Abstract
Whereas the adolescent brain is a major target for gonadal hormones, our understanding of hormonal influences on adolescent neural and behavioral development remains limited. These experiments investigated how variations in the timing of testosterone (T) exposure, relative to adolescence, alters the strength of steroid-sensitive neural circuits underlying social behavior in male Syrian hamsters. Experiment 1 simulated early, on-time, and late pubertal development by gonadectomizing males on postnatal d 10 and treating with SILASTIC brand T implants for 19 d before, during, or after adolescence. T treatment before or during, but not after, adolescence facilitated mating behavior in adulthood. In addition, preadolescent T treatments most effectively increased mating behavior overall, indicating that the timing of exposure to pubertal hormones contributes to individual differences in adult behavior. Experiment 2 examined the effects of preadolescent T treatment on behavior and brain regional volumes within the mating neural circuit of juvenile males (i.e. still preadolescent). Although preadolescent T treatment did not induce reproductive behavior in juvenile males, it did increase volumes of the bed nucleus of the stria terminalis, sexually dimorphic nucleus, posterodorsal medial amygdala, and posteroventral medial amygdala to adult-typical size. In contrast, juvenile anterodorsal medial amygdala and ventromedial hypothalamus volumes were not changed by preadolescent T treatment yet differed significantly in volume from adult controls, suggesting that further maturation of these brain regions during adolescence is required for the expression of male reproductive behavior. Thus, adolescent maturation of social behavior may involve both steroid-independent and -dependent processes, and adolescence marks the end of a postnatal period of sensitivity to steroid-dependent organization of the brain.
Adolescent maturation of social behavior may involve both steroid-independent and steroid-dependent processes; adolescence marks the end of a postnatal period of sensitivity to steroid-dependent organization of the brain.
The brain is a key target of gonadal hormones such as estradiol and testosterone (T), and numerous animal studies document the powerful effects of these hormones on a variety of behaviors ranging from sexual interest to cognitive abilities. We have known for almost 50 yr that exposure to gonadal hormones during early neural development permanently alters sex-specific behavioral responses to gonadal hormones in adulthood, a seminal finding that spawned the idea that hormones organize behavioral circuits during sensitive periods of development (1). Despite this understanding, only a handful of studies have investigated the contribution of pubertal hormones to adolescent brain development and whether hormone action in the brain during this postnatal developmental period results in enduring changes in adult behavior.
The scarcity of animal research investigating pubertal hormone influences on adolescent brain development is particularly notable considering that deviations in pubertal timing are associated with adolescent-emerging psychopathologies such as depression, anxiety, disordered eating, and conduct disorder (2,3,4,5,6,7,8,9,10). The effects of variation in pubertal timing on psychopathology have largely been attributed to psychosocial factors that come into play with the intense changes in experience that accompany sexual maturation. However, gonadal hormones secreted at pubertal onset also have wide-ranging effects on the brain. Thus, mistimed, direct hormonal influences on the brain may also skew the course of adolescent brain development toward increased risk for psychopathology (8). If so, then the timely diagnosis and treatment of disorders of pubertal timing have repercussions that extend beyond normalization of reproductive function to more global influences on social behavior.
Animal models have immense potential for elucidating the mechanisms by which gonadal hormones directly impact adolescent neural and behavioral development. For example, depriving Syrian hamsters of hormones during adolescence compromises social behaviors, even after hormones are replaced in adulthood (11). This suggests that exposure of the adolescent brain to gonadal hormones organizes behavioral circuits and programs long-lasting behavioral responses. Furthermore, these data imply that a window of sensitivity to organizational effects of gonadal steroid hormones may close after adolescence (12), a possibility we tested herein.
The current studies sought to define the temporal parameters spanning the pre- and postadolescent periods within which neural circuits mediating social behavior are sensitive to the organizational effects of gonadal steroid hormones. Although previous work has established that perinatal exposure to gonadal steroid hormones masculinizes and defeminizes behavioral neural circuits, more recent work suggests that a second window of sensitivity may open at adolescence. For example, steroid hormones fail to elicit maximal expression of male reproductive behavior immediately before adolescence (13,14) but readily activate high levels of reproductive behavior after adolescence, raising the possibility that adolescence is a second sensitive period for the organizational effects of gonadal steroid hormones on male reproductive behavior (15). Experiment 1 tested the hypothesis that brain sensitivity to hormonal influences fluctuates over the course of postnatal development. After gonadectomy (GDX) on postnatal d 10, we simulated early, on-time, or delayed onset of pubertal hormone secretion by administering SILASTIC brand T capsules (Dow Corning Corp., Midland, MI) before, during, or after adolescent development and examined the potential for sexual behavior in adulthood.
We also sought to extend the previous finding that 1 week of preadolescent T-treatment does not facilitate mating behavior in juveniles in Exp. 2 by 1) determining whether a longer duration of preadolescent T-treatment facilitates mating behavior in juveniles, 2) determining what behaviors are displayed by juveniles in the presence of estrous females, and 3) identifying which brain regions underlying social behaviors are capable of steroid-dependent organization before adolescence, and which regions undergo additional maturation during adolescence. Identifying the brain regions capable of steroid-dependent change before adolescence and those that require further maturation during adolescence is an essential first step in understanding the brain regions underlying the adolescent maturation of social behaviors.
Materials and Methods
Animals
Timed-pregnant female Syrian hamsters (Mesocricetus auratus) arrived from Harlan Sprague Dawley laboratories (Madison, WI) on gestational d 4. Pregnant females were housed in clear polycarbonate cages (37.5 × 33 × 17 cm) provided with nesting materials, food (Telkad Rodent diet no. 8640; Harlan, Madison, WI) and water. Females were exposed to a 14-h light, 10-h dark schedule, and the temperature was maintained at 21 ± 2 C. Nests were checked twice daily for births beginning on gestational d 15, 1 d before the predicted birth. On postnatal d (P) 9, litters were sexed and culled to six to eight mixed-sex pups. Males were housed with mothers and littermates until weaning and single housing on P19 (cage dimensions 30.5 × 10.2 × 20.3 cm). Animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and protocols were approved by the institutional animal care committee.
