Abstract
20-Epi-1,25-dihydroxyvitamin D3 (20-epi-1,25(OH)2D3) is a vitamin D analog that exhibits unique biologic properties. The mechanism(s) responsible for these activities remains unclear. Here we explore the ability of 20-epi-1,25(OH)2D3 to induce calcemic responses in mice in vivo and identify a potential mechanism. Surprisingly, the levels of calcemia induced at 24 h after single injections of equivalent doses of 1,25(OH)2D3 or 20-epi-1,25(OH)2D3 were similar, suggesting that both compounds were equal in both potency and efficacy. This similarity was also observed at genes involved in calcium homeostasis including, S100g (calbindin D9K), Trpv6, Cldn2 (claudin 2), Trpv5, and Tnfsf11 (Rankl) as well as Cyp24a1. Despite this, the activities of the two compounds at 48 h were strikingly different. Thus, whereas the activity of 1,25-dihydroxyvitamin D3 declined at this time point, the response to 20-epi-1,25(OH)2D3 was increased. This unique profile was not due to an exaggerated induction of calcium regulating genes in the intestine, kidney, or bone but to a sustained action on these genes in the intestine. This conclusion was supported by studies using in vivo chromatin immunoprecipitation analysis, which revealed a prolonged presence of vitamin D receptor and RNA polymerase II at the Trpv6 and Cyp24a1 promoters and a sustained increase in histone 4 acetylation in these gene regions as well. We conclude that 20-epi-1,25(OH)2D3 displays superagonist properties largely as a result of its duration of action in the intestine. This action is likely due to a decrease in the rate of intestinal-specific degradation of the ligand rather than to an increase in the functional stability of the vitamin D receptor.
The superagonist activity of 20-epi-1,25-dihydroxyvitamin D3 in vivo is not due to increased potency but rather to its prolonged biological actions in the intestine.
The biological actions of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) (1) are mediated by the vitamin D receptor (VDR), a ligand-activated transcription factor that functions as an retinoid X receptor (RXR) heterodimer to modulate the expression of specific target genes (1,2,3). Like other members of its class, the VDR binds directly to unique DNA sequence elements located within spatial proximity of the promoters for these genes and facilitates the recruitment of complex molecular machines that are essential for changing levels of transcription (4,5). Complexes known to be essential for the downstream activity of the VDR/RXR complex include the switch/sucrose nonfermentable (SWI/SNF) remodeling complexes, members of the steroid receptor coactivator family of acetyltransferases, cAMP response element-binding protein/p300 integrator complexes, components of the mediator complex and likely additional complexes as well (6,7,8). Although the molecular determinants of interaction between the VDR and these coregulator complexes have been identified, the processes that govern selectivity at individual target genes and the temporal nature of those interactions remain to be clarified.
Genes that are integral to the maintenance of systemic calcium and phosphorus homeostasis represent primary regulatory targets of the vitamin D hormone and its receptor (2). Accordingly, the products of these genes participate in the absorption of calcium from the gut lumen, the reabsorption of calcium from the renal tubular lumen, and the resorption of calcium from the skeleton (5,9,10). As a result, aberrant production of 1,25(OH)2D3 lies at the heart of a wide variety of diseases of calcium imbalance. 1,25(OH)2D3 also manifests a number of less well known biological activities including actions on cell proliferation and differentiation and cell survival and apoptotic cell death (11,12,13). At least some of these activities may be mediated via a rapid action of the ligand at the cellular membrane, in which it can trigger activation of one or more signal transduction pathways that are capable of directing key cellular responses (14,15,16). Regardless, many of the biological processes noted above are integral to 1,25(OH)2D3’s activity in skin, endothelial cells, normal cells of the immune system, and tumor cells. Thus, a potential therapeutic role for 1,25(OH)2D3 in psoriasis, arterial calcification, autoimmune disease, and cancer has emerged (2,17).
The capacity of 1,25(OH)2D3 to regulate cell growth, differentiation, and survival and maintain skeletal integrity and calcium homeostasis has prompted the development of a diverse set of analogs of vitamin D with the objective to improve potency (EC50 value), efficacy [level of maximal response relative to 1,25(OH)2D3], and/or selectivity (differential potency and/or efficacy as a function of a specific tissue) (18). Surprisingly, many of these analogs display unique blends of the above three properties. Although the mechanisms that underlie differences in analog action are generally unknown, a number of possibilities have been explored. Fundamental to these potential mechanisms is the possibility that novel ligands might promote unique VDR conformations that could result in altered biological activity (19,20,21,22), either as a result of changes in the stability of the active VDR complex at its target site or as a result of changes in the ability of the VDR to recruitment coregulators. Novel changes in activity could also be due to differences in analog susceptibility to unusual metabolic activation or degradation (23). This particular feature would be especially relevant in vivo. With several exceptions, however, the molecular mechanisms responsible for the altered biological effects of most synthetic vitamin D ligands are unknown.
20-Epi-1,25(OH)2D3 (also known as MC1288) is an especially interesting analog because its only unique feature relative to 1,25(OH)2D3 is its stereochemistry at carbon 20 (24). The primary consequence of this alteration is an apparent increase in the relative potency of the ligand in vitro and an increase in its ability to induce hypercalcemia in vivo (24,25). These features of 20-epi-1,25(OH)2D3 have prompted its characterization as a superagonist. Importantly, however, the increased potency manifested by 20-epi-1,25(OH)2D3 relative to 1,25(OH)2D3 is not due to a significant difference in its affinity for the VDR because the affinity constant for both ligands are similar (23). At the molecular level, it has been suggested that 20-epi-1,25(OH)2D3 promotes a unique VDR conformation that permits interaction with RXR at reduced ligand concentrations (26). 20-Epi-1,25(OH)2D3 is also reported to enhance the interaction of the VDR with mediator-1 (thyroid receptor-associated protein-220/vitamin D receptor-associated interacting protein-205) (22). Unfortunately, whereas these 20-epi-1,25(OH)2D3-dependent activities of the VDR may be due to an altered receptor conformation, the crystal structure of the VDR ligand binding domain in association with 20-epi-1,25(OH)2D3 does not appear to be significantly different from that observed with 1,25(OH)2D3 (27,28). Perhaps more importantly, none of the ligand-specific effects observed with 20-epi-1,25(OH)2D3 in vitro have ever been linked directly to a unique biological outcome. These results suggest that as with many other vitamin D analogs, the mechanism through which 20-epi-1,25(OH)2D3 manifests its superagonist actions in vivo requires further clarification.
