Abstract
Pain stimulates some behaviors (e.g. withdrawal responses) but depresses many other behaviors (e.g. feeding). Pain-stimulated behaviors are widely used in preclinical research on pain and analgesia, but human and veterinary medicine often rely on measures of functional impairment and pain-depressed behavior to diagnose pain or assess analgesic efficacy. In view of the clinical utility of measures of pain-depressed behaviors, our laboratory has focused on methods development for preclinical assays of pain-depressed behavior in rodents. The present study compared the effects of a chemical noxious stimulus (IP lactic acid injections) and an opioid analgesic (morphine) administered alone or in combination on the stretching response (a pain-stimulated behavior) and intracranial self-stimulation (ICSS; a behavior that may be depressed by pain) in rats. For the ICSS procedure, rats implanted with electrodes in the lateral hypothalamus responded for electrical stimulation across a range of current frequencies to permit rapid determination of frequency-rate curves and evaluation of curve shifts following treatment. Lactic acid alone produced a concentration-dependent stimulation of stretching and depression of ICSS, expressed as rightward shifts in ICSS frequency-rate curves. Morphine had little effect alone, but it produced a dose-dependent blockade of both acid-stimulated stretching and acid-depressed ICSS. Both lactic acid and morphine were equipotent in the stretching and ICSS procedures. These results suggest that ICSS may be useful as a behavioral baseline for studies of pain-depressed behavior.
Keywords: pain, analgesia, morphine, intracranial self-stimulation
INTRODUCTION
Preclinical assays of pain and analgesia necessarily include two elements: (1) a manipulation intended to produce a pain-like state (the independent variable), and (b) measurement of a response presumed to be indicative of that pain-like state (the dependent variable). In recent years, there have been significant advances in the types of manipulations used to model clinically relevant pain states [4, 27, 37]. However, the dependent measures in preclinical assays have been much slower to evolve [5, 35, 37, 49, 50]. The most widely used measures fall into a category that we have described as “pain-stimulated behaviors,” which can be defined as behaviors that increase in rate, frequency or intensity in response to a noxious stimulus [37, 46]. Common examples include withdrawal responses from stimuli that can be escaped (e.g. tail withdrawal from thermal stimuli) or stretching/flinching responses from stimuli that cannot be escaped (e.g. stretching responses elicited by intraperitoneal injection of chemical stimuli).
Although a focus on pain-stimulated behaviors can be useful for many applications, an exclusive reliance on these behaviors as dependent measures can be problematic for at least two reasons. First, drugs or other manipulations may decrease pain-stimulated behaviors not only by producing sensory effects (e.g. true analgesia, defined as a selective decrease in sensitivity to noxious stimuli), but also by producing motor effects (e.g. sedation, paralysis or other effects that decrease a subject’s ability to respond). Consequently, results obtained in assays of pain-stimulated behavior may be difficult to interpret, and assays of motor function (e.g. rotarod performance) are often conducted in parallel to aid in characterization of motor effects [44]. Second, pain states that requires clinical intervention are often associated with a depression of behavior rather than a stimulation of behavior, and in humans, pain-related depression of behavior is often accompanied by a co-morbid depression of mood [3, 28, 22, 17]. Indeed, diagnostic tools that measure pain-related depression of behavior and mood are coming to play an increasingly prominent role in human medicine [25, 10, 13], and measures of functional impairment/depressed behavior are also important in veterinary assessments of pain in animals [11].
In view of the clinical relevance and diagnostic utility of pain-depressed behaviors as dependent measures, we and others have begun to explore strategies for incorporating these behaviors into preclinical assays of pain and analgesia [32, 46, 33, 36]. Toward that end, the purpose of this study was to evaluate effects of a prototype noxious stimulus (intraperitoneal lactic acid) and analgesic drug (morphine) on intracranial self-stimulation (ICSS) in rats. Electrical brain stimulation can maintain high and stable rates of operant responding, and the present study used a type of ICSS procedure in which the frequency of stimulation varies across a wide range during daily experimental sessions to permit determination of frequency-rate curves. These curves can then be shifted both by pharmacological and non-pharmacological manipulations [34, 7, 39]. We hypothesized that IP lactic acid would depress ICSS responding, and that morphine would prevent acid-induced depression of ICSS. For comparison, effects of IP lactic acid and morphine were also evaluated on expression of the stretching response, a commonly used pain-stimulated behavior.
METHODS
Subjects
Thirty-one Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 325–375 g at the beginning of the studies were used for the studies of ICSS (n=5 per group) and lactic-acid-induced stretching (n=4 per group). Rats were individually housed and were maintained on a 12h light/dark cycle, with lights on from 7 a.m. to 7 p.m. Rats had free access to food and water except during testing. Animal maintenance and research were in compliance with NIH guidelines on care and use of animal subjects in research, and all animal use protocols were approved by the McLean Hospital Institutional Animal Care and Use Committee.
Intracranial Self-Stimulation (ICSS)
ICSS electrode implantation
Rats were anesthetized with an IP injection of a mixture of ketamine and xylazine (80 mg/kg: 12 mg/kg, Sigma, St. Louis, MO) and given subcutaneous (SC) atropine sulfate (0.25 mg/kg) to reduce bronchial secretions. Electrodes (monopolar, stainless steel; 0.25 mm in diameter; Plastics One, Roanoke, VA) were implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral from midsaggital suture, and 7.8 mm below dura). The electrodes were coated with polyamide insulation except at the flattened tip. Skull screws (one of which served as the ground) and the electrode were secured to the skull with dental acrylic. The animals were allowed to recover for at least 7 days prior to commencing ICSS training.