Experimental design
Experiment 1: manipulation of the timing of periadolescent exposure to T
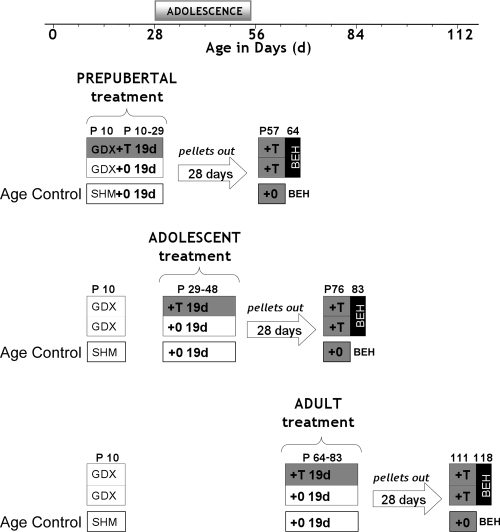
Figure 1 depicts the design of experiment 1. Males were randomly assigned to treatment groups and GDX or sham castrated at P10. To simulate early, on-time, or delayed puberty, males were exposed to blank- or T-filled SILASTIC brand capsules before, during, or after adolescence. In adulthood, 4 wk after pellet removal, both blank and T-treated males were implanted with T-filled capsules and behavior tested 1 wk later. This design kept constant the time interval between the 19-d hormone treatment (blank or T pellet) and the time of adult T treatment and behavior testing.
Figure 1.
Schematic depicting experiment 1 design. We define adolescence here as the time period encompassing not only reproductive maturation (puberty) but also cognitive, emotional, and social maturation (57), which occurs approximately between 28 and 56 d of age in male Syrian hamsters. Although puberty and adolescence are normally temporally coordinated, variations in the timing of puberty do occur and raise the question of whether brain development is similarly shaped by pubertal hormones when experienced outside the normal age range of adolescence. Here we attempted to examine the effects of temporal dissociations between puberty and adolescence on adult sexual behavior. Experimental males were gonadectomized on P10. To simulate early, on-time, or delayed puberty, males were exposed to blank- or T-filled SILASTIC brand capsules before adolescence (P10–29; row 1), during adolescence (P29–48; row 2), or after adolescence (P64–83; row 3). In adulthood, 4 wk after pellet removal, both blank and T-treated males were implanted with T-filled capsules and behavior tested 1 wk later (P64, 83, or 118). This design kept constant the time interval between the 19-d hormone treatment (blank or T pellet) and the time of adult T treatment and behavior testing (BEH). The 19- and 7-d T treatments served two different purposes. The 19-d T treatment period was intended to simulate early, on-time, or delayed onset of pubertal hormone secretion as a means of probing the ability of gonadal hormones to organize behavioral neural circuits at different ages relative to normal adolescence. The 1-wk treatment in adulthood was used to provide adult physiological levels of T as a means of probing the ability of gonadal hormones to activate sexual behavior after 1 wk of hormone priming in the groups that had experienced different histories of pubertal timing. In addition, every experimental group was raised with age-matched, sham-treated males that received all sham surgeries (sham gonadectomy and blank pellet implant) at the same time as experimental groups and were also behavior tested on the same day in adulthood. Behavioral comparisons between these groups controlled for the possible influence of age at the time of testing in young adulthood.
Because our experimental objectives required testing males at three different ages in young adulthood, sham males were included as age controls so that age-related differences in behavior could be detected independently of experimental manipulation (Fig. 1). These sham groups were chosen randomly from the same litters as GDX males and were sham GDX at P10 and tested for sexual behavior concurrently with experimental groups on P64, 83, or 118. The behaviors of these groups were statistically compared for detection of age-related changes in adult behavioral responses to T.
Experiment 2: juvenile vs. adult comparisons
Males were GDX on P10 and administered 19 d of blank or T capsules. Males were tested for sexual behavior with a hormone-primed adult female on P29 (still juvenile), and brains were collected immediately after testing. The behavior and brain region volumes of juveniles were compared with a group of males that were GDX in adulthood, after experiencing endogenous T during perinatal and pubertal development. This group was sham operated at P10, GDX at P64, treated with T 4 wk later at P92, and tested for sexual behavior on P99. Thus, the behavior of this group is representative of males that underwent normal pubertal development and whose sexual behavior was assessed in adulthood using a reinstatement paradigm (i.e. hormone replacement 4 wk after GDX).
Tissue collection, histology, and volume measurements
Male hamsters were euthanized with an overdose of sodium pentobarbital (130 mg/kg ip) after behavior testing and perfused intracardially with 4% paraformaldehyde (13). Brains were sectioned at 40 μm on a cryostat, and every fourth section was thaw mounted onto glass slides, allowed to dry, and then thionin stained and coverslipped. Hypothalamic and medial amygdalar regions were traced bilaterally at × 40 magnification by an observer blind to experimental treatment using Neurolucida (version 6; Microbrightfield, Williston, VT), and volumes were calculated by summing the traced cross-sectional areas and multiplying by the distance between sections (160 μm). The subnuclei traced for volume measurements were selected because of their steroid hormone sensitivity and/or well documented involvement in both mating and agonistic behaviors in hamsters and other rodent species (e.g. Refs. 16,17,18,19,20,21,22,23,24,25). The hypothalamic regions traced included the sexually dimorphic nucleus (SDN), posterior medial bed nucleus of the stria terminalis (BSTpm) medial preoptic nucleus (MPN), and ventromedial hypothalamus (VMH). We did not measure the MPN magnocellular in this study because our sampling interval of 160 μm did not permit accurate estimation of volume, given that the entire rostral-caudal extent of this nucleus is only approximately 150 μm (26). The medial amygdalar regions traced included the anterodorsal (MeAD), and anteroventral (MeAV) regions, and also the posterodorsal (MePD), and posteroventral (MePV) subregions. The hamster brain atlas was used as a reference for tracings (27).
Surgical procedures
GDX and sham surgeries were performed under isoflurane anesthesia and aseptic conditions (28). Two sterile SILASTIC brand T pellets (one 7 mm and one 15 mm; inner diameter 1.98 mm; outer diameter 3.18 mm) or two blank SILASTIC brand capsules were inserted sc through a 3- to 4-mm incision made on the dorsal midline, and the incision was sutured closed. Animals were returned to their mothers and littermates after surgery, and their health was monitored closely for the following week.
T RIA
Plasma concentrations of T were measured in duplicate 50-μl samples using the Coat-A-Count total T kit (Diagnostic Products, Los Angeles, CA). Samples from each group were counterbalanced across three different assays, and the intrassay coefficients of variation were 7.7, 4.0, and 8.2%. The interassay coefficient of variation was 14.1%, and the lower detection limit was 0.1 ng/ml for all assays.