In the present study, we explored the molecular actions of 20-epi-1,25(OH)2D3 in vivo after a single injection of the analog into mice. We found that the activity of 20-epi-1,25(OH)2D3 is not due to a striking increase in either potency or efficacy but rather to a sustained and selective action via the VDR on intestinal calcium-regulating genes. We concluded that whereas these actions of 20-epi-1,25(OH)2D3 may involve a unique activity at the VDR, they are more likely due to a decreased rate of degradation of the ligand or an active byproduct in the intestine.
Materials and Methods
Reagents
1,25(OH)2D3 was obtained from Solvay (da Weesp, The Netherlands). 20-Epi-1,25(OH)2D3 (MC1288) was kindly provided by Dr. Lise Binderup (Leo Pharma, Inc., Ballerup, Denmark). The structures of both compounds are documented in supplementary Fig. 1A. MEM was purchased from Cellgro (Herndon, VA). Oligonucleotide primers were obtained from IDT (Coralville, IA). Anti-VDR (C-20, sc-1008) antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-acetyl lysine histone 4 (H4; tetra, 06-866) antibody was obtained from Upstate (Charlottesville, VA). Anti-RNA polymerase II (RNA pol II; 8WG16) was obtained from Covance Research Products (Dover, PA). Lipofectamine Plus was obtained from Invitrogen (Carlsbad, CA). Tritiated 1,25(OH)2D3 (166 Ci/mmol) was purchased from PerkinElmer (Boston, MA).
Plasmids
The pCH110-β-galactosidase (βgal) reporter plasmid was previously described (29). Construction of human transient receptor potential, vanilloid (TRPV)-6 (−7 kb/+160), rat Cyp24-luc, human Cyp24a1(−6 kb/+454), and mouse TRPV6 (−4.1 kb/+213) were also documented earlier (29,30,31). All inserts were sequenced for verification.
Tritiated ligand binding assay
Human VDR (hVDR) was produced in BL21 codon Plus RIL cells (Strategene, La Jolla, CA) using the bacterial expression vector pET-hVDR (29). Soluble full-length hVDR was purified to homogeneity using sequential NiNTA and SP-Sepharose column chromatography as previously reported (29). Ligand binding was assessed as previously outlined by incubating hVDR protein (2 ng) with 0.2 nm tritiated 1,25(OH)2D3 (166 Ci/mmol) and increasing molar concentrations of either radioinert 1,25(OH)2D3 or 20-epi-1,25(OH)2D3 for 16 h at 4 C in triplicate assays (32). The separation of bound from free hormone was achieved using hydroxylapatite and quantitated by liquid scintillation spectrophotometry.
Cell culture
LS180 cells were cultured in MEM supplemented with 10% nonheat inactivated fetal bovine serum, 1% nonessential amino acids, 1% sodium pyruvate, and 1% penicillin/streptomycin (GIBCO BRL-Life Technologies, Rockville, MD).
Transfection analysis
LS180 cells were seeded into 24-well plates by dilution and transfected using Lipofectamine Plus (Invitrogen) as described by the manufacturer. Individual wells received 300 ng of DNA comprised of 250 ng of specific promoter reporter plasmid and 50 ng pCH110-βgal. After transfection, the cells were cultured in medium supplemented with 10% fetal bovine serum with or without vitamin D ligand. Cells were harvested 24 h after stimulation and the lysates assayed for luciferase and β-gal activities as described (29). Luciferase activity was normalized in all cases using β-gal activity.
Animals
All mice were obtained from Harlan (Madison, WI) and maintained in the Biochemistry Animal Care facility at the University of Wisconsin-Madison. Mice were maintained on a standard purified diet (AIN-76A) obtained from Research Diets (New Brunswick, NJ) or transiently on a vitamin D3- and calcium-deficient diet containing strontium (T.06198) obtained from Harlan Teklad (Madison, WI). This latter manipulation modestly reduced circulating levels of 1,25(OH)2D3 (<10%) and calcium (<1 mg/dl) and facilitated measurements of TRPV5 and TRPV6 mRNA induction. We used the latter diet for all of the in vivo studies wherein we examined the effects of 1,25(OH)2D3 and 20-epi-1,25(OH)2D3 on mRNA production and VDR location. All animal experiments were conducted according to protocols on file with the University of Wisconsin-Madison Research Animal Resource Center and approved by the Institutional Animal Care and Use Committee of this institution.
Serum calcium
C57BL6 wild-type female mice, 8–9 wk of age, were fed diet AIN-76A for 7 d. Mice (five per group) were treated by ip injection with either vehicle or doses of increasing concentrations of vitamin D ligand in propylene glycol (Gallipot, St. Paul, MN). Mice were anesthetized at 0, 24, and 48 h and subjected to a retroorbital bleed. Blood samples were allowed to clot at room temperature and the serum recovered after a brief centrifugation and stored at −20 C before analysis. Serum calcium levels were determined using flame atomic absorption spectrophotometry (model 3110; PerkinElmer, Norwalk, CT).
RNA isolation and analysis
C57BL6 wild-type female mice, 8–9 wk of age, were fed diet T.06198 for 7 d. Mice were treated by ip injection with either vehicle or vitamin D ligand (four or five per group) in propylene glycol (Gallipot) for the indicated time period. Animals were anesthetized with ether and 2 cm of proximal duodenum, one kidney, and the calvaria were isolated and snap frozen in TRI reagent (MRC, Cincinnati, OH). Total RNA was isolated via the TRI reagent protocol and 2 μg of the product were reverse transcribed using a SuperScript III H reverse transcriptase kit (Invitrogen). The cDNA was subjected to amplification using standard methods [see also chromatin immunoprecipitation (ChIP) analysis] (31). Total RNA was also isolated from cells in culture and analyzed using similar techniques. Primer sequences are available on request.
In vivo ChIP assays
ChIP was performed as described previously (29,30,31,33,34) with several modifications. C57BL6 wild-type female mice, 8–9 wk of age, were treated by ip injection with either vehicle or the indicated concentrations of vitamin D ligand per gram body weight (bw) (four or five per group) as described above. Seven centimeters of proximal duodenum were isolated, rinsed with cold 1× PBS, and subjected to a cross-linking reaction for 10 min with 1.5% formaldehyde. After the fixative was neutralized for 5 min with glycine (0.125 m final concentration), the intestinal tissue was homogenized briefly in PBS using a loose-fitting Dounce pestle and the cells collected via 70 μm nylon cell strainers (BD Falcon, Bedford, MA). Cells were then subjected to ChIP analysis as described previously (31). DNA fragments were purified from chromatin using QIAquick spin kits (QIAGEN, Valencia, CA) and the precipitated DNA then evaluated by either traditional PCR or a quantitative real-time PCR (qPCR) method using primers designed to amplify individual fragments of the target gene promoters of interest. DNA acquired before precipitation was used as input DNA. qPCR was accomplished using 7500 Fast Real-Time PCR instrument and Power SYBR Green master mix (Applied Biosystems, Foster City, CA). Primer sequences are available upon request. All samples were quantitated using an external standard curve and corrected for input variations.