ICSS apparatus
Experiments were conducted in sound attenuating boxes that contained modular acrylic test chambers (20.96 × 30.48 × 24.13 cm) equipped with a response lever (4.5 cm wide, extended 2.0 cm through the center of one wall, 2.5 cm off the floor), a 2-W white house light, and an ICSS stimulator (Med Associates, St. Albans, VT). Electrodes were connected to the stimulator via a swivel connector (Model SL2C, Plastics One, Roanoke, VA). The stimulator was controlled by a computer software program that also controlled all the programming parameters and data collection (Med Associates, St. Albans, VT).
Behavioral Procedure
After initial shaping of lever-press responding, rats were trained under a continuous reinforcement schedule of brain stimulation using procedures described previously [48, 7, 39], Each lever press resulted in the delivery of a 0.5-s train of square wave cathodal pulses (0.1-ms pulse duration), and stimulation was accompanied by the illumination of the house light. Responses during the 0.5-s stimulation period did not earn additional stimulation. Initially, the frequency of stimulation was held constant at 126 Hz, and the stimulation intensity for each rat was adjusted gradually to the lowest value that would sustain a high rate of responding (>30 responses/min). This intensity was then held constant for the remainder of the study, and daily sessions consisted of sequential 15-min components. During each component, a descending series of 15 current frequencies (126–25 Hz in 0.05 log increments) was presented, with a 60-s trial at each frequency. A frequency trial was initiated by a 10-s “priming” phase, during which animals received non-contingent stimulation, followed by a 50-s “response” phase during which responding produced electrical stimulation under the continuous reinforcement schedule. Test sessions consisted of up to nine consecutive components. The first component of each test session was considered to be an acclimation component, and data from this component were discarded. Data from the second and third components were used to calculate control parameters of frequency-rate curves for that session (see Data Analysis). Treatment manipulations were introduced immediately after the third component, and effects were evaluated for an additional six components (lactic acid alone, morphine alone) or three components (morphine + lactic acid; see below).
In studies of lactic acid alone, an ascending series of lactic acid concentrations (1.0–3.2%, IP) was administered across sessions to identify conditions under which lactic acid decreased ICSS. Based on these findings, a concentration of 1.8% lactic acid was chosen for use in the morphine + acid studies (see Results). In studies of morphine alone, a series of morphine doses (1.0–5.6 mg/kg IP) was tested in ascending order from inactive doses to doses that decreased response rates in some rats. In studies of morphine + lactic acid, an ascending series of morphine doses (0.1–3.2 mg/kg IP) was administered immediately after the third component of the session, and rats were placed in their holding cages for 30 min. Following the 30 min pretreatment, animals were injected with 1.8 % lactic acid and placed back in the ICSS chambers for three additional ICSS components. Each set of treatments (lactic acid alone, morphine alone, morphine + lactic acid) was conducted in a group of 5 rats.
Test sessions were separated by at least three days (morphine alone studies) or at least one week (studies with lactic acid alone and morphine + lactic acid). Training sessions consisting of three to nine components were conducted on weekdays between test sessions. In addition, vehicle treatments were administered between each set of lactic acid and/or morphine treatments to ensure that there were no residual drug effects.
Histology
At the conclusion of the experiment, rats were euthanized with pentobarbital (130 mg/kg, IP) and perfused with 4% paraformaldehyde. The fixed brains were sliced in 40-μm sections for cresyl violet staining to confirm electrode placements.
Data Analysis
The primary dependent variable in this ICSS procedure was the response rate in responses/min during each frequency trial. To normalize these data, raw response rates from each trial were converted to Percent Maximum Control Rate (%MCR), with the maximum control rate defined as the mean of the maximal rates observed during any frequency trial of the second and third “control” components of that session. Thus, %MCR values for each trial were calculated as (Response Rate During a Frequency Trial ÷ Maximum Control Rate) × 100. ICSS frequency-rate curves were then constructed for each rat during each component by plotting % MCR as a function of log frequency. The horizontal position of each curve was described by the EF50, which was defined as the log frequency that maintained 50%MCR, and EF50 values were calculated by interpolation from the linear portion of each ICSS curve. Effects of acid alone, morphine alone, and morphine + acid treatments on ICSS curves were expressed as ΔEF50 (a measure of left or right lateral shifts in ICSS curves). The ΔEF50 was calculated as Experimental EF50 - Control EF50, with the Control EF50 defined as the mean of the EF50’s obtained during the second and third “control” components of that session, and Experimental EF50 defined as the EF50 obtained during each of the subsequent test components. Positive ΔEF50s indicate right shifts in ICSS curves and depressed ICSS behavior. The vertical position of each ICSS frequency-rate curve was described by the Peak % MCR, which was defined as the highest %MCR observed during any frequency trial of that component. ΔEF50 and Peak % MCR values were determined for each rat during each component, and these values were averaged to generate mean values and standard errors. One- or two-way ANOVA was used as appropriate to compare test ΔEF50 and Peak %MCR values after treatments with a “no effect” baseline (i.e. ΔEF50=0, Peak %MCR=100). A significant ANOVA was followed by the Bonferroni post hoc test, and the criterion for significance was set at p<0.05. In addition to these procedures for statistical analysis of drug effects on ICSS curves, raw data are also shown as response rates graphed as a function of log frequency for selected control and experimental conditions.
Lactic Acid-Induced Stretching
Behavioral Procedure
The acid-induced stretching assay used a within-subjects design such that each animal received all concentrations of lactic acid alone or after pretreatment with all doses of morphine during the course of the study. Tests were separated by at least one week. In studies of lactic acid alone, an ascending series of lactic acid concentrations (0.32–3.2%, IP) was administered to determine the lactic acid concentration-effect curve. Rats were placed in an acrylic test chamber (an empty housing cage) immediately after lactic acid administration, and the number of stretches was recorded in 10 min bins for a total of 60 min. In studies of morphine + lactic acid, morphine (0.1–3.2 mg/kg IP) was administered and animals were placed in the test chamber for 30 min. After this 30 min pretreatment period, rats received an IP injection of 1.8% lactic acid, and stretching was recorded for 30 min after lactic acid administration. Scoring of stretching behavior was conducted as described previously for studies in mice [46]. Specifically, a stretch was operationally defined as a contraction of the abdomen followed by an extension of the hind limbs.