Behavioral testing
All reproductive behavior tests were conducted 1–5 h after lights out, and the timing of testing across this period was counterbalanced between groups. The male was placed in a 10-gal glass aquarium (51 × 26 × 31.5 cm) and allowed to acclimate for 5 min before the introduction of a receptive stimulus female. The behavior tests were 15 min. Ovariectomized stimulus females were brought into behavioral estrus with an injection of 10 μg estradiol benzoate (0.2 mg/ml) in sesame oil 48 h before testing, and an injection of 250 μg progesterone (5 mg/ml) in sesame oil 3 h before testing. All females were checked for behavioral receptivity before behavior tests by pairing them with colony breeder males for approximately 1 min. Females were paired with only one experimental male per test day.
The behavioral tests were videotaped under dim red light illumination and later scored to assess the number of vaginally oriented mounts, intromissions, and the latencies to mount and intromit females. The criteria for these behaviors have been described previously (13). In addition, the risk assessment behavior stretch-attend and submissive behaviors tail-up walking and escape dashes were quantified. We used the criterion established by Albers et al. (29) when scoring these behaviors. All videotapes were scored blind to experimental condition by a single observer.
Sample sizes and experimental attrition
Twelve males were assigned to each group on P9, 1 day before the first surgical procedures (GDX or sham). Some animals were lost from groups during surgeries and in the postoperative period. Videotape failures also precluded the analysis of some behavioral data. Finally, data were not analyzed if damaged or broken T pellets were found at the time of tissue collection. Therefore, final sample sizes in both experiments ranged from eight to 12 per group.
Statistics
Experiment 1: manipulation of the timing of periadolescent exposure to testosterone
Mounting behavior of males treated with blank- or T-filled capsules before, during, and after adolescence was analyzed using a 2 × 3 two-way ANOVA (±T treatment × before, during, or after adolescent age of treatment), and significant differences were probed using Fisher’s protected least significant difference test. This same analysis was used to evaluate the plasma T levels during both the organizational 19-d treatment and the 7-d adult/activational T treatment. Intromissive behavior did not pass the test for normality (skewness ≤1.96), and 2 × 2 χ2 tests were conducted to determine whether the proportion of males intromitting the female differed between blank and T-treated males. χ2 tests were conducted separately for males receiving T treatments before, during, or after adolescence.
Experiment 2: juvenile vs. adult comparisons
Social behavior.
The behaviors of blank-treated juveniles, T-treated juveniles, and adult T-treated controls were compared. Planned comparisons were conducted as opposed to a single-factor ANOVA because the three groups do not represent varying levels of a single factor.
When behavioral data were not normally distributed, 2 × 2 χ2 analyses were conducted to determine whether the proportions of males displaying a behavior differed between juvenile (blank or T treated) and adult males.
Brain regional volumes.
A repeated-measures ANOVA determined that right and left hemispheres did not significantly differ for any brain region. Thus, hemispheres were summed and planned comparisons were carried out using unpaired t tests to determine regional volume differences between untreated juveniles, T-treated juveniles, and T-treated adult control males. Planned comparisons were conducted rather than a single-factor ANOVA because the three groups do not represent varying levels of a single factor.
Results
Experiment 1: manipulation of the timing of periadolescent exposure to testosterone
Mount number and latency, and proportion of males intromitting
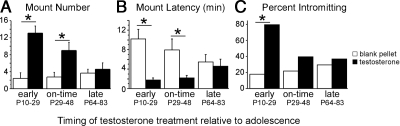
Treatment (T or blank capsule) and time (before, during, or after adolescence) interacted to influence adult mounting behavior in adulthood [Fig. 2A; F(2,52) = 5.70, P < 0.006]. T treatment before [F(2,52) = 29.65, P < 0.0001] and during adolescence [F(2,52) = 9.16, P < 0.01] significantly increased mount number relative to blank-treated males, whereas postadolescent T treatment did not [F(2,52) = 0.190, P > 0.05].
Figure 2.
Effects of periadolescent T exposure on adult reproductive behaviors. T treatments were designed to simulate early, on-time, and late pubertal development, and all behavior testing occurred in adulthood. Only pre- and midadolescent T treatments increased mounts (A) and decreased mount latencies (B) in response to T in adulthood. Adult intromissive behavior was increased only by preadolescent T treatments (C). These data suggest that early T treatments enhance behavioral responsiveness to T in adulthood. *, Significant difference (P < 0.05) between groups.
T treatment significantly reduced overall mount latencies [Fig. 2B; F(2,52) = 15.76, P < 0.0002], and a trend toward an interaction was also observed [F(1,52) = 3.03, P = 0.057]. T treatments reduced mount latencies when administered before [F(2,52) = 15.70, P < 0.001] and during adolescence [F(2,52) = 6.84, P < 0.02] but not after [F(2,52) = 0.15, P > 0.05].
Preadolescent T treatment significantly increased the proportion of males intromitting in adulthood [blank: two of 11; T: eight of 10; χ2 = 8.03, P < 0.005], whereas mid- [χ2 = 0.69, P > 0.05] and postadolescent [χ2 = 0.11, P > 0.05] treatments did not (Fig. 2C).
Behavior of sham-operated age-control groups and unmanipulated controls
One-way ANOVA was used to compare the behaviors of adult males sham-operated before, during, or after adolescence with unmanipulated controls that were not exposed to any surgical procedure. These four groups did not differ significantly in any behavior, indicating that the behavioral differences observed between the castrate groups described above are not attributable to age at the time of testing or surgical procedures [Table 1; mounts: F(3,38) = 0.890, P = 0.46; mount latency: F(3,38) = 1.9, P = 0.15; intromissions: F(3,38) = 1.91, P = 0.144; intromission latency: F(3,38) = 0.96, P = 0.423].
Table 1.
The behaviors of sham-operated age controls and unmanipulated control males
| Age at testing controls (d) | Mounts
|
lntromissions
|
||
|---|---|---|---|---|
| n | Latency (sec) | n | Latency (sec) | |
| Mean ± sem | Mean ± sem | Mean ± sem | Mean ± sem | |
| Prepubertal sham | 7.22 ±1.56 | 101.98 ± 42.39 | 17.67 ± 3.30 | 131.91 ± 39.54 |
| Adolescent sham | 7.92 ± 1.31 | 67.70 ± 15.65 | 24.92 ± 3.03 | 157.30 ± 26.02 |
| Adult sham | 8.36 ± 0.92 | 144.93 ± 35.47 | 20.00 ± 1.68 | 218.10 ± 45.72 |
| Unmanipulated | 10.20 ± 1.44 | 66.30 ± 9.36 | 24.70 ± 1.75 | 179.30 ± 34.82 |
No significant differences were found between these control groups.
