Abstract
Background
Some TGFB1 and VEGF polymorphisms are believed to be functional. Given that these genes are involved in tumor growth and progression including angiogenesis, dissemination, and invasiveness, we hypothesized that these polymorphisms would be associated with survival in patients with gastric cancer.
Methods
We genotyped TGFB1 -509 C>T, +1869 T>C, and +915 G>C and VEGF -1498T>C, -634G>C, and +936C>T in 167 patients with gastric cancer. Using the Kaplan and Meier method, log-rank tests, and Cox proportional hazard models, we evaluated associations among TGFB1 and VEGF variants with overall, 1-year, and 2-year survival rates.
Results
Although there were no significant differences in overall survival rates among all polymorphisms tested, patients with TGFB1+915CG and CC genotypes had a poorer 2-year survival (adjusted hazard ratio (HR), 3.06; 95% confidence interval (CI), 1.09–8.62; P = 0.034) than patients with the GG genotype had. In addition, patients heterozygous for VEGF -634CG also had a poorer 1-year survival (adjusted HR, 2.08; 95% CI, 1.03–4.22; P = 0.042) than patients with the -634GG genotype.
Conclusion
Our study suggested that TGFB1+915CG/CC and VEGF -634CG genotypes may be associated with short-term survival in gastric cancer patients. However, larger studies are needed to verify these findings.
Introduction
In gastric caner, patients with the same clinicopathologic characteristics and the same treatment regimens may have different clinical outcomes. Although stage is the best available clinical measure of tumor aggression and prognosis, there are clearly important differences even within the same tumor stage [1,2]. Therefore, it would be helpful to improve the prognostic accuracy by identifying readily accessible molecular markers that predict some of the variation in clinical outcomes. In recent decades, many studies have shown that genetic alterations play roles in the development and progression of gastric cancer [3]. Among these molecular markers, single nucleotide polymorphisms (SNPs) are the most commonly investigated genetic variation that may contribute to patients' clinical outcomes [4].
Epidemiologic and clinical investigations have suggested that both TGF-β1 and VEGF may play an important role in the oncogenesis of the stomach [5,6]. For example, TGFB1 and VEGF variants are associated with altered protein products, which may contribute to variation in individual susceptibility to cancer and clinical outcomes [4]. Both TGFB1 and VEGF genes are highly polymorphic, reportedly having 168 and 140 variants, respectively, but only few of these variants are within the promoter or coding regions that may be potentially functional http://www.ncbi.nlm.nih.gov/SNP/. Of these variants, several SNPs have been described as important in modulation of gene functions [7-9] and reportedly involved in the etiology of various cancers [10-13].
The TGF-β1 pathway is critically involved in tumor development and progression. In tumor cell cultures, TGF-β1 has anti-proliferative effects and can block tumor progression in its early stages, whereas it can also accelerates invasion and metastasis in the later stages of tumor progression [14,15]. One experimental study reported that TGF-β1-mediated activation of the ALK5-Smad 3 pathway is essential for the Shh protein to promote motility and invasiveness in gastric cancer cells [16]. Mouse experiments also showed that altered TGF-β1 was associated with the latent TGF-β1 binding proteins that can cause inflammation and tumors [17] and that the disrupted TGF-β1 pathway can lead to tumor growth by increasing the tumor angiogenesis induced by decreased expression of thrombospondin-1 [18]. In humans, TGF-β1 had a greater sensitivity than carcino-embryonic antigens in tumor cells from gastric cancer patients [16]. Furthermore, both experimental and clinico-pathological studies have suggested a role for the VEGF family of proteins in metastasis through the lymphatic system and in clinical outcomes in several human solid tumors, including gastric cancer [19].
In this study, we chose to genotype selected common (i.e., minor allele frequency > 0.05) TGFB1 and VEGF SNPs that either lead to non-synonymous amino acid changes [20] or have been associated with lower expression levels of these genes [8,21], which imply these SNPs may be functional. We hypothesized that potentially functional polymorphisms in TGFB1 and VEGF would be associated with clinical outcomes in patients with gastric cancer. Specifically, we evaluated the association between clinical outcomes in gastric cancer, including overall survival, and each of the following SNPs: three TGFB1 SNPs, including one promoter SNP (-509 C>T) and two exon 1 SNPs (+869 T>C and +915 G>C) and three VEGF SNPs, including one promoter SNP (-1498T>C), one 5'-untranslated region SNP (-634G>C) and one 3'-untranslated region SNP (+936 C>T).
