Abstract
Steroid hormones are synthesized in response to signaling cascades initiated by the trophic peptide hormones derived from the anterior pituitary. The mechanisms by which these peptide hormones regulate steroid hormone production are multifaceted and include controlling the transcription of steroidogenic genes, regulating cholesterol (substrate) uptake and transport, modulating steroidogenic enzyme activity, and controlling electron availability. Cytoskeletal polymers such as microfilaments and microtubules have also been implicated in regulating steroidogenesis. Of note, steroidogenesis is a multi-step process that occurs in two organelles, the endoplasmic reticulum (ER) and the mitochondrion. However, the precise mechanism by which substrates are delivered back and forth between these two organelles is unknown. In this review we will discuss the role of components of the cytoskeleton in conferring optimal steroidogenic potential. Finally, we present data that identifying a novel mechanism by which sphingosine-1-phosphate induces mitochondrial trafficking to promote steroidogenesis.
Keywords: Steroidogenesis, Cortisol, Progesterone, Adrenocorticotropin, Microtubules, Microfilaments, Sphingosine-1-phosphate, Colchicine, Taxol
Introduction
Steroid hormones are key regulators of a diverse array of physiological processes, including the maintenance of carbohydrate metabolism, sodium and fluid homeostasis, reproduction, and the development of secondary sex characteristics. These molecules allow tissues to respond in a coordinated manner to changes in the internal and external environments by functioning as ligands for both nuclear and plasma membrane receptors. Steroid hormones are synthesized from cholesterol by members of the cytochrome P450 superfamily of monooxygenases and steroid dehydrogenases [1, 2]. Because steroid hormones control the expression of numerous genes in virtually all cell types, steroidogenic cells utilize multiple mechanisms that ensure tight control of the synthesis of these molecules. Further, the central role that these molecules play in facilitating communication between different organs and tissues necessitates multiple regulatory mechanisms. Although a significant amount is known about both steroidogenesis and the effects of steroid hormones on cellular processes, many aspects of the factors that control hormone production are unknown. For example, steroid hormone biosynthesis is a step-wise process that requires several enzymes that are compartmentalized in mitochondria and the endoplasmic reticulum (ER) (Fig. 1). However, the molecular mechanisms underlying inter-organelle substrate delivery are largely unexplored. Research carried out over the past several decades have provided evidence for a key role of the cytoskeleton in mediating steroidogenesis. The goal of this review is to provide a brief summary of studies investigating the role of components of the cytoskeleton in controlling steroidogenic capacity.
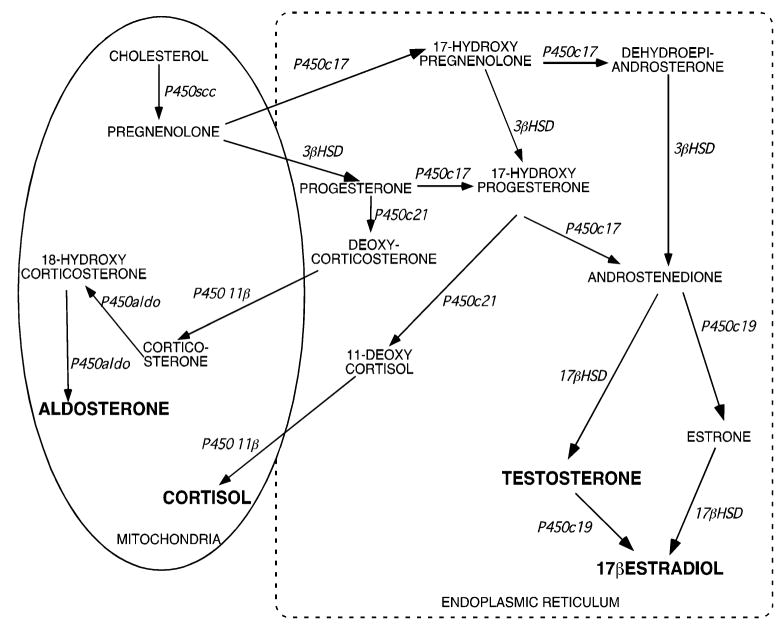
Fig. 1.