Data Analysis
For the study of lactic acid alone, the primary dependent measure was the number of stretches per 10 min bin. Data were analyzed by two-way ANOVA with lactic acid concentration and time as the two factors. For the study of morphine + lactic acid, the primary dependent measure was the total number of stretches in the 30-min observation period, and data were analyzed by a one-way ANOVA, with morphine dose as the single factor. A significant ANOVA was followed by the Bonferroni post hoc test, and the criterion for significance was set at p<0.05.
Drugs
Lactic acid was purchased from Sigma (St. Louis, MO) and was diluted in sterile water and administered IP in a volume of 1.0 ml/kg. Morphine sulfate (provided by the National Institute on Drug Abuse, Bethesda, MD) was dissolved to a final concentration of 50 mg/ml in sterile saline, and dilutions were made with 0.9% saline. Morphine was administered IP in a volume of 1 ml/kg.
RESULTS
Baseline patterns of ICSS
Under baseline conditions, there was a monotonic relationship between ICSS frequency and response rate, and illustrative ICSS frequency-rate curves are shown in the lower right panels (Panel D) of figures 1–3. In general, ICSS frequencies of 2.10 to 1.80 log Hz (126–63 Hz) maintained maximal response rates of approximately 100 responses per min. Response rates usually declined as the ICSS frequency was lowered from 1.75 to 1.65 log Hz (56–45 Hz), and ICSS frequencies below 1.65 log Hz (45 Hz) usually failed to maintain responding. Across the study, the average maximal control rate ± SEM was 99.4 ± 0.65 responses per min. The average control EF50 ± SEM was 1.80 ± 0.02 log Hz.
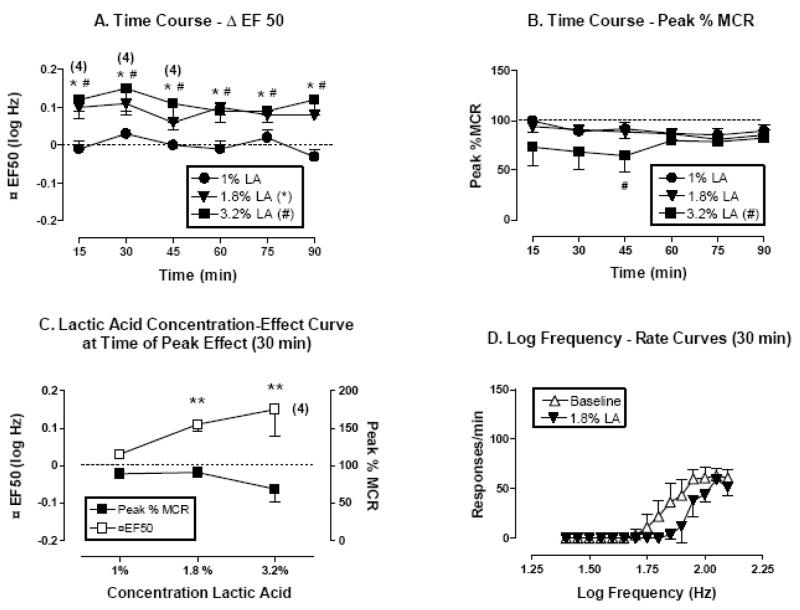
Figure 1.
Effect of lactic acid (1–3.2 %, IP) on ICSS in rats. All data points show mean data ± S.E.M. from five rats except where indicated by numbers in parentheses. In these cases, the number indicates the number of rats in which ΔEF50 values could be calculated. (A) Time course of effects on ΔEF50. Abscissa: Time in min after acid injection. Ordinate: ΔEF50 in log Hz. (B) Time course of effects on Peak % MCR. Abscissa: Time in min after acid injection. Ordinate: Peak % MCR. (C) Dose-effect curve at time of peak effect on ΔEF50 (30 min). Abscissa: Lactic acid concentration (log scale). Left ordinate: ΔEF50. Right ordinate: Peak %MCR. (D) Selected log frequency-rate curves. Abscissa: Log frequency of electrical stimulation in Hz. Ordinate: Response rate in responses per min. Curves are shown for representative baseline data and for data collected 30 min after administration of 1.8% lactic acid. *P<0.05, 1.8 % lactic acid compared to baseline, #P<0.05, 3.2 % lactic acid compared to saline; Bonferroni’s post hoc.
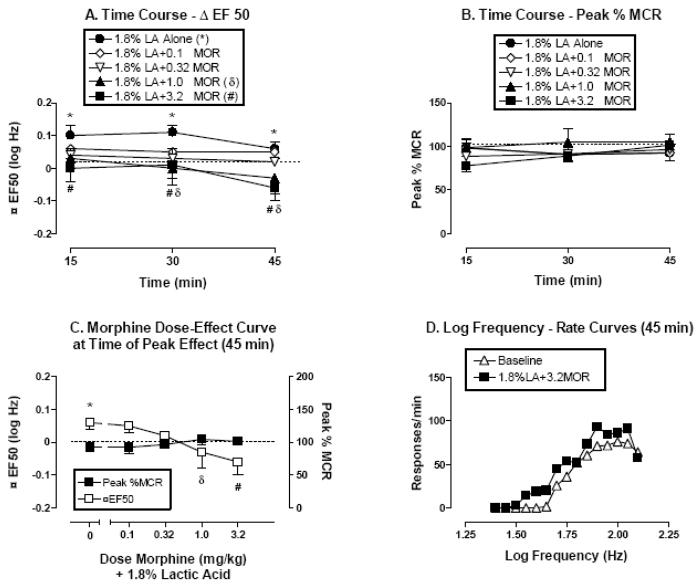
Figure 3.
