Abstract
To avoid breeding during unsuitable environmental or physiological circumstances, the reproductive axis adjusts its output in response to fluctuating internal and external conditions. The ability of the reproductive system to alter its activity appropriately in response to these cues has been well established. However, the means by which reproductively relevant cues are interpreted, integrated, and relayed to the reproductive axis remain less well specified. The neuropeptide kisspeptin has been shown to be a potent positive stimulator of the hypothalamo-pituitary-gonadal (HPG) axis, suggesting a possible neural locus for the interpretation/integration of these cues. Because a failure to inhibit reproduction during winter would be maladaptive for short-lived female rodents, female Siberian hamsters (Phodopus sungorus) housed in long and short days hamsters were examined. In long, ‘summer’ photoperiods, kisspeptin is highly expressed in the anteroventral periventricular nucleus (AVPV), with low expression in the arcuate nucleus (Arc). A striking reversal in this pattern is observed in animals held in short, ‘winter’ photoperiods, with negligible kisspeptin expression in the AVPV and marked staining in the Arc. Although all studies to date suggest that both populations act to stimulate the reproductive axis, these contrasting expression patterns of AVPV and Arc kisspeptin suggest disparate roles for these two cell populations. Additionally, we found that the stimulatory actions of exogenous kisspeptin are blocked by acyline, a gonadotropin-releasing hormone (GnRH) receptor antagonist, suggesting an action of kisspeptin on the GnRH system rather than pituitary gonadotropes. Finally, females held in short day lengths exhibit a reduced response to exogenous kisspeptin treatment relative to long-day animals. Together, these findings indicate a role for kisspeptin in the AVPV and Arc as an upstream integration center for reproductively-relevant stimuli and point to a dual mechanism of reproductive inhibition in which kisspeptin expression is reduced concomitant with reduced sensitivity of the HPG axis to this peptide.
Keywords: metastin, GPR54, photoperiod, Siberian hamster, seasonal, reproduction
Organisms inhabiting temperate climates confront numerous challenges during the winter months, when harsh weather conditions necessitate significant shifts in behavior and physiology. Many mammals have evolved strategies for partitioning energy reserves to essential physiological processes and inhibiting nonessential processes during these unfavorable climatic conditions (Bronson, 1985; Bronson, 1991; Voltura and Wunder, 1998; Wade and Schneider, 1992). One such adaptive strategy employed by many small rodent species occupying variable environments is the complete cessation of reproductive activity by inhibition of the reproductive axis (Nelson et al., 1990). This profound alteration in reproductive activity ensures offspring are not born during times when resources are scarce and weather inclement, improving the probability of both parent and offspring survival (Bronson, 1985; Bronson, 1991).
The principal environmental signal mediating seasonal reproduction is photoperiod (day length). Specifically, short, decreasing day lengths are interpreted as winter whereas long, increasing day lengths are perceived as summer. Changing day lengths accompanying the late summer to autumn transition are transduced via the pineal gland into a melatonin signal that then acts on the neural substrates mediating reproductive function (Carter and Goldman, 1983; Goldman, 2001). Specifically, long durations of melatonin are interpreted as short, winter-like days whereas short melatonin durations are interpreted as long, summer-like days. The final common neural pathway in which environmental information is integrated to modulate the reproductive axis is the gonadotropin-releasing hormone (GnRH) neuronal system (Bronson, 1985; Bronson, 1991). GnRH regulates pituitary secretion of the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which in turn act on the gonads to regulate steroidogenesis and gametogenesis, respectively. Whereas the encoding of day length into a melatonin signal has been well established as the mechanism driving seasonal changes in reproductive function, the neural loci that decode the melatonin signal and communicate this information to the reproductive axis remain largely unspecified.
The neuropeptide kisspeptin has recently been identified as a potent stimulator of GnRH release. Kisspeptin is the endogenous ligand for the (formerly orphaned) G-protein-coupled receptor 54 (GPR54) (Gottsch et al., 2006) and is a potent positive regulator of the HPG axis. Exogenous administration of kisspeptin leads to marked, dose-dependent increases in serum levels of LH in all species studied to date, including humans (Dungan et al., 2006; Seminara, 2005). The effects of kisspeptin are mediated by its actions on GnRH neurons through GPR54. For example, the immediate early gene, c-Fos, is expressed in GnRH neurons following kisspeptin administration (Irwig et al., 2004; Matsui et al., 2004). Additionally, the GnRH receptor antagonist, acyline, blocks gonadotropin release elicited by kisspeptin (Gottsch et al., 2004; Irwig et al., 2004; Shahab et al., 2005). A large proportion of GnRH cells co-express GPR54 mRNA, further supporting a direct mode of action by kisspeptin on GnRH neurons (Han et al., 2005; Irwig et al., 2004; Messager et al., 2005).