Methods
Study population
This prospective analysis consisted of 167 patients with newly diagnosed and histologically confirmed gastric cancer, who were treated at The University of Texas M.D. Anderson Cancer Center, Houston, Texas between April 2003 and July 2008. The study protocol was approved by our Institutional Review Board (IRB) and all patients gave informed consent using the IRB-approved informed consent form. Exclusion criteria included those not newly diagnosed and those having been treated elsewhere before coming to M. D. Anderson. These patients were included in this analysis because their stored blood samples were available for DNA extraction.
Genotyping
Genomic DNA was extracted from the buffy coat fraction of the blood sample of each patient by using a Blood Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. DNA purity and concentrations were determined by spectrophotometric measurement of absorbance at 260 and 280 nm by UV spectrophotometer. The three selected TGFB1 SNPs [one (-509 C>T/rs1800469) in the promoter and two (+869 T>C/rs1800470 and +915 G>C/rs1800471) in exon 1] and three promoter VEGF SNPs [one (-1498T>C/rs833061) in the promoter, one (-634G>C/rs2010963) in the 5'-untranslated region, and one (+936C>T/rs3025039) in the 3'-untranslated region] were genotyped using polymerase chain reaction(PCR) – restriction fragment length polymorphism (RFLP) method. Genotypes of the TGFB1 SNPs were determined as previously described[22], and assays on the VEGF SNPs were also previously reported [23]. For the PCR-RFLP-based genotyping assay, two research assistants independently read the gel pictures, and the repeated assays were performed, if they did not agree on the tested genotype. In addition, repeated assays were performed on a randomly selected 10% of the samples were randomly selected to perform the repeated assays with the results being 100% concordant.
Outcome data collection
All 167 gastric cancer patients had available follow-up data on outcome. The overall survival time was calculated from the date of registration at M.D. Anderson to the date of last contact or death. Patients who were still alive at the last contact were considered as a censored event in the analysis. The age at diagnosis, sex, and type of treatments (i.e., surgery and chemotherapy) were used as covariates in the analysis. The age at diagnosis was categorized into two groups according to the mean age (≤ 57 and >57 years).
Statistical Analysis
Two-sided chi-square and t tests were performed to determine any statistically significant differences in the distributions of categorical variables (e.g., the TGFB1 and VEGF alleles and genotypes) by demographic variables and clinical features and in the means of continuous variables (e.g., age and survival time), respectively. The distributions of the genotypes were tested for deviation from Hardy-Weinberg equilibrium (HWE), and the haplotypes for the variants of the same gene were reconstructed according to the PHASE program [9], by which each individual's probability of having a particular haplotype pair was estimated, and the haplotype pair with the highest estimated probability was assigned to the individual. Pearson's chi-square or global test was used to test for the survival differences among patients by all haplotypes. Overall survivals among the three genotype groups of each SNP were analyzed using the Kaplan-Meier method, and the log-rank test was used to test for the equality of the survival distributions stratified by genotypes. We used univariate and multivariate Cox proportional hazards models to estimate the effect of each genotype on survival in the presence of other covariates. Both age at diagnosis and the time interval between registration and diagnosis date (pathologic confirmation of disease) were treated as numeric covariates in the Cox model. To confirm the assumption of proportional hazards in a Cox regression model, we added a time-dependent variable to the model, and the assumption was confirmed. Hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were calculated with adjustment for other covariates in the same model. The joint effects of the TGFB1 and VEGF SNPs and their interactions with smoking and drinking on gastric cancer risk were also evaluated. All statistical tests were 2-sided, with a P value of 0.05 considered significant and all were calculated using SAS software (version 9.1; SAS Institute, Cary, NC).
Results
Characteristics of the study population
Clinical and pathological characteristics of the 167 patients enrolled in this study are shown in Table 1. There were 114 males (68.3%) and 53 females (31.7%), whose ages ranged from 32 to 89 years. Using the Cox regression analysis of the relationship between overall survival and clinicopathologic characteristics, we found that neither age, gender, ethnicity, smoking, nor alcohol status were statistically associated with overall survival (P = 0.339, 0.988, 0.297, 0.475, 0.809, respectively).
Table 1.