The effects of lactic acid alone (1.8%, IP) or 30 min after pretreatment with morphine (0.1–3.2 mg/kg, IP) on ICSS in rats. All data points show mean data ± S.E.M. from five rats. (A) Time course of effects on Δ EF50. Abscissa: Time in min after lactic acid injection. Ordinate: ΔEF50 in log Hz. (B) Time course of effects on Peak % MCR. Abscissa: Time in min after lactic acid injection. Ordinate: Peak % MCR. (C) Dose-effect curve at time of peak morphine effect on lactic acid-elevated ΔEF50 values (45 min). Abscissa: Dose of morphine in mg/kg (log scale) administered as a pretreatment to 1.8% lactic acid. Left ordinate: ΔEF50. Right ordinate: Peak %MCR. (D) Selected log frequency-rate curves. Abscissa: Log frequency of electrical stimulation in Hz. Ordinate: Response rate in responses per min. Curves are shown for representative baseline data and for data collected 45 min after administration of 3.2 mg/kg morphine + 1.8% lactic acid. *P<0.05, 1.8 % lactic acid compared to baseline, δP<0.05, 1.0 mg/kg morphine + 1.8 % lactic acid compared to 1.8% lactic acid alone, #P<0.05, 3.2 mg/kg morphine + 1.8 % lactic acid compared to 1.8% lactic acid alone; Bonferroni’s post hoc.
Treatment effects on ICSS
In figures 1–3, the top panels show the time course of treatment effects on EF50 values (expressed as ΔEF50, top left panels A) and on maximal response rates (expressed as Peak %MCR, top right panels B). The lower panels show curves that relate the experimental manipulation to ΔEF50 or Peak %MCR at the time of peak effect (lower left panels C), and illustrative frequency-rate curves for selected manipulations (lower right panels D).
Lactic acid alone (1–3.2%) produced a concentration-dependent depression of ICSS for the entire test session (figure 1A). A two-factor ANOVA for Δ EF50 indicated significant effects of lactic acid [F(3,75)=10.98, P<0.0001], but not of time course [F(5,75)=1.72, P=0.14] or the interaction [F(15,75)=1.19, P=0.30]. A concentration of 1.8% lactic acid produced a significant rightward shift in the frequency-rate curve (expressed as significant increases in ΔEF50) without a change in maximal response rates (expressed as Peak%MCR). Following treatment with a higher concentration of 3.2 % lactic acid, one of five rats did not respond at sufficiently high rates to determine an EF50 value for the first three components of the test session (15–45 min; figure 1A). A two-factor ANOVA for Peak % MCR indicated significant effects of lactic acid [F(3,80)= 4.07, P=0.03], but not of time course [F(5,80)=0.58, P=0.71] or the interaction [F(15,80)=0.81, P=0.66]. The 3.2 % lactic acid concentration tended to decrease Peak %MCR with a significant decrease at 45 min (figure 1B). Figure 1C shows summary concentration-effect curves for lactic acid at the time of peak effect on ΔEF50 (30 min). A one-factor ANOVA revealed significant effects of lactic acid on ΔEF50 [F(3,15)= 5.35, P<0.01] but not on Peak % MCR [F(3,16)=2.09, P=0.14]. Figure 1D shows the effect of 1.8 % lactic acid on the raw frequency-rate curve. This concentration of 1.8% lactic acid produced a rightward shift in the frequency-rate curve without reducing maximum response rates, and for this reason, a concentration of 1.8% lactic acid was used for subsequent studies with morphine.
Figure 2 shows the effects of saline or morphine alone (1–5.6 mg/kg) on ICSS. A two-factor ANOVA for ΔEF50 indicated no significant effects of morphine treatment (1.0–3.2 mg/kg) [F(2,60)=0.08, P=0.93], time course [F(5,60)=0.96, P=0.45], or the interaction between morphine and time [F(10,60)=1.56, P=0.14]. Similarly, a two-factor ANOVA for Peak % MCR indicated no significant effects of morphine treatment [F(3,80)= 1.86, P=0.18], time course [F(5,80)=1.12, P=0.36], or the interaction between morphine and time [F(15,80)=1.06, P=0.41]. The highest dose of 5.6 mg/kg morphine tended to reduce Peak %MCR as the session progressed (figure 2B), and rate suppression was sufficient in two of five rats that EF50 values could not be calculated for the last five components of the test session (30–90 min; figure 2A). Because of this evidence for rate suppression in 2 of 5 rats, higher morphine doses were not tested.
Figure 2.
The effects of saline or morphine (1–5.6 mg/kg, IP) on ICSS in rats. All data points show mean data ± S.E.M. from 5 rats except where indicated by numbers in parentheses. In these cases, the number indicates the number of rats in which ΔEF50 values could be calculated. (A) Time course of effects on ΔEF50. Abscissa: Time in min after drug injection. Ordinate: ΔEF50 in log Hz (B) Time course of effects on Peak % MCR. Abscissa: Time in min after drug injection. Ordinate: Peak % MCR. (C) Dose-effect curve at time of peak effect of morphine on response rates (45 min). Abscissa: Dose of morphine in mg/kg (log scale). Left ordinate: ΔEF50. Right ordinate: Peak %MCR. (D) Selected log frequency-rate curves. Abscissa: Log frequency of electrical stimulation in Hz. Ordinate: Response rate in responses per min. Curves are shown for representative baseline data and for data collected 45 min after administration of 3.2 mg/kg morphine. §Indicates that statistical analyses on ΔEF50 values could not be performed with the 5.6 mg/kg morphine treatment group between the 30–90 min time points because ΔEF50 values could not be calculated in two rats.
