Abstract
The insulin receptor (IR) isoforms and the IGF type 1 receptor (IGF-1R) share a high degree of structural homology but differ in ligand binding kinetics and functions. We developed a highly specific quantitative PCR assay to quantify and compare IR-A, IR-B, and IGF-1R expression within an RNA population. We determined receptor expression in primary murine mammary epithelial cells (MECs) during postnatal development. Both IR isoform mRNAs were 3- to 16-fold higher than IGF-1R expression at all developmental times. IR protein was also 3- to 10-fold higher than IGF-1R protein; however, significantly less IGF-1R was found in hybrid receptors at early (49%) vs. late (79%) pregnancy, indicating that the amount of hybrid receptor is developmentally regulated. Despite high IR expression, IGF ligands were more effective than insulin in stimulating the insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt pathway in acutely isolated MECs from virgin glands. Although approximately 40% of IR transcripts were the IGF-II-sensitive IR-A isoform, IGF-II failed to stimulate IR phosphorylation, and an IGF-1R-specific blocking antibody completely abrogated IGF-II-mediated Akt phosphorylation in the virgin MECs. Taken together, these data suggest that the IGF-1R is more active in signaling than the IR and is the predominant mediator of IGF actions in virgin MECs.
IGF-1R expression is lower than either insulin receptor (IR)-A or IR-B in virgin mouse mammary epithelial cells, but more active in response to ligand stimulation.
The IGF system is a complex system of ligands, binding proteins, and receptors with roles in normal development and neoplasia of mammary and breast epithelial cells. Previous studies have addressed the expression and function of the IGF ligands during normal mammary gland development and support the conclusion that IGF ligands are local regulators of ductal outgrowth and branching during puberty and of alveolar development during pregnancy (1,2,3,4,5,6,7,8,9,10). Furthermore, these data also suggest distinct functions for IGF-I and IGF-II in promoting mammary epithelial development (3,7,8,11) (Reviewed in Ref. 12).
The IGF type 1 receptor (IGF-1R) is expressed throughout the mammary epithelium during pubertal and pregnancy-induced development in mice and also in epithelium of normal human breast tissue (3,10,13). Consistent with the demonstration that IGF-I is an essential mediator of ductal outgrowth (4), the IGF-1R is also essential for normal pubertal ductal outgrowth and proliferation of terminal end bud structures (14). Conversely, constitutive activation or overexpression of the IGF-1R in the mammary epithelium disrupts pubertal development and leads to tumor formation in murine models (15,16).
The role of insulin and the insulin receptor (IR) in normal mammary development remains unclear; however, embryonic rescue and transplantation of IR knockout epithelium into wild-type mammary glands results in reduced alveolar development (17). Alternative splicing of the IR results in two isoforms: the full-length IR-B, which has high affinity for insulin and binds IGF ligands only at supraphysiological concentrations, and the IR-A splice variant, which lacks exon 11. IR-A binds insulin and IGF-II with similar high affinity but does not bind IGF-I within physiological levels (18,19,20). This particular property of IR-A may explain why IGF-I and IGF-II have distinct functions in promoting mammary epithelial development.
The complexity of the IGF system is amplified by IR/IGF-1R hybrid receptors (hybridR). HybridR formation occurs in the endoplasmic reticulum and likely occurs in a stochastic manner based on the relative expression of each receptor (21,22). Interestingly, hybridR and IGF-1R both bind IGF ligands with similar high affinity and bind insulin only at supraphysiological concentrations (18,19,23). The data summarized above support the proposal that tissue sensitivity to IGF-I, IGF-II, and insulin in vivo may be due to the receptor expression profile such that the relative levels of receptors present on the cell surface at the time of ligand stimulation may be more important in determining the cell response than the identity of the ligands (24).
Currently, there are no published reports of IR isoform mRNA quantification directly in the context of IGF-1R expression, and also no reagents are available to differentiate between IR isoforms at the protein level. Here, we report the development of a quantitative PCR (Q-PCR) assay to differentiate between and determine expression of both IR isoforms and IGF-1R mRNAs on the same scale. Moreover, we have used this assay to quantify levels of IR isoform and IGF-1R mRNA expression in acutely isolated, primary mammary epithelial cells (MECs) during postnatal development of the mammary gland. To confirm the mRNA results, we determined IGF-1R and IR protein expression in primary MECs during pregnancy stages and tested the prognostic value of receptor protein expression in determining hybridR formation. Finally, we test the predictive value of the receptor mRNA and protein expression profile in determining cell response to IGF-I, IGF-II, and insulin by analyzing acute activation of downstream signaling molecules in freshly isolated primary MECs.
Materials and Methods
Q-PCR
For detailed information on Q-PCR standards, primer design and optimization, and reaction conditions, please see supplemental Appendix (published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org).
Primary cell sample collection and purification
All animal experimentation protocols were approved by University of Medicine and Dentistry of New Jersey Institutional Animal Care and Use Committees and were conducted in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
For developmental mRNA and protein analyses, primary MECs were isolated from individual FVB female mice (10 glands pooled per mouse) at four stages of development: late puberty (LP)/10-wk virgin and age-matched females at pregnancy d 5.5 (P5), 12.5 (P12), and 17.5–18.5 (P18). For pregnancy analysis, the morning of vaginal plug detection was designated pregnancy d 0.5. Mammary glands were excised and epithelial cells were harvested using an established enzymatic digestion protocol and Percoll (GE Healthcare, Piscataway, NJ) gradient enrichment (25). After Percoll gradient enrichment, epithelial cells were washed once with complete M199 medium [M199 medium with phenol red and l-glutamine (Gibco/Invitrogen, Carlsbad, CA), 1× penicillin/streptomycin (Gibco/Invitrogen), 1 μg/ml amphotericin B (Gibco/Invitrogen), and 0.2 mg/ml BSA (Sigma Chemical Co., St. Louis, MO)] and then twice with ice-cold, sterile 1× PBS. Cell pellets from individual animals were stored at −80 C until RNA isolation or protein extraction.
Primary mouse mammary fibroblasts were obtained from a similar mammary gland dissociation protocol (26) using enzymatic digestion and differential centrifugation, and cells were stored at −80 C until RNA isolation.
RNA isolation and quantification and cDNA synthesis
Total RNA was isolated from cell pellets using the RNeasy mini kit with deoxyribonuclease digestion (QIAGEN, Valencia, CA). RNA quality and quantity was assessed using the Agilent 2100 bioanalyzer, RNA LabChip, and BioSizing software (Agilent Technologies, Santa Clara, CA).
Calibrator (universal mouse reference RNA; Stratagene, La Jolla, CA) and primary cell (epithelial and fibroblast) sample cDNA synthesis was performed using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. cDNA was quantified by spectrophotometry.
Primary MEC signaling experiments
For acute signaling analyses, 12- to14-wk-old virgin female mice were fasted for 2 h before tissue harvest to reduce endogenous receptor activation. MECs from 10–18 mice were purified as described above, and pooled after Percoll gradient enrichment. Freshly isolated primary mammary cells were maintained in three-dimensional (3D) organoid structures (supplemental Fig. 1) to preserve tissue architecture and cell-cell contact of the polarized epithelial cell layer to minimize disruption of receptor expression and localization. Attempts were made to count cells using traditional exclusion dye methods, and cell viability was consistent from experiment to experiment. However, because the signaling experiments were performed on 3D organoids rather than single cells, it was not possible to count individual cells using a hemocytometer. Therefore, in Figs. 2–5 and supplemental Fig. 2, equal volumes of pooled MEC organoids were distributed evenly into individual samples and maintained as individual samples throughout the experiments and subsequent analyses. Each treatment group contained n = 3 individual samples of organoid suspension.