Characteristics of the study population of patients with gastric cancer
| Characteristics | No. of Patients | No. of Deaths | MST (months) | P* |
|
Total subjects Age (mean) |
167 | 60 | 0.339 | |
| ≤57 years | 68 | 27 | 21.2 | |
| >57 years | 99 | 33 | 31.0 | |
| Gender | 0.988 | |||
| Male | 114 | 41 | 23.3 | |
| Female | 53 | 19 | 28.9 | |
| Ethnicity | 0.297 | |||
| White | 117 | 45 | 28.8 | |
| Non-White† | 50 | 15 | 19.1 | |
| Smoke | 0.475 | |||
| Never | 34 | 14 | 20.6 | |
| Ever | 133 | 46 | 30.1 | |
| Alcohol | 0.809 | |||
| Never | 62 | 23 | 23.2 | |
| Ever | 105 | 37 | 29.3 | |
| Location | 0.069 | |||
| Stomach | 118 | 36 | 24.3 | |
| Esophagus | 25 | 13 | 27.2 | |
| GEJ | 24 | 11 | 16.6 | |
| Histology | 0.356 | |||
| Intestinal | 118 | 45 | 28.1 | |
| Signet ring | 49 | 15 | 24.6 | |
| Differentiation | 0.694 | |||
| Poor | 96 | 37 | 21.8 | |
| Moderate-poor | 28 | 10 | 29.8 | |
| Moderate-Well | 42 | 13 | 22.6 | |
| Clinical Stage | < 0.001 | |||
| I + II | 65 | 9 | 30.4 | |
| III + IV | 101 | 51 | 22.7 | |
| Metastasis | < 0.001 | |||
| yes | 90 | 49 | 21.2 | |
| no | 77 | 11 | 34.2 | |
| Chemotherapy | < 0.001 | |||
| yes | 121 | 54 | 26.3 | |
| no | 46 | 6 | 10.4 | |
| Surgery | < 0.001 | |||
| yes | 63 | 11 | 39.2 | |
| no | 104 | 49 | 18.4 |
Abbreviations: MST, median survival time; GEJ, gastroesophageal junction.
* Chi-square test.
†Included 13 Asians, 16 blacks, 19 Hispanics, and 2 Native Indians.
The tumors of 118 (70.7%) the patients were located at the stomach and those of 49 (29.3%) patients were located at the gastroesophageal junction (GEJ). Regardless of tumor location, all the patients had adenocarcinoma. Of these, 118 (70.7%) patients were intestinal and 49 (29.3%) signet ring. We grouped the types of differentiation into the following three categories: poor, moderate-poor and moderate-well, and the number and percentage of these three groups were 96 (57.8%), 28 (16.9%) and 42 (25.3%), respectively. In all patients, clinico-pathological characteristics including tumor location, histology and differentiation status were not significantly associated with overall survival in the univariate analysis (P = 0.069, 0.356, and 0.694, respectively). Clinical tumor stages according to the International Union Against Cancer (UICC) criteria were as follows: 65 (38.9%) had stage I+II and 101 (61.1) had stage III +IV (Table 1).
Among the 167 patients, 121 (72.4%) received chemotherapy, and 63 (37.7%) received surgery; at the end of the follow-up period, 60 (35.9%) patients had died. The mean follow-up time was 18.0 ± 13.3 months for the patients who were still alive, and the mean survival time for all patients was 29.4 months. Advanced stage, metastasis, chemotherapy and surgery were all associated with overall survival (P < 0.001 for all) (Table 1). For example, the mean survival time was 34.2 months for patients without metastasis and 21.2 months for those with metastasis. Those who received chemotherapy and surgery had a longer mean survival time than those who did not (26.3 months versus 10.4 months for chemotherapy and 39.2 months versus 18.4 months for surgery).
HWE, linkage disequilibrium and haplotypes TGFB1 and VEGF
For TGFB1, one of the three SNPs (rs1800469C>T, rs1800470T>C and rs1800471G>C) was not in HWE (P < 0.05 for rs1800469C>T), suggesting a possible selection bias, but none of the VEGF SNPs (rs833061T>C, rs2010963G>C and rs3025039C>T) departed from HWE (P > 0.05 for all). None of the pairs of TGFB1 or VEGF SNPs were in high linkage disequilibrium (i.e., r2 between 0.039 and 0.541, all <0.08). Only four TGFB1 haplotypes and five VEGF haplotypes had an allele frequency of >0.05 (C-T-G, 0.570; C-C-G, 0.190; T-C-G, 0.167 and C-C-C, 0.063 for TGFB1 and C-G-C, 0.344; T-C-C, 0.287; T-G-C, 0.192; C-G-T, 0.072 and T-C-T, 0.051 for VEGF). Because of the small sample size, we did not calculate the diplotypes.
TGFB1 and VEGF genotype distributions and overall survival
When all gastric cancer patients were analyzed for overall survival, no significant difference was found in the distributions of mean survival time by genotypes for any of the polymorphisms studied. Because there were few participants in the minor homozygous variant groups, we combined the heterozygous and minor variant homozygous genotypes together for additional analysis, assuming a dominant genetic model, but there was still no association between detected polymorphisms and overall survival (see Additional file 1). Furthermore, when the gastric cancer patients were stratified by age, sex, ethnicity, and metastatic status, no difference in the distribution according to mean survival time by the six SNPs was found among the subgroups (see Additional file 1).