Steroidogenic biosynthetic pathways
The Cytoskeleton
The cytoskeleton plays a key role in varied cellular processes, including motility and migration, organelle and vesicular transport, cell–cell communication and gap junction formation, mitosis and meiosis, and maintenance of cellular morphology. In eukaryotic cells there are three major cytoskeleton components: microfilaments, intermediate filaments, and microtubules. Microfilaments are comprised of polymerized actin and are integral in the formation of cleavage furrows during cytokinesis and in cytoplasmic streaming. Intermediate filaments are larger in diameter than microfilaments and serve to maintain cellular structural architecture and spatial distribution of organelles. Intermediate filaments are comprised of lamins (form the nuclear envelope), keratin, and vimentins. Microtubules are formed when tubulin polymerizes and the dynamic assembly and disassembly of these fibers is largely regulated by the hydrolysis of GTP. Consistent with the role of GTP in microtubule function, these polymers are typically associated with motor proteins such as kinesin and dynein. Microtubules play a well-established role in the formation of cilia and flagella and are gaining much attention for their role in the intracellular transport of vesicles and organelles, particularly mitochondria in neurons.
Due to the multifaceted roles of cytoskeletal proteins in general cellular processes, it is not difficult to ascribe functions for these proteins in the biosynthesis of steroid hormones in the adrenal cortex and gonads. In fact, four decades ago observations made on Y1 mouse adrenal cells that underwent morphological changes in response to adrenocorticotropin (ACTH) provided clues into the role of the cytoskeleton architecture in controlling the response of steroidogenic cells to trophic hormone stimulation [3, 4]. These marked changes in steroidogenic cell morphology have been confirmed by several researchers [5–7] and have been subsequently attributed to cAMP-dependent rapid dephosphorylation of the focal adhesion protein paxillin [8, 9].
Cholesterol Trafficking
The first and rate-limiting step in steroid hormone biosynthesis is the delivery of the substrate cholesterol to the inner mitochondrial membrane, the site of the first enzymatic reaction. Efficient delivery of cholesterol requires the coordination of several steps. First, two receptors, low-density lipoprotein receptor (LDLR) and scavenger receptor BI (SR-BI), import lipoprotein particles containing esterified cholesterol molecules into steroidogenic cells. In humans, the primary source of cholesterol for steroid hormone production is LDL, which is imported via LDLR. However, SR-BI has been shown to be a major provider of cholesterol for hormone biosynthesis, particularly in the rodent [10, 11]. Unlike lipoproteins imported by SR-BI, both LDL and LDLR are internalized and transported to lysosomes for processing.
Studies carried out by Crivello and Jefcoate three decades ago indicated a role for cytoskeletal proteins in adrenocortical steroidogenesis [12]. Various chemical inhibitors of microfilament and microtubule formation have been shown to inhibit corticosterone biosynthesis in rat adrenal glands [12]. Notably, these studies also found that inhibitors of microtubule and microfilament polymerization decreased the delivery of cholesterol to P450 side chain cleavage enzyme (P450scc), suggesting that cytoskeleton proteins regulate steroidogenesis by facilitating the trafficking of substrate to mitochondria. A role for microtubules in steroidogenesis was also shown by Rajan and Menon where lipoprotein-stimulated progesterone synthesis was found to be inhibited by the tubulin polymerization inhibitors colchicine and nocodazole [13]. Significantly, the authors demonstrated that microtubule polymerization inhibitors decreased the degradation of radiolabeled LDL and high density lipoprotein (HDL) in cultured rat luteal cells. The role of the cytoskeleton in facilitating the uptake of HDL for steroidogenesis was also demonstrated in studies using cytochalasin B, an inhibitor of actin polymerization [14] and also in experiments using the endonuclease DNase I [15].
In a series of elegant experiments employing laser scanning coherent anti-Stokes Raman scattering (CARS) microscopy it was recently shown that lipid droplets move along microtubule tracts in Y1 mouse adrenal cells [16]. Interestingly, when CARS and two-photon fluorescence microscopy were simultaneously used to image lipid droplets and mitochondria, the investigators found that the mitochondria interacted with lipid droplets that were highly motile. In contrast to these studies establishing a role for microtubules in cholesterol transport in steroidogenic cells, it has also been found that while colchicine disrupts both lipid droplet capsules and microtubules, it stimulates corticosterone biosynthesis in rat adrenocortical cells [17]. Colchicine has also been found to stimulate corticosterone secretion from wild type Y1 protein kinase A (PKA) deficient kin-8 mouse adrenal cell lines [18], suggesting that preventing microtubule polymerization promotes steroidogenesis in a cAMP/PKA-independent manner. These studies also showed that taxol, an agent that stabilizes microtubules, inhibits ACTH- and colchicine-stimulated corticosterone biosynthesis [18].
A role for actin polymerization into microfilaments has also been demonstrated in Y1 murine adrenal cells. Treatment of Y1 cells with acrylamide to disrupt microfilaments stimulates corticosterone production in a cAMP-independent manner [19]. The stimulatory effect of acrylamide on adrenal steroidogenesis was shown to be mediated at a step prior to the conversion of cholesterol to pregnenolone [19], thus implicating the cytoskeleton in assuring efficient substrate delivery.