Plasma T concentrations
Blood samples were taken at two experimental time points to determine: 1) the plasma concentrations of T produced by SILASTIC brand capsules implanted for 19 d before, during, or after adolescence and designed to simulate early, on-time, and delayed puberty; and 2) the plasma concentrations of T produced by SILASTIC brand capsules implanted for 7 d in adulthood and designed to probe activation of mating behavior. At the end of the 19-d treatment period, males administered T-filled capsules had significantly higher concentrations of T than males given blank-filled capsules [F(1,44)=19.52, P < 0.001]. No significant differences in plasma T concentrations were found across the three 19-d treatment periods before, during, or after adolescence [F(1,44)=1.66, P = 0.20], indicating that circulating levels of T were similar during the three organizational treatment times (supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org).
The 7-d activational T treatment received by all animals in adulthood yielded small but significant differences in plasma T concentrations among groups [F(2,52) = 8.62, P < 0.001]. Specifically, the 7-d capsule implanted in adulthood yielded higher T concentrations in the two groups that had received 19-d blank or T-filled capsules as juveniles, compared with the groups that had received blank or T-filled capsules during adolescence (P < 0.003) or in adulthood (P < 0.001; supplemental Table 1). However, for all six groups, mean plasma T concentrations were within the normal adult physiological range of 2–7 ng/ml (30,31).
Experiment 2: juvenile vs. adult comparisons
Reproductive behavior
Blank and T-treated juveniles were significantly less likely to mount the female than adult controls [Fig. 3; Juv+0 (one of nine) vs. adult control (12 of 12): χ2 = 17.23, P < 0.0001; Juv+T (five of 11) vs. adult control (12 of 12): χ2 = 8.86, P < 0.003]. The proportion of males mounting the female did not significantly differ between blank- and T-treated juvenile groups [Fig. 3; one of nine vs. five of 11, χ2 = 2.780, P = 0.10].
Figure 3.
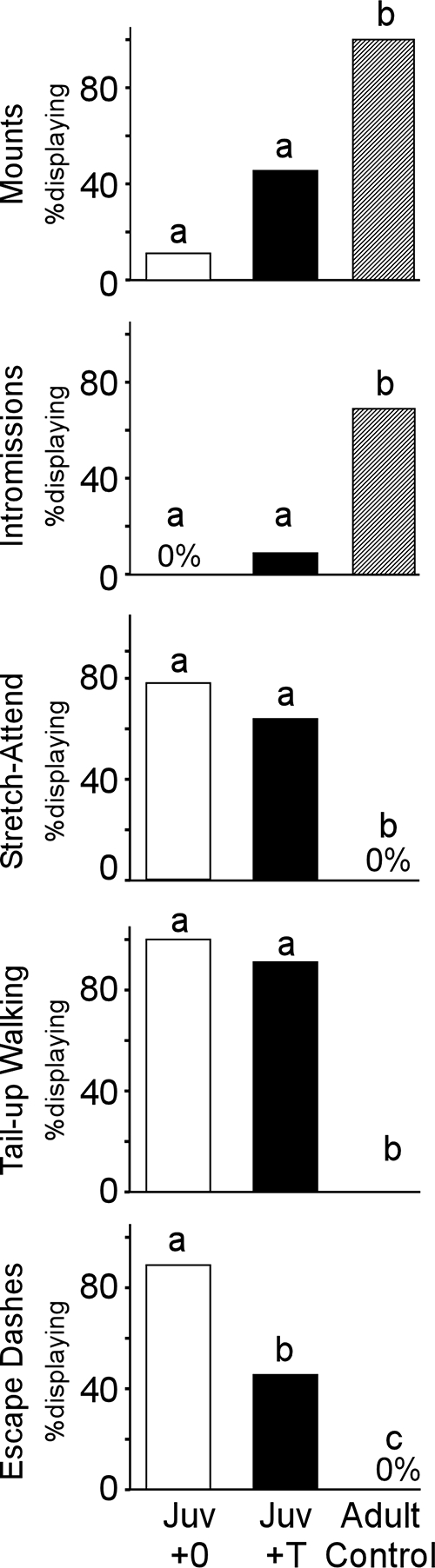
Juvenile (Juv) vs. adult behavioral responses to estrous females. Behavior testing occurred at 29 (juvenile) and 99 (adult) d of age, respectively. Preadolescent T treatment did not increase juvenile copulatory behavior to adult control levels. However, a significantly higher proportion of juvenile males displayed risk assessment (stretch attend) and submissive behaviors (tail up and escape dashes) than did adult males. Preadolescent T treatment also reduced the proportion of juvenile males displaying escape dashes. These data indicate that juveniles and adults display very different behavioral strategies during interactions with estrous females: adults engage in mating behavior, whereas juveniles engage in risk assessment and submissive behaviors. Bars sharing a letter do not differ significantly from one another.
Blank and T-treated juveniles were significantly less likely than adults to display intromissive behavior [Fig. 3; Juv+0 (none of nine) vs. adult control (nine of 12): χ2 = 11.81, P < 0.0006; Juv+T (one of 11) vs. adult control (nine of 12): χ2 = 10.15, P < 0.002], and the proportion intromitting did not differ between blank- and T-treated juvenile groups [Fig. 3; none of nine vs. one of 11; χ2 = 0.86, P > 0.05].
Risk assessment and submissive behaviors
Given the relative lack of sexual behaviors observed in juveniles, we quantified three additional behaviors displayed by these young animals during encounters with the receptive female. The proportion of males displaying stretch-attend, a behavior associated with risk assessment, was significantly higher in both blank- and T-treated juvenile groups than the adult control group [Fig. 3; Juv+0 (seven of nine) vs. adult control (0 of 12): χ2 = 14.00, P < 0.0002; Juv+T (seven of 11) vs. adult control (none of 12): χ2 = 11.00, P < 0.001]. However, the proportion of blank- and T-treated juveniles displaying stretch-attend did not significantly differ [Fig. 3; seven of nine vs. seven of 11, χ2 = 0.50, P > 0.05].
Both blank- and T-treated juvenile groups had a significantly higher proportion of males displaying tail-up walking behavior than the adult control group [Fig. 3; Juv+0 (nine of nine) vs. adult control (one of 12): χ2 = 17.33, P < 0.0001; Juv+T (10 of 11) vs. adult control (one of 12): χ2 = 15.7, P < 0.0001]. A similar proportion of blank- and T-treated juveniles displayed tail-up walking [Fig. 3; nine of nine vs. 10 of 11, χ2 = 0.86, P > 0.05].