TGFB1 and VEGF genotype distributions and 1-and 2-year survivals
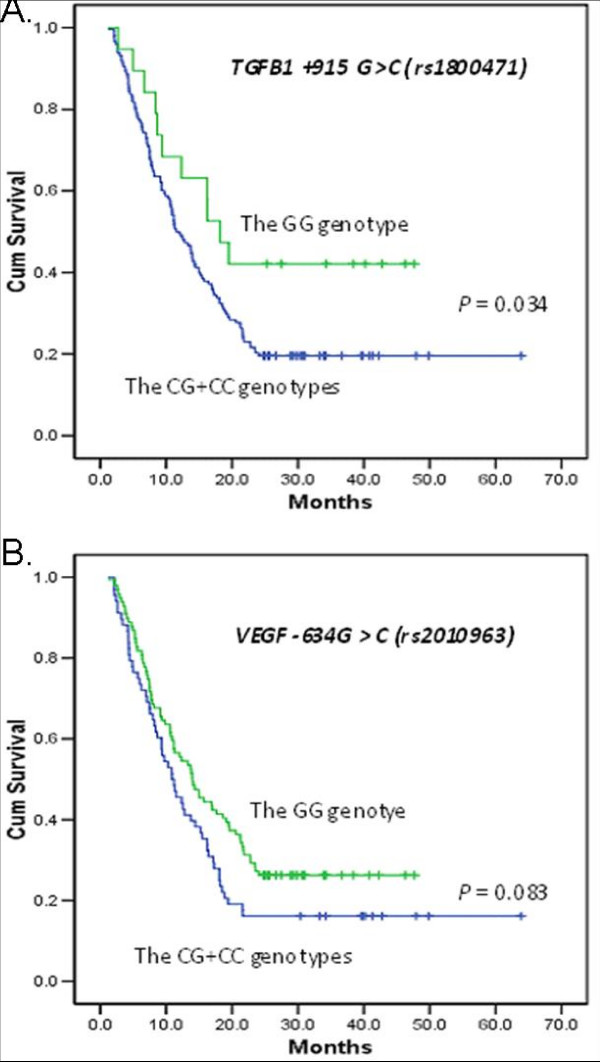
Because the prognosis is generally poor in advanced cases of gastric cancer, median survival rarely approaches 1 or 2 years [2]. In the present study, most of the cases were stage IV (101/167) with a median survival time of only 16.2 months (95% CI, 12.8–24.9). Therefore, we also calculated the 1-year and 2-year survival rates for patients with different genotypes (see Additional file 2). The overall 1-year and 2-year survivals for all patients were 51.5% and 22.1%, respectively. Although there were no significant differences in the survival rates between most genotypes, patients with TGFB1 +915CG/CC genotypes had better 1-year and 2-year survival than those with the GG genotype (adjusted HR, 2.13; 95% CI, 0.76–6.01; P = 0.122 and adjusted HR, 3.06; 95% CI, 1.09–8.62; P = 0.034, respectively) (Figure 1). Furthermore, patients heterozygous for VEGF -634CG also had a better 1-year survival rate (adjusted HR, 2.08; 95% CI, 1.03–4.22; P = 0.042) than those with the VEGF -634 GG genotype.
Figure 1.
Cumulative survival functions of the genotypes TGFB1 +915 G>C (rs1800471) and VEGF -634G>C (rs2010963).
Further analyses combining the alleles, genotypes, and haplotypes of the same gene did not substantiate the findings from the single locus analysis (data not shown), partly because of the low LD among the SNPs and the small study size that did not allow for further stratification analysis.
Discussion
The etiology of gastric cancer is multifactorial, multigenetic and multistage [24,25]. It is known that during carcinogenesis, TGF-β can switch from a tumor suppressor to a tumor enhancer in the later stages of cancer [26]. With dual role in cancer development, there is great interest in analyzing the role of genetic variation in TGFB1 in cancer progression and patient survival. For example, the TGFB1 -509C>T and rs1982073 (or rs1800470) polymorphisms have been shown to be associated with breast cancer survival in a Chinese population [27-30] and chemoradiotherapy response in 175 Finnish patients with head and neck squamous cancer[31], respectively. However, neither TGFB1 +869T>C nor +915G>C polymorphisms showed any association with tumor relapse and progression in bladder tumors without muscular invasive in a Spanish population [32]. While a Korean study showed that the variant T genotypes of the TGFB1 -509C>T SNP were associated with a reduced risk of lung cancer [33], a Chinese study of 414 patients and 414 controls [34] reported that the genotypes were not associated with an overall risk of developing gastric cancer but with a decreased risk of risk of stage I or II gastric cancer. However, no survival analyses were presented in these studies.