After lipoproteins are processed, cholesterol movement out of lysosomes is facilitated by Niemann-Pick type C1 (NPC). NPC1 is localized in late endosomes along with the lipid transfer proteins MLN64 and NPC2 that travel along microtubules tracks [20, 21]. Thus, the cytoskeleton plays a key role in directing the positioning of cholesterol in steroidogenic cells. The processing of stored cholesterol esters is mediated by hormone sensitive lipase (HSL), a neutral cholesterol ester hydrolase that cleaves the ester bonds to form free cholesterol substrate [22]. While HSL trafficking has not been studied in steroidogenic cells, the lipase is rapidly translocated to the surface of lipid droplets in 3T3-L1 adipocytes upon treatment with the beta-adrenergic receptor agonist isoproteranol [23]. This translocation, however, is not dependent on microtubules and microfilaments [23]. Collectively, these studies demonstrate the pivotal role that the cytoskeleton plays in assuring efficient uptake, processing, and transport of cholesterol in steroidogenic cells.
Steroid Hormone Biosynthesis
As mentioned above, upon delivery of cholesterol to the inner mitochondrial membrane of human adrenocortical cells, this substrate is subjected to the sequential actions of several steroid hydroxylases (cytochrome P450s) and 3β-hydroxysteroid dehydrogenase (3βHSD) leading to the production of steroid hormones (Fig. 1). In the human adrenal cortex, production of these steroid hormones occurs in specific zones, where aldosterone is formed in the outermost zone (glomerulosa), cortisol in the zona fasciculata (middle zone), and adrenal androgens in the zona reticularis (inner zone). As shown in Fig. 1, three P450s are localized in mitochondria (P450scc, P450 11β, P450aldo), while P450c17, P450c21, and P450c19 are expressed in the ER, and 3βHSD expressed in both organelles.
In the first enzymatic process, P450scc (encoded by CYP11A1) catalyzes the conversion of 27-carbon cholesterol to a 21- carbon pregnenolone. Once pregnenolone is formed, it enters the ER where it can be hydroxylated on carbon-17 by P450c17 (encoded by CYP17) to form 17-hydroxy pregnenolone or converted to progesterone by 3βHSD (Fig. 1). Importantly, optimal steroid hormone production requires the movement of pregnenolone out of mitochondria and into the ER. However, the factors that regulate inter-organelle delivery of pregnenolone to the ER are largely unexplored.
In the ER P450c17 also catalyzes a 17,20 lyase reaction to form the adrenal androgens dehydroepiandrosterone (DHEA) and androstenedione. Also, both progesterone and 17-hydroxy progesterone can be hydroxylated at the 21-carbon by P450c21 to yield deoxycorticosterone and 11-deoxycortisol, respectively (Fig. 1). The final reactions in adrenocortical steroid hormone biosynthesis occur in mitochondria, where 11-deoxycortisol and deoxycorticosterone are converted to cortisol and corticosterone, respectively, by P450 11β hydroxylase (encoded by CYP11B1). As is the case for the movement of pregnenolone into the ER, regulated transport of deoxycorticosterone and 11-deoxycortisol is also required for glucocorticoid and mineralocorticoid biosynthesis. While inter-organelle trafficking of metabolites produced in the ER back to mitochondria plays a key role in the biosynthesis of glucocorticoids and mineralocorticoids, adrenal androgens only depend on the movement of pregnenolone out of mitochondria and to ER. In the human adrenal cortex, expression of P450aldo (encoded by CYP11B2) in the zona glomerulosa allows for the conversion of corticosterone to aldosterone in a series of hydroxylation steps. In the gonads, all reactions except the initial conversion of cholesterol to pregnenolone, which takes place in the inner mitochondrial membrane, occur in the ER (Fig. 1).
Regulation of Steroid Hormone Production by the Cytoskeleton
The relationship between steroidogenesis and the cytoskeleton has been studied in several laboratories that have used microtubule depolymerization agents to demonstrate that steroid hormone production is impaired [13, 18, 24–28]. Immunostaining of microtubules in sheep follicles has revealed decreases in the amount of polymerized tubulin prior to ovulation followed by subsequent increases in microtubule formation as progesterone biosynthesis increases [29]. Moreover, colchicine prevents increased progesterone production in these follicular cells [29], providing support for the importance of dynamic changes in microtubule structure in response to changing demands for steroid hormone production. In contrast to the inhibitory effect of colchicine on progesterone production in sheep [29], this microtubule polymerization inhibitor has been shown to stimulate progesterone biosynthesis in porcine luteal cells [30]. Stabilization of microtubules in porcine granulosa cells using taxol suppresses both basal and human choriogonadotropin-stimulated progesterone and 17β-estradiol [31].