Seasonally breeding rodents provide an ideal model system for investigating the impact of external environmental cues on the reproductive axis. Siberian hamsters (Phodopus sungorus) are a cricetid rodent indigenous to the steppes and semi-arid deserts of Siberia, Mongolia, and central Asia, an environment characterized by a marked, predictable decline in ambient temperature and food availability during the fall and winter months (Gorman and Zucker, 1997). The energetic costs of reproduction under such conditions may function as an important ultimate factor that selects against individuals that attempt to breed during fall and winter and that, instead, favors individuals that time parturition to coincide with times of moderate temperatures and greater access to high-quality food (Nelson et al., 1990; Prendergast et al., 2001).
We have recently demonstrated that changes in kisspeptin expression are associated with photoperiod-induced suppression of reproductive function in male Siberian hamsters (Greives et al., 2007). Specifically, in male Siberian hamsters, we observed kisspeptin-ir cell bodies localized to the anteroventral periventricular nucleus (AVPV) and the arcuate nucleus (Arc) of the hypothalamus. High expression was observed in the AVPV of reproductively competent hamsters while the inverse state was seen in animals with gonadal involution. Notably, the opposite pattern of expression was seen in the Arc (Greives et al., 2007). Despite being reproductively quiescent, short-day males were equally sensitive to a kisspeptin challenge, suggesting that mechanisms downstream of hypothalamic kisspeptin do not differ photperiodically, at least in male hamsters.
The energetic costs of reproduction are not borne equally by the sexes; rather female mammals expend more energy than males in the form of gestation, lactation, and maternal care (Beery et al., 2006). Given this differential investment in reproductive effort, males and females have likely evolved disparate mechanisms to regulate seasonal reproduction. Most research to date has focused on the mechanisms responsible for seasonal changes in male rodents, and one goal of these experiments was to examine whether similar mechanisms underlie changes observed in females. Specifically, the goals of the present study were: 1) to identify the distribution of kisspeptin-ir neurons in the brains of reproductively active (i.e., long-day) and inactive (i.e., short-day) female Siberian hamsters, 2) examine reproductive axis sensitivity in long- and short-day females exposed to exogenous kisspeptin peptide, and 3) determine the neural substrates on which kisspeptin acts to influence reproductive axis activity.
Materials and Methods
Animals and Housing
Adult (>60 days of age), intact female Siberian hamsters (Phodopus sungorus) (n=82) were obtained from the breeding colony maintained at Indiana University. All animals were group housed with same-sex siblings in a long-day photoperiod (Light:Dark [LD] 16:8) prior to the start of the study. Animals were housed individually in polypropylene cages (27.8 × 7.5 × 13.0 cm) and placed in either long- (LD 16:8) or short-day (LD 8:16) photoperiods. Temperature was kept constant at 20 ± 2°C and relative humidity was maintained at 50 ± 5%. Food (Purina Rat Chow) and tap water were available ad libitum throughout the experiments. All animal protocols used herein were approved by the Bloomington Institutional Animal Care and Use Committee.
At the conclusion of each experiment, animals were weighed to the nearest 0.1g, euthanized and necropsies were performed. Paired ovaries and uterine horns were collected, cleaned of fat and connective tissue, and weighed together as “reproductive organ mass”.
Experiment 1: Effects of photoperiod on kisspeptin expression
To determine seasonal changes in the pattern of kisspeptin peptide expression, hamsters were held for 12 weeks in long (LD; n=10) or short (SD; n=9) photoperiods. After photoperiod treatment, hamsters were deeply anesthetized with 0.3 ml of a ketamine (20 mg/ml)/xylazine (4 mg/ml) cocktail in 0.9% saline and perfused transcardially with 50 ml of 0.9% saline, followed by 100–150 ml of 4% paraformaldehyde in 0.1 M PBS, pH 7.3. Brains were postfixed for 3 h at room temperature in 4% paraformaldehyde, and cryoprotected in 20% sucrose in 0.1 M PBS and stored at 4°C until processed. Coronal sections (40 μm) were cut on a cryostat and processed as free-floating sections beginning rostrally at the medial septum/diagonal band of Broca and extending caudally to the brainstem. Kisspeptin immunoreactive cells were labeled using a rabbit anti-kisspeptin antiserum (Penninsula Laboratories Inc, Bachem, San Carlos, CA) diluted at 1:7500 and preadsorbed with GnIH peptide to eliminate cross-reactivity with this related RFamide peptide, as previously described (Greives et al., 2007). We have previously validated this staining procedure and confirmed specificity for kisspeptin peptide (Greives et al., 2007). Amplification of the signal was accomplished by using a modified biotinylated tyramide procedure previously described (Greives et al., 2007). Sections were mounted onto gelatin-coated slides, dehydrated in a graded series of ethanol solutions (70, 95 and 100%), and cleared in xylenes (Fisher Scientific) before the application of coverslips.