As noted, we did not find any statistical evidence to support a significant association between TGFB1polymorphisms and overall survival in gastric cancer. However, the significant association between TGFB1+915 CG/CC genotypes and 2-year survival for all gastric cancer patients suggests that this TGFB1variant may have attenuated the role of TGF-β1 as a tumor suppressor in the earlier stage of tumor progression. It is also known that TGF-β1 can switch from a tumor suppressor to a tumor enhancer in the late stage of cancer [26]. Once the tumors had grown bigger and become metastatic, the resultant increase in somatic mutations or gains in the copies of oncogenes may have outweighed the role of the suppressor variants in the late stages of the tumor, leading to no difference in overall survival of the patients with different genotypes of the TGFB1+915 G>C SNP. However, this speculation needs to be validated in more rigorously designed studies with a much larger sample size and more information on the mutation spectrum in the tumors.
VEGF, as a key mediator of angiogenesis, also plays an important role in the development of cancers. VEGF polymorphisms have also been shown to be associated with survival in both gastric cancer and colorectal cancer [35,36]. However, the results from published studies remain inconsistent rather than conclusive. In a Greek gastric cancer study of five VEGF SNPs (-2578C>A, -1154G>A, -634G>C, -460T>C, and +936C>T) in 312 patients [36], VEGF -2578 AA, -634 CC and +936TT genotypes were associated with a significantly lower HR (better survival) for 6-year survival of colorectal cancers. Interestingly, another early Greek study of 100 gastric cancer patients suggested that only the VEGF -634CC/CG genotypes were associated with a decreased (poorer survival) 10-year survival, compared with the GG genotype [35]. Our data on 167 gastric cancer patients indicated that VEGF -634CC/CG carriers indeed had a poor 1-year survival than those with the VEGF -634 GG genotype. Amano et al. [37] also reported that no significant association was observed between the frequencies of the VEGF -460T>C, +405G>C, and 936C>T genotypes and 3-year disease-free survival of endometrial carcinoma patients in a Japanese study of 105 endometrial carcinoma patients. Because all these studies, including ours, have been relatively small, there was limited ability to perform the more powerful haplotype-based analysis that the analysis of a single allele or locus effect [34].
This is the first report, to our knowledge, involving TGFB1 and VEGF polymorphisms and survival in gastric cancer patients mainly consisting of a Caucasian population; however, there were some limitations to the present study. Although we tried to collect recurrence data on these patients, we could not investigate this end-point due to the lack of a pre-defined follow-up plan. A second limitation was the fact that we only included three common TGFB1 SNPs and three VEGF SNPs. It is possible that some other important SNPs were missed or that the observed associations may be due to other polymorphisms in LD with the SNPs we studied. Also, no data on serum/plasma protein levels were available for the genotype-phenotype correlation analysis, because only DNA samples were available from these patients. There are other genes in addition to TGFB1 and VEGF that also play a role in cell growth and angiogenesis, representing a complex interplay of many activating and inhibitory factors [38]. Furthermore, Helicobacter pylori infection, the presence or absence of which was not reported in the present study, is considered to be the cause of a progressive accumulation of genotypic changes in gastric cancer, which may lead to sporadic gastric cancer carcinogenesis [39]. Finally, the study size was too small to have a sufficient power to detect small HRs. For example, our post-power calculation suggested that the sample size for an equal number (n = 55) of subjects in each genotype of each SNP, the power to detect an HR of 2 was <0.4, but >0.8 for a HR of 3.4 for a follow-up time of 5 years. Therefore, only the finding of HRs for 2-year survival of TGFB1 +915G>C would have a sufficient power, suggesting a much larger study would be needed to effectively test our hypothesis for effects of the overall survival.
Conclusion
In summary, we found that some polymorphisms TGFB1 and VEGF may be associated with 1- or 2-year survival rates of gastric cancer patients. However, the present study is small, and various genetic and epigenetic events may also have led to an association between TGFB1 and VEGF polymorphisms and gastric cancer prognosis and survival. Therefore, larger and better-designed studies are required to overcome the limitations in the present study (particularly the information about Helicobacter pylori infection) and further confirm our observations.