Figure 3 shows the effects of lactic acid administered alone or 30 min after pretreatment with morphine (0.1–3.2 mg/kg). A Two-factor ANOVA for ΔEF50 indicated significant effects of treatment [F(5,48)=3.07, P=0.03] and time course [F(2,48)=9.89, P=0.0003], but not of the interaction [F(10, 48)= 1.33, P=0.24]. As in the original studies of lactic acid alone, 1.8 % lactic acid alone depressed ICSS and increased ΔEF50 values without decreasing peak response rates. A two-factor ANOVA for Peak % MCR indicated no significant effects of treatment [F(5,48)=0.99, P=0.44], time course [F(2,48)=1.36, P=0.27], or the interaction [F(10, 48)= 1.5, P=0.17]. The results suggest that morphine pretreatment produced a dose-dependent, statistically significant and complete blockade of acid-induced depression of ICSS without concomitant changes in Peak %MCR. Thus, morphine doses that had no effect on ICSS alone completely prevented lactic acid-induced depression of ICSS. The lowest morphine dose to significantly attenuate lactic acid-induced depression of ICSS was 1.0 mg/kg. A higher dose of 3.2 mg/kg morphine also significantly prevented acid-depressed ICSS while also producing a small leftward shift in the frequency-rate curve (figure 3D) and small decreases in ΔEF50 values (figures 3A and C) after 45 min. However, ΔEF50 values determined after treatment with 3.2 mg/kg morphine and lactic acid were not significantly different from zero at any time.
Treatment effects on stretching behavior
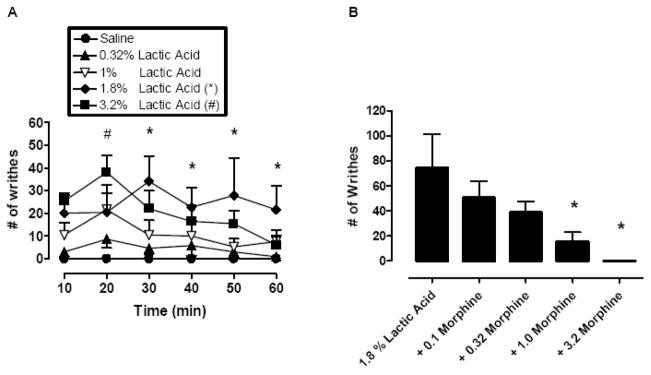
Figure 4A shows the effects of lactic acid (0.32 - 3.2 %, IP) on stretching behavior. A two-factor ANOVA indicated significant effects of lactic acid [F(4,75)=4.82, P=0.01], time course [F(5,75)=3.0, P=0.02], and the interaction between lactic acid and time [F(20,75)=1.95, P=0.02]. Lactic acid produced a concentration- and time-dependent stimulation of stretching with peak effects occurring between 20–30 min after acid administration. The lowest concentration of lactic acid to produce a significant stimulation of stretching was 1.8%. The levels of stretching produced by this acid concentration were relatively stable across the session, although effects were significantly different from saline treatment only during the last four 10-min bins (30–60 min). A higher concentration of 3.2 % lactic acid also significantly stimulated stretching, but this effect was significant only during the second 10-min bin (20 min). Stretching decreased later in the session, and rats also exhibited a marked decrease in general mobility, exploration, and grooming (data not shown). Overall, the effects of lactic acid dissipated after 60 min and animals exhibited normal behavior by 90 min after treatment (data not shown).
Figure 4.
Left panel: The effects of saline or lactic acid (0.32 – 3.2 %, IP) on the expression of stretching in rats. All data points show mean data ± S.E.M. from four rats. (A) Abscissa: Time after lactic acid injection (per 10 min bins). Ordinate: Number of stretches. *P<0.05, 1.8 % lactic acid compared to saline, #P<0.05, 3.2 % lactic acid compared to saline; Bonferroni’s post hoc. Right panel: The effects of morphine (0.1–3.2 mg/kg, IP) + lactic acid (1.8%, IP) on stretching in rats. All data points show mean data ± S.E.M. from four rats. (B) Abscissa: Dose of morphine in mg/kg administered 30 min before lactic acid. Ordinate: Number of stretches during 30 min observation period. *P<0.05, 1.0 and 3.2 mg/kg morphine + lactic acid compared to lactic acid alone; Bonferroni’s post hoc.
Figure 4B shows the effects of 30 min morphine pretreatment (0.1–3.2 mg/kg, IP) on stretching induced by 1.8 % lactic acid. Morphine alone did not elicit stretching during the pretreatment period (data not shown). However, morphine produced a dose-dependent decrease in lactic acid-induced stretching [F(3,12)=6.35, P=0.008], and the lowest dose to produce a significant effect was 1.0 mg/kg morphine
Observational studies of general health
In both the ICSS and stretching studies, animals were exposed to repeated treatments and procedures. In the ICSS studies, behavior was stable during baseline sessions between test sessions. Following repeated treatments with lactic acid, animals achieved normal weight gain, expressed normal behaviors (feeding, grooming, social interaction, and locomotor activity) and showed no observable adverse effects between test sessions and across the duration of the study.
Histology
Histological analyses of rat brain sections confirmed that ICSS electrode tips were inserted in the left medial forebrain bundle at the level of the lateral hypothalamus (data not shown). The electrode placements were indistinguishable from those depicted previously [8, 48, 30].