Microscopy, Cell Counts, and Optical Density
Slides were examined under bright field illumination on a Zeiss Z1 microscope by an independent observer naïve to the experimental conditions. Kisspeptin-immunoreactive (ir) cells were located by visually scanning the brains under 200× magnification. Cell populations were restricted to the AVPV region of the preoptic area and the arcuate nucleus (Arc). All cells were confirmed at a minimum of 400×. Cells were photographed with a Zeiss Axiocam Cooled CCD camera at 400× magnification for cell size and density analyses. All cells in every 4th section were counted through the rostro-caudal extent of the AVPV and Arc. Only those cells with a visible nucleus were counted. Soma size and optical density (OD) measurements were performed on images captured at 400×. Soma size and optical density provide a semi-quantitative measure of protein/peptide content visualized immunocytochemically (Nishio et al., 1994). Whereas this measure is unlikely to uncover subtle differences in peptide content across groups, more significant changes should be observed. Cell bodies were outlined and the two-dimensional area was calculated using NIH Image 1.61. Each pixel in the grayscale image capture has a measurable specific intensity, with values ranging from 0 (white) to 256 (black). The average value for all pixels in an outlined area is taken as the mean intensity of staining for a given region of the image. OD measures were normalized to minimize differences between replications of immunohistochemistry. First, a background measurement was taken by placing a square outline, four times, on non-overlapping, unstained areas of each section. The mean of these four measures provided the background OD for each section. The OD for each cell body was assessed by outlining the cell body, obtaining a density measure using NIH Image, and subtracting the background OD from the OD of each cell. To account for potential overcounting, an Abercrombie correction was applied to cell count data prior to analysis.
Experiment 2: Endocrine response to exogenous kisspeptin
To determine whether seasonal changes in reproduction are related to a reduction in HPG axis sensitivity to kisspeptin, we examined whether kisspeptin-induced LH expression was altered by photoperiodic treatment. Hamsters were held in long- (n=12) or short-day (n=19) photoperiods for 8 weeks prior to kisspeptin injections. Animals were injected with either kisspeptin-10 [KiSS-1 (112–121)/metastin (45–54)(human); Phoenix Pharmaceuticals, Inc. Belmont, CA], a peptide known to stimulate GPR54 (Gottsch et al., 2004), or a 0.1 M PBS vehicle injection. Injections were given between 8.5 – 11 h prior to lights out in LD animals and 4.5 – 7.0 h before lights out in SD animals. These time points were chosen to ensure low estradiol concentrations in LD animals (Dodge et al., 2002). The injection protocol has been previously described elsewhere (Messager et al., 2005). Briefly, an initial blood sample was drawn from all hamsters via the retro-orbital sinus to measure baseline hormone levels. Next, long- and short-day hamsters received i.p. injections of either 100 μl PBS (long-day: n=6)(short-day: n=9) or 100 μl of a PBS solution containing a 10 μM concentration of kisspeptin-10 (Phoenix Pharmaceuticals, Inc.)(long-day: n=6)(short-day: n=10), every 30 minutes for a total of four injections. Thirty minutes after the last injection all hamsters were again bled. Blood was centrifuged at 2500 RPM for 30 min and serum was collected and stored at −80°C until assayed for hormones.
Experiment 3: Endocrine response to exogenous kisspeptin after the administration of a GnRH antagonist
To examine whether the actions of kisspeptin are mediated at the level of the GnRH system, we examined the LH response to kisspeptin following treatment with the GnRH receptor antagonist, acyline, kindly provided by Dr. Richard Blye at the NIH/NICHD. Procedures were modified from a previously described protocol (Gottsch et al., 2004). Animals received injections of either the GnRH antagonist, acyline, or vehicle, combined with injections of kisspeptin. Briefly, an initial blood sample was drawn from all hamsters via the retro-orbital sinus to measure baseline hormone levels. Next, long-day animals were given an injection i.p. of either 50μg acyline diluted in 100μl mannitol (5 %) water (n = 6) or 100μl of vehicle mannitol water (n=6). Exactly one hour after injection, animals received single 100 μl i.p. injections of of 10 μM kisspeptin. Additionally, four animals received vehicle injections at both time points. Pilot data from our labs indicated that one injection of kisspeptin caused a comparable LH rise to that seen in animals injected following the four injection protocol described above (Greives et al., unpublished data). Thirty minutes following the final injection, a second blood sample was collected. Blood was centrifuged at 2500 RPM for 30 min. and serum was collected and stored at −80°C until assayed for hormones.
Hormone Measurements
Serum LH concentrations were measured in duplicate via a single radioimmunoassay (RIA) with reagents obtained from the National Institutes of Health based on a previous protocol (Chappell et al., 1997). The antiserum was rLH-S-11 and the standard was rLH-RP3. The sensitivity was 0.01ng/tube and the intra-assay coefficient of variation was 2.9% for the low pool and 8.5% for the high pool. The antiserum used was highly specific to LH and has been previously validated for use in Siberian hamsters (Wolfe et al., 1995).