In human adrenocortical slices, preventing microtubule polymerization has been shown to reduce ACTH-stimulated cortisol biosynthesis [32]. However, Benis and Mattson have also found that stabilizing microtubules inhibits ACTH-stimulated steroidogenesis in cultured murine adrenocortical cells [27, 28]. While collectively the studies described have demonstrated an integral role for cytoskeletal proteins in controlling hormone biosynthesis, the use of chemical inhibitors to manipulate cytoskeletal protein polymer formation has resulted in conflicting results being found with regard to the specific role of these proteins. Although it is likely that many of these inconsistencies can be attributed to species and cell specific differences, further investigation is needed to resolve these conflicting findings. Additionally, more detailed interrogation of each of the steps involved in steroidogenesis is warranted to determine if the cytoskeleton also regulates processes in hormone production that occur after cholesterol uptake and transport.
Regulation of Organelle Positioning by the Cytoskeleton
As discussed earlier, components of the cytoskeleton function in a wide variety of cellular processes. Extensive research has provided compelling evidence for the critical roles of microtubules and microfilaments in cholesterol transport and steroid hormone secretion [33, 34], however, less is known about the role of cytoskeletal proteins in the transport of substrates (pregnenolone, deoxycorticosterone, and 11-deoxycortisol) between mitochondria and the ER or increasing the proximity of mitochondria and ER.
Given the well-established role of mitochondrial transport in neurons [35–38], we sought to characterize the role of dynamic mitochondrial movements in steroidogenesis. Mitochondria have been visualized in association with microfilaments [35], microtubules [39, 40], and intermediate filaments [41, 42] in various cells types. Further, mitochondria-bound motor proteins, such as kinesins, facilitate the movement of these organelles along cytoskeletal fibers [43–47].
We postulated that components of the cytoskeleton facilitate steroidogenesis by promoting inter-organelle substrate delivery. We envisioned that agents that induce steroid hormone biosynthesis might regulate a discrete set of factors that act to promote the transport of substrates between mitochondria and the ER. To begin examining these processes, we employed time-lapsed video microscopy of H295R human adrenocortical cells. H295R cells were plated onto cover slips, labeled with MitoTracker Red, and mounted into a chamber for microscopic analysis. Cells were then treated with the bioactive lipid sphingosine-1-phosphate (S1P). S1P was used because we have previously demonstrated that S1P increases CYP17 gene expression and steroidogenesis in H295R human adrenocortical cells [48]. Several other laboratories have also shown that S1P stimulates hormone secretion from various steroidogenic cell types [49–52]. Further, S1P is a well-established inducer of cell migration [53–57] and chemotaxis [58, 59].
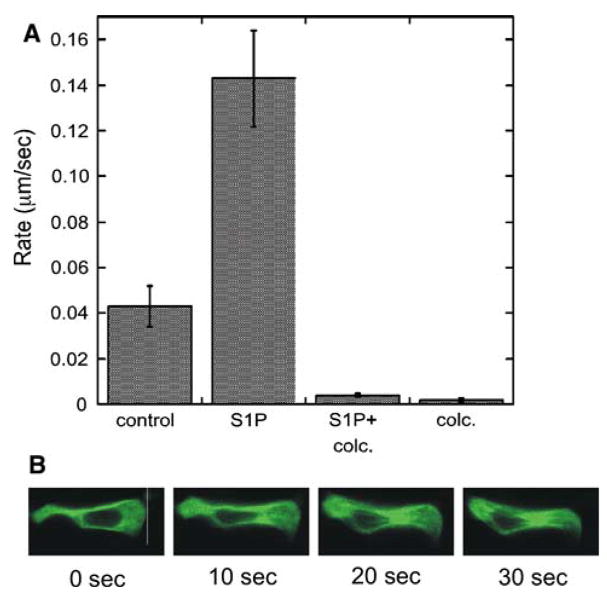
As shown in Fig. 2a, administration of S1P to H295R cells resulted in a 3-fold increase in the rate of mitochondrial movement. The increase in S1P-stimulated mitochondrial movement was attenuated when cells were pre-treated with colchicine, indicating that microtubule polymerization is required for S1P-dependent mitochondrial trafficking. In addition to an increase in the rate of mitochondrial trafficking, S1P also promoted cell migration (Fig. 2b).
Fig. 2.
