rats (34). Moreover, unilateral electrolytic lesions of the AVPV in young females produce ipsilateral reductions in cFos expression in GnRH neurons and LH release that resemble those of middle-aged female rats (34). Therefore, we hypothesized that age-related LH surge dysfunction may reflect reduced excitatory input to GnRH and other mPOA neurons from AVPV kisspeptin neurons. We first determined whether estradiol increases Kiss1 mRNA levels in the anterior hypothalamus of middle-aged rats. Although our dissection includes cells in addition to those in the AVPV, the only Kiss1-expressing cells in the dissection would be in the AVPV (for review see Ref. 51). In the absence of ovarian steroids, OVX young and middle-aged rats express equivalent levels of Kiss1 mRNA. Estradiol treatment significantly increased Kiss1 mRNA levels in both young and middle-aged rats; however, the response to estradiol was significantly reduced in middle-aged rats.
This finding suggested that the delayed and attenuated LH surge may result from reduced excitatory drive from AVPV kisspeptin neurons. If so, we hypothesized that infusion of Kp-10 directly into the mPOA (52) might rescue LH surges in middle-aged rats. Moreover, because unilateral electrolytic lesions in young females attenuate LH release (34), we predicted that unilateral infusion of kisspeptin might rescue the LH surge. Consistent with this reasoning, unilateral infusion of Kp-10 into the mPOA rescued the LH surge in middle-aged rats. Therefore, age-related LH surge changes most likely result from reduced kisspeptin availability.
Kisspeptin is permissive for generation of the LH surge
The delayed onset of the LH surge in middle-aged females is proposed to result from altered circadian inputs (for review see Ref. 53). AVPV kisspeptin neurons are hypothesized to be the node through which circadian signals initiate the LH surge (10). Therefore, we expected that Kp-10 infusion into the mPOA would advance the LH surge in both young and middle-aged rats. Interestingly, continuous infusion of Kp-10 beginning at 0730 h did not change the onset of the LH surge nor did it produce an immediate LH response in either age group. Our data are consistent with Roa et al. (54), who also showed no change in the onset of the LH surge when Kp-10 was infused intraventricularly in cycling females on the day of proestrus. These data suggest that under steroid-positive feedback conditions, kisspeptin release in the mPOA modulates the activity of GnRH neurons and other hormone-sensitive afferent inputs to GnRH neurons (55), especially GABA and glutamate neurons (Fig. 6). These findings also imply that kisspeptin plays a permissive role in generation of the LH surge.
Figure 6.
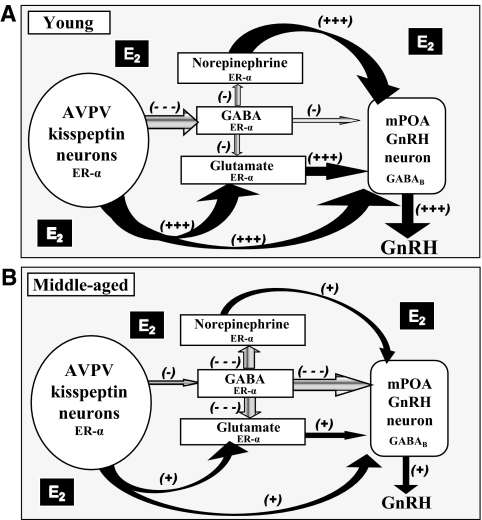
Proposed model for direct and indirect actions of kisspeptin on GnRH/LH release in young and middle-aged rats under estradiol-positive feedback conditions. A, Young adult rats: in the presence of estradiol (E2)-positive feedback environment, increased kisspeptin in AVPV neurons directly activate GnRH neurons and indirectly affects the GnRH/LH surge by increasing glutamate and decreasing GABA release in the mPOA. Kisspeptin can also inhibit GABAB receptors (28) on GnRH neurons. GnRH neurons in young females also receive E2-regulated inputs that include but are not limited to norepinephrine neurons (for review see Ref. 14). Estradiol-positive feedback is mediated by ER-α, which is expressed in the neurons indicated. B, Middle-aged rats: we propose that middle-aged rats have age-related changes in responsiveness to E2 in all ER-α expressing neurons shown. E2 induces less Kiss1 mRNA in the AVPV of middle-aged females, which may attenuate GnRH/LH release and contribute to increased GABA and GABA-mediated inhibition, and decreased glutamate in the mPOA. The increase in GABA may reduce both glutamate and norepinephrine release (82). The altered balance of glutamate and GABA neurotransmission along with reductions in norepinephrine (83,84) and alterations of other neurotransmitters in response to E2-positive feedback reduces activation of GnRH neurons on the day of the LH surge (for review see Ref. 14). Kisspeptin infusion into the mPOA rescues LH surge amplitude by direct actions on GnRH neurons and by restoring the balance of glutamate and GABA release to levels typical of young females. Black arrows, Excitatory actions; gray arrows, inhibitory actions; large arrows, robust input; small arrows, reduced input. + and −, Relative amounts of afferent excitatory and inhibitory input, respectively.