Figure 2.
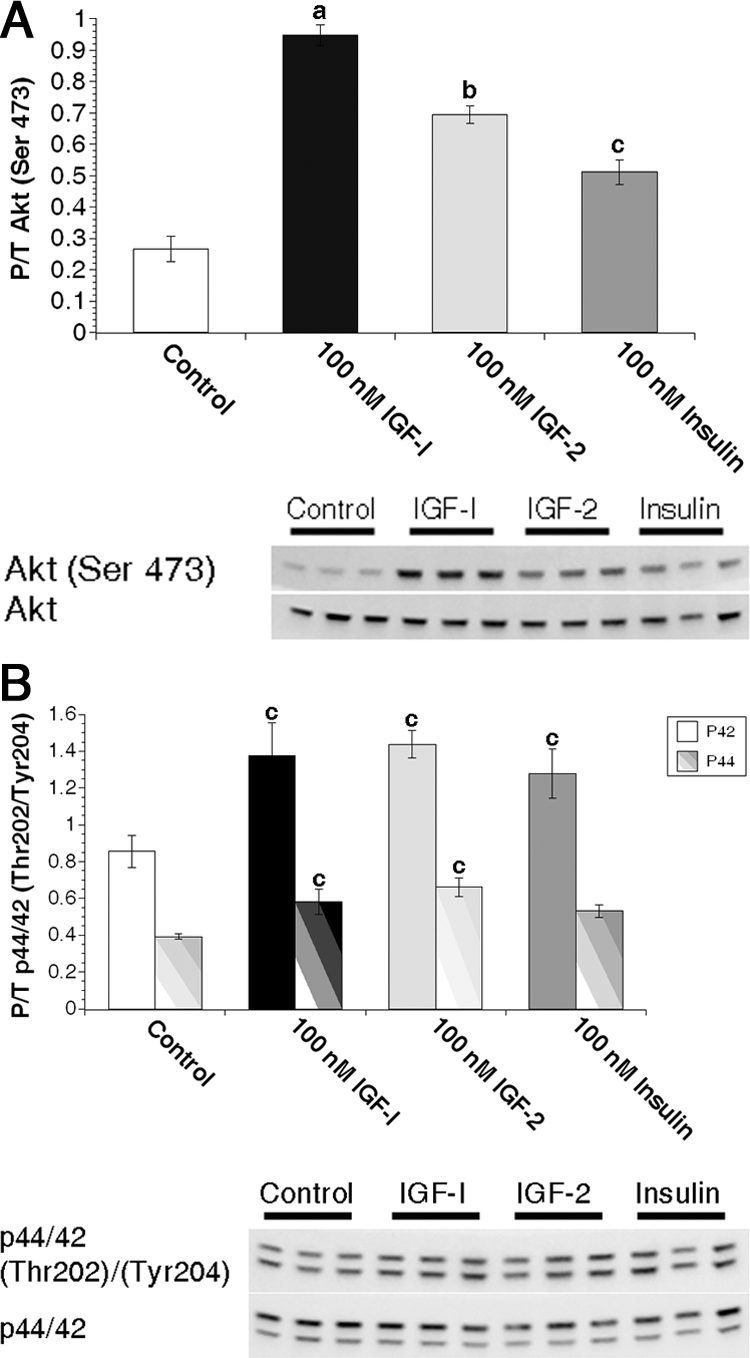
IGF ligands stimulate Akt phosphorylation more efficiently than insulin in primary MECs. Freshly isolated primary MECs from virgin animals were treated with 0 nm (control) or 100 nm IGF-I, IGF-II, or insulin for 15 min immediately followed by protein isolation. A, Graphs depict densitometric analysis of Akt Western immunoblot images. Bars indicate mean ± sem of phosphorylated Akt (Ser473) adjusted to total Akt expression. B, Graphs depict densitometric analysis of p44/p42 Western immunoblot images. Bars indicate mean ± sem of phosphorylated p42 (Tyr204, solid bars) or p44 (Thr202, striped bars) adjusted to total protein. a, P ≤ 0.001 vs. control, IGF-II, and insulin; b, P < 0.01 vs. control and insulin; c, P ≤ 0.04 vs. control. n = 3 samples per treatment.
Figure 3.
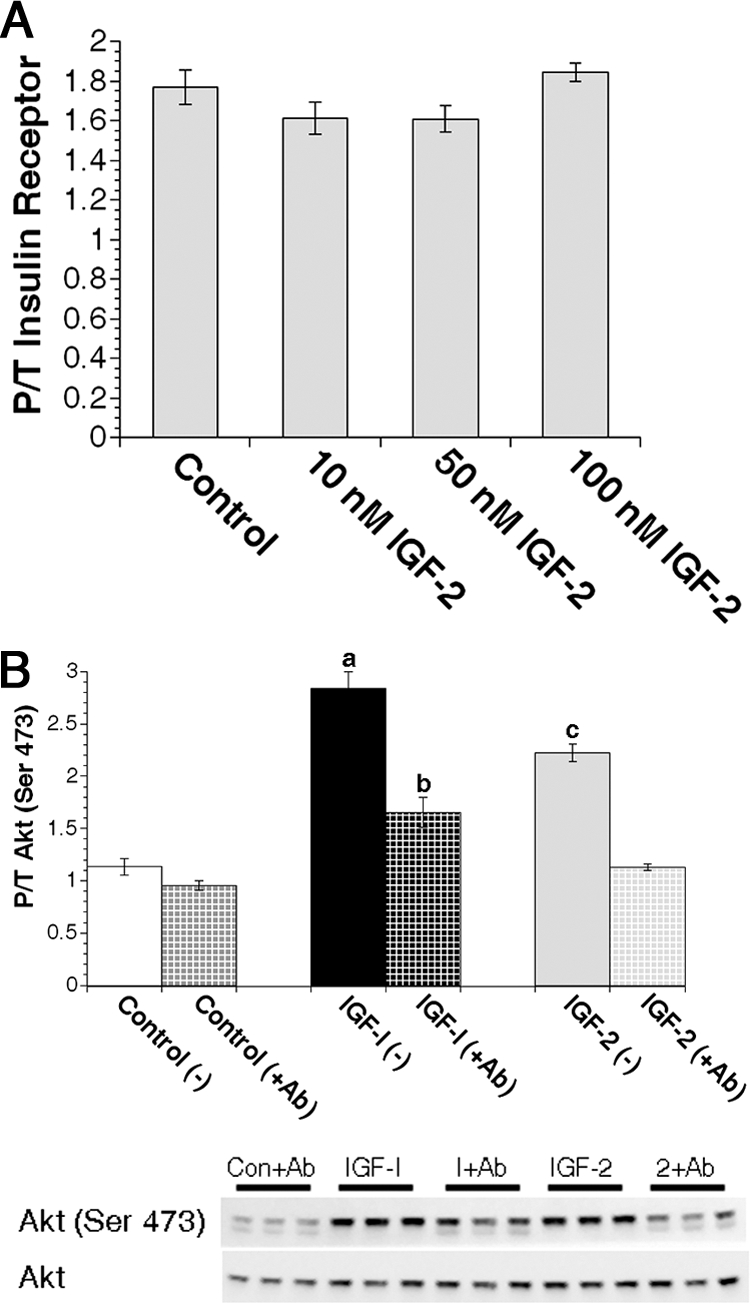
IGF-II activates the IGF-1R but not the IR in primary MECs. A, Quantification of IR activation in virgin primary MEC samples treated with 0 nm (control) or 10, 50, or 100 nm IGF-II for 15 min immediately followed by protein isolation. Bars indicate mean ± sem units of phospho (pYpY1162/1163) IRβ subunit per nanogram total IRβ subunit protein. P/T, Phospho/total. B, Freshly isolated primary MECs from virgin animals were pretreated with or without 500 nm IGF-1R blocking antibody (Ab) A12 for 30 min followed by treatment with 0 nm (control), 50 nm IGF-I, or 50 nm IGF-II with or without blocking antibody for an additional 15 min. Graphs depict densitometric analysis of Akt Western immunoblot images. Solid bars (without blocking Ab) and checked bars (with blocking Ab) indicate mean ± sem of phosphorylated Akt (Ser473) adjusted to total Akt expression. a, P ≤ 0.001 vs. control (±Ab), IGF-I (+Ab), and IGF-II (±Ab); b, P < 0.004 vs. control (±Ab), and IGF-II (+Ab); c, P < 0.002 vs. control (±Ab), IGF-I (+Ab), and IGF-II (+Ab). n = 3 samples per treatment.