Statistical Analyses
Data in Experiment 1 were analyzed using a two-tailed Student’s t-test. Specifically, the effects of photoperiod on body mass, gonad mass, kisspeptin-ir neuron number, size and optical densities were each analyzed in separate analyses. For Experiment 2, the effects of peripheral kisspeptin injections on LH were analyzed using a 2 × 2 × 2 mixed model ANOVA, with pre- and post-hormone levels as the within-subjects factor and photoperiod and injection type as the between-subjects factors. LH data for experiment 3 were analyzed with a 2 × 2 × 2 (LD vs. SD × acyline vs. vehicle × kisspeptin vs. vehicle) ANOVA. All post hoc comparisons were analyzed using Tukey tests. In all cases, differences were considered statistically significant if p < 0.05.
Results
Experiment 1: Effect of photoperiod on kisspeptin neurons
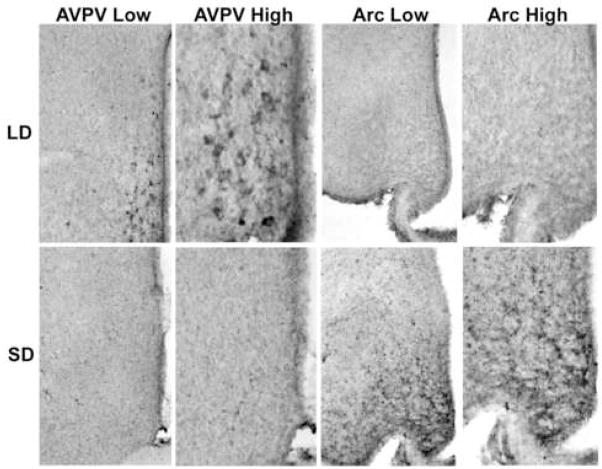
Kisspeptin-ir cell bodies were concentrated in the AVPV and Arc nuclei. In both nuclei, kisspeptin expression was significantly altered by photoperiodic condition (p<0.05 in each case) (Figures 1 and 2). LD hamsters exhibited a significantly greater number of kisspeptin-ir neurons in the AVPV compared to SD animals (p<0.05; Figures 1a and 2). The inverse was true for kisspeptin-ir expression in the Arc; females held in SD conditions had a greater number of kisspeptin-ir cells compared to LD animals (p>0.05; Figures 1a and 2). Neither cell size nor optical density differed between groups in the AVPV (p>0.05) (Figure 1b, c). As expected, photoperiod significantly affected body and reproductive organ masses, with hamsters held in short day conditions having lower body mass (mean±SEM: LD = 39.1±1.74 g, SD = 25.8±1.07 g, p<0.05) and smaller reproductive organ masses (mean±SEM: LD = 0.15±0.01g and SD = 0.048±0.004 g, p<0.05) compared to LD females.
Figure 1.
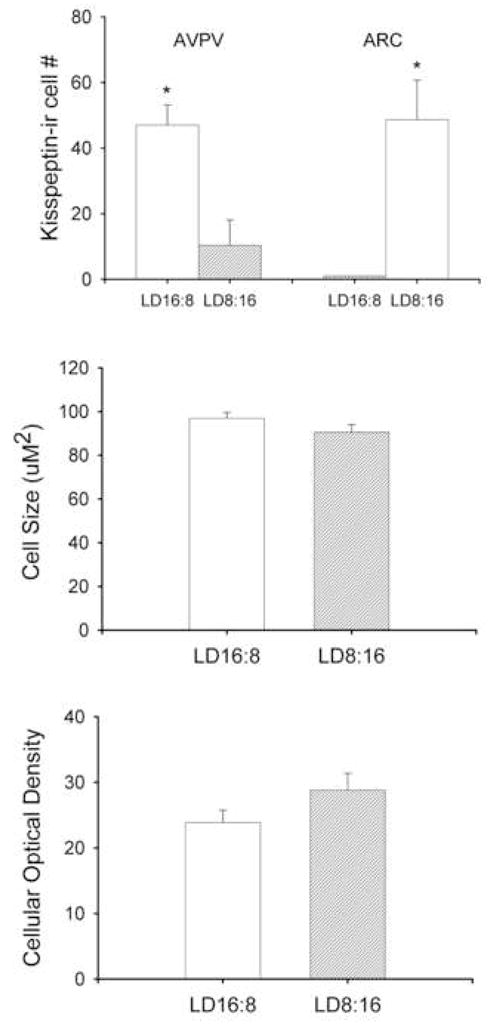
Photoperiod affects kisspeptin-ir cell numbers in the AVPV and Arc. Animals held for 8 wk in short day photoperiods display significantly fewer cells in the AVPV and significantly more cells in the Arc compared to animals held in long day photoperiods (a). Cell size (b) and optical density (c) were not affected in the AVPV by photoperiod. Because kisspeptin-ir cells were absent in the Arc of LD16:8 animals, cell size and optical density were not evaluated. * p<0.05
Figure 2.
Representative photomicrographs of kisspeptin-ir cell labeling in the AVPV and Arc of hamsters held in either long (top) or short (bottom) day lengths. Low (100×) and high power (200×) photomicrographs are shown for each condition and brain region investigated.