S1P stimulates mitochondrial movement and cell migration. a H295R cells were cultured on cover slips, incubated with MitoTracker Red (Invitrogen), and then placed in a chamber for time lapsed video microscopy. Cells were treated with S1P (1 μM) and/or 1 μM colchicine (colc.) and data was collected for 1 h using an LSM510 confocal microscope system (Carl Zeiss Inc., Thornwood, NY) equipped with a krypton-equipped with a helium-neon Coherent laser with an excitation wavelength of 543 nm MitoTracker Red. Emissions were collected with a C-apochromast 40 1.3 NA oil immersion objective (Zeiss) using a 560-nm long pass filter. b Cells expressing GFP-labeled tubulin were monitored by time lapsed video microscopy. Shown are frames during time course after exposure to 1 μM S1P
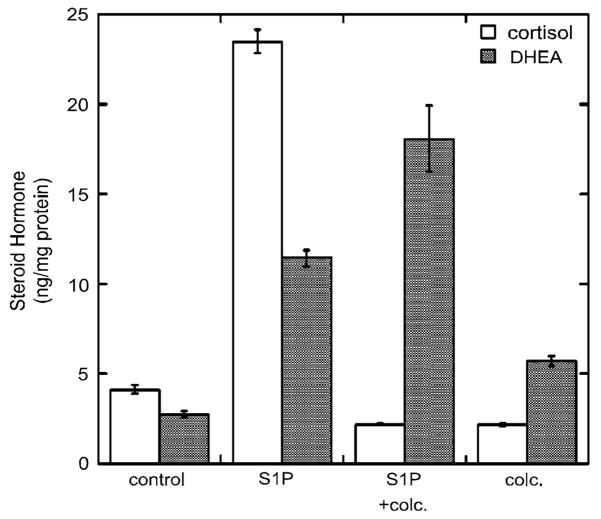
We also determined the effect of S1P and colchicine on the secretion of cortisol and DHEA into the cell culture media. S1P increased the amounts of cortisol and DHEA produced by the H295R cells while colchicine prevented this increase (Fig. 3). Interestingly, colchicine did not decrease the amount of DHEA secreted into the media, suggesting that the increase in the rate of mitochondrial trafficking evoked by S1P is dependent on microtubule polymerization and required for S1P-induced cortisol secretion. Studies are underway to determine the effect of ACTH and other agents known to stimulate adrenocortical steroidogenesis on the positioning of mitochondria in adrenal cells. Ongoing experiments are also aimed at defining the precise molecular mechanism by which S1P (and other inducers of steroidogenesis) promote mitochondrial trafficking and at identifying the factors involved in controlling the microtubule-dependent mitochondrial movement.
Fig. 3.
Colchicine alters the ratio of cortisol and DHEA secreted in response to S1P. Media was collected from cells treated with S1P (1 μM) and/or colc. (1 μM) and cortisol and DHEA released determined in triplicate against standards made up in DME/F12 medium using a 96-well plate enzyme-linked immune assay (Diagnostic Systems Corporation, Houston, TX). Results are expressed as nanomoles per milligram cellular protein
Conclusions and Future Directions
Significant advances have been made in understanding the complex and multi-faceted mechanisms used to control steroid hormone biosynthesis. Further, the use of chemical agents that perturb the polymerization of cytoskeletal proteins has shed light on a key role for microfilaments and microtubules in regulating the uptake and transport of cholesterol in steroidogenic cells. Our data presented herein describing that S1P induces rapid increases in the trafficking of mitochondria in a manner that is dependent on microtubules suggests that the cytoskeleton is also involved in steroid hormone production at steps subsequent to cholesterol delivery to mitochondria. It is tempting to speculate that the increased rate of mitochondrial movement in response to S1P promotes cortisol biosynthesis by bringing mitochondria in close proximity to the ER, thereby maximizing the delivery of 11-deoxycortisol to mitochondria for the final step of steroidogenesis. However, further studies are required to elucidate the precise role of mitochondrial trafficking in steroidogenesis. Additionally, it is equally likely that a macromolecular complex containing an unidentified 11-deoxycortisol binding transport protein may mediate this response. The use of approaches such as RNA interference, mass spectrometry, and time-lapsed video microscopy are likely to provide insight into the precise molecular parameters that underlie the role cytoskeletal proteins play in steroid hormone biosynthesis.
Acknowledgments
This work is supported by the National Institutes of Health/National Institute of General Medical Sciences (GM073241) and by a CAREER award from the National Science Foundation (MCB0347682).