Our findings do not dispute the possibility that circadian inputs activate Kiss1 neurons on the day of the LH surge. The suprachiasmatic nucleus (SCN) is considered the circadian timekeeper driving LH surges (56). SCN neurons [e.g. that express vasoactive intestinal polypeptide (VIP) or arginine vasopressin] send projections to both neurons in the AVPV and GnRH neurons and other mPOA neurons (57). Immunoneutralization of VIP with intraventricular VIP antiserum (58), infusion of VIP antisense oligonucleotides into the SCN (59), or thermal ablation of VIP neurons in the SCN (60) of young rats produces delayed and attenuated LH surges that resemble middle-aged rats. Additionally, GnRH and AVPV neurons and VIP neurons in the SCN of middle-aged rats exhibit reduced cFos expression on the day of the surge (34,48,59,61,62). Thus, both kisspeptin and GnRH neurons may receive compromised circadian inputs from VIP neurons located in the SCN of middle-aged rats (61).
It has been hypothesized that kisspeptin neurons may receive and/or transmit circadian signals to GnRH neurons (10). This hypothesis is buttressed by the observation that kisspeptin immediately induces LH release in OVX rats under negative feedback conditions (without steroid priming) or when infused into the ventricles (9,54,63). Short-latency LH release observed after iv or ventricular infusions of kisspeptin might reflect actions on GnRH terminals in the median eminence (2,51,64) and/or on pituitary gonadotropes (45). In contrast, direct application of Kp-10 into the mPOA is unlikely to reach these sites. Hence, our data are not in conflict with the hypothesis that kisspeptin neurons located in the AVPV (65) receive and then transmit circadian signals to GnRH neurons. However, our data suggest that kisspeptin does not independently drive the timing of the LH surge.
Our experiments are the first to evaluate the effects of continuous infusion of Kp-10 into the mPOA on the LH surge under positive feedback conditions. Although Roa et al. (54) evaluated Kp-10 effects on the LH surge in cycling rats on proestrus, they injected a single bolus of Kp-10 into the lateral ventricle at 1200 h, close to the onset of the LH surge in young rats. It is impossible to predict whether an earlier injection of Kp-10 on the day of proestrus would have stimulated immediate LH release. These investigators also injected a single bolus of Kp-10 into the lateral ventricle after a 4-d combined estrogen plus progesterone protocol. We cannot directly compare that study to ours because Kp-10 was injected into the lateral ventricle in a negative gonadal steroid feedback environment.
Kisspeptin infusion modulates extracellular GABA and glutamate in the mPOA
Our laboratory (21) and others (for review see Ref. 66) provide strong evidence that age-related LH surge dysfunction involves reduced excitatory input to GnRH neurons under estrogen-positive feedback conditions. We recently showed that attenuated LH surges in middle-aged rats are causally related to reduced glutamate and increased GABA release in the mPOA. When we increased glutamate on a background of reduced GABAA receptor activation in the mPOA (21), GnRH neurons in middle-aged females appeared to maintain responsiveness to these neurotransmitters and produced a robust LH surge that was comparable with that of young females.
It is unclear why middle-aged rats release more GABA and less glutamate in the mPOA than young rats under positive feedback conditions (21). Because mounting evidence suggests that kisspeptin modulates amino acid neurotransmission (26,27,28,67), we hypothesized that kisspeptin may also affect GnRH/LH release through modulation of local glutamate and GABA release. Our microdialysis results demonstrate that infusion of Kp-10 into the mPOA of middle-aged rats restores the altered balance between glutamate and GABA release on the day of the LH surge to levels observed in young controls. These findings are consistent with earlier work showing that peak and total LH release in middle-aged rats can be rescued by increasing glutamate and decreasing GABA and GABAA neurotransmission in the mPOA (21).