Experiment 2: Endocrine response to exogenous kisspeptin
The effects of kisspeptin on LH secretion were dependent upon the photoperiod to which the animals were exposed. LD hamsters responded to injections of kisspeptin with a significant increase in LH concentrations compared to animals receiving vehicle (p<0.05). In contrast, peripheral injections of kisspeptin did not elicit a rise in serum LH concentrations in SD hamsters (p>0.05 relative to baseline and vehicle controls; Figure 3). As in experiment 1, photoperiod significantly affected reproductive organ masses, with LD hamsters having significantly heavier (0.11 ± 0.017g) ovary + uterine horn masses than SD animals (0.047 ± 0.0039g) (p<0.05).
Figure 3.
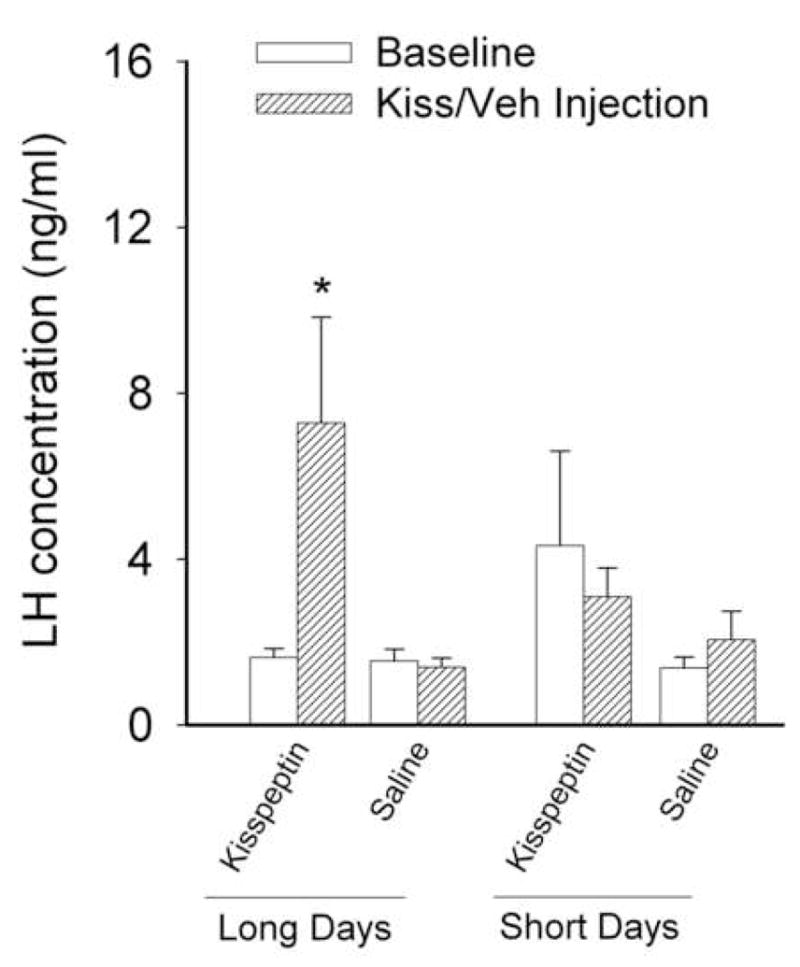
Effect of kisspeptin on gonadotropin release. Peripheral injection (i.p.) of kisspeptin significantly elevated levels of pituitary luteinizing hormone (LH) in animals held in long days relative to baseline or saline injections. Females held in short days for eight weeks did not show a significant elevation in LH concentrations after kisspeptin injection relative to baseline or saline injections. * p<0.05
Experiment 3: Endocrine response to exogenous kisspeptin after treatment with GnRH antagonist, acyline
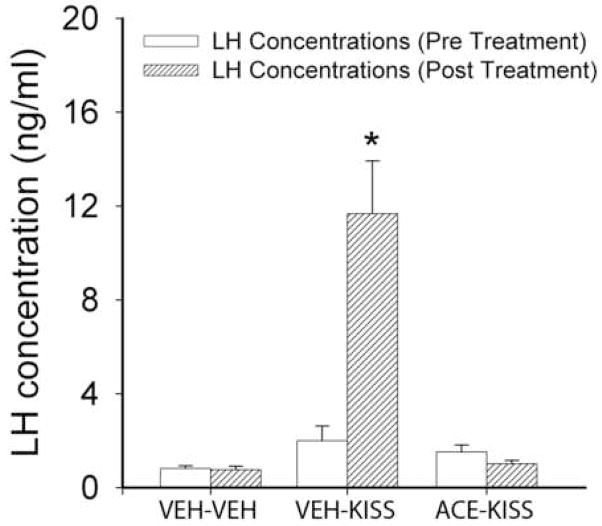
A single kisspeptin injection significantly increased LH concentrations in LD hamsters (p<0.05; Figure 4). Pre-treatment with acyline, however, prevented the rise in LH concentrations following administration of kisspeptin (p<0.05 relative to vehicle-treated animals). These findings are consistent with previous studies indicating that kisspeptin is acting at the level of the GnRH system rather than the pituitary.
Figure 4.