Abbreviations
- ACTH
Adrenocorticotropin
- ER
Endoplasmic reticulum
- LDL
Low density lipoprotein
- LDLR
Low density lipoprotein receptor
- SR-BI
Scavenger receptor type BI
- HDL
High density lipoprotein
- CARS
Coherent anti-Stokes Raman scattering
- PKA
Protein kinase A
- NPC1
Niemann-Pick type C1
- HSL
Hormone sensitive lipase
- 3βHSD
3 Beta hydroxysteroid dehydrogenase
- P450scc
P450 side chain cleavage
- DHEA
Dehydroepiandrosterone
- S1P
Sphingosine-1-phosphate
References
- 1.Sewer MB, Dammer EB, Jagarlapudi S. Transcriptional regulation of adrenocortical steroidogenic gene expression. Drug Metab Rev. 2007;39:371–388. doi: 10.1080/03602530701498828. [DOI] [PubMed] [Google Scholar]
- 2.Sewer MB, Waterman MR. ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc Res Tech. 2003;61:300–307. doi: 10.1002/jemt.10339. [DOI] [PubMed] [Google Scholar]
- 3.Yasumura Y, Buonassisi V, Sato G. Clonal analysis of differentiated function in animal cell cultures. I. Possible correlated maintenance of differentiated function and the diploid karyotype. Cancer Res. 1966;26:529–535. [PubMed] [Google Scholar]
- 4.Yasumura Y. Retention of differentiated function in clonal animal cell lines, particularly hormone-secreting cultures. Am Zool. 1968;8:285–305. doi: 10.1093/icb/8.2.285. [DOI] [PubMed] [Google Scholar]
- 5.Cuprak LJ, Lammi CJ, Bayer RC. Scanning electron microscopy of induced cell rounding of mouse adrenal cortex tumor cells in culture. Tissue Cell. 1977;9:667–680. doi: 10.1016/0040-8166(77)90034-9. [DOI] [PubMed] [Google Scholar]
- 6.Mattson P, Kowal J. The ultrastructure of functional mouse adrenal cortical tumor cells in vitro. Differentiation. 1978;11:75–88. doi: 10.1111/j.1432-0436.1978.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 7.Voorhees H, Aschenbrenner J, Carnes J, Mrotek J. Rounding and steroidogenesis of enzyme- and ACTH-treated Y-1 mouse adrenal tumor cells. Cell Biol Int Rep. 1984;8:483–497. doi: 10.1016/0309-1651(84)90169-3. [DOI] [PubMed] [Google Scholar]
- 8.Han JD, Rubin CS. Regulation of cytoskeleton organization and paxillin dephosphorylation by cAMP. J Biol Chem. 1996;271:29211–29215. doi: 10.1074/jbc.271.46.29211. [DOI] [PubMed] [Google Scholar]
- 9.Whitehouse BJ, Gyles SL, Squires PE, Sayed SB, Burns CJ, Persaud SJ, Jones PM. Interdependence of steroidogenesis and shape changes in Y1 adrenocortical cells: studies with inhibitors of phosphoprotein phosphatases. J Endocrinol. 2002;172:583–593. doi: 10.1677/joe.0.1720583. [DOI] [PubMed] [Google Scholar]
- 10.Connelly MA, Williams DL. SR-BI and HDL cholesteryl ester metabolism. Endocr Res. 2004;30:697–703. doi: 10.1081/erc-200043979. [DOI] [PubMed] [Google Scholar]
- 11.Azhar S, Leers-Sucheta S, Reaven E. Cholesterol uptake in adrenal and gonadal tissues: the SR-BI and ‘‘selective’’ pathway connection. Front Biosci. 2003;8:998–1029. doi: 10.2741/1165. [DOI] [PubMed] [Google Scholar]
- 12.Crivello JF, Jefcoate CR. Mechanisms of corticotropin action in rat adrenal cells. I. The effects of inhibitors of protein synthesis and of microfilament formation on corticosterone synthesis. Biochem Biophys Res Commun. 1978;542:315–329. doi: 10.1016/0304-4165(78)90027-2. [DOI] [PubMed] [Google Scholar]
- 13.Rajan VP, Menon KM. Involvement of microtubules in lipoprotein degradation and utilization for steroidogenesis in cultured rat luteal cells. Endocrinology. 1985;117:2408–2416. doi: 10.1210/endo-117-6-2408. [DOI] [PubMed] [Google Scholar]
- 14.Cortese F, Wolff J. Cytochalasin-stimulated steroidogenesis from high density lipoproteins. J Cell Biol. 1978;77:507–516. doi: 10.1083/jcb.77.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osawa S, Betz G, Hall PF. Role of actin in the responses of adrenal cells to ACTH and cyclic AMP: inhibition by DNase I. J Cell Biol. 1984;99:1335–1342. doi: 10.1083/jcb.99.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nan X, Potma EO, Xie XS. Nonperturbative chemical imaging of organelle transport in living cells with coherent anti-strokes Raman scattering microscopy. Biophysical J. 2006;91:728–735. doi: 10.1529/biophysj.105.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee LJ, Chen JS, Ko TL, Wang SM. Mechanism of colchicine-induced steroidogenesis in rat adrenocortical cells. J Cell Biochem. 2001;81:162–171. doi: 10.1002/1097-4644(20010401)81:1<162::aid-jcb1032>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 18.Sackett DL, Wolff J. Cyclic AMP-independent stimulation of steroidogenesis in Y-1 adrenal tumor cells by antimitotic agents. Biochim Biophys Acta. 1986;888:163–170. doi: 10.1016/0167-4889(86)90017-0. [DOI] [PubMed] [Google Scholar]
