Abstract
Graves’-like hyperthyroidism is induced by immunizing BALB/c mice with adenovirus expressing the thyrotropin receptor (TSHR) or its A-subunit. Nonantigen-specific immune strategies can block disease development and some reduce established hyperthyroidism, but these approaches may have unforeseen side effects. Without immune stimulation, antigens targeted to the mannose receptor induce tolerance. TSHR A-subunit protein generated in eukaryotic cells binds to the mannose receptor. We tested the hypothesis that eukaryotic A-subunit injected into BALB/c mice without immune stimulation would generate tolerance and protect against hyperthyroidism induced by subsequent immunization with A-subunit adenovirus. Indeed, one sc injection of eukaryotic, glycosylated A-subunit protein 1 wk before im A-subunit-adenovirus immunization reduced serum T4 levels and the proportion of thyrotoxic mice decreased from 77 to 22%. Prokaryotic A-subunit and other thyroid proteins (thyroglobulin and thyroid peroxidase) were ineffective. A-subunit pretreatment reduced thyroid-stimulating and TSH-binding inhibiting antibodies, but, surprisingly, TSHR-ELISA antibodies were increased. Rather than inducing tolerance, A-subunit pretreatment likely expanded B cells that secrete nonfunctional antibodies. Follow-up studies supported this possibility and also showed that eukaryotic A-subunit administration could not reverse hyperthyroidism in mice with established disease. In conclusion, glycosylated TSHR A-subunit is a valuable immune modulator when used before immunization. It acts by deviating responses away from pathogenic toward nonfunctional antibodies, thereby attenuating induction of hyperthyroidism. However, this protein treatment does not reverse established hyperthyroidism. Our findings suggest that prophylactic TSHR A-subunit protein administration in genetically susceptible individuals may deviate the autoantibody response away from pathogenic epitopes and provide protection against future development of Graves’ disease.
Pretreatment with eukaryotic (not prokaryotic) TSHR A-subunit attenuates Graves’ disease induced in mice using A-subunit adenovirus by deviating responses away from pathogenic thyroid-stimulating antibodies towards nonfunctional antibodies.
Autoantibodies, like TSH receptor (TSHR) autoantibodies that are responsible for Graves’ hyperthyroidism (reviewed in Ref. 1), are the outcome of a complex set of interactions between T cells, B cells, antigen-presenting cells, cytokines, and, most importantly, specific autoantigens. The interplay between cells and cytokines leading to autoimmune responses is amenable to investigation in animal models, and the outcome of such studies provides important insights into the autoimmune process and suggests targets for immune intervention. Moreover, critical information into human autoimmune disorders has come from studies of spontaneously arising disease, including type 1 diabetes mellitus in nonobese diabetic (NOD) mice and systemic lupus erythematosus in New Zealand Black (NZB) mice (2,3) as well as from investigating experimentally induced disease, notably from collagen-induced arthritis and experimental autoimmune encephalitis, models for rheumatoid arthritis and multiple sclerosis, respectively (4,5).
Graves’ disease can be induced in susceptible mouse strains such as BALB/c by immunization with adenovirus expressing the full-length human TSHR (6) or its A-subunit (7). Immune deviation away from T helper 1 toward T helper 2 type responses using cytokines (8,9) or Schistosoma infection (10) reduces the proportion of mice that become hyperthyroid, but neither of these protocols can treat animals with established hyperthyroidism. Using decoy molecules of the TNF family ligand inhibitors B cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) to target B cell proliferation or survival factors, hyperthyroidism was reduced in mice with ongoing Graves’ disease (11). Moreover, a monoclonal antibody to B cells (rituximab) is being used to treat patients with Graves’ hyperthyroidism or ophthalmopathy and likely acts by interrupting antigen presentation to T cells (e.g. Refs. 12,13,14). However, these nonantigen-specific immune manipulations carry the risk of unforeseen and potentially serious side effects (reviewed in Ref. 15).
Dendritic cells (DCs) play critical roles in antigen presentation. Immune responses are initiated by mature DCs that express major histocompatibility complex class II antigens and costimulatory molecules. For example, Graves’ disease is induced by transferring DCs infected with TSHR-expressing adenovirus (16) or the TSHR A-subunit (17) to recipient mice. However, in the absence of maturation signals, immature DCs induce antigen-specific peripheral T cell tolerance (e.g. Ref. 18).
Receptors present on macrophages and DCs, such as the mannose receptor, enhance endocytosis of glycosylated antigens and increase the efficiency of antigen presentation to T cells (19). The mannose receptor has eight carbohydrate recognition domains and an amino-terminal cysteine-rich domain that binds sulfated carbohydrates (20). All three thyroid autoantigens, the TSHR A-subunit, thyroglobulin (Tg), and thyroid peroxidase (TPO), are glycosylated and the glycan moieties of Tg are sulfated (21,22). The mannose receptor interacts with Tg via its cysteine-rich domain (23,24). More importantly, despite no interaction with TPO, the carbohydrate recognition domains of the mannose receptor bind to Tg and very strongly to the TSHR A-subunit (24).
Recently it was shown that an adaptive immune response to antigens captured by the mannose receptor on antigen-presenting cells also requires innate immune system activation, such as by coadministering endotoxin (25). Antigen presentation in the absence of the latter signal induces tolerance. Because highly glycosylated TSHR protein is avidly captured by mannose receptors on antigen-presenting cells (24), we hypothesized that preadministering such protein without activating the innate immune system would attenuate the induction of hyperthyroidism by subsequent immunization with A-subunit adenovirus. The present study confirms this hypothesis but not by the anticipated mechanism. Unexpectedly, rather than inducing tolerance, TSHR protein pretreatment diverted the antibody response away from functional thyroid-stimulating antibodies (TSAbs) toward production of nonstimulatory TSHR antibodies.
Materials and Methods
Eukaryotic TSHR A-subunit, TPO, and Tg
The recombinant A-subunit (TSHR-289) is expressed in Chinese hamster ovary (CHO) cells, purified by affinity chromatography, and dialyzed against 10 mm Tris (pH 7.4) and 50 mm NaCl (26). Two forms of this recombinant protein can be isolated. Inactive TSHR-289 is recognized by a mouse monoclonal antibody (3BD10) but not human TSHR antibodies; conversely, active TSHR-289 is recognized by and neutralizes TSHR antibody activity in Graves’ sera (27,28). To preclude the possibility of passive neutralization of TSHR antibody neutralization, we used inactive TSHR-289, hereafter referred to as A-sub-CHO. Recombinant human TPO was purified by affinity chromatography from the supernatants of transfected CHO cells (29). Human Tg (>96% pure) was purchased from Calbiochem (San Diego, CA).