Restoration of LH surge amplitude in middle-aged rats by Kp-10 was associated with an elevation of mPOA glutamate. Because NMDA receptors are expressed by GnRH neurons and they are critical in generation of the LH surge in young females (24,68), we assessed whether activation of NMDA receptors contributed to Kp-10 facilitation of GnRH/LH release in middle-aged females. The NMDA receptor antagonist MK801 completely blocked the LH surge in middle-aged female rats infused with Kp-10. MK801 blockade of the LH surge most likely represents postsynaptic actions of the drug because MK801 did not reverse the effects of Kp-10 on extracellular glutamate or GABA in the mPOA. This observation is consistent with other evidence that neurotransmitter systems in addition to kisspeptin are required for generation of the LH surge (15,55,69,70). For example, there is evidence that the Kiss1r is not essential for estradiol-induced LH surges (55). Other studies also support the hypothesis that kisspeptin indirectly affects GnRH/LH release by acting on glutamate neurons situated proximal to GnRH neurons (26). Most important, our data strongly suggest that Kiss1r activation, although necessary, is not sufficient for steroid-induced GnRH/LH surges in middle-aged rats and other neurotransmitters such as glutamate are critical.
Delayed and attenuated LH surges in middle-aged rats are also associated with increased GABA in the mPOA relative to young rats (21,32). Rescue of LH surge amplitude in middle-aged rats by kisspeptin and maintenance of the LH surge in control and kisspeptin-treated young rats correlated with low levels of extracellular GABA in the mPOA. This is consistent with our previous report that high levels of mPOA GABA are associated with low amplitude LH surges in middle-aged rats and that pharmacological antagonism of GABAA neurotransmission increases the magnitude of the LH surge (21). Although direct application of GABA onto GnRH neurons induces GABAA receptor-mediated excitation (71), we posit that the net effect of increased extracellular GABA within the mPOA is to inhibit GnRH neuron activation and that GnRH neurons are not the only target of the GABA detected by microdialysis (72). Interestingly, kisspeptin blocks GABAB receptor-mediated inhibition of GnRH neurons (28). Therefore, when the GABAB receptors are inhibited and GABA levels in the mPOA are reduced by Kp-10, the expected net result would be increased activation of GnRH neurons and enhanced GnRH/LH release.
Unexpectedly, Kp-10 infusion in young hormone-primed rats significantly decreased mPOA glutamate levels. However, this reduction in glutamate did not significantly affect the LH surge. We do not know why Kp-10 has different effects on glutamate in young and middle-aged rats. Extinction of LH release is observed after 48 h of continuous kisspeptin exposure in female rats (73). Thus, continuous infusion of exogenous Kp-10 into the mPOA of young females, which are kisspeptin replete, could reduce the ability of the peptide to activate nearby glutamate neurons, thereby decreasing synaptic glutamate levels.
There are several reasons young females with reduced mPOA glutamate still exhibit a normal LH surge. Neurotransmitters other than glutamate, especially norepinephrine, contribute to the LH surge and are implicated in LH surge dysfunction in middle-aged females (14). Because these neurotransmitter systems are intact and respond normally to estradiol in young females (Fig. 6), they may be sufficient to generate a robust LH surge despite reduced glutamate. Alternatively, LH surges in young females may have been normal because the reduction in glutamate occurred on a background of elevated Kp-10 and low GABA. In other words, as long as GABA levels are low and other excitatory neurotransmitter systems are not compromised as they are in middle-aged rats (14), GnRH neuron excitation in young rats may be sufficient to generate a normal LH surge, even if glutamate levels are reduced. GnRH neurons of middle-aged females also have altered NMDA receptor subunit stoichiometry, which may render young rats more sensitive to glutamate (29,74,75). Alternatively, glutamate neurotransmission may be more critical in generation of the LH surge in middle-aged than in young rats.
Is kisspeptin restoring the LH surge in an artificial manner?