Both blank and T-treated juvenile groups had a significantly higher proportion of males displaying escape dashes than the adult control group [Fig. 3; Juv+0 (eight of nine) vs. adult control (none of 12): χ2 = 17.23, P < 0.0001; Juv+T (five of 11) vs. adult control (none of 12): χ2 = 7.0, P < 0.01]. A significantly higher proportion of blank-treated juveniles displayed escape dashes than the T-treated juveniles [Fig. 3; eight of nine vs. five of 11, χ2 = 4.10, P < 0.05].
Brain regional volumes
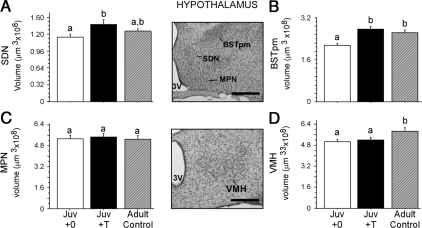
Preadolescent T treatment significantly increased the volume of the SDN in juveniles [Fig. 4A; t(1,19) = 2.25, P = 0.04] relative to blank-treated juveniles. SDN volumes of adult controls were intermediate to that of T-treated and blank-treated juveniles but not significantly different from either group [Juv+0 vs. adult: t(1,15) = 1.56, P > 0.05; Juv+T vs. adult: t(1,16) = 1.11, P > 0.05].
Figure 4.
Juvenile (Juv) vs. adult regional volumes within the hypothalamus. Brains were collected at 29 (juvenile) and 99 (adult) d of age, respectively. Preadolescent T treatment significantly increased the volumes of both the SDN (A) and BSTpm (B) in juvenile males but not the MPN (C). Preadolescent T treatment did not significantly influence VMH volume in juvenile males (D). However, adult controls had significantly greater volumes than juvenile males, suggesting that the VMH does not reach adult size until after adolescence. Bars sharing a letter do not differ significantly from one another. Middle panel, Photomicrographs of representative Nissl-stained coronal sections containing the hypothalamic brain regions traced. 3V, Third ventricle; OT, optic tract. Scale bar, 250 μm.
Preadolescent T treatment also significantly increased the volume of the BSTpm in juveniles relative to blank-treated juveniles [Fig. 4B; t(1,19) = 4.81, P = 0.0001], such that T-treated juveniles and adult controls did not differ in volume [t(1,16) = 0.89 P > 0.05], but volumes of both these groups were significantly larger than blank-treated juveniles [Juv+0 vs. adults: t(1,15) = 4.04, P = 0.001].
MPN volumes did not differ among the three groups [Fig. 4C; Juv+0 vs. Juv+T, t(1,19) = 0.29, P > 0.05; Juv+0 vs. adults, t(1,15) = 0.12, P > 0.05; Juv+T vs. adults, t(1,16) = 0.38, P > 0.05].
In the VMH, no significant differences between blank- and T-treated juveniles were observed [Fig. 4D; t (1,19) = 0.48, P > 0.05]. In contrast, the VMH of adult males was significantly larger than both blank- [t (1,15) = 2.77, P = 0.01] and T-treated juveniles [Fig. 4D; t (1,16) = 2.15, P = 0.046].
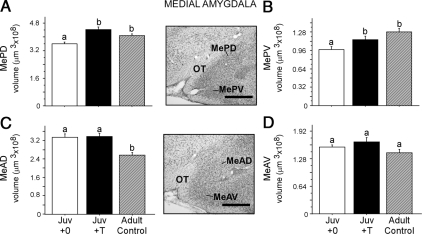
Preadolescent T treatment significantly increased the volume of both the MePD [Fig. 5A; t(1,19) = 4.20, P < 0.0006] and MePV of juveniles [Fig. 5B; t(1,19) = 2.14, P < 0.05], relative to blank-treated juveniles. T treatment increased volumes in the MePD and MePV such that no differences were observed between T-treated juveniles and adult controls [MePD: t(1,15) = 1.64, P > 0.05; MePV: t(1,15) = 1.62, P > 0.05], yet these groups had significantly greater volumes than blank-treated juveniles [MePD: Juv+0 vs. adult control, t(1,16) = 2.70, P < 0.02; MePV: Juv+0 vs. adult control, t(1,16) = 3.78, P < 0.002].
Figure 5.
Juvenile (Juv) vs. adult regional volumes within medial amygdalar brain regions. Brains were collected at 29 (juvenile) and 99 (adult) d of age, respectively. Preadolescent T treatment significantly increased the volume of subdivisions within the posterior medial amygdala (A and B) of juvenile males but not the anterior medial amygdala (C and D). However, age influenced regional volumes within the MeAD (C). Specifically, MeAD volumes were significantly larger in both blank- and T-treated juvenile groups than adult controls, suggesting that the MeAD does not reach adult size until after adolescence (C). Bars sharing a letter do not differ significantly from one another. Middle panel, Photomicrographs of representative Nissl-stained coronal sections containing the medial amygdalar brain regions traced. 3V, Third ventricle; OT, optic tract. Scale bar, 250 μm.
In contrast, preadolescent T treatment had no effect on volumes of the MeAD and MeAV subregions of the anterior medial amygdala in juveniles (Fig. 5, C and D; MeAD: t(1,19) = 0.21, P > 0.05; MeAV: t(1,19) = 1.16, P > 0.05]. However, age influenced regional volumes within the MeAD because volumes were significantly larger in both blank- [t(1,16) = 3.52, P < 0.003] and T-treated [t(1,15) = 4.42, P < 0.0005] juvenile groups than adult controls.
To summarize, preadolescent T treatment increased the size of the SDN, BSTpm, MePD, and MePV in juveniles relative to blank-treated juveniles. In contrast, preadolescent T treatment did not affect VMH or MeAD volume of juveniles, yet volumes of VMH and MeAD were, respectively, larger and smaller in adults relative to both groups of juveniles, suggesting that adolescent changes in these two structures may be independent of pubertal hormones.
Plasma T concentrations
Plasma T levels differed across the experimental groups [F(2,29) = 25.77, P < 0.0001]. T-treated juveniles and adult controls had similar plasma T concentrations [P = 0.97], and both displayed significantly higher T concentrations than blank-treated juveniles (Juv+0 vs. Juv+T, P < 0.0001; Juv+0 vs. adult controls, P < 0.0001; supplemental Table 1).