Prokaryotic TSHR-A-subunit
TSHR-289 was expressed in bacteria as a fusion protein coupled to the C terminus of thioredoxin (Trx). Briefly, the cDNA for TSHR-289 with 6-terminal histidine residues and BamHI and XhoI restriction sites (5′ and 3′, respectively) was amplified by PCR from pcDNA-TSHR289 (30). After digestion with BamHI and XhoI, the DNA fragment was ligated into the plasmid pET-32a(+); Novogen, La Jolla, CA) to generate pET-TSHR289. Escherichia coli strain Origami cells (Novogen) were transformed with this plasmid and grown at 37 C in Luria-Bertani broth (LB) medium containing 50 μg/ml ampicillin. After the OD 600 nm reached 1.0, protein expression was induced by adding 0.3 mm isopropyl-β-d-thiogalactoside (Sigma-Aldrich, St. Louis, MO) and incubation continued at 30 C for 3 h. The cell suspension was centrifuged (7,000 rpm, 10 min), the pellet dissolved in 20 ml phosphate buffer, sonicated by ultrasound, and centrifuged (4 C, 12,000 rpm, 10 min) to obtain inclusion bodies. The pellet was solubilized (6 m guanidine in phosphate buffer), the lysate centrifuged (12,000 rpm, 4 C, 10 min), and the supernatant applied to a His-Trap HP column (Amersham, GE Healthcare Bio-Sciences Corp., Piscataway, NJ) preequilibrated with 8 m urea in phosphate buffer. After extensive washing (8 m urea, 100 mm imidazole in phosphate buffer), TSHR289-Trx (subsequently referred to as A-sub-Trx) was eluted (8 m urea and 1000 mm imidazole in phosphate buffer) and dialyzed against PBS containing 1 m urea (1 h) and then against PBS (overnight). Trx protein was expressed as a soluble protein using the empty pET-32a(+) plasmid and purified without guanidine or urea. The specificity of A-sub-Trx was confirmed by immunoprecipitation (not shown) using an in-house mouse monoclonal antibody (3H9) that recognizes the N terminus of the TSHR.
Mice, pretreatment, and induction of Graves’ disease
Female BALB/c mice (6 wk old) were obtained from Jackson Laboratory (Bar Harbor, ME). Adenovirus expressing the human TSHR A-subunit (A-subunit-Ad, amino acids 1–289) and Null-adenovirus (Con-Ad) have been described (7,31). Adenoviruses were propagated in HEK293 cells (American Type Culture Collection, Manassas, VA), purified on CsCl density gradients, and viral particle concentration determined from the absorbance at 260 nm (32).
For pretreatment, mice received one sc injection on the back with A-sub-CHO (0.1–10 μg) or A-sub-Trx (8 μg) in 50 μl saline. Control injections were saline alone (0 μg) or the following proteins: Tg (50 μg), TPO (10 μg), or Trx (3 μg) (also in 50 μl saline). [Non-A-sub-CHO proteins (A-sub-Trx, Trx, Tg, TPO) were injected in approximately equivalent molar concentrations as 10 μg A-sub-CHO]. One week later, mice were immunized im in the thigh with A-sub-Ad (pAdHM4CM vector; 109 particles/injection). Three weeks later, the second A-sub-Ad immunization was performed. As controls for A-sub-Ad, mice were pretreated with saline and immunized twice with control adenovirus (Con-Ad; 109 particles/injection (31). One week after the second adenovirus injection, blood was drawn for analysis or mice were euthanized.
In the second part of the study (detailed in the text), two different protocols were used: 1) mice pretreated with A-subunit-CHO or A-subunit-Trx and immunized with A-sub-Ad received no further manipulation; and 2) mice pretreated with saline or control proteins before A-sub-Ad immunization were boosted with A-sub-CHO, A-sub-Trx, or A-sub-Ad in different combinations (detailed in the text). All mice were euthanized 12 wk after the study began.
Animal studies were approved by the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center and performed with the highest standards of care in a pathogen-free facility.
Serum T4
Total T4 was measured in undiluted mouse serum (25 μl) by RIA (Diagnostic Products Corp., Los Angeles, CA). Mice were considered hyperthyroid if their T4 levels exceeded the upper limit (mean + 2 sd) of Con-Ad immunized animals.
TSHR antibody assays
TSHR antibodies were measured as TSAbs and TSH binding inhibition (TBI) and by ELISA.
TSAb activity
The TSAb activity was determined by cAMP generated in separate assays using CHO cells expressing the human TSHR (33) and CHO cells expressing the mouse TSHR (34). The assay was performed as reported previously (35). Briefly, TSHR-CHO cells in 96-well plates were incubated (80 min, 37 C) with test sera diluted 1:20 in Ham’s F12 medium supplemented with 10 mm HEPES (pH 7.4) and 1 mm isobutyl-methylxanthine. After aspirating the medium, intracellular cAMP was extracted with ethanol, evaporated to dryness, and resuspended in Dulbecco’s PBS. Samples (20 μl) were assayed using the LANCE cAMP kit (PerkinElmer, Boston MA). TSAb activity (specific for the human or mouse TSHR) was expressed as a percentage of cAMP levels attained with sera from control, unimmunized mice.
TBI
TBI was measured using a commercial kit (Kronus, Boise, ID). Serum aliquots (25 μl) were incubated with detergent solubilized TSHR; 125I-TSH was added and the TSHR antibody complexes were precipitated with polyethylene glycol. TBI values were calculated from the formula: [1 − (TSH binding in test serum-nonspecific binding)/(TSH binding in normal serum-nonspecific binding)] × 100.
ELISA for TSHR antibody
The ELISA was performed using wells coated with A-subunit-CHO protein (see above; 5 μg/ml) as previously described (7). After incubation with test sera (duplicate aliquots, 1:100 dilution), antibody binding was detected with horseradish peroxidase-conjugated goat antimouse IgG (Sigma Chemical Co., St. Louis, MO), and the signal was developed with o-phenylenediamine and H2O2. TSHR ELISA antibodies are reported as the OD at 490 nm.
Statistical analyses
The statistical significance of differences between responses were determined by Student’s t test or ANOVA for normally distributed values or by nonparametric tests (rank sum and ANOVA on ranks). A χ2 analysis was used to determine the significance of proportional differences between groups of mice.
Results
A-subunit protein and protocol for pretreatment
We used two types of TSHR protein: TSHR A-subunit generated in eukaryotic (CHO) cells, referred to as A-sub-CHO, and the A-subunit generated in bacteria fused to Trx, abbreviated A-sub-Trx. On SDS-PAGE (Fig. 1A), A-sub-Trx and Trx (the bacterial fusion partner) run as sharply focused bands (∼47 and 20 kDa, respectively). In contrast, A-sub-CHO runs as a broad, diffuse band (∼50–70 kDa), reflecting its extensive glycosylation (e.g. Ref. 24).