Mean (±SEM) concentrations of LH before and following kisspeptin (KISS) injections preceded by vehicle (VEH) or acyline (ACE). Animals receiving kisspeptin injections show a significant elevation in LH concentrations that is blocked by pretreatment with acyline. * p<0.05
Discussion
The results of the present study demonstrate a critical role for kisspeptin in the interpretation and integration of reproductively relevant environmental signals and transmission of this information to the GnRH neuronal network. We observed striking differences in kisspeptin-ir expression following manipulation of photoperiod in two cell populations, the AVPV and Arc. Female Siberian hamsters maintained in ‘summer’ photoperiods exhibited robust kisspeptin expression in the AVPV, with virtually no labeling in the Arc. In contrast, females held in ‘winter’ day lengths had minimal kisspeptin-ir expression in the AVPV, but significant expression in the Arc. These findings suggest divergent roles for these two populations of kisspeptin neurons. Unlike male Siberian hamsters, that inhibit reproduction by reducing kisspeptin expression in the AVPV while maintaining GnRH sensitivity to this peptide (Greives et al., 2007), females housed in short days failed to show an endocrine response to exogenous kisspeptin. These findings suggest important sex differences in HPG responsivity with females exhibiting a dual mechanism of control, both reducing AVPV kisspeptin expression and reproductive axis sensitivity to this peptide. Finally, consistent with other mammalian species studied to date (rat, mouse, and macaque)(Gottsch et al., 2004; Irwig et al., 2004; Plant et al., 2006), kisspeptin produces its effects in Siberian hamsters by acting principally on the GnRH system rather than the pituitary.
The result of natural selection is organisms that are continuously adapting to their environment, and proper timing of reproduction is key to this process. Diverse selective pressures have resulted in a tightly regulated reproductive axis that is highly sensitive to a multitude of internal and external environmental cues, which provide crucial information about the present state of the environment (Clarke and Pompolo, 2005; Gore, 2004; Kriegsfeld, 2006). The fundamental role that GnRH plays in regulating reproduction and fertility has been unequivocally established, however, the upstream mechanisms that integrate the state of the organism and environmental conditions are less well understood.
The AVPV has been identified as a critical brain region mediating the positive feedback effects of estrogen, crucial to the initiation of the preovulatory LH surge (Wintermantel et al., 2006). Kiss-1 mRNA expression is at a maximum in the AVPV at the time of the LH surge and these neurons show peak activity, as measured by FOS expression, on the afternoon of proestrous (Smith et al., 2006). Results of the present study suggest an additional role for kisspeptin neurons in the AVPV, namely as an upstream center for the integration of reproductively relevant external stimuli with the steroidal signals that are necessary to trigger ovulation. Whereas inputs to kisspeptin cells have not been investigated extensively, melatonin does not act on the AVPV directly to induce reproductive quiescence. In Siberian hamsters, the SCN is a critical locus for the decoding of the short-day melatonin signal (Bartness et al., 1991; Teubner and Freeman, 2007). Because the SCN projects extensively to the AVPV (Kriegsfeld et al., 2004; Leak and Moore, 2001), it is likely that the SCN is driving seasonal changes in kisspeptin cells.
Unlike our previous findings in male Siberian hamsters (Greives et al., 2007), the present findings indicate that the effects of kisspeptin are regulated via two levels of control in females. Not only is kisspeptin expression altered in the AVPV and the Arc in response to changes in day length, but the sensitivity of the GnRH system to this peptide is also changed. In contrast to short-day male hamsters (Greives et al., 2007), the current study found that females with a regressed reproductive axis did not respond to peripheral kisspeptin injections with an elevation in LH concentrations (Figure 3). In males, LH release is elicited by exogenous kisspeptin treatment regardless of reproductive condition (Greives et al., 2007). The selective pressures impacting the timing of male and female fertility are likely to differ in significant ways and this may be reflected in the underlying mechanisms controlling seasonal reproduction (Beery et al., 2006). A winter pregnancy would likely result in death of both mother and offspring, due to the overwhelming increase in energetic demands, whereas males would not experience the pressures of gestation, lactation, and parental care. Given the minimal likelihood of offspring survival during winter, the increased energetic demand required for maintenance of the reproductive axis outweighs the potential reproductive benefits in males. Females appear to have evolved a ‘failsafe’ mechanism to ensure that pregnancy will not occur during inappropriate times of year while males may have evolved an increased sensitivity to other environmental variables to breed opportunistically as ambient conditions allow.
Whereas a role for the AVPV in direct regulation of ovulation has been well established (Wintermantel et al., 2006), the role of the Arc in this process remains less well specified. The Arc monitors current energy state and relays this information to the reproductive axis (Smith and Grove, 2002). In times of reduced energy availability, reproduction is inhibited (Bartness, 1996; Schneider et al., 2000). In addition to mediating the reproductive axis through energetic signaling, estrogen-receptor-positive Arc projections also act on GnRH terminals in the median eminence to modulate their output (Lehman, 1997). Whether kisspeptin neurons specifically mediate these Arc effects on the reproductive axis represent an interesting topic for further inquiry. That Arc kisspeptin neurons exhibit high expression during times of reproductive quiescence argues against a stimulatory role for this population of cells. Alternatively, these cells may inhibit release of kisspeptin during winter, leading to their greater detection with immunohistochemistry. Distinguishing among these competing hypotheses will require converging approaches investigating release/production rates of kisspeptin in AVPV and Arc populations. An additional possibility is that the Arc kisspeptin cells perform a function unrelated to reproductive control yet to be determined.