Figure 4.
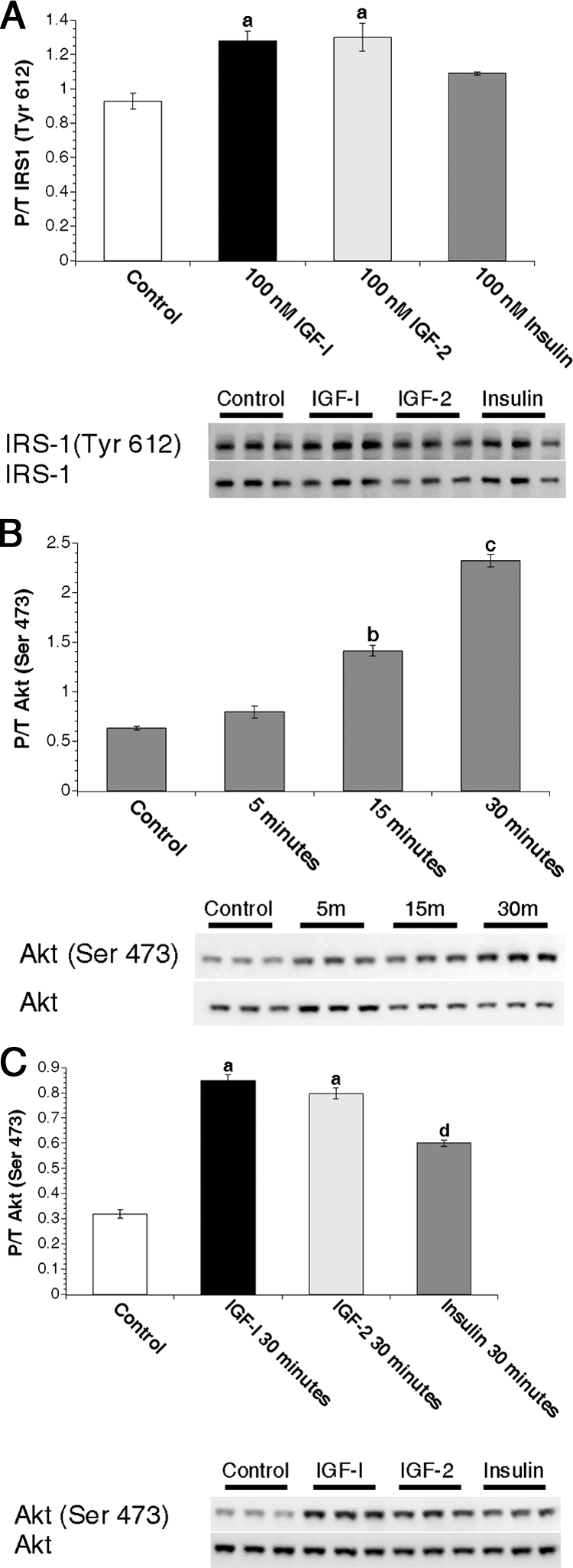
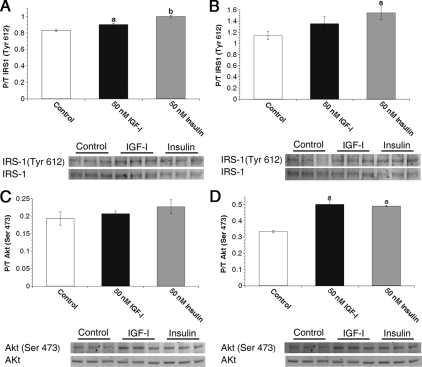
Analysis of IRS-1 and signaling kinetics in virgin MECs. A, Primary MECs from virgin animals were treated with 0 nm (control) or 100 nm IGF-I, IGF-II, or insulin for 15 min. Graphs depict densitometric analysis of IRS-1 Western images. Bars indicate mean ± sem of phosphorylated IRS-1 (Tyr612) adjusted to total IRS-1 expression. B, Freshly isolated primary MECs from virgin animals were treated with 0 nm (control) for 15 min or 50 nm insulin for 5, 15, or 30 min. Graph depicts densitometric analysis of Akt Western immunoblot images. Bars indicate mean ± sem of phosphorylated Akt (Ser473) adjusted to total Akt expression. C, Freshly isolated primary MECs from virgin animals were treated with 0 nm (control) or 50 nm IGF-I, IGF-II, or insulin for 30 min. Graph depicts densitometric analysis of Akt Western immunoblot images. Bars indicate mean ± sem of phosphorylated Akt (Ser473) adjusted to total Akt expression. a, P < 0.04 vs. control and insulin; b, P < 0.0001 vs. control and 5 min; c, P < 0.0001 vs. control and 5 and 15 min; d, P < 0.0001 vs. control. n = 3 samples per treatment. P/T, Phospho/Total.
Figure 5.
PI3K pathway activation in MECs from late pregnant females. Freshly isolated primary MECs from P16–P18 animals were treated with 0 nm (control), 50 nm IGF-I, or 50 nm insulin for 5 min (A and C) or 15 min (B and D). A and B, Graphs depict densitometric analysis of IRS-1 Western images. Bars indicate mean ± sem of phosphorylated IRS-1 (Tyr 612) adjusted to total IRS-1 expression. C and D, Graphs densitometric analysis of Akt Western images. Bars indicate mean ± sem of phosphorylated Akt (Ser473) adjusted to total Akt expression. a, P ≤ 0.04 vs. control; b, P ≤ 0.0025 vs. control and IGF-I. n = 3 samples per treatment.
Cells were then washed twice with warm 1× PBS and then re-suspended in complete M199 medium with or without IGF-I (Upstate, Lake Placid, NY), IGF-II (R&D Systems, Minneapolis, MN), or insulin (Sigma) at the indicated concentrations. Samples were incubated at 37 C with flick-mixing every 5 min. Immediately after treatment, samples were placed on ice, washed with 1 ml ice-cold 1× PBS, and lysed for protein isolation.
For analysis of MECs from late pregnant mice, six mice at pregnancy days 16.5–18.5 were fasted for 2 h before tissue harvest and processed as described for signaling analysis of virgin MECs.
The anti-IGF-1R blocking antibody, (A12, a fully human IgG1 monoclonal antibody) and a class-matched control antibody (human anti-KLH IgG1 monoclonal antibody) were obtained from ImClone Systems. For blocking antibody experiments, cells were isolated, pooled, and divided into treatment groups as described above. Samples were pretreated with or without 500 nm A12 for 30 min with flick-mixing every 10 min followed by treatment with or without 500 nm A12 with or without 50 nm IGF-I or IGF-II.