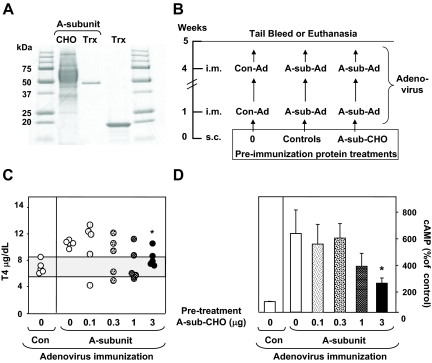
Figure 1.
A, SDS-PAGE comparison of recombinant TSHR A-subunit protein generated in CHO cells and bacteria as a fusion protein with Trx. The bacterial fusion partner (Trx) is included. B, Schedule for pretreatment before immunization with A-subunit adenovirus. BALB/c mice were injected with A-sub-CHO or controls 1 wk before the first of two A-sub-Ad immunizations. Controls include saline (0) or other proteins. A separate group of mice was immunized with Con-Ad. One week after the second immunization, sera were obtained for analysis. C, T4 levels (micrograms per deciliter) for individual mice (five per group). The shaded area represents the mean ± 2 sd levels in Con-Ad immunized mice. *, Significantly reduced vs. pretreatment without A-sub-CHO (0 μg); t test, P = 0.009. D, TSAb activity for the mice shown in C. The data were obtained using mouse TSHR-expressing CHO cells; similar observations were made with human TSHR-expressing CHO cells (not shown). TSAb values are presented as the mean + sem for cAMP release expressed as a percentage of the response from Con-Ad immunized mice. *, Significantly reduced vs. pretreatment with 0 μg A-sub-CHO (rank sum test, P = 0.032).
In a preliminary study, we first tested a wide concentration range of TSHR A-sub-CHO to examine whether pretreatment with this protein would ameliorate the induction of hyperthyroidism by A-sub-Ad immunization. Mice were injected once with TSHR A-sub-CHO (0, 0.1, 0.3, 1, and 3 μg) 1 wk before the first of two A-sub-Ad immunizations; sera were tested 1 wk after the second immunization (Fig. 1B). Baseline serum T4 values were obtained from mice immunized with Con-Ad. As expected, the majority of mice pretreated with saline (0 μg) before A-sub-Ad immunization developed elevated T4 levels. Pretreatment with A-sub-CHO did reduce the induction of hyperthyroidism but only with the highest concentration (3 μg) (Fig. 1C). Consistent with lower T4 levels, TSAb activity was decreased in mice pretreated with 3 μg of A-sub-CHO (Fig. 1D).
Specificity of A-sub-CHO and relevance of glycosylation
To expand on these observations, in two subsequent experiments, we used higher doses of A-sub-CHO (up to 10 μg) and larger numbers of mice (10 vs. five per group). In the first of these two studies, we included the thyroid antigens Tg and TPO to provide specificity controls. As mentioned, the TSHR A-subunit and Tg (but not TPO) bind to the mannose receptor (23,24). Moreover, carbohydrate is required for TSHR A-subunit binding to the mannose receptor (24). Therefore, in the second of these two studies, we compared the outcome of pretreatment with highly glycosylated A-subunit-CHO vs. nonglycosylated A-subunit expressed in bacteria as a fusion protein (A-sub-Trx). For both studies, we used the protocol outlined in Fig. 1B: a single sc injection of A-sub-CHO, A-sub-Trx, or control proteins (Tg, TPO, or Trx) a week before the first of two A-sub-Ad immunizations followed by tail bleeding 1 wk later (wk 5).
Only pretreatment with A-sub-CHO (10 μg), not Tg or TPO, significantly reduced serum T4 levels compared with saline (0 μg), Tg, or TPO (Fig. 2A). Moreover, unlike glycosylated A-sub-CHO, pretreatment with TSHR bacterial fusion protein did not lower T4 levels compared with controls (saline or Trx) (Fig. 2B). These data confirmed the specificity of A-subunit protein pretreatment, as well the requirement for glycosylated TSHR, to block hyperthyroidism induced by A-sub-Ad immunization.
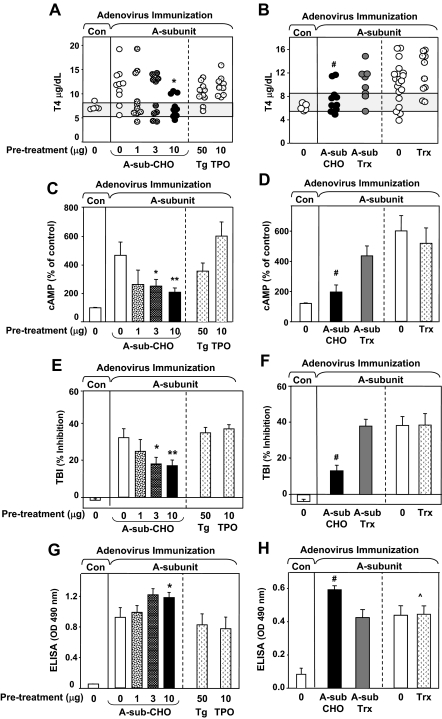
Figure 2.
Specificity of pretreatment with eukaryotic TSHR A-subunit protein in attenuating induction of hyperthyroidism. Pretreatment and immunization was performed as described in Fig. 1B. A, C, E, and G, Mice received A-sub-CHO (0–10 μg) or controls (Tg or TPO). B, D, F, and H, Mice received A-sub-CHO (10 μg), A-sub-Trx (8 μg), or controls (0 μg or Trx 3 μg). Number of mice: n = 10 with the following exceptions: Con-Ad (n = 5); TPO-treatment (n = 9); and 0 treatment (n = 20, B, D, F, and H). A and B, Serum T4 (micrograms per deciliter) in individual mice. Shaded area, the mean ± 2 sd for Con-Ad immunized mice. *, A, significantly reduced for 10 μg A-sub-CHO vs. 0 μg, Tg or TPO (ANOVA, P < 0.05); #, B, significantly reduced for A-sub-CHO vs. 0 (t test, P = 0.034). C and D, TSAb measured using mouse-TSHR expressing CHO cells and presented as the mean + sem (percentage of response in control mice, see Materials and Methods). Values are significantly different after pretreatment with: **, 10 μg vs. 0 μg A-sub-CHO (t test, P = 0.011); *, 3 μg vs. 0 μg A-sub-CHO (t test, P < 0.040); #, A-sub-CHO vs. 0 (rank sum, P = 0.001). E and F, TBI levels (mean ± sem, percent inhibition of TSH binding to the TSHR). Values significantly reduced after pretreatment with: **, 10 μg A-sub-CHO vs. 0 μg, Tg or TPO (ANOVA, P < 0.05) and *, after pretreatment with 10 μg A-sub-CHO vs. 0 (t test, P < 0.033); #, A-sub-CHO vs. A-sub-Trx, Trx, or 0 (ANOVA, P < 0.050). G and H, TSHR antibodies measured by ELISA; OD 490 nm values (mean + sem; sera diluted 1:100). Values significantly increased after pretreatment with: *, 10 μg vs. 0 μg A-sub-CHO (rank sum test, P = 0.045); #, A-sub-CHO vs. A-sub-TRX (t test, P = 0.005); ^, A-sub-CHO vs. Trx (t test, P = 0.016).