Previous studies have shown that kisspeptin expression is highly modulated by the level of circulating gonadal sex steroids. In rats, castration results in a significant increase in Kiss-1 mRNA expression in the Arc, an effect reversed by testosterone replacement (Smith et al., 2005). These results, as well as those from the present study, suggest the possibility that kisspeptin responds to levels of sex steroids rather than drives reproductive system involution. However, recent findings in Syrian hamsters (Mesocricetus auratus) indicate that photoperiod drives changes in kisspeptin directly rather than through changes in gonadal steroids (Revel et al., 2006). More specifically, Kiss-1 mRNA in short-day hamsters given testosterone replacement does not differ from short-day controls (Revel et al., 2006). Whether or not photoperiod-induced changes in kisspeptin expression in female hamsters are also independent of gonadal steroids requires further study. Whereas the estrous cycle of Syrian hamsters is remarkably precise (Dodge et al., 2002), cycles in female Siberian hamsters cannot be tracked reliably and the relationship between vaginal cell cytology and ovarian follicular development is questionable (Anand et al., 2004; Dodge et al., 2002; McMillan and Wynne-Edwards, 1999). As a result, this precluded our ability to examine the role of estrogen on kisspeptin expression in Siberian females under natural conditions. Siberian and Syrian hamsters also differ in the brain regions that appear to be critical in the interpretation of photoperiodic information, with the dorsomedial hypothalamus being essential in Syrian hamsters (Maywood et al., 1996) and the SCN being crucial in Siberians (Bartness et al., 1993; Bittman et al., 1991; Bronson, 1985). Future studies investigating the similarities and differences in melatonin signal transduction and kisspeptin control among photoperiodic species will be necessary to fully appreciate the evolution of seasonality.
In summary, kisspeptin acts as an important integration point mediating the interpretation and relay of environmental stimuli relevant for reproduction. Inhibitory photoperiods produce divergent effects on AVPV and Arc populations of cells, suggesting that these cells may have different roles in reproductive axis regulation. Female hamsters utilize a dual mechanism of control over the GnRH neuronal network, altering kisspeptin peptide levels and sensitivity of the GnRH system to kisspeptin signaling. This mode of reproductive axis suppression ensures that females will not breed during inappropriate times of year. This finding contrasts with that seen in male Siberian hamsters, where kisspeptin expression is altered without changes in sensitivity to the peptide (Greives et al., 2007). Together, these findings further place kisspeptin in a position of marked importance in the regulation of reproduction and thus reproductive success. Furthermore, these results demonstrate the utility of a photoperiod model for investigations of both the ecological and physiological factors regulating mammalian reproduction.
Acknowledgments
We thank Dr. Richard Blye of the NIH NICHD for kindly providing acyline. We also thank Stephanie Humber for technical assistance and Ilia Karatsoreos for helpful comments on an earlier version of this manuscript. Supported by NIH grant HD050470 and the UC Berkeley Committee on research grant to LJK and NSF grant IOB-0543798, a Faculty Research Support Program (FRSP) grant and a Center for the Integrative Study of Animal Behavior grant to GED.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand S, et al. Chemosensory stimulation of luteinizing hormone secretion in male Siberian hamsters (Phodopus sungorus) Biol Reprod. 2004;70:1033–40. doi: 10.1095/biolreprod.103.019380. [DOI] [PubMed] [Google Scholar]
- Bartness TJ. Photoperiod, sex, gonadal steroids, and housing density affect body fat in hamsters. Physiol Behav. 1996;60:517–29. doi: 10.1016/s0031-9384(96)80027-8. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, et al. SCN lesions block responses to systemic melatonin infusions in Siberian hamsters. Am J Physiol. 1991;260:R102–12. doi: 10.1152/ajpregu.1991.260.1.R102. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, et al. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–90. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Beery AK, et al. Sex differences in the onset of seasonal reproductive quiescence in hamsters. Proc Biol Sci. 2006 doi: 10.1098/rspb.2006.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman EL, et al. Suprachiasmatic and paraventricular control of photoperiodism in Siberian hamsters. Am J Physiol. 1991;260:R90–101. doi: 10.1152/ajpregu.1991.260.1.R90. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian reproduction: an ecological perspective. Biol Reprod. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian Reproductive Biology. University of Chicago Press; Chicago, IL: 1991. [Google Scholar]
- Carter DS, Goldman BD. Progonadal role of the pineal in the Djungarian hamster (Phodopus sungorus sungorus): mediation by melatonin. Endocrinology. 1983;113:1268–73. doi: 10.1210/endo-113-4-1268. [DOI] [PubMed] [Google Scholar]
- Chappell PE, et al. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology. 1997;138:4147–52. doi: 10.1210/endo.138.10.5456. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Pompolo S. Synthesis and secretion of GnRH. Anim Reprod Sci. 2005;88:29–55. doi: 10.1016/j.anireprosci.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Dodge JC, et al. Male-induced estrus synchronization in the female Siberian hamster (Phodopus sungorus sungorus) Physiol Behav. 2002;77:227–31. doi: 10.1016/s0031-9384(02)00851-x. [DOI] [PubMed] [Google Scholar]