ELISA
IR β-subunit, phospho-IR (pYpY1162/1163), IGF-1R β-subunit, and phospho-IGF-1R (pYpY1135/1136) ELISA kits were purchased from Biosource International, Inc. (Camarillo, CA). Protein for all analyses was extracted according to the manufacturer’s protocol. A small volume of each sample was used in the standard DC protein assay (Bio-Rad Laboratories, Hercules, CA) for quantification of total protein. We attempted to measure receptor protein levels in MECs from virgin animals, but due to the low percentage of epithelial cells in murine mammary glands at the stage, we were unable to procure enough protein to measure expression of the three receptor subtypes in those samples.
The manufacturer’s protocol was followed for quantification of IR and IGF-1R total and phosphorylated protein with the following modifications: each sample and standard was analyzed in triplicate, and each MEC sample replicate contained 10 μg total cell protein for developmental analyses or 7.5 μg total cell protein for signaling analyses. Data obtained from phospho-ELISAs were normalized to total receptor protein determined separately for each individual sample. As documented by the manufacturer, standards for all ELISAs were purified recombinant protein or cell lysates calibrated to purified recombinant protein.
It was previously demonstrated that IR- and IGF-1R-specific antibodies can bind simultaneously to hybridRs (27). Reagents from both the IRβ and IGF-1Rβ ELISA kits were used in combination to quantify hybridRs. Briefly, all IR-containing receptors (IR homodimers and hybridRs) were bound to the solid phase of the ELISA using wells coated with a specific anti-IR antibody followed by detection of hybridRs with an antibody specific for the IGF-1R, which, in this scheme, will detect only hybridRs due to the presence of an IGF-1Rβ subunit. Anti-IGF-1Rβ antibody-coated wells, IGF-1Rβ protein standards, and the anti-IGF-1Rβ detection antibody were used as standards in hybridR ELISAs because the lower detection limit of the IGF-1Rβ standards is more sensitive than that of the IRβ standards.
Western immunoblot analysis
Fifteen micrograms of protein from virgin MEC signaling experiment samples and 50 μg late pregnancy MEC signaling experiment samples were used for Western analysis. Protein samples were electrophoresed on 4–12% Bis-Tris denaturing gels, transferred to nitrocellulose membranes, and incubated for 1 h at room temperature in blocking solution (5% wt/vol nonfat powdered milk in 1× Tris-buffered saline/0.1% Tween 20).
Antibodies to phospho-Akt (serine 473), total Akt, phospho-Erk1/2 (threonine 202/tyrosine 204), total Erk1/2, and total IR substrate-1 (IRS-1) were obtained from Cell Signaling Technologies (Beverly, MA). Antibodies to phospho-IRS-1 (tyrosine 612/608-human/mouse) and β-actin were obtained from Invitrogen and Sigma, respectively. Western immunoblots were incubated with primary and secondary antibodies (horseradish peroxidase-conjugated goat antirabbit or goat antimouse; Jackson ImmunoResearch, Inc. West Grove, PA) according to the manufacturers’ instructions. Enhanced chemiluminescence plus (PerkinElmer Life Sciences, Boston, MA) was used as a substrate for detection of secondary antibody, and images were obtained using the Ultra-LUM Gel Imager System and UltraQuant Molecular Imaging and Analysis Software (Ultra-LUM, Inc., Claremont, CA).
Statistical analyses
Statistics were analyzed using Statview Software version 5.0.1 (SAS Institute, Inc., Cary, NC). Analyses of the data were performed using a one-factor ANOVA and Fisher’s protected least significant difference post hoc test. Student’s t test was used for two-group comparisons where appropriate. P values < 0.05 were considered to represent significance. All results were reproducible across multiple experiments.
Results
Relative levels of IR-A, IR-B, and IGF-1R mRNAs in MECs during differentiation
We analyzed IR isoform and IGF-1R mRNA expression in primary MEC samples from individual female mice across developmental time points from LP through late pregnancy (P18) (Fig. 1). For information on MEC sample collection and analysis of sample purity, see Materials and Methods and supplemental Fig. 1. IR-A mRNA did not vary significantly in MECs from virgin (LP) and P18 animals (Fig. 1A). IR-B mRNA expression decreased from LP to P5 but increased through midpregnancy and was highest at P18 (Fig. 1B). Although their patterns of expression were slightly different, IR-A and IR-B mRNA levels did not differ significantly at any time point analyzed (Fig. 1D). In contrast to IR-B, IGF-1R mRNA increased significantly from LP to P5 and then decreased through midpregnancy with the lowest expression at P18 (Fig. 1C). Surprisingly, IGF-1R mRNA expression was 3- to 16-fold lower than either IR isoform at all times analyzed (Fig. 1D). By summing the mRNA expression values for each IR isoform, we predict that total IR expression is highest at late pregnancy and is 6- to 24-fold higher than IGF-1R expression at all times.
Figure 1.
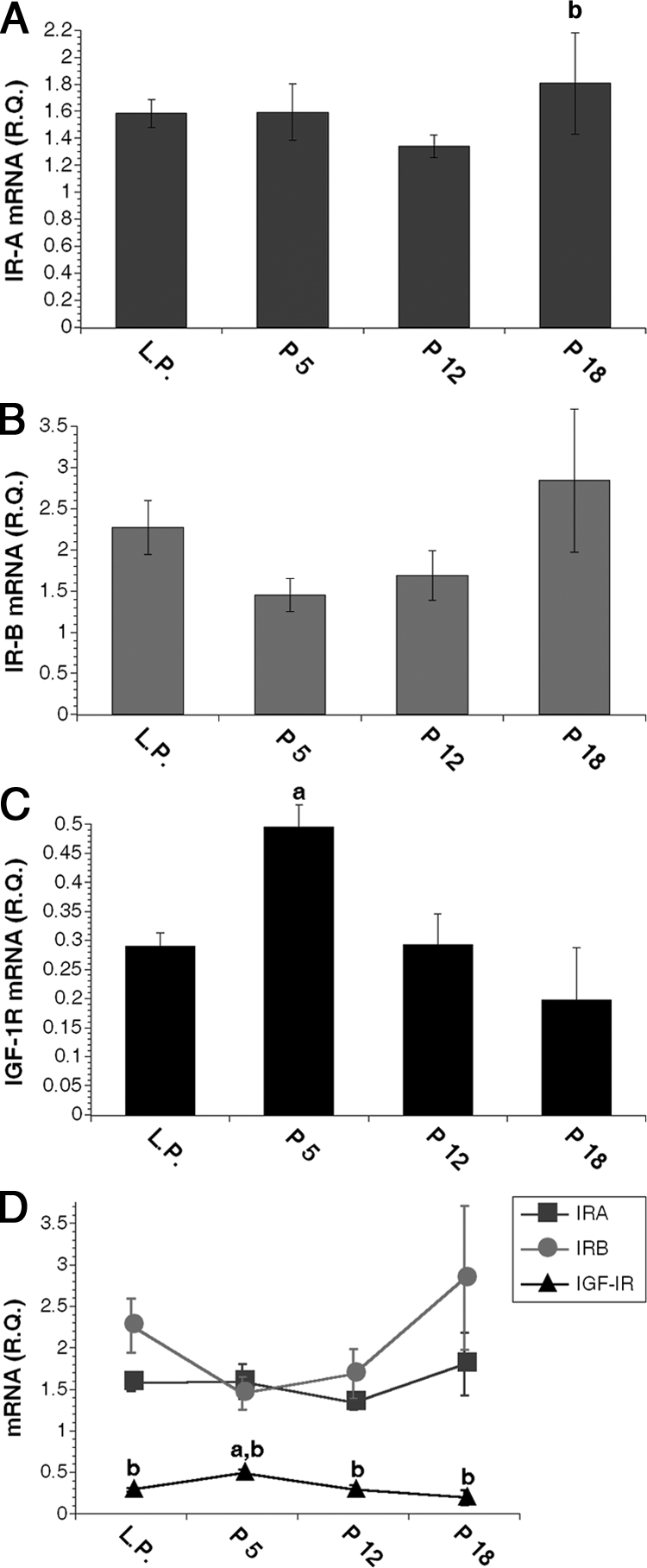
IR isoform and IGF-1R mRNA expression in MECs during development. A–C, Relative quantity (R.Q.) of IR-A (A), IR-B (B), and IGF-1R (C) mRNA molecules in purified MEC samples as measured by Q-PCR after normalization to β-actin. D, Line graph combines data from A–C to demonstrate receptor mRNA expression levels relative to each other throughout MEC differentiation. a, P < 0.02 vs. IGF-IR at LP, P12, and P18; b, P ≤ 0.05 vs. P5 in A and vs. IR-A and IR-B in D. Data are expressed as mean ± sem; n = 5 for LP and P5; n = 4 for P12 and P18.