Statistical analyses
Unpaired t tests and EC50 calculations (as determined by nonlinear regression analysis with sigmoidal dose response curves) were performed using the PRISM version 4 statistical software package from GraphPad Software Inc. (San Diego, CA). Statistical analyses of the differences noted at the mRNA level between 1,25(OH)2D3 and 20-epi-1,25(OH)2D3 were assessed in preliminary analysis using ANOVA (P < 0.05) and then using t tests at P < 0.05.
Results
1,25(OH)2D3 and 20-epi-1,25(OH)2D3 display equivalent inhibitory constants (Kis) for the VDR yet strikingly different biological EC50s in intestinal cells
As a prelude to our in vivo studies, we first determined the biochemical Kis of the VDR for both 1,25(OH)2D3 and 20-epi-1,25(OH)2D3 and then contrasted these measurements with that of biological activity in cultured cells. Supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org, documents Ki determinations for both compounds using purified VDR, radiolabeled 1,25(OH)2D3 and increasing concentrations of radioinert 1,25(OH)2D3 or 20-epi-1,25(OH)2D3. As can be seen, both ligands display high affinity for the VDR, with Kis that differ by only severalfold [1,25(OH)2D3 = 3.03 × 10−10 m; 20-epi-1,25(OH)2D3 = 7.90 × 10−10 m]. As previously observed, however, 20-epi-1,25(OH)2D3 was biologically more potent than 1,25(OH)2D3. Thus, as seen in Fig. 1, 10- to 100-fold lower concentrations of 20-epi-1,25(OH)2D3 were necessary compared with 1,25(OH)2D3 for full induction of CYP24A1 and TRPV6 mRNAs in intestinal LS180 cells and for maximal transcriptional induction from transfected promoters for these genes. These properties of 20-epi-1,25(OH)2D3 are consistent with those made previously.
Figure 1.
20-Epi-1,25(OH)2D3 demonstrates increased potency but not efficacy at the CYP24A1 and TRPV6 target genes in vitro. A, Induction of CYP24A1 and TRPV6 gene expression by 1,25(OH)2D3 or 20-epi-1,25(OH)2D3 in vitro. Human LS180 cells were treated in triplicate with increasing concentrations of either vehicle (V), 1,25(OH)2D3 or 20-epi-1,25(OH)2D3. Total RNA was collected after 6 h, reverse transcribed, and evaluated by qPCR using primers to human CYP24A1 and TRPV6 transcripts. Each point represents the fold change (vehicle set to 1) of the average unit/β-actin ± se and is representative of at least three independent experiments. B, Induction of transfected CYP24A1 and TRPV6 promoter-luciferase reporter genes by 1,25(OH)2D3 or 20-epi-1,25(OH)2D3 in vitro. Rat Cyp24a1-LUC or human CYP24A1-LUC (−6 kb/+454) reporter plasmids (left panels) and mouse TRPV6 (−4.1 kb/+213) or human TRPV6 (−7 kb/+160) reporter plasmids (right panels) were cotransfected into LS180 cells with a β-galactosidase normalization plasmid and then treated with vehicle or increasing concentrations of either 1,25(OH)2D3 or 20-epi-1,25(OH)2D3 for 24 h. Samples were then harvested and evaluated for luciferase activity and normalized to levels of β-gal. Each point represents the fold change (vehicle set to 1) of normalized light unit average ± se and is representative of at least three independent experiments. C, Linear regression analysis of the data depicted in A and B.
20-Epi-1,25(OH)2D3 stimulates an enhanced hypercalcemic response in comparison with 1,25(OH)2D3
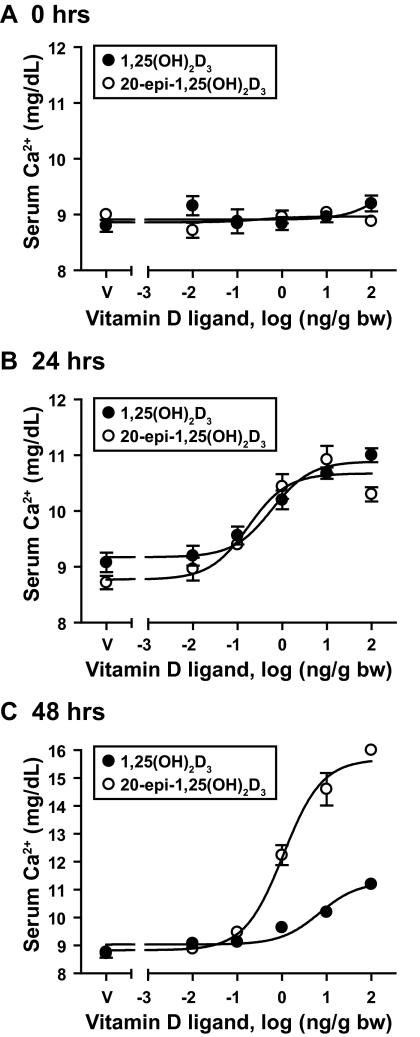
To explore whether the above feature of 20-epi-1,25(OH)2D3 contributed to or was indeed responsible for the hypercalcemic activity of this compound in vivo, we treated C57BL6 mice with increasing concentrations of vitamin D ligand (0–100 ng/g bw) and assessed levels of serum calcium after treatment as a function of both dose and time. Blood samples were collected at 0, 24, and 48 h after injection as outlined in Materials and Methods and analyzed using atomic absorption spectrophotometry. Both 1,25(OH)2D3 and 20-epi-1,25(OH)2D3 induced a similar dose-dependent rise in serum calcium at 24 h (Fig. 2, A and B), suggesting that the two compounds were equipotent. At 48 h, however, the results were much different. Thus, whereas calcemic activity in response to 1,25(OH)2D3 was beginning to diminish at the time point, activity had increased in response to 20-epi-1,25(OH)2D3 (Fig. 2C). These results suggest that in contrast to our observations in vitro, 20-epi-1,25(OH)2D3 was not more potent than 1,25(OH)2D3 but rather manifested a prolonged and perhaps enhanced accumulative action in vivo.
Figure 2.