- Dungan HM, et al. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–8. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Gore AC. Gonadotropin-releasing hormone neurons: multiple inputs, multiple outputs. Endocrinology. 2004;145:4016–7. doi: 10.1210/en.2004-0855. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Environmental induction of photononresponsiveness in the Siberian hamster, Phodopus sungorus. Am J Physiol. 1997;272:R887–95. doi: 10.1152/ajpregu.1997.272.3.R887. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, et al. Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol Cell Endocrinol. 2006;254–255:91–6. doi: 10.1016/j.mce.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Greives TJ, et al. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology. 2007;148:1158–66. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- Han SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–56. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwig MS, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ. Driving reproduction: RFamide peptides behind the wheel. Horm Behav. 2006;50:655–66. doi: 10.1016/j.yhbeh.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, et al. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J Comp Neurol. 2004;468:361–79. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol. 2001;433:312–34. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Goodman Robert L, Karsch Fred J, Jackson Gary L, Berriman Sandra J, Jansen Heiko T. The GnRH System of Seasonal Breeders: Anatomy and Plasticity. Brain Research Bulletin. 1997;44:445–457. doi: 10.1016/s0361-9230(97)00225-6. [DOI] [PubMed] [Google Scholar]
- Matsui H, et al. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–8. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- Maywood ES, et al. Lesions of the melatonin- and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biol Reprod. 1996;54:470–7. doi: 10.1095/biolreprod54.2.470. [DOI] [PubMed] [Google Scholar]
- McMillan HJ, Wynne-Edwards KE. Divergent reproductive endocrinology of the estrous cycle and pregnancy in dwarf hamsters (phodopus) Comp Biochem Physiol A Mol Integr Physiol. 1999;124:53–67. doi: 10.1016/s1095-6433(99)00090-2. [DOI] [PubMed] [Google Scholar]
- Messager S, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–6. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, et al. Mechanisms of seasonal cycles of behavior. Annu Rev Psychol. 1990;41:81–108. doi: 10.1146/annurev.ps.41.020190.000501. [DOI] [PubMed] [Google Scholar]
- Nishio T, et al. Cellular localization of nerve growth factor-like immunoreactivity in adult rat brain: quantitative and immunohistochemical study. Neuroscience. 1994;60:67–84. doi: 10.1016/0306-4522(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Plant TM, et al. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–13. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, et al. Photoperiodic polyphenisms in rodents: neuroendocrine mechanisms, costs, and functions. Q Rev Biol. 2001;76:293–325. doi: 10.1086/393989. [DOI] [PubMed] [Google Scholar]
- Revel FG, et al. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr Biol. 2006;16:1730–5. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Schneider JE, et al. Metabolic control of food intake and estrous cycles in syrian hamsters. I. Plasma insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2000;278:R476–85. doi: 10.1152/ajpregu.2000.278.2.R476. [DOI] [PubMed] [Google Scholar]
- Seminara SB. Metastin and its G protein-coupled receptor, GPR54: critical pathway modulating GnRH secretion. Front Neuroendocrinol. 2005;26:131–8. doi: 10.1016/j.yfrne.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Shahab M, et al. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–34. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–84. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Smith JT, et al. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–94. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Grove KL. Integration of the regulation of reproductive function and energy balance: lactation as a model. Front Neuroendocrinol. 2002;23:225–56. doi: 10.1016/s0091-3022(02)00002-x. [DOI] [PubMed] [Google Scholar]
- Teubner BJ, Freeman DA. Different neural melatonin-target tissues are critical for encoding and retrieving day length information in Siberian hamsters. J Neuroendocrinol. 2007;19:102–8. doi: 10.1111/j.1365-2826.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- Voltura MB, Wunder BA. Effects of ambient temperature, diet quality, and food restriction on body composition dynamics of the prairie vole, Microtus ochrogaster. Physiol Zool. 1998;71:321–8. doi: 10.1086/515929. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev. 1992;16:235–72. doi: 10.1016/s0149-7634(05)80183-6. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–80. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe AM, et al. Blockade of singular follicle-stimulating hormone secretion and testicular development in photostimulated Djungarian hamsters (Phodopus sungorus) by a gonadotropin-releasing hormone antagonist. Biol Reprod. 1995;53:724–31. doi: 10.1095/biolreprod53.3.724. [DOI] [PubMed] [Google Scholar]