IR, IGF-1R, and hybridR protein expression in MECs during pregnancy
In light of the unexpected finding that IGF-1R mRNA expression was significantly lower than IR isoform expression in MECs, we determined receptor protein expression during pregnancy (Table 1). Receptor sandwich ELISAs were obtained from Biosource/Invitrogen to specifically quantify IR and IGF-1R in primary MEC samples. The manufacturer previously established that these ELISAs accurately detect the target receptor in the presence of excess nontarget receptor, in particular, the IGF-1R ELISA demonstrated no cross-reactivity with recombinant IR or with protein from cells overexpressing an IR construct. IR and IGF-1R ELISAs are directed against receptor β-subunits; accordingly, the IR ELISA measured both IR-A and IR-B. Consistent with the mRNA data, IR protein was lowest in MECs from P5 animals but increased and remained high in MECs from P12–P18 animals (P < 0.05). IGF-1R protein was inversely expressed with the highest expression at P5-P12 and significantly less IGF-1R protein in MECs from P18 animals (P < 0.01). Furthermore, IR protein expression was 3- to 10-fold higher at all pregnancy time points (P < 0.01), demonstrating that IGF-1R and IR mRNA expression is predictive of receptor protein expression in primary MECs.
Table 1.
Summary of primary MEC receptor protein expression during pregnancy
| IRβ (ng) | IGF-1Rβ (ng) | Hybrid (ng) | % IGF-1Rβ in hybrid | |
|---|---|---|---|---|
| P5 | 15.3 ± 1.8a | 4.3 ± 0.3c,d | 2.0 ± 0.1 | 48.9 ± 2.1 |
| P12 | 27.6 ± 4.0a,b | 3.9 ± 0.4c,d | 2.4 ± 0.1 | 62.7 ± 5.8 |
| P18 | 25.7 ± 5.1a | 2.5 ± 0.1 | 2.0 ± 0.2 | 79.0 ± 9.4e |
Values are mean± sem nanograms of receptor protein per 10 μg total cell protein. Percent IGF-1R in hybrid = (nanograms hybridR/total nanograms IGF-1R) × 100.
P ≤ 0.01 vs. IGF-1R and hybrid at same time point;
P ≤ 0.05 vs. IR at P5;
P ≤ 0.03 vs. hybridR at same time point;
P ≤ 0.005 vs. IGF-1R at P18;
P = 0.01 vs. % IGF-1R at P5 by ANOVA.
Due to the large difference between IR and IGF-1R protein expression levels, we hypothesized that all IGF-1R would be present in an IR/IGF-1R hybridR based on the stochastic model of hybridR formation. It is interesting to note that even though IGF-1R levels change significantly during pregnancy, hybridR levels remained constant during pregnancy (Table 1). Moreover, IGF-1R protein levels were significantly greater than hybridR levels in MECs from P5 and P12 animals (P ≤ 0.03), even though IR protein was 3- to 7-fold higher than IGF-1R protein at these times. As mentioned above, receptor ELISAs are directed against receptor β-subunits, and therefore each IGF-1R and IR measurement included hybridRs as well as receptor homodimers. Only 49–63% of the total IGF-1R measured (homodimers plus hybrids) was present in a hybridR during the first half of pregnancy; however, at P18 as IGF-1R protein decreased and IR protein remained high, significantly more IGF-1R was present as hybrid (Table 1).
IGF-I is more effective than IGF-II or insulin in promoting Akt phosphorylation
The previous findings indicate that the IR is expressed at higher levels than the IGF-1R in MECs from virgin glands and in MECs throughout pregnancy. This was a surprising result because a role for the IR in pubertal development has not been previously described. Furthermore, these data suggest the possibility that the IR might play an important role in MEC signaling perhaps in part through IGF-II actions through the IR-A isoform. Thus, we next sought to compare the acute downstream signaling effects of the IGF ligands and insulin on freshly isolated MECs.
We examined phosphorylation of Akt and p44/p42 as indicators of phosphatidylinositol 3-kinase (PI3K) and MAPK pathway activation to assess IR and IGF-1R signaling in MECs. These pathways were used in previous analyses of IGF-I and insulin signaling activation in mammary gland (28,29). Despite higher IR expression, IGF-I was more effective than insulin at stimulating Akt phosphorylation in MECs (P ≤ 0.001; Fig. 2A). IGF-II stimulation of Akt phosphorylation was intermediate between IGF-I and insulin (P < 0.01). IGF-I, IGF-II, and insulin equally stimulated p44/p42 phosphorylation (Fig. 2B), although to a lesser extent than that seen for Akt phosphorylation. Due to the higher baseline p44/p42 phosphorylation in the 3D cultures and more robust response of Akt phosphorylation following to ligand stimulation, we used Akt phosphorylation as a readout of receptor activation in subsequent experiments. In addition, phosphorylation of Akt on Ser473 recapitulated the same dose-dependent stimulation of IR activation by insulin, whereas phosphorylation of p44/42 did not (supplemental Fig. 2). As such, we concluded that pAkt(473) is a reliable readout of IR activation in MECs.
To confirm that insulin was able to activate the IR in freshly isolated MECs, we performed an insulin dose-response study and measured phosphorylation of IR tyrosines 1162 and 1163 by ELISA as well as phospho-Akt (supplemental Fig. 2, A and B). These data indicate that surface IR is present and activated by insulin in the mammary parenchyma. Dose-response studies of IGF-I and IGF-II from 10–100 nm demonstrated that IGF-II stimulates Akt phosphorylation in a dose-dependent manner similar to insulin. In contrast, 10 nm IGF-I was equally effective as higher doses in stimulating phosphorylation of Akt (supplemental Fig. 2, C and D).
IGF-II does not stimulate IR phosphorylation in MECs
Our mRNA data demonstrated that approximately 40% of IR transcripts are the IGF-II-sensitive IR-A isoform. IR mRNA expression is predictive of total IR protein levels in MECs; thus, a significant portion of the IR may be IR-A homodimers. To determine whether IGF-II activates the IR on MECs, we analyzed the effects of IGF-II treatment on IR phosphorylation by ELISA. Although IGF-II demonstrated dose-dependent activation of Akt similar to insulin, IGF-II was unable to stimulate IR activation in MECs (Fig. 3A).
To confirm the results of the IR ELISA data, we analyzed whether the IGF-1R-specific blocking antibody, A12, could prevent Akt activation after IGF ligand treatment. The IGF-1R blocking antibody significantly reduced both IGF-I- and IGF-II-mediated Akt activation (P < 0.0001) (Fig. 3B), whereas a class-matched control antibody had no effect on IGF-I-mediated Akt phosphorylation (data not shown). Importantly, IGF-II-mediated Akt phosphorylation was reduced to control levels. These data demonstrate that IGF-II-mediated Akt phosphorylation is completely blocked by A12 and further support our conclusion that the IGF-1R mediates IGF-II as well as IGF-I ligand signaling in virgin MECs.