Abbreviations
TGF-β1: Transforming growth factor beta 1; VEGF: Vascular endothelial growth factor; LD: linkage disequilibrium; PCR-RFLP: polymerase chain reaction-restriction fragment length polymorphism; OR: odds ratio; HR: hazard ratio; CI: confidence interval; SNP: single nucleotide polymorphism.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
XG performed the laboratory work, acquisition of data, and drafted the manuscript. HZ performed statistical analysis and read the manuscript. JN assisted in performing laboratory work, statistical analysis and proofreading of the manuscript. DT and JAA performed the patient and pathological evaluation and read the manuscript. QW conceived and coordinated the study, checked statistical results, read and edited the manuscript. All the authors read and approved the final manuscript.
Supplementary Material
TGFB1 and VEGF genotype distributions and overall survival. The data provided represent the statistical analysis of TGFB1 and VEGF genotype distributions and overall survival.
TGFB1 and VEGF genotype distributions and 1-and 2-year survivals. The data provided represent the statistical analysis of TGFB1 and VEGF genotype distributions and 1-and 2-year survivals.
Acknowledgments
Acknowledgements
This study was supported in part by National Institutes of Health grants R01 ES 11740-07 and CA 131274-01 (to Q. W.) and CA 16672 (to M. D. Anderson Cancer Center). We thank Margaret Lung and Kathryn Patterson for their assistance in recruiting the subjects; Li-E Wang, Zhensheng Liu, Yawei Qiao, Min Zhao, Jianzhong He, and Kejin Xu for their laboratory assistance; and Diane Hackett and Maude Veechfor for scientific editing.
Contributor Information
Xiaoxiang Guan, Email: Xguan@mdanderson.org.
Hui Zhao, Email: huizhao@mdanderson.org.
Jiangong Niu, Email: jniu@mdanderson.org.
Dongfeng Tan, Email: Dtan@mdanderson.org.
Jaffer A Ajani, Email: jajani@mdanderson.org.
Qingyi Wei, Email: qwei@mdanderson.org.
References
- Rohde H, Gebbensleben B, Bauer P, Stutzer H, Zieschang J. Has there been any improvement in the staging of gastric cancer? Findings from the German Gastric Cancer TNM Study Group. Cancer. 1989;64:2465–2481. doi: 10.1002/1097-0142(19891215)64:12<2465::AID-CNCR2820641212>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009 doi: 10.1016/j.critrevonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Becker KF, Keller G, Hoefler H. The use of molecular biology in diagnosis and prognosis of gastric cancer. Surg Oncol. 2000;9:5–11. doi: 10.1016/S0960-7404(00)00016-5. [DOI] [PubMed] [Google Scholar]
- Wu GY, Hasenberg T, Magdeburg R, Bonninghoff R, Sturm JW, Keese M. Association between EGF, TGF-beta1, VEGF gene polymorphism and colorectal cancer. World J Surg. 2009;33:124–129. doi: 10.1007/s00268-008-9784-5. [DOI] [PubMed] [Google Scholar]
- Li T, Cao BW, Dai Y, Cui H, Yang HL, Xu CQ. Correlation of transforming growth factor beta-1 gene polymorphisms C-509T and T869C and the risk of gastric cancer in China. J Gastroenterol Hepatol. 2008;23:638–642. doi: 10.1111/j.1440-1746.2008.05324.x. [DOI] [PubMed] [Google Scholar]
- Liu DH, Zhang XY, Fan DM, Huang YX, Zhang JS, Huang WQ, Zhang YQ, Huang QS, Ma WY, Chai YB, Jin M. Expression of vascular endothelial growth factor and its role in oncogenesis of human gastric carcinoma. World J Gastroenterol. 2001;7:500–505. doi: 10.3748/wjg.v7.i4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Webb NJ, Bottomley MJ, Brenchley PE. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine. 2000;12:1232–1235. doi: 10.1006/cyto.2000.0692. [DOI] [PubMed] [Google Scholar]
- Renner W, Kotschan S, Hoffmann C, Obermayer-Pietsch B, Pilger E. A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res. 2000;37:443–448. doi: 10.1159/000054076. [DOI] [PubMed] [Google Scholar]
- Stevens A, Soden J, Brenchley PE, Ralph S, Ray DW. Haplotype analysis of the polymorphic human vascular endothelial growth factor gene promoter. Cancer Res. 2003;63:812–816. [PubMed] [Google Scholar]