1,25(OH)2D3 and 20-epi-1,25(OH)2D3 induce serum calcium levels in normal mice. C57BL6 mice were treated with increasing concentrations of 1,25(OH)2D3 or 20-epi-1,25(OH)2D3 (0–100 ng/g bw) and blood samples collected at 0 (A), 24 (B), and 48 (C) h after injection. Serum calcium levels were determined using flame atomic absorption spectrophotometry. Each point represents the average ± se, n = 5. Statistical analyses for each of the groups is as follows: EC50 = 0.59, R2 = 0.83 for 1,25(OH)2D3 and EC50 = 0.17, R2 = 0.80 for 20-epi-1,25(OH)2D3 at 24 h. The EC50 is 6.88 and R2 is 0.86 for 1,25(OH)2D3, and the EC50 is 1.08 and R2 is 0.95 for 20-epi-1,25(OH)2D3 at 48 h. These data are representative of several similar analyses.
20-Epi-1,25(OH)2D3 and 1,25(OH)2D3 induce the expression of vitamin D target genes with similar potency
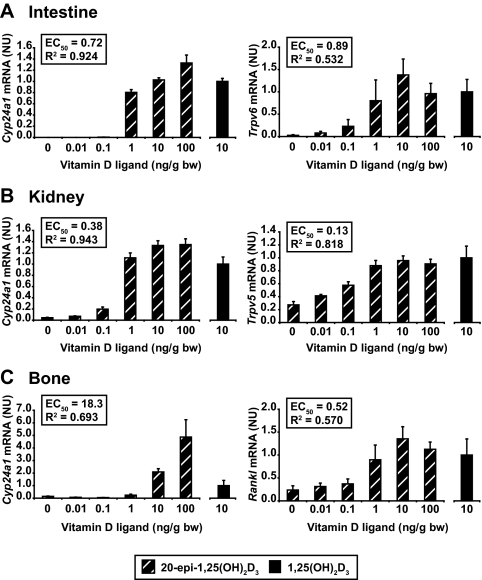
To explore the underlying mechanism behind the enhanced hypercalcemic activity of 20-epi-1,25(OH)2D3, we next assessed the ability of the analog to induce vitamin D target gene expression after a single injection of vitamin D ligand into mice. An initial experiment indicated that the maximal single dose for 1,25(OH)2D3 was 10 ng/g bw on nearly all the target genes that were analyzed (as illustrated in supplemental Fig. S2). Accordingly, C57BL6 wild-type mice were treated for 6 h with increasing concentrations of 20-epi-1,25(OH)2D3 (0–100 ng/g bw) or with 10 ng/g bw 1,25(OH)2D3 (the latter included as a relative reference point for the normalization of the 20-epi-1,25(OH)2D3 values). Each tissue sample was subjected to RNA isolation and analysis as outlined in Materials and Methods. As is documented in supplemental Fig. S2 and Fig. 3, A–C, 20-epi-1,25(OH)2D3 was at best only modestly more potent than 1,25(OH)2D3 on some but not all of the target genes examined in the intestine (Fig. 3A), kidney (Fig. 3B) and bone (Fig. 3C). These genes included Cyp24a1 in all tissues, Trpv6 in the intestine, Trpv5 in the kidney, and Tnfsf11 (Rankl) in bone. Potency differences between the two compounds were not statistically different, however, and certainly not in the range of 10- to 100-fold seen in vitro in Fig. 1. The results also indicate that with the possible exception of the skeletal Cyp24a1 target, the overall efficacy of 20-epi-1,25(OH)2D3 and 1,25(OH)2D3 was similar between the two ligands as well. These findings suggest that a striking increase in potency and efficacy is unlikely to provide an explanation for the unusual and highly elevated calcemic activity of 20-epi-1,25(OH)2D3.
Figure 3.
1,25(OH)2D3 and 20-epi-1,25(OH)2D3 induce similar levels of gene expression. C57BL6 mice were treated with increasing concentrations of 1,25(OH)2D3 (10 ng/g bw) or 20-epi-1,25(OH)2D3 (0–100 ng/g bw) and the duodenum (A), kidney (B), or bone (calvarium) (C) isolated 6 h after injection. Total RNA was isolated, reverse transcribed, and then subjected to qPCR analysis. mRNA levels for each gene were normalized to β-actin expression. Cyp24a1, Trpv6 (a calcium transport gene), Cad9k (the calcium binding protein calbindin D9K), and cldn2 (a channel protein involved in paracellular calcium transport) were examined in the intestine. Cyp24a1, Trpv6, Trpv5 (a calcium transport gene), and Cyp27b1 (the gene responsible for the production of 1,25(OH)2D3 and suppressed by its hormonal product) were examined in the kidney. Cyp24a1, Rankl (a tumor necrosis factor (TNF)-like factor involved in vitamin D-induced calcium mobilization from bone), Vdr (a key component that mediates the actions of 1,25(OH)2D3), and Lrp5 (a costimulatory receptor in bone that is involved in Wnt activation of osteoblast differentiation) were examined in bone. Each point represents the average ± se, n = 4 or 5. Levels of gene induction at a concentrations of 10 ng/g bw for both 1,25(OH)2D3 and 20-epi-1,25(OH)2D3 were not statistically different. These data are representative of several similar experiments. NU, Normalized units.
20-Epi-1,25(OH)2D3 exerts a sustained, selective action on intestinal vitamin D target genes related to calcium regulation
Based on the above findings, we next asked whether a time-dependent difference in the activation of gene expression might underlie the calcemic actions of 20-epi-1,25(OH)2D3. We injected C57BL6 mice with a single dose of 20-epi-1,25(OH)2D3 or 1,25(OH)2D3 (10 ng/g bw), killed the animals at the times indicated, and then isolated and analyzed the RNA as described in Materials and Methods. Surprisingly, the ability of 20-epi-1,25(OH)2D3 to induce calcium-regulating genes such as Trpv6, S100 g (calbindin D9K) and Cln2 (claudin 2) as well as Cyp24a1 in the intestine was dramatically different from that for 1,25(OH)2D3. Thus, as observed in Fig. 4A, peak activation of these genes was slightly delayed, significantly elevated, and sustained. As a consequence, 20-epi-1,25(OH)2D3-induced levels of mRNA for Trpv6, S100 g, Cln2, and Cyp24a1 remained significantly elevated at 24 h relative to levels induced by 1,25(OH)2D3. Interestingly, this temporal difference in response between 20-epi-1,25(OH)2D3 and 1,25(OH)2D3 was not observed for Trpv6, Trpv5, or Cyp27b1 genes in the kidney (Fig. 4B) or for the Tnfsf11, Vdr, or Lrp5 genes in bone (Fig. 4C). These findings suggest a prolonged activation by 20-epi-1,25(OH)2D3 of genes expressed primarily in the intestine. Interestingly, 20-epi-1,25(OH)2D3 also exhibited a modestly altered profile of induction at the Cyp24a1 gene in the kidney and bone as well, as seen in Fig. 4, B and C. Whereas this activity was not identical with that observed in the intestine, it suggests that in addition to its tissue-selective actions, 20-epi-1,25(OH)2D3 may also manifest a minor gene-selective component as well.