- 19.Shiver TM, Sackett DL, Knipling L, Wolff J. Intermediate filaments and steroidogenesis in adrenal Y-1 cells: acrylamide stimulation of steroid production. Endocrinology. 1992;131:201–207. doi: 10.1210/endo.131.1.1319319. [DOI] [PubMed] [Google Scholar]
- 20.Strauss JF, 3rd, Liu P, Christenson LK, Watari H. Sterols and intracellular vesicular trafficking: lessons from the study of NPC1. Steroids. 2002;67:947–951. doi: 10.1016/s0039-128x(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Liu P, Dwyer NK, Christenson LK, Fujimoto T, Martinez F, Comly M, Hanover JA, Blanchette-Mackie EJ, Strauss JF., 3rd MLN64 mediates mobilization of lysosomal cholesterol to steroidogenic mitochondria. J Biol Chem. 2002;277:33300–33310. doi: 10.1074/jbc.M200003200. [DOI] [PubMed] [Google Scholar]
- 22.Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002;43:1585–1594. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- 23.Brasaemle DL, Levin DM, Adler-Wailes DC, Londos C. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim Biophys Acta. 2000;1483(2):251–262. doi: 10.1016/s1388-1981(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 24.Denkova R, Ivanov I, Dimitrova M. Microtubules and regulation of granulosa cell steroidogenesis by porcine granulosa cell conditioned medium. Endocr Regul. 1992;26:195–199. [PubMed] [Google Scholar]
- 25.Carnegie JA, Tsang BK. Microtubules and the calcium-dependent regulation of rat granulosa cell steroidogenesis. Biol Reprod. 1987;36:1007–1015. doi: 10.1095/biolreprod36.4.1007. [DOI] [PubMed] [Google Scholar]
- 26.Carnegie JA, Dardick I, Tsang BK. Microtubules and the gonadotropic regulation of granulosa cell steroidogenesis. Endocrinology. 1987;120:819–828. doi: 10.1210/endo-120-2-819. [DOI] [PubMed] [Google Scholar]
- 27.Benis R, Mattson P. Microtubules, organelle transport, and steroidogenesis in cultured adrenocortical tumor cells. 1. An ultrastructural analysis of cells in which basal and ACTH-induced steroidogenesis was inhibited by taxol. Tissue Cell. 1989;21:479–494. doi: 10.1016/0040-8166(89)90001-3. [DOI] [PubMed] [Google Scholar]
- 28.Benis R, Mattson P. Microtubules, organelle transport, and steroidogenesis in cultured adrenocortical tumor cells. 2. Reversibility of taxol’s inhibition of basal and ACTH-induced steroidogenesis is unaccompanied by reversibility of taxol-induced changes in cell ultrastructure. Tissue Cell. 1989;21:687–698. doi: 10.1016/0040-8166(89)90079-7. [DOI] [PubMed] [Google Scholar]
- 29.Murdoch WJ. Microtubular dynamics in granulosa cells of periovulatory follicles and granulosa-derived (large) lutein cells of sheep: relationships to the steroidogenic folliculoluteal shift and functional luteolysis. Biol Reprod. 1996;54:1135–1140. doi: 10.1095/biolreprod54.5.1135. [DOI] [PubMed] [Google Scholar]
- 30.Gregoraszczuk EL, Stlomczynska M. The cytoskeleton proteins and LH-regulated steroidogenesis of porcine luteal cells. Folia Histochem Cytobiol. 1996;34:35–39. [PubMed] [Google Scholar]
- 31.Chen TT, Massey PJ, Caudle MR. The inhibitory action of taxol on granulosa cell steroidogenesis is reversible. Endocrinology. 1994;134:2178–2183. doi: 10.1210/endo.134.5.7908872. [DOI] [PubMed] [Google Scholar]
- 32.Feuilloley M, Contesse V, Lefebvre H, Delarue C, Vaudry H. Effects of selective disruption of cytoskeletal elements on steroid secretion by human adrenocortical slices. Am J Physiol. 1994;266:E202–E210. doi: 10.1152/ajpendo.1994.266.2.E202. [DOI] [PubMed] [Google Scholar]
- 33.De Loof A, Vanden J, Janssen I. Hormones and the cytoskeleton of animals and plants. Int Rev Cytol. 1996;166:1–58. doi: 10.1016/s0074-7696(08)62505-x. [DOI] [PubMed] [Google Scholar]