Kisspeptin activates GnRH neurons and Kp-10 induces GnRH/LH release independent of steroid exposure (26,54,76). Therefore, one might hypothesize that because Kp-10 can induce GnRH/LH release in the absence of ovarian steroids, then the rescue of GnRH/LH release in middle-aged rats by Kp-10 may not reflect the physiological mechanisms that normally drive the LH surge in young animals. However, we do not believe that this is the case. First, estrogen receptor (ER)-α and estradiol priming of the hypothalamic-pituitary axis are essential for the LH surge (for review see Ref. 77). Consistent with this concept, Roa et al. (54) recently demonstrated that a selective ER-α antagonist completely blocked LH surges in control and kisspeptin-treated young rats on proestrus but did not inhibit GnRH-induced LH release. Additionally when kisspeptin was infused into the ventricles of OVX rats that were not treated with estradiol, LH release was brief and did not resemble LH surges of young (9,54,63) or middle-aged rats (31). Moreover, if Kp-10 effects on GnRH/LH release do not require ovarian hormones and are unrelated to the normal LH surge mechanism, then Kp-10 should also have the same effect on GnRH/LH release in all females, regardless of age. Lastly, the effects of Kp-10 on LH release and mPOA glutamate and GABA levels are consistent with our previous work demonstrating rescue of LH surge amplitude in middle-aged rats when the balance of glutamate and GABA is restored in the mPOA (21).
Kp-10 does not desensitize GnRH neurons
Several studies suggest that continuous application of Kp-10 desensitizes GnRH neurons (26,27,67) and consequently reduces GnRH/LH release (64,73). Electrophysiology studies in OVX mice treated with estradiol (26) or intact mice killed during diestrus (67) suggest that desensitization of GnRH neurons occurs after a brief exposure to high concentrations of kisspeptin. Another study evaluated GnRH/LH release during 7 d of intraventricular Kp-10 infusion in cycling rats and suggested that GnRH neuron desensitization emerges only after 2 d of continuous Kp-10 exposure (73). We saw no evidence that Kp-10 infusion throughout the day of the LH surge desensitizes GnRH/LH release. Perhaps if we continued Kp-10 for 48 h, we would have observed desensitization (73).
Kisspeptin did not act on gonadotropes
Although unlikely, it is possible that Kp-10 diffused to the pituitary, where it acted on gonadotopes to stimulate LH release (45). Therefore, we treated Kp-10-infused, middle-aged rats with the GnRH antagonist cetrorelix. Kp-10 rescue of LH release in middle-aged rats was completely blocked by cetrorelix. These data are consistent with other studies showing that GnRH receptor antagonists block LH release induced by Kp-10 and -54 (78,79).
Summary
We demonstrate that estradiol induction of Kiss1 mRNA expression is reduced in the anterior hypothalamus of middle-aged rats and that Kp-10 infusion into the mPOA under estrogen-positive feedback conditions rescues LH surge amplitude and restores the balance of glutamate and GABA release. Kp-10 effects are blocked by a GnRH receptor antagonist, indicating that Kp-10 affects GnRH neurons (80). The NMDA receptor antagonist MK801 also blocked Kp-10 rescue of the LH surge. Taken together, our data strongly suggest that age-related changes in the LH surge reflect, in part, reduced excitatory input from AVPV kisspeptin neurons to GnRH neurons and other mPOA neurons under estrogen-positive feedback conditions. Age-related LH surge changes result from reduced Kiss1 availability rather than reduced Kiss1r expression or compromised Kiss1r function in GnRH neurons. Our findings imply that kisspeptin directly and indirectly affects GnRH neuron activity by modulating local glutamate and GABA release in the mPOA (Fig. 6).
Acknowledgments
We thank Drs. Nanette Santoro, Diana Pettit, Brigitte Todd, and Andrea Reyna for their thoughtful comments on the manuscript; Dr. Zaher Merhi and Zewei Jiang for technical support; Laura Bernard for conducting HPLC determination of glutamate and GABA; and Brigitte Mann and the Northwestern University Hormone Assay Core for conducting serum LH measurements.
Footnotes
This work was supported by Department of Health and Human Services Grants R01 HD29856 and U54 HD058155; The Robert Wood Johnson Foundation; the American Federation for Aging Research; and the Department of Obstetrics and Gynecology and Women’s Health and the D. Purpura Department of Neuroscience, Albert Einstein College of Medicine.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 7, 2009
Abbreviations: ACSF, Artificial cerebrospinal fluid; AUC, area under the curve; AVPV, anteroventral periventricular nucleus; ER, estrogen receptor; GABA, γ-aminobutyric acid; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Kp-10, kisspeptin-10 (110–119)-NH2; mPOA, medial preoptic area; NMDA, N-methyl-d-aspartate; OVX, ovariohysterectomized; RT, reverse transcription; SCN, suprachiasmatic nucleus; VIP, vasoactive intestinal polypeptide.