Combining the data from the foregoing studies, A-sub-Ad immunization induced hyperthyroidism in 77% (50 of 76) of mice pretreated with control substances (saline, Tg, TPO, or Trx) in contrast to only 22% (five of 23) of animals injected with 10 μg glycosylated A-subunit protein (P = 0.001, χ2 test). Thus, after pretreatment with a single 10 μg dose of A-subunit-CHO protein, thyrotoxicosis developed only in one third of the expected number of animals.
Characterization of TSHR antibodies after pretreatment with TSHR protein
To understand the basis for reduced hyperthyroidism described above, TSHR antibodies were measured using three assays. Consistent with findings from the preliminary experiment (Fig. 1D), TSAb activity was reduced after pretreatment with A-sub-CHO (3 and 10 μg) but not Tg, TPO, or bacterial A-subunit protein (A-sub-Trx) (Fig. 2, C and D). These TSAb data were obtained using mouse TSHR-expressing CHO cells; comparable observations were made with human TSHR-expressing CHO cells (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo. endojournals.org). TSHR antibodies measured by TBI were also decreased in mice pretreated with A-sub-CHO (10 and 3 μg) but not Tg, TPO, or bacterial A-subunit protein (Fig. 2, E and F).
Unexpectedly, measuring TSHR antibodies by ELISA revealed a different pattern. A-subunit-CHO pretreatment moderately but significantly enhanced TSHR antibody levels; no enhancement was observed in control mice pretreated with saline, Tg, TPO, A-sub-Trx, or Trx (Fig. 2, G and H). These surprising observations indicated that, contrary to our hypothesis, tolerance had not been induced. Instead, A-subunit protein injected before A-sub-Ad immunization deviated antibody generation away from those detected in TSAb and TBI assays toward those measured by ELISA. This distinction is important because functional (or pathogenic) antibodies (TSAb and TBI) in humans do not recognize TSHR A-subunits on an ELISA plate.
Persistence of the suppressive effect of A-subunit protein pretreatment
We investigated the long-term outcome of pretreatment with A-subunit protein before A-sub-Ad immunization. Mice that had received two cycles of A-subunit protein pretreatment (0, 1, 3, and 10 μg) followed by A-sub-Ad and immunization were maintained without manipulation for a further 7 wk until euthanasia (total of 12 wk). As observed at the 5-wk interval (Fig. 2), T4 levels were significantly reduced at 12 wk in mice pretreated with 10 μg A-sub-CHO vs. saline (supplemental Fig. 2A). Likewise, TBI and TSAb levels were lower after pretreatment with 10 μg or 3 μg A-sub-CHO vs. saline or A-sub-Trx (supplemental Fig. 2, B and C). Overall, serum T4, TBI, and TSAb values remained stable. Thus, these data provide no evidence for escape from the efficacy of pretreatment with A-subunit CHO and instead demonstrate its long-term effects.
Effect of TSHR A-subunit protein injection on an established TSHR immune response
A-sub-CHO protein pretreatment attenuated the hyperthyroid response to A-sub-Ad immunization, as described above. The question then arose whether A-sub-CHO protein administered for the first time after the development of hyperthyroidism would influence the course of the latter. Two experiments were performed.
In experiment A (Fig. 3, left side), we studied three groups of mice that received control pretreatment (saline, Tg, or TPO) before two A-sub-Ad immunizations. On testing at 5 wk, these groups had similar levels of T4 and TSHR antibodies (shown below). At 7 and 8 wk, these mice were challenged with A-sub-CHO protein alone, A-sub-Ad alone, or the combination of pretreatment with A-sub-CHO protein followed by A-sub-Ad. Not shown in Fig. 3, mice previously immunized twice with Con-Ad received a third injection of control adenovirus.
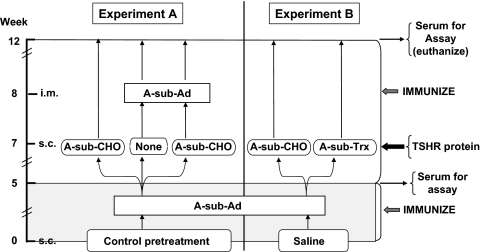
Figure 3.
Protocol to investigate the effect of TSHR A-subunit protein on established immune responses induced by immunization with A-sub-Ad. The mice studied represent the follow-up of animals tested at the 5-wk time point (Fig. 2). In experiment A mice received control pretreatment (saline, Tg, or TPO) before A-sub-Ad immunization and tail bleeding at wk 5. At wk 7, mice were injected with A-sub-CHO (10 μg) or saline. At wk 8, mice were untreated or immunized with A-sub-Ad to provide the following combinations: A-sub-CHO alone, A-sub-Ad alone, or A-sub-CHO and then A-sub-Ad. In experiment B, mice received saline pretreatment before immunization with A-sub-Ad and tail bleeding at wk 5. At wk 7, mice were injected with A-sub-CHO (10 μg) or A-sub-Trx (8 μg). For both experiments A and B, mice were euthanized 12 wk after the beginning of the study. Serum T4 and TSHR antibodies were measured at wk 5 and 12.
In experiment B (Fig. 3, right side), mice pretreated with saline before each of two A-sub-Ad immunizations were challenged at wk 7 with glycosylated or nonglycosylated A-subunit protein (A-sub-CHO protein or A-sub-Trx). Control mice for this experiment (not shown in Fig. 3) were maintained without further treatment.
For both experiments A and B, mice were euthanized at 12 wk and their sera tested for T4 and TSHR antibodies. The influence of A-sub-CHO protein on the established immune response was assessed by comparing serum T4 and TSHR antibody levels at the 5- and 12-wk time points.