- Lee KM, Park SK, Hamajima N, Tajima K, Yoo KY, Shin A, Noh DY, Ahn SH, Hirvonen A, Kang D. Genetic polymorphisms of TGF-beta1 & TNF-beta and breast cancer risk. Breast Cancer Res Treat. 2005;90:149–155. doi: 10.1007/s10549-004-3859-2. [DOI] [PubMed] [Google Scholar]
- Nikolova PN, Pawelec GP, Mihailova SM, Ivanova MI, Myhailova AP, Baltadjieva DN, Marinova DI, Ivanova SS, Naumova EJ. Association of cytokine gene polymorphisms with malignant melanoma in Caucasian population. Cancer Immunol Immunother. 2007;56:371–379. doi: 10.1007/s00262-006-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell WM, Bateman AC, Turner SJ, Collins A, Theaker JM. Influence of vascular endothelial growth factor single nucleotide polymorphisms on tumour development in cutaneous malignant melanoma. Genes Immun. 2002;3:229–232. doi: 10.1038/sj.gene.6363851. [DOI] [PubMed] [Google Scholar]
- Lin CC, Wu HC, Tsai FJ, Chen HY, Chen WC. Vascular endothelial growth factor gene-460 C/T polymorphism is a biomarker for prostate cancer. Urology. 2003;62:374–377. doi: 10.1016/S0090-4295(03)00268-1. [DOI] [PubMed] [Google Scholar]
- Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006;25:435–457. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008;29:480–490. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Obata H, Jurukovski V, Mazzieri R, Chen Y, Zilberberg L, Huso D, Melamed J, Prijatelj P, Todorovic V, Dabovic B, Rifkin DB. Perturbation of transforming growth factor (TGF)-beta1 association with latent TGF-beta binding protein yields inflammation and tumors. Proc Natl Acad Sci USA. 2008;105:18758–18763. doi: 10.1073/pnas.0805411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro A, Yashiro M, Iwata C, Morishita Y, Johansson E, Matsumoto Y, Watanabe A, Aburatani H, Miyoshi H, Kiyono K, Shirai YT, Suzuki HI, Hirakawa K, Kano MR, Miyazono K. Diffuse-type gastric carcinoma: progression, angiogenesis, and transforming growth factor beta signaling. J Natl Cancer Inst. 2009;101:592–604. doi: 10.1093/jnci/djp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirlis TD, Papastratis G, Masselou K, Tsigris C, Papachristodoulou A, Kostakis A, Nikiteas NI. Circulating lymphangiogenic growth factors in gastrointestinal solid tumors, could they be of any clinical significance? World J Gastroenterol. 2008;14:2691–2701. doi: 10.3748/wjg.14.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthanthiran M, Li B, Song JO, Ding R, Sharma VK, Schwartz JE, August P. Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: A novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci USA. 2000;97:3479–3484. doi: 10.1073/pnas.050420897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai R, Gong MN, Zhou W, Thompson TB, Kraft P, Su L, Christiani DC. Genotypes and haplotypes of the VEGF gene are associated with higher mortality and lower VEGF plasma levels in patients with ARDS. Thorax. 2007;62:718–722. doi: 10.1136/thx.2006.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Liao Z, Liu Z, Wang LE, Tucker SL, Mao L, Wang XS, Martel M, Komaki R, Cox JD, Milas L, Wei Q. Single Nucleotide Polymorphism at rs1982073:T869C of the TGF{beta}1 Gene Is Associated With the Risk of Radiation Pneumonitis in Patients With Non-Small-Cell Lung Cancer Treated With Definitive Radiotherapy. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.20.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Lee SY, Jeon HS, Park SH, Jang JS, Lee GY, Son JW, Kim CH, Lee WK, Kam S, Park RW, Park TI, Kang YM, Kim IS, Jung TH, Park JY. Vascular endothelial growth factor gene polymorphisms and risk of primary lung cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:571–575. doi: 10.1158/1055-9965.EPI-04-0472. [DOI] [PubMed] [Google Scholar]
- Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/S0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- Gao L, Nieters A, Brenner H. Meta-analysis: tumour invasion-related genetic polymorphisms and gastric cancer susceptibility. Aliment Pharmacol Ther. 2008;28:565–573. doi: 10.1111/j.1365-2036.2008.03760.x. [DOI] [PubMed] [Google Scholar]