DISCUSSION
Behavioral depression is a common sign of pain in both human and veterinary medicine, and one important goal of therapy and analgesic treatment is to restore pain-depressed behavior. The present study was conducted as part of a general effort to develop efficient and reliable assays of pain-depressed behavior for preclinical studies. The main findings of this study were that (a) a commonly used noxious stimulus (IP acid injection) produced a concentration-dependent depression of ICSS in rats, and (b) this pain-related depression of behavior was dose-dependently prevented by pretreatment with morphine doses that alone had no effect on ICSS. Lactic acid and morphine were equipotent in this ICSS assay of pain-depressed behavior and a stretching assay of pain-stimulated behavior. Thus, the putative pain state produced by IP lactic acid depressed one behavior (ICSS) while stimulating another (stretching), and the opioid analgesic morphine was equieffective in preventing both pain-related changes in behavior. These results support the hypothesis that ICSS may be useful as a sensitive behavioral baseline for preclinical studies to assess both pain-related functional impairment of motivated behavior and restoration of pain-depressed behavior by candidate analgesic drugs or other manipulations.
Effects of IP Lactic Acid on ICSS
Lactic acid depressed ICSS at concentrations that also stimulated stretching, another behavior that is commonly used to assess nociception in rodents. These results using ICSS as a behavioral baseline confirm and extend our previous study showing that IP acetic acid produced a concentration-dependent reduction in another motivated behavior, consumption of a favored food in mice [46]. Other preclinical studies have also shown that putative pain states can produce functional impairment and decreases in rates of behavior. In one early approach, for example, intra-articular injections of formalin produced inflammation and decreased use of the affected limb during a treadmill-walking task in dogs [41, 40]. In more recent studies in rats, laparotomy was found to decrease locomotor activity and rates of food-maintained operant responding [32], bilateral inflammation of the knee joints reduced spontaneous locomotion in a novel environment [33], and a chronic constriction nerve injury decreased open-arm ambulation in an elevated plus maze (an anxiety like behavior) [42]. In each case, pain-depressed behavior could be prevented or reversed with opioid or non-steroidal anti-inflammatory analgesics.
A potential confound in studies of pain-depressed behavior is that reductions in behavior may be produced by non-selective decreases in a subject’s ability to respond or by the stimulation of other behaviors (e.g. stretching) that could compete with the target behavior. The ICSS procedure used in the present study permits a direct method for evaluating this potential confound. Specifically, the procedure rapidly generates a wide range of response rates maintained by a range of different current frequencies, and multiple frequency-rate curves can be determined in each daily session. Effects of the putative pain state can then be evaluated on these full frequency-rate curves. Thus, in the present study, a concentration of 1.8% lactic acid produced a rightward shift in the frequency-rate curve and a decrease in response rates maintained by intermediate current frequencies. However, high current frequencies still maintained maximum response rates, indicating that rats were motorically capable of responding at high rates, and that acid-stimulated stretching did not prevent responding. This ability with ICSS to efficiently evaluate effects of a putative pain stimulus on a wide range of response rates maintained by a wide range of reinforcer magnitudes constitutes one key advantage of ICSS as a behavioral baseline for studies of pain-depressed behavior.
Rightward shifts in ICSS frequency-rate curves are similar to effects produced by directly reducing the intensity of electrical stimulation, and as a result, right shifts in frequency-rate curves are often interpreted as evidence that a manipulation produces an anhedonia-like or depression-like decrease in sensitivity to the positive reinforcing effects of brain stimulation [34, 7, 39]. Similar rightward shifts in ICSS frequency-rate curves or increases in ICSS thresholds can also be produced by other prodepressant manipulations including (1) drugs that produce dysphoric effects in humans (dopamine D2 receptor antagonists, kappa opioid receptor agonists; [15, 3, 6], (2) withdrawal from drugs of abuse (mu opioid agonists, cocaine, amphetamine; [43, 31, 12], (3) some stress regimens [2], (4) olfactory bulbectomy [45], and (5) experimental heart failure [16]. The degree to which shared mechanisms might mediate decreases in ICSS produced by pain states and by these other manipulations is unknown, but this could be a productive area of future research.
Effects of morphine alone on ICSS
In the present study, acute morphine alone had little effect for 90 min on ICSS frequency-rate curves up to doses that began to suppress maximal response rates in some rats. The failure of morphine alone to facilitate ICSS could not be attributed to a complete insensitivity of the procedure, because we have shown previously that amphetamine produced a dose-dependent and robust facilitation of ICSS under identical conditions [39]. Rather, this finding agrees with a large literature reporting that acute morphine produces little or no facilitation of ICSS of the medial forebrain bundle/lateral hypothalamus during the first 1–2 hr after its administration. For example, early ICSS procedures evaluated response rates maintained by stimulation of constant frequency and intensity. Under these conditions, facilitated ICSS would be indicated by an increase in response rates; however, acute morphine produced only dose-dependent decreases in response rates for the first 2 hr after its administration [1, 26, 19]. Response rate increases were observed only later in the time course of relatively large morphine doses (i.e. 3–4 hr after administration of 5–10 mg/kg morphine SC), or after repeated treatment for several consecutive days. More recent studies have employed progressive-ratio, frequency-rate or intensity-rate procedures for which response rate is not the primary dependent measure, but even in these studies, the effects of morphine have typically been small or nonsignificant, and either long pretreatment times or repeated dosing were required to reveal facilitated ICSS [18, 9, 14]. The most sensitive procedure for detection of ICSS facilitation by acute morphine has assessed ICSS thresholds by manipulating current intensity above and below the threshold intensity required to maintain responding [26, 21, 20, 23, 24]. Using this procedure, facilitation of ICSS has been observed at relatively early time points after treatment with relatively low morphine doses. However, this procedure too has failed to reveal facilitation of ICSS by acute morphine in some studies [47]. Overall, then, the results of the present study are consistent with the more general finding that acute morphine robustly facilitates ICSS during the first 1–2 hr after its administration only under a relatively narrow range of conditions, such as those described in the procedure developed by Kornetsky and colleagues.