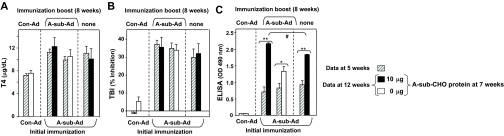
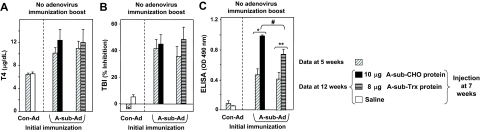
There were no major differences in the levels of T4 or TSHR antibodies measured by TBI at wk 5 and 12 in experiment A (Fig. 4, A and B). Consequently, unlike the efficacy of pretreatment, A-sub-CHO protein was unable to reverse hyperthyroidism or reduce TBI activity in mice with established immune responses to the TSHR. Remarkably, completely different observations were made for TSHR antibodies measured by ELISA (Fig. 4C). Compared with the 5-wk parameters, injecting A-subunit-CHO protein markedly enhanced TSHR ELISA antibody levels and even more so when this injection was followed 1 wk later by an immunization boost with A-sub-Ad. Without the injection of A-subunit-CHO protein, the A-subunit-Ad immunization boost only modestly increased TSHR antibodies measured by ELISA.
Figure 4.
Injecting eukaryotic TSHR A-subunit-CHO alone or in combination with A-sub-Ad in mice with established Graves’ disease enhances nonfunctional TSHR antibody levels but does not change thyroid function or TBI activity. Mice were treated according to the protocol for experiment A (Fig. 3). Data are shown as paired bar graphs (mean + sem) for sera tested at 5 and 12 wk. The 5-wk data (diagonally hatched bars) are those previously shown in Fig. 2, A, E, and G. Open bars, saline (0 μg); solid black bars, A-sub-CHO (10 μg). Number of mice: n = 10 for each test group; n = 5 for Con-Ad immunized animals. A, Serum T4 (micrograms per deciliter). B, TBI (percent inhibition of TSH binding). C, TSHR ELISA antibody (OD 490 nm). *, t test, P = 0.023, significantly different after repeat A-sub-Ad immunization; **, rank sum, P < 0.001; significantly different after boosting with A-sub-CHO+A-sub-Ad vs. A-sub-CHO; #, ANOVA, P < 0.05.
Recombinant TSHR A-subunits generated in CHO cells are very heavily glycosylated (∼40% of their mass). As demonstrated above (Fig. 2), prokaryotic, nonglycosylated TSHR A-subunits administered before A-subunit-Ad immunization did not attenuate induction of TSAb, TBI, or hyperthyroidism. Therefore, in experiment B, we compared the ability of glycosylated A-subunit-CHO vs. nonglycosylated A-subunit-Trx proteins to influence the course of an established immune response after A-sub-Ad immunization. Mice were pretreated with saline, immunized with A-sub-Ad, and then at the 7-wk time point were injected with A-sub-CHO or A-sub-Trx (Fig. 3, experiment B). As in experiment A, there were no significant differences in serum T4 or TBI levels when assayed 5 wk later (12 wk time point) (Fig. 5, A and B). These data confirmed the inability of A-sub-CHO to reverse hyperthyroidism or reduce TBI in mice with established immunity to the TSHR. In contrast, TSHR antibodies detected by ELISA were boosted by A-sub-CHO protein and, although to a lesser extent, by A-sub-Trx protein (Fig. 5C).
Figure 5.
Effect of eukaryotic A-subunit-CHO or prokaryotic A-subunit-Trx in mice with an established immune response induced using A-sub-Ad. Mice were boosted with A-subunit protein according to the protocol for experiment B (Fig. 3). Data are shown as paired bar graphs (mean + sem) for sera tested at 5 and 12 wk. The 5-wk data (diagonally hatched bars) were previously shown in Fig. 2 B, F and H. Open bars, no protein; black bars, A-sub-CHO (10 μg); horizontal striped bars, A-sub-Trx (8 μg). Number of mice: n = 10 for each group except for Con-Ad immunized animals, n = 5. A, Serum T4 (micrograms per deciliter). B, TBI (percent inhibition of TSH binding). C, TSHR ELISA antibody (OD 490 nm). **, P = 0.008, t test; significantly different after eukaryotic A-subunit injection; *, P < 0.001, t test; significantly different after prokaryotic A-subunit injection; #, P < 0.05, rank sum, significantly higher after injection with A-sub-CHO vs. A-sub-Trx.
Together, experiments A and B indicate that A-subunit protein, even if glycosylated, administered after A-subunit adenovirus immunization is unable to reduce pathogenic TSHR antibody levels and ameliorate hyperthyroidism. Unexpectedly, however, both glycosylated and prokaryotic A-subunit protein markedly boosted the levels of nonfunctional TSHR antibodies: antibodies that are detectable by ELISA but do not stimulate the TSHR or block the action of TSH.
Discussion
Antigens targeted to the mannose receptor in the absence of stimuli (like endotoxin) to the innate immune system induce tolerance (e.g. Ref. 25). Previously we observed that highly glycosylated TSHR A-subunits bind to the mannose receptor (24). We therefore tested the hypothesis that pretreatment of mice with this TSHR A-subunit protein would establish tolerance to the TSHR A-subunit and prevent or attenuate Graves’-like hyperthyroidism induced by immunization with adenovirus expressing the A-subunit. We injected TSHR A-subunit protein sc, a route known to favor peripherally located mannose receptor-expressing DCs (25).
Incidentally, we had previously attempted to block the induction of hyperthyroidism in mice using adenoviruses encoding costimulation decoys to interfere in the signaling between antigen-presenting cells and T cells. Nonspecific inhibition was an unforeseen consequence of administering a decoy adenovirus (or control adenovirus) together with low dose A-subunit adenovirus, and, therefore, we could draw only limited conclusions from these experiments (31).
In our present studies, we focused on the A-subunit protein itself as a potential tolerogen. Indeed, injecting A-subunit protein before A-sub-Ad immunization reduced serum T4 and significantly decreased the proportion of thyrotoxic mice. This reduction occurred using glycosylated eukaryotic, but not nonglycosylated prokaryotic, A-subunits. Consistent with the attenuation of hyperthyroidism, TSAbs and TBI declined in mice pretreated with A-subunit-CHO. The effect of the A-subunit was specific because neither Tg (which binds) nor TPO (which does not bind) to the mannose receptor (24) was effective. Overall, these data support the concept of tolerance induction by glycosylated A-subunits interacting with the mannose receptor (or other lectin-like receptors on antigen presenting cells) in the absence of signals to activate the innate immune system. It is of interest that Kong and colleagues induced tolerance and prevented experimentally induced thyroiditis by pretreating mice with deaggregated mouse Tg or by TSH infusion to raise circulatory mouse Tg levels (36). This tolerance induced by mouse Tg was mediated by regulatory T cells of the CD4+ CD25+ subset (37).