The IGF-1R is the primary activator of PI3K signaling in virgin MECs
To address the observed discrepancy between receptor expression levels and Akt activation, we analyzed activation of the IRS-1 docking protein as an indicator of receptor interaction with signaling intermediates in MECs. IRS-1 is important for IR-mediated Akt activation in the mammary gland during lactation (28). Thus, we hypothesized that insulin-mediated phosphorylation of IRS-1 at tyrosine 608 (human, Y612) would be equal to or greater than IGF ligand-mediated phosphorylation of IRS-1 due to the increased levels of IR protein. However, IRS-1 phosphorylation induced by IGF-I and IGF-II was modest but significantly higher than in insulin-treated virgin MECs (P < 0.04; Fig. 4A).
We next hypothesized that the kinetics of IR-mediated Akt phosphorylation might be slower than for IGF-1R-mediated Akt phosphorylation. To test this hypothesis, we analyzed insulin-mediated Akt phosphorylation from 5–30 min (Fig. 4B). Insulin-mediated Akt phosphorylation was stimulated by 15 min and continued to increase at 30 min. Comparison of insulin- with IGF-I- and IGF-II-mediated Akt phosphorylation at 30 min demonstrates that the IGF ligands were still more effective than insulin at stimulating Akt phosphorylation at this time (Fig. 4C).
IGF-I and insulin equally activate Akt phosphorylation in late-pregnancy MECs
Our signaling data demonstrate that the IGF-1R is the primary activator of PI3K signaling in virgin MECs, even though IR expression is more than 10-fold higher than IGF-1R at this time. We next analyzed the effects of IGF-I and insulin on PI3K signaling during late pregnancy, the stage when the difference in protein levels between these two receptors is greatest and IRS-1 expression increases in MECs (30). Because IRS-1 phosphorylation has been detected at 5 min after ligand treatment in mammary glands of mice at lactation d 3 (28), we analyzed IRS-1 and Akt phosphorylation at 5 and 15 min after ligand treatment in acutely isolated MECs from mice at pregnancy d 16–18.
In contrast to our data in virgin MECs, insulin was more effective than IGF-I at stimulating IRS-1 phosphorylation at both 5 and 15 min (Fig. 5, A and B) in MECs from late pregnant mice. Although no significant stimulation of Akt phosphorylation was seen at 5 min in MECs at this stage (Fig. 5C), insulin was equally as effective as IGF-I at stimulating Akt phosphorylation by 15 min (Fig. 5D).
Discussion
Here, we present data supporting a model of highly regulated expression and function of IGF and insulin receptors in MECs during mammary development. Results from these studies demonstrate regulation of IR, IGF-1R, and hybridR expression in MECs during postnatal developmental stages and provide the first report of the mouse IR-B sequence. Furthermore, there is an abundance of IR expression relative to IGF-1R expression in MECs at all times. Despite the high levels of IR expression, stimulation of the IGF-1R resulted in more robust activation of downstream signaling compared with IR activation in virgin MECs and equivalent activation in MECs from late pregnant glands.
A major finding from these studies is the differential regulation of the IR and IGF-1R receptor subtypes in MECs throughout postnatal development. We found steady-state expression of IR-A mRNA in MECs from virgin animals throughout pregnancy with no significant increase in expression during times of luminal or alveolar cell proliferation. The lack of stimulation of the IR by IGF-II in our signaling assays may reflect the developmental stage of the cells, which were isolated from virgin glands. We predict that IGF-II may activate IR-A in MECs during pregnancy stages consistent with demonstrated functions for IGF-II and the IR in alveolar development (8,17).
IR-B mRNA expression increased in MECs during late pregnancy (P = 0.05 vs. P5). Previous analysis of IR isoform expression in other cell types demonstrated preferential expression of IR-A in undifferentiated and/or transformed tissues where it transmits proliferation signals and expression of IR-B in differentiated cells and tissues where it is proposed to function primarily in stimulating metabolic pathways (31,32,33,34,35,36,37,38,39,40) (reviewed in Ref. 41). However, in addition to its metabolic effects, IR-B has also been shown to promote differentiation signals in hematopoietic cells (32). Consistent with these analyses, IR-B expression increased coincident with terminal alveolar cell differentiation and the need for increased metabolic output necessary for lactation. Based on ligand binding kinetics, it is likely that the increase in IR-B reflects a role for insulin actions through IR-B in lactation. Our data also are consistent with a very recent report showing increased IR-B mRNA in whole-gland extracts at late pregnancy and in HC11 cells differentiated in vitro (42). Although the whole-gland analyses in this study included stroma and fat cell receptor mRNA expression, the data support our conclusions in epithelial cell extracts.
Our findings implicate the IGF-1R as a central player in MEC proliferation, particularly during early alveolar proliferation. IGF-1R expression in MECs peaked at early pregnancy and decreased by late pregnancy. A role for the IGF-1R in proliferation of pubertal epithelium was demonstrated previously (14). A similar role for the IGF-1R in alveolar epithelial proliferation was suggested by studies showing decreased alveolar budding at pregnancy d 5 in glands with a heterozygous deletion of IGF-I (7). Because the IGF-1R is the primary signaling receptor for IGF-I, this suggests that its peak expression in early pregnancy is necessary for IGF-I-mediated alveolar budding.
In contrast to previous analyses of IGF-1R and IR isoform mRNA expression, our Q-PCR assay was optimized to allow comparison of all three mRNAs on the same scale. We determined that expression of either IR isoform is 3- to 16-fold higher than IGF-1R mRNA in MECs at all time points studied. Consistent with these data, we found that total IR protein expression was at least 3- to 10-fold higher than IGF-1R protein expression in primary MECs during pregnancy.
Based on the vast difference between IR and IGF-1R protein expression and a stochastic model of hybridR formation, we hypothesized that the measured quantities of IGF-1R and hybridRs would be equivalent, an indication that all IGF-1R β-subunits are present in a hybridR. Unexpectedly, we found that a significant proportion (∼40–50%) of IGF-1R β-subunits are present as classic IGF-1R homotetramers at early and midpregnancy, decreasing to 20% at late pregnancy, even though the total number of hybridRs did not vary during pregnancy. Based on our previous studies showing uniform expression of IGF-1R in MECs (3), we propose there is a threshold of IGF-1R protein expression in MECs below which the stochastic model is preserved and hybridR formation is preferred and above which formation of classical IGF-1R is favored.
HybridRs have higher affinity for IGF ligands than insulin, irrespective of the IR isoform present (19,23) and are accepted as functional IGF-1R in terms of kinase activity (20). However, Nakae et al. (43) proposed that the distinct phenotypes of IGF-1R and IR null animals indicate, albeit circumstantially, that hybridRs do not play a role in embryonic development. Moreover, evidence from breast cancer cell lines with small interfering RNA-mediated knockdown of IGF-1R and consequent knockdown of hybridR expression demonstrate that the loss of hybridR increased insulin sensitivity in these cells (36). These data can be interpreted as an indication that hybridR formation is a mechanism for reducing IR signaling by decreasing the number of insulin-sensitive receptors. Alternatively, we hypothesize that IGF ligand binding to a hybridR may be a mechanism to reduce IGF-1R signaling by acting as a sink for IGF action. The downstream biological effects from these receptors in a physiological context are unknown, and their presence may shift signaling and activation of downstream targets to something distinct from the homodimers. As such, the higher proportion of IGF-1R homodimer formation during early and midpregnancy may indicate that IGF-1R signaling is necessary for MEC development at these time points, whereas increased IGF-1R presence in hybridR during late pregnancy may serve as a mechanism to suppress IGF-1R signaling at this time (supplemental Fig. 3).