Figure 4.
20-Epi-1,25(OH)2D3 exhibits an enhanced and sustained activity at selected target genes in the intestine in vivo. C57BL6 mice were treated with either 1,25(OH)2D3 or 20-epi-1,25(OH)2D3 (10 ng/g bw) and duodenum (A), kidney (B), or bone (C) harvested at the indicated time points and used to prepare RNA. RNA was reverse transcribed and then subjected to qPCR analysis. mRNA levels for each gene were normalized to β-actin expression. Each point represents the average ± se, n = 5. An unpaired t test was used to compare 1,25(OH)2D3 and 20-epi-1,25(OH)2D3 at each time point (*, P < 0.05). NU, Normalized units.
20-Epi-1,25(OH)2D3 promotes VDR binding, H4 acetylation, and RNA pol II recruitment at the Cyp24a1 and Trpv6 gene loci in intestine
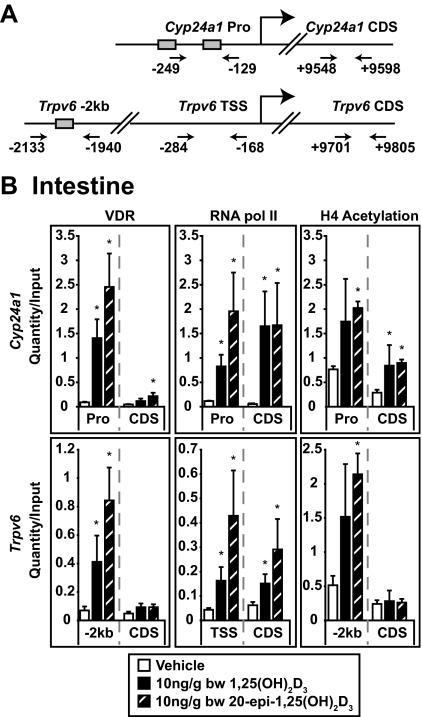
Previous studies using ChIP analysis indicated that VDR occupancy at the regulatory elements for both the Cyp24a1 and Trpv6 genes was increased immediately before their induction by 1,25(OH)2D3 in the kidney and intestine (3). We therefore used this approach to determine whether the modest increase in efficacy and prolonged activation of these two genes by 20-epi-1,25(OH)2D3 in the intestine were indeed a function of altered VDR binding. Using this technique, we also contrasted the ability of 20-epi-1,25(OH)2D3 and 1,25(OH)2D3 to induce H4 acetylation and promote the recruitment of RNA pol II. Mice were treated with either vehicle or the individual vitamin D ligands (10 ng/g bw) for 1 h and then subjected to ChIP analysis as outlined in Materials and Methods. Figure 5A depicts the location and numerical position of the primer sets used to detect immunoprecipitated DNA fragments derived from the Cyp24a1 and Trpv6 genes. As can be seen in Fig. 5B (left panels), both 1,25(OH)2D3 and 20-epi-1,25(OH)2D3 strongly induced an increase in the level of VDR detected at the regulatory elements [promoter (Pro) and −2 kb] but not in the downstream coding regions for either gene. This increase in VDR occupancy correlated directly with an elevation in RNA pol II recruitment at transcriptional start sites for both genes (Fig. 5B, center panels) and an increase in H4 acetylation that was most pronounced in the vicinity of VDR binding (Fig. 5B, right panels). RNA pol II as well as H4 acetylation was also increased near the coding regions of both genes (downstream coding regions) as previously observed (31). Interestingly, the increases observed at the level of VDR binding and RNA pol II occupancy at the Cyp24a1 and Trpv6 genes were always greater with 20-epi-1,25(OH)2D3 than with 1,25(OH)2D3. Although not statistically significant at this early time point, this trend was highly reproducible, suggesting that the modest increases in efficacy of 20-epi-1,25(OH)2D3 at target genes in the two tissues might be due to slight increases in VDR activity.
Figure 5.
20-Epi-1,25(OH)2D3 induces VDR binding, H4 acetylation, and RNA pol II recruitment to the Cyp24a1 and Trpv6 genes in the intestine. A, Regulatory regions of the Cyp24a1 and Trpv6 genes. Depicted is the location of regulatory elements and positions of the primer sets used to evaluate the presence of Cyp24a1 and Trpv6 gene fragments. All numbering is relative to the transcriptional start site (TSS). Shaded boxes refer to the presence of an established vitamin D response element. B, Detection of VDR binding, H4 acetylation, and RNA pol II recruitment at the Cyp24a1 and Trpv6 genes. C57BL6 mice were treated with vehicle, 1,25(OH)2D3, or 20-epi-1,25(OH)2D3 (10 ng/g bw) and the duodenum isolated 1 h later and subjected to ChIP analysis using antibodies to VDR, tetraacetylated H4, and RNA pol II. Samples were analyzed by qPCR using the primer sets documented in A. For all samples, each point represents the average quantity/input ± se (n = 4) with an unpaired t test compared with vehicle. Statistical groups were created within each primer set and antibody (*, P < 0.05). These experimental results are representative of three to five similar experiments. CDS, Coding region control; Pro, promoter.