Surprisingly, however, our deduction that we had induced tolerance by pretreatment with A-subunit-CHO protein was overturned in subsequent analysis of the type of TSHR antibodies generated. There are a number of assays for TSHR antibodies. In the context of hyperthyroidism, the most relevant are a bioassay for thyroid stimulation (TSAb) and a competitive inhibition assay for TSH binding to the native receptor on the cell surface (TBI). In Graves’ disease and hyperthyroidism induced in mice, serum TBI and TSAb are neutralized by a highly conformational form of recombinant, glycosylated TSHR A-subunits generated in eukaryotic cells (27,28,38). Coating of ELISA wells with this same antigen abolishes recognition by Graves’ sera. However, nonfunctional TSHR antibodies readily bind to this material on ELISA wells (e.g. Ref. 39). Consequently, in our numerous studies on induced hyperthyroidism in mice, we do not always measure TSHR antibodies by ELISA. Fortuitously, in the present study, we also assayed TSHR antibodies by ELISA. We observed that although pretreatment with A-subunit-CHO protein reduced the subsequent serum T4, TSAb, and TBI responses to A-subunit adenovirus immunization, TSHR antibody levels measured by ELISA were moderately increased. Therefore, contrary to our expectations, rather than inducing tolerance (such as via the mannose receptor), the injected A-subunit protein diverted the immune response toward the generation of nonfunctional TSHR antibodies.
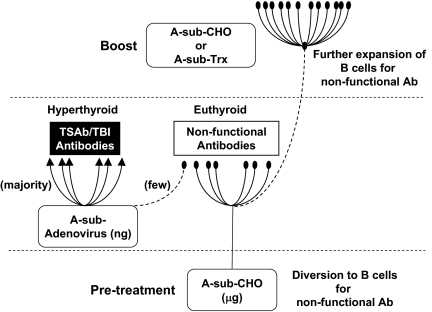
We propose a mechanism to explain our unexpected findings. Recombinant A-subunits generated in CHO cells are purified in two conformational forms: active, recognized by functional TSAb and TBI, and inactive, recognized only by antibodies without functional activity (27). Active A-subunits are conformationally unstable and spontaneously convert to the inactive form. Injecting microgram amounts of A-subunit protein (largely inactive) targets B cells bearing receptors for nonfunctional (ELISA type) antibodies (Fig. 6). Unlike injection of TSHR protein generated in vitro, immunization with adenovirus generates very small (nanogram) amounts of TSHR A-subunits in vivo, much of which is in the active conformation and therefore captured and processed by B cell precursors that ultimately secrete pathogenic antibodies (measured by TBI and TSAb). The adenovirus immunization provides the immune signals required to activate both B cell populations. However, because A-subunit protein pretreatment administers a much larger dose of A-subunit protein than A-subunit adenovirus, B cells secreting ELISA-type TSHR antibodies dominate and attenuate the induction of functional (or pathogenic) antibodies.
Figure 6.
Schematic illustration of the impact of glycosylated A-subunit protein (A-sub-CHO) on the immune system, which protects against Graves’ hyperthyroidism induced by A-subunit adenovirus immunization. For detailed explanation, see text.
Support for the foregoing concept is provided by follow-up studies. Animals not pretreated with A-subunit-CHO protein before A-subunit adenovirus immunization develop mainly pathogenic and some nonfunctional antibodies. In these mice, once the immune response to A-subunit immunization has been established, subsequent injection of A-subunit-CHO protein markedly enhances generation of antibodies detected by TSHR ELISA but not TBI or TSAb. Moreover, although ineffective in the pretreatment phase, prokaryotic A-subunits also enhance ELISA TSHR antibody generation in mice with an established immune response (Fig. 6). Taking all our data together, only glycosylated A-subunits are capable of initially redirecting pathogenic antibodies toward nonfunctional antibodies and attenuating hyperthyroidism. However, once the immune response to the TSHR is established, both glycosylated and nonglycosylated A-subunit proteins can expand the population of TSHR ELISA antibody-secreting B cells. At this time point and despite high levels, these nonfunctional antibodies cannot reduce the production or ongoing effects of TSAb.
Observations in mice transgenic for a hapten-specific antibody provide parallels for our study. Transgenic monoclonal B cells are fixed and unable to undergo antibody affinity maturation that occurs after immunization with the hapten conjugate in nontransgenic normal B cells. Adoptive transfer of transgenic B cells to nontransgenic animals dramatically impairs the affinity maturation process (40). By analogy, B cells that recognize the A-subunit protein that we used for pretreatment correspond to the low-affinity monoclonal hapten-specific population because their antibodies have a low affinity for (that is, do not recognize) the functional TSHR. In contrast, B cells activated by the A-subunit expressed in vivo (by adenovirus) secrete antibodies that recognize (and activate) the TSHR. This B cell population corresponds to the normal B cells that are capable of undergoing affinity maturation after hapten immunization.
Our observations have implications for the murine model of Graves’ disease. First, it is possible to redirect (deviate) antibodies by pretreatment with microgram amounts of TSHR protein. Second, A-sub-CHO treatment cannot reverse hyperthyroidism in mice with ongoing immune responses to the TSHR. Third, we provide insight into the time frame during which TSHR antibodies and/or their effects persist. After two TSHR A-sub-Ad immunizations, regardless of the A-sub-CHO concentration used for pretreatment, TBI and TSAb levels were essentially unchanged for the following 7 wk. Because the mice were maintained in pathogen-free conditions, environmental antigen exposure could not provide bystander stimulation to maintain antibody responses. The half-life of antibodies is of the order of weeks (e.g. Ref. 41). Therefore, the persistent TSHR antibody levels are a testament to the extended time period (>1 yr) for which antibody-secreting plasma cells can survive (42).
Unlike many autoimmune conditions with multiple and/or unknown autoantigens, the TSHR (particularly the shed A-subunit) is the unequivocal immune target in Graves’ disease. Even for antibody-mediated diseases with recognized autoantigens, stimulatory autoantibodies are rare. An important question arising from our studies is whether A-subunit deviation toward nonfunctional antibodies could be applied therapeutically in humans. Because we were unable to block hyperthyroidism in mice with an established immune response, this treatment is unlikely to succeed in Graves’ patients. Instead, it may be possible to vaccinate against pathogenic antibodies in individuals at risk of developing Graves’ hyperthyroidism, namely the relatives of Graves’ patients. However, apart from the practical hurdles of generating sufficient A-subunit protein, redirecting antibody epitopes may not preclude activating TSHR-specific T cells. Clearly, before the A-subunit protein vaccination approach could be used in humans, it would be essential to ensure that the process did not activate TSHR-specific T cells with the potential to home to the orbit and precipitate or enhance Graves’ ophthalmopathy.