Prevention of lactic acid-induced depression of ICSS by morphine
Although morphine had little effect on ICSS in the absence of noxious stimulation, it dose-dependently prevented lactic acid-induced depression of ICSS at doses that also prevented acid-stimulated stretching. There are two implications of this finding for the potential utility of ICSS in preclinical studies of pain and analgesia. First, these findings illustrate a useful feature of preclinical assays designed to evaluate candidate analgesic effects on pain-depressed behavior. In these assays, the putative pain stimulus produces a decrease in rates of the target behavior, and as demonstrated with morphine in the present study, analgesics would be expected to increase rates of pain-depressed behavior. As a result, drugs that produce non-selective suppression of behavior would not be expected to produce false-positive analgesia-like effects (e.g. see [46]). Of course, assays of pain-depressed behavior may be vulnerable to false-positive analgesic effects related to non-selective stimulation of the target behavior; however, this possibility can be directly evaluated as in the present study by examining effects of the candidate analgesic on the target behavior in the absence of a putative pain stimulus. Overall, assays of pain-depressed behavior might best be conceived as useful complements to more established assays of pain-stimulated behavior, and optimal analgesics might be those that counteract pain-related effects in both types of procedure [37].
A second implication of the present study is that ICSS may be useful to model effects of putative pain states and candidate analgesics on physical functioning, which is a clinically relevant and translationally accessible domain of pain. Measurement of pain and analgesia in humans relies heavily on verbal reports, but verbal reports can be difficult to interpret in humans and cannot be collected at all in preclinical studies with laboratory animals. However, pain and analgesia are associated with other, non-verbal behaviors in both humans and research animals, and a recent consensus report issued by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) identified physical functioning as 1 of 2 outcome domains that should be included as core components in all clinical trials of treatments for chronic pain [13]. The report further recommended that physical functioning be assessed with such well-established instruments as the Interference Scale of the Multidimensional Pain Inventory, which includes questions such as “How much has your pain changed your ability to take part in recreational … activities?” [10]. ICSS may serve as a sensitive, stable and quantifiable baseline of behavior that can be used to detect pain- or analgesia-related changes in physical functioning in preclinical studies.
Acknowledgments
This work was supported in part by R01-DA11460 and R01-DA12736 from NIDA, NIH. The authors would like to thank Katrina Schrode and Samuel McWilliams for expert technical assistance. None of the authors have professional or financial relationships that could result in conflicts of interest related to work described in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams WJ, Lorens SA, Mitchell CL. Morphine enhances lateral hypothalamic self-stimulation in the rat. Proc Soc Exp Biol Med. 1972;140(3):770–771. doi: 10.3181/00379727-140-36549. [DOI] [PubMed] [Google Scholar]
- 2.Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29(4–5):525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 4.Bennett GJ. Animal Models of Pain. In: Kruger L, editor. Methods in Pain Research. Vol. 4. Boca Raton, FL: CRC Press; 2001. pp. 67–91. [Google Scholar]
- 5.Blackburn-Munro G. Pain-like behaviours in animals - how human are they? Trends Pharmacol Sci. 2004;25(6):299–305. doi: 10.1016/j.tips.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316(1):440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 7.Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2(11):2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 8.Carlezon WA, Jr, Todtenkopf MS, McPhie DL, Pimentel P, Pliakas AM, Stellar JR, Trzcinska M. Repeated exposure to rewarding brain stimulation downregulates GluR1 expression in the ventral tegmental area. Neuropsychopharmacology. 2001;25(2):234–241. doi: 10.1016/S0893-133X(01)00232-9. [DOI] [PubMed] [Google Scholar]
- 9.Carlezon WA, Jr, Wise RA. Morphine-induced potentiation of brain stimulation reward is enhanced by MK-801. Brain Res. 1993;620(2):339–342. doi: 10.1016/0006-8993(93)90177-o. [DOI] [PubMed] [Google Scholar]
- 10.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 11.Council National Research. Guide for the Care and Use of Laboratory Animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- 12.Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry. 2003;54(1):49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- 13.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Easterling KW, Plovnick RM, Holtzman SG. Acute opioid but not benzodiazepine dependence in rats responding for intracranial self-stimulation. Psychopharmacology (Berl) 2000;148(3):263–271. doi: 10.1007/s002130050050. [DOI] [PubMed] [Google Scholar]
- 15.Gallistel CR, Freyd G. Quantitative determination of the effects of catecholaminergic agonists and antagonists on the rewarding efficacy of brain stimulation. Pharmacol Biochem Behav. 1987;26(4):731–741. doi: 10.1016/0091-3057(87)90605-8. [DOI] [PubMed] [Google Scholar]
- 16.Grippo AJ, Francis J, Weiss RM, Felder RB, Johnson AK. Cytokine mediation of experimental heart failure-induced anhedonia. Am J Physiol Regul Integr Comp Physiol. 2003;284(3):666–673. doi: 10.1152/ajpregu.00430.2002. [DOI] [PubMed] [Google Scholar]
- 17.Gureje O, Von Korff M, Kola L, Demyttenaere K, He Y, Posada-Villa J, Lepine JP, Angermeyer MC, Levinson D, de Girolamo G, Iwata N, Karam A, Guimaraes Borges GL, de Graaf R, Browne MO, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J. The relation between multiple pains and mental disorders: results from the World Mental Health Surveys. Pain. 2008;135(1–2):82–91. doi: 10.1016/j.pain.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Hand TH, Franklin KB. Associative factors in the effects of morphine on self-stimulation. Psychopharmacology (Berl) 1986;88(4):472–479. doi: 10.1007/BF00178509. [DOI] [PubMed] [Google Scholar]