Analysis of transgenic animals has demonstrated the requirement for controlled IGF-1R expression and signaling in the mammary gland. Constitutive activation of IGF-1R signaling disrupts ductal patterning and growth in puberty due to hyperproliferation/hyperplasia of terminal end buds, respectively (15). Additionally, transgenic lines with particularly high levels of transgene expression developed mammary tumors as early as 8 wk of age. Finally, data here demonstrate that the EC50 for IGF-I stimulation of P-Akt is clearly lower than that of insulin (supplemental Fig. 2). This is a further indication of the potency of IGF ligand activation of PI3K signaling in virgin MECs, providing an additional reason for tight regulation of IGF-1R signaling during puberty. However, these data do not explain why the IGF ligands are more potent than insulin at activating the PI3K pathway in the presence of high levels of the IR in virgin MECs. Two possible explanations for this finding are that all of the IR is not localized to the cell surface during ligand treatment or that signaling intermediates required for IR signal transduction are not present in virgin MECs. We do not address the first hypothesis with the studies here; however, in vivo data on receptors and on IRS signaling intermediates support the second hypothesis.
In the mammary gland, extensive data on IRS-1 and IRS-2 expression and function suggest that these proteins may mediate differences in IGF-1R and IR activity during different stages of development. Both IRS proteins are expressed at low levels during puberty and are significantly increased during pregnancy and lactation (30). IRS-1 and IRS-2 null animals have decreased lactation capacity resulting in decreased pup weight (28), a phenotype that complements the reduced alveolar development seen in IR null epithelium. In contrast to IGF-1R null epithelium, IRS-1 null mammary glands have normal ductal patterning during pubertal development, indicating that this signaling intermediate is not required for IGF-1R activity. Furthermore, it has been proposed that IRS-2 can compensate for IRS-1 (28) and that IRS-2 may be the preferential signaling intermediate for IGF-1R (30).
Hadsell et al. (28) demonstrated that IRS-1 is required for insulin-mediated stimulation of Akt during lactation and that insulin was more potent than IGF-I at stimulating Akt phosphorylation in mammary glands from lactation d-3 mice. With these data in mind, we hypothesized that at late pregnancy, when IRS-1 expression begins to increase, insulin stimulation of the PI3K pathway will increase. Stimulation of IRS-1 and Akt phosphorylation in MECs from late pregnant mice was not as robust as that seen in MECs from virgin animals, perhaps because of high baseline levels of signaling pathway activity at this stage and/or due to a dilutional effect from high levels of milk protein expression. Nonetheless, as predicted, insulin was more effective at stimulating IRS-1 phosphorylation and equal to IGF-I in stimulating Akt phosphorylation in MECs from late pregnant animals in contrast to virgin MECs.
Taken together with data from transgenic animal models, the data presented here provide the basis for a new model of IGF-1R and IR signaling in MECs during postnatal mammary development (summarized in supplemental Fig. 3). As part of this study, we developed a unique Q-PCR assay that is both specific and sensitive to accurately measure mRNA expression of the IGF-1R, IR-A, and IR-B receptors on the same scale. We have used this assay also to provide a signature for IGF signaling receptors in MECs during different developmental stages.
Supplementary Material
Acknowledgments
We thank Rob Brucklacher and Dan Krissinger of The Pennsylvania State University College of Medicine, Functional Genomics Core Facility, for assistance with the Q-PCR assay design and implementation, the laboratories of Dr. David Alland of the University of Medicine and Dentistry of New Jersey and Dr. Bruce Baum of the National Institutes of Health for the use of their equipment, Dr. William Tyler for helpful discussions of experimental design, Zhaoyu Sun for assistance with pregnancy experiments, and Dr. Paul Kelly for helpful comments during the preparation of the manuscript.
Footnotes
This work was supported by Public Health Service Grant DK-060612 from the National Institute of Diabetes and Digestive and Kidney Diseases (to T.L.W.).
Disclosure Summary: A.R. and T.W. are inventors on Provisional Patent Application Ref. No. NJMS-07-23A. D.L. is an employee of ImClone Systems, a wholly owned subsidiary of Eli Lilly and Co., who generated the drug A12 used in this study. D.L. is an inventor on PCT WO 2005016970.
First Published Online April 30, 2009
Abbreviations: 3D, Three-dimensional; hybridR, IR/IGF-IR hybrid receptor; IGF-1R, IGF type 1 receptor; IR, insulin receptor; IRS-1, IR substrate-1; LP, late puberty; MEC, mammary epithelial cell; P5, pregnancy d 5.5; PI3K, phosphatidylinositol 3-kinase; Q-PCR, quantitative PCR.
References
- Paik S 1992 Expression of IGF-I and IGF-II mRNA in breast tissue. Breast Cancer Res Treat 22:31–38 [DOI] [PubMed] [Google Scholar]
- Yee D, Paik S, Lebovic GS, Marcus RR, Favoni RE, Cullen KJ, Lippman ME, Rosen N 1989 Analysis of insulin-like growth factor I gene expression in malignancy: evidence for a paracrine role in human breast cancer. Mol Endocrinol 3:509–517 [DOI] [PubMed] [Google Scholar]
- Richert MM, Wood TL 1999 The insulin-like growth factors (IGF) and IGF type I receptor during postnatal growth of the murine mammary gland: sites of messenger ribonucleic acid expression and potential functions. Endocrinology 140:454–461 [DOI] [PubMed] [Google Scholar]
- Ruan W, Kleinberg DL 1999 Insulin-like growth factor I is essential for terminal end bud formation and ductal morphogenesis during mammary development. Endocrinology 140:5075–5081 [DOI] [PubMed] [Google Scholar]
- Kleinberg DL, Feldman M, Ruan W 2000 IGF-I: an essential factor in terminal end bud formation and ductal morphogenesis. J Mammary Gland Biol Neoplasia 5:7–17 [DOI] [PubMed] [Google Scholar]
- Richards RG, Klotz DM, Walker MP, Diaugustine RP 2004 Mammary gland branching morphogenesis is diminished in mice with a deficiency of insulin-like growth factor-I (IGF-I), but not in mice with a liver-specific deletion of IGF-I. Endocrinology 145:3106–3110 [DOI] [PubMed] [Google Scholar]
- Loladze AV, Stull MA, Rowzee AM, Demarco J, Lantry 3rd JH, Rosen CJ, Leroith D, Wagner KU, Hennighausen L, Wood TL 2006 Epithelial-specific and stage-specific functions of insulin-like growth factor-I during postnatal mammary development. Endocrinology 147:5412–5423 [DOI] [PubMed] [Google Scholar]
- Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Tan J, Dey SK, Dotto GP, Weinberg RA, Jan T 2002 IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell 3:877–887 [DOI] [PubMed] [Google Scholar]
- Boutinaud M, Shand JH, Park MA, Phillips K, Beattie J, Flint DJ, Allan GJ 2004 A quantitative RT-PCR study of the mRNA expression profile of the IGF axis during mammary gland development. J Mol Endocrinol 33:195–207 [DOI] [PubMed] [Google Scholar]
- Modha G, Blanchard A, Iwasiow B, Mao XJ, Troup S, Adeyinka A, Watson P, Shiu R, Myal Y 2004 Developmental changes in insulin-like growth factor I receptor gene expression in the mouse mammary gland. Endocr Res 30:127–140 [DOI] [PubMed] [Google Scholar]