20-Epi-1,25(OH)2D3 elicits prolonged VDR binding, histone 4 (H4) acetylation, and RNA pol II recruitment at the Cyp24a1 and Trpv6 gene loci in intestine
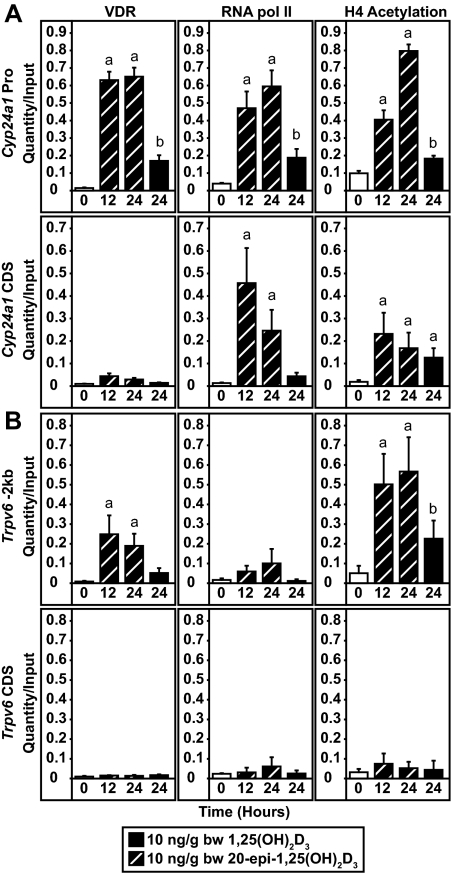
In view of the prolonged activity of 20-epi-1,25(OH)2D3 at the Cyp24a1 and Trpv6 genes at the mRNA level seen in Fig. 4, we next assessed the level of VDR binding 24 h after treatment with either 1,25(OH)2D3 or 20-epi-1,25(OH)2D3. Mice were dosed for 0, 12, or 24 h with 10 ng/g bw with 20-epi-1,25(OH)2D3 or 24 h with 10 ng/g bw 1,25(OH)2D3 and the abundance of the VDR at the Cyp24a1 and Trpv6 genes in the intestine determined by ChIP analysis using the primer sets described previously in Fig. 5. We also measured the levels of H4 acetylation and the relative presence of RNA pol II. 20-Epi-1,25(OH)2D3 induced a significant level of VDR binding at the regulatory regions of the Cyp24a1 (Fig. 6A) and Trpv6 (Fig. 6B) genes, which was detectable at both 12 and 24 h after injection. As above, these increases in VDR binding correlated directly with elevations at the two genes in both H4 acetylation and RNA pol II recruitment. In contrast, the level of VDR binding induced by 1,25(OH)2D3 at 24 h was substantially less than that seen with 20-epi-1,25(OH)2D3 and consistent with previous studies (31). This reduction in activity was also manifested at the level of H4 acetylation and RNA pol II recruitment. These observations suggest that the prolonged activity of 20-epi-1,25(OH)2D3 correlates directly with the presence and accompanying activity of the VDR.
Figure 6.
20-Epi-1,25(OH)2D3 induces prolonged VDR binding, H4 acetylation, and RNA pol II recruitment to the Cyp24a1 and Trpv6 genes in the intestine. C57BL6 mice were treated with 1,25(OH)2D3 or 20-epi-1,25(OH)2D3 (10 ng/g bw) for 0, 12, or 24 h and the isolated duodenum subjected to ChIP analysis using antibodies to VDR, tetraacetylated H4, or RNA pol II. Samples were then analyzed by qPCR using the primers for either Cyp24a1 (A) or Trpv6 (B) as depicted in Fig. 5A. Each point represents the average quantity/input ± se (n = 5) with an unpaired t test compared with vehicle. Statistical groups (a and b) were created within each primer set and antibody (P < 0.05). These experimental results are representative of two similar experiments. CDS, Coding region control; Pro, promoter.
Discussion
Studies of the biological and molecular actions of 20-epi-1,25(OH)2D3 have been conducted for almost 2 decades using both in vitro and in vivo models (23,25). These studies have suggested that 20-epi-1,25(OH)2D3 manifests unexpected properties, features that have prompted the term superagonist (19,24,26,35). Because the relative affinity of the VDR for 20-epi-1,25(OH)2D3 is similar to that for 1,25(OH)2D3, the unusual biological properties of 20-epi-1,25(OH)2D3 are believed to arise from its capacity to provoke an unusual conformation within the VDR that results in increased VDR-ligand stability or altered interaction with copartners such as RXR or coactivators such as mediator-1. Unfortunately, whereas the ability of 20-epi-1,25(OH)2D3 to induce a unique conformation within the VDR is supported by extensive protease digestion studies, it is not supported by x-ray diffraction studies of 20-epi-1,25(OH)2D3 in complex with the VDR ligand binding domain (19,27,28). There is also evidence, however, to indicate that 20-epi-1,25(OH)2D3 is not as efficiently degraded by CYP24a1, the enzyme that is primarily responsible for vitamin D ligand catabolism (23). Thus, these observations suggest that the unusual activity of 20-epi-1,25(OH)2D3 in cells may be due to a prolonged half-life of the analog itself or to the extended life span of a metabolic by-product (23). In view of these uncertainties, we contrasted the biological effects of 20-epi-1,25(OH)2D3 with that of 1,25(OH)2D3 in vivo with a focus on the activities of these compounds on calcium regulation and the genes responsible for these processes.
20-Epi-1,25(OH)2D3 displayed a striking increase in potency when its biological activity was examined in cell culture experiments in vitro. In vivo, however, 20-epi-1,25(OH)2D3 was neither more potent nor strikingly more efficacious than 1,25(OH)2D3 at raising either blood calcium levels or inducing genes in the intestine, kidney, or bone that are responsible for calcium homeostasis. Instead, we found that 20-epi-1,25(OH)2D3 appeared to be selective for the prolonged induction of genes involved in the transepithelial uptake of calcium from the gut lumen to the blood. Importantly, these responses at the mRNA level were confirmed by molecular studies using ChIP analysis, which revealed a modest increase and a sustained presence of VDR in the intestine at the Trpv6 and Cyp24a1 genes. These differences correlated directly with changes observed in gene activity, as measured by both increased site-specific H4 acetylation and the recruitment of RNA pol II. Our results suggest therefore that the hypercalcemic activity of 20-epi-1,25(OH)2D3 is not due to a substantial increase in ligand potency and efficacy but rather to an ability to sustain the activation of calcium-regulating target genes in the intestine. One potential consideration relevant to these conclusions, nevertheless, is the rapid turnover of epithelial cells that occurs at the intestinal mucosa. Thus, measurements of compound activity at the level of mRNA production (or protein) in the intestine may be influenced, diluted, or in fact altered as newly minted epithelial cells emerge from the basal intestinal crypt region of the mucosa. Whereas it is likely that this process impacts our results to some degree, the sustained activation of mRNA expression and VDR binding at the target gene in the presence of 20-epi-1,25(OH)2D3 is evident within 12–24 h, thus minimizing the potential effect of a continuously changing epithelial cell population. In addition, we would expect, if anything, that this process would curb the actions 20-epi-1,25(OH)2D3 rather than delay its actions. We therefore conclude that although important, it is unlikely that rapid intestinal epithelial turnover can account for the results observed with 20-epi-1,25(OH)2D3.