A recent study in humans provides a potential parallel with our findings. As mentioned in the introductory text, anti-CD20 antibody (rituximab) is currently being explored to treat Graves’ disease (12,13,14). When added to methimazole therapy, rituximab reduced TSAb but not TBI activity when compared with methimazole treatment alone (43). In contrast, in patients with mild relapsing Graves’ disease, TBI levels decreased after rituximab treatment (44). Differences between the two studies include disease severity as well as time frame. However, if the TSAb (but not TBI) reduction by rituximab is confirmed, these findings will demonstrate the feasibility of deviating away from pathogenic TSHR antibodies in humans.
In conclusion, a single injection of glycosylated TSHR A-subunit before TSHR immunization deviates immune responses away from pathogenic, and toward nonfunctional, antibodies, thereby protecting mice from developing Graves’ hyperthyroidism. The effect is antigen specific and is sustained, at least in the short term, but this approach does not reverse established hyperthyroidism in mice. Consequently, although TSHR A-subunit protein is a potential immune modulator, it cannot be used to treat immunized animals and is unlikely to be useful in patients diagnosed with Graves’ disease. However, in genetically susceptible individuals, vaccination with TSHR A-subunit protein has the potential for deviating away from pathogenic antibodies and providing long-term protection against the development of Graves’ disease.
Supplementary Material
Acknowledgments
We are grateful for contributions by Dr. Boris Catz (Los Angeles, CA).
Footnotes
This work was supported by National Institutes of Health Grants DK54684 (to S.M.M.) and DK19289 (to B.R).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 23, 2009
Abbreviations: CHO, Chinese hamster ovary; DC, dendritic cell; NOD, nonobese diabetic; TBI, TSH binding inhibition; Tg, thyroglobulin; TPO, thyroid peroxidase; Trx, thioredoxin; TSAb, thyroid-stimulating antibody; TSHR, TSH receptor.
References
- Rapoport B, McLachlan SM 2007 The thyrotropin receptor in Graves’ disease. Thyroid 17:911–922 [DOI] [PubMed] [Google Scholar]
- Uibo R, Lernmark A 2008 GAD65 autoimmunity—clinical studies. Adv Immunol 100:39–78 [DOI] [PubMed] [Google Scholar]
- Kanta H, Mohan C, Three checkpoints in lupus development: central tolerance in adaptive immunity, peripheral amplification by innate immunity and end-organ inflammation. Genes Immun, in press [DOI] [PubMed] [Google Scholar]
- Rowley MJ, Nandakumar KS, Holmdahl R 2008 The role of collagen antibodies in mediating arthritis. Mod Rheumatol 18:429–441 [DOI] [PubMed] [Google Scholar]
- Kuerten S, Angelov DN 2008 Comparing the CNS morphology and immunobiology of different EAE models in C57BL/6 mice—a step toward understanding the complexity of multiple sclerosis. Ann Anat 190:1–15 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Kita-Furuyama M, Ando T, Nakao K, Mizuguchi H, Hayakawa T, Eguchi K, Niwa M 2002 A novel murine model of Graves’ hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol 168:2789–2794 [DOI] [PubMed] [Google Scholar]
- Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM 2003 The thyrotropin receptor autoantigen in Graves’ disease is the culprit as well as the victim. J Clin Invest 111:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama Y, Mizuguchi H, Hayakawa T, Niwa M, McLachlan SM, Rapoport B 2003 Prevention of autoantibody-mediated Graves’-like hyperthyroidism in mice with IL-4, a Th2 cytokine. J Immunol 170:3522–3527 [DOI] [PubMed] [Google Scholar]
- Saitoh O, Mizutori Y, Takamura N, Yamasaki H, Kita A, Kuwahara H, Nagayama Y 2005 Adenovirus-mediated gene delivery of interleukin-10, but not transforming growth factor β, ameliorates the induction of Graves’ hyperthyroidism in BALB/c mice. Clin Exp Immunol 141:405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama Y, Watanabe K, Niwa M, McLachlan SM, Rapoport B 2004 Schistosoma mansoni and α-galactosylceramide: prophylactic effect of Th1 immune suppression in a mouse model of Graves’ hyperthyroidism. J Immunol 173:2167–2173 [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Kalled SL, Moorhead J, Hess DM, Rennert P, Li Z, Khan MZ, Banga JP 2006 Treatment of autoimmune hyperthyroidism in a murine model of Graves’ disease with TNF-family ligand inhibitors suggests a key role for BAFF in disease pathology. Endocrinology 147:4561–4568 [DOI] [PubMed] [Google Scholar]
- El Fassi D, Nielsen CH, Hasselbalch HC, Hegedüs L 2006 Treatment-resistant severe, active Graves’ ophthalmopathy successfully treated with B lymphocyte depletion. Thyroid 16:709–710 [DOI] [PubMed] [Google Scholar]
- Salvi M, Vannucchi G, Campi I, Currò N, Dazzi D, Simonetta S, Bonara P, Rossi S, Sina C, Guastella C, Ratiglia R, Beck-Peccoz P 2007 Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol 156:33–40 [DOI] [PubMed] [Google Scholar]
- El Fassi D, Nielsen CH, Bonnema SJ, Hasselbalch HC, Hegedüs L 2007 B lymphocyte depletion with the monoclonal antibody rituximab in Graves’ disease: a controlled pilot study. J Clin Endocrinol Metab 92:1769–1772 [DOI] [PubMed] [Google Scholar]
- Feldmann M, Steinman L 2005 Design of effective immunotherapy for human autoimmunity. Nature 435:612–619 [DOI] [PubMed] [Google Scholar]
- Kita-Furuyama M, Nagayama Y, Pichurin P, McLachlan SM, Rapoport B, Eguchi K 2003 Dendritic cells infected with adenovirus expressing the thyrotropin receptor induce Graves’ hyperthyroidism in BALB/c mice. Clin Exp Immunol 131:234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutori Y, Saitoh O, Eguchi K, Nagayama Y 2006 Adenovirus encoding the thyrotropin receptor A-subunit improves the efficacy of dendritic cell-induced Graves’ hyperthyroidism in mice. J Autoimmun 26:32–36 [DOI] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC 2001 Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 194:769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engering AJ, Cella M, Fluitsma D, Brockhaus M, Hoefsmit EC, Lanzavecchia A, Pieters J 1997 The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol 27:2417–2425 [DOI] [PubMed] [Google Scholar]