Discussion
These data support the hypothesis that adolescence marks the end of a single, protracted postnatal period of nervous system sensitivity to the organizing actions of T, which is likely an extension of the perinatal period of hormone-dependent sexual differentiation. When T exposure after P10 was restricted to a discrete 19-d treatment period either before, during, or after adolescence, both pre- and midadolescent T treatment, but not postadolescent T treatment, enhanced T-activated reproductive behavior later in adulthood, demonstrating that adolescence is not an isolated sensitive period for the organizing actions of T on adult reproductive behavior. Moreover, the potential for T to organize behavioral circuits appears to decrease across postnatal development because activation of behavior by T in adulthood was enhanced when exposure to T occurred earlier rather than later. Thus, the classical view of organizational and activational mechanisms of steroid action should be revised to incorporate an extended window of decreasing postnatal sensitivity to the organization of adult mating behavior by steroid hormones. Furthermore, these results have implications for the treatment of delayed puberty because they indicate that the later hormone therapy is begun, the less organizational influences hormone replacement will have on behavioral circuits.
Perinatal hormone secretions masculinize and defeminize neural circuits, but relatively few have investigated whether similar effects are possible before (32) or during adolescence (11,33,34,35). Furthermore, studies have not directly compared whether T exposure across the pre-, mid-, and postadolescent periods has differential effects on neural circuits underlying social behavior. Bloch and Mills (32) demonstrated that 15 d of juvenile T treatment masculinizes and defeminizes adult reproductive behavior in neonatally GDX male rats. The same hormone treatment also defeminizes adult lordosis behavior in females (36). Our data support their conclusion that organizing actions of T are possible well beyond the neonatal period and extend their findings by demonstrating that the window for organization by T closes in early adulthood. We note, however, that under normal developmental circumstances, nervous system organization by T is driven by marked elevations in endogenous testicular secretions during two distinct phases of life: the perinatal and adolescent periods.
Many other social behaviors have organizational links to the adolescent period (for review see Ref. 37), but whether these behaviors also exhibit an overall postnatal decline in sensitivity to the organizing actions of gonadal steroid hormones is unknown and likely depends on both the sex of the subject and the particular behavior (37). Thus, additional studies are needed that carefully control for the timing of steroid hormone exposure before, during, and after adolescence before adult behavioral assessment.
An intriguing quandary is why preadolescent T treatments facilitate the adult display of mating behavior, yet preadolescent T treatments fail to elicit mating behavior in juvenile males. The primary objective of experiment 2 was to address this question. Extending the duration of preadolescent T treatment to 19 d was no more successful in activating juvenile mating behavior than were previously used 7-d T treatments (13), suggesting that regardless of the duration of T treatment, juveniles are not capable of displaying adult-like mating behavior. In contrast, juveniles displayed very high levels of defensive behavior during testing, even though hormone-primed female Syrian hamsters assume a stationary lordosis posture during much of behavior testing (even in the absence of tactile stimuli) and therefore pose very little threat to males. Adult males, however, displayed little or no defensive behavior in the presence of the receptive female, even when their mating behavior was negligible. These data raise the possibility that female-elicited defensive behavioral displays may, at least in part, inhibit the ability of T to activate mating behavior in juveniles.
Adolescent changes in volume of brain regions mediating social behaviors may help to explain the transition from defensive to mating interactions with estrous females. Our aim was to distinguish brain regions capable of steroid-dependent organization before adolescence from those that continue to develop during adolescence. The MeAD and VMH differed significantly between juvenile and adult males, irrespective of T treatment, suggesting these regions may require additional maturation during adolescence to facilitate behavioral activation by T in adulthood. Specifically, both blank- and T-treated juveniles exhibited smaller VMH volumes and larger MeAD volumes than adults. Thus, age-dependent and possibly steroid-independent maturation of these regions occurs during adolescence. In addition, the MeAD and VMH are key regions within a neural circuit responsible for suppressing mating behavior in the face of threatening stimuli in mice (38). Thus, the differences in VMH and MeAD volumes found between juvenile and adult males coupled with the highly defensive behaviors only juveniles display toward estrous females suggest that development of the medial amygdala and VMH during adolescence may facilitate the appropriate assessment of estrous females as nonthreatening potential mates, but this possibility awaits further experimental verification.
In contrast to the MeA and VMH, preadolescent T treatment significantly increased volumes of the SDN, BST, MePD, and MePV in juveniles relative to blank-treated juveniles. These regions may be the neural targets for steroid-dependent organization of mating behavior across the pre- and midadolescent periods, as indicated by the behavioral results of experiment 1. Although we cannot exclude the possibility that the observed volume increases reflect transient activational rather than long-term organizational effects of T on regional volumes, these results clearly indicate that regional volumes of several brain areas important for mating are responsive to T before adolescence, even in the absence of behavioral responses to T at this age. Although the MePD and SDN are known for their plasticity in response to the presence or absence of gonadal hormones in adulthood (16,23,39,40), volumes of the BST and MePV are not as sensitive to fluctuations in adult gonadal hormones (16). Thus, the T-induced increases in regional volumes in juveniles likely reflect both long-term organizational and transient activational effects of T. Future studies will be aimed at determining whether the observed volume increases are due to increases in cell number, cell soma size, or changes in dendritic morphology.
The mechanism by which the postnatal sensitive period to organizational effects of T closes at the end of adolescence is unknown. However, if the sensitive period for exposure to T shares features common to other well-known sensitive periods, it may close as a consequence of circuit reorganization or consolidation during adolescence (41,42,43,44). Specifically, particular experiences occurring during adolescence (e.g. the presence or absence of T) may organize circuits in a fashion that render them resistant to further modification or change. For example, patterns of synaptic connectivity change across adolescence within the hamster MePD, a key region within the neural circuit underlying male reproductive behavior (17). Dendrites, spine densities, and spinophilin protein all decrease substantially across adolescence, concomitant with dramatic rises in gonadal steroid hormones (45). These changes in dendritic morphology may reflect organizational changes induced by T during the adolescent sensitive period. Indeed, decreased forebrain spine densities are the physiological manifestation of sexual imprinting in zebra finches (reviewed in Ref. 46). Furthermore, in the absence of imprinting stimuli, spine densities eventually decrease to the point at which subsequent imprinting stimuli can no longer influence spine densities, and the timing is contemporaneous with diminished behavioral sensitivity to imprinting stimuli (42,47). Whether time-dependent reductions in MePD dendritic branches and spine densities reflect both the organizational influence of T and the closing of the sensitive period remains to be determined, and experiments testing these possibilities are underway.