References
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE 2008 Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH 2008 Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 149:3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE 2005 Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2004 Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE 2006 Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology 147:1166–1174 [DOI] [PubMed] [Google Scholar]
- Le WW, Berghorn KA, Rassnick S, Hoffman GE 1999 Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology 140:510–519 [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE 1978 Persistent estrus and blockade of progesterone-induced LH release follows lesions which do not damage the suprachiasmatic nucleus. Endocrinology 102:1645–1648 [DOI] [PubMed] [Google Scholar]
- Moguilevsky JA, Wuttke W 2001 Changes in the control of gonadotrophin secretion by neurotransmitters during sexual development in rats. Exp Clin Endocrinol Diabetes 109:188–195 [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K 2005 Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146:4431–4436 [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA 2006 Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K 2007 Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378 [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2009 Oestrogen, kisspeptin, GPR54 and the preovulatory luteinising hormone surge. J Neuroendocrinol 21:305–311 [DOI] [PubMed] [Google Scholar]
- Plant TM, Ramaswamy S 2009 Kisspeptin and the regulation of the hypothalamic-pituitary-gonadal axis in the rhesus monkey (Macaca mulatta). Peptides 30:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JL, Wise PM 2009 The role of the brain in female reproductive aging. Mol Cell Endocrinol 299:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsushima D, Tin-Tin-Win-Shwe, Kimura F 2003 Sexual dimorphism in the GABAergic control of gonadotropin release in intact rats. Neurosci Res 46:399–405 [DOI] [PubMed] [Google Scholar]
- Ping L, Mahesh VB, Wiedmeier VT, Brann DW 1994 Release of glutamate and aspartate from the preoptic area during the progesterone-induced LH surge: in vivo microdialysis studies. Neuroendocrinology 59:318–324 [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Shwe TT, Funabashi T, Shinohara K, Kimura F 2002 GABA release in the medial preoptic area of cyclic female rats. Neuroscience 113:109–114 [DOI] [PubMed] [Google Scholar]
- Brann DW 1995 Glutamate: a major excitatory transmitter in neuroendocrine regulation. Neuroendocrinology 61:213–225 [DOI] [PubMed] [Google Scholar]
- Funabashi T, Jinnai K, Kimura F 1997 Bicuculline infusion advances the timing of Fos expression in LHRH neurons in the preoptic area of proestrous rats. Neuroreport 8:771–774 [DOI] [PubMed] [Google Scholar]
- Neal-Perry GS ZG, Shu J, Santoro NF, Etgen AM, Reduced medial preoptic area glutamate and increased GABA levels on the day of the LH surge: a hypothalamic cause for reproductive senescence. Program of the 89th Annual Meeting of The Endocrine Society, San Francisco, CA, 2007 p686 (Abstract P4-76) [Google Scholar]
- Neal-Perry GS, Zeevalk GD, Shu J, Etgen AM 2008 Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Biol Reprod 79:878–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EJ, Ronnekleiv OK, Bosch MA, Kelly MJ 2001 Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. J Neurosci 21:2085–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB 1991 Endogenous excitatory amino acid involvement in the preovulatory and steroid-induced surge of gonadotropins in the female rat. Endocrinology 128:1541–1547 [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Ojeda SR 1990 A role for N-methyl-d-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology 126:1774–1776 [DOI] [PubMed] [Google Scholar]
- Seltzer AM, Donoso AO 1992 Restraining action of GABA on estradiol-induced LH surge in the rat: GABA activity in brain nuclei and effects of GABA mimetics in the medial preoptic nucleus. Neuroendocrinology 55:28–34 [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM 2008 Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M 2008 Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci 28:8003–8013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Ronnekleiv OK, Kelly MJ 2009 GABAB receptor mediated inhibition of GnRH neurons is suppressed by kisspeptin-GPR54 signaling. Endocrinology 150:2388–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias P, Carbone S, Szwarcfarb B, Feleder C, Rodríguez M, Scacchi P, Moguilevsky JA 1996 Effects of aging on N-methyl-d-aspartate (NMDA)-induced GnRH and LH release in female rats. Brain Res 740:234–238 [DOI] [PubMed] [Google Scholar]
- Cashion AB, Smith MJ, Wise PM 2004 Glutamic acid decarboxylase 67 (GAD67) gene expression in discrete regions of the rostral preoptic area change during the oestrous cycle and with age. J Neuroendocrinol 16:711–716 [DOI] [PubMed] [Google Scholar]
- Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM 2005 Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology 146:4331–4339 [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Jimenez-Linan M, Rubin BS 2007 Middle-aged female rats lack the dynamic changes in GAD(67) mRNA levels observed in young females on the day of a luteinising hormone surge. J Neuroendocrinol 19:708–716 [DOI] [PubMed] [Google Scholar]
- Maffucci JA, Walker DM, Ikegami A, Woller MJ, Gore AC 2008 NMDA receptor subunit NR2b: effects on LH release and GnRH gene expression in young and middle-aged female rats, with modulation by estradiol. Neuroendocrinology 87:129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le WW, Wise PM, Murphy AZ, Coolen LM, Hoffman GE 2001 Parallel declines in Fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology 142:4976–4982 [DOI] [PubMed] [Google Scholar]
- Kim W, Jessen HM, Auger AP, Terasawa E 2009 Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides 30:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE 2007 Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750 [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M 2001 The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276:34631–34636 [DOI] [PubMed] [Google Scholar]
- Lee WS, Abbud R, Hoffman GE, Smith MS 1993 Effects of N-methyl-d-aspartate receptor activation on cFos expression in luteinizing hormone-releasing hormone neurons in female rats. Endocrinology 133:2248–2254 [DOI] [PubMed] [Google Scholar]
- Horvath JE, Bajo AM, Schally AV, Kovacs M, Herbert F, Groot K 2002 Effects of long-term treatment with the luteinizing hormone-releasing hormone (LHRH) agonist Decapeptyl and the LHRH antagonist Cetrorelix on the levels of pituitary LHRH receptors and their mRNA expression in rats. Proc Natl Acad Sci USA 99:15048–15053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Schally AV 2001 Comparison of mechanisms of action of luteinizing hormone-releasing hormone (LHRH) antagonist cetrorelix and LHRH agonist triptorelin on the gene expression of pituitary LHRH receptors in rats. Proc Natl Acad Sci USA 98:12197–12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML 2008 KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol 20:381–393 [DOI] [PubMed] [Google Scholar]
- Pellegrino LJ 1979 A stereotaxic atlas of the rat brain. 2nd ed. New York: Plenum Press [Google Scholar]
- Freeman MC, Dupke KC, Croteau CM 1976 Extinction of the estrogen-induced daily signal for LH release in the rat: a role for the proestrous surge of progesterone. Endocrinology 99:223–229 [DOI] [PubMed] [Google Scholar]
- Smith JT 2009 Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides 30:94–102 [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Pascual E, Martínez-Fuentes AJ, Pinilla L, Tena-Sempere M, Malagón MM, Castaño JP 2007 Direct pituitary effects of kisspeptin: activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J Neuroendocrinol 19:521–530 [DOI] [PubMed] [Google Scholar]
- Carbone S, Szwarcfarb B, Otero Losada ME, Moguilevsky JA 1992 Effects of ovarian steroids on the gonadotropin response to N-methyl-d-aspartate and on hypothalamic excitatory amino acid levels during sexual maturation in female rats. Endocrinology 130:1365–1370 [DOI] [PubMed] [Google Scholar]
- Lloyd JM, Hoffman GE, Wise PM 1994 Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling, middle-aged rats. Endocrinology 134:1800–1805 [DOI] [PubMed] [Google Scholar]
- Krajnak K, Rosewell KL, Wise PM 2001 Fos-induction in gonadotropin-releasing hormone neurons receiving vasoactive intestinal polypeptide innervation is reduced in middle-aged female rats. Biol Reprod 64:1160–1164 [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW 1980 Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 31:147–157 [DOI] [PubMed] [Google Scholar]
- Hahn JD, Coen CW 2006 Comparative study of the sources of neuronal projections to the site of gonadotrophin-releasing hormone perikarya and to the anteroventral periventricular nucleus in female rats. J Comp Neurol 494:190–214 [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Bentsen AH, Ansel L, Simonneaux V, Juul A 2009 Comparison of the effects of peripherally administered kisspeptins. Regul Peptides 152:95–100 [DOI] [PubMed] [Google Scholar]