- 19.Holtzman SG. Comparison of the effects of morphine, pentazocine, cyclazocine and amphetamine on intracranial self-stimulation in the rat. Psychopharmacologia. 1976;46(3):223–227. doi: 10.1007/BF00421106. [DOI] [PubMed] [Google Scholar]
- 20.Hubner CB, Kornetsky C. Heroin, 6-acetylmorphine and morphine effects on threshold for rewarding and aversive brain stimulation. J Pharmacol Exp Ther. 1992;260(2):562–567. [PubMed] [Google Scholar]
- 21.Izenwasser SE, Kornetsky C. Pharmacological effects of morphine on brain-stimulation reward. Psychopharmacology. 1987;93(1):136–137. doi: 10.1007/BF02439601. [DOI] [PubMed] [Google Scholar]
- 22.Jann MW, Slade JH. Antidepressant agents for the treatment of chronic pain and depression. Pharmacotherapy. 2007;27(11):1571–1587. doi: 10.1592/phco.27.11.1571. [DOI] [PubMed] [Google Scholar]
- 23.Jha SH, Knapp CM, Kornetsky C. Effects of morphine on brain-stimulation reward thresholds in young and aged rats. Pharmacol Biochem Behav. 2004;79(3):483–490. doi: 10.1016/j.pbb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26(22):5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI) Pain. 1985;23(4):345–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 26.Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38(11):2473–2476. [PubMed] [Google Scholar]
- 27.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53(4):597–652. [PubMed] [Google Scholar]
- 28.Lepine JP, Briley M. The epidemiology of pain in depression. Human psychopharmacology. 2004;19 (Suppl 1):S3–7. doi: 10.1002/hup.618. [DOI] [PubMed] [Google Scholar]
- 29.Lorens SA, Mitchell CL. Influence of morphine on lateral hypothalamic self-stimulation in the rat. Psychopharmacologia. 1973;32(3):271–277. doi: 10.1007/BF00422149. [DOI] [PubMed] [Google Scholar]
- 30.Mague SD, Andersen SL, Carlezon WA., Jr Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol Psychiatry. 2005;57(2):120–125. doi: 10.1016/j.biopsych.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 31.Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4(1):17–26. [PubMed] [Google Scholar]
- 32.Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101(1):191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 33.Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther. 2007;320(1):194–201. doi: 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- 34.Miliaressis E, Rompre PP, Laviolette P, Philippe L, Coulombe D. The curve-shift paradigm in self-stimulation. Physiol Behav. 1986;37(1):85–91. doi: 10.1016/0031-9384(86)90388-4. [DOI] [PubMed] [Google Scholar]
- 35.Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain. 2004;112(1–2):12–15. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 36.Morgan D, Carter CS, Dupree JP, Yezierski RP, Vierck CJ. Evaluation of prescription opioids using operant-based pain measures in rats. Exp Clin Psychopharmacol. 2008;16(5):367–375. doi: 10.1037/a0013520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical Assessment of Candidate Analgesic Drugs: Recent Advances and Future Challenges. J Pharmacol Exp Ther. 2006;319(2):507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- 38.Pacharinsak C, Beitz A. Animal models of cancer pain. Comparative medicine. 2008;58(3):220–233. [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira Do, Carmo G, Folk JE, Rice KC, Chartoff EH, Carlezon WA, Jr, Negus SS. Effects of the selective delta opioid agonist SNC80 on intracranial self-stimulation in rats: comparison to other pharmacological and non-pharmacological manipulations. Eur J Pharmacol. In Press. [Google Scholar]
- 40.Rodriguez L, Pardo EG. Reversal by narcotics and narcotic antagonists of pain-induced functional impairment. In: Braude MC, Harris LS, May EL, Smith JP, Villarreal JE, editors. Narcotic Antagonists. New York: Raven Press; 1974. pp. 213–223. [Google Scholar]
- 41.Rodriguez R, Pardo EG. Drug reversal of pain induced functional impairment. Arch Int Pharmacodyn Ther. 1968;172(1):148–160. [PubMed] [Google Scholar]
- 42.Roeska K, Doods H, Arndt K, Treede RD, Ceci A. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain. 2008;139(2):349–357. doi: 10.1016/j.pain.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Schaefer GJ, Michael RP. Morphine withdrawal produces differential effects on the rate of lever-pressing for brain self-stimulation in the hypothalamus and midbrain in rats. Pharmacol Biochem Behav. 1983;18(4):571–577. doi: 10.1016/0091-3057(83)90283-6. [DOI] [PubMed] [Google Scholar]
- 44.Seguin L, Le Marouille-Girardon S, Millan MJ. Antinociceptive profiles of non-peptidergic neurokinin1 and neurokinin2 receptor antagonists: a comparison to other classes of antinociceptive agent. Pain. 1995;61(2):325–343. doi: 10.1016/0304-3959(94)00194-J. [DOI] [PubMed] [Google Scholar]
- 45.Slattery DA, Markou A, Cryan JF. Evaluation of reward processes in an animal model of depression. Psychopharmacology (Berl) 2007;190(4):555–568. doi: 10.1007/s00213-006-0630-x. [DOI] [PubMed] [Google Scholar]
- 46.Stevenson GW, Bilsky EJ, Negus SS. Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain. 2006;7:408–416. doi: 10.1016/j.jpain.2006.01.447. [DOI] [PubMed] [Google Scholar]
- 47.Stratmann JA, Craft RM. Intracranial self-stimulation in female and male rats: no sex differences using a rate-independent procedure. Drug Alcohol Depend. 1997;46(1–2):31–40. doi: 10.1016/s0376-8716(97)00040-9. [DOI] [PubMed] [Google Scholar]
- 48.Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172(4):463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 49.Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135(1–2):7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Whiteside GT, Adedoyin A, Leventhal L. Predictive validity of animal pain models? A comparison of the pharmacokinetic-pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology. 2008;54(5):767–775. doi: 10.1016/j.neuropharm.2008.01.001. [DOI] [PubMed] [Google Scholar]