Human adolescents exhibit substantial individual variability in pubertal onset (48,49), and extremes in pubertal timing (early or late) have been associated with a range of human psychopathologies such as depression (4,10,50,51), anxiety (5,8), conduct disorder (52,53), and increased alcohol and tobacco use (54,55). Although these studies demonstrate that pubertal timing influences adolescent and adult psychopathology, many questions remain regarding the underlying mechanisms. For example, it is not clear whether the perception of undergoing puberty earlier or later than peers is more important than an individual’s actual pubertal status in predicting later psychopathology or whether mistimed direct hormonal influences on the brain also influence behavioral psychopathology. To our knowledge, this is the first demonstration in an animal model of how variations in pubertal timing during adolescence (i.e. when hormone exposure occurs relative to adolescent brain development) program variations in adult brain morphology and behavior. Although this research did not directly model a psychiatric disease state, the finding that adult behaviors vary, depending on the timing of hormone exposure across the adolescent period, speaks to the possibility that direct hormone-brain interactions moderate the effects of pubertal timing on many behaviors, even in humans. However, the effects of gonadal hormones in humans on the developing brain may also be modulated by social and environmental factors that change dramatically across the pre-, mid-, and postadolescent periods (10,56). Additional research using animal models is needed to elucidate how gonadal hormones and an individual’s unique experience impact the developing adolescent brain to drive individual differences in behavior.
Supplementary Material
Acknowledgments
We thank Sara Hunter, Pamela Montalto, Andrew Poole, and Elizabeth Hingst for their valuable assistance with surgeries, behavior testing, and general animal care. We also thank Lisa Rogers for tissue sectioning and Jane Venier and Lisa Rogers for tissue histology. Thanks also go to Jane Venier for assistance with RIAs. A special thank you goes to Heather Molenda-Figueira for helpful feedback on early manuscript drafts. A portion of manuscript editing was done as part of Julia Zehr’s official duties as a federal government employee.
Footnotes
This work was supported by National Institutes of Health Grants R01-MH068764 (to C.L.S.), F31-MH070125 (to K.M.S.), and F32-MH068975 (to J.L.Z.) and the American Psychological Association Diversity in Neuroscience Program (to K.Y.S.-R.).
Disclosure summary: The authors have nothing to disclose. The views expressed in this article do not necessarily represent the views of the National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services, or the United States government.
First Published Online May 7, 2009
Abbreviations: BSTpm, Posterior medial bed nucleus of the stria terminalis; GDX, gonadectomy; MeAD, medial anterodorsal; MeAV, medial anteroventral; MePD, medial posterodorsal; MePV, medial posteroventral; MPN, medial preoptic nucleus; P, postnatal day; SDN, sexually dimorphic nucleus; T, testosterone; VMH, ventromedial hypothalamus.
References
- Phoenix CH, Goy RW, Gerall AA, Young WC 1959 Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382 [DOI] [PubMed] [Google Scholar]
- McCabe MP, Ricciardelli LA 2004 Body image dissatisfaction among males across the lifespan—a review of past literature. J Psychosom Res 56:675–685 [DOI] [PubMed] [Google Scholar]
- Ge XJ, Brody GH, Conger RD, Simons RL 2006 Pubertal maturation and African American children’s internalizing and externalizing symptoms. J Youth Adolesc 35:531–540 [Google Scholar]
- Ge XJ, Kim IJ, Brody GH, Conger RD, Simons RL, Gibbons FX, Cutrona CE 2003 It’s about timing and change: pubertal transition effects on symptoms of major depression among African American youths. Dev Psychol 39:430–439 [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Marttunen M, Rantanen P, Rimpelä M 2003 Early puberty is associated with mental health problems in middle adolescence. Soc Sci Med 57:1055–1064 [DOI] [PubMed] [Google Scholar]
- Laitinen-Krispijn S, Van der Ende J, Hazebroek-Kampschreur AA, Verhulst FC 1999 Pubertal maturation and the development of behavioural and emotional problems in early adolescence. Acta Psychiatr Scand 99:16–25 [DOI] [PubMed] [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J 1997 Is psychopathology associated with the timing of pubertal development? J Am Acad Child Adolesc Psychiatry 36:1768–1776 [DOI] [PubMed] [Google Scholar]
- Zehr JL, Culbert KM, Sisk CL, Klump KL 2007 An association of early puberty with disordered eating and anxiety in a population of undergraduate women and men. Horm Behav 52:427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardelli LA, McCabe MP 2004 A biopsychosocial model of disordered eating and the pursuit of muscularity in adolescent boys. Psychological Bulletin 130:179–205 [DOI] [PubMed] [Google Scholar]
- Conley CS, Rudolph KD 2009 The emerging sex difference in adolescent depression: Interacting contributions of puberty and peer stress. Development and Psychopathology 21:593–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL 2004 Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav 45:242–249 [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL 2006 Pubertal hormones, the adolescent brain, and the maturation of social behaviors: lessons from the Syrian hamster. Mol Cell Endocrinol 254–255:120–126 [DOI] [PubMed] [Google Scholar]
- Meek LR, Romeo RD, Novak CM, Sisk CL 1997 Actions of testosterone in prepubertal and postpubertal male hamsters: dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Horm Behav 31:75–88 [DOI] [PubMed] [Google Scholar]
- Romeo RD, Wagner CK, Jansen HT, Diedrich SL, Sisk CL 2002 Estradiol induces hypothalamic progesterone receptors but does not activate mating behavior in male hamsters (Mesocricetus auratus) before puberty. Behav Neurosci 116:198–205 [DOI] [PubMed] [Google Scholar]
- Sisk CL, Schulz KM, Zehr JL 2003 Puberty: a finishing school for male social behavior. Ann NY Acad Sci 1007:189–198 [DOI] [PubMed] [Google Scholar]
- Romeo RD, Sisk CL 2001 Pubertal and seasonal plasticity in the amygdala. Brain Res 889:71–77 [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW 1995 Integration of chemosensory and hormonal cues is essential for mating in the male Syrian hamster. J Neurosci 15:7261–7269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Newman SW 1995 The medial amygdaloid nucleus and medial preoptic area mediate steroidal control of sexual behavior in the male Syrian hamster. Horm Behav 29:338–353 [DOI] [PubMed] [Google Scholar]