- Hovey RC, Harris J, Hadsell DL, Lee AV, Ormandy CJ, Vonderhaar BK 2003 Local insulin-like growth factor-II mediates prolactin-induced mammary gland development. Mol Endocrinol 17:460–471 [DOI] [PubMed] [Google Scholar]
- Rowzee AM, Lazzarino DA, Rota L, Sun Z, Wood TL 2008 IGF ligand and receptor regulation of mammary development. J Mammary Gland Biol Neoplasia 13:361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happerfield LC, Miles DW, Barnes DM, Thomsen LL, Smith P, Hanby A 1997 The localization of the insulin-like growth factor receptor 1 (IGFR-1) in benign and malignant breast tissue. J Pathol 183:412–417 [DOI] [PubMed] [Google Scholar]
- Bonnette SG, Hadsell DL 2001 Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology 142:4937–4945 [DOI] [PubMed] [Google Scholar]
- Carboni JM, Lee AV, Hadsell DL, Rowley BR, Lee FY, Bol DK, Camuso AE, Gottardis M, Greer AF, Ho CP, Hurlburt W, Li A, Saulnier M, Velaparthi U, Wang C, Wen ML, Westhouse RA, Wittman M, Zimmermann K, Rupnow BA, Wong TW 2005 Tumor development by transgenic expression of a constitutively active insulin-like growth factor I receptor. Cancer Res 65:3781–3787 [DOI] [PubMed] [Google Scholar]
- Jones RA, Campbell CI, Gunther EJ, Chodosh LA, Petrik JJ, Khokha R, Moorehead RA 2007 Transgenic overexpression of IGF-IR disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene 26:1636–1644 [DOI] [PubMed] [Google Scholar]
- Robinson G, Accili D, Hennighausen L 2000 Rescue of mammary epithelium of early lethal phenotypes by embryonic mammary gland transplantation as exemplified with insulin receptor null mice. In: Ip MM, Asch BB, eds. Methods in mammary gland biology and breast cancer research. New York: Kluwer Academic/Plenum Publishers; 307–316 [Google Scholar]
- Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A 2002 Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem 277:39684–39695 [DOI] [PubMed] [Google Scholar]
- Benyoucef S, Surinya KH, Hadaschik D, Siddle K 2007 Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation. Biochem J 403:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya J, Paz IB, Maddux BA, Goldfine ID, Hefta SA, Fujita-Yamaguchi Y 1993 Characterization of human placental insulin-like growth factor-I/insulin hybrid receptors by protein microsequencing and purification. Biochemistry 32:13531–13536 [DOI] [PubMed] [Google Scholar]
- Bailyes EM, Nave BT, Soos MA, Orr SR, Hayward AC, Siddle K 1997 Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem J 327(Pt 1):209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frattali AL, Pessin JE 1993 Relationship between α-subunit ligand occupancy and β-subunit autophosphorylation in insulin/insulin-like growth factor-1 hybrid receptors. J Biol Chem 268:7393–7400 [PubMed] [Google Scholar]
- Slaaby R, Schäffer L, Lautrup-Larsen I, Andersen AS, Shaw AC, Mathiasen IS, Brandt J 2006 Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J Biol Chem 281:25869–25874 [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR 1995 Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16:3–34 [DOI] [PubMed] [Google Scholar]
- Imagawa W, Yang J, Guzman RC, Nandi S 2000 Collagen gel method for the primary culture of mouse mammary epithelium. In: Ip MM, Asch BB, eds. Methods in mammary gland biology and breast cancer research. New York: Kluwer Academic/Plenum Publishers; 111–123 [Google Scholar]
- Stingl J, Eaves CJ, Kuusk U, Emerman JT 1998 Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation 63:201–213 [DOI] [PubMed] [Google Scholar]
- Soos MA, Field CE, Siddle K 1993 Purified hybrid insulin/insulin-like growth factor-I receptors bind insulin-like growth factor-I, but not insulin, with high affinity. Biochem J 290(Pt 2):419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadsell DL, Olea W, Lawrence N, George J, Torres D, Kadowaki T, Lee AV 2007 Decreased lactation capacity and altered milk composition in insulin receptor substrate null mice is associated with decreased maternal body mass and reduced insulin-dependent phosphorylation of mammary Akt. J Endocrinol 194:327–336 [DOI] [PubMed] [Google Scholar]
- Lee AV, Taylor ST, Greenall J, Mills JD, Tonge DW, Zhang P, George J, Fiorotto ML, Hadsell DL 2003 Rapid induction of IGF-IR signaling in normal and tumor tissue following intravenous injection of IGF-I in mice. Horm Metab Res 35:651–655 [DOI] [PubMed] [Google Scholar]
- Lee AV, Zhang P, Ivanova M, Bonnette S, Oesterreich S, Rosen JM, Grimm S, Hovey RC, Vonderhaar BK, Kahn CR, Torres D, George J, Mohsin S, Allred DC, Hadsell DL 2003 Developmental and hormonal signals dramatically alter the localization and abundance of insulin receptor substrate proteins in the mammary gland. Endocrinology 144:2683–2694 [DOI] [PubMed] [Google Scholar]
- Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R 1999 Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol 19:3278–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciacca L, Prisco M, Wu A, Belfiore A, Vigneri R, Baserga R 2003 Signaling differences from the A and B isoforms of the insulin receptor (IR) in 32D cells in the presence or absence of IR substrate-1. Endocrinology 144:2650–2658 [DOI] [PubMed] [Google Scholar]
- Kalli KR, Falowo OI, Bale LK, Zschunke MA, Roche PC, Conover CA 2002 Functional insulin receptors on human epithelial ovarian carcinoma cells: implications for IGF-II mitogenic signaling. Endocrinology 143:3259–3267 [DOI] [PubMed] [Google Scholar]
- Neuvians TP, Pfaffl MW, Berisha B, Schams D 2003 The mRNA expression of insulin receptor isoforms (IR-A and IR-B) and IGFR-2 in the bovine corpus luteum during the estrous cycle, pregnancy, and induced luteolysis. Endocrine 22:93–100 [DOI] [PubMed] [Google Scholar]
- Kosaki A, Webster NJ 1993 Effect of dexamethasone on the alternative splicing of the insulin receptor mRNA and insulin action in HepG2 hepatoma cells. J Biol Chem 268:21990–21996 [PubMed] [Google Scholar]
- Zhang H, Pelzer AM, Kiang DT, Yee D 2007 Down-regulation of type I insulin-like growth factor receptor increases sensitivity of breast cancer cells to insulin. Cancer Res 67:391–397 [DOI] [PubMed] [Google Scholar]
- Louvi A, Accili D, Efstratiadis A 1997 Growth-promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Dev Biol 189:33–48 [DOI] [PubMed] [Google Scholar]
- Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA 1990 Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J 9:2409–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrione A, Valentinis B, Xu SQ, Yumet G, Louvi A, Efstratiadis A, Baserga R 1997 Insulin-like growth factor II stimulates cell proliferation through the insulin receptor. Proc Natl Acad Sci USA 94:3777–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entingh AJ, Taniguchi CM, Kahn CR 2003 Bi-directional regulation of brown fat adipogenesis by the insulin receptor. J Biol Chem 278:33377–33383 [DOI] [PubMed] [Google Scholar]
- Belfiore A, Frasca F 2008 IGF and insulin receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia 13:381–406 [DOI] [PubMed] [Google Scholar]
- Berlato C, Doppler W 2009 Selective response to insulin versus IGF-I and IGF-II and upregulation of insulin-receptor splice variant B in the differentiated mouse mammary epithelium. Endocrinology 150:2924–2933 [DOI] [PubMed] [Google Scholar]
- Nakae J, Kido Y, Accili D 2001 Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev 22:818–835 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.