- Taylor ME, Conary JT, Lennartz MR, Stahl PD, Drickamer K 1990 Primary structure of the mannose receptor contains multiple motifs resembling carbohydrate-recognition domains. J Biol Chem 265:12156–12162 [PubMed] [Google Scholar]
- Baumeister FA, Herzog V 1988 Sulfation of thyroglobulin: a ubiquitous modification in vertebrates. Cell Tissue Res 252:349–358 [DOI] [PubMed] [Google Scholar]
- Spiro RG, Bhoyroo VD 1988 Occurrence of sulfate in the asparagine-linked complex carbohydrate units of thyroglobulin. Identification and localization of galactose 3-sulfate and N-acetylglucosamine 6-sulfate residues in the human and calf proteins. J Biol Chem 263:14351–14358 [PubMed] [Google Scholar]
- Linehan SA, Martínez-Pomares L, da Silva RP, Gordon S 2001 Endogenous ligands of carbohydrate recognition domains of the mannose receptor in murine macrophages, endothelial cells and secretory cells; potential relevance to inflammation and immunity. Eur J Immunol 31:1857–1866 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Pichurin PN, Guo J, Rapoport B, McLachlan SM 2005 Interactions between the mannose receptor and thyroid autoimmunity. Clin Exp Immunol 139:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie EJ, Taylor PR, Stillion RJ, Lucas AD, Harris J, Gordon S, Martinez-Pomares L 2007 Mannose receptor expression and function define a new population of murine dendritic cells. J Immunol 178:4975–4983 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Wang Y, Guo J, Hutchison JS, Segal D, Jaume JC, McLachlan SM, Rapoport B 1999 A mouse monoclonal antibody to a thyrotropin receptor ectodomain variant provides insight into the exquisite antigenic conformational requirement, epitopes and in vivo concentration of human autoantibodies. J Clin Endocrinol Metab 84:702–710 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, McLachlan SM, Pichurin P, Yan XM, Rapoport B 2001 A “prion-like” shift between two conformational forms of a recombinant thyrotropin receptor A subunit module: purification and stabilization using chemical chaperones of the form reactive with Graves’ autoantibodies. J Clin Endocrinol Metab 86:1287–1293 [DOI] [PubMed] [Google Scholar]
- Schwarz-Lauer L, Chazenbalk GD, Mclachlan SM, Ochi Y, Nagayama Y, Rapoport B 2002 Evidence for a simplified view of autoantibody interactions with the TSH receptor. Thyroid 12:115–120 [DOI] [PubMed] [Google Scholar]
- Guo J, McLachlan SM, Hutchison S, Rapoport B 1998 The greater glycan content of recombinant human thyroid peroxidase of mammalian than on insect cell origin facilitates purification to homogeneity of enzymatically active protein remaining soluble at high concentration. Endocrinology 139:999–1005 [DOI] [PubMed] [Google Scholar]
- Pichurin P, Yan XM, Farilla L, Guo J, Chazenbalk GD, Rapoport B, McLachlan SM 2001 Naked thyrotropin receptor DNA vaccination: A TH1 T cell response in which interferon-γ production, rather than antibody, dominates the immune response in mice. Endocrinology 142:3530–3536 [DOI] [PubMed] [Google Scholar]
- Chen CR, Aliesky HA, Guo J, Rapoport B, McLachlan SM 2006 Blockade of costimulation between T cells and antigen-presenting cells: an approach to suppress murine Graves’ disease induced using thyrotropin receptor-expressing adenovirus. Thyroid 16:427–434 [DOI] [PubMed] [Google Scholar]
- Mittereder N, March KL, Trapnell BC 1996 Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol 70:7498–7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma A, Chazenbalk G, Filetti S, McLachlan SM, Rapoport B 1996 Both the 5′ and 3′ non-coding regions of the thyrotropin receptor messenger RNA influence the level of receptor protein expression in transfected mammalian cells. Endocrinology 137:2664–2669 [DOI] [PubMed] [Google Scholar]
- Misharin A, Hewison M, Chen CR, Lagishetty V, Aliesky HA, Mizutori Y, Rapoport B, McLachlan SM 2009 Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology 150:1051–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan SM, Aliesky HA, Pichurin PN, Chen CR, Williams RW, Rapoport B 2008 Shared and unique susceptibility genes in a mouse model of Graves’ disease determined in BXH and CXB recombinant inbred mice. Endocrinology 149:2001–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Giraldo AA, Kong YC 1987 Resistance to experimental autoimmune thyroiditis induced by physiologic manipulation of thyroglobulin level. Clin Immunol Immunopathol 45:92–104 [DOI] [PubMed] [Google Scholar]
- Morris GP, Kong YC 2006 Tolerance to autoimmune thyroiditis: (CD4+)CD25+ regulatory T cells influence susceptibility but do not supersede MHC class II restriction. Front Biosci 11:1234–1243 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Jaume JC, McLachlan SM, Rapoport B 1997 Engineering the human thyrotropin receptor ectodomain from a non-secreted form to a secreted, highly immunoreactive glycoprotein that neutralizes autoantibodies in Graves’ patients’ sera. J Biol Chem 272:18959–18965 [DOI] [PubMed] [Google Scholar]
- Schwarz-Lauer L, Pichurin PN, Chen CR, Nagayama Y, Paras C, Morris JC, Rapoport B, McLachlan SM 2003 The cysteine-rich amino terminus of the thyrotropin receptor is the immunodominant linear antibody epitope in mice immunized using naked DNA or adenovirus vectors. Endocrinology 144:1718–1725 [DOI] [PubMed] [Google Scholar]
- Le TV, Kim TH, Chaplin DD 2008 Intraclonal competition inhibits the formation of high-affinity antibody-secreting cells. J Immunol 181:6027–6037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot PJ, Buchmeier MJ 1987 Catabolism of homologous murine monoclonal hybridoma IgG antibodies in mice. Immunology 60:485–489 [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Antia R, Whitmire JK, Ahmed R 1998 Humoral immunity due to long-lived plasma cells. Immunity 8:363–372 [DOI] [PubMed] [Google Scholar]
- El Fassi D, Banga JP, Gilbert JA, Padoa C, Hegedüs L, Nielsen CH 2009 Treatment of Graves’ disease with rituximab specifically reduces the production of thyroid stimulating autoantibodies. Clin Immunol 130:252–258 [DOI] [PubMed] [Google Scholar]
- Heemstra KA, Toes RE, Sepers J, Pereira AM, Corssmit EP, Huizinga TW, Romijn JA, Smit JW 2008 Rituximab in relapsing Graves’ disease, a phase II study. Eur J Endocrinol 159:609–615 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.