- 34.Hall PF, Almahbobi G. Roles of microfilaments and intermediate filaments in adrenal steroidogenesis. Microsc Res Tech. 1997;36:463–479. doi: 10.1002/(SICI)1097-0029(19970315)36:6<463::AID-JEMT4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 35.Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J Cell Biol. 1995;131:1315–1326. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris RL, Hollenbeck PJ. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci. 1993;104:917–927. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- 37.Davis AF, Clayton DA. In situ localization of mitochondrial DNA replication in intact mammalian cells. J Cell Biol. 1996;135:883–893. doi: 10.1083/jcb.135.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 39.Ball EH, Singer SJ. Mitochondria are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proc Natl Acad Sci. 1982;79:123–126. doi: 10.1073/pnas.79.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Summerhayes IC, Wong D, Chen LB. Effect of microtubules and intermediate filaments on mitochondrial distribution. J Cell Sci. 1983;61:87–105. doi: 10.1242/jcs.61.1.87. [DOI] [PubMed] [Google Scholar]
- 42.Stromer MH, Bendayan M. Immunocytochemical identification of cytoskeletal linkages to smooth muscle cell nuclei and mitochondria. Cell Motil Cytoskeleton. 1990;17:11–18. doi: 10.1002/cm.970170104. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- 44.Miki H, Okada Y, Hirokawa N. Analysis of the kinesin superfamily: insights into the structure and function. Trends Cell Biol. 2005;15:467–476. doi: 10.1016/j.tcb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Kwok BH, Kapoor TM. Microtubule flux: drivers wanted. Curr Opin Cell Biol. 2007;19:36–42. doi: 10.1016/j.ceb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gross SP. Hither and yon: a review of bi-directional microtubule-based transport. Phys Biol. 2004;1:R1–R11. doi: 10.1088/1478-3967/1/2/R01. [DOI] [PubMed] [Google Scholar]
- 48.Ozbay T, Rowan A, Leon A, Patel P, Sewer MB. Cyclic adenosine 5′-monophosphate-dependent sphingosine-1-phosphate biosynthesis induces human CYP17 gene transcription by activating cleavage of sterol regulatory element binding protein 1. Endocrinology. 2006;147:1427–1437. doi: 10.1210/en.2005-1091. [DOI] [PubMed] [Google Scholar]
- 49.Rabano M, Pena A, Brizuela L, Marino A, Macarulla JM, Trueba M, Gomez-Munoz A. Sphingosine-1-phosphate stimulates cortisol secretion. FEBS Lett. 2003;535:101–105. doi: 10.1016/s0014-5793(02)03882-6. [DOI] [PubMed] [Google Scholar]
- 50.Cai Z, Kwintkiewicz J, Young M, Stocco D. Prostaglandin E2 increases CYP19 expression in rat granulosa cells: implication of GATA-4. Mol Cell Endocrinol. 2007;263:181–189. doi: 10.1016/j.mce.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brizuela L, Rabano M, Pena A, Gangoiti P, Macarulla JM, Trueba M, Gomez-Munoz A. Sphingosine-1-phosphate: a novel stimulator of aldosterone secretion. J Lipid Res. 2006;47:1238–1249. doi: 10.1194/jlr.M500510-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Brizuela L, Rabano M, Gangoiti P, Narbona N, Macarulla JM, Trueba M, Gomez-Munoz A. Sphingosine-1-phosphate stimulates aldosterone secretion through a mechanism involving the PI3 K/PKB and MEK/ERK1/2 pathways. J Lipid Res. 2007;48:2264–2274. doi: 10.1194/jlr.M700291-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol. 2005;25:4237–4249. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 55.Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 56.Hait NC, Sarkar S, Le Stunff H, Mikami A, Maceyka M, Milstien S, Spiegel S. Role of sphingosine kinase 2 in cell migration toward epidermal growth factor. J Biol Chem. 2005;280:29462–29469. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- 57.Rosenfeldt HM, Hobson JP, Maceyka M, Olivera A, Nava VE, Milstien S, Spiegel S. EDG-1 links the PDGF receptor to Src and focal adhesion kinase activation leading to lamellipodia formation and cell migration. FASEB J. 2001;15:2649–2659. doi: 10.1096/fj.01-0523com. [DOI] [PubMed] [Google Scholar]
- 58.Le Stunff H, Mikami A, Giussani P, Hobson JP, Jolly PS, Milstien S, Spiegel S. Role of sphingosine-1-phosphate in epidermal growth factor-induced chemotaxis. J Biol Chem. 2004;279:34290–34297. doi: 10.1074/jbc.M404907200. [DOI] [PubMed] [Google Scholar]
- 59.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]