- Romeo RD, Diedrich SL, Sisk CL 2000 Effects of gonadal steroids during pubertal development on androgen and estrogen receptor-α immunoreactivity in the hypothalamus and amygdala. J Neurobiol 44:361–368 [PubMed] [Google Scholar]
- Joppa MA, Meisel RL, Garber MA 1995 C-fos expression in female hamster brain following sexual and aggressive behaviors. Neuroscience 68:783–792 [DOI] [PubMed] [Google Scholar]
- Robertson GS, Pfaus JG, Atkinson LJ, Matsumura H, Phillips AG, Fibiger HC 1991 Sexual behavior increases c-fos expression in the forebrain of the male rat. Brain Res 564:352–357 [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, King ZA, Northcutt KV, Breedlove SM 2008 Sexual dimorphism and steroid responsiveness of the posterodorsal medial amygdala in adult mice. Brain Res 1190:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Breedlove SM, Jordan CL 2003 Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav 43:336–346 [DOI] [PubMed] [Google Scholar]
- Cooke BM, Stokas MR, Woolley CS 2007 Morphological sex differences and laterality in the prepubertal medial amygdala. J Comp Neurol 501:904–915 [DOI] [PubMed] [Google Scholar]
- Bleier R, Byne W, Siggelkow I 1982 Cytoarchitectonic sexual dimorphisms of the medial preoptic and anterior hypothalamic areas in guinea-pig, rat, hamster, and mouse. J Comp Neurol 212:118–130 [DOI] [PubMed] [Google Scholar]
- Wang J, Swann JM 2006 The magnocellular medial preoptic nucleus I. Sources of afferent input. Neuroscience 141:1437–1456 [DOI] [PubMed] [Google Scholar]
- Morin LP, Wood RI 2001 A stereotaxic atlas of the golden hamster brain. San Diego: Academic Press [Google Scholar]
- Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL 2006 Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Horm Behav 50:477–483 [DOI] [PubMed] [Google Scholar]
- Albers HE, Huhman KL, Meisel RL 2002 Hormonal basis of social conflict and communication. In: Pfaff D, ed. Hormones, brain and behavior. San Diego: Academic Press; 393–433 [Google Scholar]
- Sisk CL, Turek FW 1983 Developmental time course of pubertal and photoperiodic changes in testosterone negative feedback on gonadotropin secretion in the golden hamster. Endocrinology 112:1208–1216 [DOI] [PubMed] [Google Scholar]
- Vomachka AJ, Greenwald GS 1979 The development of gonadotropin and steroid hormone patterns in male and female hamsters from birth to puberty. Endocrinology 105:960–966 [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Mills R 1995 Prepubertal testosterone treatment of neonatally gonadectomized male rats: defeminization and masculinization of behavioral and endocrine function in adulthood. Neurosci Biobehav Rev 19:187–200 [DOI] [PubMed] [Google Scholar]
- Edwards DA 1970 Post-neonatal androgenization and adult aggressive behavior in female mice. Physiol Behav 5:465–467 [DOI] [PubMed] [Google Scholar]
- Costantini RM, Park JH, Beery AK, Paul MJ, Ko JJ, Zucker I 2007 Post-castration retention of reproductive behavior and olfactory preferences in male Siberian hamsters: role of prior experience. Horm Behav 51:149–155 [DOI] [PubMed] [Google Scholar]
- Ford JJ 1990 Differentiation of sexual behaviour in pigs. J Reprod Fertil Suppl 40:311–321 [PubMed] [Google Scholar]
- Bloch GJ, Mills R, Gale S 1995 Prepubertal testosterone treatment of female rats: defeminization of behavioral and endocrine function in adulthood. Neurosci Biobehav Rev 19:177–186 [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HN, Sisk CL 2009 Back to the future: the organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav 55:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ 2005 Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron 46:647–660 [DOI] [PubMed] [Google Scholar]
- Davis EC, Shryne JE, Gorski RA 1995 A revised critical period for the sexual differentiation of the sexually dimorphic nucleus of the preoptic area in the rat. Neuroendocrinology 62:579–585 [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM 1999 A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci USA 96:7538–7540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK 2004 Critical period regulation. Annu Rev Neurosci 27:549–579 [DOI] [PubMed] [Google Scholar]
- Bischof HJ 2007 Behavioral and neuronal aspects of developmental sensitive periods. Neuroreport 18:461–465 [DOI] [PubMed] [Google Scholar]
- Knudsen EI 2004 Sensitive periods in the development of the brain and behavior. J Cogn Neurosci 16:1412–1425 [DOI] [PubMed] [Google Scholar]
- Hensch TK 2003 Controlling the critical period. Neurosci Res 47:17–22 [DOI] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL 2006 Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol 66:578–590 [DOI] [PubMed] [Google Scholar]
- Bischof HJ, Rollenhagen A 1999 Behavioural and neurophysiological aspects of sexual imprinting in zebra finches. Behav Brain Res 98:267–276 [DOI] [PubMed] [Google Scholar]
- Bischof HJ 2003 Neural mechanisms of sexual imprinting. Anim Biol 53:89–112 [Google Scholar]
- Dubas JS 1991 Cognitive abilities and physical maturation. In: Petersen AC, Brooks-Gunn J, eds. Encyclopedia of adolescence. New York: Garland Publishing; 133–138 [Google Scholar]
- Tanner J 1962 Growth at adolescence. Oxford, UK: Blackwell Scientific [Google Scholar]
- Michaud PA, Suris JC, Deppen A 2006 Gender-related psychological and behavioural correlates of pubertal timing in a national sample of Swiss adolescents. Mol Cell Endocrinol 254:172–178 [DOI] [PubMed] [Google Scholar]
- Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM 2004 Is pubertal timing associated with psychopathology in young adulthood? J Am Acad Child Adolesc Psychiatry 43:718–726 [DOI] [PubMed] [Google Scholar]
- Burt SA, McGue M, DeMarte JA, Krueger RF, Iacono WG 2006 Timing of menarche and the origins of conduct disorder. Arch Gen Psychiatry 63:890–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio M, Karnik NS, Steiner H 2006 Early maturation as a risk factor for aggression and delinquency in adolescent girls: a review. Int J Clin Pract 60:1254–1262 [DOI] [PubMed] [Google Scholar]
- Bratberg GH, Nilsen TI, Holmen TL, Vatten LJ 2007 Perceived pubertal timing, pubertal status and the prevalence of alcohol drinking and cigarette smoking in early and late adolescence: a population based study of 8950 Norwegian boys and girls. Acta Paediatr 96:292–295 [DOI] [PubMed] [Google Scholar]
- Biehl MC, Natsuaki MN, Ge XJ 2007 The influence of pubertal timing on alcohol use and heavy drinking trajectories. J Youth Adolesc 36:153–167 [Google Scholar]
- Spear L 2000 Modeling adolescent development and alcohol use in animals. Alcohol Res Health 24:115–123 [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL 2005 Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 26:163–174 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.