- Gu G, Varoqueaux F, Simerly RB 1999 Hormonal regulation of glutamate receptor gene expression in the anteroventral periventricular nucleus of the hypothalamus. J Neurosci 19:3213–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion AB, Böttner M, Rosewell KL 2002 Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog Horm Res 57:235–256 [DOI] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M 2008 Opposite roles of estrogen receptor (ER)-α and ERβ in the modulation of luteinizing hormone responses to kisspeptin in the female rat: implications for the generation of the preovulatory surge. Endocrinology 149:1627–1637 [DOI] [PubMed] [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA 2007 The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 27:12088–12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Schwartz WJ 2006 Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 147:1148–1153 [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM 2008 Vasoactive intestinal polypeptide can excite gonadotropin-releasing hormone neurons in a manner dependent on estradiol and gated by time of day. Endocrinology 149:3130–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Beek EM, Swarts HJ, Wiegant VM 1999 Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats. Neuroendocrinology 69:227–237 [DOI] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, Rosewell KL, Wise PM 1996 In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology 137:3696–3701 [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, van der Donk HA, van den Hurk R, Buijs RM 1993 Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J Neuroendocrinol 5:137–144 [DOI] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM 1998 Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci 18:4767–4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM 1998 Sex differences in the daily rhythm of vasoactive intestinal polypeptide but not arginine vasopressin messenger ribonucleic acid in the suprachiasmatic nuclei. Endocrinology 139:4189–4196 [DOI] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Navarro VM, Fernández-Fernández R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M 2006 Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female rat. Endocrinology 147:2864–2878 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Dipietro MJ, Ramaswamy S, Crowley Jr WF, Plant TM 2006 Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology 147:2122–2126 [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB 2005 The aging reproductive neuroendocrine axis. Steroids 70:273–283 [DOI] [PubMed] [Google Scholar]
- Liu X, Lee K, Herbison AE 2008 Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149:4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Wu TJ, Rosenberg JJ, Roberts JL 1996 Gonadotropin-releasing hormone and NMDA receptor gene expression and colocalization change during puberty in female rats. J Neurosci 16:5281–5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW 2000 The role of glutamate and nitric oxide in the reproductive neuroendocrine system. Biochem Cell Biol 78:165–179 [PubMed] [Google Scholar]
- Herbison AE 1997 Noradrenergic regulation of cyclic GnRH secretion. Rev Reprod 2:1–6 [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA 2005 Endogenous γ-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 146:5374–5379 [DOI] [PubMed] [Google Scholar]
- Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH 1999 GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19:2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa J, Vigo E, García-Galiano D, Castellano JM, Navarro VM, Pineda R, Diéguez C, Aguilar E, Pinilla L, Tena-Sempere M 2008 Desensitization of gonadotropin responses to kisspeptin in the female rat: analyses of LH and FSH secretion at different developmental and metabolic states. Am J Physiol Endocrinol Metab 294:E1088–E1096 [DOI] [PubMed] [Google Scholar]
- Miller BH, Gore AC 2002 N-methyl-d-aspartate receptor subunit expression in GnRH neurons changes during reproductive senescence in the female rat. Endocrinology 143:3568–3574 [DOI] [PubMed] [Google Scholar]
- Adjan V, Centers A, Jennes L 2008 Expression and activation of NMDAR1 receptor subunits in gonadotrophin releasing hormone (GnRH) neurones of young and middle-aged mice during the LH surge. J Neuroendocrinol 20:1147–1154 [DOI] [PubMed] [Google Scholar]
- Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK 2008 Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28:4423–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD 2003 Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod 69:1771–1778 [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA 2004 Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA 2004 A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR 2004 Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 16:850–858 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C 2007 The rat brain in stereotaxic coordinates. Burlington, MA: Academic Press of Elsevier [Google Scholar]
- Adler BA, Crowley WR 1986 Evidence for gamma-aminobutyric acid modulation of ovarian hormonal effects on luteinizing hormone secretion and hypothalamic catecholamine activity in the female rat. Endocrinology 118:91–97 [DOI] [PubMed] [Google Scholar]
- Mohankumar PS, Thyagarajan S, Quadri SK 1995 Cyclic and age-related changes in norepinephrine concentrations in the medial preoptic area and arcuate nucleus. Brain Res Bull 38:561–564 [DOI] [PubMed] [Google Scholar]
- Wise PM 1982 Norepinephrine and dopamine activity in microdissected brain areas of the middle-aged and young rat on proestrus. Biol Reprod 27:562–574 [DOI] [PubMed] [Google Scholar]