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- Shu XO, Gao YT, Cai Q, Pierce L, Cai H, Ruan ZX, Yang G, Jin F, Zheng W. Genetic polymorphisms in the TGF-beta 1 gene and breast cancer survival: a report from the Shanghai Breast Cancer Study. Cancer Res. 2004;64:836–839. doi: 10.1158/0008-5472.CAN-03-3492. [DOI] [PubMed] [Google Scholar]
- Dunning AM, Ellis PD, McBride S, Kirschenlohr HL, Healey CS, Kemp PR, Luben RN, Chang-Claude J, Mannermaa A, Kataja V, Pharoah PD, Easton DF, Ponder BA, Metcalfe JC. A transforming growth factorbeta1 signal peptide variant increases secretion in vitro and is associated with increased incidence of invasive breast cancer. Cancer Res. 2003;63:2610–2615. [PubMed] [Google Scholar]
- Le Marchand L, Haiman CA, Berg D van den, Wilkens LR, Kolonel LN, Henderson BE. T29C polymorphism in the transforming growth factor beta1 gene and postmenopausal breast cancer risk: the Multiethnic Cohort Study. Cancer Epidemiol Biomarkers Prev. 2004;13:412–415. [PubMed] [Google Scholar]
- Shin A, Shu XO, Cai Q, Gao YT, Zheng W. Genetic polymorphisms of the transforming growth factor-beta1 gene and breast cancer risk: a possible dual role at different cancer stages. Cancer Epidemiol Biomarkers Prev. 2005;14:1567–1570. doi: 10.1158/1055-9965.EPI-05-0078. [DOI] [PubMed] [Google Scholar]
- Lundberg M, Pajusto M, Koskinen WJ, Makitie AA, Aaltonen LM, Mattila PS. Association between transforming growth factor beta1 genetic polymorphism and response to chemoradiotherapy in head and neck squamous cell cancer. Head Neck. 2009;31:664–672. doi: 10.1002/hed.21014. [DOI] [PubMed] [Google Scholar]
- Castillejo A, Rothman N, Murta-Nascimento C, Malats N, Garcia-Closas M, Gomez-Martinez A, Lloreta J, Tardon A, Serra C, Garcia-Closas R, Chanock S, Silverman DT, Dosemeci M, Kogevinas M, Carrato A, Soto JL, Real FX. TGFB1 and TGFBR1 polymorphic variants in relationship to bladder cancer risk and prognosis. Int J Cancer. 2009;124:608–613. doi: 10.1002/ijc.24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HG, Chae MH, Park JM, Kim EJ, Park JH, Kam S, Cha SI, Kim CH, Park RW, Park SH, Kim YL, Kim IS, Jung TH, Park JY. Polymorphisms in TGF-beta1 gene and the risk of lung cancer. Lung Cancer. 2006;52:1–7. doi: 10.1016/j.lungcan.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Jin G, Wang L, Chen W, Hu Z, Zhou Y, Tan Y, Wang J, Hua Z, Ding W, Shen J, Zhang Z, Wang X, Xu Y, Shen H. Variant alleles of TGFB1 and TGFBR2 are associated with a decreased risk of gastric cancer in a Chinese population. Int J Cancer. 2007;120:1330–1335. doi: 10.1002/ijc.22443. [DOI] [PubMed] [Google Scholar]
- Tzanakis N, Gazouli M, Rallis G, Giannopoulos G, Papaconstantinou I, Theodoropoulos G, Pikoulis E, Tsigris C, Karakitsos P, Peros G, Nikiteas N. Vascular endothelial growth factor polymorphisms in gastric cancer development, prognosis, and survival. J Surg Oncol. 2006;94:624–630. doi: 10.1002/jso.20619. [DOI] [PubMed] [Google Scholar]
- Dassoulas K, Gazouli M, Rizos S, Theodoropoulos G, Christoni Z, Nikiteas N, Karakitsos P. Common polymorphisms in the vascular endothelial growth factor gene and colorectal cancer development, prognosis, and survival. Mol Carcinog. 2009;48:563–569. doi: 10.1002/mc.20495. [DOI] [PubMed] [Google Scholar]
- Amano M, Yoshida S, Kennedy S, Takemura N, Deguchi M, Ohara N, Maruo T. Association study of vascular endothelial growth factor gene polymorphisms in endometrial carcinomas in a Japanese population. Eur J Gynaecol Oncol. 2008;29:333–337. [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/S0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Nardone G, Compare D. Epigenetic alterations due to diet and Helicobacter pylori infection in gastric carcinogenesis. Expert Rev Gastroenterol Hepatol. 2008;2:243–248. doi: 10.1586/17474124.2.2.243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TGFB1 and VEGF genotype distributions and overall survival. The data provided represent the statistical analysis of TGFB1 and VEGF genotype distributions and overall survival.
TGFB1 and VEGF genotype distributions and 1-and 2-year survivals. The data provided represent the statistical analysis of TGFB1 and VEGF genotype distributions and 1-and 2-year survivals.