Interestingly, whereas 20-epi-1,25(OH)2D3 sustained the activation of genes in the intestine, this action was not generally replicated in either the kidney or bone (with the possible exception of Cyp24a1 induction). Thus, 1,25(OH)2D3 and 20-epi-1,25(OH)2D3 were temporally equivalent in their capacity to induce genes such as Trpv5 and Trpv6 in the kidney and Tnfsf11 in the bone (36,37,38). The unique actions of 20-epi-1,25(OH)2D3 in the intestine are particularly apparent at the Trpv6 gene, in which prolonged activation of Trpv6 was observed only in the intestine and not in the kidney. Several explanations could account for this prolonged activity of 20-epi-1,25(OH)2D3 on target genes in the intestine. First, sustained activity of 20-epi-1,25(OH)2D3 could arise as a result of the ability of 20-epi-1,25(OH)2D3 to create and maintain a more stable VDR/RXR/DNA complex exclusively in the intestine. Whereas this explanation is consistent with our results, it is difficult to understand why this same biochemical stability would not occur in all target tissues and on all target genes. Second, the prolonged activity of 20-epi-1,25(OH)2D3 could arise from a selective decrease in the metabolic degradation of 20-epi-1,25(OH)2D3 (or the metabolic stability of a catabolic by product) in the intestine. This possibility could arise as a result of the limited or absent expression of an enzyme in this tissue responsible for 20-epi-1,25(OH)2D3 catabolism. One consequence would be a prolonged ligand activation of the VDR, prolonged residence time of the VDR on target genes, and sustained target gene induction. It is worth noting here that 20-epi-1,25(OH)2D3, due to its side-chain configuration, is not a significant substrate for CYP24A1 (23). Otherwise, the striking and sustained up-regulation that occurs at the Cyp24a1 gene in response to 20-epi-1,25(OH)2D3 action would almost certainly limit rather than enhance the activity of 20-epi-1,25(OH)2D3. Finally, it is possible that the prolonged activity of 20-epi-1,25(OH)2D3 in the intestine could be influenced by the strong influx of calcium that occurs across the mucosal epithelial cell. Calcium is known to influence directly the expression of S100 g and perhaps Trpv6 (39,40).
The results obtained herein raise the question as to why 20-epi-1,25(OH)2D3 is more potent than 1,25(OH)2D3 in vitro but not in vivo. Interestingly, our recent findings suggest that the increased potency of 20-epi-1,25(OH)2D3 in vitro is not due to an unusual property of the analog but rather to the ability of 1,25(OH)2D3 to strongly interact with the vitamin D binding protein (DBP) found in serum (41). This interaction reduces the effective concentration of 1,25(OH)2D3 that can enter cells, thus reducing the potency of the ligand in vitro. Indeed, 1,25(OH)2D3 and 20-epi-1,25(OH)2D3 manifest an equivalent biological potency in vitro on removal of DBP from the culture medium (41). 20-Epi-1,25(OH)2D3, on the other hand, does not interact with DBP. Thus, its potency remains unaltered. Surprisingly, DBP does not affect the biological potency of 1,25(OH)2D3 in vivo (41). It is currently unclear why this is so.
In conclusion, we find that the superagonist activity of 20-epi-1,25(OH)2D3 in normal mice in vivo is likely to arise from a prolonged activation of the expression of calcium-regulating genes in the intestine but not in kidney or bone. Given the nature of this response, these effects are unlikely to be due to a unique pharmacokinetic effect but rather to a selective decrease in the degradation of 20-epi-1,25(OH)2D3 in the intestine or selective activation of long lived by-products. Unfortunately, the unusual biological effects of 20-epi-1,25(OH)2D3 in vitro are not reflective of those that occur in vivo for the reasons outlined above. This discrepancy suggests that significant caution should be taken when evaluating vitamin D3 analogs in vitro for prospective therapeutic properties in vivo.
Supplementary Material
Acknowledgments
We thank the members of the Pike laboratory for helpful contributions to this work. We also thank Laura Vanderploeg for facilitating preparation of the illustrations contained in this manuscript and Wendy Hellwig for conducting the serum calcium measurements reported herein. We thank Professor Brian S. Yandell (Department of Horticulture Biostatistics and Medical Informatics, University of Wisconsin) for conducting statistical analyses.
Footnotes
This work was supported by National Institutes of Health Grant DK-73995 (to J.W.P.).
Disclosure Summary: The authors have nothing to declare.
First Published Online May 7, 2009
Abbreviations: bw, Body weight; ChIP, chromatin immunoprecipitation; DBP, vitamin D binding protein; β-gal, β-galactosidase; H4, histone 4; hVDR, human vitamin D receptor; Ki, inhibitory constant; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; qPCR, quantitative real-time PCR; RNA pol II, RNA polymerase II; RXR, retinoid X receptor; SWI/SNF, switch/sucrose nonfermentable; TRPV, transient receptor potential, vanilloid; VDR, vitamin D receptor.
References
- DeLuca HF 1988 The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J 2:224–236 [PubMed] [Google Scholar]
- Jones G, Strugnell SA, DeLuca HF 1998 Current understanding of the molecular actions of vitamin D. Physiol Rev 78:1193–1231 [DOI] [PubMed] [Google Scholar]
- Sutton AL, MacDonald PN 2003 Vitamin D: more than a “bone-a-fide” hormone. Mol Endocrinol 17:777–791 [DOI] [PubMed] [Google Scholar]
- Glass CK 1994 Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr Rev 15:391–407 [DOI] [PubMed] [Google Scholar]
- Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR 2001 Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord 2:203–216 [DOI] [PubMed] [Google Scholar]
- Kingston RE, Narlikar GJ 1999 ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev 13:2339–2352 [DOI] [PubMed] [Google Scholar]
- Rachez C, Suldan Z, Ward J, Chang CP, Burakov D, Erdjument-Bromage H, Tempst P, Freedman LP 1998 A novel protein complex that interacts with the vitamin D3 receptor in a ligand-dependent manner and enhances VDR transactivation in a cell-free system. Genes Dev 12:1787–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, O'Malley BW 2002 Molecular mechanisms and cellular biology of the steroid receptor coactivator (SRC) family in steroid receptor function. Rev Endocr Metab Disord 3:185–192 [DOI] [PubMed] [Google Scholar]
- Wasserman RH 1997 Vitamin D and the intestinal absorption of calcium and phosphorus. In: Feldman D, Glorieux FH, Pike JW, eds. Vitamin D. 1st ed. San Diego: Academic Press; 259–273 [Google Scholar]
- Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW 1998 The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 13:325–349 [DOI] [PubMed] [Google Scholar]
- Inoue T, Kamiyama J, Sakai T 1999 Sp1 and NF-Y synergistically mediate the effect of vitamin D(3) in the p27(Kip1) gene promoter that lacks vitamin D response elements. J Biol Chem 274:32309–32317 [DOI] [PubMed] [Google Scholar]
- Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP 1996 Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev 10:142–153 [DOI] [PubMed] [Google Scholar]
- Pan P, Reddy K, Lee S, Studzinski GP 1991 Differentiation-related regulation of 1,25-dihydroxyvitamin D3 receptor mRNA in human leukaemia cells HL-60. Cell Prolif 24:159–170 [DOI] [PubMed] [Google Scholar]
- Zanello LP, Norman AW 1997 Stimulation by 1alpha,25(OH)2-vitamin D3 of whole cell chloride currents in osteoblastic ROS 17/2.8 cells. A structure-function study. J Biol Chem 272:22617–22622 [DOI] [PubMed] [Google Scholar]
- Zanello LP, Norman AW 2004 Electrical responses to 1α,25(OH)2-Vitamin D3 and their physiological significance in osteoblasts. Steroids 69:561–565 [DOI] [PubMed] [Google Scholar]
- Zhou LX, Nemere I, Norman AW 1992 1,25-Dihydroxyvitamin D3 analog structure-function assessment of the rapid stimulation of intestinal calcium absorption (transcaltachia). J Bone Miner Res 7:457–463 [DOI] [PubMed] [Google Scholar]
- Deluca HF, Cantorna MT 2001 Vitamin D: its role and uses in immunology. FASEB J 15:2579–2585 [DOI] [PubMed] [Google Scholar]
- Pike JW, Yamamoto H, Shevde NK 2002 Vitamin D receptor-mediated gene regulation mechanisms and current concepts of vitamin D analog selectivity. Adv Ren Replace Ther 9:168–174 [DOI] [PubMed] [Google Scholar]
- Liu YY, Collins ED, Norman AW, Peleg S 1997 Differential interaction of 1α,25-dihydroxyvitamin D3 analogues and their 20-epi homologues with the vitamin D receptor. J Biol Chem 272:3336–3345 [DOI] [PubMed] [Google Scholar]
- Peleg S, Nguyen C, Woodard BT, Lee JK, Posner GH 1998 Differential use of transcription activation function 2 domain of the vitamin D receptor by 1,25-dihydroxyvitamin D3 and its A ring-modified analogs. Mol Endocrinol 12:525–535 [DOI] [PubMed] [Google Scholar]
- Takeyama K, Masuhiro Y, Fuse H, Endoh H, Murayama A, Kitanaka S, Suzawa M, Yanagisawa J, Kato S 1999 Selective interaction of vitamin D receptor with transcriptional coactivators by a vitamin D analog. Mol Cell Biol 19:1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Freedman LP 1999 20-Epi analogues of 1,25-dihydroxyvitamin D3 are highly potent inducers of DRIP coactivator complex binding to the vitamin D3 receptor. J Biol Chem 274:16838–16845 [DOI] [PubMed] [Google Scholar]
- Dilworth FJ, Calverley MJ, Makin HL, Jones G 1994 Increased biological activity of 20-epi-1,25-dihydroxyvitamin D3 is due to reduced catabolism and altered protein binding. Biochem Pharmacol 47:987–993 [DOI] [PubMed] [Google Scholar]
- Binderup L, Latini S, Binderup E, Bretting C, Calverley M, Hansen K 1991 20-Epi-vitamin D3 analogues: a novel class of potent regulators of cell growth and immune responses. Biochem Pharmacol 42:1569–1575 [DOI] [PubMed] [Google Scholar]
- Binderup L, Binderup E, Godtfredsen W, Kissmeyer A 2005 Development of new vitamin D analogs. In: Feldman D, Pike JW, Glorieux F, eds. Vitamin D. 2nd ed. New York: Elsevier/Academic Press; 1489–1510 [Google Scholar]
- Peleg S, Sastry M, Collins ED, Bishop JE, Norman AW 1995 Distinct conformational changes induced by 20-epi analogues of 1α,25-dihydroxyvitamin D3 are associated with enhanced activation of the vitamin D receptor. J Biol Chem 270:10551–10558 [DOI] [PubMed] [Google Scholar]
- Tocchini-Valentini G, Rochel N, Wurtz JM, Mitschler A, Moras D 2001 Crystal structures of the vitamin D receptor complexed to superagonist 20-epi ligands. Proc Natl Acad Sci USA 98:5491–5496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D 2000 The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell 5:173–179 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Shevde NK, Warrier A, Plum LA, DeLuca HF, Pike JW 2003 2-Methylene-19-nor-(20S)-1,25-dihydroxyvitamin D3 potently stimulates gene-specific DNA binding of the vitamin D receptor in osteoblasts. J Biol Chem 278:31756–31765 [DOI] [PubMed] [Google Scholar]
- Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW 2006 The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol 20:1447–1461 [DOI] [PubMed] [Google Scholar]
- Meyer MB, Zella LA, Nerenz RD, Pike JW 2007 Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J Biol Chem 282:22344–22352 [DOI] [PubMed] [Google Scholar]
- Pike JW, Haussler MR 1983 Association of 1,25-dihydroxyvitamin D3 with cultured 3T6 mouse fibroblasts. Cellular uptake and receptor-mediated migration to the nucleus. J Biol Chem 258:8554–8560 [PubMed] [Google Scholar]
- Kim S, Shevde NK, Pike JW 2005 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J Bone Miner Res 20:305–317 [DOI] [PubMed] [Google Scholar]
- Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M 2000 Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- Ryhanen S, Mahonen A, Jaaskelainen T, Maenpaa PH 1996 Synthetic 20-epi analogs of calcitriol are potent inducers of target-gene activation in osteoblastic cells. Eur J Biochem 238:97–103 [DOI] [PubMed] [Google Scholar]
- den Dekker E, Hoenderop JG, Nilius B, Bindels RJ 2003 The epithelial calcium channels, TRPV5, TRPV6: from identification towards regulation. Cell Calcium 33:497–507 [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Muller D, Van Der Kemp AW, Hartog A, Suzuki M, Ishibashi K, Imai M, Sweep F, Willems PH, Van Os CH, Bindels RJ 2001 Calcitriol controls the epithelial calcium channel in kidney. J Am Soc Nephrol 12:1342–1349 [DOI] [PubMed] [Google Scholar]
- Yasuda H, Higashio K, Suda T 2005 Vitamin D and osteoclastogenesis. In: Feldman D, Pike JW, Glorieux F, eds. Vitamin D. 2nd ed. New York: Elsevier/Academic Press; 665–685 [Google Scholar]
- van Abel M, Hoenderop JG, van der Kemp AW, van Leeuwen JP, Bindels RJ 2003 Regulation of the epithelial Ca2+ channels in small intestine as studied by quantitative mRNA detection. Am J Physiol Gastrointest Liver Physiol 285:G78–G85 [DOI] [PubMed] [Google Scholar]
- Van Cromphaut SJ, Dewerchin M, Hoenderop JGJ, Stockmans I, Van Herck E, Kato S, Bindels RJM, Collen D, Carmeliet P, Bouillon R, Carmeliet G 2001 Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA 98:13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zella LA, Shevde NK, Hollis BW, Cooke NE, Pike JW 2008 Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology 149:3656–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.